Abstract
Background
The 4th European Conference on Infections in Leukemia recommends early adaptation of empirical antibiotic therapy (EAT) for febrile neutropenia in stable patients.
Objectives
To assess the efficacy of an antimicrobial stewardship (AMS) intervention promoting early de-escalation and discontinuation of EAT in high-risk neutropenic patients.
Methods
This before-after study was conducted in the hematology department of the University Hospital of Nice, France. The AMS intervention included the development of clinical decision support algorithms, a twice-weekly face-to-face review of all antibiotic prescriptions and monthly feedback on the intervention. The primary endpoint was overall antibiotic consumption during hospital stay, expressed as days of therapy (DOT).
Results
A total of 113 admissions were included: 56 during the pre-intervention period and 57 during the intervention period. Induction chemotherapy and conditioning for allogeneic stem cell transplantation were the most frequent reasons for admission. In the intervention period, there was a significant decrease in overall antibiotic consumption (median DOT 20 vs. 28 days, p = 0.006), carbapenem consumption (median DOT 5.5 vs. 9 days, p = 0.017) and anti-resistant Gram-positive agents consumption (median DOT 8 vs. 11.5 days, p = 0.017). We found no statistical difference in the rates of intensive care unit admission (9% in each period) and 30-day mortality (5% vs. 0%, p = 0.243). Compliance with de-escalation and discontinuation strategies was significantly higher in the intervention period (77% vs. 8%, p < 0.001).
Conclusion
A multifaceted AMS intervention led to high compliance with early de-escalation and discontinuation of EAT and lower overall antibiotic consumption, without negatively affecting clinical outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-023-01354-5.
Keywords: Antibiotic, Antimicrobial stewardship, Hematology, Febrile neutropenia
Introduction
Febrile neutropenia (FN) is a frequent and serious complication in patients with hematological malignancies undergoing intensive chemotherapy [1]. FN episodes are responsible for repeated and prolonged antibiotic therapy, leading to an increased risk of antibiotic resistance [2], Clostridioides difficile infections [3], fungal infections [4] and adverse drug events [5]. The spread of antibiotic resistance is a major threat in high-risk neutropenic patients given that a delay in introduction of appropriate empirical antibiotic therapy (EAT) in this population is associated with increased morbidity and mortality [6].
Excessive and inappropriate antibiotic use are major drivers of the emergence and spread of antibiotic resistance [7, 8]. Antimicrobial stewardship (AMS) interventions have therefore been introduced to optimize antibiotic use, in order to decrease unintended consequences of antibiotic use, such as growing antibiotic resistance and excessive healthcare costs [9, 10]. Given the threat of antibiotic resistance and the extensive use of antibiotics in high-risk neutropenic patients, there is an urgent need to implement AMS interventions in hematology departments.
In 2013, the 4th European Conference on Infections in Leukemia (ECIL) group published new guidelines for the management of FN, prompting early adaptation of EAT in stable afebrile patients, regardless of neutrophil count and expected duration of neutropenia [11]. Despite these evidence-based guidelines, de-escalation and discontinuation strategies are not widely implemented in hematology departments [12]. Recent studies have found that early adaptation of EAT is feasible and safe and could lead to reduced antibiotic consumption [13–23]. However, most of these studies solely investigated the effects of one form of adaptation (i.e. de-escalation or discontinuation) [13, 15, 16, 18–21] or focused on specific presentations of FN [13, 15, 18, 19, 21, 24, 25] or patient profiles [14–16, 18, 24]. One randomized controlled trial has found that discontinuation of EAT after 72 h of apyrexia and clinical recovery in high-risk neutropenic patients without microbiological documentation is safe and can significantly reduce unnecessary exposure to antimicrobials [13]. Several interrupted time series studies have demonstrated a significant reduction in carbapenem consumption following implementation of early adaptation of EAT but not a significant reduction in total antibiotic consumption [17, 20, 23].
In response to growing antibiotic resistance and low compliance with ECIL guidelines in the hematology department in our center, we have developed and implemented a multifaceted AMS intervention. This intervention aimed to improve the quality of FN management and to promote the adoption of early de-escalation and discontinuation strategies in high-risk neutropenic patients by the hematology team.
The goal of this before-after study was to assess the impact of a multifaceted AMS intervention on antibiotic consumption and clinical outcomes in high-risk neutropenic patients. Moreover, we sought to assess the applicability of de-escalation and discontinuation strategies as well as the compliance of the hematology team with these strategies.
Methods
Study population
This study was conducted in the hematology department of the 1800-bed University Hospital of Nice, France for two 6-month periods (October 2019–March 2020 and December 2021–May 2022). The hematology department is divided into two separate units: an 8-bed hematologic intensive care unit with laminar air flow rooms and a 16-bed conventional hospitalization unit.
During these periods, all consecutive admissions to the hematology department for intensive chemotherapy, with chemotherapy-induced neutropenia lasting seven days or more, and occurrence of at least one febrile episode, were eligible for inclusion. Admissions were excluded if patients were younger than 18 years old, had chemotherapy-induced neutropenia for less than seven days or received corticosteroids.
Definitions
High-risk neutropenia was defined as neutropenia lasting seven days or more. Fever recurrence was defined as relapse of fever during neutropenia in patients who had been afebrile for at least 48 h. FN episodes were classified as fever of unknown origin (FUO), clinically documented infection (CDI) or microbiologically documented infection (MDI), according to ECIL guidelines [11].
Discontinuation was defined as the cessation of all antibiotic therapy. De-escalation was defined as either switching to a narrower-spectrum beta-lactam according to the consensual ranking by Weiss et al. [26] or stopping one antibiotic of a combination therapy (except for the discontinuation of aminoglycosides after a single injection). Escalation was defined as either switching to a broader-spectrum beta-lactam or adding an antibiotic from a different class.
Design and implementation of the AMS intervention
The pre-intervention period lasted six months and spanned from October 2019 to March 2020. A pre-intervention period prior to the COVID-19 pandemic was chosen due to a decrease of the AMS team activity during the first wave of the pandemic, which may have impacted antibiotic prescribing practices in the hematology department. During the pre-intervention period, a dedicated hotline for antimicrobial prescribing guidance was provided but there was no systematic review of all antibiotic prescriptions. During that period, early discontinuation of EAT was not performed, while early de-escalation was rarely performed. Laboratory surveillance of antimicrobial resistance among Gram-negative bacteria isolated from blood cultures in the hematology department between January 2018 and December 2020 showed that out of the 29 Pseudomonas aeruginosa isolates, 17.2% were resistant to imipenem, 10.3% were resistant to piperacillin-tazobactam and 6.9% were resistant to cefepime. Out of the 141 Enterobacterales isolates, 7.8% produced extended spectrum beta-lactamase and 4.3% produced high level cephalosporinase, while 2.8% produced carbapenemase. As for Gram-positive bacteria, out of the 11 Staphylococcus aureus and 130 coagulase-negative staphylococci isolates, 9.1% and 70.8% were respectively resistant to methicillin. No vancomycin-resistant enterococci were isolated among the 37 isolates of enterococci.
In early 2021, we designed a persuasive multifaceted AMS intervention, which we implemented in November 2021. This intervention included the development of new local clinical guidelines in the form of visual decision algorithms. These decision algorithms were subsequently discussed and reviewed with the hematology medical team until consensus was reached. These visual decision aids were then displayed in all medical offices of the hematology department. Educational meetings were held with medical residents and paramedical staff during the implementation phase. Additionally, a patient-level review of all antibiotic prescriptions was conducted twice a week for a period of six months in the presence of the hematology medical team and the AMS team. Patient-specific recommendations regarding antibiotic therapy were provided by the AMS team based on clinical presentation and results of microbiological samples. Moreover, the AMS team offered day-to-day guidance via a dedicated hotline.
The intervention phase started in December 2021 and lasted for the subsequent six months, until May 2022. During that time, antibiotic consumption, clinical outcomes and compliance with ECIL guidelines were prospectively assessed and face-to-face meetings were organized monthly by the AMS team to provide feedback on the progress of the intervention and to discuss opportunities for improvement with the hematology team.
Febrile neutropenia guidelines
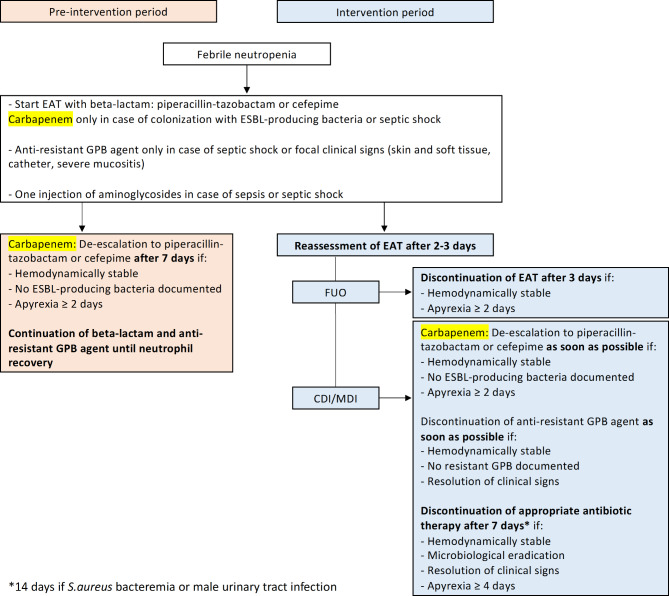
During the intervention period, EAT was systematically reassessed between 48 and 72 h after introduction. Early adaptation of EAT was encouraged in stable patients according to ECIL criteria, as shown in Fig. 1. If fever persisted in stable patients without new clinical signs, changes in antibiotic therapy were discouraged and the diagnostic work-up was continued. If fever recurred after antibiotic discontinuation, EAT was immediately reintroduced, using the same class of antibiotics as before, after new blood cultures were taken. Detailed FN guidelines and decision algorithms are available in the Supplementary material (Figure S1 to S3).
Fig. 1.
Summary of febrile neutropenia guidelines during the pre-intervention and the intervention periods. Abbreviations: EAT: Empirical antibiotic therapy; ESBL: Extended-spectrum beta-lactamase; GPB: Gram-positive bacteria
Neither gut decontamination nor bacterial prophylaxis were used during the study. Antifungal prophylaxis was used according to ECIL guidelines.
Data collection
Individual data on the pre-intervention period were retrospectively collected through a review of electronic health records (EHRs) while data on the intervention period were prospectively collected. All data on antibiotic consumption were retrieved from EHRs and were then double checked for accuracy by the pharmacy department. The applicability of de-escalation and discontinuation strategies and clinician compliance with these strategies were assessed for all FN episodes by the AMS team. Clinician compliance was defined as the application of de-escalation or discontinuation of EAT within 48 h of applicability according to the algorithms.
The primary endpoint was overall antibiotic consumption during hospital stay, expressed as days of therapy (DOT). All doses of a specific antibiotic administered on a given day were counted as one DOT [27]. The DOT for a given stay was the sum of DOT for each antibiotic [27]. Secondary endpoints included length of therapy (LOT), antibiotic-free days (AFD), 30-day mortality, intensive care unit (ICU) admission, C. difficile infection and length of stay. LOT was defined as the number of days that a patient received antibiotic therapy, irrespective of the number of drugs [27]. AFD were defined as the number of days that a patient did not receive antibiotic therapy between the onset of the first FN episode and neutrophil recovery. We defined 30-day mortality as death occurring within 30 days of the onset of neutropenia.
Statistical analysis
Continuous variables are reported as median (interquartile range) and categorical variables as number (percentage). Continuous variables were compared using the Student’s t-test or the Mann-Whitney test, when appropriate. Categorical variables were compared using the Chi-2 test or the Fisher’s exact test, when appropriate. All tests were two-tailed and p-values ≤ 0.05 were considered statistically significant. Univariate and multivariate logistic regression was performed to identify factors associated with clinician compliance with early de-escalation and discontinuation strategies. Only variables with p-values < 0.1 in the univariate analysis were included in the multivariate logistic regression model. Sample size was calculated using a power of 80% and an alpha value of 0.05. Based on preliminary data from our hematology department, mean DOT per stay was 29 days (standard deviation = 13.5 days) and a reduction of DOT by 25% in the intervention period was considered significant. Based on these assumptions, a sample size of 55 per period was required. The open-source software R Foundation for Statistical Computing (Vienna, Austria) was used for statistical analysis.
Results
A total of 113 admissions were included, according to the inclusion criteria: 56 during the pre-intervention period and 57 during the intervention period, involving 47 and 48 patients respectively.
Four educational meetings were held with medical residents and paramedical staff during the implementation phase. Forty reviews of antibiotic prescriptions were carried out by the AMS team during the implementation and the intervention phases, while 4 face-to-face meetings were held with the hematology medical team during the intervention phase to provide feedback on the intervention.
Sample characteristics
Patient and admission characteristics were similar between the two periods, as displayed in Table 1. FN episodes characteristics are summarized in Table 2. Patients experienced more febrile episodes per stay during the intervention period, although this difference was not statistically significant. The median time between the first episode of FN and neutrophil recovery was 19 days in both periods. Overall, distribution of etiologies of FN episodes did not differ between the two periods. Bacterial species and their antibiotic susceptibility isolated from blood cultures in bacteremia during the two periods are described in the Supplementary material (Table S1). Frequency of fever recurrence and time to recurrence were also similar during the two periods. Moreover, there was no statistical difference in the occurrence of sepsis and septic shock between the two periods.
Table 1.
Main characteristics of patients and admissions
| Patient characteristics | Pre-intervention period (n = 47) | Intervention period (n = 48) | p value |
|---|---|---|---|
| Age (years) | 61 (44.5–66) | 60.5 (54–67.25) | 0.099 |
| Sex (female), n (%) | 26 (55) | 18 (38) | 0.082 |
| Underlying hematologic disease, n (%) | 0.065 | ||
| Acute myeloid leukemia | 22 (47) | 29 (61) | |
| Acute lymphoblastic leukemia | 3 (6) | 0 (0) | |
| Multiple myeloma | 7 (15) | 5 (10) | |
| Lymphoma | 9 (20) | 2 (4) | |
| Myelodysplastic syndrome | 3 (6) | 3 (6) | |
| Myelofibrosis | 2 (4) | 5 (10) | |
| Other | 1 (2) | 4 (9) | |
| Charlson Comorbidity Index | 4 (3–4) | 4 (3–5) | 0.126 |
| Colonization with MDR bacteria, n (%) | 5 (11) | 3 (6) | 0.486 |
| Admission characteristics | Pre-intervention period (n = 56) | Intervention period (n = 57) | p value |
| Reason for admission, n (%) | 0.095 | ||
| Induction | 14 (25) | 26 (46) | |
| Consolidation | 8 (14) | 4 (7) | |
| Conditioning for autologous HSCT | 11 (20) | 6 (10) | |
| Conditioning for allogeneic HSCT | 22 (39) | 21 (37) | |
| Microtransplantation | 1 (2) | 0 (0) | |
| Duration of neutropenia (days) | 14 (10–22) | 18 (13–24) | 0.121 |
Data are presented as median (interquartile range) unless otherwise indicated
Colonization with MDR bacteria was defined as colonization with methicillin-resistant Staphylococcus aureus or extended-spectrum beta-lactamase producing Enterobacterales
Abbreviations: HSCT: Hematopoietic stem cell transplantation; MDR: Multidrug-resistant
Table 2.
Main characteristics of febrile neutropenia episodes
| Pre-intervention period (n = 56) | Intervention period (n = 57) | p value | |
|---|---|---|---|
| Total number of fever episodes, n | 87 | 103 | |
| Number of fever episodes per admission | 1 (1–2) | 2 (1–2) | 0.08 |
| Number of fever episodes per admission, n (%) | |||
| 1 | 33 (59) | 24 (42) | |
| 2 | 15 (27) | 20 (35) | |
| 3 | 8 (14) | 13 (23) | |
| Type of fever episodes, n (%) | 0.35 | ||
| MDI | 19 (22) | 32 (31) | |
| Bacteremia | 9 (48) | 18 (57) | |
| Urinary tract infection | 4 (21) | 3 (9) | |
| CVC-related infection | 4 (21) | 8 (25) | |
| Clostridioides difficile infection | 1 (5) | 0 (0) | |
| Fungal infection | 1 (5) | 3 (9) | |
| CDI | 36 (41) | 39 (38) | |
| Oral mucositis/dental | 9 (25) | 6 (15) | |
| Abdominal | 9 (25) | 8 (21) | |
| Skin and soft tissue | 0 (0) | 6 (15) | |
| Pulmonary | 7 (20) | 8 (21) | |
| Perianal | 4 (11) | 3 (8) | |
| CVC | 3 (8) | 3 (8) | |
| Multiple | 4 (11) | 5 (12) | |
| FUO | 32 (37) | 32 (31) | |
| Duration of fever episode (days) | 4 (2–7) | 3 (2–5) | 0.326 |
| Fever recurrence, n (%) | 31 (36) | 46 (45) | 0.207 |
| Time to fever recurrence (days) | 6 (4–10.5) | 7 (4–10) | 0.896 |
| Sepsis, n (%) | 7 (8) | 9 (9) | 0.864 |
| Septic shock, n (%) | 2 (2) | 3 (3) | 1 |
Data are presented as median (interquartile range) unless otherwise indicated
Abbreviations: CDI: Clinically documented infection; CVC: Central venous catheter; FUO: Fever of unknown origin; MDI: Microbiologically documented infection
Applicability and compliance with de-escalation and discontinuation strategies
Applicability and compliance with early de-escalation and discontinuation strategies are presented in Table 3. Early de-escalation or discontinuation of EAT was applicable in 56% of FN episodes in the pre-intervention period and 69% of FN episodes in the intervention period. Reasons for non-applicability included presentation with septic shock, failure to achieve the required duration of treatment and apyrexia or failure to obtain clinical recovery before neutrophil recovery. Compliance with early de-escalation and discontinuation strategies was significantly higher in the intervention period than in the pre-intervention period.
Table 3.
Applicability and compliance with early de-escalation and discontinuation strategies
| Pre-intervention period (n = 87) | Intervention period (n = 103) | |
|---|---|---|
| Episodes when strategies applicable, n (%) | 49 (56) | 71 (69) |
| Compliance*, n (%) | 4 (8) | 55 (77) |
| De-escalation | 4 (8) | 12 (17) |
| De-escalation followed by discontinuation | 0 (0) | 6 (8) |
| Discontinuation | 0 (0) | 37 (52) |
| Non-compliance*, n (%) | 45 (92) | 16 (23) |
| Lack of timelinessa | 2 (4) | 11 (16) |
| Continuation | 40 (82) | 3 (4) |
| Escalation | 3 (6) | 2 (3) |
| Episodes when strategies not applicable, n (%) | 38 (44) | 32 (31) |
*p < 0.001 for the comparison of compliance between pre-intervention and intervention periods
aLack of timeliness was defined as a delay of more than 48 h in the application of early discontinuation or de-escalation strategies
In univariate analysis, compliance with early de-escalation and discontinuation of EAT was more frequent in patients with acute myeloid leukemia (OR 2.62, 95% CI [1.25–5.5], p = 0.011) and in patients from the intervention period (OR 38.67, 95% CI [12.07–123.9], p < 0.001). In multivariate analysis, inclusion in the intervention period was the only factor associated with compliance with early de-escalation and discontinuation strategies (OR 49.27, 95% CI [12.53–193.68], p < 0.001). The logistic regression model is included in the Supplementary material (Table S2).
Antibiotic consumption
Antibiotic consumption data are presented in Table 4. In the intervention period, the total number of DOT per 1000 hospital days decreased by 24%. Following the implementation of the intervention, there was a significant decrease in overall antibiotic consumption: overall antibiotic DOT per stay decreased by a median of 8 days while LOT decreased by a median of 6 days. The number of AFD between the onset of the first FN episode and neutrophil recovery was significantly greater in the intervention period. When assessing specific antibacterial agents, carbapenem DOT and anti-resistant Gram-positive agents DOT (e.g., glycopeptides, lipopeptides and oxazolidinones) were significantly lower in the intervention period. The number of days of therapy with piperacillin-tazobactam, cefepime, aminoglycosides and quinolones did not differ significantly between the two periods.
Table 4.
Antibiotic consumption
| Pre-intervention period (n = 56) | Intervention period (n = 57) | p value | |
|---|---|---|---|
| DOT/1000 hospital days, n | 860 | 648 | |
| DDD/1000 hospital days, n | 1216 | 946 | |
| Overall antibiotic DOT | 28 (20.75–40) | 20 (10–32) | 0.006 |
| LOT | 21 (14–24.25) | 15 (8–23) | 0.006 |
| Antibiotic-free days | 0 (0–0) | 2 (0–8) | < 0.001 |
| Aminoglycosides, n (%) | 20 (36) | 16 (28) | |
| DOT | 1 (1–1) | 1 (1–1) | 0.553 |
| Carbapenems, n (%) | 26 (46) | 20 (35) | |
| DOT | 9 (6.25–15.25) | 5.5 (4–8) | 0.017 |
| Piperacillin-tazobactam, n (%) | 55 (98) | 49 (86) | |
| DOT | 14 (8–21.5) | 10 (6–17) | 0.084 |
| Cefepime, n (%) | 4 (7) | 20 (35) | |
| DOT | 6.5 (5–10.5) | 7.5 (4–10) | 0.953 |
| Piperacillin-tazobactam/Cefepime/Aztreonam, n (%) | 56 (100) | 56 (98) | |
| DOT | 14.5 (8–22) | 12.5 (8–20) | 0.61 |
| Quinolones, n (%) | 5 (9) | 5 (9) | |
| DOT | 3 (2–8) | 4 (2–8) | 0.733 |
| Anti-resistant Gram-positive agents, n (%) | 36 (64) | 36 (63) | |
| DOT | 11.5 (7–18) | 8 (4–12) | 0.017 |
Data are presented as median (interquartile range) unless otherwise indicated
DOT and LOT are expressed in days
Anti-resistant Gram-positive agents include glycopeptides, lipopeptides and oxazolidinones
Abbreviations: DDD: Defined daily dose; DOT: Days of therapy; LOT: Length of therapy
Clinical outcomes
Clinical outcomes are displayed in Table 5. We found no statistical difference in the rates of ICU admission (9% during each period) and 30-day mortality (0% vs. 5%) between the two periods. However, none of the three deaths in the intervention period were related to bacterial complications (i.e., hepatic veno-occlusive disease following allogeneic HSCT, coronavirus pneumonia, invasive pulmonary aspergillosis). Two Clostridioides difficile infections were diagnosed during the pre-intervention period and none during the intervention period. Finally, length of stay did not differ significantly between the two periods.
Table 5.
Clinical outcomes
| Outcomes | Pre-intervention period (n = 56) | Intervention period (n = 57) | p value |
|---|---|---|---|
| Clostridioides difficile infection | 2* (4) | 0 (0) | 0.243 |
| ICU admission | 5 (9) | 5 (9) | 1 |
| Bacterial infection-related ICU admission | 2 (4) | 2 (4) | 1 |
| 30-day mortality | 0 (0) | 3 (5) | 0.243 |
| Bacterial infection-related 30-day mortality | 0 (0) | 0 (0) | 1 |
| In-hospital mortality | 2 (4) | 3 (5) | 1 |
| Length of stay (days), median (IQR) | 30.5 (27–41) | 34 (29–39) | 0.308 |
Data are presented as number (%) unless otherwise indicated
Abbreviations: ICU: Intensive care unit; IQR: Interquartile range
*One Clostridioides difficile infection occurred after neutrophil recovery and antibiotic discontinuation
Discussion
These findings suggest that the implementation of an AMS intervention is a safe and effective tool to optimize antibiotic consumption in high-risk neutropenic patients. Implementation of early de-escalation and discontinuation strategies resulted in lower overall antibiotic consumption as well as lower carbapenem and anti-resistant Gram-positive agents consumption. The AMS intervention did not significatively affect aminoglycosides and quinolones consumption, which may likely be explained by the low consumption of these molecules prior to the implementation of this intervention. It should be noted that cefepime use was more frequent during the intervention period due to growing resistance to piperacillin-tazobactam in the department between 2015 and 2020. Despite decreased overall antibiotic consumption, we found no detrimental effects of the AMS intervention on 30-day mortality, ICU admission and length of stay. Assessing clinical outcomes is key to reassure clinicians that AMS interventions do not negatively affect patient safety, in order to increase adherence to these interventions. Patients receiving corticosteroids were excluded from the intervention due to the potential masking effect of corticosteroids on fever, which may have delayed the reintroduction of antibiotics in these patients. Acceptability of early de-escalation or discontinuation of EAT was high following the implementation of the AMS intervention. Indeed, clinician compliance was observed in more than 75% of episodes when strategies were applicable. It is worth mentioning that late adaptation of EAT was the most frequent form of non-compliance in the intervention period whereas continuation of EAT was the most frequent form of non-compliance in the pre-intervention period. High compliance to early adaptation of EAT in this study can likely be attributed to the collaborative and persuasive approach throughout the implementation of this AMS intervention, which was maintained through regular face-to-face meetings and ongoing feedback. Handshake stewardship has been shown to be an effective and sustainable approach to decrease anti-infective use [28]. Exploring the barriers and facilitators to appropriate fever management and antibiotic prescribing by hematologists is key to enhance the adoption of early adaptation of EAT in high-risk neutropenic patients.
In line with previous studies, early de-escalation and discontinuation of EAT was safe in this study. Two prior larger studies found that the implementation of an AMS intervention resulted in improved clinical outcomes, including decreased ICU admission or overall mortality [22, 23]. We believe that our study did not have sufficient power to detect such difference. Given the growing evidence on the safety of early adaptation of EAT, ECIL guidelines should be more widely implemented in hematology departments. In contrast to previous studies [17, 23], we observed a significant decrease in overall antibiotic consumption after implementation of the intervention. This may be explained by the fact that despite lower carbapenem consumption in the intervention phase, there was no increase in the consumption of other beta-lactams, indicating a high uptake of both de-escalation and discontinuation strategies. Previous studies have reported discordant results regarding the occurrence of fever recurrence after early adaptation of EAT [13, 14, 21, 22, 25]. In our study, fever recurred after more than 35% of febrile episodes, without significant variation between the two periods. Moreover, the frequency of microbiological documentation did not differ significantly between the two periods. These results suggest that early adaptation of EAT, as recommended by ECIL guidelines, does not increase the risk of infection relapse. Given the frequency of fever recurrence and its occurrence independent of EAT adaptation, de-escalation and discontinuation strategies should be applied in the same manner after fever recurrence.
One of the strengths of this study is the use of a validated metric to measure antibiotic consumption [27, 29]. Antibiotic consumption was expressed as DOT to account for the use of combination therapy, in order to reflect total antibiotic exposure. Moreover, antibiotic consumption was measured at the level of the study population and not for the whole department and therefore more directly reflects the effects of the AMS intervention. Finally, the required study sample size, based on an expected reduction of overall antibiotic consumption by 25%, was achieved.
This study has several limits, including the retrospective inclusion of patients in the pre-intervention period which may have introduced confounding factors to the analysis, such as the type of chemotherapy used. Evaluation of the impact of the AMS intervention was performed according to a before-after design, which did not account for pre-existing trends unlike interrupted time series (ITS) analysis [30]. However, ITS analysis could not be performed due to insufficient time points during the two periods. Furthermore, the before-after design without a concomitant control group did not account for potential confounding external factors, which may have affected our results. This study could also have benefited from assessing the impact of the AMS intervention on bacterial antibiotic resistance. Indeed, reducing selective pressure on bacterial flora by decreasing unnecessary antibiotic exposure in high-risk neutropenic patients may positively impact local antimicrobial resistance patterns and should be the subject of future research. Moreover, some AMS interventions have been shown to have unsustainable long-term effects after removal of the intervention, despite initial positive effects on antibiotic consumption [31]. Considering that AMS interventions are labor-intensive and cost-intensive, studies on the cost-effectiveness and sustainability of these interventions in high-risk neutropenic patients are needed.
Conclusion
The implementation of a multifaceted AMS intervention led to high compliance with early de-escalation and discontinuation of EAT by the hematology team. High application of de-escalation and discontinuation of EAT resulted in lower overall antibiotic consumption, without negatively affecting clinical outcomes. These results suggest that AMS interventions, based on multidisciplinary collaboration and personalized clinical recommendations, are a safe and effective tool to optimize the quality of antibiotic prescribing and fever management in high-risk neutropenic patients. Prospective studies are needed to evaluate the sustainability and long-term impact of AMS interventions on antibiotic consumption, clinical outcomes and antibiotic resistance in high-risk hematological patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: File S1. Febrile neutropenia guidelines. Figure S1. Decision algorithm for introduction of empirical antibiotic therapy for febrile neutropenia. Figure S2. Decision algorithm for reassessment of empirical antibiotic therapy with piperacillin-tazobactam or cefepime. Figure S3. Decision algorithm for reassessment of empirical antibiotic therapy with carbapenem. Table S1. Description of bacterial species and antibiotic susceptibility isolated from blood cultures in bacteremia. Table S2. Logistic regression model: factors associated with compliance with early de-escalation and discontinuation strategies
Acknowledgements
The authors would like to thank the Pharmacy Department and the Bacteriology Department of the University Hospital of Nice for their collaboration on this study. Preliminary results of this work were presented at the 2022 Thematic Days of the French Infectious Diseases Society (SPILF).
Author contributions
C.D. and K.R. wrote the febrile neutropenia guidelines. M.L. and T.C. reviewed the febrile neutropenia guidelines. C.D., K.R. and M.C. designed the intervention. C.D., N.R. and A.E. collected the data. C.D. performed statistical analyses. M.C. supervised the study. C.D., K.R. and J.C. drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was not required since all procedures were routine. The study was approved by the Institutional Review Board (IRB00011642) of the French Infectious Diseases Society (CER-MIT n° 2021 − 1101).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klastersky J. Management of Fever in neutropenic patients with different risks of Complications. Clin Infect Dis. 2004;39:32–7. doi: 10.1086/383050. [DOI] [PubMed] [Google Scholar]
- 2.Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sánchez-Ortega I, et al. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother. 2011;66:657–63. doi: 10.1093/jac/dkq494. [DOI] [PubMed] [Google Scholar]
- 3.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over Time and the risk of Clostridium difficile Infection. Clin Infect Dis. 2011;53:42–8. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 4.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Risk factors for hospital-acquired candidemia. A matched case-control study. Arch Intern Med. 1989;149:2349–53. doi: 10.1001/archinte.1989.00390100145030. [DOI] [PubMed] [Google Scholar]
- 5.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177:1308–15. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58:3799–803. doi: 10.1128/AAC.02561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 8.Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet Lond Engl. 2016;387:176–87. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 9.Tamma PD, Cosgrove SE. Antimicrobial stewardship. Infect Dis Clin North Am. 2011;25:245–60. doi: 10.1016/j.idc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Dyar OJ, Huttner B, Schouten J, Pulcini C, ESGAP (ESCMID Study Group for Antimicrobial stewardshiP) What is antimicrobial stewardship? Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2017;23:793–8. doi: 10.1016/j.cmi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica. 2013;98:1826–35. [DOI] [PMC free article] [PubMed]
- 12.Verlinden A, Mikulska M, Knelange NS, Averbuch D, Styczynski J, Infectious Diseases Working Party (IDWP) of the European Group for Blood and Marrow Transplantation Group (EBMT) Current antimicrobial practice in febrile neutropenia across Europe and Asia: the EBMT Infectious Disease Working Party survey. Bone Marrow Transplant. 2020;55:1588–94. doi: 10.1038/s41409-020-0811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilar-Guisado M, Espigado I, Martín-Peña A, Gudiol C, Royo-Cebrecos C, Falantes J, et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (how long study): an open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017;4:e573–83. doi: 10.1016/S2352-3026(17)30211-9. [DOI] [PubMed] [Google Scholar]
- 14.Gustinetti G, Raiola AM, Varaldo R, Galaverna F, Gualandi F, Del Bono V, et al. De-escalation and discontinuation of empirical antibiotic treatment in a cohort of allogeneic hematopoietic stem cell transplantation recipients during the Pre-engraftment Period. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2018;24:1721–6. doi: 10.1016/j.bbmt.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Schauwvlieghe A, Dunbar A, Storme E, Vlak A, Aerts R, Maertens J, et al. Stopping antibiotic therapy after 72 h in patients with febrile neutropenia following intensive chemotherapy for AML/MDS (safe study): a retrospective comparative cohort study. EClinicalMedicine. 2021;35:100855. doi: 10.1016/j.eclinm.2021.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Wyngaert Z, Berthon C, Debarri H, Bories C, Bonnet S, Nudel M, et al. Discontinuation of antimicrobial therapy in adult neutropenic haematology patients: a prospective cohort. Int J Antimicrob Agents. 2019;53:781–8. doi: 10.1016/j.ijantimicag.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 17.la Martire G, Robin C, Oubaya N, Lepeule R, Beckerich F, Leclerc M, et al. De-escalation and discontinuation strategies in high-risk neutropenic patients: an interrupted time series analyses of antimicrobial consumption and impact on outcome. Eur J Clin Microbiol Infect Dis. 2018;37:1931–40. doi: 10.1007/s10096-018-3328-1. [DOI] [PubMed] [Google Scholar]
- 18.Snyder M, Pasikhova Y, Baluch A. Early antimicrobial de-escalation and stewardship in adult hematopoietic stem cell transplantation recipients: Retrospective Review. Open Forum Infect Dis. 2017;4:ofx226. doi: 10.1093/ofid/ofx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Clech L, Talarmin J-P, Couturier M-A, Ianotto J-C, Nicol C, Le Calloch R, et al. Early discontinuation of empirical antibacterial therapy in febrile neutropenia: the ANTIBIOSTOP study. Infect Dis Lond Engl. 2018;50:539–49. doi: 10.1080/23744235.2018.1438649. [DOI] [PubMed] [Google Scholar]
- 20.Niessen FA, van Mourik MSM, Bruns AHW, Raijmakers Ra, de Groot P, van der Bruggen MCH. Early discontinuation of empirical antibiotic treatment in neutropenic patients with acute myeloid Leukaemia and high-risk Myelodysplastic Syndrome. Antimicrob Resist Infect Control. 2020;9:74. doi: 10.1186/s13756-020-00729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paret R, Le Bourgeois A, Guillerm G, Tessoulin B, Rezig S, Gastinne T, et al. Safety and risk of febrile recurrence after early antibiotic discontinuation in high-risk neutropenic patients with haematological malignancies: a multicentre observational study. J Antimicrob Chemother. 2022;77:2546–56. doi: 10.1093/jac/dkac190. [DOI] [PubMed] [Google Scholar]
- 22.Verlinden A, Jansens H, Goossens H, Anguille S, Berneman ZN, Schroyens WA, et al. Safety and efficacy of antibiotic de-escalation and discontinuation in high-risk hematological patients with febrile Neutropenia: a single-center experience. Open Forum Infect Dis. 2022;9:ofab624. doi: 10.1093/ofid/ofab624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contejean A, Abbara S, Chentouh R, Alviset S, Grignano E, Gastli N, et al. Antimicrobial stewardship in high-risk febrile neutropenia patients. Antimicrob Resist Infect Control. 2022;11:52. doi: 10.1186/s13756-022-01084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petteys MM, Kachur E, Pillinger KE, He J, Copelan EA, Shahid Z. Antimicrobial de-escalation in adult hematopoietic cell transplantation recipients with febrile neutropenia of unknown origin. J Oncol Pharm Pract off Publ Int Soc Oncol Pharm Pract. 2020;26:632–40. doi: 10.1177/1078155219865303. [DOI] [PubMed] [Google Scholar]
- 25.Rearigh L, Stohs E, Freifeld A, Zimmer A. De-escalation of empiric broad spectrum antibiotics in hematopoietic stem cell transplant recipients with febrile neutropenia. Ann Hematol. 2020;99:1917–24. doi: 10.1007/s00277-020-04132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss E, Zahar J-R, Lesprit P, Ruppe E, Leone M, Chastre J, et al. Elaboration of a consensual definition of de-escalation allowing a ranking of β-lactams. Clin Microbiol Infect. 2015;21:649e1–10. doi: 10.1016/j.cmi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Stanić Benić M, Milanič R, Monnier AA, Gyssens IC, Adriaenssens N, Versporten A, et al. Metrics for quantifying antibiotic use in the hospital setting: results from a systematic review and international multidisciplinary consensus procedure. J Antimicrob Chemother. 2018;73:vi50–8. doi: 10.1093/jac/dky118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacBrayne CE, Williams MC, Levek C, Child J, Pearce K, Birkholz M, et al. Sustainability of handshake stewardship: extending a hand is effective years later. Clin Infect Dis off Publ Infect Dis Soc Am. 2020;70:2325–32. doi: 10.1093/cid/ciz650. [DOI] [PubMed] [Google Scholar]
- 29.Moehring RW, Anderson DJ, Cochran RL, Hicks LA, Srinivasan A, Dodds Ashley ES, et al. Expert Consensus on Metrics to assess the impact of patient-level antimicrobial stewardship interventions in Acute-Care settings. Clin Infect Dis. 2017;64:377–83. doi: 10.1093/cid/ciw787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweitzer VA, van Werkhoven CH, Rodríguez Baño J, Bielicki J, Harbarth S, Hulscher M, et al. Optimizing design of research to evaluate antibiotic stewardship interventions: consensus recommendations of a multinational working group. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2020;26:41–50. doi: 10.1016/j.cmi.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Effective Practice and Organisation of Care Group, editor. Cochrane Database Syst Rev [Internet]. 2017 [cited 2023 Apr 20]; 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: File S1. Febrile neutropenia guidelines. Figure S1. Decision algorithm for introduction of empirical antibiotic therapy for febrile neutropenia. Figure S2. Decision algorithm for reassessment of empirical antibiotic therapy with piperacillin-tazobactam or cefepime. Figure S3. Decision algorithm for reassessment of empirical antibiotic therapy with carbapenem. Table S1. Description of bacterial species and antibiotic susceptibility isolated from blood cultures in bacteremia. Table S2. Logistic regression model: factors associated with compliance with early de-escalation and discontinuation strategies
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.