Abstract

The production of syngas (i.e., a mixture of CO and H2) via the electrochemical reduction of CO2 and water can contribute to the green transition of various industrial sectors. Here we provide a joint academic–industrial perspective on the key technical and economical differences of the concurrent (i.e., CO and H2 are generated in the same electrolyzer cell) and separated (i.e., CO and H2 are electrogenerated in different electrolyzers) production of syngas. Using a combination of literature analysis, experimental data, and techno-economic analysis, we demonstrate that the production of synthesis gas is notably less expensive if we operate a CO2 electrolyzer in a CO-selective mode and combine it with a separate PEM electrolyzer for H2 generation. We also conclude that by the further decrease of the cost of renewable electricity and the increase of CO2 emission taxes, such prepared renewable syngas will become cost competitive.
The Importance of Syngas
For decades, hydrogen (H2) and carbon monoxide (CO) have been used in a variety of ways as building blocks for chemical and fuel production. Depending on the final product and the type of process, H2 and CO can be utilized separately or together. When combined, their mixture is commonly known as syngas. Nowadays, syngas is mainly produced via either reforming or partial oxidation of fossil resources, such as natural gas, naphtha, and heavy residual fuel oils. Other methods include gasification of coal, and most recently of municipal solid waste and biomass.1 The methods using fossil resources are energy intensive with huge environmental footprint, including significant carbon dioxide (CO2) emissions.2 The biomass-based processes, on the other hand, are limited in scale. The type of feedstock and the associated production process determine the syngas ratio (i.e., the ratio of H2 and CO). A specific ratio is needed depending on the application, such as the production of pharmaceuticals, plastics, chemicals, or synthetic fuels (Tables 1 and S1).3−6
Table 1. Illustration of the H2:CO Ratio Required for the Main Syngas Applications.
| H2:CO Ratio | Application |
|---|---|
| <1 | Polyurethanes, polycarbonates, acetic acid |
| 1 | Oxo alcohols, dimethyl ether |
| 2 | Methanol, Fischer–Tropsch liquid fuels |
The global syngas market has been estimated to have a size of 218 MM Nm3/h in 2022 including hydrogen and ammonia, using syngas as an intermediary.7 The market is projected to increase notably in the coming decades (with an approximate CAGR of 6% by 2028).7 This increase is rooted in the projected growth of industries consuming syngas today, as well as the fact that novel pathways of utilization are expanding.8,9 To fight global warming and to comply with the Paris Agreement (COP-21) and consequent regulatory changes (e.g., RED III in Europe10), massive greenhouse gas emission reductions in all sectors are required by 2050.11According to The International Energy Agency (2021), to meet current net-zero targets, the rapid deployment of appropriate carbon capture and utilization (CCU) technologies is required to stay on track with carbon emissions by 2050. Consequently, the production of low-carbon syngas on CO2 basis, together with electrification and hydrogen mobility, is expected to significantly contribute to the decarbonization of both industrial and transportation sectors.12−16
In Figure 1 we illustrate the growth potential of syngas for the different chemical and fuel markets (excluding all hydrogen and ammonia production and energy applications). We specifically show that the growth is expected to come mainly from the synthetic sustainable fuels market which currently is in its infancy. Notably, it is very difficult to estimate the current syngas (CO + H2) market size because most statistics and analysis include all sorts of synthetic gases in this category (e.g., N2 + H2 mixtures for ammonia synthesis). Our approach was to analyze the markets and market trends of the most important syngas-derived products (see the Supporting Information).
Figure 1.

Long-term prediction of the syngas market size (own estimation based on the growth of the key market segments, see section S5 in the Supporting Information).
Electrochemical Syngas Generation
Traditionally, CO has been produced in large, centralized plants that profit from the economy of scale. Its toxicity and flammability, however, make the transportation of pressurized CO gas cylinders or tube trailers very hazardous and therefore expensive. Electrolysis, when using electricity from renewable sources, is one of the most prominent and environmentally friendly solutions to produce such chemicals.17 Water electrolysis to produce renewable hydrogen has reached commercial maturity, and large projects have been announced to get into operation in the coming years.18 CO2 electrolysis is another promising method, allowing the electrochemical conversion of CO2 to chemicals.19 From an energetic point of view, the production of CO is the most favorable large market chemical that can be obtained via CO2 electrolysis.20,21 Electrochemical processes can be operated at low temperatures and pressures, as opposed to other chemical or catalytic processes. Furthermore, it allows decentralized operation, because the scale has little effect on the system cost and efficiency. Another key differentiator compared to traditional methods is the possibility of dynamic operation, as we have recently demonstrated.22 Overall, the electrochemical route would make CO production not only more sustainable but also less centralized/more distributed and therefore suitable for a larger number of applications.
According to different techno-economic assessments (TEAs), electrochemical production of CO can already be profitable, if the technology is available at scale.20,21,23 Furthermore, for applications where a steady supply of syngas is not needed, the electrochemical reduction of CO2 provides a unique opportunity to convert intermittent but abundant renewable energy source into chemical fuels.24 What is equally important, different life cycle assessment (LCA) studies confirmed that such electrochemical routes can have a significant reduction in CO2 footprint, compared to the traditional methods.25,26 Meeting these three conditions together (i.e., economic viability, CO2 footprint decrease, and large market size) predicts a great promise for electrochemical CO2-to-CO conversion. At the same time, several challenges, such as low energy efficiency, short demonstrated system lifetime, and consequently high capital and operating costs, must be overcome prior to commercialization. Such efforts are underway at several companies.19,27
Due to the presence of water in the electrolyzer system (vapor or liquid, depending on the reaction conditions and the electrolyzer type), part of the electrical current applied for the CO2 electrolysis may generate H2 instead of CO, lowering the Faradaic efficiency (FE) of CO formation. This opens the opportunity for the one-step generation of syngas, which is often claimed as a benefit of such technologies. A recent article analyzed the economics of electrochemical syngas production, comparing different processes and cell configurations, with particular emphasis on the integration of direct air capture.28 Our Perspective focuses on a different angle of the story, namely, the separate vs concurrent production of the two components of syngas (i.e., H2 and CO).
Options for Green Syngas Generation
Several types of CO2 electrolyzer setups can be used for renewable syngas production, each of them having its own advantages and limitations.29 High-temperature solid-oxide electrolyzers have been recently benchmarked against conventional CO-generating processes.30 This Perspective focuses on low-temperature electrolysis because, as opposed to the high-temperature processes, such systems can be operated dynamically and under similar conditions as proton exchange membrane (PEM), alkaline, and anion exchange membrane (AEM) water electrolyzers. More specifically, we investigated the AEM containing gas diffusion electrode (GDE) system for both CO- and syngas production from CO2. Such cells employ a GDE to enhance the mass transport of CO2 to the catalyst, resulting in higher current density and single-pass conversion.31,32 AEM CO2 electrolyzers show better energy efficiencies and often use less expensive materials, compared to their PEM-based and bipolar membrane-containing counterparts.33 Carbonate formation, however, is a challenge for this technology, as carbonate/bicarbonate ions are formed at the cathode when CO2 comes into contact with OH– ions.34 Due to the unintended cation crossovers from the anolyte,32,35 carbonates can precipitate, leading to accumulation and poisoning/flooding of the cathode GDE. Carbonate ions also migrate to the anode, leading to the coevolution of CO2 and O2 and thus to more purification efforts.34,36
In this study, we studied three options to produce syngas with an H2:CO ratio of 2:1 (Figure 2):
-
A:
AEM CO2 electrolyzer with a FE of 98% toward CO. The final syngas is obtained by mixing the CO with H2 produced from a PEM water electrolyzer. The syngas composition can then be controlled by adjusting the relative proportions of CO and H2.
-
B:
AEM CO2 electrolyzer with a FE of 50% toward CO. The resulting H2:CO ratio of 1:1 is then modified by the addition of H2 coming from a PEM water electrolyzer.
-
C:
AEM CO2 electrolyzer with a FE of 33% toward CO. The desired syngas composition with an H2:CO ratio of 2:1 is obtained using only the AEM CO2 electrolyzer. The FE and consequently the syngas composition can be adjusted by varying the operational parameters of the electrolyzer (e.g., cell voltage, CO2 and H2O feed rate).
Figure 2.
Illustration of the three different syngas production scenarios analyzed in this Perspective.
Technical Considerations
CO2 electrolysis literature has grown massively during the past two decades. Many of these papers claim “the production of syngas with tunable composition” as their key selling point. This has been seen for Ag, Au, Zn, different bimetallic, Pd hydride, and metal–nitrogen doped carbon catalysts.37−42 These catalysts are known to form CO as the predominant CO2 reduction (CO2R) product, with the concurrent formation of H2. In Table S1, we summarize selected examples from the literature, where the H2:CO ratio varied between the broad range of 1:4 to 25:1 (a range of 2 orders of magnitude(!)). This variation was mostly attributed to the catalyst surface composition and the applied electrode potential. While these exploratory studies are interesting, they were performed in H-cells, in the presence of one or more aqueous electrolytes (i.e., one electrolyte in membrane-less cells and two electrolytes (anolyte and catholyte) in membrane-separated cells), at low current density (up to 20 mA/cm2), for short time periods (typically up to a few hours).
From a practical perspective, the most promising studies for CO and syngas generation have been reported on GDE-containing cells and stacks.19,43 The results are massively different from those obtained in H-cells. This is mainly because the selectivity (i.e., HER vs CO2R ratio) is dictated by multiple parameters beyond the catalyst itself: components of the membrane electrode assembly (MEA), local chemical environment (pH, water content), and operational parameters (gas flow rate, etc.). There are only a very few long-term studies for CO formation,44−47 and no long-term data is available for syngas generation. We think this is partly because the most important fading mechanism in such systems is flooding when too much electrolyte accumulates in the cathode GDE. This in turn results in increased HER, which ultimately leads to cell fading. Some studies indicate the intricate connection among precipitate formation, flooding, and increased HER,48,49 but it is not within the scope of this Perspective. There is, however, reason to believe that process conditions in which large amounts of H2 are generated favor the eventual flooding of the cathode.
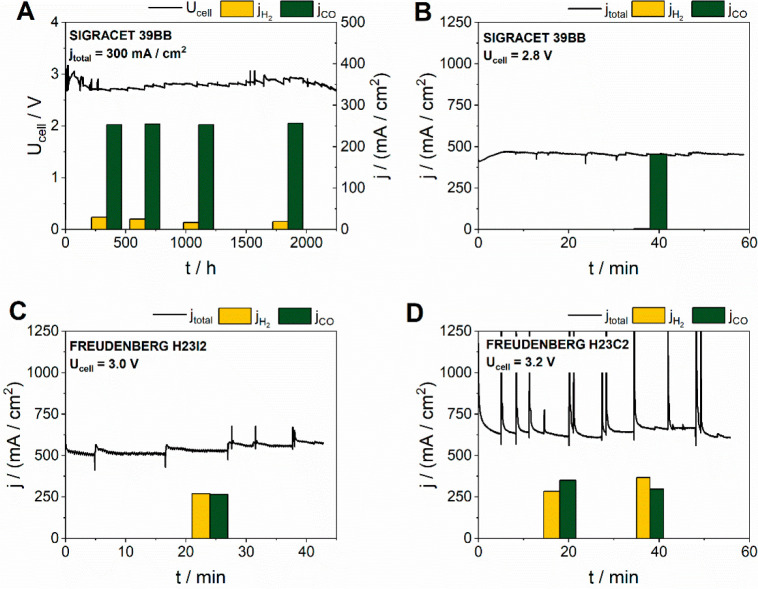
In Figure 3A we show our own data as an example of the long-term stable operation of a CO-selective CO2 electrolyzer (with over 90% FE for CO formation for over 2000 h, with an approximate degradation rate of ∼50 μV/h) and examples for the other target gas compositions, obtained using GDEs with different structures. Clearly, the use of Sigracet 39BB carbon GDL, together with proper electrolyte management (to keep steady-state local pH and cation concentration), results in a CO-selective operation (Figure 3B), while the use of Freudenberg H23I2 and Freudenberg H23C2 carbon GDLs results in an increased HER activity (see more measurements on different GDLs37). Overall, depending on the cell components and the applied cell voltage, we can get a similar product composition as in the three targeted syngas production pathways previously defined (Figure 2). At the same time, the more frequent and larger spikes on the current curves with the increasing H2 content indicate pronounced water accumulation and release in the cell, which is typically the first step on the way to flooding. In addition to our own data, we present a brief comparison of selected studies from the literature where high current densities were obtained (Table S1).
Figure 3.
Illustrative electrochemical data for the formation of CO and syngas formation. (A, B) An optimized zero-gap electrolyzer cell; (C, D) nonoptimal carbon GDLs. Results were obtained with a zero-gap electrolyzer cell with Ag cathode, Ir anode, and PiperION anion exchange membrane, using 0.05 M (A) or 0.1 M (B–D) CsHCO3 anolyte.
Based on the above literature analysis, our own data, the historic learning curve of PEM and AEM water electrolyzers, and future predictions, we defined today’s and future (2030) operational parameters for the three studied scenarios (Table 2). These numbers also reflect the expected effects of ongoing engineering efforts, integrating state-of-the art cell components, implementing methods established by allied fields (e.g., MEA production in the fuel cell industry). Please note that with regard to the single-pass conversion, an exclusive carbonate ion transport from the cathode to the anode has been assumed for all the cases, including scenarios “B” and “C” where the HER becomes prevalent (i.e., the charge carrier species are still the carbonate ions; see more discussion in Table S2).
Table 2. Electrochemical Performance Parameters for Different Cases.
| CO2 Electrolyzer |
|||||||
|---|---|---|---|---|---|---|---|
| Case A |
Case B |
Case C |
PEM Electrolyzer | ||||
| Today | 2030 | Today | 2030 | Today | 2030 | Cases A and B | |
| FECO (%) | 98% | 98% | 50% | 50% | 33% | 33% | 0% |
| Current density (mA/cm2) | 500 | 600 | 800 | 900 | 1000 | 1100 | 2000 |
| Voltage (V) | 2.6 | 2.4 | 2.9 | 2.8 | 3.1 | 3.0 | 1.9 |
| Single-pass CO2 conversion (%) | 40% | 40% | 25% | 28% | 15% | 18% | 0% |
| Degradation rate (μV/hours) | 30 | 10 | 50 | 25 | 80 | 40 | 2.6 |
Technoeconomic Comparison
To evaluate the viability of the three different green syngas production routes, and to provide guidance to the R&D community, the total cost of ownership (TCO) of the syngas (expressed in €/kgSG) has been calculated. The main methods and governing equations used in this paper are in accordance with the ones described in previous CO2 electrolysis TEA studies (see details in the Supporting Information).20,23 Nowadays, a typical industrial capacity for CO production only is 10,000 Nm3/h and is usually supplied through reforming of natural gas in which large quantities of hydrogen are also coproduced.50 The same CO capacity has therefore been set for the CO2 electrolysis as benchmark. As the desired H2:CO ratio of syngas in this study is 2:1, the final syngas quantity to be produced will be 30,000 Nm3/h (342 t/day).
As low-temperature CO2 electrolysis has not reached commercial availability yet, the CAPEX of the CO2 electrolyzer stack has been extrapolated from the cost of the more mature water electrolysis (see Table S3 for our literature review).51 In parallel, a bottom-up approach has also been performed to estimate stack component prices (Table S4). The final cost of the CO2 stack used for this study was 2587 €/m2. This value should be seen as a target cost in 2030 for a 10 MW stack (at a reference voltage and current of 2.6 V and 500 mA/cm2) rather than as a current stack cost. The overall cost of the system is then obtained by adding the balance of plant costs (the stack contributes ca. 30% to the total system cost, with the other 70% being the balance of the plant52). An installation factor of 1.6 is used for the electrolyzer system.53 The resulting total installed cost of a CO2 electrolyzer is therefore 13797 €/m2. The complementary PEM water electrolysis system used to produce any hydrogen needed depending on the route considered has a total installed cost (TIC) of 1300 €/kW with an expected cost decrease of 20% by 2030.54 This system produces hydrogen at 56 kWh/kgH2 with an estimated degradation rate of 2.6 μV/hour.55,56 Due to degradation, the stacks need to be replaced over the lifetime of the system.56,57 For each configuration and for both water and CO2 electrolyzers, the optimum number of stack replacements was calculated to minimize the cost (see the Supporting Information). What is often neglected in academic TEA studies is the fact that the rectifiers and power electronics are designed to function within specific voltage limits.58 A significant voltage increase beyond this range can induce various technical challenges, including reduced system efficiency, increased wear and tear on equipment, and potential safety risks.59 To avoid these issues, the electrolyzer stack in this study is assumed to be replaced before a 50% voltage increase due to degradation is reached.
To limit CO2 consumption and therefore variable costs (and also to achieve the maximum CO2 emission avoidance), CO2 capture and recirculation is needed both at the cathode stream (because of the incomplete CO2 single-pass conversion) and at the anode stream (because of carbonate crossover and subsequent CO2 liberation).34,60 In CO2 capture from gaseous streams, various methods can be employed, including chemical absorption, cryogenic, and membrane separation.61 Pressure swing adsorption (PSA), in which CO2 is selectively removed from the gaseous mixture using solid adsorbents, has been used for this study at both the anode and cathode sides. This is due to its low energy consumption, its broad adaptability to different capture needs, and its ability to achieve high purity levels.62 For this study the technology taken as a reference is the PSA used in biogas upgrade.63 A scaling factor of 0.7 was used to adjust the reference costs to our system.
The two main variable costs considered are renewable electricity and the CO2 feedstock. As the Fischer–Tropsch process requires a continuous and stable feed supply to produce liquid fuels, the renewable electricity used to produce H2 and CO must be purchased at a very high availability. Different studies project levelized costs of energy (LCOE) for utility-scale PV and wind in 2030 between 20 and 35 €/MWh; however, such power comes with high intermittency and low capacity factors (20–40%).64−66 To maintain a high availability, a combination of PV, wind, and energy storage is necessary.67 This configuration, however, will increase the overall electricity cost due to the need for excess capacity and storage infrastructure.68
For renewable syngas to be considered a real carbon sink, the CO2 must come from direct air capture (DAC) or biogenic sources. DAC’s current high cost makes it less economically attractive for large-scale fuel production.69 On the other hand, biogenic CO2 comes at lower costs with various sources such as fermentation, anaerobic digestion, and biomass postcombustion processes, and we used such data (purification needs of different sources may vary (e.g., SOx/NOx); that is why a relatively high average CO2 cost is considered).70 The use of CO2 from an industrial point-source, although largely available at a low price, would only lead to delayed emissions in the case of fuel production, and it is therefore not considered in this study.
Although use cases may exist where CO and O2 could be utilized by one or different end users at the same location (i.e., in oxyfuel combustion), the oxygen produced at the anode side is not valorized in the model because its cost would depend on its final purity, which in turn would need a more detailed assessment. In addition, the oxygen sale is not expected to affect the final TCO significantly. No CO2 tax savings or subsidies have been taken into consideration. Any other operating costs such as water consumption, adsorbent costs, and other various utilities are not considered in this study, since they would account for a very minor cost share of the TCO. The list of assumptions is summarized in Table 3.
Table 3. Main Process, Market, and Production Assumptions.
TEA Results
Clearly, the most economical way of producing syngas by means of CO2 electrolysis is to couple a very CO-selective CO2 electrolyzer with a water electrolyzer delivering the required hydrogen (Figure 4). This configuration results in a final syngas price of 1 €/kgSG, which is 30% lower than the configuration in which the syngas is fully produced from a single CO2 electrolyzer operated at low FECO. The TCO of syngas is mainly driven by the variable costs (i.e., electricity), and their contribution increases when decreasing the FECO of the CO2 electrolyzer. In fact, while case A produces syngas at 12.6 kWh/kgSG, the direct syngas production requires 20.5 kWh/kgSG (throughout the lifetime of the plant; Figure S3a). This difference is mainly due to hydrogen production, highlighting that the hydrogen produced during the electrolysis of CO2 cannot be considered to be free. The notable difference in the overpotential between AEM CO2 and PEM water electrolyzers (2.4 V in 2030 instead of 1.9 V) causes the hydrogen coming from a CO2 electrolyzer to have a higher energy cost (also reflected in Figure S3b). A PEM electrolyzer produces hydrogen over the lifetime of the system at 60 kWh/kgH2 while a direct syngas CO2 electrolyzer does it at 110 kWh/kgH2.
Figure 4.

Syngas (H2:CO = 2:1) TCO [€/kg] for CO2-CO (A), CO2-SG-50 (B), and CO2-SG (C) cases in 2030.
The degradation rate of the electrolyzer also plays an important role in the energy efficiency of the system (Figure S3b,c). At the beginning of life, there is not a huge difference among the three different syngas production configurations. The degradation rate of the CO2 electrolysis, at this stage of technological immaturity, however, is much higher than that of a PEM water electrolyzer over the entire lifetime of the system. As a result, the energy consumption of hydrogen of system C (CO2 electrolyzer only) ends up being 90% higher than that of case A and 36% higher than case B. Notably, this is a parameter where the largest improvement is expected beyond 2030 and also needed for commercialization. The impact of the higher degradation rate of the CO2 electrolysis on the final syngas TCO is also reflected in CAPEX, as the stack must be replaced more often. In addition, the increased capacity requirement of PSAs brings a non-negligible additional cost to recirculate CO2 which gets more prominent as hydrogen is produced with the CO2 electrolyzer.
Sensitivity Analysis and Pessimistic–Realistic–Optimistic Scenarios
A sensitivity analysis on the operating conditions has been carried out for this best approach (Table S7). Figure 5 shows that the price of syngas using case A can decrease to values below 1 €/kgSG. Furthermore, even assuming a pessimistic performance, Case A would remain more advantageous from an economical point of view than the base case standalone CO2 electrolyzer (Case C) where a syngas TCO of 1.4 €/kgSG had been estimated (Figure 4).
Figure 5.

Syngas (H2:CO = 2:1) TCO [€/kg] for CO2-CO in the pessimistic, base, and optimistic cases.
Finally, a study on the TCO sensitivity toward electricity and CO2 costs was carried out (Figure 6a,b, respectively). The gap between the different cases would be minimized for electricity prices below 10 €/MWh for which a syngas TCO of 0.7 €/kgSG can be achieved (Figure 6a). This price may become a reality for specific geographies and highly intermittent power supply. It seems, however, unrealistic in the short to medium term if high renewable power availability is required. As opposed to the electricity price, the CO2 purchase cost is shown to have a significantly lower impact on the final TCO of the syngas, which is an important observation for the future adoption of DAC technologies (Figure 6b). Even under optimistic conditions, the syngas produced by means of CO2 electrolysis is still less competitive from an economic standpoint than traditional methods. Syngas produced through coal gasification or methane reforming leads to prices between 0.5 and 0.7 €/kgSG.71 This difference calls for legislation and initiatives to promote the use of renewable syngas and bridge the gap of the green premium.
Figure 6.
Sensitivity of syngas TCO [€/kg] to electricity (A) and CO2 (B) purchase price.
Summary and Outlook
Since many industries rely on a consistent supply of syngas, the electrochemical reduction of CO2 and water to syngas can contribute to their transformation to become more sustainable and eventually carbon negative.72 Electrochemical approaches can also alter the dynamics of the syngas market, as smaller, decentralized solutions can emerge to be deployed at a customer’s facility, converting CO2 emission into value, saving on transportation cost, as well as reducing emission.29 By transforming syngas into synthetic fuels by Fischer–Tropsch or other catalytic processes, it can be integrated into existing infrastructure, significantly reducing investment costs compared to other approaches of power-to-gas solutions.73
In this Perspective, we have shown that syngas production from a stand-alone CO2 electrolyzer system would be possible from a technical point of view. However, it appears that it would not be reasonable from an energetic and therefore economical point of view. Through the combination of experimental data and techno-economic analysis, we have shown that the production of a syngas is always significantly less expensive when a CO2 electrolyzer is operated with the final goal of having only CO as the final product and then coupled with a PEM electrolyzer for H2 supply. We conclude that future studies shall focus on achieving high CO Faradaic efficiencies (over 98%). This selectivity shall be achieved at industrially relevant current densities (>400 mA/cm2), and degradation rates must be further minimized. To reach these key performance indicators, research and development on MEAs and electrolyzer cell/stacks shall go hand-in-hand because they mutually affect their applicability. Our study also indicated that downstream separation of O2 and CO from residual CO2 significantly contributes to the final investment costs (about 7–14%). Therefore, development, optimization, and/or integration of the gas treatment at the anode and cathode sides will also play an important role in further decreasing the total TCO of CO. Gaining substantial operational experience at a relevant scale will be key to allow the commercialization of CO2 electrolysis to CO. Large demonstration projects supported by funding agencies would therefore be the next natural step in the commercial development of CO2 electrolysis for CO and syngas when coupled with water electrolysis.
Acknowledgments
This project has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No. 101099284. Supported by the KDP-2021 program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation fund (A.A.S.).
Biographies
Andrés Raya-Imbernón holds a French degree in materials engineering from the Institut National Polytechnique de Toulouse. He works at Air Liquide R&D focusing on sustainable approaches to the production of key molecules. Engaged in the Air Liquide Leading Excellence graduate program, he combines academic acuity with practical and business insights.
Angelika A. Samu is a Ph.D. student at the University of Szeged. She is working on CO2 electroreduction in zero gap electrolyzer cells. Her research focuses on investigating the operating parameters and characterizing the main components (gas diffusion layer, anion exchange membrane, and porous transport layer) of the electrolyzer cell.
Stefan Barwe holds a Ph.D. in Chemistry from the Ruhr-University Bochum with a strong track record in electrochemistry and electrocatalysis. He currently is a senior local expert for electrochemical processes at the Air Liquide Innovation Campus Frankfurt and leads a team focusing on techno-economic and life cycle assessments.
Giuseppe Cusati holds a Ph.D. in Heterogeneous Catalysis from CNRS Lyon. He started his career at Air Liquide Innovation Campus Frankfurt in 2011 and currently is Group Manager Process & Chemical Engineering. He leads the definition of R&D projects in the field of Energy Transition & Digitalization.
Tamás Fődi holds a Ph.D. in Chemical Engineering from the Budapest University of Technology and Economics. He currently is a senior expert at eChemicles on electrolyser system design, system integration, and techno-economic assessment.
Balázs M. Hepp is a product manager at eChemicles following a transition from the oil industry. He obtained master level degrees from Jönköping University in 2021 and University of Pannonia in 2023 in Strategic Entrepreneurship and Nuclear Engineering, respectively.
Csaba Janáky is an Associate Professor at the University of Szeged, where he is the founding director of the Greennovation Center, an interdisciplinary research unit supporting the green transition of industry. He is also cofounder of eChemicles, a company scaling-up CO2 electrolysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsenergylett.3c02446.
Literature review of a CO2 electrolyzer stack costs, selected examples from CO2 electrolysis studies, TEA methodology, additional parameters for market analysis, details of the purification model, and energy consumption data in the different scenarios (PDF)
The authors declare the following competing financial interest(s): eChemicles is scaling up its patented CO2 electrolyzer technology to provide an environmentally and economically sustainable alternative to fossil fuel-based chemicals. Air Liquide offers and operates large-scale PEM water electrolysis solutions for the production of low-carbon hydrogen.
Supplementary Material
References
- Bolívar Caballero J. J.; Zaini I. N.; Yang W. Reforming Processes for Syngas Production: A Mini-Review on the Current Status, Challenges, and Prospects for Biomass Conversion to Fuels. Applications in Energy and Combustion Science 2022, 10, 100064. 10.1016/j.jaecs.2022.100064. [DOI] [Google Scholar]
- Rashid R. T.; Chen Y.; Liu X.; Chowdhury F. A.; Liu M.; Song J.; Mi Z.; Zhou B.; Bell A. Tunable Green Syngas Generation from CO2 and H2O with Sunlight as the Only Energy Input. Proc. Natl. Acad. Sci. U. S. A. 2022, 119 (26), e2121174119 10.1073/pnas.2121174119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D. J.; Simbeck D. R.; Karp A. D.; Dickenson R. L. Syngas Production for Gas-to-Liquids Applications: Technologies, Issues and Outlook. Fuel Process. Technol. 2001, 71 (1–3), 139–148. 10.1016/S0378-3820(01)00140-0. [DOI] [Google Scholar]
- Bertau M.; Offermanns H.; Plass L.; Schmidt F.; Wernickez H.-J.. Methanol: The Basic Chemical and Energy Feedstock of the Future; Springer: Heidelberg, 2014. [Google Scholar]
- de Klerk A.Fischer–Tropsch Refining; Wiley-VCH Verlag GmbH & Co. KGaA, 2011. [Google Scholar]
- Hernández S.; Farkhondehfal M. A.; Sastre F.; Makkee M.; Saracco G.; Russo N. Syngas Production from Electrochemical Reduction of CO2: Current Status and Prospective Implementation. Green Chem. 2017, 19 (10), 2326–2346. 10.1039/C7GC00398F. [DOI] [Google Scholar]
- Syngas Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023–2028; Report 2022, https://www.imarcgroup.com/syngas-market (accessed 2023-08-18).
- Syngas Market by Gasifier (Moving Bed Gasifier, Fluidized Bed Gasifier, Entrained Flow Gasifier, and Others), Technology (Steam Reforming, Partial Oxidation, Auto-Thermal Reforming, Combined or Two-Step Reforming, and Biomass Gasification), Feedstock (Natural Gas, Coal, Biomass/Waste, and Others), Application (Power Generation, Chemicals, and Others), and Regional Analysis (North America, Europe, Asia-Pacific, and LAMEA): Global Opportunity Analysis and Industry Forecast, 2020–2027; Report 2021, https://www.researchdive.com/8407/syngas-market (accessed 2023-08-18).
- Syngas Market Size, Share, Trends, By Production Technology (Steam Reforming, Partial Oxidation, Auto-Thermal Reforming, Two-Step Reforming, and Biomass Gasification), Gasifier Type, Feedstock, Application, and By Region Forecast to 2030; Report 2022, https://www.emergenresearch.com/industry-report/syngas-market (accessed 2023-08-18).
- Farkas-Csamangó E.; Gyenes P.; Janáky C.; Kádár K.; Lukovics M.; Sziebig O. J.; Törőcsikné Görög M.. Carbon Capture and Storage/Carbon Capture and Utilization 2023. https://greennovation.hu/feherkonyv.pdf (accessed 2023-09-18). [Google Scholar]
- United Nations. Population. https://www.un.org/en/global-issues/population (accessed 2023-08-09).
- Chris Barrington . The Iron Ore Challenge for Direct Reduction On Road to Carbon-Neutral Steelmaking. https://www.midrex.com/tech-article/the-iron-ore-challenge-for-direct-reduction-on-road-to-carbon-neutral-steelmaking/ (accessed 2023-12-08).
- Wood Mackenzie . Power facilities to potentially use 100 Mt of low-carbon ammonia as feedstock by 2050. https://www.woodmac.com/press-releases/power-facilities-to-potentially-use-100-mt-of-low-carbon-ammonia-as-feedstock-by-2050/ (accessed 2023-12-18).
- Sick V.; Stokes G.; Mason F.; Yu Y.-S.; Van Berkel A.; Daliah R.; Gamez O.; Gee C.; Kaushik M.. Implementing CO2 Capture and Utilization at Scale and Speed; Report 2022, https://deepblue.lib.umich.edu/handle/2027.42/174094 (accessed 2023-12-08).
- Rozzi E.; Minuto F. D.; Lanzini A.; Leone P. Green Synthetic Fuels: Renewable Routes for the Conversion of Non-Fossil Feedstocks into Gaseous Fuels and Their End Uses. Energies 2020, 13 (2), 420. 10.3390/en13020420. [DOI] [Google Scholar]
- Bachmann M.; Völker S.; Kleinekorte J.; Bardow A. Syngas from What? Comparative Life-Cycle Assessment for Syngas Production from Biomass, CO2, and Steel Mill Off-Gases. ACS Sustain Chem. Eng. 2023, 11 (14), 5356–5366. 10.1021/acssuschemeng.2c05390. [DOI] [Google Scholar]
- Foit S. R.; Vinke I. C.; de Haart L. G. J.; Eichel R.-A. Power-to-Syngas: An Enabling Technology for the Transition of the Energy System?. Angew. Chem. 2017, 56 (20), 5402–5411. 10.1002/anie.201607552. [DOI] [PubMed] [Google Scholar]
- Air Liquide Normand’Hy . Air Liquide receives support from French State to its 200 MW electrolyzer project in Normandy and accelerates the development of the hydrogen sector in Europe. https://normandhy.airliquide.com/en (accessed 2023-08-09).
- Stephens I. E. L.; Chan K.; Bagger A.; Boettcher S. W.; Bonin J.; Boutin E.; Buckley A. K.; Buonsanti R.; Cave E. R.; Chang X.; Chee S. W.; da Silva A. H. M.; de Luna P.; Einsle O.; Endrődi B.; Escudero-Escribano M.; Ferreira de Araujo J. V.; Figueiredo M. C.; Hahn C.; Hansen K. U.; Haussener S.; Hunegnaw S.; Huo Z.; Hwang Y. J.; Janáky C.; Jayathilake B. S.; Jiao F.; Jovanov Z. P.; Karimi P.; Koper M. T. M.; Kuhl K. P.; Lee W. H.; Liang Z.; Liu X.; Ma S.; Ma M.; Oh H.-S.; Robert M.; Cuenya B. R.; Rossmeisl J.; Roy C.; Ryan M. P.; Sargent E. H.; Sebastián-Pascual P.; Seger B.; Steier L.; Strasser P.; Varela A. S.; Vos R. E.; Wang X.; Xu B.; Yadegari H.; Zhou Y. 2022 Roadmap on Low Temperature Electrochemical CO2 Reduction. Journal of Physics: Energy 2022, 4 (4), 042003. 10.1088/2515-7655/ac7823. [DOI] [Google Scholar]
- Shin H.; Hansen K. U.; Jiao F. Techno-Economic Assessment of Low-Temperature Carbon Dioxide Electrolysis. Nat. Sustain 2021, 4 (10), 911–919. 10.1038/s41893-021-00739-x. [DOI] [Google Scholar]
- De Luna P.; Hahn C.; Higgins D.; Jaffer S. A.; Jaramillo T. F.; Sargent E. H. What Would It Take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes?. Science 2019, 364 (6438), eaav3506 10.1126/science.aav3506. [DOI] [PubMed] [Google Scholar]
- Samu A. A.; Kormányos A.; Kecsenovity E.; Szilágyi N.; Endrődi B.; Janáky C. Intermittent Operation of CO2 Electrolyzers at Industrially Relevant Current Densities. ACS Energy Lett. 2022, 7 (5), 1859–1861. 10.1021/acsenergylett.2c00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouny M.; Luc W.; Jiao F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57 (6), 2165–2177. 10.1021/acs.iecr.7b03514. [DOI] [Google Scholar]
- Leverett J.; Khan M. H. A.; Tran-Phu T.; Tricoli A.; Hocking R. K.; Yun S. L. J.; Dai L.; Daiyan R.; Amal R. Renewable Power for Electrocatalytic Generation of Syngas: Tuning the Syngas Ratio by Manipulating the Active Sites and System Design. ChemCatChem. 2022, 14 (24), e202200981 10.1002/cctc.202200981. [DOI] [Google Scholar]
- Kibria Nabil S.; McCoy S.; Kibria M. G. Comparative Life Cycle Assessment of Electrochemical Upgrading of CO2 to Fuels and Feedstocks. Green Chem. 2021, 23 (2), 867–880. 10.1039/D0GC02831B. [DOI] [Google Scholar]
- Somoza-Tornos A.; Guerra O. J.; Crow A. M.; Smith W. A.; Hodge B.-M. Process Modeling, Techno-Economic Assessment, and Life Cycle Assessment of the Electrochemical Reduction of CO2: A Review. iScience 2021, 24 (7), 102813. 10.1016/j.isci.2021.102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.; Shi Y.; Meng N.; Lu S.; Yu Y.; Zhang B. Electrosynthesis of Syngas via the Co-Reduction of CO2 and H2O. Cell Rep. Phys. Sci. 2020, 1 (11), 100237. 10.1016/j.xcrp.2020.100237. [DOI] [Google Scholar]
- Debergh P.; Gutiérrez-Sánchez O.; Khan M. N.; Birdja Y. Y.; Pant D.; Bulut M. The Economics of Electrochemical Syngas Production via Direct Air Capture. ACS Energy Lett. 2023, 8 (8), 3398–3403. 10.1021/acsenergylett.3c00885. [DOI] [Google Scholar]
- Küngas R. Review - Electrochemical CO2 Reduction for CO Production: Comparison of Low- and High-Temperature Electrolysis Technologies. J. Electrochem. Soc. 2020, 167 (4), 044508. 10.1149/1945-7111/ab7099. [DOI] [Google Scholar]
- van Berkel F.; van ’t Noordende H.; Stodolny M.. Next Level Solid Oxide Electrolysis; Report 2023, https://ispt.eu/publications/next-level-solid-oxide-electrolysis/ (accessed 2023-12-08).
- Endrődi B.; Kecsenovity E.; Samu A.; Darvas F.; Jones R. V.; Török V.; Danyi A.; Janáky C. Multilayer Electrolyzer Stack Converts Carbon Dioxide to Gas Products at High Pressure with High Efficiency. ACS Energy Lett. 2019, 4 (7), 1770–1777. 10.1021/acsenergylett.9b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrődi B.; Samu A.; Kecsenovity E.; Halmágyi T.; Sebők D.; Janáky C. Operando Cathode Activation with Alkali Metal Cations for High Current Density Operation of Water-Fed Zero-Gap Carbon Dioxide Electrolysers. Nat. Energy 2021, 6 (4), 439–448. 10.1038/s41560-021-00813-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore D. A.; Gabardo C. M.; Reyes A.; O’Brien C. P.; Holdcroft S.; Pintauro P.; Bahar B.; Hickner M.; Bae C.; Sinton D.; Sargent E. H.; Berlinguette C. P. Designing Anion Exchange Membranes for CO2 Electrolysers. Nat. Energy 2021, 6 (4), 339–348. 10.1038/s41560-020-00761-x. [DOI] [Google Scholar]
- Endrődi B.; Kecsenovity E.; Samu A.; Halmágyi T.; Rojas-Carbonell S.; Wang L.; Yan Y.; Janáky C. High Carbonate Ion Conductance of a Robust PiperION Membrane Allows Industrial Current Density and Conversion in a Zero-Gap Carbon Dioxide Electrolyzer Cell. Energy Environ. Sci. 2020, 13 (11), 4098–4105. 10.1039/D0EE02589E. [DOI] [Google Scholar]
- El-Nagar G. A.; Haun F.; Gupta S.; Stojkovikj S.; Mayer M. T. Unintended Cation Crossover Influences CO2 Reduction Selectivity in Cu-Based Zero-Gap Electrolysers. Nat. Commun. 2023, 14, 2062. 10.1038/s41467-023-37520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M.; Clark E. L.; Therkildsen K. T.; Dalsgaard S.; Chorkendorff I.; Seger B. Insights into the Carbon Balance for CO2 Electroreduction on Cu Using Gas Diffusion Electrode Reactor Designs. Energy Environ. Sci. 2020, 13 (3), 977–985. 10.1039/D0EE00047G. [DOI] [Google Scholar]
- Samu A. A.; Szenti I.; Kukovecz A.; Endrődi B.; Janáky C. Systematic Screening of Gas Diffusion Layers for High Performance CO2 Electrolysis. Commun. Chem. 2023, 6 (1), 41. 10.1038/s42004-023-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. B.; Li Y.; De Luna P.; Kim D.; Sargent E. H.; Yang P. Electrocatalytic Rate Alignment Enhances Syngas Generation. Joule 2019, 3 (1), 257–264. 10.1016/j.joule.2018.09.013. [DOI] [Google Scholar]
- Qin B.; Li Y.; Fu H.; Wang H.; Chen S.; Liu Z.; Peng F. Electrochemical Reduction of CO2 into Tunable Syngas Production by Regulating the Crystal Facets of Earth-Abundant Zn Catalyst. ACS Appl. Mater. Interfaces 2018, 10 (24), 20530–20539. 10.1021/acsami.8b04809. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Tian D.; Biswas A. N.; Xie Z.; Hwang S.; Lee J. H.; Meng H.; Chen J. G. Transition Metal Nitrides as Promising Catalyst Supports for Tuning CO/H2 Syngas Production from Electrochemical CO2 Reduction. Angewandte Chemie - International Edition 2020, 59 (28), 11345–11348. 10.1002/anie.202003625. [DOI] [PubMed] [Google Scholar]
- Tackett B. M.; Lee J. H.; Chen J. G. Electrochemical Conversion of CO2 to Syngas with Palladium-Based Electrocatalysts. Acc. Chem. Res. 2020, 53 (8), 1535–1544. 10.1021/acs.accounts.0c00277. [DOI] [PubMed] [Google Scholar]
- Ju W.; Bagger A.; Hao G. P.; Varela A. S.; Sinev I.; Bon V.; Roldan Cuenya B.; Kaskel S.; Rossmeisl J.; Strasser P. Understanding Activity and Selectivity of Metal-Nitrogen-Doped Carbon Catalysts for Electrochemical Reduction of CO2. Nat. Commun. 2017, 8 (1), 944. 10.1038/s41467-017-01035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyny T.; Smith W. A. CO2 Reduction on Gas-Diffusion Electrodes and Why Catalytic Performance Must Be Assessed at Commercially-Relevant Conditions. Energy Environ. Sci. 2019, 12 (5), 1442–1453. 10.1039/C8EE03134G. [DOI] [Google Scholar]
- Yin Z.; Peng H.; Wei X.; Zhou H.; Gong J.; Huai M.; Xiao L.; Wang G.; Lu J.; Zhuang L. An Alkaline Polymer Electrolyte CO2 Electrolyzer Operated with Pure Water. Energy Environ. Sci. 2019, 12 (8), 2455–2462. 10.1039/C9EE01204D. [DOI] [Google Scholar]
- Lee W. H.; Ko Y. J.; Choi Y.; Lee S. Y.; Choi C. H.; Hwang Y. J.; Min B. K.; Strasser P.; Oh H. S. Highly Selective and Scalable CO2 to CO - Electrolysis Using Coral-Nanostructured Ag Catalysts in Zero-Gap Configuration. Nano Energy 2020, 76, 105030. 10.1016/j.nanoen.2020.105030. [DOI] [Google Scholar]
- Liu Z.; Yang H.; Kutz R.; Masel R. I. CO2 Electrolysis to CO and O2 at High Selectivity, Stability and Efficiency Using Sustainion Membranes. J. Electrochem. Soc. 2018, 165 (15), J3371–J3377. 10.1149/2.0501815jes. [DOI] [Google Scholar]
- Bagger A.; Christensen O.; Ivaništšev V.; Rossmeisl J. Catalytic CO2/CO Reduction: Gas, Aqueous, and Aprotic Phases. ACS Catal. 2022, 12 (4), 2561–2568. 10.1021/acscatal.1c05358. [DOI] [Google Scholar]
- Moss A. B.; Garg S.; Mirolo M.; Giron Rodriguez C. A.; Ilvonen R.; Chorkendorff I.; Drnec J.; Seger B. In Operando Investigations of Oscillatory Water and Carbonate Effects in MEA-Based CO2 Electrolysis Devices. Joule 2023, 7 (2), 350–365. 10.1016/j.joule.2023.01.013. [DOI] [Google Scholar]
- Reyes A.; Jansonius R. P.; Mowbray B. A. W.; Cao Y.; Wheeler D. G.; Chau J.; Dvorak D. J.; Berlinguette C. P. Managing Hydration at the Cathode Enables Efficient CO2 Electrolysis at Commercially Relevant Current Densities. ACS Energy Lett. 2020, 5 (5), 1612–1618. 10.1021/acsenergylett.0c00637. [DOI] [Google Scholar]
- Air Liquide Normand’Hy . Air Liquide starts up a large hydrogen production unit in Germany. https://www.airliquide.com/group/press-releases-news/2015-04-17/air-liquide-starts-large-hydrogen-production-unit-germany (accessed 2023-08-09).
- Taibi E.; Blanco H.; Miranda R.; Carmo M.. Green Hydrogen Cost Reduction Scaling Up Electrolysers to Meet The 1.5°C Climate Goal; International Renewable Energy Agency; https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Dec/IRENA_Green_hydrogen_cost_2020.pdf (accessed 2023-10-09).
- Holst M.; Aschbrenner S.; Smolinka T.; Voglstätter C.; Grimm G.. Cost Forecast for Low-Temperature Electrolysis-Technology Driven Bottom-Up Prognosis for PEM and Alkaline Water Electrolysis Systems; Report 2021, https://publica.fraunhofer.de/entities/publication/5a0b888c-453c-449b-9dbb-d18f61c3abee/details (accessed 2023-12-08).
- Van’t Noordende H.; Ripson P.. Baseline Design and Total Installed Costs of a GW Green Hydrogen Plant. State-of-the-Art Design and Total Installed Capital Costs; Report 2020, https://ispt.eu/publications/public-report-gigawatt-green-hydrogen-plant/ (accessed 2023-08-18).
- Randolph K.; Vickers J.; Peterson D.; Hubert M.; Miller E.. Historical Cost Reduction of PEM Electrolyzers; DOE Hydrogen Program Record; 2022. https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/22002-historical-cost-reduction-pem-electrolyzers.pdf (accessed 2023-08-18).
- Fragiacomo P.; Genovese M. Numerical Simulations of the Energy Performance of a PEM Water Electrolysis Based High-Pressure Hydrogen Refueling Station. Int. J. Hydrogen Energy 2020, 45 (51), 27457–27470. 10.1016/j.ijhydene.2020.07.007. [DOI] [Google Scholar]
- Papakonstantinou G.; Algara-Siller G.; Teschner D.; Vidaković-Koch T.; Schlögl R.; Sundmacher K. Degradation Study of a Proton Exchange Membrane Water Electrolyzer under Dynamic Operation Conditions. Appl. Energy 2020, 280, 115911. 10.1016/j.apenergy.2020.115911. [DOI] [Google Scholar]
- Samani A. E.; D’Amicis A.; de Kooning J. D. M.; Bozalakov D.; Silva P.; Vandevelde L. Grid Balancing with a Large-Scale Electrolyser Providing Primary Reserve. IET Renewable Power Generation 2020, 14 (16), 3070–3078. 10.1049/iet-rpg.2020.0453. [DOI] [Google Scholar]
- Hysa G.; Ruuskanen V.; Kosonen A.; Niemelä M.; Aarniovuori L.; Guilbert D.; Ahola J. Effect of Voltage Elevation on Cost and Energy Efficiency of Power Electronics in Water Electrolyzers. J. Power Sources 2023, 574, 233108. 10.1016/j.jpowsour.2023.233108. [DOI] [Google Scholar]
- Chatenet M.; Pollet B. G.; Dekel D. R.; Dionigi F.; Deseure J.; Millet P.; Braatz R. D.; Bazant M. Z.; Eikerling M.; Staffell I.; Balcombe P.; Shao-Horn Y.; Schäfer H. Water Electrolysis: From Textbook Knowledge to the Latest Scientific Strategies and Industrial Developments. Chem. Soc. Rev. 2022, 51 (11), 4583–4762. 10.1039/D0CS01079K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M.; Clark E. L.; Therkildsen K. T.; Dalsgaard S.; Chorkendorff I.; Seger B. Insights into the Carbon Balance for CO2 Electroreduction on Cu Using Gas Diffusion Electrode Reactor Designs. Energy Environ. Sci. 2020, 13 (3), 977–985. 10.1039/D0EE00047G. [DOI] [Google Scholar]
- Yousef A. M.; Eldrainy Y. A.; El-Maghlany W. M.; Attia A. Biogas Upgrading Process via Low-Temperature CO2 Liquefaction and Separation. J. Nat. Gas Sci. Eng. 2017, 45, 812–824. 10.1016/j.jngse.2017.07.001. [DOI] [Google Scholar]
- Moore T.; Oyarzun D. I.; Li W.; Lin T. Y.; Goldman M.; Wong A. A.; Jaffer S. A.; Sarkar A.; Baker S. E.; Duoss E. B.; Hahn C. Electrolyzer Energy Dominates Separation Costs in State-of-the-Art CO2 Electrolyzers: Implications for Single-Pass CO2 Utilization. Joule 2023, 7 (4), 782–796. 10.1016/j.joule.2023.03.015. [DOI] [Google Scholar]
- Fredric B.; Christian H.; Tobias P.; Daniel T.. Biogas Upgrading - Review of Commercial Technologies; Report 2012, https://portal.research.lu.se/en/publications/biogas-upgrading-review-of-commercial-technologies (accessed 2023-12-08).
- Fraunhofer Institute for Wind Energy and Energy System Technology (IWES) . The European Power System in 2030: Flexibility Challenges and Integration Benefits; Report 2015, https://www.agora-energiewende.org/publications/the-european-power-system-in-2030-flexibility-challenges-and-integration-benefits-1 (accessed 2023-12-08).
- Fraunhofer Institute for Solar Energy Systems ISE . Levelized Cost of Electricity- Renewable Energy Technologies; Report 2021, https://www.ise.fraunhofer.de/en/publications/studies/cost-of-electricity.html (accessed 2023-12-08).
- International Renewable Energy Agency (IRENA) . Future of Wind - A Global Energy Transformation Paper; Report 2019, https://www.irena.org/publications/2019/Oct/Future-of-wind (accessed 2023-12-08).
- International Renewable Energy Agency (IRENA) . Electricity Storage and Renewables: Costs and Markets to 2023; Report 2019, https://www.irena.org/publications/2017/Oct/Electricity-storage-and-renewables-costs-and-markets (accessed 2023-12-08).
- Haegel N.; Margolis R.; Buonassisi T.; Feldman D.; Froitzheim A.; Garabedian R.; Green M.; Glunz S.; Henning H.-M.; Holder B.; Kaizuka I.; Kroposki B.; Matsubara K.; Niki S.; Sakurai K.; Schindler R. A.; Tumas W.; Weber E. R.; Wilson G.; Woodhouse M.; Kurtz S. Terawatt-Scale Photovoltaics: Trajectories and Challenges. Science (1979) 2017, 356 (6334), 141–143. 10.1126/science.aal1288. [DOI] [PubMed] [Google Scholar]
- Fasihi M.; Efimova O.; Breyer C. Techno-Economic Assessment of CO2 Direct Air Capture Plants. J. Clean Prod 2019, 224, 957–980. 10.1016/j.jclepro.2019.03.086. [DOI] [Google Scholar]
- Rodin V.; Lindorfer J.; Böhm H.; Vieira L. Assessing the Potential of Carbon Dioxide Valorisation in Europe with Focus on Biogenic CO2. Journal of CO2 Utilization 2020, 41, 101219. 10.1016/j.jcou.2020.101219. [DOI] [Google Scholar]
- Nakyai T.; Saebea D. Exergoeconomic Comparison of Syngas Production from Biomass, Coal, and Natural Gas for Dimethyl Ether Synthesis in Single-Step and Two-Step Processes. J. Clean Prod 2019, 241, 118334. 10.1016/j.jclepro.2019.118334. [DOI] [Google Scholar]
- Bushuyev O. S.; De Luna P.; Dinh C. T.; Tao L.; Saur G.; van de Lagemaat J.; Kelley S. O.; Sargent E. H. What Should We Make with CO2 and How Can We Make It?. Joule 2018, 2 (5), 825–832. 10.1016/j.joule.2017.09.003. [DOI] [Google Scholar]
- Bernadet L.; Laurencin J.; Roux G.; Montinaro D.; Mauvy F.; Reytier M. Effects of Pressure on High Temperature Steam and Carbon Dioxide Co-Electrolysis. Electrochim. Acta 2017, 253, 114–127. 10.1016/j.electacta.2017.09.037. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.