Abstract
This report examines the effect of the major capsular polysaccharide of Cryptococcus neoformans, glucuronoxylomannan (GXM), on the antigen-presenting capability of human monocytes treated with acapsular cells of C. neoformans. We found that pretreatment of acapsular cryptococci with GXM downregulates, in a dose-dependent manner, the antigen-presenting capacity of monocytes, leading to reduced proliferative T-lymphocyte responses. Similar levels of suppression occurred when monocytes were exposed to encapsulated cryptococci or acapsular cryptococci that were pretreated with GXM. The magnitude of the T-cell response correlated with the ability of monocytes to ingest the yeast. Supernatant fluids from cocultures of monocytes and T cells cultured with encapsulated cryptococci contained higher levels of interleukin-10 (IL-10) than supernatant fluids of cells with acapsular cryptococci. Addition of anti-IL-10 monoclonal antibodies to the incubation medium of monocytes and T cells cultured with encapsulated cryptococci restored proliferative T-cell responses to levels observed during culture with acapsular cryptococci. Finally, treatment of monocytes with encapsulated cryptococci or GXM-treated acapsular cryptococci suppressed expression of class II major histocompatibility complex (MHC) molecules in a manner consistent with previous reports of IL-10-mediated suppression of class II MHC molecules and suppression of proliferative T-cell responses. These results suggest a link between GXM encapsulation, increased IL-10 synthesis by monocytes, decreased expression of class II MHC molecules on monocytes, and reduced proliferative T-cell responses.
Cryptococcus neoformans is an opportunistic fungus that produces a life-threatening meningoencephalitis in AIDS patients (10, 11, 25, 38). This fungus is believed to enter the body via inhalation of airborne yeast cells or conidia from the environment. During initial exposure to the microorganism, the capsule may be absent or small, but it is rapidly synthesized in vivo. The capsule represents a major virulence factor endowed with antiphagocytic and toleragenic properties (16, 17, 23, 31). Glucuronoxylomannan (GXM) is the major component of the capsule of C. neoformans, and studies focusing on capsular material have identified many regulatory effects on phagocytic cells (16, 19, 21, 24, 28, 39, 45). The large amount of this inhibitory substance found in the body fluids of patients with cryptococcosis (8, 36) raises the possibility that GXM exerts regulatory effects during a cryptococcal infection. Several reports indicate that GXM or products of C. neoformans that are rich in GXM induce or regulate the cell-mediated response to C. neoformans. Murphy and Cox found that intravenous injection of sera with high GXM titers from C. neoformans-infected mice into recipient mice suppresses the ability of the recipient mice to produce a normal delayed hypersensitivity response to cryptococcal antigens (30). Several studies from the Murphy laboratory found that intravenous injection of cryptococcal polysaccharides induces a cascade of suppressor cells and soluble factors that downregulate the anticryptococcal delayed hypersensitivity response (13, 29, 32–34). More recently, Blackstock reported that GXM stimulates an antigen-presenting cell (APC) to induce secretion by T cells of a T-suppressor factor that is specific for GXM (1).
Collins and Bancroft were the first to report that encapsulation of C. neoformans greatly reduces the ability of C. neoformans-treated monocytes/macrophages to induce a T lymphoproliferative response (5). This report was followed by one presenting similar observations by Mody and Syme (26), as well as ours (43). Recently we found that GXM is a potent downregulator of proinflammatory cytokine release by human monocytes (45). This inhibitory effect is due, in part, to an increased secretion of interleukin-10 (IL-10) that appears, in turn, to be responsible for reduced secretion of tumor necrosis factor alpha (TNF-α), and/or IL-1β (6, 22, 46). A role for GXM encapsulation in suppression of the T lymphoproliferative response is supported by our observation that opsonization of encapsulated cryptococci with anti-GXM monoclonal antibodies (MAbs) enhances the ability of monocytes to process C. neoformans yeast cells, leading to an enhanced T proliferative response (44).
The objectives of our study were (i) to provide further evidence for the central role that encapsulation with GXM plays in reducing the ability of monocytes to process C. neoformans yeast cells, leading to a proliferative response by T lymphocytes, (ii) to determine the effect of GXM encapsulation on expression of major histocompatibility complex (MHC) class II molecules by monocytes, and (iii) to assess the importance of autologous IL-10 in altered expression of MHC class II molecules by monocytes and suppressed T lymphoproliferation. Our results confirmed the role of GXM in suppression of the T lymphoproliferative response and provide a link between the capability of GXM encapsulation to perturb processing by APC and suppression of the cell-mediated response to acapsular cryptococci.
MATERIALS AND METHODS
Reagents and media.
RPMI 1640 medium and fetal calf serum (FCS) were obtained from Eurobio Laboratories (Paris, France). Human serum (HS) was obtained from Biosource International (Camarillo, Calif.). GXM was isolated from culture supernatant fluid of a serotype A strain (ATCC 24064) that was grown on a liquid synthetic medium (4) on a gyratory shaker for 4 days at 30°C. GXM was isolated by use of differential precipitation with ethanol and hexadecyl trimethyl ammonium bromide (CTAB; Sigma Chemical Co., St. Louis, Mo.) (3). The isolation procedure has been described in detail elsewhere (12). Lipopolysaccharide (LPS) from Escherichia coli O55:135 was obtained from Difco Laboratories (Detroit, Mich.). Concanavalin A (ConA) and polymyxin B were purchased from Sigma. Anti-IL-10 MAb was obtained from Genzyme Corp. (Boston, Mass.). RPMI 1640, FCS, C. neoformans cells (approximately 5 × 108), ConA, anti-IL-10 MAb, and HS were tested for endotoxin contamination by a Limulus amebocyte lysate (LAL) assay (Sigma) which had a sensitivity of approximately 0.05 to 0.1 ng of E. coli LPS/ml. All reagents tested negative. The GXM was not examined by LAL because GXM preparations, even those made under stringent conditions designed to minimize or eliminate LPS contamination, routinely test positive in LAL assays, suggesting that contamination of GXM with endotoxin cannot be accurately assessed by LAL.
Preparation of peripheral blood monocytes (PBM) and lymphocytes.
Heparinized venous blood, obtained from healthy donors, was diluted with RPMI 1640 plus 5% FCS (cRPMI), and the mononuclear cells were separated by density gradient centrifugation on Ficoll-Hypaque (35). The mononuclear cells were washed twice in cRPMI and incubated for 1 h at a concentration of 2 × 106 to 3 × 106/ml in cell culture petri dishes (Nunc Inter Med, Roskilde, Denmark). The remaining adherent cells (approximately 2 × 104/well) were >98% esterase positive and >98% viable as evaluated by trypan blue dye exclusion. Nonadherent cells were E rosetted as previously described (37). The cells recovered were T lymphocytes [T(E+), >98% CD3+ as evaluated by flow cytometry analysis].
Microorganisms.
The two strains of C. neoformans examined in this study were obtained from J. Orendi (Central Bureau Schimmel Cultures [CBS], Delft, The Netherlands). C. neoformans var. neoformans 6995 (CBS 6995; also known as NIH 37) is a thinly encapsulated isolate of serotype A. C. neoformans var. neoformans 7698 (CBS 7698; also known as NIH B-4131) is an acapsular mutant. Candida albicans PCA-2 was kindly supplied by D. Kerridge, Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom. This is an agerminative strain which grows as a pure yeast form in vitro at both 28 and 37°C in conventional mycological media. The morphological characteristics and conditions for growth of the two strains of C. neoformans and the C. albicans isolate have been described previously (41–43). The cultures were maintained by serial passage on Sabouraud agar (BioMerieux, Lyon, France) and harvested by suspending a single colony in RPMI 1640. The cells were washed twice, counted on a hematocytometer, and adjusted to the desired concentration. Cells of C. neoformans 6995 and 7698 and C. albicans were killed by autoclaving.
Phagocytosis.
Phagocytosis assays were performed as previously described (45). Briefly, live C. neoformans 6995 or 7698 or C. albicans cells were added to PBM monolayers cultured in RPMI 1640 plus 10% unheated HS at an effector-to-target cell ratio of 1:1. After 1 h of incubation at 37°C in 5% CO2, nonadherent microorganisms were removed by extensive washing. The percent phagocytosis was calculated as the proportion of PBM containing one or more yeast cells per 100 PBM counted.
Lymphocyte proliferation assay.
Monolayers of PBM (2 × 104), adherent in flat-bottom 96-well plates, were incubated with or without heat-inactivated C. neoformans (2 × 105) for 2 h at 37°C in 5% CO2 in RPMI 1640 plus 10% HS and used throughout as APC. The PBM monolayers were washed to remove nonbound microorganisms, and autologous T(E+) cells (105) in RPMI 1640 plus 10% HS were added to the cultures. In selected experiments, ConA (5 μg/ml) was added to cocultures of PBM and T(E+) autologous lymphocytes. In selected experiments, supernatant fluids were harvested after 3 or 7 days of culture for IL-10 determination. At various days, cultures were pulsed for 6 h with 0.5 μCi of [3H]thymidine (Amersham International, Amersham, United Kingdom), and the nonadherent contents of the wells were collected onto filter paper by using a cell harvester (Flow Laboratories, McLean, Va.). The dried filters were counted directly in a β counter (Packard Instruments, Downers Grove, Ill.). In all experiments, the proliferating cell population was >98% CD3 positive as evaluated by flow cytometry analysis. Mouse anti-human CD3 (immunoglobulin G2a [IgG2a]) was obtained from Caltag Laboratories (South San Francisco, Calif.). The viability of the lymphocytes and the adherent cells after 3 and 7 days was >98% in each experimental group, as evaluated by trypan blue dye exclusion. Proliferation was expressed as mean values of thymidine uptake of the indicated number of replicates ± standard error of the mean (SEM).
Flow cytometry analysis.
PBM untreated or treated with various stimuli were harvested by scraping into phosphate-buffered saline containing 0.5% bovine serum albumin and 0.4% sodium azide. A total of 106 cells in 50 μl were mixed with 10 μl of a fluorescein isothiocyanate-conjugated mouse MAb that is specific for human IgG1 (Sigma) or 10 μl of a fluorescein isothiocyanate-conjugated mouse MAb that is specific for human HLA-DR (Boehringer, Mannheim, Germany). After 45 min of incubation, the cells were washed three times and stained with phycoerythrin-conjugated anti-human CD4 (Caltag). Mouse monoclonal anti-human CD14R (IgG2b)-phycoerythrin conjugate was purchased from Ancell Corporation (Bayport, Minn.). For each sample, MHC class II expression was measured on the surface of CD14 positive cells by using a fluorescence-activated cell sorter (Becton Dickinson).
Cytokine determination.
IL-10 was determined with a human IL-10 enzyme-linked immunosorbent assay (ELISA) kit purchased from Genzyme. The Predicta IL-10 kit is a solid-phase ELISA based on the antibody sandwich principle; its sensitivity is 5 pg/ml. Specificity was determined by measuring IL-10 with no detectable cross-reaction with human IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, gamma interferon, α-2-microglobulin, granulocyte-macrophage colony-stimulating factor, leukocyte inhibiting factor, stem cell factor, TNF-α, TNF-β in IL-10, and bovine serum.
Statistical analysis.
Statistical analysis was performed with Student’s t test.
RESULTS
Previous reports by ourselves (42, 43) and others (5, 26) demonstrated that encapsulation of C. neoformans influences T-cell proliferation in response to C. neoformans-treated monocytes. To determine whether the GXM component of the cryptococcal capsule is responsible for the observed phenomenon, we added various amounts of GXM and acapsular cells of C. neoformans 7698 (2 × 105) to monocytes (2 × 104) at the time of monocyte preparation. Subsequently, autologous T lymphocytes (105) were added, and lymphoproliferation was measured after 3 or 7 days of culture. The results (Table 1) showed that GXM inhibited the T-cell blastogenic response in a dose-dependent manner. To exclude the possibility that the GXM-mediated inhibition was due to contamination by LPS, we performed a similar experiment in the presence or absence of polymyxin B. The results (Table 2) showed that the inhibitory effect of GXM is not attributable to LPS contamination because (i) incorporation of polymyxin B into the medium had no significant effect on the subsequent T-cell response and (ii) addition of LPS to cells of strain 7698 caused an increase in the T-cell lymphoproliferative response rather than the decreased response observed in the presence of GXM.
TABLE 1.
Effect of GXM on the proliferative response of T(E+) to PBM pretreated with acapsular C. neoformans 7698
| Treatmenta | Mean cpm ± SEMc
|
|
|---|---|---|
| Day 3 | Day 7 | |
| T(E+) | 270 ± 25 | 315 ± 25 |
| T(E+) + 7698 | 305 ± 30 | 350 ± 30 |
| T(E+)+7698 + GXM (500 μg/ml) | 310 ± 30 | 355 ± 30 |
| PBM + T(E+) | 360 ± 20 | 490 ± 40 |
| PBM + T(E+) + 7698 | 21,500 ± 1,800 | 32,800 ± 2,740 |
| PBM + T(E+) + 7698 + GXM (500 μg/ml)b | 9,200 ± 1,000d | 11,000 ± 1,900d |
| PBM + T(E+) + 7698 + GXM (250 μg/ml)b | 13,500 ± 1,000d | 16,400 ± 950d |
| PBM + T(E+) + 7698 + GXM (25 μg/ml)b | 14,400 ± 1,200 | 20,700 ± 1,850d |
| PBM + T(E+) + 7698 + GXM (2.5 μg/ml)b | 23,000 ± 840 | 29,300 ± 1,000 |
| PBM + T(E+) + 7698 + GXM (0.1 μg/ml)b | 21,900 ± 1,600 | 30,100 ± 2,900 |
| PBM + T(E+) + 6995 | 10,800 ± 1,000 | 19,800 ± 1,800 |
Monocyte monolayers (PBM, 105) were treated with encapsulated C. neoformans 6995 (2 × 105) or acapsular C. neoformans 7698 (2 × 105) plus GXM at the indicated doses. After 2 h of incubation, the monolayers were washed and then autologous T(E+) (2 × 105) were added. Lymphoproliferation was measured after 3 or 7 days of incubation.
GXM was added to monocytes at the time of 7698 treatment.
Mean ± SEM of three separate experiments from three different donors.
P < 0.01 (GXM-treated versus untreated cells).
TABLE 2.
Effect of polymyxin B on the T-cell proliferative response to monocytes pretreated with LPS or with acapsular C. neoformans 7698 plus GXM
| Treatmenta | Polymyxin B additionb | Mean cpm ± SEMc on day 7 |
|---|---|---|
| None | − | 400 ± 35 |
| None | + | 390 ± 30 |
| 7698 | − | 34,900 ± 2,000 |
| 7698 | + | 32,000 ± 1,000 |
| 7698 + GXM (250 μg/ml) | − | 13,500 ± 1,600 |
| 7698 + GXM (250 μg/ml) | + | 12,500 ± 850 |
| LPS (10 μg/ml) + 7698 | − | 43,600 ± 3,600 |
| LPS (10 μg/ml) + 7698 | + | 30,900 ± 2,900d |
GXM or LPS was added to monocytes at the time of 7698 treatment. For experimental details, see the footnotes to Table 1.
Polymyxin B (50 μg/ml) was added at the time of 7698, 7698 plus GXM, or LPS treatment.
Mean ± SEM of three separate experiments from three different donors.
P < 0.01 (polymyxin B-treated versus untreated cells).
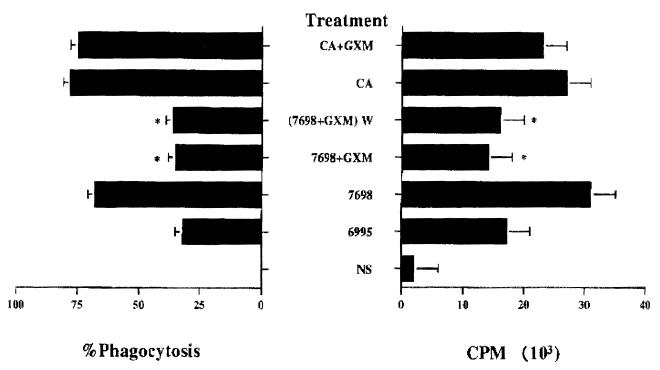
Incubation of acapsular cryptococci with purified GXM leads to binding of GXM to the yeast surface (14, 16, 40) and renders the acapsular cryptococci resistant to phagocytosis (2, 14). As a consequence, we considered the possibility that the inhibitory activity of GXM is related to the ability of GXM to coat the surface of the acapsular cells. GXM (50 μg in a total volume of 0.2 ml) was mixed with cells of strain 7698 (2 × 105) for 30 min, and then the cells were washed to remove nonadherent GXM and added to APC. We also evaluated the effect of GXM on APC functions in assays using an unrelated antigen (C. albicans). APC (2 × 104) were treated with C. albicans (2 × 105) and GXM (50 μg in a total volume of 0.2 ml), and then T lymphocytes (105) were added. The results (Fig. 1) showed significant (P < 0.01) and similar inhibition of T-cell proliferation regardless of whether (i) GXM was added simultaneously with the acapsular cells, (ii) acapsular cells were pretreated with GXM and washed [Fig. 1, (7698 + GXM) W] and then added to APC in the absence of free GXM, (iii) GXM was present in the form of naturally encapsulated cryptococci. In contrast, GXM had little effect on the proliferative response to C. albicans. In every instance, inhibition of proliferation was accompanied by an inhibition of phagocytosis [Fig. 1, (7698 + GXM) W, 7698 + GXM, or 6995]. In contrast, treatments that elicited a strong proliferative response (Fig. 1, CA+GXM, CA, or 7698) were also characterized by high levels of phagocytosis.
FIG. 1.
Correlation between T-cell proliferative response to monocytes pretreated with the acapsular strain of C. neoformans (7698) in the absence or presence of GXM and phagocytic capability of monocytes. Percent phagocytosis was calculated as the percentage of PBM containing 1 or more yeast cells per 100 cells counted as described in Materials and Methods. GXM (250 μg/ml) was added at the time of acapsular C. neoformans 7698 or C. albicans (CA) treatment. GXM (250 μg/ml) was also preincubated with acapsular C. neoformans 7698 for 30 min at 37°C, and then the cells were washed twice to remove nonadherent GXM and added to PBM [(7698 + GXM) W]. Values represent the mean counts per minute ± SEM of three separate experiments from three different donors. For experimental details, see Table 1. ∗, P < 0.001 (GXM-treated versus untreated cells). NS, not stimulated.
IL-10 has been found to completely prevent antigen-specific T-cell proliferation by inhibiting monocyte antigen-presenting capacity (6, 7). As a consequence, we examined IL-10 production during coculture of C. neoformans-treated monocytes with T lymphocytes under the same conditions as used in the experiment described above. Small samples were collected on days 3 and 7, and IL-10 concentrations were determined. The results (Table 3) showed that IL-10 production after coculture with strain 7698 was only slightly greater than levels observed in the absence of the cryptococcal cells. In contrast, coculture in the presence of encapsulated cryptococci (strain 6995) resulted in a significant (P = 0.008) elevation in extracellular IL-10 levels. Peak levels of IL-10 were observed after 3 days incubation, with a slight decrease in IL-10 levels occurring at 7 days.
TABLE 3.
IL-10 levels in supernatant fluids of cocultures of T lymphocytes plus C. neoformans-treated monocytes
| Treatment | Mean IL-10 (pg/ml) ± SEMa
|
|
|---|---|---|
| Day 3 | Day 7 | |
| None | 8 ± 2 | 7 ± 1 |
| 7698 | 6 ± 1 | 7 ± 0 |
| 6995 | 25 ± 3b | 18 ± 5 |
IL-10 was determined in supernatant fluids from cocultures of Cryptococcus-treated monocytes plus T lymphocytes after 3 or 7 days of culture. Values represent mean ± SEM of three separate experiments from three different donors.
P < 0.01 (treated versus untreated cells).
IL-10 secreted in response to encapsulated C. neoformans represents an inhibitory factor that could, in part, explain the downregulation of T-cell proliferation. As a consequence, we evaluated the effect of addition of anti-IL-10 MAbs on the proliferative response to encapsulated (strain 6995) or nonencapsulated (strain 7698) cryptococci. The results (Table 4) showed that addition of anti-IL-10 upregulated (P < 0.01) lymphoproliferation induced by the encapsulated strain (6995) but had no significant effect (P > 0.05) on the proliferative response to nonencapsulated C. neoformans. An alternative explanation for upregulation by the anti-IL-10 MAb is the presence of LPS contamination of MAb preparation. A role for contaminating LPS was excluded by LAL assay and by a parallel experiment in which polymyxin B (50 μg/ml) was added at the time of coculture. The results were unchanged in the presence of polymyxin B (data not shown). Additionally, a role for LPS contamination of the MAb preparation in upregulation of the proliferative response to encapsulated cryptococci is unlikely because the anti-IL-10 MAb had no effect on the proliferative response to acapsular cryptococci (strain 7698).
TABLE 4.
Effect of the addition of anti-IL-10 MAb on proliferative response of T lymphocytes to C. neoformans-treated monocytes
| Treatment | Mean cpm ± SEMb
|
|
|---|---|---|
| Day 3 | Day 7 | |
| None | 500 ± 25 | 389 ± 29 |
| 7698 | 18,800 ± 1,600 | 32,000 ± 1,800 |
| 7698 + anti-IL-10 MAba | 17,000 ± 1,300 | 36,900 ± 900 |
| 6995 | 12,900 ± 1,000 | 21,600 ± 1,200 |
| 6995 + anti-IL-10 MAba | 18,000 ± 1,000c | 36,700 ± 2,500c |
Anti-IL-10 MAb (10 μg/ml) was added at the time of culture preparation.
Mean ± SEM of three separate experiments from three different donors.
P < 0.01 (6995-plus-anti-IL-10 MAb-treated versus 6995-treated cells).
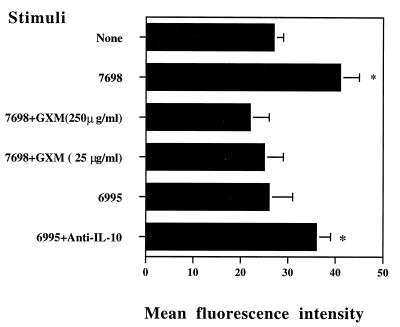
Previous studies by de Waal Malefyt found that IL-10 reduces antigen-specific T-cell proliferation by diminishing the antigen-presenting capacity of monocytes via a downregulation of class II MHC molecules (6, 7). Our recent results show that exogenous IL-10 regulates APC function of monocytes exposed to C. neoformans (27). The observation that endogenous IL-10 downregulates the proliferative response to encapsulated cryptococci (Table 4) suggested a paracrine role for endogenous IL-10 in reducing antigen-specific T-cell proliferation by diminishing the accessory function of monocytes via downregulation of MHC class II molecule expression. Thus, we analyzed class II MHC molecular expression in monocytes treated with acapsular C. neoformans 7698 in the presence or absence of various amounts of GXM, as well as after challenge with encapsulated strain 6995 in the absence or presence of MAb anti-IL-10. The results (Fig. 2) show that acapsular strain 7698 upregulates constitutive HLA-DR expression on monocytes. The addition of GXM inhibits, in a dose-dependent fashion, HLA-DR expression induced by treatment with strain 7698. Moreover, the absence of increased HLA-DR expression observed when monocytes were stimulated with encapsulated cryptococci was reversed by incorporation of an anti-IL-10 MAb into the incubation mixture. These results indicate a role for GXM and encapsulation in inhibiting expression of MHC class II molecules on monocytes, via an IL-10-dependent process.
FIG. 2.
HLA-DR molecule expression on monocytes untreated (None) or treated with the acapsular strain of C. neoformans (7698) in the absence or presence of various concentrations of GXM or treated with the encapsulated strain of C. neoformans (6995) in the absence or presence of anti-IL-10 MAb. Monocytes were cultured for 24 h and analyzed for HLA-DR expression. The results are the mean fluorescence intensity ± SEM of three experiments performed. The mean fluorescence intensity of monocytes treated with isotype-matched MAb was 8 ± 2. ∗, P < 0.05 (Cryptococcus-treated versus untreated cells).
DISCUSSION
Results from this study show that T lymphocytes proliferate in response to incubation with APC that have been pretreated with acapsular cryptococci. In contrast, APC pretreated with encapsulated cryptococci have little or no ability to stimulate T-cell proliferation. Thus, the presence of a capsule is associated with decreased lymphoproliferation. The cryptococcal capsule is undoubtedly comprised of several constituents. The observation that acapsular cryptococci can be incubated with purified GXM and washed and acquire a diminished ability to stimulate lymphoproliferation (Table 1) identifies GXM as the capsular component which mediates the suppression. These results confirm similar observations by ourselves (39, 40, 43) and others (5, 26). A role for GXM in the suppressive phenomenon is further supported by our more recent study which found that anti-GXM MAbs are able to upregulate APC function of monocytes treated with encapsulated cryptococci (44). Effects of MAb treatment of encapsulated cryptococci included enhancement of T-cell lymphoproliferative response and a reduction of endogenous IL-10 production.
Our studies provide new insight into the mechanisms for capsule-mediated suppression of T lymphoproliferation by identifying a role for endogenous IL-10 in the suppressive phenomenon and correlating GXM-dependent reduced APC function with downregulation of HLA-DR expression on monocytes. Coculture of encapsulated cryptococci with monocytes and T lymphocytes led to elevated levels of IL-10 in culture supernatant fluids. We have previously reported that supernatant fluids of monocytes cultured with encapsulated cryptococci have higher levels of IL-10 than supernatant fluids of monocytes cultured with acapsular cryptococci (46). The present study directly links the increased levels of IL-10 with GXM-dependent suppressed T lymphoproliferation because addition of anti-IL-10 MAbs to the culture medium reversed the suppression.
Previous studies found that IL-10 reduces antigen-specific proliferation of human T cells by reducing the antigen-presenting capacity of monocytes via downregulation of class II MHC expression (7). Given our observation that IL-10 plays a critical role in suppression of the lymphoproliferative response to C. neoformans antigens (27), we assessed the effect of GXM encapsulation on expression of HLA-DR on monocytes. The finding that expression of HLA-DR is suppressed on monocytes exposed to encapsulated cryptococci or GXM-treated acapsular cryptococci is consistent with our recent observations that establish a clear link between IL-10-induced downregulation of class II MHC expression on monocytes exposed to C. neoformans (27). The present results confirm and extend our previous data (27) indicating an autocrine/paracrine role for endogenous IL-10 in dampening APC capacity of monocytes, with consequent reduction of T-cell response.
Our conclusions regarding the effects of encapsulation on the antigen-presenting capacity of monocytes are based on comparisons of encapsulated and acapsular cryptococci as well as the effect of experimental GXM encapsulation of acapsular cryptococci. Several possible caveats should be noted. First, the encapsulated and acapsular isolates are not isogenic; therefore, we cannot rule out a phenotypic difference that accounts for the observed results. However, the similarity in the effect of GXM-treated acapsular cryptococci and the effect of encapsulated cryptococci argues for a GXM-biased response. Second, the possibility of LPS contamination of the GXM preparation must be considered. It is unlikely that LPS contamination accounts for inhibition of the T-cell lymphoproliferative response because (i) inhibition was also observed with encapsulated cryptococci that were not subjected to the prolonged processing inherent in purification of GXM, (ii) inhibition was observed with acapsular cryptococci that were treated with GXM and washed to remove nonadherent polysaccharide, (iii) GXM did not suppress the T-cell proliferative response to C. albicans, (iv) inhibition was not blocked by incorporation of polymyxin B into the medium, and (v) the T-cell proliferative response to acapsular cryptococci was enhanced (rather than inhibited) by incorporation of LPS into the incubation medium. Finally, there is the possibility that suppression of the T-cell proliferative response is due to contamination of GXM by CTAB used for isolation of GXM. It is unlikely that CTAB accounts for the observed suppression because (i) similar levels of suppression were observed with encapsulated cryptococci that were not treated with CTAB and (ii) GXM did not suppress the T-cell proliferative response to C. albicans.
The mechanism by which encapsulation with GXM mediates suppression of the T lymphoproliferative response remains to be determined. GXM binds to the surface of acapsular cryptococci, leading to inhibition of phagocytosis (14, 15). The observation that decreased T lymphoproliferation paralleled decreased phagocytosis (Fig. 1) suggests that suppression might be due to nothing more than an inability to be ingested. Such inhibition of phagocytosis could reduce processing by APC and subsequent stimulation of T-cell proliferation. This passive role by GXM encapsulation is not consistent with the observation that coincubation of monocytes and T lymphocytes with encapsulated cryptococci leads to increased levels of IL-10 in the culture supernatant fluids. Moreover, addition of anti-IL-10 MAbs to the incubation mixture restored the T lymphoproliferative response to encapsulated cryptococci to levels observed with acapsular cryptococci. This finding suggests that GXM encapsulation plays an active role in suppression of APC-dependent lymphoproliferation.
There are at least two mechanisms by which GXM encapsulation could actively modulate the lymphoproliferative response. First, GXM could directly bind to monocytes, leading to release of IL-10. In support of this mechanism, Blackstock reported that APC treated with GXM can be washed and still retain the ability to induce release of a T-suppressor factor from an inducible T-T hybridoma (1). Also in agreement with a direct stimulation of monocytes by GXM, Dong and Murphy have reported evidence that GXM binds CD18 on human neutrophils (9). An alternative mechanism for an active role in stimulation of monocytes is the possibility that GXM encapsulation leads to enhanced activation of the complement system. The cryptococcal capsule is a powerful activator of the complement system (18, 20). Complement cleavage fragments such as C3a or C5a could stimulate monocytes, with a consequent release of IL-10. Studies in progress in our laboratories are examining both the direct interaction between GXM and monocytes and the role of complement cleavage fragments in GXM-dependent modulation of monocyte function.
ACKNOWLEDGMENTS
We are grateful to Eileen Mahoney Zannetti for excellent technical assistance.
This study was supported by IX Progetto AIDS (contract 9404-39), Italy, and by Public Health Service grant AI-14209 (to T.R.K.).
REFERENCES
- 1.Blackstock R. Cryptococcal capsular polysaccharide utilizes an antigen-presenting cell to induce a T-suppressor cell to secrete TsF. J Med Vet Mycol. 1996;34:19–30. doi: 10.1080/02681219680000041. [DOI] [PubMed] [Google Scholar]
- 2.Bulmer G S, Sans M D. Cryptococcus neoformans. III. Inhibition of phagocytosis. J Bacteriol. 1968;95:5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherniak R, Reiss E, Slodki M E, Plattner R D, Blumer S O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans. Mol Immunol. 1980;17:1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 4.Cherniak R, Reiss E, Turner S H. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;103:239–250. [Google Scholar]
- 5.Collins H L, Bancroft G I. Encapsulation of Cryptococcus neoformans impairs antigen-specific T-cell responses. Infect Immun. 1991;59:3883–3888. doi: 10.1128/iai.59.11.3883-3888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Waal Malefyt R, Haanen J, Spits H, Roncarolo M G, Te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond R D, Bennett J E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974;80:176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 9.Dong Z M, Murphy J W. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect Immun. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng R H K, Bishburg E, Smith S M, Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986;81:19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- 11.Good C B, Coax W A. Cryptococcal infections in patients with AIDS. N Engl J Med. 1990;322:701–702. doi: 10.1056/nejm199003083221017. [DOI] [PubMed] [Google Scholar]
- 12.Houpt D C, Pfrommer G S T, Young B J, Larson T A, Kozel T R. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:2857–2864. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khakpour F R, Murphy J W. Characterization of a third-order suppressor T cell (Ts3) induced by cryptococcal antigen(s) Infect Immun. 1987;55:1657–1662. doi: 10.1128/iai.55.7.1657-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozel T R. Nonencapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977;16:99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozel T R, Gotschlich E C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 16.Kozel T R, Hermerath C A. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984;43:879–886. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozel T R, Mastroianni R P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976;14:62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozel T R, Pfrommer G S T. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986;52:1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozel T R, Pfrommer G S T, Redelman D. Activated neutrophils exhibit enhanced phagocytosis of Cryptococcus neoformans opsonized with normal human serum. Clin Exp Immunol. 1987;70:238–246. [PMC free article] [PubMed] [Google Scholar]
- 20.Kozel T R, Wilson M A, Pfrommer G S T, Schlageter A M. Activation and binding of opsonic fragments of C3 onto encapsulated Cryptococcus neoformans using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989;57:1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levitz S M, Tabuni A, Kornfield H, Reardon C C, Golenbock D T. Production of tumor necrosis factor alpha in human leukocytes stimulated by Cryptococcus neoformans. Infect Immun. 1994;62:1975–1981. doi: 10.1128/iai.62.5.1975-1981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levitz S M, Tabuni A, Nang S-H, Golenbock D T. Effects of interleukin-10 on human peripheral blood mononuclear cell responses to Cryptococcus neoformans, Candida albicans, and lipopolysaccharide. Infect Immun. 1996;64:945–951. doi: 10.1128/iai.64.3.945-951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller G P G, Kohl S. Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol. 1983;131:1455–1459. [PubMed] [Google Scholar]
- 24.Miller M F, Mitchell T G. Killing of Cryptococcus neoformans strain by human neutrophils and monocytes. Infect Immun. 1991;59:24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS: 100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mody C H, Syme R M. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464–468. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monari C, Retini C, Palazzetti B, Bistoni F, Vecchiarelli A. Regulatory role of exogenous IL-10 in the development of immune response versus Cryptococcus neoformans. Clin Exp Immunol. 1997;109:242–248. doi: 10.1046/j.1365-2249.1997.4021303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy J W. Effects of first-order Cryptococcus-specific suppressor cells on induction of cells responsible for delayed-type hypersensitivity. Infect Immun. 1985;48:439–445. doi: 10.1128/iai.48.2.439-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy J W, Cox R A. Induction of antigen-specific suppression by circulating Cryptococcus neoformans antigen. Clin Exp Immunol. 1988;73:174–180. [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy J W, Cozad G C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy J W, Moorhead J W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982;128:276–282. [PubMed] [Google Scholar]
- 33.Murphy J W, Mosley R L. Regulation of cell-mediated immunity to cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2) J Immunol. 1985;134:577–584. [PubMed] [Google Scholar]
- 34.Murphy J W, Mosley R L, Moorhead J W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells and induction of second-order suppressor cells. J Immunol. 1983;130:2876–2881. [PubMed] [Google Scholar]
- 35.Orendi J M, Nottet H S L M, Visser M R, Verheul A F M, Snippe H, Verhoef J. Enhancement of HIV-1 replication in peripheral blood mononuclear cells by Cryptococcus neoformans is monocyte-dependent but tumor necrosis factor-independent. AIDS. 1994;8:423–429. doi: 10.1097/00002030-199404000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Pettoello-Mantovani M, Casadevall A, Smarnoworawong P, Goldstein H. Enhancement of HIV type 1 infectivity in vitro by capsular polysaccharide of Cryptococcus neoformans and Haemophilus influenzae. AIDS Res Hum Retroviruses. 1994;10:1079–1084. doi: 10.1089/aid.1994.10.1079. [DOI] [PubMed] [Google Scholar]
- 37.Pfeffer K, Shoel B, Gies H, Kaufmann S H E, Wagner H. Primary responses of human mycobacteria: a frequent set of γ/δ T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 38.Powderly, W. G. 1992. Therapy for cryptococcal meningitis in patients with AIDS Clin. Infect. Dis. 14(Suppl. 1):S54–S59. [DOI] [PubMed]
- 39.Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel T R. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Small J M, Mitchell T G. Binding of purified and radioiodinated capsular polysaccharides from Cryptococcus neoformans serotype A strains to capsule-free mutants. Infect Immun. 1986;54:742–750. doi: 10.1128/iai.54.3.742-750.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vecchiarelli A, Dottorini M, Beccari T, Cociani C, Todisco T, Bistoni F. Inhibition of candidacidal activity of polymorphonuclear cells by alveolar macrophage-derived factor from lung cancer patients. Am Rev Respir Dis. 1993;147:414–419. doi: 10.1164/ajrccm/147.2.414. [DOI] [PubMed] [Google Scholar]
- 42.Vecchiarelli A, Dottorini M, Pietrella D, Monari C, Retini C, Todisco T, Bistoni F. Role of alveolar macrophages as antigen presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994;11:130–137. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 43.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vecchiarelli A, Retini C, Casadevall A, Bistoni F. Third International Conference on Cryptococcus & Cryptococcosis. Paris, France. 1996. Monoclonal antibody to Cryptococcus neoformans glucuronoxylomannan enhances antigen presenting capacity of human monocytes, abstr. 2.4; p. 172. [Google Scholar]
- 45.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation of cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]