Abstract
Persons with fragile X syndrome (FXS) with cooccurring autism spectrum disorder (ASD) are at risk for poorer educational, medical, employment, and independent living outcomes. Thus, the identification of ASD in those with FXS is fundamental to ensuring access to appropriate supports to achieve good quality of life. Yet, optimal diagnostic methods and the exact rate of ASD comorbidity remains controversial, and description of ASD identification in the community in FXS has been limited. This study characterized ASD in a sample of 49 male youth with FXS across multiple diagnostic sources: parent-reported community diagnoses, classification derived from ADOS-2 and ADI-R thresholds, and clinical best-estimate classifications from an expert multidisciplinary team. High concordance was found between ADOS-2/ADI-R and clinical best estimate classifications, with both methods supporting ASD in ~75% of male youth with FXS. In contrast, 31% had a community diagnosis. Findings supported gross under-identification of ASD in male youth with FXS in community settings; 60% of those who met clinical best estimate criteria for ASD had not received a diagnosis in the community. Moreover, community diagnoses were poorly aligned with the presence of ASD symptoms as perceived by parents and professionals and, unlike clinical best estimate diagnoses, were not associated with cognitive, behavioral, or language features. Findings highlight under-identification of ASD in community settings as a significant barrier to service access for male youth with FXS. Clinical recommendations should emphasize the benefits of seeking a professional ASD evaluation for children with FXS who are noted to display key ASD symptoms.
Keywords: intellectual disability, neurodevelopmental disorder, community identification, diagnostic overshadowing, FMR1
Fragile X syndrome (FXS) is caused by an expanded CGG sequence on the Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene that results in a reduction or absence of an essential protein for brain development and function, FMRP (Crawford et al., 2001). Reduced FMRP is the cause of intellectual disability in FXS, which occurs in the majority of males with the syndrome (females with FXS, who benefit from the neuroprotective effects of the second X chromosome, are less likely to have IQs within the range of intellectual disability; Abbeduto et al., 2021). Reduced FMRP is also thought to underlie the high likelihood that persons with FXS will develop ASD, with reported ASD rates ranging from 25-75% (Bailey et al., 1998; Clifford et al., 2007; Garcia-Nonell et al., 2008; Harris et al., 2008; Hatton et al., 2006; Klusek et al., 2014b). In fact, recent research has highlighted the major role of FMRP in controlling the translational regulation of the products of a multitude of other genes, which include one-third to one-half of candidate ASD susceptibility genes (Darnell et al., 2011; Iossifov et al., 2012; Li et al., 2020; Waltes et al., 2014). However, despite widespread agreement that FXS is associated with an increased likelihood of cooccurring ASD, the diagnosis of ASD in persons with FXS has represented a source of great controversy.
Differential diagnosis of ASD within the context of FXS is clinically complex because of the inherent difficulty in teasing apart overlapping symptoms of ASD, intellectual disability, anxiety, and attention problems that characterize the FXS phenotype (Abbeduto et al., 2014). There has also been significant controversy surrounding the validity of the ASD diagnosis within the context of FXS (Abbeduto et al., 2014; Hall et al., 2010). For example, it has been argued that ASD traits in FXS can be accounted for, in part, by intellectual disability or social anxiety aspects of the FXS phenotype (Budimirovic et al., 2006; Hall et al., 2010), and therefore reflect the continuum of FXS-associated behavior rather than “true” ASD. Yet, others have contended that cooccurring ASD is an entity that is distinct from the FXS phenotype and arises from mechanistic pathways that are shared with non-syndromic forms of ASD (Belmonte & Bourgeron, 2006; Rogers et al., 2001).
Also complicating ASD identification in FXS are issues related to the measurement of ASD characteristics that have resulted in wide variation in the estimated rate that ASD occurs within males with FXS. Early studies relying on the Childhood Autism Rating Scale (CARS; Schopler et al., 1988) reported rates of ASD in males with FXS of around 25% (Bailey et al., 1998; Hatton et al., 2006). Later studies using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) or the Autism Diagnostic Interview- Revised (ADI-R; Lord et al., 1994) have suggested rates closer to 50-75% (Clifford et al., 2007; Garcia-Nonell et al., 2008; Harris et al., 2008; Klusek et al., 2014b). However, reliance on cut-off scores of standardized autism diagnostic assessments has limitations, as these tools show reduced accuracy when applied to persons with intellectual disability or emotional/behavioral problems (Havdahl et al., 2016; Risi et al., 2006).
Some studies have attempted to circumvent these diagnostic challenges by using the criteria outlined by the Diagnostic and Statistical Manual of Mental Disorders (DSM) to classify ASD. These methods suggest rates of ASD comorbidity in boys with FXS of 28-51%. A limitation of these latter studies is their reliance on retrospective endorsement of DSM criteria from medical records or parent-report questionnaire data (Fielding-Gebhardt et al., 2021; Kaufmann et al., 2017; Wheeler et al., 2015), which may also have led to failure to capture clinically important nuances in symptom presentation. Importantly, none of the above approaches to diagnosing ASD in persons with FXS incorporate expert clinical judgment or apply rule-out criteria as specified in the DSM-5 to confirm that symptoms are not better accounted for by intellectual disability (American Psychiatric Association, 2013).
In the scientific investigation of nonsyndromic ASD, clinical best-estimate (CBE) procedures have long been accepted as the diagnostic gold-standard (Charman & Baird, 2002; Lord, Petkova, et al., 2012). CBE diagnostic determination of ASD involves the integration of the results from standardized ASD diagnostic instruments along with expert clinical opinion and often with expertise from multiple disciplines represented. The judgment of experienced clinicians has been consistently shown to increase the accuracy of ASD diagnoses (Klin et al., 2000; Le Couteur et al., 2008; Lord et al., 2006), and expert clinical opinion is particularly essential when clinical presentations are complex, such as when intellectual disability must be considered as a competing diagnosis (Thurm et al., 2019). Yet, the CBE diagnostic approach has rarely been adopted in FXS research. In the only report of this type, CBE diagnostic procedures yielded a rate of ASD cooccurrence of 73% in a sample of 37 preschool boys with FXS (Roberts et al., 2020).
Considering the challenges associated with identifying ASD in persons with FXS within the context of a specialized research setting, it is not surprising that ASD identification within community settings also presents with complications. Indeed, research suggests that ASD in FXS is significantly under-identified in community settings. Klusek et al. (2014b) applied ADOS and ADI-R thresholds to determine the ASD status of 51 boys with FXS aged 6-17 years. Of the boys who exceeded diagnostic thresholds for ASD as part of the research protocol, 67% had not been previously identified as having ASD in the community, per their caregiver’s report. In other words, over two-thirds of ASD cases in the boys with FXS in that sample have been missed in clinical/educational settings. Klusek et al. also found that the likelihood of community ASD identification was not linked to the child’s cognitive or language skills or socioeconomic factors such as household income or maternal education level. However, boys with greater executive deficits were more likely to be identified with ASD in the community, suggesting that executive difficulties may prompt health professionals to suspect ASD. The Klusek et al. report did not explore additional factors that could explain low rates of community diagnoses, such as whether the boys with FXS had ever been evaluated for ASD, or caregiver beliefs about the nature of ASD symptoms in FXS that might influence the likelihood of seeking out a diagnosis.
This striking mismatch in the translation of ASD diagnoses into community settings, documented by the Klusek et al. (2014b) study, is notable because a specific ASD diagnosis is often required to access high-intensity and specialized intervention services in community and educational settings (Barton et al., 2016; Callaghan & Sylvester, 2019; Dimian et al., 2021; Shea et al., 2021). ASD cooccurrence in FXS is associated with poorer cognitive, language, academic, and daily living skills. Therefore, those with FXS and cooccurring ASD are highly likely to need more intensive treatment than their non-ASD counterparts to achieve successful community employment, independent living, and good quality of life (Klusek et al., 2014a; Lewis et al., 2006; Raspa et al., 2018; Roberts et al., 2007; Roberts et al., 2005). Additionally, persons with FXS and cooccurring ASD are at heightened risk for specific medical problems that may require additional monitoring or specialized care, such as seizures, sleep disorders, and attention problems (Garcia-Nonell et al., 2008; Kaufmann et al., 2017; Talisa et al., 2014). Consequently, understanding patterns and challenges to community identification of ASD in persons with FXS is critical to ensuring access to necessary educational and medical services.
In the present study, we sought to address variability in the rate of ASD cooccurrence in males with FXS across three diagnostic sources: CBE, ADOS-2 and ADI-R thresholds, and parent-reported community diagnoses. In an attempt to achieve more reliable estimates of ASD cooccurrence, we focused on an older sample of adolescent and young adult males, given evidence to support fluctuating symptoms during childhood and relative stability of ASD symptoms during adulthood in FXS (Hartley et al., 2015; Hatton et al., 2006; Lee et al., 2016). We also sought to identify the factors related to community identification of ASD, including associations with the cognitive, language, anxiety, and attention/hyperactivity deficits, that are common in persons with FXS and might result in behavioral manifestations that increase the likelihood for referral for community evaluation. Because parents are the primary advocates (and gatekeepers) for receiving diagnostic and therapeutic services for their children, we also surveyed parental views on ASD comorbidity in FXS as a previously unexplored but potentially central factor that may influence the receipt of an ASD diagnosis in the community. ASD interferes with social, academic, and occupational outcomes for individuals with FXS, and its identification has major implications for access to treatment and community services. Our research aims were as follows:
Determine the rate and concordance of ASD in adolescent and young adult males with FXS, as indicated by three diagnostic methods: (1) CBE, (2) combined thresholds of the ADOS-2 and ADI-R, and (3) ASD diagnoses obtained in the community. We hypothesized that CBE and ADOS-2/ADI-R diagnostic methods would yield higher rates of ASD than community diagnostic rates. We anticipated high concordance between CBE and ADOS-2/ADI-R classification, and low concordance between these methods and community diagnoses.
Identify cognitive, language, and behavioral characteristics that predict ASD classification across diagnostic sources. We hypothesized that lower IQ, lower vocabulary, and increased inattention/hyperactivity and anxiety symptoms would be associated with higher likelihood of ASD per CBE and ADOS-2/ADI-R diagnostic methods. Consistent with Klusek et al. (2014b), who found that executive and behavioral/emotional difficulties were associated with increased likelihood of community ASD diagnosis, we hypothesized that the related constructs of inattention/hyperactivity and anxiety, but not IQ or language skills, would be associated with community diagnoses.
Describe parental views and other factors related to the diagnosis of comorbid ASD in males with FXS in community settings. We anticipated poor alignment between community diagnosis and the presence of ASD symptoms per parental report, the identification of ASD characteristics by health/educational professionals, and parental belief that their son meets criteria for ASD.
Methods
Participants
Participants were 49 males with FXS aged 15-24 years (M =18.85) who were recruited from a larger multi-site study of language development in adolescents and young adults with FXS (Abbeduto et al., 2019). We focused on males because they show higher rates of ASD than females with FXS (Klusek et al., 2014b). Inclusion criteria for the larger project required that the participant with FXS: (a) had the FMR1 full mutation as confirmed via review of genetic testing report; (b) was aged 15-22 years at study entry; (c) resided with biological mother at study entry; (d) used speech as the primary means of communication, with regular use of phrase speech per parent report; (e) displayed no uncorrected sensory of physical impairments that would prevent participation in assessments according to the parent; and (f) was a native speaker of English. The presence of the FMR1 full mutation (>200 CGG repeats on 5’UTR FMR1) was also confirmed via molecular genetic testing conducted through the larger study. All participants enrolled in the larger study who had available ASD diagnostic data were included in the present paper. Recruitment for the study was conducted nationally, with enrollment and testing occurring at one of two university sites (University of South Carolina, MIND Institute at University California-Davis). Recruitment sources included social media, word of mouth, advertisements through the National Fragile X Foundation, and the Research Registries of the Carolina Institute for Developmental Disabilities at the University of North Carolina at Chapel Hill and the UC Davis Mind Institute IDDRC Clinical Translational Core. Demographic variables are presented in Table 1.
Table 1.
Demographic Characteristics
| Characteristic | % |
|---|---|
| Maternal Education | |
| High school graduate | 12% |
| Some college | 22% |
| Associates degree | 12% |
| Bachelor’s degree | 21% |
| Some graduate work | 12% |
| Graduate degree | 21% |
| Race | |
| White | 89% |
| Black | 7% |
| Asian | 2% |
| Not reported | 2% |
| Income | |
| $<40,000 | 23% |
| $40 – 79,000 | 23% |
| $>80,000 | 54% |
Measures
ASD Diagnostic Sources
ADOS-2 and ADI-R Thresholds.
The Autism Diagnostic Observation Schedule-Second Edition (ADOS-2; Lord et al., 2012) and Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994) were administered by graduate or doctoral-level professionals who had achieved research reliability standards. The ADOS-2 is a semi-structured observational measure in which a trained examiner observes social communication skills, play, and restricted and repetitive behaviors exhibited during a semi-structured interaction with the participant. A diagnostic algorithm provides standardized cut-off scores to if determine diagnostic thresholds for ASD are exceeded. The ADI-R is a diagnostic caregiver interview that evaluates the presence of language, communication, social, and restricted/repetitive/stereotyped behaviors consistent with ASD. The diagnostic algorithm is based on symptoms exhibited during early childhood and participants must meet thresholds across social interaction, communication, restricted/repetitive/stereotyped behavior symptoms domains, as well as exhibit symptom onset before three years of age. Inter-rater reliability was evaluated on 10% of randomly selected administrations. All examiners across both sites assessed the videotaped administration and consensus codes were achieved through group discussion. The mean percent agreement of each individual examiner relative to the consensus averaged at 80% across ADOS-2 items and 91% across ADI-R items. ADOS-2 module was determined as specified in the administration manual, with Module 1 (n=2), Module 2 (n=27), and Module 3 (n=20) represented in the sample.
The Risi et al. (2006) caseness criteria was used to combine diagnostic information from the ADOS-2 and ADI-R as this method maximizes sensitivity and specificity when combining threshold scores from these diagnostic assessments in samples with intellectual disability, consistent with prior studies (e.g., Abbeduto et al., 2019; McDuffie et al., 2015; Roberts et al., 2018). Participants were considered to have exceeded thresholds for ASD if they met cut-offs on the ADOS-2 and one of the following conditions on the ADI-R: (1) met cut-offs for the social reciprocity domain and either the communication or the repetitive behavior domains, (2) scored within one point of the cut-offs for the social reciprocity and communication domains, or (3) met the cut-off for either the social reciprocity or communication domains and scored within two points of the cut-off for the repetitive behavior domain.
Clinical Best Estimate (CBE).
CBE diagnoses of ASD were established, following procedures adapted from Lord, Petkova, et al. (2012) and Lord et al. (2006). CBE diagnoses were determined by a multidisciplinary team that included a licensed psychologist, a licensed speech-language pathologist, and at least one other graduate or Ph.D.-level examiner involved in data collection. Procedures were led by the licensed psychologist (K.H.), who is a certified trainer for the ADOS-2. All CBE team members, in addition to being well-versed in FXS from a clinical/research perspective, had extensive training in differential ASD diagnosis and had established research reliability on the ADOS-2 and ADI-R. Diagnostic procedures involved review of videotaped behavioral samples, scores from standardized cognitive, language, psychiatric, behavioral assessments, and ASD assessments collected at the study visit, including the ADOS-2 and ADI-R. CBE diagnostic outcomes considered were “ASD”, “non-ASD developmental delay”, and “no developmental concerns”. ASD was not diagnosed if symptoms were better accounted for by intellectual ability or other psychiatric disorders. Diagnostic certainty estimates, reflecting the CBE team’s consensus on their certainty in the assigned CBE diagnostic classification, expressed on a percentage scale (with 100% being completely certain), were recorded for each case. If certainty dipped below 40% the case was referred to an independent clinical psychologist with specific expertise in ASD diagnostics, including trainer qualification for both the ADOS-2 and ADI-R (Dr. Somer Bishop) for additional review, and CBE diagnosis was determined through group consensus. Only one case required such referral; diagnostic certainty for the other 48 cases was at 40-59% for 10% of the sample, 60-79% for 29%, and 80-100% for 59%.
Community ASD Diagnoses.
We developed the Community ASD Diagnosis Questionnaire to obtain information about ASD diagnoses received in the community. Questionnaires were administered by a trained examiner and completed by the participants’ mothers during the study visit. The presence of a community ASD diagnosis was determined with the following item: “Does your son currently have an ASD diagnosis (including educational diagnoses)?”, with response options of “yes” and “no”. Participants who responded “yes” were provided with an open field to write-in the age of diagnosis. The term “ASD” was defined at the top of the questionnaire with the following statement “***Autism Spectrum Disorder (ASD), includes: autism, autism spectrum, pervasive developmental disorder-not otherwise specified (PDD-NOS), and Asperger’s syndrome”. The administering examiner monitored caregiver comprehension and provided clarification as needed. The questionnaire also included questions about ASD diagnostic evaluations and parental beliefs about their son’s ASD diagnostic status. Qualitative data were also gathered via an open text field following the item “In your opinion, do you believe that your son has ASD? Why or why not?” to allow for parents to provide written commentary. Questionnaire items can be viewed in Table 2.
Table 2.
Parental Responses on the Community ASD Diagnosis Questionnaire
| Question | Response | Full Sample | Sample Stratified by CBE ASD Diagnosis |
Sample Stratified by Community ASD Diagnosis |
||
|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |||
|
|
|
|
||||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| 1. Have health/educational professionals ever told you that your son shows symptoms of ASD?a | Yes | 30 (61%) | 7 (14%) | 23 (47%) | 14 (29%) | 16 (33%) |
| No | 19 (39%) | 6 (12%) | 13 (27%) | 18 (37%) | 1 (2%) | |
|
| ||||||
| 2. In your opinion, do you feel that your son shows symptoms of ASD? | Yes | 34 (69%) | 7 (14%) | 27 (55%) | 18 (37%) | 16 (33%) |
| No | 15 (31%) | 6 (12%) | 9 (18%) | 14 (29%) | 1 (2%) | |
|
| ||||||
| 3. Does your son currently have an ASD diagnosis?b | Yes | 17 (35%) | 2 (4%) | 15 (31%) | 0 (0%) | 17 (35%) |
| No | 32 (65%) | 11 (23%) | 21 (43%) | 32 (65%) | 0 (0%) | |
|
| ||||||
| 4. In your opinion, do you believe that your son has ASD? | Yes | 22 (45%) | 3 (6%) | 19 (39%) | 6 (12%) | 16 (33%) |
| No | 27 (55%) | 10 (20%) | 17 (35%) | 26 (53%) | 1 (2%) | |
|
| ||||||
| 5. Has your son ever been formally evaluated for ASD, either recently or when he was younger? | Yes | 29 (59%) | 8 (16%) | 21 (43%) | 14 (29%) | 15 (31%) |
| No | 20 (41%) | 5 (10%) | 15 (31%) | 18 (37%) | 2 (4%) | |
|
| ||||||
| 6. In what type of setting was your son’s ASD evaluation conducted? | Educational | 9 (20%) | 1 (2%) | 8 (18%) | 3 (7%) | 6 (13%) |
| Clinical or medical | 16 (35%) | 4 (9%) | 12 (27%) | 7 (16%) | 9 (20%) | |
| Research | 5 (11%) | 3 (7%) | 2 (4%) | 4 (9%) | 1 (2%) | |
| FXS specialty clinic | 12 (27%) | 4 (9%) | 8 (18%) | 7 (15%) | 5 (11%) | |
| ASD specialty clinic | 3 (7%) | 2 (4%) | 1 (2%) | 0 (0%) | 3 (7%) | |
|
| ||||||
| 7. Do you feel that the clinician(s) who conducted your son’s ASD evaluation had adequate knowledge about fragile X syndrome to make an accurate diagnosis? | Yes | 19 (65%) | 4 (14%) | 15 (51%) | 9 (31%) | 10 (35%) |
| No | 6 (21%) | 2 (7%) | 4 (14%) | 3 (10%) | 3 (10%) | |
| I don’t know | 4 (14%) | 2 (7%) | 2 (7%) | 2 (7%) | 2 (7%) | |
|
| ||||||
| 8. Do you feel that knowing whether or not your son has ASD is helpful when making medical decisions? | Yes | 24 (57%) | 6 (14%) | 18 (43%) | 11 (26%) | 13 (31%) |
| No | 18 (43%) | 6 (14%) | 12 (29%) | 15 (36%) | 3 (7%) | |
|
| ||||||
| 9. Do you feel that knowing whether or not your son has ASD is helpful when making educational decisions? | Yes | 29 (71%) | 6 (15%) | 23 (56%) | 15 (37%) | 14 (34%) |
| No | 12 (29%) | 5 (12%) | 7 (17%) | 11 (27%) | 1 (2%) | |
|
| ||||||
| 10. Do you feel that you have adequate knowledge about how ASD co-occurs with FXS to make informed medical/educational decisions about your son’s care? | Yes | 38 (90%) | 11 (26%) | 27 (65%) | 23 (55%) | 15 (36%) |
| No | 4 (10%) | 1 (2%) | 3 (7%) | 3 (7%) | 1 (2%) | |
|
| ||||||
| 11. Does your son receive therapies/services that are designed for individuals with ASD (such as ABA therapy or participating in an autism-support classroom)? | Yes | 16 (36%) | 2 (5%) | 14 (32%) | 7 (16%) | 9 (20%) |
| No | 24 (55%) | 8 (18%) | 16 (35%) | 19 (43%) | 5 (11%) | |
| I don’t know | 4 (9%) | 2 (5%) | 2 (5%) | 2 (5%) | 2 (5%) | |
|
| ||||||
| 12. Do you feel that your son would benefit from services that are tailored towards individuals with ASD? | Yes | 23 (47%) | 3 (6%) | 20 (41%) | 9 (18%) | 14 (29%) |
| No | 10 (20%) | 4 (8%) | 6 (12%) | 9 (18%) | 1 (2%) | |
| I don’t know | 16 (33%) | 6 (12%) | 10 (20%) | 14 (29%) | 2 (4%) | |
Note.
ASD was defined in the questionnaire header, prior to the first item, with the following statement: “***Autism Spectrum Disorder (ASD), includes: autism, autism spectrum, pervasive developmental disorder-not otherwise specified (PDD-NOS), and Asperger’s syndrome”.
Parents who responded “yes” were provided with an open field to write in the age of diagnosis in years.
Cognitive, Language, and Behavioral Characteristics
Nonverbal IQ.
The Brief IQ scale of the Leiter International Performance Scale-Revised (Leiter-R; Roid & Miller, 1997) provided an index of nonverbal intelligence. Given that all participants had intellectual disability, Growth Scale Values (GSV) scores were used in the analysis. These equal-interval Rasch-model scores are derived from raw scores to provide an estimate of absolute level of ability rather than ability relative to same-age individuals and thus are less subject to floor effects than standard scores and, unlike raw scores, have the advantage of interval scaling (Roid & Miller, 1997). Additionally, GSV scores are derived from the same normative samples as are the standard scores.
Vocabulary.
The Peabody Picture Vocabulary Test-4 (PPVT-4; Dunn & Dunn, 2007), and the Expressive Vocabulary Test-2 (EVT-2; Williams, 2007) indexed receptive and expressive vocabulary, respectively. GSV scores were used in analysis.
Inattention/hyperactivity.
The Attention Problems subscale of the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001) measured inattention and hyperactivity symptoms. The Attention Problems scale of this caregiver-report form measures consists of 10 items evaluating symptoms of ADHD such as inattention, impulsivity, and difficulty concentrating. The 6-18-year-old form of the CBCL was used for all participants, given that the items of the adult from were deemed inappropriate for the developmental level of the sample. Because some participants were older than the CBCL norming sample, raw scores were used in analysis; raw scores have a possible range of 0-20, with higher scores indicating increased symptoms.
Anxiety.
The General Anxiety subscale of the Anxiety Depression and Mood Scale (ADAMS; Esbensen et al., 2003) was administered. This 28-item caregiver report measure is specifically normed for populations with intellectual disability. The General Anxiety subscale consists of 7 items related to worry and nervousness that are rated on a 4-point Likert scale corresponding to the severity of symptoms. Raw scores are tallied yielding a total score with a possible range of 0-28.
Procedures
Data were gathered using direct assessments and parent-completed interviews and questionnaires that were conducted as part of a larger, two-day research protocol that consisted of standardized and experimental assessments of language, cognition, and behavior. Assessments took place in a research laboratory setting at the respective university site in which the participant was enrolled. Assessments were administered in a standardized order, with the ADOS-2 completed on the morning of the second day. ADI-R interviews were completed with the participants’ mothers, who accompanied them on the research visit. Questionnaires were mailed to families and completed by the participants’ mothers generally in the two weeks prior to the study visit. Data for the present study were drawn from the first annual time point of the larger longitudinal study, with the exception of the Community ASD Diagnosis Questionnaire, which reflected a refinement to the protocol and was added at Time 2. Notably, no participants received a diagnosis of ASD from a community provider in the time elapsed between Time 1 and Time 2. All procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments and were approved by the Institutional Review Boards of the respective university sites. Informed consent was obtained from all participants or their legal guardian as appropriate.
Analytic Strategy
First, descriptive statistics were computed for the study variables; see Table 3. Descriptive statistics were focused on the rates of ASD across the three primary diagnostic indicators (e.g., ADOS-2/ADI-R thresholds, CBE, and community diagnoses). Concordance was examined descriptively with percent agreement, and with Cohen’s kappa (κ; Cohen, 1960), a measure of nominal scale agreement commonly used as a measure of classification accuracy (Kwiecien et al., 2011). Because high concordance was observed across the CBE and ADOS-2/ADI-R diagnoses as described in Results, the remaining analyses focused only on CBE and community diagnoses in order to reduce the number of statistical comparisons. A series of binary logistic regression models examined IQ, receptive and expressive vocabulary, attention/hyperactivity, and anxiety as predictors of CBE and community diagnoses. Separate logistic models were estimated across predictors due to the degree of collinearity between the independent variables. Odds ratios (ORs) were calculated to describe the association between the predictor variable and the likelihood of ASD diagnosis; an OR equal to 1 indicates no association between the predictor and likelihood of an ASD diagnosis while ORs higher than 1 indicate higher odds associated with the predictor and ORs lower than 1 indicate lower odds. Finally, parental views and other factors related to the diagnosis of ASD in community settings were examined. Responses on the Community ASD Diagnosis Questionnaire were examined descriptively within the full sample and within the sample stratified by CBE and community ASD diagnostic outcomes. Open field responses on the questionnaire were analyzed using standard qualitative thematic analyses (Frankel et al., 2014; Patton, 2002).
Table 3.
Descriptive Characteristics
| Characteristic | M (SD), Range |
|---|---|
| Leiter-R, Brief IQ Standard score | 38.65 (4.56), 36–56 |
| Leiter-R, GSV score | 461.91 (11.99), 420–479 |
| EVT-2, GSV score | 152.13 (12.31), 120 – 177 |
| EVT-2, Standard score | 58.74 (13.89), 21 – 84 |
| PPVT-4, GSV score | 148.79 (24.40), 94 – 195 |
| PPVT-4, Standard score | 48.68 (18.22), 20 – 93 |
| CBCL Attention Problems subscale, raw score | 7.17 (3.38), 1–15 |
| ADAMS Anxiety subscale, raw score | 5.59 (3.30), 0–14 |
Note. ADAMS = Anxiety Depression and Mood Scale; CBCL = Child Behavior Checklist; EVT-2 = Expressive Vocabulary Test-2; GSV= growth scale value; Leiter-R = Leiter International Performance Scale-Revised; PPVT-4 = Peabody Picture Vocabulary Test-4.
Results
Rates of ASD across Diagnostic Methods
According to the combined ADOS-2/ADI-R thresholds, 73% (n=36) of the sample met criteria for ASD. Similarly, 76% (n=37) met criteria for ASD per CBE diagnostic procedures. In contrast, only 31% (n=17) of the sample had received a diagnosis of ASD in the community.
Concordance across Diagnostic Sources
Concordance between CBE and ADOS-2/ADI-R Classification.
High concordance was observed across ADOS-2/ADI-R thresholds and the CBE diagnostic outcome, with Cohen’s kappa calculated at κ = 0.84 (95% CI 0.64, 1.00), which is consistent with “almost perfect” levels of agreement (Cohen, 1960). Percent agreement was 94%. Diagnostic outcome differed for only three participants. Two of these participants exceeded the Risi et al. (2006) caseness criteria for combined ADOS-2/ADI-R thresholds but obtained a CBE diagnosis of “non-ASD developmental delay”. CBE diagnostic certainty was at 20-39% for one of these participants (this individual had received an ADOS-2 calibrated severity score of 4 and exceeded ADI-R cut-offs for all domains) and at 60-79% for the other participant, who had received an ADOS-2 calibrated severity score of 7 and exceeded all ADI-R cut-offs. The third participant was negative for ASD per the ADOS-2/ADI-R (ADOS-2 calibrated severity score of 3; missed the ADI-R Communication domain cut-off of 8 with a score of 3 but exceeded cut-offs in all other domains). This participant received a CBE diagnosis of “ASD” with 80-100% certainty.
Concordance between Community, CBE, and ADOS-2/ADI-R Classification
Concordance between community diagnoses and CBE classification was consistent with “none to slight” agreement, κ = 0.16 (95% CI −0.03, 0.35), with percent agreement near chance-level at 53%. Similarly, concordance between the ADOS-2/ADI-R classifications and community diagnoses was at κ = 0.14 (95% CI −0.05, 0.32), with percent agreement at 51%. Discordance was most often due to the lack of a community diagnosis despite CBE diagnostic classification of ASD; of the 36 youth who received a CBE diagnosis of ASD, 21 (58%) had not been identified as having a diagnosis of ASD in the community. In contrast, only two participants (4%) had received a community diagnosis but did not meet CBE criteria (see Figure 1).
Figure 1.
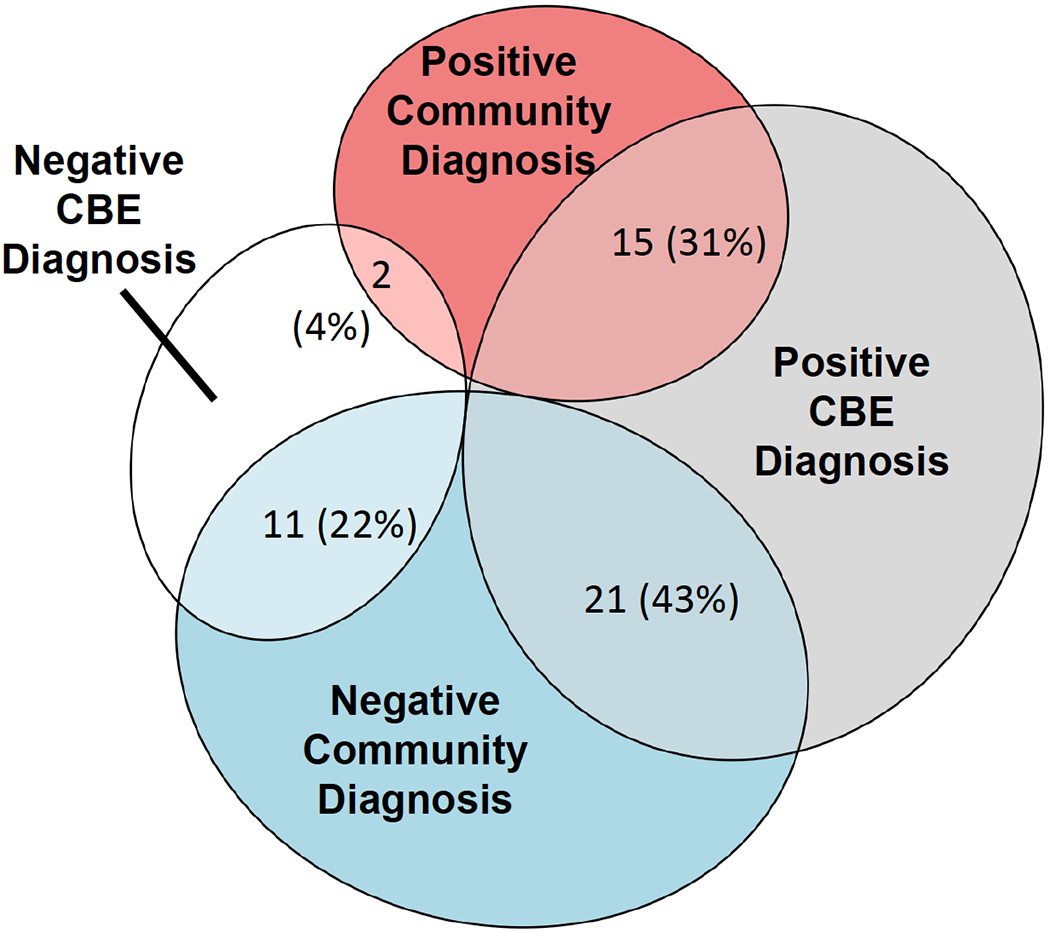
Concordance across CBE and Community ASD Diagnoses.
Note.Percentages reflect the number of participants falling within each category out of the full sample. Concordance with ADOS-2/ADI-R rates are not depicted given the strong agreement between the CBE and ADOS-2/ADI-R diagnostic methods.
Cognitive, Language, and Behavioral Predictors of ASD Diagnoses
Predictors of CBE ASD Classification.
Logistic regression identified several predictors of CBE classification of ASD, including lower nonverbal IQ (OR = 0.89; p= .016) such that the odds of having a CBE ASD classification decreased by 11% with each unit increase in nonverbal IQ. Expressive vocabulary (OR = 0.88; p = .003) and receptive vocabulary (OR = 0.93; p = .005) were also significant predictors; the odds of having a CBE ASD classification decreased by 12% with each unit increase in expressive language, and by 7% with each unit increase in receptive language. Symptoms of inattention/hyperactivity and anxiety were not predictive of CBE categorization (see Table 4).
Table 4.
Logistic Regression Results Examining Predictors of CBE and Community ASD Diagnoses
| Clinical Best Estimate |
Community ASD Diagnosis |
|||
|---|---|---|---|---|
| B (SE) | OR (95% CI) | B (SE) | OR (95% CI) | |
| IQ | −0.12 (0.05)* | 0.89 (0.80 – 0.98) | −0.01(0.02) | 0.99 (0.94 – 1.04) |
| Expressive Language | −0.13 (0.05)** | 0.88 (0.80 – 0.96) | −0.02 (0.03) | 0.98 (0.94 – 1.03) |
| Receptive Language | −0.07 (0.02)** | 0.93 (0.89 – 0.98) | −0.01 (0.02) | 0.99 (0.97 – 1.02) |
| Inattention/Hyperactivity | 0.01 (0.10) | 1.01 (0.83 – 1.23) | 0.03 (0.09) | 1.03 (0.86 – 1.23) |
| Anxiety | 0.07 (0.10) | 1.07 (0.87 – 1.31) | −0.01 (0.09) | 0.99 (0.83 – 1.19) |
p<.050
p<.010
Predictors of Community ASD Diagnosis
As shown in Table 4, none of the examined cognitive, language, or behavioral characteristics were significant predictors of community ASD diagnoses.
Parental Views and Other Factors Related to Community ASD Diagnoses
Table 2 summarizes the responses on the Community ASD Diagnosis Questionnaire. The average age of community ASD diagnosis was 7 years. Although only 35% of participants with FXS were reported to have a current ASD diagnosis, 69% of parents felt that their son showed signs of ASD and 61% had been told by professionals that their son exhibited signs of ASD. Parental beliefs about their son’s ASD status had near-chance alignment with the CBE ASD diagnostic classification; of those with a positive CBE diagnosis, 53% of parents believed their son had ASD and 47% did not.
Forty-one percent of the sample had never been formally evaluated for ASD in a community setting. Of those who had never been evaluated, 75% (15/20) exhibited sufficient ASD symptoms to meet diagnostic criteria for ASD per the CBE procedures. The majority (65%) of families who participated in an ASD evaluation believed that the clinician who conducted their son’s ASD evaluation had adequate knowledge about FXS to make an accurate diagnosis, 21% did not have this belief, and 14% were unsure. The majority of parents believed that knowledge about their son’s ASD status would be helpful in making educational (71%) or medical decisions (57%). Forty-seven percent of parents believed that their son would benefit from services that were tailored towards individuals with ASD, whereas 20% did not believe that their son would benefit and 33% were unsure. Thirty-six percent of parents reported that their son was receiving services designed for individuals with ASD (9% were unsure and 55% reported that their son does not receive this type of service). Youth who were reported to receive ASD therapies were only slightly more likely to be identified as having ASD in the community (56% had a community ASD diagnosis and 43% did not).
Qualitative Analysis of Parental Views on ASD Cooccurrence in FXS
Qualitative analysis of the open-ended response option following the item “In your opinion, do you believe that your son has ASD? Why or why not?” was conducted to better understand parental views on ASD diagnoses within the context of FXS. As we were particularly interested in the low rates of community diagnoses relative to CBE diagnoses, we focused on the written commentary provided by the parents whose sons met CBE criteria for ASD but did not have a community diagnosis. Of the 21 parents whose sons met these criteria, a total of 12 mothers provided such commentary. Using standard qualitative thematic analyses (Frankel et al., 2014; Patton, 2002), we evaluated responses to identify common themes and then categorized responses across the common themes that emerged. A second team member then conferred on the categorization to confirm thematic content and/or establish inter-rater agreement (Frankel et al., 2014; Patton, 2002). Three prominent themes emerged; the three themes were equally represented with about one-third of responses reflecting each of the themes. The first theme reflected the belief that social interest/motivation precludes an ASD diagnosis (e.g., “Too social”; “Very social and wants to engage with others”). The second theme reflected an attribution of features to FXS rather than ASD and was observed in one-third of the responses analyzed (e.g., “Symptoms are typical of FXS”; “Socially behind, great difficulty with eye contact. Could just be from the fragile X”; “Chief symptom is MR [intellectual disability]”). The third theme reflected uncertainty about whether their son may have comorbid ASD; this theme was observed in one-third of the responses (e.g., “All the symptoms of ASD as well as FXS”; “Not sure. He has autistic tendencies”; “Become[s] overstimulated with visual/auditory stimuli, similar to ASD”).
Discussion
Persons with FXS who also meet criteria for ASD are at heightened risk for poor outcomes. The detection and diagnosis of ASD in this group is necessary to ensure access to specialized treatment and community services. Yet, the diagnosis of ASD in persons with FXS has represented a source of great controversy, with wide variation in the estimated rates of ASD comorbidity and limited understanding of diagnostic practices in the community settings. In this study, we set out to describe the rate of ASD in adolescent and young adult males with FXS across multiple diagnostic sources, and to describe predictors and parental views surrounding ASD diagnoses that may inform barriers to identification. Findings supported high concordance between the ADOS-2/ADI-R and CBE ASD classifications, with both diagnostic methods supporting rates of ASD comorbidity of about 75% in male youth with FXS. In contrast, only 31% of the sample had received a diagnosis of ASD in the community, and there was limited concordance between community diagnoses and those determined through the research protocol. A parental survey revealed community ASD diagnoses that were poorly aligned with presence of ASD symptoms as perceived by parents and health/educational professionals, and parental beliefs about their son’s ASD status. In contrast to CBE ASD classification, receipt of a community diagnosis was not predicted by cognition, language, inattention/hyperactivity, or anxiety. Findings inform ASD diagnostic practices for male youth with FXS and highlight under-identification of ASD in community settings as a potentially significant barrier to service access for this group.
Clinical Best Estimate Diagnostic Classification of ASD
Our findings support cooccurring ASD in about three-fourths of male adolescents and young adults with FXS. This study helps address controversies concerning the “validity” of ASD in FXS, which partially stem from concerns about the accuracy of standardized ASD diagnostic tools when applied within the complex clinical presentation of FXS (Abbeduto et al., 2014). Our estimates are derived from rigorous CBE diagnostic classification procedures, which involve the consensus of a multidisciplinary team of expert ASD clinicians informed by standardized diagnostic, cognitive, and behavioral testing. Although CBE diagnostic procedures are accepted as the gold standard in studies of nonsyndromic ASD, they have been rarely applied to FXS and thus this study provides a more precise estimate of the expected rates of ASD comorbidity in male youth with FXS.
We observed very high concordance between CBE diagnostic classification and the ADOS-2/ADI-R, with Cohen’s kappa statistic supporting high levels of agreement between these two methods. CBE diagnostic procedures included review of all available test data, including ADOS-2 and ADI-R scores, so in some respects the high concordance is expected. However, the present data on concordance reflect novel empirical data that informs an ongoing debate regarding the validity of standardized ASD tools when used in intellectual and developmental disability populations (e.g., Abbeduto et al., 2014; Thurm et al., 2019). This high concordance indicates that the ADOS-2 and ADI-R are highly effective tools in characterizing features and severity of ASD in male youth with FXS, at least when applied within those of adolescent/young adult age who exhibit characteristics similar to those of the present sample (e.g., have phrase speech). It is important to note, however, that while standardized tools provide valuable information a comprehensive clinical diagnostic approach is always a requirement for diagnosis. Additionally, these standardized tools may prove to be less accurate in capturing both presence and severity of ASD features in FXS in characteristically different groups, such as younger, minimally verbal, or female children with FXS. This has been demonstrated in an existing study which yielded 82% agreement between CBE and ADOS-2 cutoff scores alone (Roberts et al., 2020) versus 94% agreement in the current project. In the Roberts et al. (2020) study, 61% were diagnosed with ASD using CBE which would have been inflated to 78% if only score cutoffs had been used. The samples across these studies and this current one are fairly different with the Roberts study focused on preschool-aged boys and girls without a minimal language level while the current study includes only males of late adolescent and early adult age who have at least phrase speech; age, sex, and language/cognitive level are all factors known to influence the accuracy of standardized ASD diagnostic tools (Lord, Rutter, et al., 2012; Rea et al., 2022) .
ASD is Under-identified in Community Settings
In contrast to the 75% rate of ASD per CBE diagnostic classifications, only 31% of the sample had been identified as having ASD in the community. Concordance between CBE and community diagnosis was very poor. Sixty percent of those who received a CBE diagnosis were not identified in the community, indicating significant under-identification of ASD in youth with FXS. Notably, in an independent sample of adolescents and young adults, the present study closely replicates Klusek et al. (2014)’s earlier report focused on school-aged of boys with FXS that documented similarly low rates of ASD community diagnoses (26%). Focus on an older cohort in the present study rules out the possibility that the low rate of community diagnosis in prior reports is due to the inclusion of younger children who had not yet been identified in the community. Considering that 41% of parents reported that their son had never been formally evaluated for ASD in the community, our data suggest failure to refer children with FXS for ASD diagnostics may be a contributing factor. In the majority of cases, ASD evaluation had not taken place despite the presence of significant ASD symptoms; 75% of those who had never been evaluated exhibited sufficient symptoms for a CBE classification of ASD.
Implications of Missed ASD Diagnosis for Service Access and Tailoring of Therapeutic Services
The failure to clinically identify ASD in persons with FXS represents a major barrier to meeting the service needs of this population. A diagnosis is often a prerequisite for service eligibility, and there can be clear benefits of establishing a comorbid diagnosis of ASD in persons with FXS. For example, all 50 US states have passed laws requiring commercial insurance plans to cover certain evidence-based treatments for those with ASD, which may include services such as physical therapy, occupational therapy, speech therapy, and Applied Behavior Analysis (ABA) therapy (Choi et al., 2020); these “autism mandates” only ensure coverage for those with an ASD diagnosis. In addition, many states have enacted Medicaid waivers to expand service coverage for those with ASD, including the expansion of services for adults (Shea et al., 2021). Without a diagnosis, persons with FXS and cooccurring ASD are unable to access these benefits.
Beyond implications for service access, professionals also rely on diagnostic information to tailor the type and intensity of therapeutic services. Behavioral intervention techniques that are effective for those with nonsyndromic ASD, such as ABA, also appear to be successful at targeting behavioral difficulties in those with FXS, including aggression (Moskowitz et al., 2011; Wheeler et al., 2016), problem behavior (Hall et al., 2022; Kurtz et al., 2015; Monlux et al., 2019), and specific social skills such as eye contact (Gannon et al., 2018; Hall et al., 2009). At the same time, however, many of these services, including ABA, are provided at very low rates to individuals with FXS (Kaufmann et al., 2017). There is also emerging evidence that children with FXS may benefit from Naturalistic Developmental-Behavioral Interventions (NDBIs), originally developed to target the specific developmental challenges seen in children with nonsyndromic ASD (Vismara et al., 2019). Failure to diagnose concomitant ASD will limit access to high-intensity and specialized intervention services that are tailored toward the profile of ASD (Barton et al., 2016; Callaghan & Sylvester, 2019; Dimian et al., 2021; Shea et al., 2021). At the same time, however, it is important to recognize that there may be important differences between the support and treatment needs of individuals with FXS and cooccurring ASD relative to those with nonsyndromic ASD (Abbeduto et al., 2014), and there is a need for further research to guide treatment. Nonetheless, the identification of cooccurring ASD is a critical starting point for the development of an appropriately individualized treatment plan.
Why is ASD Missed in FXS?
What factors, then, led to some youth being identified in the community and others being missed? Our data did not reveal any specific skill deficits, behavioral markers, or parental views that were clearly associated with an increased likelihood of community identification. Specifically, community diagnoses were not associated with the degree of intellectual impairment, the level of vocabulary delay, anxiety symptoms, or inattention/hyperactivity problems. Similarly, parental survey results showed a puzzling lack of alignment between receipt of a community ASD diagnosis and factors that would be expected to promote identification, such as parental report that their son exhibits ASD symptoms, parental belief that their son has ASD, and parental report health/educational professionals have told them that their son exhibits ASD symptoms. For example, among families whose sons were unidentified in the community, 57% of parents thought their son showed ASD symptoms, 27% believed that their son had ASD, and nearly one-half (47%) had been told by professionals that their son showed symptoms of ASD.
Our qualitative analysis sheds light on potential contributors to missed diagnoses, by exploring the views held by families whose son lacked a community diagnosis despite meeting CBE criteria for ASD. One theme that emerged included attributing ASD symptoms to FXS rather than considering them as part of a distinct cooccurring condition. This theme reflects diagnostic overshadowing, which occurs when a salient primary diagnosis interferes with the clinical recognition of other health conditions, resulting in the failure to diagnose and treat concomitant disorders (Reiss et al., 1982). People with intellectual disability (Mason & Scior, 2004; White et al., 1995) and neurogenetic syndromes (Minnes & Steiner, 2009; Reilly et al., 2015) are particularly likely to face diagnostic overshadowing bias, resulting in unmet health needs. For example, clinicians working with persons with FXS may overlook ASD symptoms or attribute them to intellectual disability rather than considering these features as a distinct condition. Given the relatively low incidence of FXS, families may experience difficulty in locating clinical providers who are sufficiently knowledgeable about the phenotypic and developmental presentation of FXS and thus able to discern cooccurring ASD symptoms. Indeed, a substantial minority of parents surveyed (35%) had doubts about whether their diagnosing clinician had adequate knowledge about FXS to make an accurate ASD diagnosis. In future research, a direct survey of practitioners may further our understanding of the potential role of diagnostic overshadowing in ASD diagnostics in FXS.
Another theme associated with missed ASD diagnosis was the belief that social interest/motivation precludes an ASD diagnosis. Although this viewpoint is common in the FXS field (e.g., Fragile X Clinical & Research Consortium, 2020), it is not universally accepted and its application to differential diagnosis is not black-and-white. Social profiles of ASD characterized by social approach that is active but unusual in its one-sided, odd, naïve, or inappropriate quality (i.e., “active-but-odd”), have been recognized since the earliest characterizations of ASD (Wing & Gould, 1979) and are captured in the diagnostic criteria for ASD (American Psychiatric Association, 2013). The final theme that emerged from parental responses was uncertainty.
Recommendations to Improve Identification of ASD in FXS
In many ways, the views expressed by parents whose sons had a missed community ASD diagnosis mirrored the key controversies in the fragile X field regarding the nature of ASD in FXS (Abbeduto et al., 2014; Hall et al., 2010). This suggests a need for clearer messaging from the FXS clinical/research community to ensure that internal debate about the nature of ASD symptoms within the context of FXS does not overshadow clear communication of the practical benefits of seeking professional ASD evaluation when it is indicated. Indeed, given the complexity of differential ASD diagnosis within FXS, a comprehensive professional ASD evaluation should be viewed as a vital tool for understanding a child’s symptom profile and arriving at an accurate diagnosis that will inform services. The fact that 40% of the youth in our sample had never been evaluated for ASD in a community setting suggests that professional ASD evaluation has either not been recommended by the youth’s clinical/educational care team, or that families did not to proceed with the evaluation if it was recommended. Clarifying fragile X practice guidelines to highlight the potential therapeutic and service advantages associated with ASD co-diagnosis will provide needed guidance for practitioners and families who are noting ASD symptoms but are unsure whether pursuing a formal evaluation would be beneficial.
Strengths, Limitations, and Directions
A notable strength of this study is our multifaceted and rigorous characterization of ASD. Our use of CBE diagnostic procedures increases confidence in the accuracy of ASD characterization. These procedures have rarely been applied in FXS research, given that they are very time-intensive and require involvement of an expert multidisciplinary diagnostic team, as well as access to extensive cognitive, behavioral, and diagnostic testing. Our battery of standardized ASD diagnostic assessments was also comprehensive, gleaning information from both the ADOS-2 and ADI-R. Finally, a novel aspect of this study is the inclusion of information on community ASD diagnoses, which provides another source of diagnostic information while also allowing us to begin to describe how ASD comorbidity in FXS is being handled community settings. We did not verify diagnostic information provided by parents with review of medical or educational records, which may be perceived as a weakness. However, parents’ reports on their son’s diagnostic status arguably hold enhanced clinical significance relative to record review (regardless of what is stated in a medical or educational record, a parent unaware that their child has been identified as having ASD is unlikely to advocate for ASD-related services for their child).
Another strength is our sample of 49 participants with FXS which is relatively large for the field. Our focus on an older sample of adolescents and young adults may have yielded more reliable estimates given the relative stability of ASD symptoms during adulthood in FXS (Hartley et al., 2015) and the fact we would expect community diagnoses to occur earlier in childhood. It is notable that our inclusion criteria required the ability to use phrase speech, as was a requirement for the larger language-focused study. Thus, while detected ASD rate of 75% may seem remarkably high, this figure may actually be an underestimate as it does not account for potentially heightened rates of ASD that may be observed in minimally verbal persons.
Our sample size was more limited for the qualitative analyses that focused only on the subset of youth who met CBE criteria but did not have a community diagnosis. While this subgroup consisted of 21 participants, only 12 parents chose to provide commentary in response to the open field inquiring about their beliefs about their son’s ASD status. The small sample may have limited our ability to achieve thematic saturation, and we also cannot rule out the possibility that the parents who chose to provide commentary held different or stronger views than those who chose not to comment. Thus, while the qualitative analyses provide insight into family views and experiences that can help inform the patterns observed in our primary analyses, they should be viewed as preliminary as they may not capture the full range of parental views.
Our sample was also predominately White, which may limit generalization of findings to people of color. And, while a range of incomes and education levels were represented in the sample, the socioeconomic and educational characteristics of participating families were perhaps higher than the general population average; thus, inclusion of better-resourced families could have inflated the rates of community ASD diagnosis (note, however, that Klusek et al., 2014, tested this hypothesis and found no relationship between community ASD identification in FXS and either income or maternal education level). The challenges to engaging people from economically disadvantaged and historically marginalized racial groups in research are well-documented and FXS recruitment poses particular challenges given reliance on parent organization and specialty clinics that have majority White participation (Kidd et al., 2017). Use of evidence-based strategies to reduce barriers to research participation may improve representation of historically disadvantaged groups in future research, such as the use of personalized community-based and linguistically appropriate recruitment, employment of culturally matched research personnel, offering childcare and adequate remuneration, cultivating personal relationships, and use of research to help families and communities (Austin-Wells et al., 2006; Chechi et al., 2014; George et al., 2014; Johnson et al., 2009), in addition to the development of linguistically and culturally appropriate measures for non-English speakers (e.g., del Hoyo Soriano et al., 2021). Finally, future studies would benefit from focus on younger children and females to determine if similar patterns hold.
Conclusion
This study documents high rates of ASD cooccurrence in male youth with FXS at ~75%, with estimates supported by both CBE diagnostic procedures and ADOS-2/ADI-R thresholds and high concordance between the two classification methods. We also detected evidence of substantial under-identification of ASD in youth with FXS in community settings. Sixty percent of youth who exhibited sufficient ASD symptoms for a CBE diagnosis of ASD had not been identified in the community. The failure to clinically identify ASD in persons with FXS in the community may contribute to difficulties accessing services and unmet health needs for this group. Our findings underscore the need for clearer messaging from the FXS clinical/research community on the benefits of seeking professional ASD evaluation for those with FXS who have been noted to display ASD symptoms.
Supplementary Material
Acknowledgments:
This research was supported by the This research was supported by the National Institutes of Health (R01HD024356; P50HD103526, PI: Abbeduto; UL1TR001860, PI: Wun; R21DC017804; PI: Jessica Klusek) and the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities (P50HD103573). We appreciate the time and support of the families who participated in this research study. We thank Dr. Somer Bishop for her consultation on the clinical best estimate diagnoses.
Footnotes
Competing Interests: The authors have no relevant financial or non-financial interests to disclose.
Ethics: All procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments and approved by the Institutional Review Boards of the respective university sites.
Consent to Participate: Informed consent was obtained from all participants or their legal guardian as appropriate.
References
- Abbeduto L, McDuffie A, & Thurman AJ (2014). The fragile X syndrome–autism comorbidity: what do we really know? Frontiers in Genetics, 5, 355. 10.3389/fgene.2014.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbeduto L, Thurman AJ, del Hoyo Soriano L, & Klusek J (2021). Fragile X syndrome and associated disorders. In Glidden LM, Abbeduto L, McIntyre L, & Tasse J (Eds.), APA Handbook of Intellectual and Developmental Disabilities (pp. 151–185). American Psychological Association. 10.1037/0000194-007 [DOI] [Google Scholar]
- Abbeduto L, Thurman AJ, McDuffie A, Klusek J, Feigles RT, Brown WT, Harvey DJ, Adayev T, LaFauci G, & Dobkins C (2019). ASD comorbidity in fragile X syndrome: symptom profile and predictors of symptom severity in adolescent and young adult males. Journal of Autism and Developmental Disorders, 49(3), 960–977. 10.1007/s10803-018-3796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed. ed.). American Psychiatric Publishing, Incorporated. [Google Scholar]
- Austin-Wells V, McDougall GJ, & Becker H (2006). Recruiting and retaining an ethnically diverse sample of older adults in a longitudinal intervention study. Educational Gerontology, 32(2), 159–170. 10.1080/03601270500388190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Mesibov G, Hatton DD, Clark RD, Roberts JE, & Mayhew L (1998). Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders, 28(6), 499–508. 10.1023/A:1026048027397 [DOI] [PubMed] [Google Scholar]
- Barton EE, Harris B, Leech N, Stiff L, Choi G, & Joel T (2016). An analysis of state autism educational assessment practices and requirements. Journal of Autism and Developmental Disorders, 46(3), 737–748. 10.1007/s10803-015-2589-0 [DOI] [PubMed] [Google Scholar]
- Belmonte MK, & Bourgeron T (2006). Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience, 9(10), 1221–1225. 10.1038/nn1765 [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, & Kaufmann WE (2006). Autism spectrum disorder in fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. American Journal of Medical Genetics Part A, 9999, 1–13. [DOI] [PubMed] [Google Scholar]
- Callaghan T, & Sylvester S (2019). Autism spectrum disorder, politics, and the generosity of insurance mandates in the United States. PLOS ONE, 14(5), e0217064. 10.1371/journal.pone.0217064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, & Baird G (2002). Practitioner review: Diagnosis of autism spectrum disorder in 2-and 3-year-old children. Journal of Child Psychology and Psychiatry, 43(3), 289–305. 10.1111/1469-7610.00022 [DOI] [PubMed] [Google Scholar]
- Chechi T, Siyahian S, Lucy T, Randi H, & Reymundo L (2014). Participation of underrepresented minority children in clinical trials for fragile X syndrome and other neurodevelopmental disorders. Intractable and Rare Diseases Research, 3(4), 147–152. 10.5582/irdr.2014.01025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KR, Knight EA, Stein BD, & Coleman KJ (2020). Autism insurance mandates in the US: Comparison of mandated commercial insurance benefits across states. Maternal and Child Health Journal, 24(7), 894–900. 10.1007/s10995-020-02950-2 [DOI] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, & Loesch DZ (2007). Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders, 37(4), 738–747. 10.1007/s10803-006-0205-z [DOI] [PubMed] [Google Scholar]
- Cohen J (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20(1), 37–46. [Google Scholar]
- Crawford DC, Acuna JM, & Sherman SL (2001). FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine, 3, 359–371. 10.1097/00125817-200109000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, & Darnell RB (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell, 146(2), 247–261. 10.1016/j.cell.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Hoyo Soriano L, Bullard L, Hoyos Alvarez C, Thurman AJ, & Abbeduto L (2021). Using telehealth-delivered procedures to collect a parent-implemented expressive language sampling narrative task in monolingual and bilingual families with Autism Spectrum Disorder: A pilot study. Frontiers in rehabilitation sciences, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimian AF, Symons FJ, & Wolff JJ (2021). Delay to early intensive behavioral intervention and educational outcomes for a medicaid-enrolled cohort of children with autism. Journal of Autism and Developmental Disorders, 51(4), 1054–1066. 10.1007/s10803-020-04586-1 [DOI] [PubMed] [Google Scholar]
- Dunn LM, & Dunn DM (2007). Peabody Picture Vocabulary Test, Fourth Edition. Pearson Assessments. [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, & Ruedrich S (2003). Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders, 33(6), 617–629. 10.1023/B:JADD.0000005999.27178.55 [DOI] [PubMed] [Google Scholar]
- Fielding-Gebhardt H, Bredin-Oja SL, Warren SF, & Brady NC (2021). Rethinking measurement standards of autism symptomology in adolescents with fragile X syndrome. Journal of Autism and Developmental Disorders, 51(12), 4520–4533. 10.1007/s10803-021-04892-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragile X Clinical & Research Consortium. (2020, Dec.). Consensus of the Fragile X Clinical & Research Consortium: Understanding autism spectrum disorder in fragile X syndrome. Retrieved April 19 from https://fragilex.org/our-research/treatment-recommendations/understanding-asd-fxs/
- Frankel EB, Hutchinson NL, Burbidge J, & Minnes P (2014). Preservice early childhood educators’ and elementary teachers’ perspectives on including young children with developmental disabilities: A mixed methods analysis. Journal of Early Childhood Teacher Education, 35(4), 373–391. 10.1080/10901027.2014.968300 [DOI] [Google Scholar]
- Gannon CE, Britton TC, Wilkinson EH, & Hall SS (2018). Improving social gaze behavior in fragile X syndrome using a behavioral skills training approach: a proof of concept study. Journal of Neurodevelopmental Disorders, 10(1), 25. 10.1186/s11689-018-9243-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nonell C, Ratera ER, Harris S, Hessl D, Ono MY, Tartaglia N, Marvin E, Tassone F, & Hagerman RJ (2008). Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. American Journal of Medical Genetics Part A, 146A(15), 1911–1916. 10.1002/ajmg.a.32290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Duran N, & Norris K (2014). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American journal of public health, 104(2), e16–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, & Reiss AL (2010). Autism in fragile X syndrome: a category mistake? Journal of the American Academy of Child and Adolescent Psychiatry, 49(9), 921–933. 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Maynes NP, & Reiss AL (2009). Using percentile schedule to increase eye contact in children with fragile X syndrome. Journal of Applied Behavior Analysis, 42(1), 171–176. 10.1901/jaba.2009.42-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Rodriguez AB, Jo B, & Pollard JS (2022). Long-term follow-up of telehealth-enabled behavioral treatment for challenging behaviors in boys with fragile X syndrome. Journal of Neurodevelopmental Disorders, 14(1), 53. 10.1186/s11689-022-09463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones BL, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman K, Hagerman R. i. J., MacLean WE Jr., & Abbeduto L (2008). Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation, 113(6), 427–438. 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Wheeler AC, Mailick MR, Raspa M, Mihaila I, Bishop E, & Bailey DB (2015). Autism symptoms across adulthood in men with fragile X syndrome: A cross-sectional analysis. Journal of Autism and Developmental Disorders, 45(11), 3668–3679. 10.1007/s10803-015-2513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Skinner M, Sideris J, Mankowski JB, Bailey DB, Roberts JE, & Mirrett P (2006). Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics 140(17), 1804–1813. 10.1002/ajmg.a.31286 [DOI] [PubMed] [Google Scholar]
- Havdahl KA, Hus Bal V, Huerta M, Pickles A, Øyen A-S, Stoltenberg C, Lord C, & Bishop SL (2016). Multidimensional influences on autism symptom measures: Implications for use in etiological research. Journal of the American Academy of Child & Adolescent Psychiatry, 55(12), 1054–1063.e1053. 10.1016/j.jaac.2016.09.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton LL, Fulton RS, Magrini VJ, Ye K, Darnell JC, Darnell RB, Mardis ER, Wilson RK, Schatz MC, McCombie WR, & Wigler M (2012). De novo gene disruptions in children on the autistic spectrum. Neuron, 74(2), 285–299. 10.1016/j.neuron.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VA, Edwards KKA, Sherman SL, Stephens LD, & Deer-Smith MH (2009). Decisions to participate in fragile X and other genomics-related research: Native American and African American voices. Journal of Cultural Diversity, 16(3), 127. [PubMed] [Google Scholar]
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, Stackhouse T, Riley C, Peacock G, & Sherman SL (2017). Autism spectrum disorder in fragile X syndrome: Cooccurring conditions and current treatment. Pediatrics, 139(Suppl 3), S194. 10.1542/peds.2016-1159F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd SA, Raspa M, Clark R, Usrey-Roos H, Wheeler AC, Liu JA, Wylie A, & Sherman SL (2017). Attendance at fragile X specialty clinics: Facilitators and barriers. American Journal on Intellectual and Developmental Disabilities, 122(6), 457–475. 10.1352/1944-7558-122.6.457 [DOI] [PubMed] [Google Scholar]
- Klin A, Lang J, Cicchetti DV, & Volkmar FR (2000). Brief report: Interrater reliability of clinical diagnosis and DSM-IV criteria for autistic disorder: Results of the DSM-IV autism field trial. Journal of Autism and Developmental Disorders, 30(2), 163. 10.1023/A:1005415823867 [DOI] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M (2014a). A comparison of pragmatic language in boys with autism and fragile X syndrome. Journal of Speech, Language, and Hearing Research, 57, 1692–1707. 10.1044/2014_JSLHR-L-13-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M (2014b). Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. Journal of Intellectual Disability Reasearch, 58, 940–952. 10.1111/jir.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz PF, Chin MD, Robinson AN, O’Connor JT, & Hagopian LP (2015). Functional analysis and treatment of problem behavior exhibited by children with fragile X syndrome. Research in Developmental Disabilities, 43-44, 150–166. 10.1016/j.ridd.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Kwiecien R, Kopp-Schneider A, & Blettner M (2011). Concordance analysis: Part 16 of a series on evaluation of scientific publications. Deutsches Arzteblatt international, 108(30), 515–521. 10.3238/arztebl.2011.0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Haden G, Hammal D, & McConachie H (2008). Diagnosing autism spectrum disorders in pre-school children using two standardised assessment instruments: the ADI-R and the ADOS. Journal of Autism and Developmental Disorders, 38(2), 362–372. 10.1007/s10803-007-0403-3 [DOI] [PubMed] [Google Scholar]
- Lee M, Martin GE, Berry-Kravis E, & Losh M (2016). A developmental, longitudinal investigation of autism phenotypic profiles in fragile X syndrome. Journal of Neurodevelopmental Disorders, 8(1), 47. 10.1186/s11689-016-9179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, & Schroeder S (2006). Cognitive, language and social-cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research, 50, 532–545. 10.1111/j.1365-2788.2006.00803.x [DOI] [PubMed] [Google Scholar]
- Li M, Shin J, Risgaard RD, Parries MJ, Wang J, Chasman D, Liu S, Roy S, Bhattacharyya A, & Zhao X (2020). Identification of FMR1-regulated molecular networks in human neurodevelopment. Genome Research, 30(3), 361–374. 10.1101/gr.251405.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Petkova E, Hus V, Gan W, Lu F, Martin DM, Ousley O, Guy L, Bernier R, & Gerdts J (2012). A multisite study of the clinical diagnosis of different autism spectrum disorders. Archives of General Psychiatry, 69(3), 306–313. 10.1001/archgenpsychiatry.2011.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, & Pickles A (2006). Autism from 2 to 9 years of age. Archives of General Psychiatry, 63(6), 694–701. 10.1001/archpsyc.63.6.694 [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, & Rutter M (2000). The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Mason J, & Scior K (2004). ‘Diagnostic overshadowing’ amongst clinicians working with people with intellectual disabilities in the UK. Journal of Applied Research in Intellectual Disabilities, 17(2), 85–90. 10.1111/j.1360-2322.2004.00184.x [DOI] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, & Abbeduto L (2015). Symptoms of autism in males with fragile X syndrome: A comparison to nonsyndromic ASD using current ADI-R scores. Journal of Autism and Developmental Disorders, 45(7), 1925–1937. 10.1007/s10803-013-2013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes P, & Steiner K (2009). Parent views on enhancing the quality of health care for their children with fragile X syndrome, autism or Down syndrome. Child: Care, Health and Development, 35(2), 250–256. 10.1111/j.1365-2214.2008.00931.x [DOI] [PubMed] [Google Scholar]
- Monlux KD, Pollard JS, Bujanda Rodriguez AY, & Hall SS (2019). Telehealth delivery of function-based behavioral treatment for problem behaviors exhibited by boys with fragile X syndrome. Journal of Autism and Developmental Disorders, 49(6), 2461–2475. 10.1007/s10803-019-03963-9 [DOI] [PubMed] [Google Scholar]
- Moskowitz LJ, Carr EG, & Durand VM (2011). Behavioral intervention for problem behavior in children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 116(6), 457–478. 10.1352/1944-7558-116.6.457 [DOI] [PubMed] [Google Scholar]
- Patton MQ (2002). Qualitative Research and Evaluation Methods (3rd ed.). Sage Publications. [Google Scholar]
- Raspa M, Franco V, Bishop E, Wheeler AC, Wylie A, & Bailey DB (2018). A comparison of functional academic and daily living skills in males with fragile X syndrome with and without autism. Research in Developmental Disabilities, 78, 1–14. 10.1016/j.ridd.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Rea HM, Øien RA, Shic F, Webb SJ, & Ratto AB (2022). Sex Differences on the ADOS-2. Journal of Autism and Developmental Disorders. 10.1007/s10803-022-05566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C, Senior J, & Murtagh L (2015). ASD, ADHD, mental health conditions and psychopharmacology in neurogenetic syndromes: Parent survey. Journal of Intellectual Disability Research, 59(4), 307–318. 10.1111/jir.12147 [DOI] [PubMed] [Google Scholar]
- Reiss S, Levitan GW, & Szyszko J (1982). Emotional disturbance and mental retardation: Diagnostic overshadowing. American Journal of Mental Deficiency. [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH Jr, Leventhal BL, & Pickles A (2006). Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 45(9), 1094–1103. 10.1097/01.chi.0000227880.42780.0e [DOI] [PubMed] [Google Scholar]
- Roberts JE, Bradshaw J, Will E, Hogan AL, McQuillin S, & Hills K (2020). Emergence and rate of autism in fragile X syndrome across the first years of life. Development and Psychopathology, 32(4), 1335–1352. 10.1017/S0954579420000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Ezell JE, Fairchild AJ, Klusek J, Thurman AJ, McDuffie A, & Abbeduto L (2018). Biobehavioral composite of social aspects of anxiety in young adults with fragile X syndrome contrasted to autism spectrum disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 10.1002/ajmg.b.32674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Martin GE, Moskowitz L, Harris AA, Foreman J, & Nelson L (2007). Discourse skills of boys with fragile X syndrome in comparison to boys with Down syndrome. Journal of Speech, Language, and Hearing Research, 50, 475–492. 10.1044/1092-4388(2007/033) [DOI] [PubMed] [Google Scholar]
- Roberts JE, Schaaf JM, Skinner M, Wheeler A, Hooper S, Hatton DD, & Bailey DB Jr (2005). Academic skills of boys with fragile X syndrome: Profiles and predictors. American Journal of Intellectual and Developmental Disabilities, 110(2), 107–120. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, & Hagerman R (2001). The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental Behavioral Pediatrics, 22(6), 409–417. [DOI] [PubMed] [Google Scholar]
- Roid GH, & Miller LJ (1997). Leiter International Performance Scale-Revised. Stoelting. [Google Scholar]
- Schopler E, Reichler J, & Renner B (1988). The Childhood Autism Rating Scale (CARS). Western Psychological Services. [Google Scholar]
- Shea LL, Koffer Miller KH, Verstreate K, Tao S, & Mandell D (2021). States’ use of Medicaid to meet the needs of autistic individuals. Health Services Research, 56(6), 1207–1214. 10.1111/1475-6773.13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talisa VB, Boyle L, Crafa D, & Kaufmann WE (2014). Autism and anxiety in males with fragile X syndrome: An exploratory analysis of neurobehavioral profiles from a parent survey. American Journal of Medical Genetics Part A, 164(5), 1198–1203. 10.1002/ajmg.a.36468 [DOI] [PubMed] [Google Scholar]
- Thurm A, Farmer C, Salzman E, Lord C, & Bishop S (2019). State of the field: Differentiating intellectual disability from autism spectrum disorder. Frontiers in Psychiatry, 526. 10.3389/fpsyt.2019.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara LA, McCormick CEB, Shields R, & Hessl D (2019). Extending the parent-delivered Early Start Denver Model to young children with fragile X syndrome. Journal of Autism and Developmental Disorders, 49(3), 1250–1266. 10.1007/s10803-018-3833-1 [DOI] [PubMed] [Google Scholar]
- Waltes R, Duketis E, Knapp M, Anney RJ, Huguet G, Schlitt S, Jarczok TA, Sachse M, Kämpfer LM, & Kleinböck T (2014). Common variants in genes of the postsynaptic FMRP signalling pathway are risk factors for autism spectrum disorders. Human Genetics, 133(6), 781–792. 10.1007/s00439-013-1416-y [DOI] [PubMed] [Google Scholar]
- Wheeler AC, Mussey J, Villagomez A, Bishop E, Raspa M, Edwards A, Bodfish J, Bann C, & Bailey DB (2015). DSM-5 changes and the prevalence of parent-reported autism spectrum symptoms in fragile X syndrome. Journal of Autism and Developmental Disorders, 45(3), 816–829. 10.1007/s10803-014-2246-z [DOI] [PubMed] [Google Scholar]
- Wheeler AC, Raspa M, Bishop E, & Bailey DB Jr (2016). Aggression in fragile X syndrome. Journal of Intellectual Disability Research, 60(2), 113–125. 10.1111/jir.12238 [DOI] [PubMed] [Google Scholar]
- White MJ, Nichols CN, Cook RS, & Spengler PM (1995). Diagnostic overshadowing and mental retardation: A meta-analysis. American Journal on Mental Retardation, 3(100), 293–298. [PubMed] [Google Scholar]
- Williams KT (2007). Expressive Vocabulary Test, Second Edition. American Guidance Service. [Google Scholar]
- Wing L, & Gould J (1979). Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of Autism and Developmental Disorders, 9(1), 11–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


