Abstract
Adherence of Haemophilus influenzae to epithelial cells plays a central role in colonization and is the first step in infection with this organism. Pili, which are large polymorphic surface proteins, have been shown to mediate the binding of H. influenzae to cells of the human respiratory tract. Earlier experiments have demonstrated that the major epitopes of H. influenzae pili are highly conformational and immunologically heterogenous; their subunit pilins are, however, immunologically homogenous. To define the extent of structural variation in pilins, which polymerize to form pili, the pilin genes (hifA) of 26 type a to f and 16 nontypeable strains of H. influenzae were amplified by PCR and subjected to restriction fragment length polymorphism (RFLP) analysis with AluI and RsaI. Six different RFLP patterns were identified. Four further RFLP patterns were identified from published hifA sequences from five nontypeable H. influenzae strains. Two patterns contained only nontypeable isolates; one of these contained H. influenzae biotype aegyptius strains F3031 and F3037. Another pattern contained predominantly H. influenzae type f strains. All other patterns were displayed by a variety of capsular and noncapsular types. Sequence analysis of selected hifA genes confirmed the 10 RFLP patterns and showed strong identity among representatives displaying the same RFLP patterns. In addition, the immunologic reactivity of pili with antipilus antisera correlated with the groupings of strains based on hifA RFLP patterns. Those strains that show greater reactivity with antiserum directed against H. influenzae type b strain M43 pili tend to fall into one RFLP pattern (pattern 3); while those strains that show equal or greater reactivity with antiserum directed against H. influenzae type b strain Eagan pili tend to fall in a different RFLP pattern (pattern 1). Sequence analysis of representative HifA pilins from typeable and nontypeable H. influenzae identified several highly conserved regions that play a role in bacterial pilus assembly and other regions with considerable amino acid heterogeneity. These regions of HifA amino acid sequence heterogeneity may explain the immunologic diversity seen in intact pili.
Haemophilus influenzae is a fastidious, gram-negative bacterium that is commonly found as a commensal organism in the human nasopharynx (28). H. influenzae is characterized as encapsulated (possessing one of six chemically and immunologically distinct polysaccharide capsules, i.e., types a to f) or nonencapsulated (i.e., nontypeable H. influenzae). Invasive infections, such as bacteremia, cellulitis, septic arthritis, and meningitis, occur in nonimmune hosts and are usually caused by organisms possessing the type b capsule. In children, immunocompromised individuals, and individuals with underlying pulmonary disease (e.g., cystic fibrosis, chronic bronchitis, and chronic obstructive pulmonary disease), H. influenzae can cause localized respiratory infections, such as otitis media, sinusitis, conjunctivitis, and pneumonia, and acute exacerbations of chronic lung diseases (16, 28, 30, 34).
Colonization of the upper respiratory tract is an essential step in the pathogenesis of H. influenzae disease and is a likely target for therapeutic intervention. Both typeable and nontypeable H. influenzae organisms have been shown to adhere to cultured epithelial cells and human nasopharyngeal tissues (33). One of the cell surface molecules shown to mediate attachment to epithelial cells is the polymeric hemagglutinating pilus found on both typeable and nontypeable H. influenzae (15).
Five genes (hifA, hifB, hifC, hifD, and hifE) are required for the synthesis of mature H. influenzae pili, and they are located on an approximately 6-kb chromosomal locus (15, 26, 40). hifA encodes the major pilin subunit and lies on one end of the pilus gene cluster (26, 40). The HifA pilin is approximately 24 kDa and comprises the primary structural component of the shaft of the mature pilus (9, 27, 35). The hifA pilin genes of 11 H. influenzae strains, including 5 type b strains and 6 nontypeable strains (including 2 H. influenzae biotype aegyptius strains), have been cloned and their nucleotide sequences have been determined in earlier studies by several investigators (3, 10, 12, 20, 22, 37, 39, 43).
Immunologic characterizations of intact H. influenzae pili and the HifA pilins have been complicated by the fact that intact pili are highly conformational and are immunologically diverse while denatured pilins are immunologically homogeneous (11, 13). Further, polyclonal antisera raised against native pili from type b strains Eagan and M43 bind to homologous piliated type b H. influenzae but do not bind to homologous denatured HifA pilins, suggesting that epitopes defined by these sera may be assembled by protein folding or by protein-protein interactions and are not available on denatured pilins (13). Similarily, polyclonal antisera raised against pilins of strains M43 and Eagan do not bind to intact pili of the homologous strains (11, 13).
The two goals of this work were (i) to identify differences in the HifA sequences from several different typeable and nontypeable H. influenzae isolates that might explain the pilus immunologic heterogeniety and (ii) to identify sequence similarities that might relate to functional importance in bacterial pilus assembly. To do this analysis, the hifA genes from 26 typeable and 16 nontypeable strains were amplified by PCR and subjected to restriction fragment length polymorphism (RFLP) analysis with AluI and RsaI. Six different RFLP patterns were displayed with this analysis, and four more patterns were revealed from the nucleotide sequences of cloned hifA genes. Cloning and sequencing of representative hifA genes from each of the six RFLP patterns were performed and used for further analysis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The H. influenzae strains used in this study are presented in Table 1. Except for strains AAr108 and AA61, the strains listed were isolated from individuals in a variety of geographical areas over a number of years and thus probably represent different bacterial clones. Strains AAr108 and AA61 were isolated from a mother and her son and may be the same strain. Bacterial strains designated AA and AAr were obtained from the clinical laboratories at the University of Michigan from 1983 to 1988, while strains designated M and Mr were obtained from the clinical laboratories at the University of Minnesota from 1979 to 1982. Bacterial strains were grown on Levinthal agar (37 g of brain heart infusion broth [Difco Laboratories, Detroit, Mich.], 18 g of Bacto agar [Difco], 2,000 μg of NAD [Sigma Chemical Co., St. Louis, Mo.], and 2,000 μg of hemin [Sigma] in 1,000 ml of deionized water) at 37°C with 5% CO2 for 18 to 24 h (13). The H. influenzae strains were classified by using type-specific anticapsular antisera (for types a to f [Difco]) in a slide agglutination test.
TABLE 1.
hifA RFLP comparison of various H. influenzae clinical isolates by using AluI and RsaI
| hifA RFLP patterna | Strainb | Source and/or referencec | Capsular type |
|---|---|---|---|
| 1 | Eagan (E1a) | CSF (10) | b |
| AAr108 | Throat (13) | b | |
| AA61 | Blood (13) | b | |
| R9 | (13) | b | |
| AAr120 | Trachea (this study) | f | |
| AAr39 | Throat (11) | NTf | |
| AAr176d | Nasopharynx (11) | NT | |
| AAr64 | Trachea (13) | b (lost capsule) | |
| AAr169 | Sinus (11) | NT | |
| AAr117 | Nasopharynx (this study) | b (lost capsule) | |
| 2 | Mr31 | Middle ear (11) | NT |
| AAr49d | Throat (11) | NT | |
| 1712MEE (LB5)d | Middle ear (11) | NT | |
| AAr91 | Throat (11) | NT | |
| 3 | M96 | CSF (this study) | a |
| M43e | Throat (12) | b | |
| AM30 (770235) | CSF (39) | b | |
| MinnA | CSF (3) | b | |
| AO2 | Throat (22) | b | |
| AAr122 | Nasopharynx (13) | b | |
| SL2 | Throat (13) | b | |
| AAr7 | Nasopharynx (23) | b | |
| AAr103 | Eye (23) | b | |
| AAr119 | Nasopharynx (13) | b | |
| ATCC 9007d | ATCC | c | |
| AAr45 | Nasopharynx (11) | NT | |
| AAr154 | Nasopharynx (11) | NT | |
| AAr60 | Peritonsillar abscess (this study) | NT | |
| 86-1249 | Middle ear (1) | NT | |
| 4 | ATCC 9008 | Throat (ATCC) | d |
| AAr191 | Throat (this study) | e | |
| AAr73d | Nasopharynx (11) | NT | |
| 86-042NP (LB1) | Nasopharynx (11) | NT | |
| 1128MEE (LB2) | Middle ear (11) | NT | |
| AAr160d | Trachea (11) | NT | |
| 5 | AAr107 | Nasopharynx (this study) | e |
| AA18d | Blood (this study) | f | |
| AAr9 | Throat (this study) | f | |
| AAr32d | Throat (this study) | f | |
| AAr80 | Nasopharynx (this study) | f | |
| AA12 | Blood (this study) | f | |
| AA58 | Blood (this study) | f | |
| AA195 | Blood (this study) | f | |
| 6 | ATCC 9006d | ATCC | a |
| AAr157d | Nasopharynx (11) | NT | |
| AAr180 | Sputum (11) | NT | |
| 7 | 86-0295 | Middle ear (1) | NT |
| 8 | 81-0384 | Middle ear (1) | NT |
| 9 | M37 | Nasopharynx (3) | NT |
| 10 | F3037 | Blood (37) | NT |
| F3031 | Blood (43) | NT |
hifA RFLP fragment sizes (in base pairs) based on DNA sequences. Pattern 1: AluI, 374, 213, 41, 23; RsaI, 411, 117, 86, 37. Pattern 2: AluI, 288, 243, 41, 29, 23; RsaI, 243, 189, 155, 37. Pattern 3: AluI, 365, 159, 95, 23; RsaI, 264, 144, 111, 68, 37, 18. Pattern 4: AluI, 320, 112, 80, 48, 41, 23; RsaI, 501, 86, 37. Pattern 5: AluI, 314, 235, 55, 23, 10; RsaI, 263, 254, 83, 37. Pattern 6: AluI, 262, 146, 100, 54, 41, 23; RsaI, 589, 37. Pattern 7: AluI, 272, 127, 98, 60, 47, 23, 12; RsaI, 339, 228, 72. Pattern 8: AluI, 613, 23; RsaI, 264, 228, 84, 48, 12. Pattern 9: AluI, 380, 236, 23; RsaI, 335, 267, 37. Pattern 10: AluI, 284, 165, 164, 23; RsaI, none.
Strains with published hifA sequences are in boldface type. Database accession numbers or references are as follows: Eagan (E1a), M64334; M43, reference 12; AO2, X52419; AM30 (770235), X16991; 86-1249, U19761; 86-0295, U19730; 81-0384, U19795; M37 and MinnA, reference 3; F3037, S59288; F3031, L08606.
CSF, cerebrospinal fluid; ATCC, American Type Culture Collection.
H. influenzae strains whose hifA genes were sequenced in this study.
The hifA sequences from strains M43, AM30 (770235), and MinnA are identical and are treated as one sequence throughout this article.
NT, nontypeable H. influenzae.
Competent Escherichia coli DH5α (Gibco BRL, Gaithersburg, Md.) was grown in Luria-Bertani (LB) broth or on LB agar (Gibco BRL) at 37°C for transformation. Transformants were screened on LB agar containing 100 μg of ampicillin (Sigma) per ml and 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal [Sigma]) per ml.
Isolation of genomic DNA from H. influenzae.
Genomic DNA was isolated from H. influenzae either by a modification of the Marmur procedure (25, 42) or by the Wizard genomic DNA purification kit (Promega, Madison, Wis.).
Amplification of hifA from H. influenzae by PCR.
PCR was used for the amplification of hifA from H. influenzae genomic DNA. Primers used were based on the conserved 5′ and 3′ regions of six hifA genes from strains Eagan, M43, AM30, 86-1249, 86-0295, and 81-0384 (3, 10, 12, 20, 22, 37, 39, 43). The primer sequences were derived from the 5′ and 3′ regions in the hifA gene that show significant nucleotide sequence identity among the six H. influenzae pilin sequences. The nucleotide sequences of these primers were 5′-ATGAAAAAAACACT(AT)CTTGGTAGC-3′ and 5′-TTAT(CT)CGTAAGCAATT(GT)GGAAACC-3′. Fifty nanograms of H. influenzae genomic DNA was mixed with 20 pmol of each primer and 45 μl of PCR SuperMix (Gibco BRL) to a final volume of 50 μl and overlayed with mineral oil (Sigma). The final PCR mixture contained 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphates (dATP, dCTP, dGTP, and dTTP), and 1 U of recombinant Taq DNA polymerase along with the H. influenzae genomic DNA and primers. The published error frequency for Taq DNA polymerase ranges from 1.1 × 10−4 errors/bp to 8.9 × 10−5 errors/bp (2, 38). The mixture was first incubated for 1 min at 95°C and then for 35 cycles of 95°C for 1 min, 50°C for 1 min, and 72°C for 2 min, followed by a final elongation step for 3 min at 72°C in a model PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, Mass.). After amplification, samples were separated on 1% agarose gels and bands were visualized after staining with ethidium bromide (Sigma) and illumination by UV light. Molecular weight markers were run to estimate PCR fragment sizes (50- and 100-bp ladders [Gibco BRL]).
RFLP analysis of hifA PCR fragments.
Amplified hifA PCR fragments were digested with restriction endonucleases AluI and RsaI according to the manufacturer’s directions (Gibco BRL). Digestion products were resolved on 1% agarose gels and were visualized on a UV transilluminator after ethidium bromide staining. Molecular weight markers were run to estimate AluI and RsaI fragment sizes (50- and 100-bp ladders [Gibco BRL]). H. influenzae strains were grouped according to the hifA AluI and RsaI digestion patterns displayed after agarose gel electrophoresis.
Cloning of representative hifA genes.
Two representative strains from each RFLP group were selected for cloning and sequencing of their hifA genes (Table 1). Amplified hifA fragments were electrophoresed on a preparative 1% agarose gel and purified with the GeneClean II kit (Bio 101, Inc., La Jolla, Calif.). The purified hifA PCR fragments were ligated into the SrfI site of pCR-Script Amp SK(+) after generation of blunt ends with a PCR Polishing Kit (Stratagene Cloning Systems, La Jolla, Calif.) and transformed into competent E. coli DH5α (Gibco BRL). Transformants were selected on LB agar containing 100 μg of ampicillin per ml and 40 μg of X-Gal per ml. Plasmid DNA from putative transformants was isolated with Qiagen Minipreps (Qiagen, Chatsworth, Calif.). BamHI/NotI (Gibco BRL) double digests were used to confirm DNA inserts in the isolated recombinant plasmids.
Sequencing of cloned representative hifA PCR fragments.
Cloned representative hifA genes were sequenced at the University of Michigan Medical School DNA Core Facility with an Applied Biosystems model 373A automated sequencer (Applied Biosystems, Inc., Foster City, Calif.). Sequencing primers were purchased from Stratagene (M13 −20 and reverse primers) and synthesized at the University of Michigan Medical School DNA Core Facility with the hifA DNA sequences from the representative cloned genes. DNA and protein sequences were analyzed with Lasergene Biocomputing software for the Macintosh from DNASTAR, Inc. (Madison, Wis.) and the Wisconsin Package, version 9.0, from the Genetics Computer Group (GCG) (Madison, Wis.) (5).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the hifA DNA sequences and the derived protein sequences determined in this study are as follows: AAr176, AF020908; AAr49, AF020909; 1712MEE (LB5), AF020910; ATCC 9007, AF020911; AAr73, AF020912; AAr160, AF020913; AA18, AF020914; AAr32, AF020915; ATCC 9006, AF020916; AAr157, AF020917.
RESULTS
PCR amplification and RFLP analysis of hifA from typeable and nontypeable H. influenzae.
The hifA genes from representative typeable (n = 26) and nontypeable (n = 16) H. influenzae (Table 1) were amplified directly from genomic DNA. The resultant hifA PCR products were approximately 650 bp in length (data not shown). H. influenzae Rd, which lacks the hif gene cluster (8), was used as a negative control and did not yield PCR products with the hifA primers.
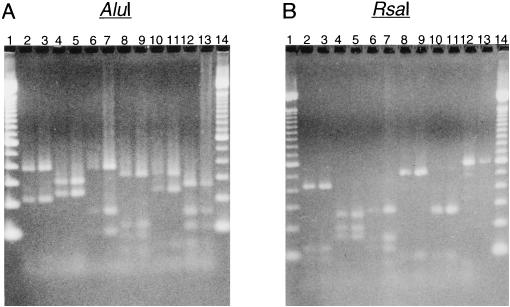
RFLP analysis was performed on the amplified hifA fragments with restriction endonucleases AluI and RsaI. Six different RFLP patterns were displayed with each enzyme by this analysis (groups 1 to 6 [Fig. 1 and Table 1]). Pattern 2 contained all nontypeable isolates (n = 4), while pattern 5 contained predominantly type f isolates (7 of 8 isolates). Both H. influenzae biotype aegyptius hifA sequences displayed the same RFLP patterns (pattern 10 [Table 1]). In considering a possible association of capsular type (types b and f and nontypeable strains) with hifA RFLP patterns (patterns 1, 3, and 5), capsular type f was significantly associated with RFLP pattern 5 (χ2 = 22.339; P ≤ 0.001); no association was seen between the other capsular types and RFLP patterns (χ2 = 0.02; 0.80 < P ≤ 0.90).
FIG. 1.
AluI and RsaI RFLP analysis of PCR amplified hifA from genomic DNAs of representative H. influenzae strains. (A) hifA samples digested with AluI. (B) hifA samples digested with RsaI. Lanes: 1 and 14, 100-bp ladder; 2, Eagan (E1a; LKP3); 3, AAr176; 4, 1712MEE (LB5); 5, AAr49; 6, M43; 7, ATCC 9007; 8, AAr73; 9, AAr160; 10, AAr32; 11, AA18; 12, ATCC 9006; 13, AAr157.
Eleven different hifA genes have been cloned and sequenced previously and are included in Table 1. Five of these hifA genes fall into RFLP pattern 3 [M43, AM30 (770235), MinnA, AO2, and 86-1249 (LKP4) (Table 1)], while one falls into pattern 1 [Eagan (E1a; LKP3) (Table 1)]. The hifA genes from H. influenzae biogroup aegyptius strains F3031 and F3037 display the same RFLP pattern (pattern 10) and are distinct from the six RFLP groups defined in this study (Table 1). The three remaining hifA sequences each display new RFLP patterns that are different from the six RFLP patterns identified in this study (patterns 7 to 9 [Table 1]).
Correlation between the hifA RFLP grouping of strains and immunoreactivity with antipilus polyclonal antisera.
Gilsdorf et al. (13) have shown that polyclonal antisera raised against intact pili from H. influenzae type b strains Eagan and M43 each reacted with a different subset of 22 piliated type b isolates. Table 2 presents representative type b strains reactive with each of the antipilus sera and representative nontypeable strains that do not react with either antipilus serum (13). Those type b strains that demonstrate greater reactivity with antiserum directed against strain M43 pili (i.e., 4+) tend to fall into RFLP pattern 3, while those type b strains that react equally (i.e., 2+) or greater (2+ to 4+) with antiserum directed against strain Eagan pili tend to fall into RFLP pattern 1 (χ2 = 6.875; 0.02 < P ≤ 0.05). Strain AAr103p+ is the exception, in that it shows greater reactivity with anti-Eagan pilus serum and yet falls into RFLP pattern 3 (Table 2).
TABLE 2.
Reactivity of H. influenzae strains with antipilus antisera in a dot blot assaya
| hifA RFLP pattern | Strain (capsular type)b | Hemagglu- tination titer | Reactivity with antiserumc
|
|
|---|---|---|---|---|
| R1 (anti-M43 pili) | R19 (anti-Eagan pili) | |||
| 1 | E1ap+ (b) | 1:16 | 0 | 4+ |
| E1ap− (b)d | 0 | 0 | ||
| R9p+ (b) | 1:16 | 3+ | 4+ | |
| AA61p+ (b) | 1:16 | 2+ | 2+ | |
| AAr108p+ (b) | 1:16 | 0 | 2+ | |
| AAr176 (NT) | 1:16 | 0 | 0 | |
| AAr39 (NT) | 1:16 | 0 | 0 | |
| AAr169 (NT) | 1:16 | 0 | 0 | |
| 3 | M43p+ (b) | 1:16 | 4+ | 1+ |
| M42p− (b)e | 0 | 0 | 0 | |
| AAr122p+ (b) | 1:32 | 4+ | 0 | |
| AAr7p+ (b) | 1:16 | 4+ | 2+ | |
| AAr119p+ (b) | 1:32 | 4+ | 0 | |
| AAr103p+ (b) | 1:32 | 2+ | 4+ | |
| SL2p+ (b) | 1:32 | 4+ | 0 | |
| AAr45 (NT) | 1:64 | 0 | 0 | |
An enrichment technique that depends upon erythrocyte agglutination by piliated H. influenzae type b strains was used to select piliated variants from the clinical isolates. NT, nontypeable H. influenzae.
The reactivities of the antibodies with native pili on the surfaces of intact bacteria were assessed visually with a dot blot assay and compared with reactivities of positive (homologous strains) and negative (nonpiliated variants) controls included in each assay by using a grading scale ranging from 0 (identical to negative controls) to 4+ (identical to homologous strains).
Nonpiliated phase variant of E1ap+.
Nonpiliated variant of strain M43p+; used as a negative control.
Table 2 also includes four nontypeable H. influenzae strains that display either hifA RFLP pattern 1 or pattern 3; these strains demonstrated no reactivity with either antipilus serum (11). Thus, H. influenzae type b strains reacted with either antipilus serum while nontypeable H. influenzae strains reacted with neither serum, irrespective of hifA RFLP type (11, 13, 23).
Sequence analysis of representative hifA genes.
In order to confirm the validity of the RFLP analysis and to explore sequence differences between each RFLP group, we chose 10 different representative hifA genes to clone and sequence from the H. influenzae strains in Table 1 to complement the existing hifA sequence database. The strains chosen were from several different sources and represent the six RFLP patterns defined in this study.
Analysis of the derived HifA amino acid sequences (Fig. 2) revealed that all 19 representative pilins have a highly conserved 18- to 20-amino-acid leader sequence and contain such pilin signatures as strong C-terminal amino acid homologies, conserved tyrosines and glycines at 2 and 14 residues, respectively, from the C terminus, and a similarly spaced pair of cysteine residues at positions 45 and 85 (Fig. 2). Further analysis of the C-terminal amino acids showed a range of 60 to 100% identity in the terminal 16 residues, with 8 of the 16 residues being absolutely conserved (Fig. 2). These pilin signature sequences are shared among a wide variety of bacterial pilus proteins that are assembled by periplasmic chaperones (17, 21). Recent studies by St. Geme III et al. (36) demonstrated that the biogenesis of H. influenzae pili is dependent upon the periplasmic chaperone HifB, which belongs to the PapD family of immunoglobulin-like chaperones (17, 21). Several regions of amino acid identity which are distributed throughout the HifA sequence are evident in the sequence comparison (e.g., residues 29 to 85, 99 to 120, 127 to 136, 145 to 155, 176 to 178, and 191 to 200 [Fig. 2]).
FIG. 2.
Comparison of predicted amino acid sequences of HifA from representative H. influenzae strains. Identical residues throughout all HifA sequences are in boldface type and are shown in the bottom line as “Consensus.” Arrowheads denote the conserved cysteine residues; asterisks denote the positions of the conserved tyrosine and glycine residues 2 and 14 amino acids, respectively, from the C terminus. Hydrophilic regions I, II, and III are underlined (10), and pilin motifs “Segment S3” and “Segment S6” (17) are in shaded boxes. The comparison was performed with the Pileup program of the Wisconsin Package, version 9.0, from GCG (5).
The validity of the RFLP groups for identifying like hifA genes is confirmed by comparisons of the amino acid sequence identities of the derived, representative HifA pilins (Table 3). The amino acid identities were stronger within RFLP groups than between groups (P < 0.0001 [by two-way analysis of variance). For example, pairwise comparison of HifA sequences within a specific RFLP group yielded between 87 and 100% identities, whereas pairwise comparisons of HifA sequences between members of different RFLP groups showed between 59 and 81% identities. The HifA amino acid sequences of RFLP groups 1 and 3 have between 78 and 81% identities.
TABLE 3.
Amino acid identities of HifA based on DNA sequences from various strains confirm relationships defined by RFLP analysisa
| hifA RFLP pattern | Strain | % Amino acid identityb
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattern 1
|
Pattern 2
|
Pattern 3
|
Pattern 4
|
Pattern 5
|
Pattern 6
|
Pattern 7
|
Pattern 8
|
Pattern 9
|
Pattern 10
|
|||||||||||
| Eagan | AAr176 | AAr49 | LB5 | AO2 | M43c | ATCC 9007 | 86-1249 | AAr73 | AAr160 | AA18 | AAr32 | ATCC 9006 | AAr157 | 86-0295 | 81-0384 | M37 | F3031 | F3037 | ||
| 1 | Eagan | 99 | 69 | 69 | 79 | 79 | 81 | 79 | 68 | 68 | 65 | 65 | 67 | 69 | 60 | 62 | 65 | 75 | 75 | |
| AAr176 | 69 | 68 | 78 | 78 | 80 | 78 | 68 | 68 | 65 | 65 | 67 | 69 | 59 | 62 | 64 | 74 | 74 | |||
| 2 | AAr49 | 99 | 71 | 72 | 71 | 72 | 68 | 68 | 77 | 77 | 69 | 67 | 67 | 62 | 64 | 71 | 71 | |||
| LB5 | 71 | 72 | 71 | 72 | 67 | 67 | 76 | 76 | 69 | 67 | 67 | 62 | 64 | 72 | 72 | |||||
| AO2 | 99 | 87 | 99 | 68 | 68 | 66 | 66 | 69 | 70 | 66 | 63 | 69 | 72 | 72 | ||||||
| 3 | M43c | 88 | 99 | 69 | 69 | 66 | 66 | 69 | 70 | 66 | 64 | 69 | 73 | 73 | ||||||
| ATCC 9007 | 87 | 70 | 70 | 68 | 68 | 72 | 72 | 62 | 62 | 68 | 73 | 73 | ||||||||
| 86-1249 | 69 | 69 | 66 | 66 | 69 | 70 | 66 | 64 | 69 | 73 | 73 | |||||||||
| 4 | AAr73 | 98 | 74 | 74 | 80 | 70 | 63 | 71 | 64 | 70 | 70 | |||||||||
| AAr160 | 74 | 74 | 80 | 70 | 63 | 71 | 64 | 70 | 70 | |||||||||||
| 5 | AA18 | 100 | 77 | 71 | 64 | 63 | 65 | 68 | 68 | |||||||||||
| AAr32 | 77 | 71 | 64 | 63 | 65 | 68 | 68 | |||||||||||||
| 6 | ATCC 9006 | 89 | 64 | 66 | 73 | 73 | 73 | |||||||||||||
| AAr157 | 63 | 66 | 74 | 72 | 72 | |||||||||||||||
| 7 | 86-0295 | 62 | 65 | 66 | 66 | |||||||||||||||
| 8 | 81-0384 | 66 | 66 | 66 | ||||||||||||||||
| 9 | M37 | 74 | 74 | |||||||||||||||||
| 10 | F3031 | 99 | ||||||||||||||||||
| F3037 | ||||||||||||||||||||
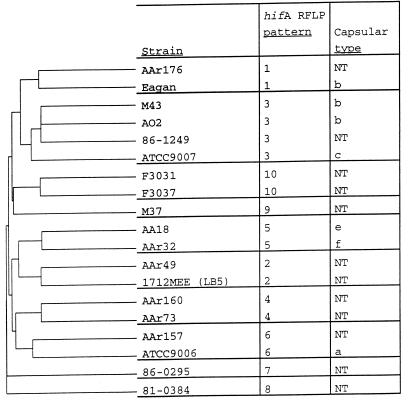
A dendrogram (Fig. 3) depicting the groupings of strains based upon the complete pileup of derived amino acid sequences (Fig. 2) further confirms the groupings based on hifA RFLP patterns and shows the relationships between these groups.
FIG. 3.
Dendrogram showing the relationships of the predicted amino acid sequences of HifA from the 19 representative H. influenzae strains. The dendrogram was generated with the Pileup program of the Wisconsin Package, version 9.0, from GCG (5). NT, nontypeable H. influenzae.
DISCUSSION
The goal of this work was to compare the hifA genes from several independent typeable and nontypeable H. influenzae isolates and identify any sequence differences that might explain the pilus immunologic heterogeneity. Further, these hifA genes were compared to pilins from other bacteria to identify conserved regions potentially important for pilus assembly.
The relationships of H. influenzae strains based upon hifA RFLP analysis (Table 1; Fig. 1) were confirmed by analysis of the derived amino acid sequences (Fig. 2 and 3; Table 3). All of the strains contain highly conserved 18- to 20-residue signal sequences (Fig. 2) and several regions of high sequence identity. Of note are the equally spaced pairs of cysteine residues at positions 45 and 85 in all of the HifA pilin sequences (Fig. 2). This Cys-Cys loop is conserved among pilins from several different bacteria, is postulated to play a role in the maintenance of protein structure, and is thought to be a dominant immunogenic epitope in the PapA pilins of uropathogenic E. coli (4, 17). The amino acids contained within the region of the Cys-Cys loop (residues 58 to 121 [Fig. 2]) of H. influenzae M37 were demonstrated by Palmer and Munson, Jr. (31), to possess a significant part of the epitope defined by the pilin-specific monoclonal antibody 3H12, emphasizing the immunogenic potential of this region in H. influenzae pilins.
The region in HifA from amino acids 156 to 205, which is the most variable region within the pilin sequences, is analogous to the variable region found in the PapA pilins of uropathogenic E. coli and the pilins from other bacteria and may account for the immunologic diversity in H. influenzae pili (4, 17). For PapA, strain-to-strain differences in the variable region and the Cys-Cys loop are thought to constitute the basis for the serological diversity of these pili (4).
In an effort to identify common regions of type b pili that are surface exposed and represent antigenic epitopes, Forney et al. (10) analyzed the hydrophilicity of the pilin proteins expressed by the type b strains M43 and Eagan. They identified three hydrophilic regions within the HifA sequence (regions I, II, and III [Fig. 2]) and proposed that these regions might constitute conserved antigenic epitopes. The present study shows that these three regions are highly conserved within all HifA sequences (Fig. 2), with 10 of 19 residues being absolutely conserved in region I. Further, 10 of 18 and 4 of 12 residues are absolutely conserved in regions II and III, respectively, in the HifA comparison (Fig. 2). Along with the absolutely conserved amino acids in each region, several conserved amino acid substitutions result in high degrees of sequence similarity in these regions. To determine if these regions contain surface-exposed, immunogenic epitopes, Gilsdorf et al. (14) constructed 14- to 15-amino-acid peptides corresponding to regions I, II, and III and raised polyclonal rabbit antisera to these peptides. Sera to these peptides demonstrated poor to no reactivity to native pili and moderate to strong reactivity to denatured pili, suggesting that the epitopes determined by these peptides are not present on assembled pili in a conformation that can be recognized by the antipeptide antibodies (14). These results were supported by the previous observation that antibodies raised against denatured pilin and an internal peptide of strain M43 HifA recognized epitopes on denatured pilins of both type b and nontypeable H. influenzae better than native pili on the same strains (11, 13). Therefore, although these regions are highly conserved on denatured pilins, they are not available for antipeptide or antipilin antibody binding on native pili.
Several conserved features characteristic of pilus proteins assembled by E. coli PapD-like molecular chaperones are seen in the C-terminal sequences of the HifA pilins (Fig. 2). For example, the derived HifA sequences have strong amino acid homology to one another in the C terminus (Fig. 2) and contain absolutely conserved tyrosines and glycines at 2 and 14 residues, respectively, from the C terminus (3, 10, 19, 21, 22, 37, 39, 43). These conserved HifA sequence features are shared with the minor pilin HifD and the C terminus of the putative adhesin, HifE (26, 40). Recently, HifB-HifA and HifB-HifD chaperone-pilin complexes have been isolated, demonstrating that the biogenesis of H. influenzae pili is dependent upon the periplasmic chaperone HifB (36).
Recently, Girardeau and Bertin (17), using two-dimensional sequence analysis, described other conserved markers of the bacterial pilin family; these features are conserved within the representative HifA sequences (Fig. 2). Among the motifs identified, segments S3 (FxlxLxxC [where x is any residue]) and S6 (Ax[G/N]VGVQi [where i is a hydrophobic residue]) were the most conserved. Girardeau and Bertin (17) suggest that the S3 and S6 motifs, along with the conserved Cys-Cys loop and C-terminal homology, play a role in the function or maintenance of the structural integrity of the protein.
The intact pili of H. influenzae demonstrate a high degree of immunologic heterogeneity with both polyclonal and monoclonal antipilus sera (1, 11, 13, 23, 31). Brinton et al. (1) used polyclonal anti-LKP pilus sera to differentiate clinical isolates of H. influenzae into seven different LKP pilus types (LKP1 to LKP7). Four LKP serotypes are represented in this study [LKP3, Eagan (E1a); LKP4, 86-1249; LKP1, 86-0295; LKP5, 81-0384] and each displays a different RFLP pattern (Table 1).
Polyclonal antipilus sera raised against the pili of type b strains Eagan and M43 each reacted with a different subset of 22 type b H. influenzae strains (13, 23). Those type b strains showing equal or greater reactivity with Eagan antipilus serum displayed hifA RFLP pattern 1, while those strains showing greater reactivity with M43 antipilus serum displayed hifA RFLP pattern 3 (Table 2). Several strains, though, show reactivity with both antipilus sera, suggesting that epitopes defined by these antisera are shared by some strains and not others. These findings support those of Denich et al. (4), who found common immunogenic domains among PapA pilins from different strains of uropathogenic E. coli.
The amino acid sequences of representative HifA pilins from RFLP patterns 1 and 3 range between 78 and 81% identity (Table 3). Further, strain AAr103p+ reacts more strongly with Eagan antipilus serum yet displays hifA RFLP pattern 3, to which strain M43 belongs (Table 2). This result demonstrates that although the HifA pilin sequence of strain AAr103p+ has an overall amino acid sequence identity closer to that of M43, it shares certain critical antigenic residues with that of Eagan. Few amino acid differences between HifA pilins could explain the varying reactivities with antipilus antisera. For example, H. influenzae AAr176 bacteria do not react with Eagan antipilus serum (Table 2) (11). This serum appears to be specific for the Eagan HifA pilin and decorates the entire pilus shaft on whole bacteria subjected to immunoelectron microscopy (9). The derived HifA sequences from strains Eagan and AAr176 are 99% identical and differ by only three amino acids (residues 43, 217, and 221) (Table 3; Fig. 2).
The first amino acid difference (residue 43 [Fig. 2]) lies within the predicted hydrophilic region I originally identified by Forney et al. (10) and near a pair of conserved cysteines that define the Cys-Cys loop that may represent a major surface-exposed antigenic region in HifA. The second pair of amino acid differences between the Eagan and AAr176 HifA sequences (residues 217 and 221) are at the C terminus, a region that tends to be conserved among pilins and is thought to play a role in pilus subunit interactions (17, 21, 26). However, Palmer and Munson, Jr. (31), observed that monoclonal antibody 3H12 reactivity with M37 HifA was enhanced by the addition of M37 C-terminal sequences to the M37-MinnA HifA chimeras, suggesting that this region may itself be antigenic or that amino acids in this region contribute to nonlinearly assembled epitopes.
The rationale for the immunological heterogeneity of H. influenzae pili is not well understood. Limited studies have shown that humans can produce serum antibodies directed against H. influenzae pili (7, 32). Further, H. influenzae has been shown to undergo pilus phase variation and antigenic variation in such cell surface molecules as major outer membrane protein P2, immunoglobulin A1 proteases, and lipopolysaccharide (6, 18, 24, 29, 41). Taken together, the antigenic diversity of H. influenzae pili may be due to small changes in immunodominant surface-exposed epitopes in HifA and may play a role, along with phase variation and antigenic drift of other surface molecules, in the organism’s ability to evade the host immune system.
ACKNOWLEDGMENTS
We thank Gregory Russell for designing the hifA primers used in PCR.
This work was supported by Public Health Service grant AI25630 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Brinton C C, Carter M J, Derber D B, Kar S, Kramarik J A, To A C-C, To S C-M, Wood S W. Design and development of pilus vaccines for Haemophilus influenzae diseases. Pediatr Infect Dis J. 1989;8:S54–S61. [PubMed] [Google Scholar]
- 2.Cariello N F, Swenberg J A, Skopek T R. Fidelity of Thermococcus litoralis DNA polymerase (Vent) in PCR determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1991;19:4193–4198. doi: 10.1093/nar/19.15.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman T, Grass S, Munson R., Jr Molecular cloning, expression, and sequence of the pilin gene from nontypeable Haemophilus influenzae M37. Infect Immun. 1991;59:1716–1722. doi: 10.1128/iai.59.5.1716-1722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denich K, Blyn L B, Craiu A, Braaten B A, Hardy J, Low D A, O’Hanley P D. DNA sequences of three papA genes from uropathogenic Escherichia coli strains: evidence of structural and serological conservation. Infect Immun. 1991;59:3849–3858. doi: 10.1128/iai.59.11.3849-3858.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duim B, Vogel L, Puijk W, Jansen H M, Meloen R H, Dankert J, van Alphen L. Fine mapping of outer membrane protein P2 antigenic sites which vary during persistent infection by Haemophilus influenzae. Infect Immun. 1996;64:4673–4679. doi: 10.1128/iai.64.11.4673-4679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erwin A L, Kenny G E, Smith A L, Stull T L. Human antibody response to outer membrane proteins and fimbriae of Haemophilus influenzae type b. Can J Microbiol. 1988;34:723–729. doi: 10.1139/m88-123. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritch J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 9.Forney L J, Gilsdorf J R, Wong D C L. Effect of pili-specific antibodies on the adherence of Haemophilus influenzae type b to human buccal cells. J Infect Dis. 1992;165:464–470. doi: 10.1093/infdis/165.3.464. [DOI] [PubMed] [Google Scholar]
- 10.Forney L J, Marrs C F, Bektesh S L, Gilsdorf J R. Comparison and analysis of the nucleotide sequences of pilin genes from Haemophilus influenzae type b strains Eagan and M43. Infect Immun. 1991;59:1991–1996. doi: 10.1128/iai.59.6.1991-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilsdorf J R, Chang H, McCrea K W, Bakaletz L. Comparison of hemagglutinating pili of Haemophilus influenzae type b with similar structures of nontypeable H. influenzae. Infect Immun. 1992;60:374–379. doi: 10.1128/iai.60.2.374-379.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilsdorf J R, Marrs C F, McCrea K W, Forney L J. Cloning, expression, and sequence analysis of the Haemophilus influenzae type b strain M43p+ pilin gene. Infect Immun. 1990;58:1065–1072. doi: 10.1128/iai.58.4.1065-1072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilsdorf J R, McCrea K W, Forney L J. Conserved and nonconserved epitopes among Haemophilus influenzae type b pili. Infect Immun. 1990;58:2252–2257. doi: 10.1128/iai.58.7.2252-2257.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilsdorf J R, Forney L J, McCrea K W. Reactivity of antibodies against conserved regions of pilins of Haemophilus influenzae type b. J Infect Dis. 1993;167:962–965. doi: 10.1093/infdis/167.4.962. [DOI] [PubMed] [Google Scholar]
- 15.Gilsdorf J R, McCrea K W, Marrs C F. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilsdorf J R. Haemophilus influenzae non-type b infections in children. Am J Dis Child. 1987;141:1063–1065. doi: 10.1001/archpedi.141.10.1063. [DOI] [PubMed] [Google Scholar]
- 17.Girardeau J-P, Bertin Y. Pilins of fimbrial adhesins of different member species of enterobacteriaceae are structurally similar to the C-terminal half of adhesin proteins. FEBS Lett. 1995;357:103–108. doi: 10.1016/0014-5793(94)01340-7. [DOI] [PubMed] [Google Scholar]
- 18.Groeneveld K, van Alphen L, Voorter C, Eijk P P, Jansen H M, Zanen H C. Antigenic drift of Haemophilus influenzae in patients with chronic obstructive pulmonary disease. Infect Immun. 1989;57:3038–3044. doi: 10.1128/iai.57.10.3038-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultgren S J, Jones C H. Utility of the immunoglobulin-like fold of chaperones in shaping organelles of attachment in pathogenic bacteria. ASM News. 1995;61:457–464. [Google Scholar]
- 20.Kar S, To S C-M, Brinton C C., Jr Cloning and expression in Escherichia coli of LKP pilus genes from a nontypeable Haemophilus influenzae strain. Infect Immun. 1990;58:903–908. doi: 10.1128/iai.58.4.903-908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuehn M J, Ogg D J, Kihlberg J, Slonim L N, Flemmer K, Bergfors T, Hultgren S J. Structural basis of pilus subunit recognition by the PapD chaperone. Science. 1993;262:1234–1241. doi: 10.1126/science.7901913. [DOI] [PubMed] [Google Scholar]
- 22.Langermann S, Wright A. Molecular analysis of the Haemophilus influenzae type b pilin gene. Mol Microbiol. 1990;4:221–230. doi: 10.1111/j.1365-2958.1990.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 23.LiPuma J J, Gilsdorf J R. Structural and serological relatedness of Haemophilus influenzae type b pili. Infect Immun. 1988;56:1051–1056. doi: 10.1128/iai.56.5.1051-1056.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomholt H, van Alphen L, Kilian M. Antigenic variation of immunoglobulin A1 proteases among sequential isolates of Haemophilus influenzae from healthy children and patients with chronic obstructive pulmonary disease. Infect Immun. 1993;61:4575–4581. doi: 10.1128/iai.61.11.4575-4581.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 26.McCrea K W, Watson W J, Gilsdorf J R, Marrs C F. Identification of hifD and hifE in the pilus gene cluster of Haemophilus influenzae type b strain Eagan. Infect Immun. 1994;62:4922–4928. doi: 10.1128/iai.62.11.4922-4928.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrea K W, Watson W J, Gilsdorf J R, Marrs C F. Identification of two minor subunits in the pilus of Haemophilus influenzae. J Bacteriol. 1997;179:4227–4231. doi: 10.1128/jb.179.13.4227-4231.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moxon, E. R. 1986. The carrier state: Haemophilus influenzae. J. Antimicrob. Chemother. 18(Suppl A):17–24. [DOI] [PubMed]
- 29.Moxon E R, Rainey P B, Nowak M A, Lenski R E. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol. 1994;4:24–33. doi: 10.1016/s0960-9822(00)00005-1. [DOI] [PubMed] [Google Scholar]
- 30.Murphy T, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 31.Palmer K L, Munson R S., Jr Construction of chimaeric genes for mapping a surface-exposed epitope on the pilus of non-typeable Haemophilus influenzae. Mol Microbiol. 1992;6:2583–2588. doi: 10.1111/j.1365-2958.1992.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 32.Pichichero M E, Anderson P, Loeb M, Smith D H. Do pili play a role in pathogenicity of Haemophilus influenzae type b? Lancet. 1982;ii:960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- 33.St. Geme J W., III Molecular determinants of the interaction between Haemophilus influenzae and human cells. Am J Respir Crit Care Med. 1996;154:S192–S196. doi: 10.1164/ajrccm/154.4_Pt_2.S192. [DOI] [PubMed] [Google Scholar]
- 34.St. Geme J W., III Nontypeable Haemophilus influenzae disease: epidemiology, pathogenesis, and prospects for prevention. Infect Agents Dis. 1993;2:1–16. [PubMed] [Google Scholar]
- 35.St. Geme J W, III, Cutter D, Barenkamp S J. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281–6287. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St. Geme J W, III, Pinkner J S, Krasan G P, Heuser J, Bullitt E, Smith A L, Hultgren S J. Haemophilus influenzae pili are composite structures assembled via the HifB chaperone. Proc Natl Acad Sci USA. 1996;93:11913–11918. doi: 10.1073/pnas.93.21.11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St. Geme J W, III, Falkow S. Isolation, expression, and nucleotide sequencing of the pilin structural gene of the Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius. Infect Immun. 1993;61:2233–2237. doi: 10.1128/iai.61.5.2233-2237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tindall K R, Kunkel T A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988;27:6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- 39.van Ham S M, Mooi F R, Sindhunata M G, Maris W R, van Alphen L. Cloning and expression in Escherichia coli of Haemophilus influenzae fimbrial genes establishes adherence to oropharyngeal epithelial cells. EMBO J. 1989;8:3535–3540. doi: 10.1002/j.1460-2075.1989.tb08519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ham S M, van Alphen L, Mooi F R, van Putten J P M. The fimbrial gene cluster of Haemophilus influenzae type b. Mol Microbiol. 1994;13:673–684. doi: 10.1111/j.1365-2958.1994.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 41.van Ham S M, van Alphen L, Mooi F R, van Putten J P M. Phase variation of H. influenzae fimbriae: transcriptional control of two divergent genes through a variable combined promoter region. Cell. 1993;73:1187–1196. doi: 10.1016/0092-8674(93)90647-9. [DOI] [PubMed] [Google Scholar]
- 42.Watson W J, Gilsdorf J R, Tucci M A, McCrea K W, Forney L J, Marrs C F. Identification of a gene essential for piliation in Haemophilus influenzae type b with homology to the pilus assembly platform genes of gram-negative bacteria. Infect Immun. 1994;62:468–475. doi: 10.1128/iai.62.2.468-475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitney A M, Farley M M. Cloning and sequence analysis of the structural pilin gene of Brazilian purpuric fever-associated Haemophilus influenzae biogroup aegyptius. Infect Immun. 1993;61:1559–1562. doi: 10.1128/iai.61.4.1559-1562.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]