Abstract
The different ways of expressing concentrations of drugs in solution, as ratios or percentages or mass per unit volume, are a potential cause of confusion that may contribute to dose errors. To assess doctors' understanding of what they signify, all active subscribers to doctors.net.uk, an online community exclusively for UK doctors, were invited to complete a brief web-based multiple-choice questionnaire that explored their familiarity with solutions of adrenaline (expressed as a ratio), lidocaine (expressed as a percentage) and atropine (expressed in mg per mL), and their ability to calculate the correct volume to administer in clinical scenarios relevant to all specialties.
2974 (24.6%) replied. The mean score achieved was 4.80 out of 6 (SD 1.38). Only 85.2% and 65.8% correctly identified the mass of drug in the adrenaline and lidocaine solutions, respectively, whilst 93.1% identified the correct concentration of atropine. More would have administered the correct volume of adrenaline and lidocaine in clinical scenarios (89.4% and 81.0%, respectively) but only 65.5% identified the correct volume of atropine.
The labelling of drug solutions as ratios or percentages is antiquated and confusing. Labelling should be standardized to mass per unit volume.
INTRODUCTION
The concentration of drugs in solution is expressed in many different ways, but most frequently as mass per unit volume (e.g. mg per mL, mg.mL-1), ratios (e.g. 1:1000), or percentages. As the ratio system is based on thousands whilst percentages are based on hundreds, there is potential for confusion and order-of-magnitude errors. This is compounded when mixtures such as 1% lidocaine with 1:200 000 adrenaline are used for infiltration anaesthesia.1 Heparin, lidocaine, adrenaline, and potassium chloride are most commonly associated with drug error; lidocaine causing the most fatalities;2 that these drugs are presented in solution be relevant.
We used a web-based questionnaire to study doctors' awareness of the mass of active drug in solutions that any doctor might need to give. As examples we chose adrenaline (expressed as a ratio), lidocaine (expressed as a percentage), and atropine (expressed in mg.mL-1).
METHODS
Subscribers to doctors.net.uk [http://www.doctors.net.uk], an online community exclusively for UK doctors, were invited to answer six multiple-choice questions about the three drug solutions in common clinical scenarios (Figure 1). All respondents were entered into a prize draw funded by Abbott Laboratories to encourage participation. Answers were submitted anonymously, but respondents' age, specialty and seniority were recorded. We did not try to eliminate the use of calculators or formularies. The study was conducted over three weeks in September 2003. Afterwards participants were sent the correct answers and directed towards an online continuing medical education module about drug administration.
Figure 1.
Questions and images in the online questionnaire (correct answers in bold)
| Q1: The first picture shows a vial of epinephrine (adrenaline). It contains 1 mL of 1 in 1000 epinephrine. How much epinephrine is there in the vial? | 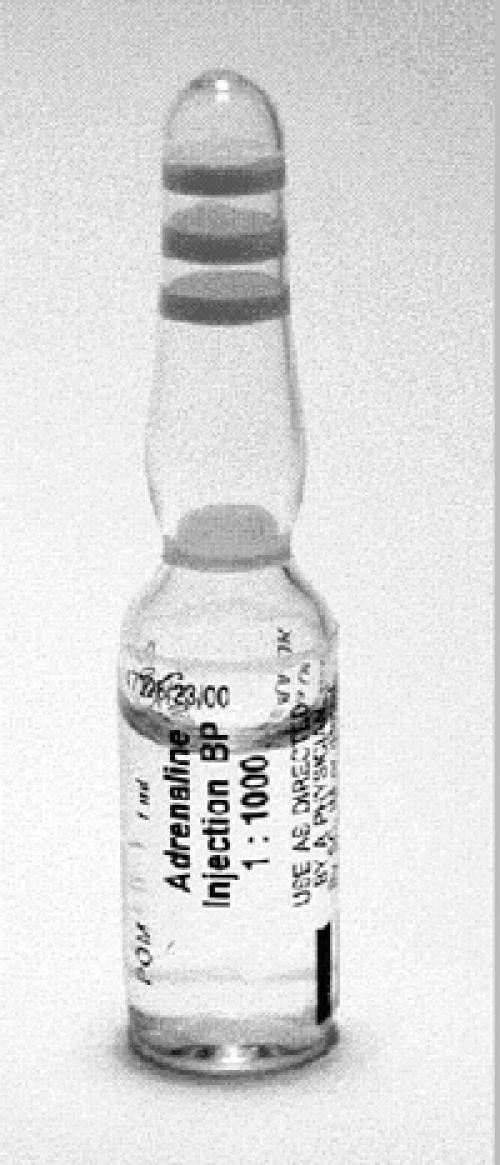 |
| A. 10 μg (microgram) | |
| B. 100 μg | |
| C. 10 mg | |
| D. 1 mg | |
| E. 1000 mg | |
| Q2: You are treating a ten-year-old whom you suspect is in anaphylactic shock. The protocol says the recommended intramuscular dose of epinephrine is 250 μg (micrograms). What volume of solution in the picture will you give? | |
| A. 2.5 mL | |
| B. 0.25 mL | |
| C. 0.025 μL | |
| D. 2.5 μL | |
| E. 25 μL | |
| Q3: The second picture shows a vial of lidocaine. It contains 10 mL of 1% w/v lidocaine. How much lidocaine is there in the vial? |  |
| A. 100 μg | |
| B. 10 g | |
| C. 10 mg | |
| D. 100 mg | |
| E. 1000 mg | |
| Q4: You find yourself treating a 60 kg patient with a laceration that you will need to suture under local anaesthetic. Given that the maximum safe dose of lidocaine is 3 mg/kg, what is the maximum volume of the solution in the picture (lidocaine 1% w/v) that can be administered safely? | |
| A. 60 mL | |
| B. 6 mL | |
| C. 180 mL | |
| D. 18 mL | |
| E. 180 μL | |
| Q5: Here is a Mini-Jet of atropine as found on emergency drugs trolleys. There is 1 mg in 10 mL. What is the concentration of the solution? | 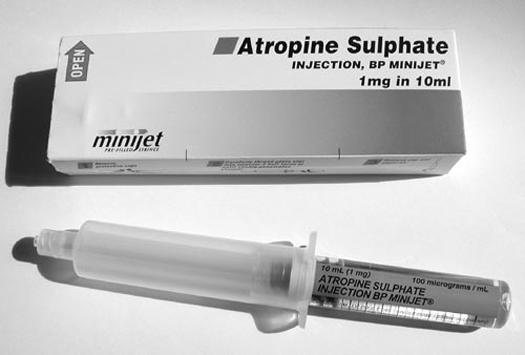 |
| 1 mg/mL | |
| 10 μg/mL | |
| C. 0.1 mg/mL | |
| D. 1 μg/mL | |
| E. 0.1 μg/mL | |
| Q6: At work, you come across a patient with an acute symptomatic bradycardia. A pulse is present and the blood pressure is 85 systolic. You estimate the weight at 60 kg. You choose to treat this with atropine 20 μg/kg. How much of this solution will you need to give? | |
| A. 12 mL | |
| B. 1.2 mL | |
| C. 6 mL | |
| D. 8.5 mL |
The extent to which the study population represented the UK medical workforce was assessed by comparison with data supplied by the UK Department of Health.3
Statistical analysis
Answers were analysed by use of the Kruskal–Wallis test; P values are corrected for ties. The Komolgorov–Smirnov test was used to compare study population with Department of Health workforce data (Statview, SAS Institute Inc, North Carolina, USA).3
RESULTS
There were 2975 participants. The web page containing the link to the study was viewed by 12 096 subscribers, so the response rate was 24.6%. The study population did not differ significantly from the medical workforce by specialty, age or seniority.
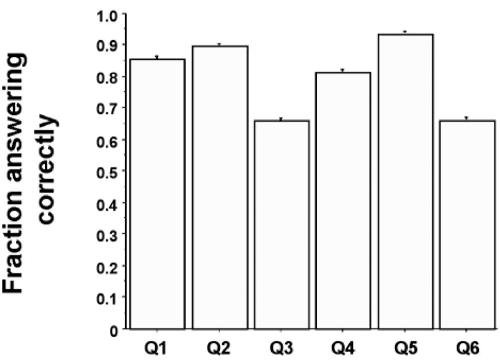
The mean score was 4.80 (SD 1.38) out of 6. Figure 2 shows the proportion of participants answering each question correctly. The question concerning the mass of adrenaline was answered correctly by 85.2% of participants (2535). Only 65.8% (1958) chose the correct amount of lidocaine in the vial. However, 93.1% (2768) identified the correct concentration of atropine in mg.mL-1 (P<0.0001 for all comparisons).
Figure 2.
Proportion of participants answering each question correctly (error bars 1 SD, n=2975)
Participants found two of the clinical scenarios easier than the raw calculations. Whilst 85.2% had correctly identified the mass of adrenaline in the vial, a larger proportion, 89.4%, would have given the correct volume in the ensuing clinical scenario. Similarly, whilst only 65.8% had correctly identified the mass of lidocaine in the vial, 81.0% identified the correct volume. Although 93.1% had calculated the concentration of the atropine solution correctly, only 65.5% would have administered the correct volume (P<0.0001 for all comparisons).
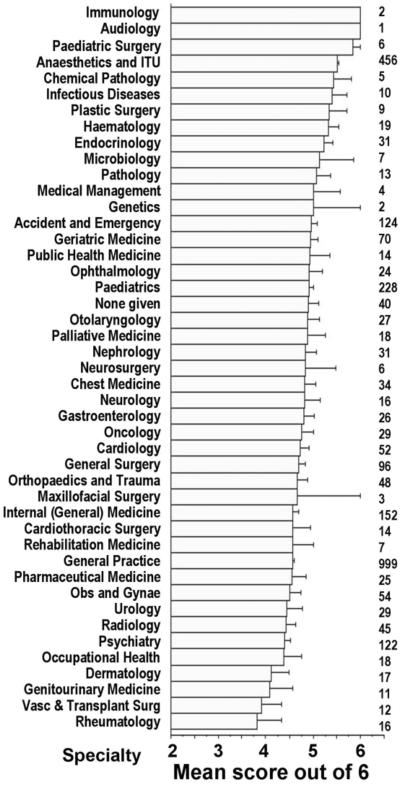
Figure 3 represents the mean score for each specialty. For some the samples were too small to be representative; of those represented by sizeable numbers, anaesthesia seemed to perform best.
Figure 3.
Mean score for each specialty (error bars 1 SD, n=2975). Numbers to right of graph indicate number of participants for that specialty. Note truncation of horizontal bars at 2
DISCUSSION
A substantial number of doctors are confused by the ways in which drug concentrations in solution are expressed, percentages being particularly troublesome, and our study shows how this confusion could translate into clinical errors. Most doctors are familiar with adrenaline and lidocaine, and fewer would have administered the wrong volume than calculated the concentration incorrectly; doctors know what is 'about the right amount', and the quantity in the container gives a clue (except, for example, in paediatrics). This approach did not work with atropine. Although the great majority calculated the concentration in milligrams per millilitre correctly, many would have still given the wrong volume—perhaps because atropine is a less familiar drug, or because a conversion from micrograms to milligrams was required.
A weakness of this survey was the low response rate, under 25%. However, the use of online questionnaires for such research is relatively new, and what constitutes an 'adequate' response is not yet agreed. Our results compare well with similarly constructed published work,4 and we are reassured by the close similarity of our participants with the UK medical population as a whole. An alternative approach would be to conduct observational studies in the workplace, but this would be time-consuming and cumbersome, and would inevitably concentrate on acute hospital specialties.
We do not believe that further research is required before action is taken. The difficulties of converting micrograms to milligrams can only be addressed by better education.5 Our key conclusion concerns the labelling of drugs. The use of ratios and percentages is a relic of the imperial system, and conversion to mass per unit volume is an extra step that increases the likelihood of error. Experience in the chemical, nuclear and aviation industries has shown how risk is lessened by reducing the number of actions required to complete a process.6-9 There would be little extra cost in labelling drug solutions solely as mass per unit volume. In the interests of patient safety, this change should be introduced without delay.
Acknowledgments
We thank the Association of Anaesthetists of Great Britain and Ireland for funding the study. Mr Andrew MacLaughlin of doctors.net.uk gave valuable technical assistance with the databases. Dr Basil Matta of Addenbrooke's Hospital, Cambridge and Dr Emma Crabtree of Abbott Laboratories, Queenborough, Kent, sought and provided funding for the prize draw. DWW is in receipt of a Raymond and Beverly Sackler studentship from the School of Clinical Medicine, University of Cambridge.
References
- 1.Lawrence C. Drug management in skin surgery. Drugs 1996;52: 805-17 [DOI] [PubMed] [Google Scholar]
- 2.Edgar TA, Lee DS, Cousins DD. Experience with a national medication error reporting program. Am J Hosp Pharm 1994;51: 1335-8 [PubMed] [Google Scholar]
- 3.NHS Hospital, Public Health Medicine and Community Health Service: Medical and Dental Workforce Census, England at 30 September 2002. [www.doh.gov.uk/public/work_workforce.htm]
- 4.Braithwaite D, Emery J, de Lusignan S, Sutton S. Using the internet to conduct surveys of health professionals: a valid alternative? Fam Pract 2003;20: 545-51 [DOI] [PubMed] [Google Scholar]
- 5.Wheeler DW, Remoundos DD, Whittlestone KD, House TP, Menon DK. Medical students are confused by different means of expressing the concentration of drugs in solution. Drug Safety (in press) [DOI] [PubMed]
- 6.Reason J. Human error: models and management. BMJ 2000;320: 768-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagan SD. The Limits of Safety—Organizations, Accidents and Nuclear Weapons. Princeton, NJ: Princeton University Press, 1993
- 8.Takano K, Reason J. Psychological biases affecting human cognitive performance in dynamic operational environments. J Nucl Sci Technol 1999;36: 1041-51 [Google Scholar]
- 9.Chiles JR. Inviting Disaster—Lessons from the Edge of Technology. New York: Harper Collins, 2001