Abstract
Background
Physical activity is known to influence the symptoms of a variety of pain disorders including fibromyalgia and osteoarthritis although the underlying mechanism is not fully understood. In spite of the high prevalence of temporomandibular disorders (TMD), no previous study has objectively evaluated the relationship between TMD and general physical activity. This study aims to investigate the influence of physical activity on pain and disability from TMD, considering various confounders including sleep, systemic inflammation, psychosocial disturbances, and widespread pain.
Methods
This observational cross-sectional study is based on consecutive samples of 100 TMD patients (22 with high pain disability and 78 with low pain disability level). Physical activity levels were assessed with actigraph. Level of pain and disability were evaluated using the Graded Chronic Pain Scale. Hematologic examinations including inflammatory biomarkers were assessed and comorbidities were investigated with validated questionnaires. Differences were analyzed according to disability level.
Results
Patients with high disability level spent significantly more time doing both moderate (p = 0.033) and vigorous (p = 0.039) level physical activity. Light physical activity, on the other hand, was associated with low disability but the difference did not reach statistical significance. Time spent in light physical activity was significantly associated with high levels of pain and disability (p = 0.026, β = −0.001) and time spent in vigorous physical activity had significant predictive power (cutoff value 2.5 min per week, AUC 0.643, p = 0.041). Scores of the Jaw Function Limitation Score-20 (p = 0.001), present McGill Pain Score (p = 0.010), and number of people potentially diagnosed with fibromyalgia (p = 0.033) were significantly higher in the high disability group.
Conclusions
Moderate or vigorous physical activity is associated with worse TMD symptoms while light physical activity may be beneficial. Further research related to the amount and frequency of physical activity is necessary to establish clinical guidelines for TMD.
Trial registration
clinical trial registration of the Clinical Research Information Service of Republic of Korea (number KCT0007107).
Keywords: Temporomandibular disorders, Physical activity, Actigraphy, Pain, Comorbidity
Introduction
Temporomandibular disorders (TMD) is characterized by pain and functional problems involving the temporomandibular joint (TMJ) and masticatory musculature, which is the second most common cause of nonodontogenic pain in the orofacial area with a prevalence of 5–12% of the adult population [1]. Many factors including genetic, anatomic, hormonal, sleep quality, and psychosocial conditions are known to be involved in its initiation and exacerbation [2, 3].
Physical activity defined as “any bodily movement produced by skeletal muscles that requires energy expenditure” by World Health Organization (WHO) [4] is known to influence the symptoms of a variety of pain disorders. Fibromyalgia patients reported significantly lower perceived pain levels when exercise was implemented [5]. Similarly, it was reported that physical activity can relieve pain and enhance joint function of patients with knee or hip osteoarthritis [6]. On the other hand, high intensity exercise was correlated to increased pain of patients with fibromyalgia and low back pain [5, 7]. The underlying mechanism of such interactions are not fully understood and specific guidelines regarding physical activity intensity and duration related to distinct disease entities are yet to be provided.
In spite of its high prevalence and impact on quality of life with prolonged symptoms, no previous study has objectively evaluated the relationship between TMD and general physical activity while considering established confounders such as psychological problems and sleep. Sleep quantity and quality have major influences on pain characteristics, and the proper management of related issues are known to result in favorable treatment response [8–10]. Physical activity, sleep, and pain show a strong interrelationship with one affecting the one through overlapping mechanisms [11–13]. One common underlying factor may be the presence of nonspecific inflammation that is known to be involved in all three physiological states. The association between certain hematological markers indicating systemic inflammation and pain levels have been reported, however the results were inconsistent. High-intensity exercise may produce inflammatory mediators, which in turn could increase pain levels [14]. Nevertheless, another study reported that moderate to vigorous physical activity was significantly correlated with lower inflammatory biomarker levels such as high sensitivity C-reactive protein (CRP) [15]. Another confounder, psychologic disturbances including anxiety and depression are also crucial when interpreting the effect of physical activity but often omitted in analysis.
Therefore, the objective of this study was to investigate the correlation of physical activity and pain in a well-defined group of TMD patients, taking into account various confounders including sleep, systemic inflammation, and psychosocial disturbances. Also, the value of physical activity level as a predictive index of TMD severity was analyzed to provide a guideline in TMD.
Patients and methods
Study design
The protocol of this cross-sectional study can be found in a previous paper [16]. Those who visited the Orofacial Pain Clinic of Seoul National University Dental Hospital with the chief complaint of discomfort of the TMJ area were recruited from May, 2021 to February 2022. To prevent selection bias, patients were recruited sequentially in the order they arrived. The Institutional Review Board (IRB) of the same hospital (CRI21007) approved the study protocol and written informed consent was obtained from all participants. The study was registered in the Clinical Research Information Service of Republic of Korea (KCT0007107). All procedures complied with multiple ethical standards of the institutional research committee, the Helsinki declaration in 1964, and its later amendments or other equivalent ethical standards.
Subjects
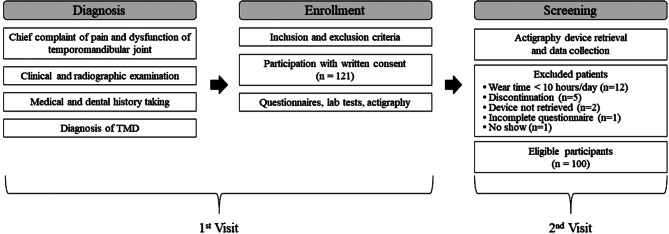
Participants with Korean nationality who were ≥ 18 years old were included. TMD was diagnosed according to the Diagnostic Criteria for TMD (DC/TMD) [17]. Patients with previously diagnosed systemic musculoskeletal disorders, uncontrolled endocrine, liver, or kidney disorder, autoimmune disease, trauma within the last 6 months, psychiatric disorder that may affect the study, and primary sleep disorder diagnosis were excluded. A total of 121 patients provided written consent. The flowchart of the whole study process is illustrated in Fig. 1. The participants were grouped according to pain disability level of the Graded Chronic Pain Scale (GCPS) of DC/TMD Axis II as low (low disability-low intensity pain and low disability-high intensity pain) and high (high disability-moderately limiting pain and high disability-severely limiting pain) disability groups for statistical analysis [18].
Fig. 1.
Flowchart of the study procedure
Clinical assessment
On the first visit, hematologic examinations including complete blood cell count (CBC), erythrocyte sedimentation rate (ESR), and high sensitivity CRP, rheumatoid factor (RF), fluorescent antinuclear antibody (FANA), and anti-cyclic citrullinated peptide (Anti-CCP) were implemented. Several inflammatory markers showing significant correlation with disease activity and mortality, such as platelet-to-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), and derived neutrophil-lymphocyte ratio (dNLR) were calculated [19–22].
Confounding factors were investigated with well-known validated questionnaires applied in various previous studies, which are summarized in Table 1. Validated Korean versions were completed on the first visit. Also, questionnaires of the DC/TMD axis II including Jaw Function Limitation Scale-20 (JFLS-20), Oral Behavior Checklist (OBC), General Anxiety Disorder-7 (GAD-7), Patient Health Questionnaire-9 (PHQ-9), and Patient Health Questionnaire-15 (PHQ-15) were implied [18].
Table 1.
List of structured questionnaires to measure comorbidities
| Section | Questionnaire | Description | Score range |
|---|---|---|---|
| Physical activity | International physical activity questionnaire [23] | Self-reported physical activity level | 1–3 |
| Tampa Scale of Kinesiophobia for Temporomandibular Disorders (TSK-TMD) [24] | Fear of movement and activity | 18–72 | |
| General health | Short Form 36 (SF-36) [25] | Difficulties in various activities | 0–100 |
| Composite Autonomic Symptom Score 31 (COMPASS 31) [26] | Autonomic symptoms | 0-100 | |
| Short form McGill Pain Questionnaire (MPQ) [27] | Quality and intensity of pain | 0–45 | |
| Sleep disturbance and fatigue | Pittsburgh Sleep Quality Index (PSQI) [28] | Quality of sleep | 0–21 |
| Epworth Sleepiness Scale (ESS) [29] | Daytime sleepiness | 0–24 | |
| Fatigue Assessment Instrument (FAI) [30] | Fatigue and related medical disorders | 1–7 | |
| Insomnia Severity Index (ISI) [31] | Severity of insomnia | 0–28 | |
| Morningness-eveningness questionnaire (MEQ) [32] | Preference of when to start sleep or wake up | 16–86 | |
| Widespread pain | Symptom Severity (SS) scale | Widespread body pain and centralized pain characteristics | 0–12/0–19 |
| Widespread Pain Index (WPI) [33] | |||
| Fibromyalgia Impact Questionnaire (FIQ) [34] | Pain and functional impact of fibromyalgia | 0-100 | |
| Psychologic disturbance | Symptom Checklist-90-Revised (SCL-90-R) [35] | Psychological problems | |
| Beck Depression Index (BDI) [36] | Emotional and behavioral depression | 0–63 | |
| Beck Anxiety Index (BAI) [37] | Emotional and physical anxiety | 0–63 | |
| Pain Catastrophizing Scale (PCS) [38] | Negative thoughts and emotions about pain | 0–52 | |
| Central Sensitization Inventory (CSI) [39] | Symptoms related to central sensitization | 0-100 | |
| Pennebaker Index of Limbic Languidness (PILL) [40] | Tendency to notice physical symptoms and sensations | 0-216 | |
| Perceived Stress Scale (PSS) [41] | Nonspecific perceived stress | 0–40 |
Physical activity assessment
Actigraph wGT3X-BT (Fig. 2) with proven reliability for assessing continuous physical activity data was used to objectively monitor the periods of sleep, rest, and activity [42–45]. Participants wore the device 24 h a day for 7 consecutive days starting from the day of the first visit. Recording ended automatically after this period. It was worn on the wrist, either dominating or non-dominating side. An epoch was 60 s, and the bout setting was customized to count bouts regardless of length according to the most recent WHO recommendation [46]. The device was collected on the next visit, and data were downloaded using ActiLife v6.13.4 (ActiGraph, Florida, USA). Since wear time of more than 10 h a day is considered as compliant according to Choi’s wear time validation, only participants who wore the device for an average of 10 h or more per day were included [47]. Choi’s wear time validation has been proven to be reliable and superior to Troiano technique, accurately reflecting actual wear time with less error for wrist-worn devices in a free-living setting [48, 49]. Cutoff values of 232, 4,514, and 15,044 vector magnitude counts were applied for light, moderate, and vigorous activity, respectively [50].
Fig. 2.

Actigraph wGT3X-BT (ActiGraph, Florida, USA)
Statistical analysis
Shapiro-Wilk test was used to test normality of data with following methods selected accordingly. Differences in outcomes between groups were analyzed with Student’s t-test or Mann–Whitney U test for continuous variables and chi-square test or Fisher’s test for discrete variables. After evaluation of multicollinearity, a limited number of variables were selected to enter logistic regression analysis based on presence of significance in independent comparison analysis and also clinical relevance. The eight variables chosen displayed a correlation coefficient of less than 0.8 with respect to each other. Additionally, collinearity diagnostic analysis was conducted individually for each of the 8 variables, confirming variance inflation factor values below 5 and tolerance values above 0.2. Logistic regression with backward elimination was used to investigate actigraphy indices associated with clinical outcomes according to groups. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were analyzed to evaluate the predictive ability of physical activity for TMD disability level. Complete case analysis, a method which involves excluding any observations with missing data was used. All data were analyzed using IBM Corp. released 2019. IBM SPSS Statistics for Windows, Version 26.0. (Armonk, NY: IBM Corp). The significance level was set at 0.05.
Results
Clinical TMD indices
As shown in Table 2, there was no significant difference in age, body mass index (BMI), and sex between the two groups. Those in the high disability group reported significantly more limitation in jaw function (p = 0.001) and higher pain intensity based on MPQ (p = 0.010) and 0–10 numeric rating scale (NRS, p = 0.021). All other clinical indices consistently reflected a more severe state of TMD symptoms in the high disability group however, the difference was not statistically significant.
Table 2.
Clinical characteristics according to different disability level groups
| Low (n = 78) | High (n = 22) | P-value | |
|---|---|---|---|
| Age†† | 29.00 (24.00, 45.25) | 27.00 (24.00, 35.75) | 0.386 |
| Sex (Female)‡‡ | 79.49% (62/78) | 86.36% (19/22) | 0.555 |
| BMI† | 22.39 (3.31) | 21.94 (3.23) | 0.578 |
| BMI ≥ 25‡‡ | 17.95% (14/78) | 13.64% (3/22) | 0.757 |
| JFLS-20† | 1.65 (1.33) | 2.83 (1.75) | 0.001* |
| OBC† | 17.17 (10.47) | 21.55 (9.91) | 0.099 |
| OBC > 20‡ | 32.47% (25/77) | 54.55% (12/22) | 0.080 |
| CMO (mm)† | 40.01 (9.88) | 37.55 (11.47) | 0.296 |
| MMO (mm)† | 43.08 (8.92) | 43.32 (10.15) | 0.956 |
|
Masticatory muscle palpation (number of positive sites)†† |
1.00 (0.00, 5.50) | 1 (0.00, 9.75) | 0.262 |
|
Cervical muscle palpation (number of positive sites)†† |
0.00 (0.00, 1.00) | 0.00 (0.00, 0.00) | 0.583 |
|
Capsule palpation (number of positive sites)†† |
0.00 (0.00, 1.00) | 0 (0.00, 2.00) | 0.214 |
| Pain on mouth opening‡ | 46.15% (36/78) | 68.18% (15/22) | 0.091 |
| Pain on lateral movement‡ | 34.62% (27/78) | 40.91% (9/22) | 0.620 |
| Pain on protrusive movement‡ | 25.64% (20/78) | 27.27% (6/22) | 1.000 |
| Tooth attrition‡‡ | 16.88% (13/77) | 28.57% (6/21) | 0.230 |
| Tongue ridging‡ | 43.42% (33/76) | 57.14% (12/21) | 0.326 |
| Mucosal ridging‡ | 59.21% (45/76) | 80.95% (17/21) | 0.077 |
| MPQ | |||
| Total†† | 5.00 (2.00, 8.00) | 7.00 (4.25, 10.75) | 0.154 |
| Average† | 0.34 (0.25) | 0.43 (0.19) | 0.126 |
| Now†† | 1.00 (1.00, 2.00) | 2.00 (2.00, 2.00) | 0.010* |
| Initial NRS† | 3.78 (2.27) | 5.05 (1.80) | 0.021* |
| DJD‡‡ | 85.90% (67/78) | 81.82% (18/22) | 0.736 |
BMI: Body mass index, JFLS-20: Jaw function limitation scale-20, OBC: Oral behavior checklist, CMO: Comfortable mouth opening, MMO: Maximum mouth opening, MPQ: McGill pain questionnaire, NRS: Numeric rating scale, DJD: Degenerative joint disease
†Differences between groups were tested with independent t-test: mean (standard deviation)
††Differences between groups were tested with Mann-Whitney test: median (lower quartile, upper quartile)
‡Differences between groups were tested with chi-square test: number of subjects or positive palpation sites (%)
‡‡Differences between groups were tested with Fisher’s exact test: number of subjects or positive palpation sites (%)
*Significant difference, p < 0.05
Physical activity and sleep indices
Unlike subjective levels of physical activity based on IPAQ which did not show a difference, certain objective measurements were significantly different between groups as shown in Table 3. High disability TMD patients participated in more moderate (p = 0.033) and vigorous (p = 0.039) physical activities. Those with low disability spent more time doing light physical activity although the difference was not significant from the high disability group.
Table 3.
Physical activity and sleep indices according to different disability level groups
| Low (n = 78) | High (n = 22) | P-value | |
|---|---|---|---|
| IPAQ | |||
| Categorical‡ | 24.36% (19/78) | 23.81% (5/21) | 1.000 |
| Continuous†† |
2006.25 (717.75, 4129.50) |
2112.00 (717.75, 5482.50) |
0.635 |
| Average kcals per day† | 1226.12 (471.73) | 1173.86 (508.32) | 0.656 |
| METs† | 2.15 (0.22) | 2.11 (0.24) | 0.422 |
| Physical activity per week (min) | |||
| Light† | 3790.28 (726.11) | 3462.86 (965.89) | 0.090 |
| Moderate† | 1185.19 (556.88) | 1203.86 (571.11) | 0.033* |
| Vigorous†† | 3.00 (1.00, 9.00) | 7.00 (3.00, 11.00) | 0.039* |
| Total MVPA† | 1195.62 (561.02) | 1219.27 (587.09) | 0.035* |
| Time in sedentary bouts per day† | 313.20 (87.95) | 324.19 (69.76) | 0.594 |
| Time in sedentary breaks per day† | 1036.40 (103.12) | 1030.42 (77.53) | 0.803 |
| Sedentary bouts per day† | 14.64 (4.06) | 15.23 (3.90) | 0.553 |
| Sedentary bouts average length† | 21.44 (1.88) | 21.80 (2.73) | 0.582 |
| Sedentary breaks average length†† | 74.10 (60.75, 84.70) | 66.15 (56.80, 75.53) | 0.241 |
| Axis 1 CPM† | 864.64 (272.28) | 847.50 (265.39) | 0.796 |
| Axis 2 CPM† | 888.85 (260.35) | 896.08 (257.81) | 0.860 |
| Axis 3 CPM† | 981.29 (319.55) | 988.28 (308.45) | 0.928 |
| Vector magnitude | |||
| Average counts† | 1619.22 (470.77) | 1629.19 (482.40) | 0.931 |
| CPM† | 1581.05 (487.66) | 1582.45 (475.18) | 0.991 |
| Step counts per day† | 10383.96 (3080.31) | 10225.73 (3578.22) | 0.840 |
| Lux average counts†† | 25.30 (7.18, 55.40) | 16.55 (4.65, 50.05) | 0.446 |
| Minutes in bed†† | 449.81 (417.89, 513.33) | 480.92 (424.01, 529.54) | 0.318 |
| Minutes in bed < 420‡ | 26.92% (21/78) | 22.73% (5/22) | 0.695 |
| Total Sleep Time (TST)†† | 391.86 (362.96, 463.44) | 424.74 (388.32, 458.64) | 0.191 |
| Total Sleep Time (TST) < 420‡ | 60.26% (47/78) | 50.00% (11/22) | 0.466 |
| Wake After Sleep Onset (WASO)†† | 52.46 (42.32, 66.48) | 48.67 (41.13, 59.33) | 0.621 |
| Sleep efficiency†† | 88.41 (85.57, 90.86) | 90.22 (87.85, 91.11) | 0.190 |
| Sleep fragmentation index† | 25.13 (7.20) | 23.00 (4.81) | 0.198 |
| Movement index† | 13.04 (3.21) | 11.96 (2.66) | 0.156 |
| Fragmentation index† | 12.09 (4.88) | 11.03 (3.56) | 0.352 |
IPAQ: International physical activity questionnaire, METs: Metabolic equivalent of tasks, MVPA: Moderate-to-vigorous physical activity, CPM: Counts per minute
†Differences between groups were tested with independent t-test: mean (standard deviation)
††Differences between groups were tested with Mann-Whitney test: median (lower quartile, upper quartile)
‡Differences between groups were tested with chi-square test: number of subjects or positive palpation sites (%)
‡‡Differences between groups were tested with Fisher’s exact test: number of subjects or positive palpation sites (%)
*Significant difference, p < 0.05
There was no significant difference in sleep indices between the two groups.
Comorbidity levels
Table 4 shows comorbidity levels of both groups. Although the results of FIQ had no significant difference, the number of people diagnosed with fibromyalgia based on the SS scale and WPI according to the 2016 American College of Rheumatology diagnostic criteria was higher in the high disability group (p = 0.033).
Table 4.
Comorbidity levels according to different disability level groups
| Low (n = 78) | High (n = 22) | P-value | |
|---|---|---|---|
| ESS† | 6.77 (4.49) | 6.23 (3.38) | 0.544 |
| ESS > 10‡‡ | 18.18% (14/77) | 13.64% (3/22) | 0.756 |
| PSQI† | 7.90 (3.41) | 7.91 (2.73) | 0.987 |
| PSQI ≥ 5‡ | 85.71% (66/77) | 90.91% (20/22) | 0.727 |
| FAI† | 4.14 (1.01) | 4.43 (0.71) | 0.207 |
| FIQ† | 29.39 (19.54) | 31.78 (16.26) | 0.605 |
| FM diagnosis‡‡ | 1.30% (1/77) | 13.64% (3/22) | 0.033* |
| WPI†† | 3.00 (2.00, 6.00) | 4.00 (2.00, 5.00) | 0.809 |
| SS† | 4.71 (2.24) | 5.05 (2.25) | 0.546 |
| COMPASS 31 | |||
| Orthostatic intolerance†† | 20.00 (8.00, 28.00) | 22.00 (8.00, 28.00) | 0.920 |
| Vasomotor†† | 1.67 (1.67, 1.67) | 1.67 (1.67, 1.67) | 0.871 |
| Secretomotor† | 18.34 (3.19) | 19.29 (2.69) | 0.208 |
| Gastrointestinal†† | 15.18 (10.71, 16.96) | 12.95 (9.15, 16.74) | 0.247 |
| Bladder†† | 3.33 (3.33, 4.44) | 3.33 (3.33, 3.33) | 0.296 |
| Pupillomotor†† | 2.67 (1.00, 3.33) | 1.00 (1.00, 3.25) | 0.210 |
| Total† | 60.85 (15.26) | 59.58 (12.11) | 0.723 |
| CSI† | 30.38 (17.59) | 31.68 (15.43) | 0.756 |
| CSI ≥ 30‡ | 41.56% (38/77) | 50% (11/22) | 1.000 |
| BDI† | 8.45 (8.14) | 9.41 (7.01) | 0.622 |
| BDI ≥ 30‡ | 1.30% (1/77) | 0.00% (0/22) | 1.000 |
| BAI†† | 4.00 (2.00, 11.00) | 5.00 (2.00, 10.25) | 0.847 |
| BAI ≥ 26‡‡ | 3.90% (3/77) | 4.55% (1/22) | 1.000 |
| GAD-7† | 4.03 (4.89) | 4.09 (3.30) | 0.982 |
| PILL† | 52.71 (31.98) | 57.36 (31.87) | 0.553 |
| PILL > 84‡‡ | 18.18% (14/77) | 13.64% (3/22) | 0.756 |
| PSS† | 16.10 (7.37) | 14.68 (7.05) | 0.427 |
| PSS > 13‡ | 62.34% (48/77) | 50% (11/22) | 0.332 |
| TSK-TMD† | 26.76 (6.71) | 29.59 (5.01) | 0.082 |
| TSK-TMD ≥ 23‡‡ | 78.21% (61/78) | 86.36% (19/22) | 0.551 |
| SF-36 | |||
| Physical functioning†† | 95.00 (80.00, 100.00) | 95.00 (76.25, 100.00) | 0.973 |
| Role physical†† | 100.00 (50.00, 100.00) | 75 (31.25, 100.00) | 0.080 |
| Role emotional†† | 100.00 (66.67, 100.00) | 66.67 (41.67, 100.00) | 0.073 |
| Energy/fatigue† | 49.68 (20.39) | 46.82 (19.75) | 0.565 |
| Emotional well-being† | 67.90 (20.02) | 66.55 (14.46) | 0.924 |
| Social functioning†† | 87.50 (67.50, 100.00) | 87.50 (69.38, 90.00) | 0.646 |
| Pain† | 64.51 (21.98) | 56.59 (19.81) | 0.196 |
| General health† | 53.44 (22.71) | 54.09 (16.56) | 0.804 |
| PHQ-9† | 4.41 (4.95) | 5.50 (4.24) | 0.354 |
| PHQ-9 ≥ 20‡‡ | 1.28% (1/78) | 0.00% (0/22) | 1.000 |
| PHQ-15† | 5.76 (4.61) | 6.45 (3.96) | 0.524 |
| PHQ-15 ≥ 15‡‡ | 6.41% (5/78) | 4.55% (1/22) | 1.000 |
| Headache‡ | 41.56% (32/77) | 63.64% (14/22) | 0.408 |
ESS: Epworth sleepiness scale, PSQI: Pittsburgh sleep quality index, FAI: Fatigue assessment instrument, FIQ: Fibromyalgia impact questionnaire, FM: Fibromyalgia, WPI: Widespread pain index, SS: Symptom severity scale, COMPASS 31: Composite autonomic symptom score 31, CSI: Central sensitization index, BDI: Beck depression index, BAI: Beck anxiety index, GAD-7: General anxiety disorder-7, PILL: Pennebaker index of limbic languidness, PSS: Perceived stress scale, TSK-TMD: Tampa scale of kinesiophobia for TMD, SF-36: Short form-36, PHQ-9: Patient health questionnaire-9, PHQ-15: Patient health questionnaire-15
†Differences between groups were tested with independent t-test: mean (standard deviation)
††Differences between groups were tested with Mann-Whitney test: median (lower quartile, upper quartile)
‡Differences between groups were tested with chi-square test: number of subjects or positive palpation sites (%)
‡‡Differences between groups were tested with Fisher’s exact test: number of subjects or positive palpation sites (%)
*Significant difference, p < 0.05
Hematologic indices
As shown in Table 5, none of the examined indices was significantly different between the two groups. Indices such as PLR, NLR, dNLR, SII, ESR, and hs-CRP were higher while LMR was lower in the high disability group, which reflects the possibility of systemic inflammation although the difference was not statistically significant.
Table 5.
Hematologic indices of systemic inflammation according to different disability level groups
| Low (n = 77) | High (n = 22) | P-value | |
|---|---|---|---|
| WBC† | 5.83 (1.42) | 6.02 (1.39) | 0.584 |
| WBC group (≥ 10)‡‡ | 1.30% (1/77) | 0.00% (0/22) | 1.000 |
| RBC† | 4.51 (0.37) | 4.43 (0.34) | 0.355 |
| RBC group (≥ 5.40)‡‡ | 6.49% (5/77) | 9.09% (2/22) | 0.650 |
| Hgb†† | 13.70 (12.80, 14.30) | 13.60 (12.65, 14.05) | 0.426 |
| Hgb group (< 12)‡‡ | 2.60% (2/77) | 4.55% (1/22) | 0.534 |
| Hct† | 40.71 (3.11) | 39.96 (2.74) | 0.315 |
| Hct group (≤ 36)‡‡ | 3.90% (3/77) | 9.09% (2/22) | 0.307 |
| Platelet† | 262.90 (47.60) | 269.32 (51.68) | 0.589 |
| PLR†† | 82.87 (30.38) | 86.88 (32.68) | 0.749 |
| PLR group (≥ 142.76 (F), ≥ 122.73 (M))‡‡ | 3.90% (3/77) | 4.55% (1/22) | 1.000 |
| NLR†† | 1.13 (0.76) | 1.25 (0.83) | 0.614 |
| NLR group (≥ 1.662 (F), ≥ 1.634 (M))‡‡ | 15.58% (12/77) | 27.27% (6/22) | 0.222 |
| dNLR† | 1.40 (0.53) | 1.47 (0.62) | 0.601 |
| LMR†† | 5.10 (3.93, 6.26) | 4.83 (3.84, 5.68) | 0.711 |
| LMR group (≤ 5.598 (F), ≤ 5.048 (M))‡ | 33.77% (26/77) | 31.82% (7/22) | 1.000 |
| SII†† | 249.58 (171.78, 356.45) | 255.39 (181.97, 399.05) | 0.668 |
| ESR (mm/h)†† | 5.00 (2.00, 10.00) | 9.00 (4.25, 14.75) | 0.440 |
| CRP (mg/L)†† | 0.04 (0.02, 0.08) | 0.05 (0.04, 0.08) | 0.354 |
| RF‡‡ | 1.30% (1/76) | 4.50% (1/22) | 0.400 |
| FANA‡ | 29.90% (23/77) | 18.20% (4/22) | 0.416 |
| Anti-CCP‡‡ | 3.90% (3/77) | 0.00% (0/22) | 1.000 |
| Total protein (g/dL)†† | 7.40 (7.1, 7.7) | 7.30 (7.0, 7.58) | 0.660 |
WBC: White blood cell, RBC: Red blood cell, Hgb: Hemoglobin, Hct: Hematocrit, PLR: Platelet-to-lymphocyte ratio, NLR: Neutrophil-lymphocyte ratio, dNLR: derived neutrophil-lymphocyte ratio, LMR: Lymphocyte-monocyte ratio, SII: Systemic inflammatory index, ESR: Erythrocyte sedimentation rate, CRP: C-reactive protein, RF: Rheumatoid factor, FANA: Fluorescent antinuclear antibody, anti-CCP: anti-cyclic citrullinated peptide
†Differences between groups were tested with independent t-test: mean (standard deviation)
††Differences between groups were tested with Mann-Whitney test: median (lower quartile, upper quartile)
‡Differences between groups were tested with chi-square test: number of subjects or positive palpation sites (%)
‡‡Differences between groups were tested with Fisher’s exact test: number of subjects or positive palpation sites (%)
*Significant difference, p < 0.05
Physical activity indices indicating high disability TMD
Logistic regression analysis results are shown in Table 6. As a result of analyzing VIF and tolerance for all variables, all values were less than 5 and exceeded 0.2, respectively. All values derived from Pearson bivariate correlation analysis were less than 0.8. No significant multicollinearity between any variables was found. Time spent in light physical activity (p = 0.026, β=-0.001), mucosal ridging (p = 0.015, β=-1.608), PHQ-9 (p = 0.038, β = 0.196), and pain on mouth opening (p = 0.038, β = 1.248) were variables significantly associated with high levels of pain and disability. With the equation using the variables, the classification accuracy was estimated to be 84.5% and Nagelkerke R-squared value was 0.258 (p = 0.007).
Table 6.
Logistic regression analysis of physical activity and sleep indices associated with disability level
| Variable | Standardized β | Standard error | 95% CI | P-value |
|---|---|---|---|---|
| Time of light physical activity | −0.001 | 0.000 | 0.999-1.000 | 0.026* |
| Age | −0.035 | 0.021 | 0.926–1.007 | 0.100 |
| Mucosal ridging | −1.608 | 0.664 | 0.055–0.735 | 0.015* |
| GAD-7 | −0.197 | 0.103 | 0.671–1.005 | 0.056 |
| PHQ-9 | 0.196 | 0.094 | 1.011–1.463 | 0.038* |
| Pain on mouth opening | 1.248 | 0.603 | 1.069–11.357 | 0.038* |
| Constant | 2.227 | 1.543 | - | 0.149 |
Results were obtained from logistic regression analysis
GAD-7: General anxiety disorder-7, PHQ-9: Patient health questionnaire-9
*Significant difference: p < 0.05
Amount of physical activity predictive of high disability TMD
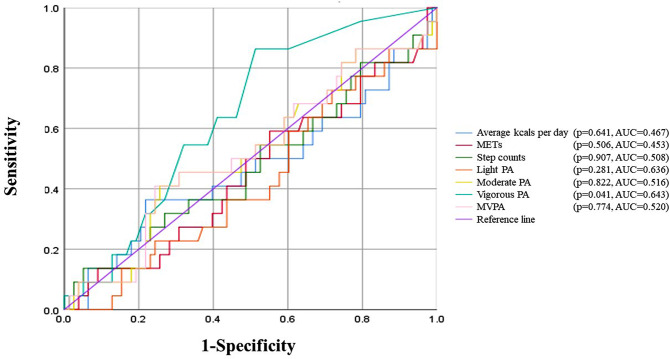
As shown in Fig. 3 and Table 7, the time spent in vigorous physical activity had significant predictive power (p = 0.041). The ROC curve analysis shows that vigorous physical activity with a cutoff value of 2.5 min per week leads to an AUC of 0.643 for high disability due to TMD. By using the cutoff value, the patients in this study were classified with sensitivity, specificity, and accuracy of 86.4%, 48.7%, 57.0% respectively.
Fig. 3.
Receiver operating characteristic (ROC) curve. METs, metabolic equivalent of tasks; PA, physical activity; MVPA, moderate-to-vigorous physical activity; AUC, area under the curve
Table 7.
Receiver operating characteristics (ROC) curve analyses for evaluation scores to assess influence on disability from Temporomandibular disorders (TMD)
| AUC (95% CI) | Cut off value | Sensitivity | Specificity | PPV | NPV | LR+ | LR- | Error rate (%) | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Average kcals per day |
0.467 (0.318–0.617) |
1511.446 | 0.364 | 0.782 | 0.320 | 0.813 | 1.670 | 0.813 | 31.00 | 0.641 |
| METs |
0.453 (0.315–0.592) |
2.441 | 0.136 | 0.718 | 0.120 | 0.747 | 0.482 | 1.203 | 41.00 | 0.506 |
| Step counts |
0.508 (0.370–0.646) |
6.900 | 0.727 | 0.397 | 0.640 | 0.920 | 1.206 | 0.688 | 15.00 | 0.907 |
| Light PA |
0.636 (0.442–0.708) |
3770.000 | 0.636 | 0.551 | 0.560 | 0.893 | 1.416 | 0.661 | 31.00 | 0.281 |
| Moderate PA |
0.516 (0.375–0.656) |
1409.000 | 0.409 | 0.744 | 0.360 | 0.827 | 1.598 | 0.794 | 29.00 | 0.822 |
| Vigorous PA |
0.643 (0.526–0.761) |
2.500 | 0.864 | 0.487 | 0.760 | 0.960 | 1.684 | 0.279 | 9.00 | 0.041* |
| MVPA |
0.520 (0.379–0.661) |
1447.500 | 0.409 | 0.756 | 0.360 | 0.827 | 1.676 | 0.782 | 29.00 | 0.774 |
AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR-, negative likelihood ratio; METs, metabolic equivalents of task; PA, physical activity; MVPA, moderate to vigorous physical activity
Sensitivity was obtained from TP/(TP + FN) × 100, Specificity was obtained from TN/(TN + FP) × 100, PPV was obtained from TP/(TP + FP) × 100, NPV was obtained from TN/(TN + FN) × 100, Error rate was obtained from (FN + FP)/(TN + TP + FN + FP)
*Significant difference, p < 0.05
Discussion
This study is the first attempt to elucidate the relationship between objective levels of physical activity and clinical symptoms in a well-defined group of TMD patients based on DC/TMD. The results of this study showed that moderate to vigorous levels of physical activity was associated with high disability TMD accompanied by higher pain intensity and widespread pain. Such results are in line with a recent study based on a large-scale national database in South Korea reporting that moderate-intensity physical activity was associated with more pain in those with TMD symptoms [51]. Unfortunately, direct comparison of results is limited due to the fact that diagnostic criteria used in the previous study was different as it followed the WHO criteria generally applied in oral health surveys, which examines requires only one item of TMD sign and symptom for diagnosis. Also, only subjective information from questionnaires was gathered to assess physical activity levels. On the other hand, another study based on 8,685 Finnish conscripts reported that those who exercised less frequently showed more TMD symptoms. The evaluation of TMD symptoms was based on 6 self-reporting questions and physical activity level was also assessed with 2 questions [52]. As far as the authors are aware of these studies are the only investigations that have analyzed the relationship between general physical activity level and TMD symptoms but, interpretation of results is restricted due to the subjective nature of gathered data and ambiguity in defining TMD. Therefore, this study based on a TMD patient group defined through a standardized diagnostic process by a calibrated orofacial pain specialist and objectively measured physical activity data holds significance as the results have higher generalizability and reproducibility. The contradictory results from the studies may have originated from the variation in assessment approaches, however aspects of physical activity such as intensity and frequency were handled differently according to the study which may partly explain the discrepant conclusions. Findings based on other musculoskeletal diseases also report inconsistent results. According to the recommendation of the European League Against Rheumatism, physical activity can be beneficial on pain, function, and quality of life to people with rheumatic and musculoskeletal diseases especially, those with osteoarthritis and axial spondyloarthritis suggesting that physical inactivity should be avoided [53, 54]. Another umbrella review also concluded that exercise can reduce pain from fibromyalgia by analyzing thirty-seven recent systematic reviews. On the other hand, an earlier review reported that certain exercise types do help improve function in fibromyalgia patients but some experience an increase in symptoms with higher than moderate intensity exercise that eventually led to incompliance to their exercise regimen [5]. For low back pain, a systematic review and meta-analysis also showed that certain studies comparing high with low or moderate activity reported an elevation in pain level with high-intensity exercise [7]. Such discrepancies may result from the difference in study population, lack of controlling confounding factors, and application of different cut-off levels to separate low, moderate, and high intensity physical activity, all aspects to be considered in future studies to accumulate data that may support the establishment of a standardized physical activity protocol for a certain disease population.
According to the WHO recommendation on physical activity announced in 2020, 150 to 300 min of moderate intensity or 75 to 150 min of vigorous intensity per week, or an equivalent combination of moderate-to-vigorous physical activity can provide many health benefits for both normal and diseased populations [42]. An interesting point is that the past WHO recommendation of 2010 only suggested the minimum amount of activities required for general health improvement. This may reflect the recent realization that more physical activity does not always guarantee better health outcomes and excessive amounts of physical activity may even be harmful in specific situations [55]. Regarding TMD, a recent review paper investigating the effect of competitive sports in this context implied that the incidence of TMD appeared to be increased in athletes compared to non-athletes suggesting an association with vigorous levels of physical activity [56]. The results of our study also support the need of specific guidelines including both the minimum and maximum amount of physical activity for optimal prognosis in TMD patients. It appears that it is crucial to implement exercise only up to the point where symptoms do not exacerbate. ROC analysis of our data showed that as little as 2.5 min/week of vigorous activity was associated with high disability TMD. The equivalent of vigorous activity would be jogging, running, carrying heavy loads upstairs [57].
Based on the obtained logistic regression model, one could easily correlate high pain level and interference of daily life in TMD patients with less light-intensity physical activity. Also, depressive symptoms evaluated with PHQ-9 showed significant correlation with high disability TMD. Such clinical indices could be easily applied in a clinical setting to discern TMD patients with worse symptoms and more comorbidities.
Another point to consider is that objective sleep data was collected in this study. People with poor general health and psychologic problems are more likely to report discrepant subjective sleep information [58]. Although statistically different results could not be found, total sleep time and minutes in bed were both longer and sleep efficiency was higher in the high pain disability group. This does not fall in line with the prevalent knowledge that sufficient sleep time is associated with less TMD pain [59]. Patients in the high disability group got in bed 51 min earlier in average, which was 11:27 PM. This is also contradictory to results from a recent systematic review revealing that later sleep timing was generally associated with worse health outcomes [60]. Some studies do imply that excessive sleep is detrimental to health [61]. The findings of our study place emphasis on a previous report that showed only self-report sleep questionnaire but not actigraphy-measured results were able to differentiate those with TMD from health controls [62].
The relationship between high intensity physical activity and high disability TMD may be mediated by elevated systemic inflammation accompanied by high intensity activities [63]. During high-intensity exercise, skeletal muscles secrete interleukin-6, which are known to influence platelet activation, lymphocyte modulation, and neutrophil function in the dynamic interplay of such blood cells during inflammatory responses [62, 64–66]. Such an increased state of systemic inflammation may directly aggravate TMD related pain however, specific evidence quantitatively linking specific levels of physical activity, inflammation level, and TMD are yet to come. The inflammatory markers investigated in this study were ratio values selected based on previous studies showing there higher accuracy in reflecting systemic inflammatory status and long-term disease prognosis compared to absolute values [67, 68]. All investigated hematological inflammatory indices showed a trend of increased inflammation however, the difference between groups was not statistically significant. Systemic inflammation is generally known to be associated with sleep disturbance and obesity [69, 70]. The lack of distinction between groups may have arisen from the absence of distinct differences in causal factors, such as deprivation of sleep and BMI. Future studies based on different grouping criteria are necessary to further investigate the correlation among physical activity, sleep, pain, and inflammation.
There are some limitations of this study to consider. Due to the cross-sectional design of this study, the causal relationship between physical activity level and TMD cannot be derived. Therefore, experimental and longitudinally designed studies are needed to confirm the findings. Second, despite applying a validated accelerometer for physical activity evaluation, measurements have limitations in reflecting the true amount of physical activity. For instance, basal metabolic rate is not taken into account and resistance exercise or leg exercise are prone to underestimation [71]. Objective measurements alone are not consistently related to results from questionnaires, so adopting both subject and object investigations would be more effective [72]. Third, there is no consensus on cut-off values of vector magnitude for classifying physical activity intensity with a triaxial accelerometer worn on the wrist. A calibration study has been conducted only recently [50]. This study used cut-offs from the calibration study instead of using values derived from studies with devices worn on the hip for more accurate classification. Fourth, the shortcomings of actigraphy measured sleep data compared to polysomnography should be considered in interpreting results. Furthermore, sleep data was not supported by sleep dairy logging. Lastly, potential biases may exist due to the sequential recruitment of patients. Subject recruitment occurred over an 8-month period and seasonal effects on psychological condition and physical activity may be present. Also those who were excluded from the study may have an inherent characteristic that is essential for investigation of the study subject. Future studies should consider recruiting subjects considering such aspects [71, 73–76]. In the present study, the association between TMD and everyday physical activity was investigated. However, it is noteworthy that certain types of exercises may aggravate or alleviate TMD [56] and additional studies are called upon to assess the effect of certain sports activities based on objective measurements of intensity and frequency to be able to provide recommendations to the patients.
Conclusions
TMD patients showed a clear difference in terms of physical activity according to disability level. Both moderate- and high-intensity physical activity was positively associated with high disability. This study showed that the time of vigorous activity associated with high disability TMD was 2.5 min per week. Based on such findings, TMD patients are recommended to minimize vigorous activities while engaging in more light-intensity physical activity to avoid symptom aggravation. Clinicians should objectively evaluate general physical activity level in TMD patients and be able to provide recommendations for better treatment outcomes. Further investigations are necessary to provide more detailed guidelines regarding the optimum intensity and frequency of physical activity for TMD patients.
Acknowledgements
The paper is based on a Master’s thesis.
Abbreviations
- TMD
temporomandibular disorders
- DC/TMD
diagnostic criteria for TMD
- IPAQ
international physical activity questionnaire
- TSK-TMD
Tampa scale of kinesiophobia for TMD
- SF-36
Short form-36
- COMPASS 31
composite autonomic symptom score 31
- MPQ
McGill pain questionnaire
- PSQI
Pittsburgh sleep quality index
- ESS
Epworth sleepiness scale
- FAI
fatigue assessment instrument
- BDI
Beck depression index
- BAI
Beck anxiety index
- CSI
central sensitization index
- PILL
Pennebaker index of limbic languidness
- PSS
perceived stress scale
- SS
symptom severity scale
- WPI
widespread pain index
- FIQ
fibromyalgia impact questionnaire
- JFLS-20
jaw function limitation scale-20
- OBC
oral behavior checklist
- GAD-7
general anxiety disorder-7
- PHQ-9
patient health questionnaire-9
- PHQ-15
patient health questionnaire-15
- CBC
complete blood cell count
- ESR
erythrocyte sedimentation rate
- CRP
C-reactive protein
- RF
rheumatoid factor
- FANA
fluorescent antinuclear antibody
- anti-CCP
anti-cyclic citrullinated peptide
- PLR
platelet-to-lymphocyte ratio
- NLR
neutrophil-lymphocyte ratio
- dNLR
derived neutrophil-lymphocyte ratio
- LMR
lymphocyte-monocyte ratio
- SII
systemic inflammatory index
- GCPS
graded chronic pain scale
Author contributions
YC conducted data curation and authored the original draft of the manuscript. JHJ conducted data curation and validation. JWP was involved in conceptualizing the project, performing data curation, acquiring funding, providing supervision, and authoring the original draft of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the New Faculty Startup Fund from Seoul National University (860-20190102). The funding body did not participate in any activities related to study design, data collection, analysis, and interpretation, and writing the manuscript.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due ethical reasons but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Institutional Review Board (IRB) of Seoul National University Dental Hospital (CRI21007) approved the study protocol and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prevalence of TMJD and its Signs and Symptoms, National Institute of Dental and Craniofacial Research. 2018. https://www.nidcr.nih.gov/research/data-statistics/facial-pain/prevalence. Accessed 13 Mar 2023.
- 2.Chisnoiu AM, Picos AM, Popa S, et al. Factors involved in the etiology of temporomandibular disorders - a literature review. Clujul Med. 2015;88(4):473–8. doi: 10.15386/cjmed-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rener-Sitar K, John MT, Pusalavidyasagar SS, Bandyopadhyay D, Schiffman EL. Sleep quality in temporomandibular disorder cases. Sleep Med. 2016;25:105–12. doi: 10.1016/j.sleep.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Physical, activity, World Health Organization. 2022. https://www.who.int/news-room/fact-sheets/detail/physical-activity. Accessed 13 Mar 2023.
- 5.Busch AJ, Overend TJ, Schachter CL. Fibromyalgia treatment: the role of exercise and physical activity. Int J Clin Rheumtol. 2009;4:343–80. [Google Scholar]
- 6.Kraus VB, Sprow K, Powell KE, Buchner D, Bloodgood B, Piercy K, George SM, Kraus WE, PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE*.; 2018. Effects of Physical Activity in Knee and Hip Osteoarthritis: A Systematic Umbrella Review. Med Sci Sports Exerc. 2019;51(6):1324–1339. doi: 10.1249/MSS.0000000000001944. PMID: 31095089; PMCID: PMC6527143. [DOI] [PMC free article] [PubMed]
- 7.Shiri R, Falah-Hassani K. Does leisure time physical activity protect against low back pain? Systematic review and meta-analysis of 36 prospective cohort studies. Br J Sports Med. 2017;51(19):1410–8. doi: 10.1136/bjsports-2016-097352. [DOI] [PubMed] [Google Scholar]
- 8.Benoliel R, Zini A, Zakuto A et al. Subjective sleep quality in temporomandibular disorder patients and association with disease characteristics and oral health-related quality of life. J Oral Facial Pain Headache. 2017 Fall;31(4):313–322. 10.11607/ofph.1824. Epub 2017 Oct 3. PMID: 28973048. [DOI] [PubMed]
- 9.Yatani H, Studts J, Cordova M, Carlson CR, Okeson JP. Comparison of sleep quality and clinical and psychologic characteristics in patients with temporomandibular disorders. J Orofac Pain. 2002;16(3):221–8. [PubMed] [Google Scholar]
- 10.Lerman SF, Mun CJ, Hunt CA, et al. Insomnia with objective short sleep duration in women with temporomandibular joint disorder: quantitative sensory testing, inflammation and clinical pain profiles. Sleep Med. 2022;90:26–35. doi: 10.1016/j.sleep.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberger ME, Buman MP, Haskell WL, McConnell MV, Carstensen LL. Twenty-four hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med Sci Sports Exerc. 2016;48(3):457–65. doi: 10.1249/MSS.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolezal BA, Neufeld EV, Boland DM, Martin JL, Cooper CB. Interrelationship between sleep and exercise: A aystematic review. Adv Prev Med. 2017;2017:1364387. doi: 10.1155/2017/1364387. Epub 2017 Mar 26. Erratum in: Adv Prev Med. 2017;2017:5979510. PMID: 28458924; PMCID: PMC5385214. [DOI] [PMC free article] [PubMed]
- 13.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4(4):CD011279. doi: 10.1002/14651858.CD011279.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hojman P, Brolin C, Nørgaard-Christensen N, et al. IL-6 release from muscles during exercise is stimulated by lactate-dependent protease activity. Am J Physiol Endocrinol Metab. 2019;316(5):E940–7. doi: 10.1152/ajpendo.00414.2018. [DOI] [PubMed] [Google Scholar]
- 15.Majka DS, Chang RW, Vu TH, et al. Physical activity and high-sensitivity C-reactive protein: the multi-ethnic study of atherosclerosis. Am J Prev Med. 2009;36(1):56–62. doi: 10.1016/j.amepre.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun Y, Jo JH, Park JW. Does physical activity level have an impact on long-term treatment response in temporomandibular disorders: protocol for a prospective study. BMC Oral Health. 2022;22:401. doi: 10.1186/s12903-022-02428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohrbach R, Gonzalez Y, List T, Michelotti A, Schiffman E. Diagnostic Criteria for Temporomandibular disorders (DC/TMD) Clinical Examination Protocol: Version 02June2013. www.rdc-tmdinternational.org, Accessed on March 13, 2023.
- 18.Ohrbach R, editor. Diagnostic Criteria for Temporomandibular Disorders: Assessment Instruments. Version 15May2016. www.rdc-tmdinternational.org Accessed on February 25, 2023.
- 19.Aktürk S, Büyükavcı R. Evaluation of blood neutrophil-lymphocyte ratio and platelet distribution width as inflammatory markers in patients with fibromyalgia. Clin Rheumatol. 2017;36(8):1885–9. doi: 10.1007/s10067-017-3647-0. [DOI] [PubMed] [Google Scholar]
- 20.Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372–6. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Chen Y, Yang X, Chen L, Yang Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol. 2016;36:94–9. doi: 10.1016/j.intimp.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Mercan R, Bitik B, Tufan A, et al. The Association between Neutrophil/Lymphocyte Ratio and Disease Activity in Rheumatoid Arthritis and Ankylosing Spondylitis. J Clin Lab Anal. 2016;30(5):597–601. doi: 10.1002/jcla.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun MY. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med. 2012;33(3):144–51. doi: 10.4082/kjfm.2012.33.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visscher CM, Ohrbach R, van Wijk AJ, Wilkosz M, Naeije M. The Tampa Scale for Kinesiophobia for Temporomandibular disorders (TSK-TMD) Pain. 2010;150(3):492–500. doi: 10.1016/j.pain.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 26.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87(12):1196–1201. 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed]
- 27.Melzack R. The McGill pain questionnaire: from description to measurement. Anesthesiology. 2005;103(1):199–202. doi: 10.1097/00000542-200507000-00028. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: a new instrument. J Psychosom Res. 1993;37(7):753–62. doi: 10.1016/0022-3999(93)90104-n. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM. Insomnia: psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 32.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 33.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 34.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–33. [PubMed] [Google Scholar]
- 35.Derogatis LR. SCL-90-R: Administration, Scoring of procedures Manual-II for the R (evised) Version and Other Instruments of the psychopathology rating Scale Series. Clinical Psychometric Research Incorporated; 1992.
- 36.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 37.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524. [Google Scholar]
- 39.Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12(4):276–85. doi: 10.1111/j.1533-2500.2011.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The psychology of physical symptoms, Pennebaker JW. (1982). The psychology of physical symptoms. Springer-Verlag Publishing. 10.1007/978-1-4613-8196-9.
- 41.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 42.NHANES 2003–2004 Pubic Data General Release File Documentation. Centers for Disease Control Prevention. 2005. https://www.cdc.gov/NCHS/data/nhanes/nhanes_03_04/general_data_release_doc_03-04.pdf. Accessed 28 Nov 2022.
- 43.O’Donovan G, Hillsdon M, Ukoumunne OC, et al. Objectively measured physical activity, cardiorespiratory fitness and cardiometabolic risk factors in the Health Survey for England. Prev Med. 2013;57:201–5. doi: 10.1016/j.ypmed.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Colley RC, Garriguet D, Janssen I, et al. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian Health measures Survey. Health Rep. 2011;22:7–14. [PubMed] [Google Scholar]
- 45.da Silva IC, van Hees VT, Ramires VV, et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol. 2014;43(6):1959–68. doi: 10.1093/ije/dyu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee IM, Shiroma EJ, Evenson KR, et al. Accelerometer-measured physical activity and sedentary behavior in relation to all-cause mortality: the women’s Health Study. Circulation. 2018;137(2):203–5. doi: 10.1161/CIRCULATIONAHA.117.031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knaier R, Höchsmann C, Infanger D, Hinrichs T, Schmidt-Trucksäss A. Validation of automatic wear-time detection algorithms in a free-living setting of wrist-worn and hip-worn ActiGraph GT3X. BMC Public Health. 2019;19(1):244. doi: 10.1186/s12889-019-6568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee P, Tse CY. Calibration of wrist-worn ActiWatch 2 and ActiGraph wGT3X for assessment of physical activity in young adults. Gait Posture. 2019;68:141–9. doi: 10.1016/j.gaitpost.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 51.Cho HJ, Kim SJ, Park SE, Park JW. Physical activity level and temporomandibular disorders in south koreans. Community Dent Oral Epidemiol. 2020;48(3):225–31. doi: 10.1111/cdoe.12519. [DOI] [PubMed] [Google Scholar]
- 52.Miettinen O, Kämppi A, Tanner T Association of temporomandibular disorder symptoms with physical fitness among Finnish conscripts. Int J Environ Res Public Health. 2021 Mar 16;18(6):3032. 10.3390/ijerph18063032. PMID: 33809450; PMCID: PMC7998271.53. [DOI] [PMC free article] [PubMed]
- 53.Rausch Osthoff AK, Niedermann K, Braun J, et al. EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018 Sep;77(9):1251–1260. 10.1136/annrheumdis-2018-213585. Epub 2018 Jul 11. PMID: 29997112. [DOI] [PubMed]
- 54.Gwinnutt JM, Wieczorek M, Balanescu A, et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseasesAnnals. Of the Rheumatic Diseases. 2023;82:48–56. doi: 10.1136/annrheumdis-2021-222020. [DOI] [PubMed] [Google Scholar]
- 55.Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. PMID: 26180873. [PubMed]
- 56.Freiwald HC, Schwarzbach NP, Wolowski A. Effects of competitive sports on temporomandibular dysfunction: a literature review. Clin Oral Investig. 2021;25(1):55–65. 10.1007/s00784-020-03742-2. PMCID: PMC7785544. [DOI] [PMC free article] [PubMed]
- 57.Physical Activity Guidelines for Americans, 2nd edition. U.S. Department of Health and Human Services. 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed 23 Feb 2023.
- 58.Matthews KA, Patel SR, Pantesco EJ, et al. Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health. 2018 Feb;4(1):96–103. 10.1016/j.sleh.2017.10.011. Epub 2017 Dec 13. PMID: 29332687; PMCID: PMC5771411. [DOI] [PMC free article] [PubMed]
- 59.Al-Jewair T, Shibeika D, Ohrbach R. Temporomandibular disorders and their association with sleep disorders in adults: A systematic review. J Oral Facial Pain Headache. 2021 Winter;35(1):41–53. 10.11607/ofph.2780. PMID: 33730126. [DOI] [PubMed]
- 60.Chaput JP, Dutil C, Featherstone R et al. Sleep timing, sleep consistency, and health in adults: a systematic review. Appl Physiol Nutr Metab. 2020;45(10 (Suppl. 2)):S232-S247. 10.1139/apnm-2020-0032. PMID: 33054339. [DOI] [PubMed]
- 61.Choi H, Sim HY, Han K, Yun KI. Association between sleeping time and temporomandibular disorders in a sample of the South Korean population. Cranio. 2021;39(2):107–12. doi: 10.1080/08869634.2019.1587243. [DOI] [PubMed] [Google Scholar]
- 62.Boggero IA, Schneider VJ, Thomas PL, Nahman-Averbuch H, King CD. Associations of self-report and actigraphy sleep measures with experimental pain outcomes in patients with temporomandibular disorder and healthy controls. J Psychosom Res. 2019;123:109730. doi: 10.1016/j.jpsychores.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hojman P, Brolin C, Norgaard-Christensen N, et al. IL-6 release from muscles during exercise is stimulated by lactate-dependent protease activity. Am J Physiol Endocrinol Metab. 2019;316:E940–7. doi: 10.1152/ajpendo.00414.2018. [DOI] [PubMed] [Google Scholar]
- 64.Ishibashi T, Kimura H, Uchida T, Kariyone S, Friese P, Burstein SA. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989;86(15):5953–7. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25(5):241 – 53. 10.1089/jir.2005.25.241. PMID: 15871661. [DOI] [PubMed]
- 66.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181(3):2189-95. 10.4049/jimmunol.181.3.2189. PMID: 18641358. [DOI] [PubMed]
- 67.Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 68.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in Rheumatic diseases. Ann Lab Med. 2019;39(4):345–57. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Artemniak-Wojtowicz D, Kucharska AM, Pyrżak B. Obesity and chronic inflammation crosslinking. Cent Eur J Immunol. 2020;45(4):461–8. doi: 10.5114/ceji.2020.103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stec MJ, Rawson ES. Estimation of resistance exercise energy expenditure using triaxial accelerometry. J Strength Cond Res. 2012;26(5):1413-22. 10.1519/JSC.0b013e318248d7b4. PMID: 22222328. [DOI] [PubMed]
- 72.Skender S, Ose J, Chang-Claude J, et al. Accelerometry and physical activity questionnaires - a systematic review. BMC Public Health. 2016;16:515. doi: 10.1186/s12889-016-3172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ranatunga KW. Temperature effects on force and actin-myosin interaction in muscle: a look back on some experimental findings. Int J Mol Sci. 2018;19(5):1538. doi: 10.3390/ijms19051538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harmatz MG, Well AD, Overtree CE, Kawamura KY, Rosal M, Ockene IS. Seasonal variation of depression and other moods: a longitudinal approach. J Biol Rhythms. 2000;15(4):344 – 50. 10.1177/074873000129001350. PMID: 10942266. [DOI] [PubMed]
- 75.Choi JC, Lee JH, Choi E, Chung MI, Seo SM, Lim HK. Effects of seasonal differences in testosterone and cortisol levels on pain responses under resting and anxiety conditions. Yonsei Med J. 2014;55(1):216–23. doi: 10.3349/ymj.2014.55.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shephard RJ, Aoyagi Y. Seasonal variations in physical activity and implications for human health. Eur J Appl Physiol. 2009;107(3):251 – 71. 10.1007/s00421-009-1127-1. Epub 2009 Jul 16. PMID: 19609553. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due ethical reasons but are available from the corresponding author on reasonable request.