ABSTRACT
Background
Treatment mechanisms involve the steps or processes through which an intervention unfolds and produces change in an outcome variable. Treatment mechanisms can be specific to the intervention provided (i.e. pain modulation) or shared with other treatments (i.e. reduced fear of movement). Whether specific and shared treatment mechanisms are different across interventions and whether they lead to the outcomes seen in trials is largely unknown. The management of individuals with chronic neck pain routinely include manual therapy (MT) and resistance exercise (RE), as both approaches are included in clinical practice guidelines and both yield similar outcomes.
Objectives
Our study plans to answer two research questions: 1) what are the specific mechanisms associated with MT versus interventions (and are these different), and 2) what are the shared mechanisms associated with these interventions, and do specific or shared mechanisms mediate clinical outcomes?
Methods
This study will involve a 2-group parallel (1:1) single-blinded randomized trial to compare the specific and potential shared treatment mechanisms between these two approaches. We will enroll individuals with a history of chronic neck pain and evaluate whether specific or shared mechanisms mediate clinical outcomes.
Results
We hypothesize that MT and RE approaches will both exhibit different specific treatment mechanisms, and that both approaches will exhibit shared treatment mechanisms, which will notably influence outcomes at both discharge and 6-months.
Conclusions
This study is important because it will help identify what specific or shared treatment mechanisms are associated with different interventions and, how different treatment mechanisms influence clinical outcomes.
KEYWORDS: Manual therapy, Physical therapy, Neck pain, Mechanisms
1. Introduction
Chronic neck pain is a notable personal and societal burden, affecting 30% to 50% of adults in the general population in any given year [1]. Approximately 50%–85% of individuals with acute neck pain do not experience complete resolution of symptoms, and some continue to experience chronic, impairing pain [1]. In 2016, among the 154 conditions examined, low back and neck pain were associated with the highest healthcare costs in the United States, with an estimated $134.5 billion spent [2]. In 2012, neck pain was responsible for job absences among 25.5 million Americans, who missed an average of 11.4 days of work [2]. Increasing population awareness about risk factors, preventive strategies, and targeting appropriate treatment for neck pain is warranted to reduce the future burden associated with this condition [3].
Chronic neck pain is characterized by pain, weakness, and alteration of cervical spine proprioception, which may contribute to symptoms such as pain, fatigue, stiffness, dizziness, vertigo, and benign paroxysmal vertigo [4]. Psychological distress is common in individuals with chronic neck pain, including a known higher prevalence of both anxiety and depression [5]. What remains alarming is that 50% to 75% of individuals who currently report neck pain will report additional episodes of pain (recurrence) within 1 to 5 years in the future. Physical activity appears to play only a nominal role, as participation in a general exercise program is unassociated with outcome [6]. Psychosocial and contextual factors, including psychological health, coping patterns (worrying, fear avoidance, getting angry or frustrated), and the need to socialize, are the strongest prognostic factors-all presenting negative associations with clinical outcomes [6].
Clinical practice guidelines and meta-analyses recommend manual therapy as a necessary component of a multi-modal approach for management of individuals with chronic neck pain [7–9]. Manual therapy has been described as the passive application of mechanical force to the outside of the body with therapeutic intent, often as part of comprehensive pain management care (e.g. low-back pain), rehabilitation care, or general wellness and disease prevention [10]. Exercise may take many forms; however, it is generally associated with resistance training, endurance training, flexibility, aerobic activity, or motor control activities including postural coordination [7].
Treatment mechanisms associated with interventions such as manual therapy or exercise refer to the underlying processes or methods through which a particular treatment or intervention produces its therapeutic effects. The treatment mechanisms associated with manual therapy techniques are poorly understood but are hypothesized to involve mechanical, neurophysiological, circulatory, psychological, and reflexive effects [11]. In preclinical research, manual therapy techniques exhibit both peripheral and central influences, which are theorized to modulate pain and improve range of motion quantity and quality [11]. In preclinical research, resistance exercise is associated with increases in muscle fiber size, neural adaptations in strength, and improvements in cognition tasks, which are conjectured to lead to improved strength and endurance [12]. Treatment mechanisms can be specific to an intervention (e.g. leading to a unique set of physiological/psychological processes within the body) or shared (e.g. suggesting that two seemingly different forms of treatment actually lead to the same physiological/psychological processes).
To date, the specific and shared mechanisms associated with manual therapy and exercise that lead to changes in clinical outcomes are unexplored within the context of chronic neck pain. Further study is needed to understand how treatment mechanisms influence outcomes associated with chronic neck pain to improve our management of patients with this common condition. In clinical practice, both manual therapy and exercise lead to similar overall clinical outcomes [7,13–18], despite the thought that both interventions exhibit separate specific treatment effects. This paper presents the protocol for a clinical trial that explores mechanisms outcomes comparisons between manual therapy and exercise and examines whether specific or shared mechanisms are most likely to influence and shape clinical outcomes in individuals with chronic neck pain. In this protocol, we will outline our design, our objectives, hypotheses, and suspected outcomes.
2. Materials and methods
2.1. Study design
This study will involve a 2-group parallel (1:1) single-blinded randomized trial to compare the specific and potential shared treatment mechanisms between manual therapy and resistance exercise approaches (Figure 1). Individuals with chronic neck pain will be randomized to one of the study arms and each participant will remain in their assigned treatment arm for the study duration. An advantage of a parallel group design is that it allows the running of simultaneous experiments in two or more groups in separate locations. The randomized participants in parallel groups will unlikely contaminate the other group by unplanned co-interventions or cross-overs.
Figure 1.
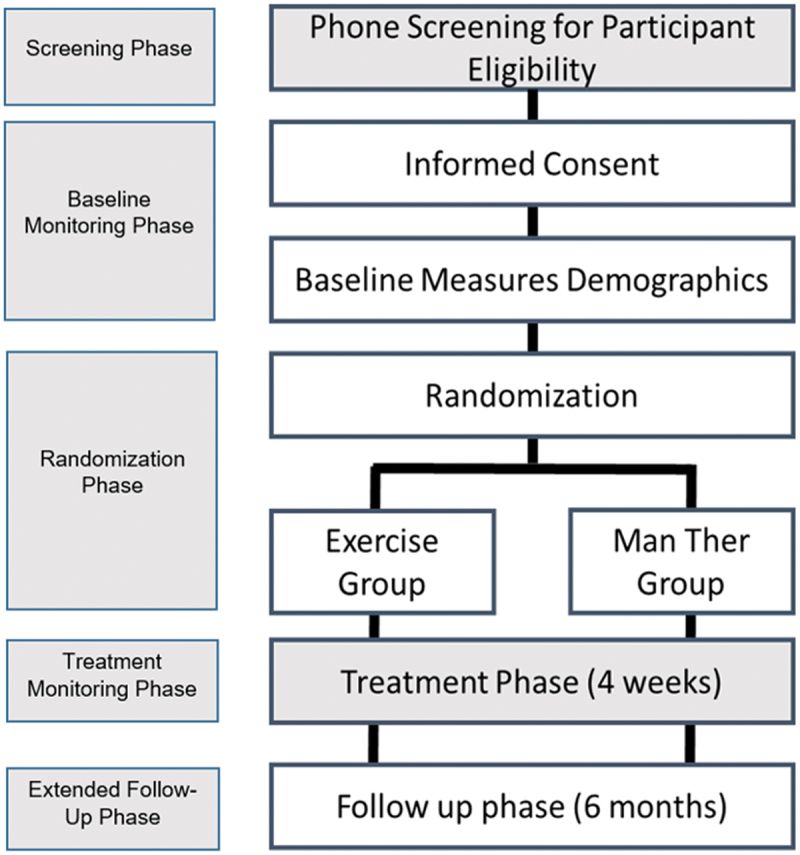
Study randomization and timelines.
2.2. Specific aims and hypotheses
Our study plans to answer two research questions: 1) what are the specific treatment mechanisms associated with manual therapy and resistance exercise interventions, and 2) what are the potential shared treatment mechanisms associated with these interventions, and subsequently, which mechanisms (specific or shared) are the most important mediators of clinical outcomes? Understanding mechanisms of treatment is a priority of the National Institutes of Health (NIH) [10], and further, has been described as an underexplored focus of translational research [19–22]. The research team plans to address this focus area by targeting two specific aims.
2.2.1. Specific aim one
Compare differences in specific treatment mechanisms between manual therapy and resistance exercise. Hypotheses: We hypothesize that manual therapy and exercise will lead to similar changes in clinical outcomes; however, manual therapy will lead to specific treatment mechanisms i.e. improved quantity of range of motion (and not endurance), whereas resistance exercise will lead to improved endurance (and not range of motion).
2.2.2. Specific aim two
Uncover potential shared treatment mechanisms between manual therapy and resistance exercise approaches (secondary aim 1) and determine the extent to which specific or shared treatment mechanisms mediate clinical outcomes at 4 weeks and 6 months (secondary aim 2). Hypotheses: We hypothesize that both treatments will result in notable shared treatment mechanisms (e.g. therapeutic alliance, pain self-efficacy), and these shared treatment mechanisms will mediate pain interference and functional outcomes of both groups at a higher rate than specific mechanisms at 4 weeks and 6 months.
2.3. Study sites and treating therapists
2.3.1. Study sites
All assessments, treatments and outcomes will be captured at one of three physical locations: 1) The Doctor of Physical Therapy Division at Duke University in Durham, North Carolina; 2) University of Colorado Physical Therapy Program in Aurora, Colorado; or 3) the Physical Therapy Department and clinical designations from SaintJoseph University in Philadelphia, Pennsylvania. The trial is undergoing approval by the three designations’ institutional review boards and is undergoing registration at ClinicalTrials.gov.
2.3.2. Treating physical therapists
Physical therapists (PTs) with a range of clinical experience will participate in the recruitment, examination, and treatment of all participants in this study. All PTs will undergo a standardized training regimen, which will include studying a manual of standard procedures with operational definitions of each examination and treatment procedure and flow of study procedures. Participating PTs will view a 60-minute training video and undergo a 1-hour hands-on training session provided by one of the investigators. Examination and intervention techniques will be reviewed and assessed for accuracy in the training sessions. Further, research fidelity checklists will be used at every visit to assure compliance with the research protocol. Treating clinicians will be provided access to online training materials containing video footage of examination and intervention procedures. Competency will be assessed using a standardized test in which all participating PTs have to score ≥ 80% to be included on the data collection team.
Training across each site will be standardized to ensure harmonization of procedures. To ensure the three sites are communicating vital study information, a formal meeting will be held every other week. A single clinical research coordinator, located at Duke University, will assure regulatory aspects of the study.
2.4. Study participants
Individuals with chronic neck pain who are 18 years of age and older and who experience ongoing neck pain of ≥ 3 on a 10-point scale for most days of the previous 3 months will be screened for eligibility. We operationally define chronic pain using the International Association of the Study of Pain (IASP) pragmatic criteria of pain lasting for 3 months or more that cannot be attributed to another diagnosis or condition [23]. Those with chronic neck pain and ≥ 3 on an 11-point scale for neck pain are necessary to measure whether differences occur from baseline to medium and long-term follow up, and are necessary for the mediation analyses goal of the study. Individuals with neck pain combined with suspected radicular symptoms, a history of neck surgery, current or suspected red flags, and those who are unable to speak or write in English will be excluded.
2.5. Recruitment, screening and enrollment
Subjects will be recruited using MyChart™ invitations (or locations’ E-chart equivalent), direct marketing efforts, and through recommendations from the partner clinics (e.g. Duke PT/OT department). Individuals who enroll and complete the study will be eligible for a $100 research stipend.
2.6. Randomization and scheduling
Randomization will be based on a single sequence of random assignments using a random number generator and concealed allocation will be performed before participant recruitment by an individual not involved in data collection. Individual, sequentially numbered index cards will be placed in sealed opaque envelopes with the random assignment prepared. After initial phone screening for eligibility, and after obtaining informed consent, randomization will occur after baseline measures are captured in the clinic. Each group will be randomized to receive either four weeks of a manual therapy-based or resistance exercise approach (a total of five visits), administered using guideline-oriented parameters.
2.7. Blinding
The design is a single-blinded parallel trial. We are unable to blind the clinicians who provide the care since the treatment approaches are unique and specific to either resistance exercise or a manual therapy-based approach.
2.8. Interventions
2.8.1. Standardized process
To reduce the risk of biasing one treatment arm, a standardized set of instructions regarding the evidence of each approach will be provided to each participant. Individuals in both the manual therapy and resistance exercise treatment arms will be educated that the treatment approach is supported by clinical practice guidelines and the approaches are designed to create changes in their report of pain, pain interference, and functional outcomes. For both groups, the supervising PTs will adopt a series of principles that emphasize a collaborative therapeutic relationship in which the autonomy of the participant is respected and the patient’s intrinsic resources for change are elicited by the therapist [24]. Both approaches will require similar amounts of in-clinic time to supervise the full set of treatments, and will have similarly weighted time dedicated to the home exercise program (HEP).
Physical interventions will be prescribed using both prescriptive and pragmatic strategies, however, a standardized approach to interventions, aligning with clinical practice guidelines, will be utilized across physical therapists. Therefore, all treatment options utilized (regardless of arm assignment) are supported by monodisciplinary clinical practice guidelines for neck pain [7]. The prescriptive aspect of each arm will allow reproduction and a firmer understanding of treatment dosage parameters. Pragmatic applications may occur in the event of patient specific variabilities in pain, change in symptoms, or capacity. Treatment will consist of five individual ‘on-site’ supervised sessions and an assigned (prescriptive) HEP that will be performed daily. All sessions will be overseen by a licensed physical therapist who is either a principal investigator or a co-investigator in the grant. In total, supervised treatment will occur over a 4-week period.
2.8.2. Manual therapy treatment (see appendix A)
All treatments associated with the manual therapy approaches are theorized to exhibit both peripheral and central influences, and reduce muscle spasm, which can lead to pain modulation and improved mobility [11]. All manual therapy techniques incorporated in this trial are considered standards of practice in the management of individuals with chronic neck pain. All passive movements will be performed with the purpose of pain provocation to determine the most likely involved segment of the neck [25]. Although dedicated passive movements have been shown to lack specificity in terms of segmental movement in the spine [25], pain provocation has been suggested to be the most reliable method when isolating the site of the disorder [25]. The type of cervical non-thrust mobilization and dosage will be based on the participant’s presentation and feedback (pragmatic), as well as the therapist’s clinical decisions, however, it will only be applied to the most symptomatic level (prescriptive) with consistent repetitions and set parameters. Any form of non-thrust mobilization will be permitted and the therapist will be able to provide the technique anywhere in range and for any duration thought to produce a positive patient response.
All of the manual therapy techniques are commonly used procedures that would qualify in the overview of a previous monodisciplinary clinical practice guideline for physical therapists [7]. Manual therapy treatments will consist of global soft tissue stretching of the upper trapezius, occipital muscles, levator scapula, and scalene muscles as the participant lies supine. Soft-tissue mobilization will be performed as needed. Non-thrust manipulation (mobilization) will consist of unilateral or central posterior-anterior accessory movements (PAIVMs) to the cervical and upper thoracic segments (in prone) at the most symptomatic levels. Passive physiological intervertebral movements of rotation will be performed in supine, as a mechanism to reduce pain and increase range of motion.
Thrust manipulation techniques likely exhibit similar clinical outcomes [7,26] and are both recommended in a summary [7]. Because there is some risk associated with cervical manipulation, and because mechanisms and outcomes appear similar between thrust manipulation and non-thrust manipulation, we elected to exclude the technique in this study [27].
Participants with chronic neck pain randomized to the manual therapy arm will be assigned a HEP twice daily that will consist of cervical rotations with belt or equivalent, side flexion with belt or equivalent, self-stretching exercises that are designed to target the upper thoracic musculature, and corner wall stretches. During each onsite session, the supervising PT will evaluate the replication capacity of the HEP by the participant and will verbally emphasize the importance of adherence to the assigned program.
2.8.3. Resistance exercise treatment (see appendix B)
All assigned resistance or endurance-based exercises are selected as they are theoretically associated with increases in muscle fiber size and neural adaptations, which can lead to improved endurance and strength [12]. All exercises incorporated in this trial are considered standards of practice in management of chronic neck pain. Appendix B outlines each of the exercises, including the instructions. All of the resistance exercises are commonly used procedures that would qualify in the overview of a previous monodisciplinary clinical practice guideline for PTs [7]. In-clinic exercises will consist of chin retractions in sitting, supine clock isometric resistance, supine anterior neck flexion exercises that target the deep neck flexors, prone neck extensor exercises (with concurrent chin retraction), and lateral neck raises (bilaterally). We will also target the mid and upper thoracic region by performing upright rows, supine chest raises that target the mid-scapular muscles and the paraspinal muscles, prone ‘I, T, and Y’ exercises, and proprioceptive neuromuscular facilitation exercises using a bar or a cane. Individuals randomized to the resistance exercise arm will be assigned a HEP twice daily that will consist of chin retractions in sitting, supine anterior neck flexion exercises, and elastic band rows that replicate the upright rows performed in the clinic. During each onsite session, the supervising physical therapist will evaluate the replication capacity of the HEP by the patient and will emphasize the importance of adherence to the assigned program.
2.8.4. Fidelity checks
Within our study, we will use a treatment fidelity checklist and will perform quarterly fidelity evaluations of our treatments to assure we do not experience therapeutic drift (Table 1). A treatment fidelity checklist is a progress-monitoring tool that we will use as a guide for planning, implementing, and sustaining best practice in the application of our designed interventions. Monthly review will be performed by a trained clinical research coordinator at one site and designees at the other sites and will be scored and shared with each provider.
Table 1.
Research fidelity checklist.
| Item | Yes | No | Measurement Criteria |
|---|---|---|---|
| 1 | Did the therapist emphasize a collaborative therapeutic relationship in which the autonomy of the patient is respected and the patient’s intrinsic resources for change are elicited? | ||
| 2 | Did the therapist emphasize the importance of attending all five of the therapy sessions? | ||
| 3 | Did the therapist explain the reasoning behind the procedures to the patient? | ||
| 4 | Did the therapist indicate that the treatment received is considered ‘best practice’ and is part of the clinical practice guidelines for treatment of chronic neck pain? | ||
| 5 | Did the therapist query the patient on their adherence and abilities with the home exercise program? | ||
| 6 | Did the therapist complete all of the prescriptively assigned treatment interventions? | ||
| 7 | Did the therapist avoid cross-contamination by restricting care that qualifies under the opposite treatment arm? |
Reviewer _____________________ Date _______________________
Patient Code _________________ Quarter/Year ________________.
The following fidelity checklist is a progress-monitoring tool that is used as a guide for planning, implementing, and sustaining best practice in the application of our designed interventions.
3. Assessment and measures
3.1. Primary outcomes
3.1.1. Specific mechanisms
Specific treatment mechanisms refer to the unique features of an intervention and are considered the primary reason the intervention is effective. Another way of understanding mechanistic research is by acknowledging that the purpose of the study is to explore ‘how does this treatment work?’ Mechanisms include specific measures of the physiological changes that occur in the body once an intervention is applied.
Methodologically, specific mechanisms are logically divided into direct and indirect mechanisms (Table 2). In almost all cases, direct mechanisms are captured in laboratory or preclinical environments. Examples include changes in cortisol, fMRI finding, neuro-immune (central/peripheral inflammatory cytokines), neurovascular, and neurotransmitters concentrations. In contrast, indirect mechanisms are proxy measurements of an underlying direct mechanistic change that occurs after an intervention. Examples include psychological (i.e. self-report of cognitions or fear) or performance-oriented (i.e. range of motion, strength changes) variables, which are captured after a specific treatment intervention. Indirect mechanisms have potential value since they are often captured in a clinical setting, are less time consuming and costly to administer, and can be captured concurrently with clinical outcomes. Clinical outcomes include self-reported patient reported outcomes, physical performance measures, cost analysis, and/or self-reported patient experiences after a dedicated treatment regimen.
Table 2.
Direct and indirect mechanisms and treatment outcomes.
| Variable | Direct Measures of Mechanisms | Indirect Measures of Mechanisms | Treatment Outcomes |
|---|---|---|---|
| Definition | Mechanisms reflect the steps or processes through which an intervention (or some independent variable) unfolds and produces the change (outcome variable). | A clinical finding that is consequence of a specific technique, which serves as a proxy measure to an underlying direct mechanism. | A patient-reported or a physical performance measured outcome that is reflective of a clinical change seen in a patient at a given time. |
| Measurements | Measures such as cortisol, fMRI, neuro-immune (inflammatory mediators), neurovascular, and neurotransmitters, etc. | Measures which may include contextual factors (e.g. placebo/nocebo effect, expectations), muscle spindle activation and/or performance, tissue extensibility, and/or selected tests for psychological variables such as fear, distress, etc. | Pen and paper test findings for pain, function, interference (tradition PROMs), and physical performance measures such as gait speed, timed up and go, etc. |
| Study Setting | Generally captured in a basic science/pre-clinical setting | Captured in a pre-clinical or clinical setting | Captured in a clinical setting |
We will capture the indirect mechanisms in both whole numbers and measures of change (Δ) in Cervical Range of motion (ROM) from baseline [28] Δ in Deep Neck Flexor Endurance from baseline [29] Δ in Cervical Extensor Endurance from baseline [30] and the Δ in Lateral Neck Musculature Endurance from baseline [31] (Table 3).
Table 3.
Key measures for specific and potential shared treatment mechanisms.
| Specific or Shared | Variable Domain | Tool and Reference |
|---|---|---|
| Specific Treatment Mechanisms-Manual therapy | Range of motion quantity Left and Right Rotation | Δ Cervical Range of motion device (CROM)54 |
| Range of motion quantity Left and Right Side-Flexion | Δ Cervical Range of motion device (CROM)54 | |
| Range of motion quantity Extension | Δ Cervical Range of motion device (CROM)54 | |
| Quantitative Sensory Testing (QST) | Δ Pain Pressure Threshold with Algometry51 | |
| Specific Treatment Mechanisms-Resistance exercise | Neck flexor endurance | Δ Deep Neck Flexor Endurance Test55 |
| Neck extensor endurance | Δ Cervical Extensor Endurance Test56 | |
| Side neck flexor endurance left | Δ Lateral Neck Flexor Endurance Test57 | |
| Side neck flexor endurance right | Δ Lateral Neck Flexor Endurance Test57 | |
| Shared Treatment Mechanisms | Therapeutic alliance | Δ Working Alliance Inventory58 |
| Fear of movement | Δ OSPRO-YF59 | |
| Pain self-efficacy | Δ UW Pain-Related Self Efficacy Scale60 | |
| Patient engagement | Δ Clinician rated Patient engagement61 |
In addition, although not a primary objective, we will capture Δ in Pain Pressure Threshold (PPT) with Algometry from baseline [32]. PPT will be tested locally and distally. Local measures include PPT kg/cm testing at the first point of pain on the left (L) and right (R) zygapophyseal joints, L and R at the midpoint between the tip of the acromion and the C5 spinous process, and L and R at the tibialis anterior (2.5 CM lateral to the tibial tubercle and 5 CM down at the belly of the tibialis anterior). Cervical ROM and PPT are hypothetical indirect mechanisms of manual therapy, whereas deep neck flexor endurance, extensor endurance, and lateral neck musculature endurance are hypothetical indirect mechanisms of resistance exercise.
3.2. Secondary outcomes
3.2.1. Shared mechanisms
Shared treatment mechanisms occur when two seemingly different treatments (e.g. manual therapy and resistance exercise) are found to exert their effects on clinical outcomes via similar or common mechanisms (e.g. therapeutic alliance) [15]. Potential shared mechanisms will include Δ in Working Alliance Inventory [15], Δ in OSPRO-YF [33], Δ in UW Pain-Related Self Efficacy Scale [34], and the Δ in Clinician Rated Patient Engagement [35] (Table 3). These indirect shared measures are designed to reflect the psychological and/or social mechanisms associated with changes in Therapeutic alliance, Fear of movement, Pain self-efficacy and Patient engagement.
3.2.2. Clinical outcomes
We will capture clinical outcomes using selected items from the Patient-Reported Outcomes Measurement Information System (PROMIS) 29.2 [36]. PROMIS measures were developed with support from the NIH [37]. There are several PROMIS instruments, each with validated individual items, which has led to several versions of PROMIS instruments. Each version is person-centered and is designed to evaluate and monitor physical, mental, and social health in adults and children, with and without chronic conditions. Our primary clinical outcome variable for our study is the PROMIS pain interference score. Our secondary clinical outcome variables include the PROMIS pain intensity item and the PROMIS physical function measures. Each of the primary and secondary ‘scales’ are part of the PROMIS 29.2 and include four items, with the exception of the PROMIS pain intensity, which has one item [36]. Data will be gathered using pen and paper scoring and transferred to the RedCAP database where raw data will be converted. Raw concept index score conversion tables are available on the HealthMeasures website and are unique for each domain [36]. For PROMIS measures, higher scores are more reflective of the concept being measured (e.g. sleep, anxiety) in which 50 is the mean of a relevant reference population and 10 is the standard deviation (SD) of that population. Consequently, scores of 40 or 60 are both 1 standard deviation below and above the mean, which could be a desirable or undesirable outcome, depending on how the item is coded [36].
3.3. Timeline of capture
Specific mechanisms (i.e. endurance, range of motion, etc.) will be captured at baseline, 2 weeks, and 3 weeks. Shared mechanisms (i.e. Therapeutic alliance, Fear of movement, Pain self-efficacy and Patient engagement) will also be captured at baseline, 2 weeks, and 3 weeks as well. These findings will be used in our comparative analyses between the manual therapy and exercise groups. We will capture baseline, 4 weeks, and 6-month values of PROMIS pain intensity, PROMIS pain interference and PROMIS physical function measures [35] (Figure 2). These clinical outcomes will be used to measure the mediating effect of specific and shared mechanisms on 4-week and 6-months outcomes for pain interference and function.
Figure 2.
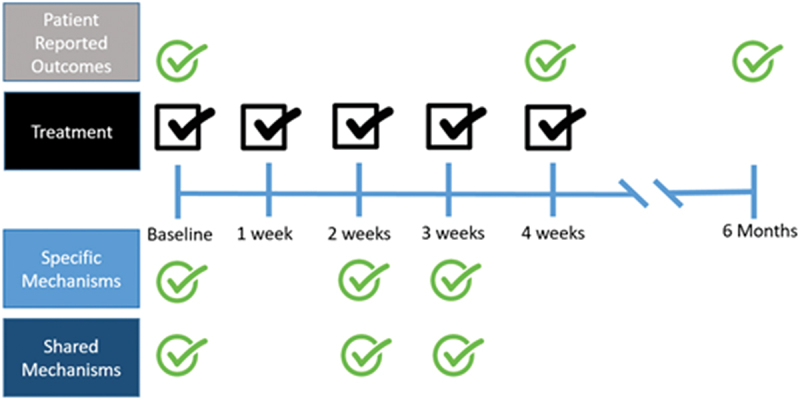
Timeline for Data Capture.
4. Data and safety monitoring
Study data are always accessible for the clinical research coordinator (CRC) to review. A clinical research coordinator will assure regulatory elements of the study are followed as well. The CRC and the lead authors will ensure that all protocol deviations, adverse events (AEs), and severe adverse events (SAEs) are reported to the funding source and the corresponding institutional review boards according to the applicable regulatory requirements.
For this study, AEs are defined as any unfavorable and unintended sign, symptom or disease temporally associated with the use of medical treatment or procedure, regardless of whether it is considered related to the medical treatment or procedure. SAEs are defined as any AE that results in: death, is life-threatening, results in inpatient hospitalization or prolongation of existing hospitalization, or persistent or significant disability/incapacity [37].
AEs will be graded as: Mild-An experience that is transient, and requires no special treatment or intervention; Moderate-An experience that is alleviated with simple therapeutic treatments; or Severe-An experience that requires formal intervention or referral to a medical doctor and interrupts usual daily activities [37]. We will attribute the AE as: ‘not related’ if it is clearly not related to the study procedures (i.e. another cause of the event is most plausible and/or a clinically plausible temporal sequence is inconsistent with the onset of the event); ‘possibly related’ if it follows a reasonable temporal sequence from the initiation of the study procedures, but that could readily have been produced by several other factors; or ‘related’ if the AE is clearly related to the study procedures [38]. After discharge, AEs will be assessed formally at six months, but the patient will have the opportunity to communicate within the discharge to six-month follow up by text or e-mail.
5. Management of missing values
We will endeavor to capture data accordingly at all dedicated time intervals. We powered the study by anticipating 30% dropout before the six-month completion of follow-up. Additionally, we expect some incidental missingness at various follow-up points in the study, due to a patient’s inability to attend an interim visit (i.e. 2-week or 3-week). For mediation analyses, we will use listwise deletion, which will restrict our focus to only those patients who completed the study at all follow-up points. The use of linear mixed-effects models permits some tolerance of missingness at interim follow-ups, assuming data are missing at random (MAR). However, we intend to acknowledge the use of listwise as a limitation; data may be missing not at random (MNAR), which would undermine our approach and bias the results of our analysis [39].
6. Sample size estimation and analysis
6.1. Sample size
Sample size estimation: The study is powered (estimated sample size) on its primary aim, which compares differences in eight specific mechanisms between the manual therapy and resistance exercise approaches. We assume a summative medium effect size in differences in the eight specific mechanisms, and at 80% power and a p value of 0.05, two-tails, three specific measurement points, two groups, and a correlation of 0.5 among measures, with the use of a linear mixed effects model for testing between groups differences, we would require a sample size of 86 to meet statistical significance. We project a potential loss of several subjects across the three sites at the six months data collection point and will oversample by 40 to account for this loss. Our final sample size that we will target will be 126 subjects.
6.2. Statistical analysis plan
6.2.1. Statistical analyses for specific aim 1 and 2
For our statistical analyses, we will report descriptive characteristics between the two groups. For both the primary and secondary aims involving continuous measures (e.g. specific mechanisms and shared mechanisms), we will explore differences in specific and potential shared treatment mechanisms between groups using repeated measures, linear mixed effects modeling. Time points will include baseline measures, two weeks, and three weeks. Baseline measures will serve as covariate controls. Advantages to using linear mixed effects in our analysis are that observations within a participant may be correlated and that in addition to estimation of the model parameters, between- and within-subject variability may be estimated [40]. We will include the three data collection sites as potential random. Differences across PPT values will also be measured but this is not a primary objective. Effect sizes for the linear mixed effects models will be conducted using a Cohen’s f2 effect size measure, which can be interpreted as small, medium, and large for values of 0.02, 0.15, and ≥ 0.35, respectively [41]. A p value of < 0.05 will be set for statistical significance testing.
6.2.2. Statistical analysis for specific aim 2
This study endeavors to following the AGReMA reporting recommendations for mediation analyses [42]. The mediating role of specific and shared mechanisms will be explored using a parallel mediation analysis to evaluate the influences of specific and shared treatment mechanisms. In this type of mediation analysis, there are multiple mediators that operate in parallel, meaning they all mediate the relationship between the independent and dependent variables independently [43,44]. In our model, which is represented by a directed acyclic graph (Figure 3), our independent variable (X) is baseline pain intensity and our dependent variables (Y) will be PROMIS pain interference (primary), PROMIS function (secondary), and PROMIS pain intensity (secondary). Our direct effects (C1 and C2) will be evaluated in distinct models and will include a) sum of changes in endurance from baseline and b) sum of changes in ROM from baseline. Our four indirect mediation variables (M1–4) will include the Δ in Working Alliance Inventory, Δ in OSPRO-YF, Δ in UW Pain-Related Self Efficacy Scale, and the Δ in Clinician Rated Patient Engagement from baseline. We plan to run mediation analyses at both 4 weeks and 6-months and will measure the indirect effects of at both 2 weeks and 3 weeks. We plan to collapse treatment groups in the mediation analyses; however, if we identify group differences in our primary aim, we acknowledge the potential for inclusion of a group variable as a covariate.
Figure 3.
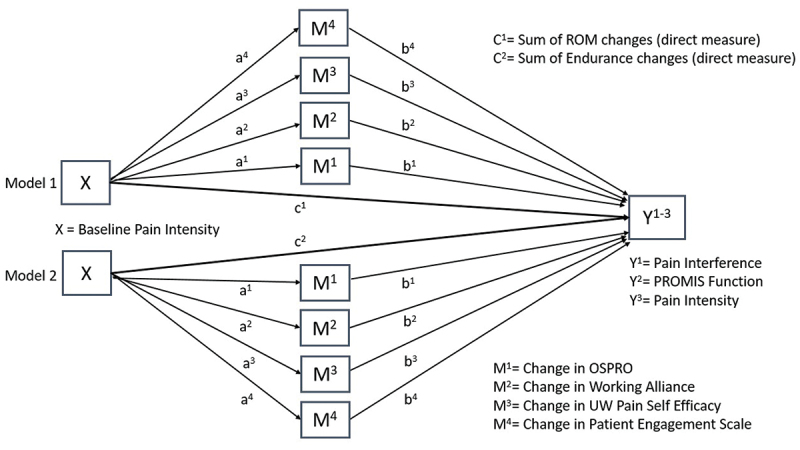
The Directed Acyclic Graph for the Mediation Analysis.
Mediation will be confirmed if the 95% CI for the indirect effect does not include zero, and complete mediation will be confirmed if the 95% CI for the direct effect includes zero [43]. We realize that most mediation analyses involve larger sample sizes (although the exact sample size is controversial) thus we are at risk of being underpowered for our second aim [44]. Nonetheless, we feel the findings may be useful when powering a future study involving mediation of specific and potential shared effects.
7. Conclusion
In this proposal, we outline the protocol for measurement of specific and shared indirect mechanisms for manual therapy and exercise. The results of the study are important for several reasons. First, it will l identify if specific indirect mechanisms are different between manual therapy and exercise and it also will identify which form of mechanism (specific or shared) is most responsible for the clinical outcomes seen after an intervention for chronic neck pain. These insights could lead to new conceptual models and enhance our theoretical understanding of how manual therapy and exercise work to improve clinical outcomes. Second, the findings of this study could inform future research studies. These might include studies designed to examine the specific components of manual therapy protocols (or exercise protocols) that produce the largest improvements in key treatment mechanisms. Findings will inform clinicians about treatment mechanisms and better enable them to tailor different treatment protocols (e.g. manual therapy and exercise) to optimize change in relevant mechanisms. This approach fits well with the clinical goal of personalizing treatment to meet the needs of each patient.
Future studies could also examine other indicators of direct mechanisms associated with manual therapy and exercise, yielding more granular results than indirect measures of mechanisms. Finally, this study has implications for public policy. For example, the findings could inform clinical practice guidelines and health policy recommendations aimed at treating and preventing disability in persons with neck pain by potentially increasing our options for care.
Supplementary Material
Biographies
Chad E. Cook PT, PhD, FAPTA is a Professor in the Department of Orthopaedic Surgery in Duke University. He also has appointments in Population Health Sciences and the DUke Clinical Research Instutute. Dr Cook is an NIH and DoD funded researcher and is the Director of the Duke Center for Excellence in Manual and Manipulative Therapy.
Bryan O’Halloran PT, DPT, DSc has been a clinician practice owner and clinician researcher prior to joining the Doctor of Physical Therapy program at St Joseph’s University in Philadelpia as an Asistant Professor. He completed his initial degree in Physiotherapy at Latrobe University in Melbourne, his DPT from MGH-IHP in Boston and his DSc from Andrews University in Michigan. He is a board certified orthopaedic and sports specialist and a Fellow of the American Academy of Orthopaedic Manual Physical Therapists.
Amy McDevitt PT, DPT, PhD is an Associate Professor in the Doctor of Physical Therapy program at the University of Colorado School of Medicine. She completed her Bachelor of Science in Biology from Fairfield University, her Doctor of Physical Therapy from the University of St Augustine in Florida, and her Doctor of Philosophy in Physiotherapy from the University of Newcastle in Callahan, Australia. She is a board-certified orthopaedic physical therapist and Fellow of the American Academy of Orthopaedic Manual Physical Therapists with over 20 years of clinical experience in the managment of acute and chronic musculoskeletal conditions.
Francis J. Keefe is a Professor in the Department of Psychiatry and Behavioural Sciences at Duke University. He also holds appointments in Psychology and Neuroscience, Anesthesiology, Medicine and is a Member of the Duke Cancer Institute. He is an NIH funded researcher and has published over 500 peer reviewed papers.
Funding Statement
The study is funded by the Foundation for Physical Therapy, Paris/Patla Grant.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10669817.2023.2267391.
References
- [1].Goode AP, Freburger J, Carey T.. Prevalence, practice patterns, and evidence for chronic neck pain. Arthritis Care Res (Hoboken). 2010. Nov;62(11):1594–1601. doi: 10.1002/acr.20270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kazeminasab S, Nejadghaderi SA, Amiri P, et al. Neck pain: global epidemiology, trends and risk factors. BMC Musculoskelet Disord. 2022. Jan 3;23(1):26. doi: 10.1186/s12891-021-04957-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Safiri S, Kolahi A, Hoy D, et al. Global, regional, and national burden of neck pain in the general population, 1990-2017: systematic analysis of the global burden of disease study 2017. BMJ. 2020;368:m791. doi: 10.1136/bmj.m791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bernal-Utrera C, González-Gerez JJ, Saavedra-Hernandez M, et al. Manual therapy versus therapeutic exercise in non-specific chronic neck pain: study protocol for a randomized controlled trial. Trials. 2019. Aug 9;20(1):487. doi: 10.1186/s13063-019-3598-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Elbinoune I, Amine B, Shyen S, et al. Chronic neck pain and anxiety-depression: prevalence and associated risk factors. Pan Afr Med J. 2016. May 27;24:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carroll LJ, Hogg-Johnson S, van der Velde G, et al. Course and prognostic factors for neck pain in the general population: results of the bone and joint Decade 2000 –2010 task force on neck pain and its associated disorders. Eur Spine J. 2008. Apr;17(Suppl 1):75–82. doi: 10.1007/s00586-008-0627-8 [DOI] [PubMed] [Google Scholar]
- [7].Blanpied PR, Gross AR, Elliott JM, et al. Neck pain: revision 2017. J Orthop Sports Phys Ther. 2017. Jul;47(7):A1–A83. doi: 10.2519/jospt.2017.0302 [DOI] [PubMed] [Google Scholar]
- [8].Castellini G, Pillastrini P, Vanti C, et al. Some conservative interventions are more effective than others for people with chronic non-specific neck pain: a systematic review and network meta-analysis. J Physiother. 2022. Oct;68(4):244–254. doi: 10.1016/j.jphys.2022.09.007 [DOI] [PubMed] [Google Scholar]
- [9].Wang SQ, Jiang AY, Gao Q. Effect of manual soft tissue therapy on the pain in patients with chronic neck pain: a systematic review and meta-analysis. Complement Ther Clin Pract. 2022. Nov;49:101619. doi: 10.1016/j.ctcp.2022.101619 [DOI] [PubMed] [Google Scholar]
- [10].National Institutes Of Health. Neural Mechanisms Of Force-Based Manipulations: High Priority Research Networks (U24 Clinical Trial Optional). RFA-AT-21-006. [cited 2022 7 January]. Available from: https://grants.nih.gov/grants/guide/rfa-files/RFA-AT-21-006.html
- [11].Bialosky JE, Beneciuk JM, Bishop MD, et al. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther. 2018;48(1):8–18. doi: 10.2519/jospt.2018.7476 [DOI] [PubMed] [Google Scholar]
- [12].Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36(2):133–149. doi: 10.2165/00007256-200636020-00004 [DOI] [PubMed] [Google Scholar]
- [13].Chmielewski TL, George SZ, Tillman SM, et al. Low- versus high-intensity plyometric exercise during rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(3):609–617. doi: 10.1177/0363546515620583 [DOI] [PubMed] [Google Scholar]
- [14].Cook CE, George SZ, Keefe F. Different interventions, same outcomes? Here are four good reasons. Br J Sports Med. 2018;52(15):951–952. doi: 10.1136/bjsports-2017-098978 [DOI] [PubMed] [Google Scholar]
- [15].Day MA, Ehde DM, Burns J, et al. A randomized trial to examine the mechanisms of cognitive, behavioral and mindfulness-based psychosocial treatments for chronic pain: study protocol. Contemp Clin Trials. 2020;93:106000. doi: 10.1016/j.cct.2020.106000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Di Blasi Z, Harkness E, Ernst E, et al. Influence of context effects on health outcomes: a systematic review. Lancet. 2001;357(9258):757–762. doi: 10.1016/S0140-6736(00)04169-6 [DOI] [PubMed] [Google Scholar]
- [17].O’Keeffe M, Purtill H, Kennedy N, et al. Comparative effectiveness of conservative interventions for nonspecific chronic spinal pain: physical, behavioral/psychologically informed, or combined? A systematic review and meta-analysis. J Pain. 2016. Jul;17(7):755–774. doi: 10.1016/j.jpain.2016.01.473 [DOI] [PubMed] [Google Scholar]
- [18].Veehof MM, Oskam MJ, Schreurs KMG, et al. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533–542. doi: 10.1016/j.pain.2010.11.002 [DOI] [PubMed] [Google Scholar]
- [19].Voogt L, de Vries J, Meeus M, et al. Analgesic effects of manual therapy in patients with musculoskeletal pain: a systematic review. Man Ther. 2015. Apr;20(2):250–256. doi: 10.1016/j.math.2014.09.001 [DOI] [PubMed] [Google Scholar]
- [20].Meyer AL, Amorim MA, Schubert M, et al. Unravelling functional neurology: does spinal manipulation have an effect on the brain? - a systematic literature review. Chiropr Man Therap. 2019. Oct 2;27(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reed WR, Little JW, Lima CR, et al. Spinal mobilization prevents NGF-Induced trunk mechanical hyperalgesia and attenuates expression of CGRP. Front Neurosci. 2020. Apr 30;14:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sabri M, Gnadt J. Neurocircuitry of force-based manipulations workshop. 2021. [2019 September 17-18 meeting report]. https://files.nccih.nih.gov/force-workshop-summary-110920-508-updated.pdf
- [23].Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the international association for the study of pain, subcommittee on taxonomy. Pain Suppl. 1986;3:S1–226. [PubMed] [Google Scholar]
- [24].Arrieta Valero I. Autonomies in interaction: dimensions of patient autonomy and non-adherence to treatment. Frontiers In Psychology. 2019. Aug 14;10:1857. doi: 10.3389/fpsyg.2019.01857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Griswold D, Learman K, O’Halloran B, et al. A preliminary study comparing the use of cervical/upper thoracic mobilization and manipulation for individuals with mechanical neck pain. J Man Manip Ther. 2015. May;23(2):75–83. doi: 10.1179/2042618614Y.0000000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Minnucci S, Innocenti T, Salvioli S, et al. Benefits and harms of spinal manipulative therapy for treating recent and persistent nonspecific neck pain: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2023. Aug;10(9):1–53. doi: 10.2519/jospt.2023.11708 [DOI] [PubMed] [Google Scholar]
- [27].Chaibi A, Russell MB. A risk-benefit assessment strategy to exclude cervical artery dissection in spinal manual-therapy: a comprehensive review. Ann Med. 2019. Mar;51(2):118–127. doi: 10.1080/07853890.2019.1590627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gugliotti M, Tau J, Gallo K, et al. Between-week reliability of the cervical range of motion (CROM) device for upper cervical rotation. J Man Manip Ther. 2021. Jun;29(3):176–180. doi: 10.1080/10669817.2020.1805691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Domenech MA, Sizer PS, Dedrick GS, et al. The deep neck flexor endurance test: normative data scores in healthy adults. PM R. 2011. Feb;3(2):105–110. doi: 10.1016/j.pmrj.2010.10.023 [DOI] [PubMed] [Google Scholar]
- [30].Sebastian D, Chovvath R, Malladi R. Cervical extensor endurance test: a reliability study. J Bodyw Mov Ther. 2015. Apr;19(2):213–216. doi: 10.1016/j.jbmt.2014.04.014 [DOI] [PubMed] [Google Scholar]
- [31].Swanson BT, Bromaghin HM, Bubacy N, et al. The lateral neck flexor endurance test: normative values in the young adult population. J Bodyw Mov Ther. 2020. Jul;24(3):242–245. doi: 10.1016/j.jbmt.2020.03.002 [DOI] [PubMed] [Google Scholar]
- [32].Walton DM, Levesque L, Payne M, et al. Clinical pressure pain threshold testing in neck pain: comparing protocols, responsiveness, and association with psychological variables. Phys Ther. 2014. Jun;94(6):827–837. doi: 10.2522/ptj.20130369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Butera KA, George SZ, Lentz TA. Psychometric evaluation of the optimal screening for prediction of referral and outcome yellow flag (OSPRO-YF) tool: factor structure, reliability, and validity. J Pain. 2020. May-Jun;21(5–6):557–569. doi: 10.1016/j.jpain.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miles CL, Pincus T, Carnes D, et al. Measuring pain self-efficacy. Clin J Pain. 2011. Jun;27(5):461–470. doi: 10.1097/AJP.0b013e318208c8a2 [DOI] [PubMed] [Google Scholar]
- [35].Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89–99. doi: 10.1037/a0031242 [DOI] [PubMed] [Google Scholar]
- [36].Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853. doi: 10.1016/j.jval.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cook CE, Rethorn ZD, Chiarotto A, et al. Construct validity and item response theory analysis of the PROMIS-29 v2.0 in recipients of lumbar spine surgery. Spine (Phila Pa 1976). 2021. Dec 15;46(24):1721–1728. [DOI] [PubMed] [Google Scholar]
- [38].US Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE). [cited 2023 June 9]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf
- [39].Heymans MW, Twisk JWR. Handling missing data in clinical research. J Clin Epidemiol. 2022. Nov;151:185–188. doi: 10.1016/j.jclinepi.2022.08.016 [DOI] [PubMed] [Google Scholar]
- [40].Jiang J, Nguyen T. Linear and generalized linear mixed models and their applications. New York: Springer; 2022. [Google Scholar]
- [41].Selya AS, Rose JS, Dierker LC, et al. A practical guide to calculating Cohen’s f(2), a measure of local effect size, from PROC MIXED. Frontiers In Psychology. 2012. Apr 17;3:111. doi: 10.3389/fpsyg.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cashin AG, McAuley JH, Lee H. Advancing the reporting of mechanisms in implementation science: a guideline for reporting mediation analyses (AGReMA). Implement Res Pract. 2022. Jun 6;3:26334895221105568. doi: 10.1177/26334895221105568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- [44].Lentz TA, Rhon DA, George SZ. Predicting opioid use, increased health care utilization and high costs for musculoskeletal pain: what factors mediate pain intensity and disability? J Pain. 2020;21(1–2):135–145. doi: 10.1016/j.jpain.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


