Abstract
This study demonstrates that the therapeutic effect of a nitric oxide inhibitor in a murine model of fecal peritonitis is mediated in part by increased levels of interleukin-10 (IL-10) and monocyte chemoattractant protein 1 (MCP-1). Female CD1 mice were subjected to cecal ligation and puncture (CLP) with a 21-gauge needle and, immediately following surgery, were injected intraperitoneally with saline, NG-nitro-l-arginine methyl ester (l-NAME; 8 mg/kg), or NG-nitro-d-arginine methyl ester (d-NAME; 8 mg/kg). At 96 h after surgery and drug treatment, 20% of mice that received d-NAME had survived whereas 60% of mice that received l-NAME were alive. To elucidate the effect of l-NAME treatment on chemokine and cytokine production during fecal peritonitis, the levels of macrophage inflammatory protein 2 (MIP-2), IL-10, and MCP-1 were measured in peritoneal washings from additional groups of mice 24 h after the CLP surgery. Peritoneal fluids from l-NAME-treated mice contained significantly higher levels of IL-10 and MCP-1 than did those from d-NAME-treated mice. To elucidate the effect of nitric oxide inhibition on potential cellular sources of IL-10 and MCP-1 in the CLP model, cultured alveolar and peritoneal macrophages were activated with bacterial lipopolysaccharide in the presence of l-NAME; these macrophages produced significantly more MCP-1 than did similarly activated macrophages in the presence of d-NAME. In the CLP surgery model, immunoneutralization of IL-10 alone or IL-10 and MCP-1 together with polyclonal antibodies prior to surgery significantly reduced the survival rates in l-NAME-treated groups compared with l-NAME-treated groups that received preimmune serum. Taken together, these data demonstrate that the inhibition of nitric oxide following experimental CLP fecal peritonitis is therapeutic, in part through the modulatory effect of this treatment on the synthesis of IL-10 and MCP-1.
Following exposure to bacterial by-products and/or secondarily elicited inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), and gamma interferon, macrophages, neutrophils, endothelial and smooth muscle cells express the inducible isoform of nitric oxide synthase type II (NOS II) (16). In contrast to constitutively expressed isoforms of NOS found in neurons and endothelial cells, NOS II is a calcium-independent NOS which generates large quantities of nitric oxide over extended periods. Increased nitric oxide synthesis following NOS II induction exerts a critical role in host defense against viral and bacterial pathogens and plays a significant role in the containment of tumor growth, but elevated levels of nitric oxide also exert deleterious effects in many acute inflammatory responses and chronic diseases (15). For example, increased nitric oxide synthesis during sepsis contributes significantly to the hypotension that accompanies sepsis syndrome (12, 13, 26). However, NOS inhibitor treatment of sepsis has yielded mixed results, possibly because normal vasoregulation requires constitutive nitric oxide production and this physiologic role of nitric oxide is also impaired by NOS inhibitors (for a review, see reference 11). Although augmented nitric oxide synthesis during sepsis exerts marked effects on the cardiovascular system, little is known about the role of increased nitric oxide levels on the inflammatory cascade associated with sepsis.
Previous studies have demonstrated that the selective targeting of proinflammatory cytokines such as IL-1 and TNF-α fail to attenuate the sequlae associated with sepsis (25). More recently, it has been postulated that effective therapy for sepsis syndrome may be found in manipulating the inflammatory response so as to restore the balance between proinflammatory and anti-inflammatory cytokines. A key cytokine that appears to restore this balance during sepsis is interleukin-10 (IL-10). IL-10 has been shown to enhance survival in a number of experimental toxin-induced shock models through its inhibition of the synthesis of many proinflammatory cytokines (4, 10, 24). Interestingly, IL-10 also has a very potent inhibitory effect on NOS II expression in macrophages (9). The chemoattractant cytokines or chemokines also exert potent modulatory roles in models of sepsis. The C-X-C chemokine, macrophage inflammatory protein 2 (MIP-2), is a strong chemoattractant for polymorphonuclear cells and is greatly upregulated following murine fecal peritonitis induced by cecal ligation and puncture (CLP) (29). Further, immunoneutralization of MIP-2 largely prevented the mortality observed in this model, presumably through a reduction in neutrophil extravasation into the inflamed peritoneal cavity. Recent data suggests that the exogenous administration of the C-C chemokine, monocyte chemoattractant protein 1 (MCP-1), markedly reduces mortality in a murine model of endotoxemia (31). Overall, the protective effects afforded by exogenous addition or immunoneutralization of modulatory cytokines or chemokines may be related to the fact that these interventions restore the balance between pro- and anti-inflammatory mediators.
The aims of the present study were threefold: (i) to determine whether the inhibition of nitric oxide affects mouse survival following CLP surgery; (ii) to characterize changes in the levels of MIP-2, IL-10, and MCP-1 following NG-nitro-l-arginine methyl ester (l-NAME) treatment in this sepsis model, and (iii) to assess the role of IL-10 and MCP-1 in mice treated with either l-NAME or NG-nitro-d-arginine methyl ester (d-NAME) after CLP surgery. The CLP surgery model was used because it provides many advantages over the commonly used endotoxemia models since it recapitulates the clinical septic situation in which bowel contents escape into the peritoneal cavity following trauma or surgical manipulation and it exhibits the bacterial infectious process associated with clinical sepsis (3).
MATERIALS AND METHODS
CLP model.
Female CD-1 mice (6 to 8 weeks of age) were purchased from Jackson Laboratory (Bar Harbor, Maine) and maintained under specific-pathogen-free conditions with free access to water and food. As previously described in detail (28), the mice were lightly anesthetized with ketamine HCl (Ketaset; Fort Dodge Laboratories) and placed in the prone position. Methoxyflurane (Metafane; Pitman-Moore Inc.) was used to deepen the anesthesia, and the cecum was exteriorized through a 2-cm midline incision. The distal one-third of the cecum was loosely ligated with 3-0 silk suture so that a patent opening remained between the distal portion of the cecum and the remainder of the bowel. The wall of the ligated cecum was compromised by a through-and-through puncture with a 21-gauge needle. The ligated and perforated cecum was restored to the peritoneal cavity, and the surgical incision was closed with stainless steel wound clips (Becton Dickinson and Co., Sparks, Md.). Finally, all the mice received normal saline subcutaneously and were placed in clean cages that were situated next to a heating lamp during their recovery from surgery. The mice that failed to recover from surgery (<1%) were excluded from the remainder of the study.
l-NAME treatment.
Immediately after the CLP surgery, groups of 7 to 10 mice received saline, l-NAME, or d-NAME via an intraperitoneal (i.p.) injection. The mode of administration and an approximate concentration of NOS inhibitor that could be administered in vivo were obtained from a previous study by Chan et al. (6). While Chan et al. (6) used NG-monomethyl-l-arginine (l-NMMA), we opted to use l-NAME because of its greater in vivo potency (20). Our objective in the inhibition of nitric oxide in the CLP model was to target primarily the area of increased nitric oxide production (i.e., the peritoneal cavity) during the time point when the nitric oxide level is markedly enhanced (i.e., the first 24 h). In our pilot study, we observed that l-NAME at 8 mg/kg was tolerated by the mice and that this dose of l-NAME reduced nitrite and nitrate levels (see below) in the peritoneal cavity by 75%. d-NAME at 8 mg/kg was administered via the same route; d-NAME is a structural enantiomer of l-NAME that lacks NOS-inhibitory actions (20). Over the subsequent 96 h, the mice were monitored daily for changes in survival rates. In a separate experiment, mice (five per treatment group) were euthanized after 24 h and the peritoneal cavity was lavaged with 2 ml of sterile saline containing 25 mM EDTA. Peritoneal samples were collected because the peritoneal cavity is the focal point of the inflammatory response and previous studies have shown that peritoneal cytokine levels peak at 24 h in this model (28, 29). Peritoneal washings were collected into Eppendorf tubes and stored at −20°C prior to determination of nitrite and nitrate levels by the Griess reaction and determination of MIP-2, IL-10, and MCP-1 levels by enzyme-linked immunosorbent assay (ELISA).
Isolation of alveolar and peritoneal macrophages.
We next determined whether the inhibition of nitric oxide directly affected the synthesis of IL-10 and MCP-1 by macrophages. Previous studies have shown that macrophages produce IL-10 and MCP-1 following lipopolysaccharide (LPS) stimulation (2, 7, 21). Normal CD-1 mice were killed with a Metafane overdose, and 2 ml of cold (4°C) sterile saline containing 25 mM EDTA (saline-EDTA) was injected into the lungs. Approximately 10 ml of bronochoalveolar lavage fluid was collected from each mouse by multiple lung washings with saline-EDTA. Peritoneal macrophages were obtained from the same mice by injecting 2 ml of saline-EDTA into the abdomen, and this solution was removed by sterile pipetting through a midline incision. The alveolar and peritoneal washes were separately distributed to 150-mm tissue culture plates and were placed into a 37°C CO2 incubator for 1 h. All nonadherent cells were subsequently removed, and the adherent cells were briefly treated with 0.250% trypsin. The latter cells were diluted to 5.0 × 105 cells/ml of RPMI 1640 containing 10% fetal bovine serum and plated into six-well tissue culture plates. By this method of isolation and purification, more than 95% of the adherent cells were macrophages as shown by nonspecific esterase staining. The macrophages were subsequently exposed to lipopolysaccharide (LPS; 400 ng/ml) in the presence of either l-NAME or d-NAME (both at 500 μM), and supernatants were removed 24 h later for cytokine analysis and nitrite and nitrate assay.
Nitrite and nitrate assay.
Measurement of nitrate and nitrite levels in peritoneal washings and supernatants from cultured macrophages was used as an indirect method of determining nitric oxide synthesis. Nitrite and nitrate are stable end products of nitric oxide metabolism and were measured in this study by a modified Griess reaction, described in detail elsewhere (27). Briefly, 50-μl aliquots of sample were incubated with Aspergillus nitrate reductase (Sigma Chemical Co., St. Louis, Mo.) and reduced β-NADPH (Sigma) for 1 h at 37°C. To these samples, 50 μl of Griess reagent, containing equal parts of 1% sulfanilamide in 25% (vol/vol) phosphoric acid and 0.0133 N-1-naphthylethylenediamine dihydrochloride in distilled water, was added for 10 min. The nitrite and nitrate concentrations were calculated from a standard curve with sodium nitrite, and the sensitivity of this assay consistently reached 1 μM.
Cytokine and chemokine measurements.
Cytokine and chemokine measurements were made by using a double-ligand ELISA system as previously described in detail (28). Briefly, Nunc-immuno ELISA plates (MaxiSorp) were coated with cytokine capture antibody at a dilution of 1 μg/ml of coating buffer (0.6 M NaCl, 0.26 M H3BO4, 0.08 M NaOH [pH 9.6]) for 16 h at 4°C. Excess capture antibody was washed away, and each plate was blocked for 90 min with 2% bovine serum albumin in phosphate-buffered saline (PBS) at 37°C. After the blocking period, each ELISA plate was washed with PBS-Tween 20 (0.05%, vol/vol), and samples (no dilution or 1:10; 50-μl volume) were added to wells in duplicate for 1 h at 37°C. Recombinant murine MIP-2, IL-10, and MCP-1 standard curves were used to calculate the cytokine concentrations. The plates were then thoroughly washed, and the appropriate biotinylated polyclonal rabbit anti-cytokine antibody (3.5 μg/ml) was added (14). The plates were washed 30 min later, streptavidin-peroxidase (Bio-Rad Laboratories, Richmond, Calif.) was added to each well for 30 min, and each plate was thoroughly washed again. Chromagen substrate (Bio-Rad Laboratories) was added, and the plates were read on an ELISA plate scanner at 492 nm. The limit of detection for each cytokine was consistently above 10 pg/ml.
In vivo immunoneutralization of IL-10 and MCP-1.
Polyclonal antibodies to murine IL-10 and MCP-1 were developed in multiple-site immunized New Zealand White rabbits as previously described in detail (5). The specificity of these antibodies was screened, and it was found that they all lacked cross-reactivity with other cytokines. In passive-immunization experiments, groups of 10 mice received either 0.5 ml of preimmune normal rabbit serum or a similar volume of anti-IL-10 or anti-MCP-1 immune serum 2 h prior to the CLP surgery. This protocol has been previously used in this laboratory to successfully neutralize the in vivo activity of IL-10 (24) and MCP-1 (31) in an LPS-induced endotoxemia model. In two additional groups (seven mice per group), IL-10 and MCP-1 were both neutralized by the combined administration of 0.5 ml of anti-IL-10 antibody and 0.5 ml of anti-MCP-1 antibody. Immediately following surgery, the preimmune serum and antibody treatment groups received either l-NAME or d-NAME via i.p. injection. The mice were monitored for changes in survival rates over the subsequent 96 h.
Statistical analysis.
Survival curves were generated with Prism computer software (Graphpad Software, Inc., San Diego, Calif.), and comparisons between curves were made with the log-rank test. ELISA data are expressed as the mean ± standard error of the mean (SEM) for 10 mice/group, and statistical analysis of these samples was performed by a one-way analysis of variance. P ≤ 0.05 was considered statistically significant.
RESULTS
Nitric oxide inhibition markedly increases the survival rate in the CLP model.
The effects of l-NAME treatment in mice subjected to CLP surgery were determined by using the Griess reaction. Nitrite and nitrate levels in peritoneal washings from normal mice were below the limit of detection for this assay (i.e., 1 μM). In contrast, approximately 100 μM nitrite and nitrate was measured in peritoneal washings from either saline- or d-NAME-treated mice 24 h after CLP surgery while l-NAME treatment reduced peritoneal levels of nitrite and nitrate by about 75% (data not shown).
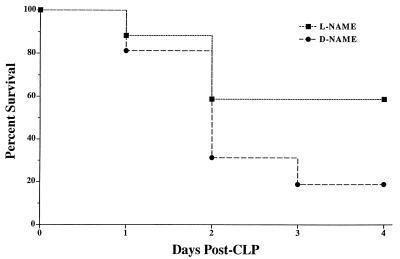
No differences between the survival rates of the l-NAME and d-NAME treatment groups were observed on day 1 post-CLP (Fig. 1). However, on day 2 post-CLP, 60% of l-NAME-treated mice were alive compared to 30% in the d-NAME-treated group. On day 3 post-CLP, 60% of the mice in the l-NAME treatment group remained viable whereas the d-NAME group contained 2 survivors from the initial 10 mice in this group. By day 4 post-CLP, the survival rates were not changed from day 3 in either treatment group. Using the log rank statistical test, a significant (P = 0.033) difference was detected between these survival curves.
FIG. 1.
Survival rates in groups of CLP mice which received saline, d-NAME, or l-NAME. Each mouse received an i.p. injection of either l-NAME or d-NAME immediately following CLP surgery. l-NAME, but not d-NAME, treatment markedly increased survival in CLP mice. According to the log-rank test, a statistically significant (P = 0.033) difference exists between these survival curves. Each CLP treatment group contained 10 mice at the beginning of the experiment shown.
Nitric oxide inhibition augments IL-10 and MCP-1 in peritoneal fluid in mice 24 h after CLP surgery.
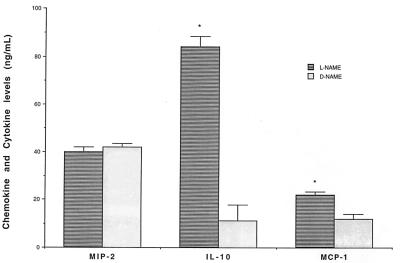
The purpose of the following experiment was to determine whether l-NAME treatment affected the endogenous production of MIP-2, IL-10, and MCP-1 in the peritoneal cavity after CLP surgery. Since peak elevations in the levels of these three chemokines/cytokines were previously shown to occur at 24 h post-CLP (28), levels were measured in peritoneal fluids from mice that had undergone CLP surgery and had been treated with l-NAME or d-NAME 24 h previously. The data from this experiment is summarized in Fig. 2. No differences in MIP-2 levels were observed between the l-NAME and d-NAME treatment groups. However, significant (P ≤ 0.05) increases in IL-10 and MCP-1 levels were apparent in peritoneal washings removed from l-NAME-treated mice compared to d-NAME-treated mice (Fig. 2).
FIG. 2.
l-NAME treatment in mice experiencing fecal peritonitis after CLP augmented immunoreactive levels of IL-10 and MCP-1 in peritoneal washings removed 24 h after surgery. Each animal received an i.p. bolus injection of either l-NAME or d-NAME immediately following CLP surgery. MIP-2, IL-10, and MCP-1 levels were measured in cell-free supernatants by specific ELISAs (see Methods and Materials). Data shown are mean ± SEM of a minimum of 10 mice/group. ∗, P ≤ 0.05 compared to the d-NAME-treated group.
Inhibition of nitric oxide production by LPS-activated peritoneal and alveolar macrophages promotes MCP-1 production.
A potential source of IL-10 and MCP-is the macrophage (2, 7), and previous studies have shown that macrophage activation is directly regulated by nitric oxide (18). In the present experiment, we examined alveolar and peritoneal macrophages from normal CD-1 mice for IL-10 and MCP-1 production following LPS stimulation in the presence of either l-NAME or d-NAME (both at 500 μM) for 24 h. As assessed by measurement of nitrate and nitrite levels, the addition of l-NAME at 500 μM completely inhibited nitric oxide generation by both macrophage types (data not shown). LPS-activated alveolar macrophages treated with l-NAME released twofold more MCP-1 than did similarly activated macrophages treated with d-NAME (Table 1). In contrast, no differences in IL-10 generation by alveolar macrophages were observed between the treatment groups. LPS-activated peritoneal macrophages released fivefold more MCP-1 following l-NAME treatment than did similar cultures treated with d-NAME, but IL-10 generation by peritoneal macrophages was not affected by l-NAME treatment (Table 1). These data suggested that the inhibition of nitric oxide synthesis augmented MCP-1 synthesis by LPS-activated macrophages.
TABLE 1.
Changes in IL-10 and MCP-1 production by LPS-elicited alveolar and peritoneal macrophages following either l-NAME or d-NAME treatmenta
| Macrophage type | Treatment (500 μM) | Cytokine level (ng/ml)
|
|
|---|---|---|---|
| IL-10 | MCP-1 | ||
| Alveolar | d-NAME | 0.5 ± 0.001 | 0.06 ± 0.005 |
| l-NAME | 0.6 ± 0.001 | 0.1 ± 0.001* | |
| Peritoneal | d-NAME | 2.4 ± 0.02 | 0.1 ± 0.01 |
| l-NAME | 1.7 ± 0.4 | 0.5 ± 0.06* | |
Peritoneal and alveolar macrophages were isolated from normal mice and stimulated with LPS (400 ng/ml) in the presence of either l-NAME or d-NAME (each at 500 μM) for 24 h. IL-10 and MCP-1 levels were measured in cell free supernatants by specific ELISAs (see Methods and Materials). Data represent the mean ± SEM of three separate experiments. ∗, P ≤ 0.05 compared with d-NAME treatment.
Individual contributions of IL-10 and MCP-1 to the protective effect of l-NAME treatment in the CLP model.
Previous studies have shown that endogenous IL-10 and MCP-1 play important immunomodulatory roles during CLP (28) and endotoxemia (31). To determine the relative contributions of IL-10 and MCP-1 to the therapeutic effects of l-NAME treatment in the CLP model, either preimmune rabbit serum or rabbit polyclonal neutralizing antibodies to IL-10 or MCP-1 were injected intraperitoneally 2 h prior to surgery and drug treatment. The results of these experiments are shown in Fig. 3 and 4.
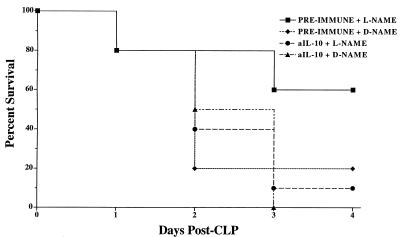
FIG. 3.
Mouse survival following immunoneutralization of IL-10 during CLP-induced fecal peritonitis in d-NAME- and l-NAME-treated mice. The mice were pretreated with 0.5 ml of preimmune rabbit serum or an equivalent amount of rabbit polyclonal anti-IL-10 antibody 2 h prior to surgery. These mice subsequently received either l-NAME or d-NAME immediately following CLP surgery. Anti-IL-10 antibody pretreatment significantly (P = 0.0296; log-rank test) reduced the therapeutic effect of l-NAME in mice experiencing fecal peritonitis. Ten mice were included in each treatment group at the start of the experiment shown.
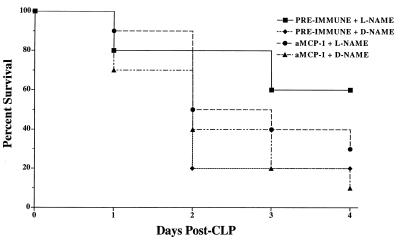
FIG. 4.
Mouse survival following immunoneutralization of MCP-1 during CLP-induced fecal peritonitis in d-NAME- or l-NAME-treated mice. Mice were pretreated with 0.5 ml of preimmune rabbit serum or an equivalent amount of rabbit polyclonal anti-MCP-1 antibody 2 h prior to surgery. These mice subsequently received either l-NAME or d-NAME immediately following CLP surgery. Anti-MCP-1 antibody pretreatment reduced by twofold the therapeutic effect (i.e., mouse survival) of l-NAME in mice experiencing fecal peritonitis, but according to the log-rank test, this was not statistically different from the results for l-NAME treated mice that received preimmune serum. Ten mice were included in each treatment group at the beginning of the experiment shown.
For the first 2 days after CLP surgery, the survival rate in the anti-IL-10 antibody- and d-NAME-treated mice was similar to that in mice that received preimmune serum and d-NAME (Fig. 3). However, on day 3 post-CLP, no mice were alive in the anti-IL-10 antibody-treated group that received d-NAME whereas 20% of the mice that underwent CLP surgery and received preimmune serum and d-NAME were alive. The immunoneutralization of IL-10 completely abolished the therapeutic effect of l-NAME treatment following CLP. After the first 2 days of this experiment, 50% of the mice that received anti-IL-10 antibody and l-NAME were alive compared with 80% of mice that received preimmune serum and l-NAME. On day 3 post-CLP surgery, 1 of the initial 10 mice in the anti-IL-10 antibody and l-NAME treatment group was alive compared with 6 of the original 10 mice in the preimmune serum and l-NAME treatment group (Fig. 3). According to the log rank test, there was a statistically significant (P = 0.0296) difference between the survival curves of the two l-NAME groups.
The effects of either pre-immune serum or anti-MCP-1 antibody administration to mice prior to surgery and drug treatment are shown in Fig. 4. Survival rates in the two d-NAME-treated groups were similar on day 1 post-CLP. On day 2, a 20% survival rate was observed in the d-NAME-treated group that received preimmune serum but 5 of the 10 mice that received anti-MCP-1 antibody and d-NAME were alive. On day 4 post-CLP, twice as many mice were alive in the d-NAME group that received anti-MCP-1 antibody as in the d-NAME group that received preimmune serum. Mice that received anti-MCP-1 antibody treatment prior to CLP surgery and l-NAME treatment had a lower survival rate than those in the preimmune serum and l-NAME treatment group. On day 2 post-CLP, 8 of the initial 10 mice in the preimmune serum and l-NAME treatment group were alive but only 5 of 10 mice in the CLP surgery group that received anti-MCP-1 antibody and l-NAME were alive. Similarly, on day 4 post-CLP, there was a twofold difference between the survival rates of these two groups: 60% of the preimmune serum- and l-NAME-treated mice that had undergone CLP surgery were alive compared with 30% of the mice in the anti-MCP-1 antibody and l-NAME treatment group. However, by the log-rank test, no statistically significant difference was detected between the two l-NAME treatment groups.
Combined effects of anti-IL-10 and anti-MCP-1 on mouse survival during fecal peritonitis.
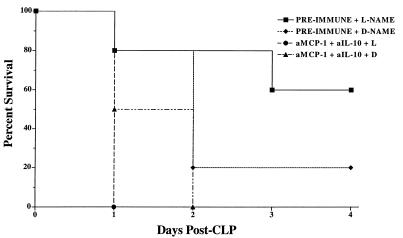
To address the over all contribution of IL-10 and MCP-1 to the protective effects of l-NAME treatment in the CLP model, both mediators were inhibited by the i.p. administration of both polyclonal antibodies (i.e., a total of 1 ml of neutralizing serum). The findings from this experiment are summarized in Fig. 5. The survival rates in groups of CLP mice that received 1 ml of preimmune serum prior to l-NAME or d-NAME treatment were identical to those observed in the previous experiments (Fig. 3 and 4). However, all CLP mice that received the combination of anti-IL-10 and anti-MCP-1 and l-NAME were dead by 24 h after surgery (Fig. 5). All the d-NAME-treated CLP mice that received anti-IL-10 and anti-MCP-1 antibodies were dead by 48 h after surgery. By the log-rank test, a statistically significant difference (P = 0.0047) was detected between the preimmune serum and the dual-antibody treatment groups that received l-NAME.
FIG. 5.
Mouse survival following immunoneutralization of IL-10 and MCP-1 during CLP-induced fecal peritonitis in d-NAME- or l-NAME-treated mice. Mice were pretreated with 1.0 ml of preimmune rabbit serum or a combination of 0.5 ml of rabbit polyclonal anti-IL-10 antibody and 0.5 ml of rabbit polyclonal anti-MCP-1 antibody 2 h prior to surgery. These mice subsequently received either l-NAME or d-NAME immediately following CLP surgery. The antibody combination pretreatment resulted in the death of all CLP mice given l-NAME (P = 0.0047; log-rank test). Seven mice were included in each treatment group at the beginning of the experiment shown.
DISCUSSION
Enhanced nitric oxide production by macrophages and neutrophils is a critical defense mechanism against many forms of fungal and bacterial infection and tumorogenesis (16). However, the excessive production of nitric oxide by immune cells and nonimmune cells such as endothelial, epithelial, and smooth muscle cells can also lead to tissue damage, as demonstrated by the protective effects of NOS inhibitors in many inflammatory and autoimmune disease models (15). The role of nitric oxide in sepsis has been hotly contested, since pharmacological manipulation of nitric oxide synthesis in septic patients has been shown to be both deleterious and beneficial (11, 19, 23). The deleterious effects of NOS inhibition are related in part to the inability of NOS substrate inhibitors to discriminate between the constitutive and inducible isoforms of NOS, whereas the beneficial effects of these compounds have been attributed to the ability of NOS inhibitors to restore normal cardiovascular function in septic patients (11). From the present study, it appears that the inhibition of nitric oxide synthesis following the initiation of experimental fecal peritonitis enhances mouse survival. On day 4 after CLP surgery, 60% of l-NAME-treated mice were alive compared to 20 and 30%, respectively, in the saline and d-NAME treatment groups. Compared to the results in CLP mice that received either saline or d-NAME, significant elevations (i.e., two- to fourfold) in IL-10 and MCP-1 levels in the peritoneal cavity were measured in CLP mice that received l-NAME. Further, l-NAME treatment of LPS-stimulated macrophages significantly increased MCP-1 production by these cells, suggesting that nitric oxide may directly alter the MCP-1 synthetic capacity of macrophages. As demonstrated by immunoneutralization of IL-10 with rabbit polyclonal antibodies in the CLP model, the increase in the level of IL-10 appeared to be responsible for the increased survival following l-NAME treatment. The combined protective importance of IL-10 and MCP-1 following CLP surgery was supported by the observation that the combined immunoneutralization of these mediators resulted in complete mortality by 24 h in the l-NAME group and by 48 h in the d-NAME group. Thus, the data from the present study suggest that nitric oxide may be a key modulator of IL-10 and MCP-1 production during fecal peritonitis.
The introduction of bacteria or bacterial by-products into the peritoneal cavity elicits a strong and often overwhelming inflammatory response that has only been partly characterized (3). Early studies established a role for IL-1 and TNF-α in sepsis, but it is now evident that multiple secondary cytokine pathways exist to perpetuate a robust inflammatory response even in the absence of these proinflammatory mediators. As a consequence, strategies aimed at specifically neutralizing IL-1 or TNF-α have had a limited therapeutic impact in the treatment of sepsis (1). Recent evidence suggests that the successful treatment of sepsis may depend on the restoration of the balance between pro- and anti-inflammatory cytokines (28). For example, it has been shown that IL-10 exerts a protective effect during conditions of experimental endotoxemia (4, 10, 24) and during septic peritonitis (29) due to its generalized downregulation of proinflammatory cytokines such as IL-1, TNF-α, and IL-6 (7) and adhesion molecules (8). The CLP model is associated with marked increases in the levels of IL-1, TNF, and MIP-2 over the first 6 h, which is followed by increases in the levels of IL-10 and soluble TNF receptor (p75) by 24 h. In this model, death occurs rapidly when the latter increases in IL-10 and soluble TNF receptor levels are diminished or inhibited (28). Death in the CLP model has been shown to be the result of the eventual failure of various organs associated with the renal and gastrointestinal systems (3). The mechanisms that regulate the subsequent production of anti-inflammatory cytokines in this model are not entirely clear, but the results of the present study suggest that nitric oxide is involved.
The ability of nitric oxide to modulate the synthesis of immunomodulatory cytokines such as IL-10 and MCP-1 following CLP may have important implications for treating sepsis. IL-10 is a potent immunoregulatory cytokine, which is produced by macrophages, T cells, B cells, epithelial cells, and mast cells and regulates the expression of the inducible NOS (9) and TNF-α, IL-1, and IL-6 production (7) in many cells. Neutralization of IL-10 during experimental endotoxemia has been shown to exacerbate death, in part due to increased MIP-2 and TNF-α production. In the present study, IL-10 levels in the peritoneal cavity were augmented fourfold in CLP mice treated with l-NAME, but these increases did not appear to be due to a direct action of l-NAME on the macrophage. As mentioned above, many other cellular sources of IL-10 exist in the peritoneal cavity; therefore, it is possible that the inhibition of nitric oxide directly alters the production of IL-10 by these cell populations.
IL-10 and MCP-1 appear to work in a complementary fashion to exert protective effects in the CLP model, as evidenced by the accelerated mortality observed in CLP mice that received both neutralizing antibodies. Indeed, the inhibition of nitric oxide in the context of the dual immunoneutralization of IL-10 and MCP-1 resulted in complete mortality by 24 h after CLP surgery. IL-10 is a potent stimulus for the production of MCP-1 by endothelial cells and monocytes (22). Exogenous MCP-1 administration is protective in murine models of lethal Pseudomonas aeroginosa or Salmonella typhimurium infection, where it promotes the phagocytic and killing activity of monocytes (17). More recently, endogenous MCP-1 synthesis has been shown to be necessary for survival in a model of endotoxemia (31). One potential source of MCP-1 is the macrophage, and in the present study it was observed that both alveolar and peritoneal macrophages elicited with LPS in the presence of l-NAME released more than twice as much MCP-1 than did similar macrophages treated with d-NAME. These results coincide with previous findings that showed that the inhibition of nitric oxide generation by endothelial cells increases MCP-1 mRNA levels through increased nuclear factor κB (NF-κB) binding activity (30). Further studies are necessary to determine whether nitric oxide exerts a suppressive effect on MCP-1 generation by macrophages through a similar mechanism.
In summary, the present findings demonstrate a therapeutic effect of nitric oxide inhibition during fecal peritonitis in mice that is mediated, in part, through the promotion of anti-inflammatory mediators such as IL-10 and MCP-1. The data further support the postulate that maintenance of a balance between pro- and anti-inflammatory factors during sepsis is necessary for survival in the CLP model, and they provide a novel mechanism through which nitric oxide participates in septic responses.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grants HL31237, HL31963, and HL35276.
REFERENCES
- 1.Bagby G J, Plessala K J, Wilson L A, Thompson J J, Nelson S. Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis. 1991;163:83–88. doi: 10.1093/infdis/163.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dahinden C A. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 3.Baker C C, Chaudry I H, Gaines H O, Bauer A E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 4.Bean A G D, Freiberg R A, Andrade S, Menon S, Zlotnick A. Interleukin 10 protects mice against staphylococcal enterotoxin B-induced lethal shock. Infect Immun. 1993;61:4937–4939. doi: 10.1128/iai.61.11.4937-4939.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdick M D, Kunkel S L, Lincoln P M, Wilke C A, Strieter R M. Specific ELISAs for the detection of human macrophage inflammatory protein-1 alpha and beta. Immunol Invest. 1993;226:441–446. doi: 10.3109/08820139309063422. [DOI] [PubMed] [Google Scholar]
- 6.Chan J, Tanaka K, Carroll D, Flynn J, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorentino D F, Zlotnik A, Mosmann T, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3820. [PubMed] [Google Scholar]
- 8.Fiorentino D F, Zlotnik A, Vieira P, Mosmann T R, Howard M, Moore K, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3454. [PubMed] [Google Scholar]
- 9.Gazzinelli R T, Oswald I P, James S L, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 10.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilbourn R G, Szabo C, Traber D L. Beneficial versus detrimental effects of nitric oxide inhibitors in circulatory shock: lessons learned from experimental and clinical studies. Shock. 1997;7:235–246. doi: 10.1097/00024382-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Liaudet L, Feihl F, Rosselet A, Markert M, Hurni J M, Perret C. Beneficial effects of l-canavanine, a selective inhibitor of inducible nitric oxide synthase, during rodent endotoxemia. Clin Sci. 1996;90:369–377. doi: 10.1042/cs0900369. [DOI] [PubMed] [Google Scholar]
- 13.Lin P J, Chang C H, Chang J P. Reversal of refractory hypotension in septic shock by inhibitor of nitric oxide synthase. Chest. 1994;106:626–629. doi: 10.1378/chest.106.2.626. [DOI] [PubMed] [Google Scholar]
- 14.Lukacs N W, Chensue S W, Smith R E, Strieter R M, Warmington K, Wilke C, Kunkel S L. Production of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 alpha by inflammatory granuloma fibroblasts. Am J Pathol. 1994;144:711–718. [PMC free article] [PubMed] [Google Scholar]
- 15.Moilanen E, Vapaatalo H. Nitric oxide in inflammation and immune responses. Ann Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- 16.Moncada S. The l-arginine:nitric oxide pathway. Acta Physiol Scand. 1992;145:201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakono Y, Kasahara T, Mukaida N, Ko Y-C, Nakano M, Matsushima K. Protection against lethal bacterial infection in mice by monocyte-chemotactic and activating-factor. Infect Immun. 1994;62:377–383. doi: 10.1128/iai.62.2.377-383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 19.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Valance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994;28:34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 20.Rees D D, Palmer R M J, Shulz R, Hodson H F, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollins B J. JE/MCP-1: an early-response gene encodes a monocyte-specific cytokine. Cancer Cells. 1991;3:517–524. [PubMed] [Google Scholar]
- 22.Rollins B J, Pober J S. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991;138:1315–1319. [PMC free article] [PubMed] [Google Scholar]
- 23.Spain D A, Wilson M A, Garrison R N. Nitric oxide synthase inhibition exacerbates sepsis-induced renal hypoperfusion. Surgery. 1994;116:322–330. [PubMed] [Google Scholar]
- 24.Standiford T J, Strieter R M, Lukacs N W, Kunkel S L. Neutralization of IL-10 increases lethality in endotoxemia. J Immunol. 1995;155:2222–2229. [PubMed] [Google Scholar]
- 25.Stone R. Search for sepsis drugs goes on despite past failures. Science. 1994;264:365–367. doi: 10.1126/science.8153620. [DOI] [PubMed] [Google Scholar]
- 26.Szabo C, Southan G J, Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci USA. 1994;91:12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai W C, Strieter R M, Zisman D A, Wilkowski J M, Bucknell K A, Chen G-W, Standiford T J. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun. 1997;65:1870–1875. doi: 10.1128/iai.65.5.1870-1875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel K L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Elevated levels of macrophage inflammatory protein-2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847–3851. doi: 10.1128/iai.65.9.3847-3851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeiher A M, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein-1 in cultured human endothelial cells. Circ Res. 1995;76:980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- 31.Zisman D A, Kunkel S L, Strieter R M, Tsai W C, Bucknell K, Wilkowski J, Standiford T J. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]