Abstract
Background:
While much research has been done to identify individual workplace lung carcinogens, little is known about joint effects on risk when workers are exposed to multiple agents.
Objectives:
We investigated the pairwise joint effects of occupational exposures to asbestos, respirable crystalline silica, metals (i.e., nickel, chromium-VI), and polycyclic aromatic hydrocarbons (PAH) on lung cancer risk, overall and by major histologic subtype, while accounting for cigarette smoking.
Methods:
In the international 14-center SYNERGY project, occupational exposures were assigned to 16,901 lung cancer cases and 20,965 control subjects using a quantitative job-exposure matrix (SYN-JEM). Odds ratios (ORs) and 95% confidence intervals (CIs) were computed for ever vs. never exposure using logistic regression models stratified by sex and adjusted for study center, age, and smoking habits. Joint effects among pairs of agents were assessed on multiplicative and additive scales, the latter by calculating the relative excess risk due to interaction (RERI).
Results:
All pairwise joint effects of lung carcinogens in men were associated with an increased risk of lung cancer. However, asbestos/metals and metals/PAH resulted in less than additive effects; while the chromium-VI/silica pair showed marginally synergistic effect in relation to adenocarcinoma (RERI: 0.24; CI: 0.02, 0.46; = 0.05). In women, several pairwise joint effects were observed for small cell lung cancer including exposure to PAH/silica (OR = 5.12; CI: 1.77, 8.48), and to asbestos/silica (OR = 4.32; CI: 1.35, 7.29), where exposure to PAH/silica resulted in a synergistic effect (RERI: 3.45; CI: 0.10, 6.8).
Discussion:
Small or no deviation from additive or multiplicative effects was observed, but co-exposure to the selected lung carcinogens resulted generally in higher risk than exposure to individual agents, highlighting the importance to reduce and control exposure to carcinogens in workplaces and the general environment. https://doi.org/10.1289/EHP13380
Introduction
Occupational carcinogens represent a significant threat on worker’s health, and exposed workers may be (simultaneously) exposed to more than one carcinogen. The European CAREX project estimated that 23% of those working in the European Union (EU) in the 1990s were exposed to at least one of the occupational exposures classified by the International Agency for Research on Cancer (IARC) Monographs program as carcinogenic to humans (group 1), probably carcinogenic (group 2A), and possibly carcinogenic to humans (group 2B) as of February 1995, and the exposed workers were exposed on average to 1.3 of these agents.1 More recently (2011–2012) it has been estimated that 38% of workers in the Australian Work Exposures Study (AWES) were exposed to at least one carcinogen in their current job, 31% were exposed to multiple carcinogens, and 15% of men and 3% of women were exposed to more than five carcinogens. The average number of carcinogens among exposed workers were 3.7 in men and 2.7 in women.2 Yet, little is known about potential joint effects between occupational exposures on health.
There has been a long-standing interest and controversy regarding the effect on lung cancer risk of joint exposure to asbestos and tobacco smoking, and in particular whether the joint effect is closer to additive or multiplicative.3–8 In the SYNERGY project—a pooled analysis of lung cancer case-control studies from Europe and Canada on the joint effects of occupational carcinogens in the risk of lung cancer—we have in addition shown joint effects between tobacco smoking and occupational exposure to respirable crystalline silica,9 polycyclic aromatic hydrocarbons (PAH),10 diesel engine exhaust,11 hexavalent chromium (chromium-VI) and/or nickel,12 and between smoking and working as bricklayer,13 welder,14 or painter15 on the risk of lung cancer. In most countries, there is no official compensation scheme for occupational diseases due to multiple exposures, yet. However, in Germany, lung cancer can be recognized as an occupational disease following combined exposure to PAH and asbestos, according to the German occupational disease scheme BK 4114.16
Individual epidemiological studies are usually unable to investigate joint effects, for one or more of the following reasons: too low prevalence of exposure to combinations of agents, and thus low power, and absence of exposure data for multiple agents. The SYNERGY project was established to overcome this limitation of single studies.
The aim of the present analysis was to investigate the joint effects of occupational exposure to asbestos, respirable crystalline silica, nickel, chromium-VI, and PAH on lung cancer risk, in a pairwise fashion, while accounting for cigarette smoking using data from the SYNERGY lung cancer case-control studies. We formally assessed the pairwise interactions both on an additive and on a multiplicative scale for lung cancer overall and by major histological subtypes, as well as by smoking status.
Methods
The SYNERGY project was established in 2007 to study joint effects of occupational carcinogens and smoking in the development of lung cancer and has been described previously.17–19 The original studies obtained ethical approvals from their respective countries (Canada, Czech Republic, France, Germany, Hungary, Italy, the Netherlands, Poland, Romania, Russia, Slovakia, Spain, Sweden, and United Kingdom) at the time of their study, and the SYNERGY project (pooled analyses) received ethical clearance from the International Agency for Research on Cancer (IARC) Ethics Committee in 2007 (IEC 07-05). Five occupational agents were a priori considered in the current study: asbestos [abbreviation, unit: asbestos, fibers (f)/mL-years], respirable crystalline silica (silica, ), hexavalent chromium (chromium-VI, ), nickel (nickel, ), and PAHs by its proxy benzo[a]pyrene (BaP, ). The main reasons for selecting these exposures were their group 1 classification by the IARC Monographs program, the relatively high prevalence of joint exposure in the SYNERGY study population, the availability of quantitative exposure data, relevance both for prevention and for compensation, and the possibility to disentangle correlated occupational exposures from exposures in the general population. More information about the SYNERGY project is available online at https://synergy.iarc.who.int/.
Study Population
Case-control studies on lung cancer were required to have data on lifetime tobacco smoking and occupational history to be included in the SYNERGY project. Therefore, study participants with incomplete information on covariates (804 cases, 848 controls) were excluded in the final dataset. In the present analysis, data of the SYNERGY project were pooled from 14 population- or hospital-based case–control studies of lung cancer from Europe and Canada, conducted between 1985 and 2010. Table 1 shows numbers of study participants, response-rates among cases and controls, calendar years when the data was collected, the range of calendar years when the study participants worked, the source of control subjects, and who was interviewed (mostly face-to-face) by study. Moreover, the LUCAS and LUCA studies were restricted to men and the PARIS study included only regular smokers. All studies, except MORGEN, provided data on lifetime smoking habits and self-reported complete occupational history until diagnosis or recruitment. MORGEN is a case–control study nested in the European Prospective Investigation into Cancer and Nutrition (EPIC) study in the Netherlands, where study participants completed a questionnaire at recruitment, i.e., on average 5.3 years before diagnosis or end of follow-up.
Table 1.
Description of the case-control studies on lung cancer included in these analyses in the SYNERGY project.
| Study | Country | Data collection | Cases | Controls | Occupational history range | Source of controlsa | Intervieweeb | ||
|---|---|---|---|---|---|---|---|---|---|
| Response rate (%) | Response rate (%) | ||||||||
| AUT-Munich | Germany | 1990–1995 | 3,180 | 77 | 3,249 | 41 | 1931–1995 | P | S |
| CAPUA | Spain | 2000–2010 | 559 | 91 | 512 | 96 | 1926–2010 | H | S |
| EAGLE | Italy | 2002–2005 | 1,908 | 87 | 2,065 | 72 | 1931–2005 | P | S |
| HdA | Germany | 1988–1993 | 1,004 | 69 | 1,002 | 68 | 1926–1993 | P | S |
| ICARE | France | 2001–2007 | 2,739 | 87 | 3,449 | 81 | 1937–2007 | P | S & NOK |
| INCO | Czech Republic | 1999–2002 | 304 | 94 | 452 | 80 | 1932–2002 | H | S |
| INCO | Hungary | 1998–2001 | 391 | 90 | 305 | 100 | 1931–1999 | H | S |
| INCO | Poland | 1998–2002 | 793 | 88 | 835 | 88 | 1933–2002 | P & H | S |
| INCO | Romania | 1998–2002 | 179 | 90 | 225 | 99 | 1936–2002 | H | S |
| INCO | Russia | 1998–2001 | 599 | 96 | 580 | 90 | 1931–2001 | H | S |
| INCO | Slovakia | 1998–2002 | 345 | 90 | 285 | 84 | 1935–2002 | H | S |
| INCO/LLP | United Kingdom | 1998–2005 | 441 | 78 | 916 | 84 | 1932–2005 | P | S |
| LUCA | France | 1989–1992 | 280 | 98 | 282 | 98 | 1927–1992 | H | S |
| LUCAS | Sweden | 1985–1990 | 1,014 | 87 | 2,307 | 85 | 1923–1990 | P | S & NOK |
| MONTREAL | Canada | 1996–2002 | 1,176 | 85 | 1,505 | 69 | 1934–2002 | P | S & NOK |
| MORGENc | The Netherlands | 1993–1997 | 43 | N/A | 115 | N/A | 1945–1997 | P | S |
| PARIS | France | 1988–1992 | 169 | 95 | 227 | 95 | 1923–1992 | H | S |
| ROME | Italy | 1993–1996 | 326 | 74 | 321 | 63 | 1926–1996 | H | S |
| TORONTO | Canada | 1997–2002 | 365 | 62 | 844 | 71 | 1923–2002 | P & H | S |
| TURIN/VENETO | Italy | 1990–1994 | 1,086 | 79 | 1,489 | 80 | 1922–1994 | P | S |
| Overall | 14 countries | 1985–2010 | 16,901 | 83% | 20,965 | 70% | 1922–2010 | ||
Note: AUT-Munich, Arbeit und Technik–Munich; CAPUA, Cancer de Pulmon en Asturias; EAGLE, Environment and Genetics in Lung Cancer Etiology; HdA, Humanisierung des Arbeitslebens; ICARE, Investigations Cancers Respiratoires et Environnement; INCO, International Agency for Research on Cancer Multicenter Case-Control Study of Occupation, Environment, and Lung Cancer in Central and Eastern Europe; LLP, Liverpool Lung Project; LUCA, study of lung cancer in France; LUCAS, Lungcancer i Stockholm; MONTREAL, Montreal case-control study of environmental causes of lung cancer; MORGEN, Monitoring van Risicofactoren en Gezondheid in the Netherlands; N/A, not applicable; PARIS, lung cancer study in Paris; ROME, Rome lung cancer case-control study; TORONTO, Toronto lung cancer (case-control) study; TURIN/VENETO, population-based case-control study of lung cancer in the city of Turin and in the Eastern part of Veneto Region.
aP, population; H, hospital.
S, subject; NOK, next-of-kin.
Nested case-control study.
Occupational Exposure Assessment
Occupational data from the original studies were coded or recoded from national classifications into the International Standard Classification of Occupations (ISCO-68). Personal quantitative exposure measurements, i.e., samples collected with a personal portable pump in the breathing zone of a worker, of the five agents from 18 European countries and Canada were assembled in an exposure database called ExpoSYN.19 Empirical linear models were developed using the personal occupational exposure measurements, as well as auxiliary information including job (industry), year of sampling, region, an a priori exposure rating of each job (none, low, and high exposed), sampling and analytical methods, and sampling duration. The model outcomes were used to create SYN-JEM with a quantitative estimate of the level of exposure by job, year, and region.20 The quantitative job-exposure matrix for general population studies (SYN-JEM), linkable to ISCO-68, was used to assign occupational exposures to workers’ lifelong occupational histories. The minimum duration of employment to be considered was 6 or 12 months.
Statistical Analyses
Ever vs. never exposure metrics were obtained for the selected occupational lung carcinogens. The prevalence of both individual occupational exposure and of exposure to multiple carcinogens were assessed separately by sex. Multiple exposure was restricted to two carcinogens (or hereinafter called co-exposures) since the combinations of exposure to more than two carcinogens were very numerous and involved few study participants in each exposure combination subgroup. Indeed, a total of 284 women (3.6%) and 7,265 men (24.2%) had been exposed to more than two (i.e., 3–5) of the selected occupational lung carcinogens (Table 2). We did not distinguish if the two carcinogens occurred simultaneously (at the same time) or successively (one after the other), and we did not account for exposures beyond pairwise combinations in the logistic regression models.
Table 2.
Study population characteristics of the SYNERGY pooled analysis by lung cancer status and sex.
| Characteristics | Male controls | Male cases | Female controls | Female cases |
|---|---|---|---|---|
| Age at inclusion () | 61.9 (9.4) | 62.4 (8.9) | 60.0 (11.4) | 60.5 (10.2) |
| Age group (years) | ||||
| 895 (5.4%) | 486 (3.6%) | 476 (10.5%) | 229 (6.9%) | |
| 45–49 | 949 (5.8%) | 756 (5.6%) | 366 (8.1%) | 316 (9.6%) |
| 50–54 | 1,616 (9.8%) | 1,428 (10.5%) | 519 (11.5%) | 386 (11.7%) |
| 55–59 | 2,510 (15.3%) | 2,141 (15.7%) | 640 (14.2%) | 537 (16.3%) |
| 60–64 | 3,107 (18.9%) | 2,660 (19.6%) | 647 (14.3%) | 539 (16.4%) |
| 65–69 | 3,568 (21.7%) | 2,903 (21.3%) | 813 (18.0%) | 567 (17.2%) |
| 70–74 | 3,056 (18.6%) | 2,431 (17.9%) | 791 (17.5%) | 534 (16.2%) |
| 750 (4.6%) | 800 (5.9%) | 262 (5.8%) | 188 (5.7%) | |
| Smoking status and years since quitting smoking among former smokers | ||||
| Never-smokers | 4,437 (27.0%) | 490 (3.6%) | 2,716 (60.2%) | 879 (26.7%) |
| 2,324 (14.1%) | 642 (4.7%) | 230 (5.1%) | 62 (1.9%) | |
| 16–25 | 2,095 (12.7%) | 1,044 (7.7%) | 251 (5.6%) | 127 (3.9%) |
| 8–15 | 1,691 (10.3%) | 1,355 (10.0%) | 207 (4.6%) | 176 (5.3%) |
| 1,218 (7.4%) | 1,746 (12.8%) | 204 (4.5%) | 280 (8.5%) | |
| Current smokers | 4,686 (28.5%) | 8,328 (61.2%) | 906 (20.1%) | 1,772 (53.8%) |
| Cigarette pack-years among ever smokers | ||||
| 2,724 (16.6%) | 692 (5.1%) | 649 (14.4%) | 231 (7.0%) | |
| 10–19 | 2,332 (14.2%) | 1,248 (9.2%) | 420 (9.3%) | 396 (12.0%) |
| 20–40 | 4,147 (25.2%) | 4,994 (36.7%) | 499 (11.1%) | 979 (29.7%) |
| 2,811 (17.1%) | 6,181 (45.4%) | 230 (5.1%) | 811 (24.6%) | |
| Pack-years [median (IQR)] | 23.80 (10.80, 39.00) | 39.00 (26.50, 54.00) | 15.50 (5.55, 28.75) | 31.50 (19.50, 45.30) |
| Ever occupational exposure to | ||||
| Asbestos yes | 6,802 (41.3%) | 6,958 (51.1%) | 510 (11.3%) | 482 (14.6%) |
| Asbestos no | 9,649 (58.7%) | 6,647 (48.9%) | 4,004 (88.7%) | 2,814 (85.4%) |
| Chromium-VI yes | 3,820 (23.2%) | 4,131 (30.4%) | 146 (3.2%) | 159 (4.8%) |
| Chromium-VI no | 12,631 (76.8%) | 9,474 (69.6%) | 4,368 (96.8%) | 3,137 (95.2%) |
| Nickel yes | 3,140 (19.1%) | 3,216 (23.6%) | 131 (2.9%) | 151 (4.6%) |
| Nickel no | 13,311 (80.9%) | 10,389 (76.4%) | 4,383 (97.1%) | 3,145 (95.4%) |
| PAH yes | 4,077 (24.8%) | 4,021 (29.6%) | 636 (14.1%) | 618 (18.8) |
| PAH no | 12,374 (75.2%) | 9,584 (70.4%) | 3,878 (85.9%) | 2,678 (81.2%) |
| Silica yes | 4,140 (25.2%) | 4,649 (34.2%) | 348 (7.7%) | 274 (8.3%) |
| Silica no | 12,311 (74.8%) | 8,956 (65.8%) | 4,166 (92.3%) | 3,022 (91.7%) |
| Cumulative occupational exposure among ever exposed workers [median (IQR)] | ||||
| Asbestos (-years) | 1.21 (0.52, 2.96) | 1.61 (0.66, 3.63) | 0.57 (0.2, 1.65) | 0.71 (0.26, 1.74) |
| Chromium-VI (-years) | 0.04 (0.02, 0.1) | 0.04 (0.02, 0.11) | 0.03 (0.01, 0.06) | 0.03 (0.01, 0.06) |
| Nickel (-years) | 0.03 (0.01, 0.08) | 0.04 (0.01, 0.08) | 0.02 (0.01, 0.04) | 0.02 (0.01, 0.04) |
| PAH (BaP -years) | 0.26 (0.1, 0.56) | 0.27 (0.11, 0.58) | 0.14 (0.08, 0.31) | 0.17 (0.08, 0.36) |
| Silica (-years) | 1.14 (0.43, 2.46) | 1.27 (0.51, 2.66) | 0.86 (0.37, 1.78) | 0.55 (0.25, 1.41) |
| Duration in years of occupational exposure among ever exposed workers () | ||||
| Asbestos | 18.4 (14.0) | 19.5 (14.0) | 10.8 (9.5) | 10.2 (9.2) |
| Chromium-VI | 17.8 (14.4) | 19.2 (14.4) | 9.9 (9.0) | 8.7 (8.7) |
| Nickel | 17.1 (14.2) | 18.2 (14.1) | 10.1 (9.0) | 9.0 (8.8) |
| PAH | 16.8 (13.7) | 17.1 (13.6) | 11.5 (10.0) | 12.3 (10.3) |
| Silica | 18.2 (15.5) | 19.0 (15.5) | 14.8 (14.4) | 12.3 (13.3) |
| Ever exposed to number of selected exposures | ||||
| 0 | 6,525 (39.7%) | 3,868 (28.4%) | 3,262 (72.3%) | 2,191 (66.5%) |
| 1 | 3,710 (22.6%) | 3,079 (22.6%) | 923 (20.4%) | 749 (22.7%) |
| 2 | 2,757 (16.8%) | 2,852 (21.0%) | 192 (4.3%) | 209 (6.3%) |
| 3 | 1,623 (9.9%) | 1,739 (12.8%) | 87 (1.9%) | 83 (2.5%) |
| 4 | 1,294 (7.9%) | 1,360 (10.0%) | 47 (1.0%) | 52 (1.6%) |
| 5 | 542 (3.3%) | 707 (5.2%) | 3 (0.1%) | 12 (0.4%) |
| Lung cancer subtype | ||||
| SqC | — | 5,828 (42.8%) | — | 675 (20.5%) |
| SCC | — | 2,200 (16.2%) | — | 530 (16.1%) |
| AC | — | 3,325 (24.4%) | — | 1,427 (43.3%) |
| LCC | — | 619 (4.5%) | — | 191 (5.8%) |
| Other | — | 1,554 (11.4%) | — | 458 (13.9%) |
| “Unknown” | — | 79 (0.6%) | — | 15 (0.5%) |
Note: AC, adenocarcinoma; IQR, interquartile range; LCC, large cell carcinoma; SCC, small cell lung cancer; SD, standard deviation; SqC, squamous cell cancers.
Associations between pairwise ever/never occupational exposures and lung cancer risk were evaluated using unconditional logistic regression models, both overall and separately by major histological subtype: adenocarcinoma (AC), squamous cell carcinoma (SqC), and small cell lung cancer (SCC). Odds ratios (ORs) and 95% confidence intervals (CIs) were computed for the ever/never exposure metric. Subjects who were never exposed to the agents in each pair of interest were considered as the reference category. Cigarette pack-years (cPY) were calculated: [duration(years) × (average cigarette smoked per day/20 (cigarettes per pack))]. Other types of tobacco consumption (e.g., cigars and pipes) were not considered because all studies did not collect this information consistently, and most study participants who smoked cigars and/or pipes also smoked cigarettes. All models were stratified by sex to account for potential sex differences in job tasks and subsequent exposures and were adjusted for study center and age group (, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and years), cigarette pack-years (log[]) and time-since-quitting smoking cigarettes (categorized as current smokers; former smokers who have stopped smoking 2–7, 8–15, 16–25, years before interview/diagnosis; and never-smokers). Cut points used among former smokers in the “time-since-quitting smoking” variable were based on the quartile distribution of the “years since quitting cigarette smoking” distribution among control subjects. Stratified analyses were also performed by smoking status (never, former, current smokers). In most studies, smokers were defined as having smoked at least one cigarette per day for 6 months or more, and current smokers included those who had stopped smoking within the last 2 years before diagnosis or interview.
Pairwise joint effects between occupational exposures on lung cancer risks were estimated both on an additive scale and a multiplicative scale, adjusted for study, age group, cigarette pack-years, and time-since-quitting smoking. Interactions on a multiplicative scale were assessed using an interaction term between never vs. ever occupational exposures under consideration in logistic regression models. Interactions on an additive scale were assessed by fitting linear OR models and calculating the relative excess risk due to interaction () in order to estimate the departure from additivity of the effects of both risk factors combined.21 Linear OR models were adjusted for the same risk factors described previously in the logistic regression models. RERI estimates along with 95% CIs based on the delta method are reported.22,23 A indicates a positive departure from additivity where the effect of both exposures together (e.g., ever being occupationally exposed to both asbestos and silica) exceeds the sum of the effects of the two exposures considered (asbestos and silica) separately, while a indicates a less than additive effect.
As secondary analyses, RERI-based analyses stratified by smoking status were also conducted and restricted to men due to the low numbers of smoking women and the relatively low prevalence of occupational exposures among women.
Figure S1 shows heatmaps of Cramer’s V statistics between the never/ever occupational exposures by lag time in years. Since exposure to chromium-VI and to nickel were found highly correlated (Cramer’s ), the corresponding joint effects of the combination of these two heavy metals was not investigated. Analyses were performed using R statistical software (version 3.6.1). -Values are two-sided, and a significance level was set to 0.05.
Results
Table 2 shows the main characteristics of the study participants by sex and lung cancer status. A total of 16,901 lung cancer cases (4,752 AC, 6,503 SqC, 2,730 SCC, 2,822 other/unspecified lung cancers, and 94 cases with no histological data) and 20,965 control subjects were included in the present analysis.
Prevalence of Occupational Exposures
The five agents of interest in this paper are: asbestos, silica, PAH, chromium-VI, and nickel. The prevalence of ever occupational exposure to each of these is shown in Table 2, while Figure 1 also illustrates both single and co-occurring exposures in study participants exposed to one or two of the agents. Among men, asbestos was by far the most frequent single exposure; about 40% of male controls and 11% of female controls were considered to have had exposure to asbestos in at least one of their jobs. About half of those who were exposed to asbestos were also exposed to either silica or PAH. Exposure to chromium-VI and nickel almost always co-occurred with each other, although chromium-VI also co-occurred with silica. PAH co-occurred to a large extent with asbestos, 70% of those exposed to PAH were also exposed to asbestos. Sixty-five percent of the men were exposed to at least one of the five agents during their working life, and among those men they were exposed to an average of 2.3 of the five agents. Women were most often exposed to PAH (16%), followed by asbestos (13%) and silica (8%), and they also co-occurred to some extent with each other, while exposure to both chromium-VI and nickel was rare. Thirty percent of women were exposed to at least one of the five agents, and the average number of the five selected lung carcinogens among the exposed was 1.5.
Figure 1.
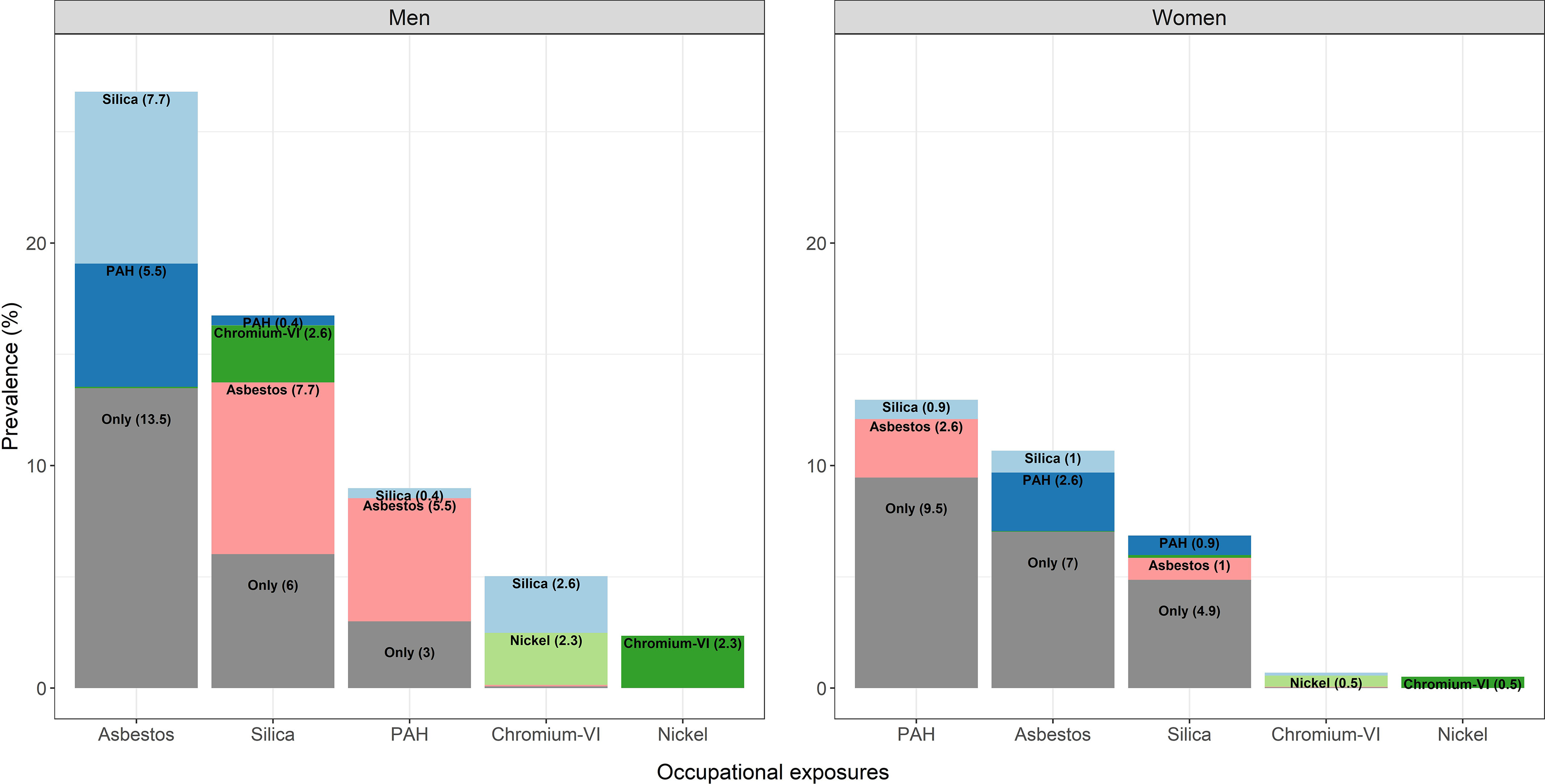
Prevalence of occupational exposure to asbestos, silica, PAH, chromium-VI, and nickel among SYNERGY study participants with one or two exposures ( men, women). The total of the columns represents the overall—individual plus co-exposed—prevalence of each exposure, while within the column the proportion of “co-exposure” to each of the other exposures is also shown. Percentages lower than 0.4% are not displayed because of lack of visibility. Note: PAH, polycyclic aromatic hydrocarbons.
Pairwise Interactions between Occupational Exposures and Risk of Lung Cancer: Overall and by Histological Subtype
Main and joint effects (ORs) of occupational exposures, RERIs, and tests for multiplicative interaction (p-values) between the occupational exposures are illustrated for lung cancer overall in Figure 2 and by major lung cancer subtype (AC, SqC, SCC) in Figure 3 for men, while the corresponding results for women are shown in Figure 4 and Figure 5. These results are presented in numbers in Tables S1 and S2 for men and women, respectively.
Figure 2.
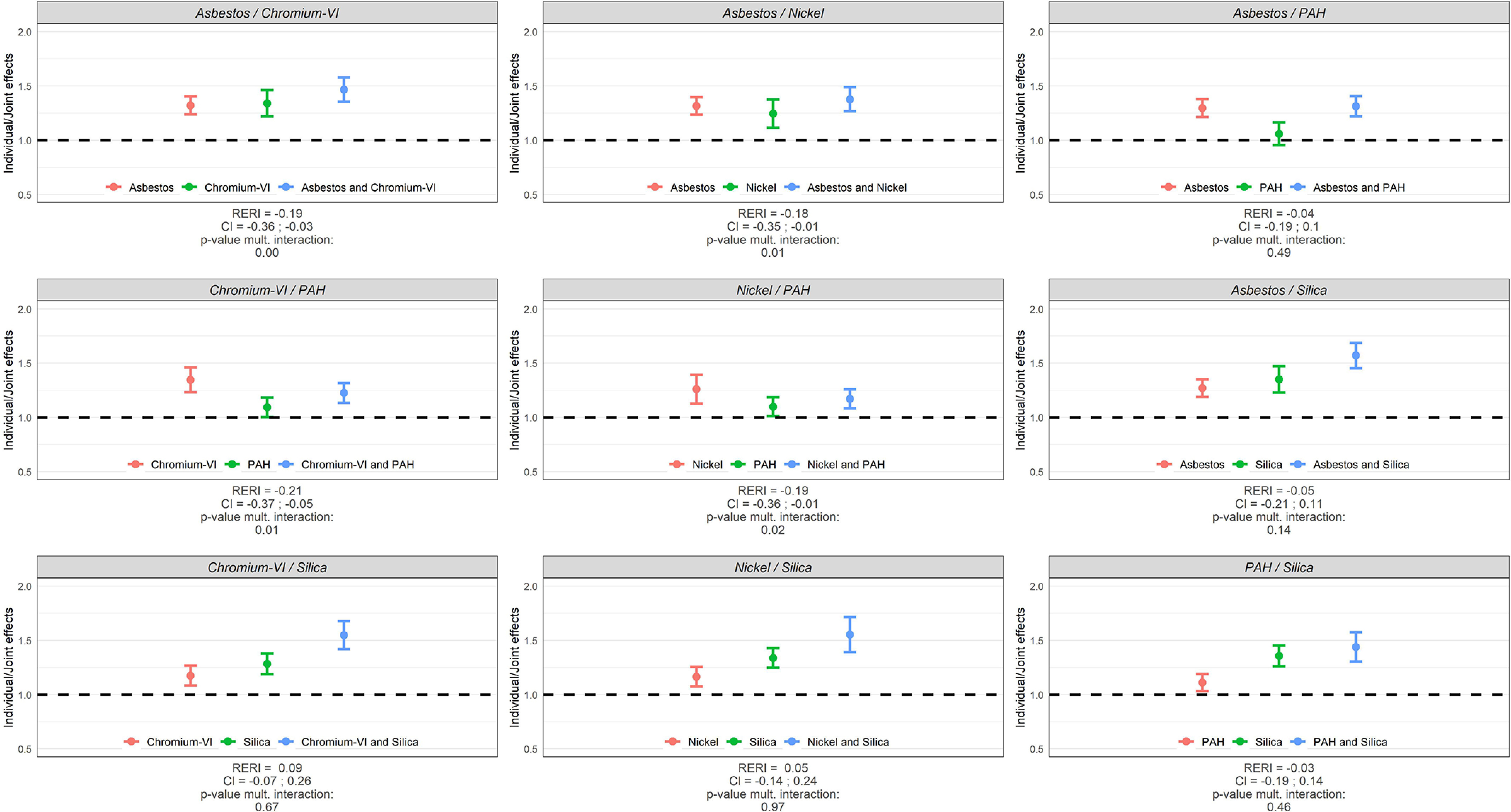
Individual and joint effects (ORs) of occupational exposures, RERIs, and tests for multiplicative interaction (p-values) between occupational exposures in relation to lung cancer risk in SYNERGY for men ( lung cancer cases and 16,451 control subjects). Note: CI, confidence interval; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; RERI, relative excess risk due to interaction.
Figure 3.
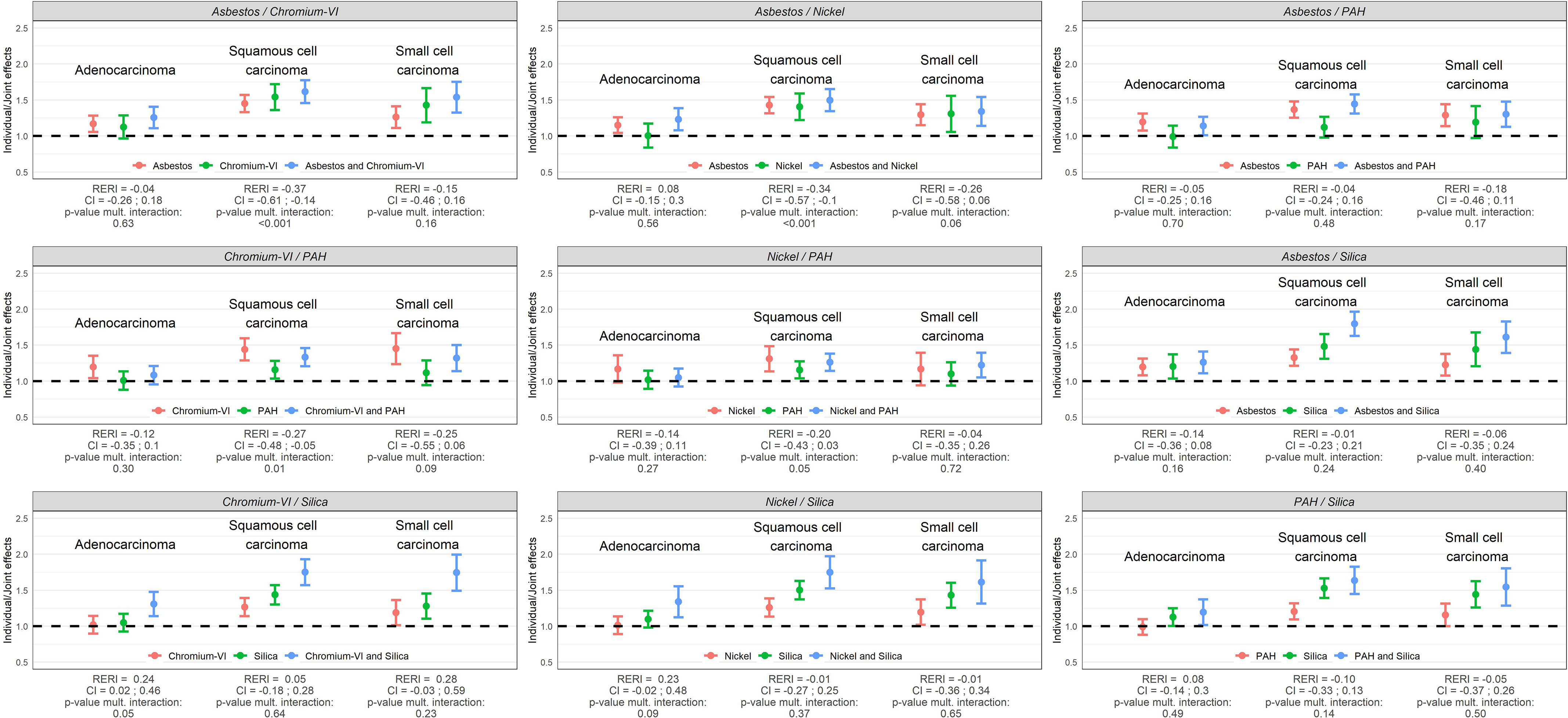
Individual and joint effects (ORs) of occupational exposures, RERIs, and tests for multiplicative interaction (p-values) between occupational exposures in relation to major histological subtypes of lung cancer in SYNERGY for men ( lung cancer cases and control subjects). Note: CI, confidence interval; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; RERI, relative excess risk due to interaction.
Figure 4.
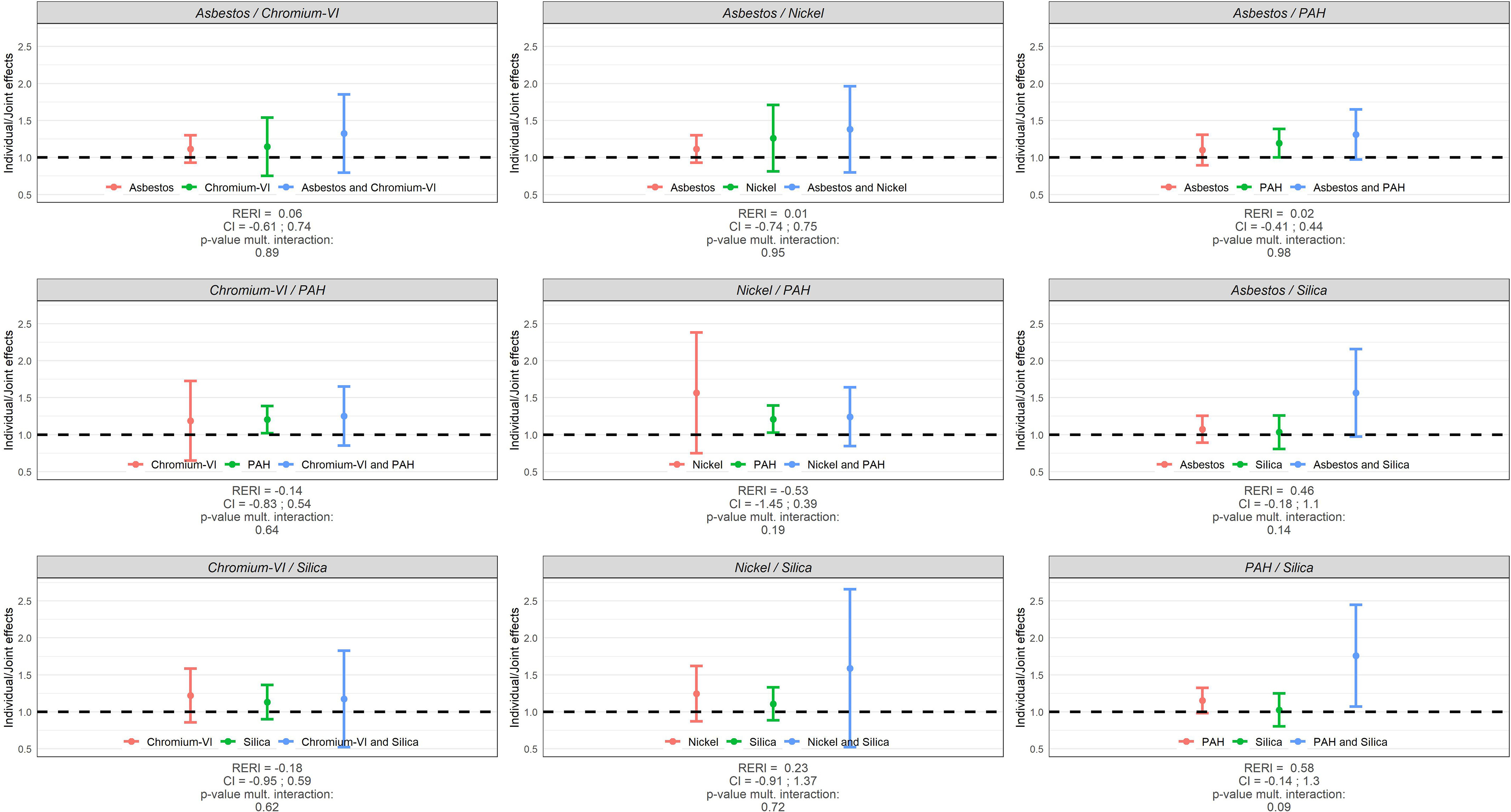
Individual and joint effects (ORs) of occupational exposures, RERIs, and tests for multiplicative interaction (p-values) between occupational exposures in relation to lung cancer risk in SYNERGY for women ( lung cancer cases and control subjects). Note: CI, confidence interval; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; RERI, relative excess risk due to interaction.
Figure 5.
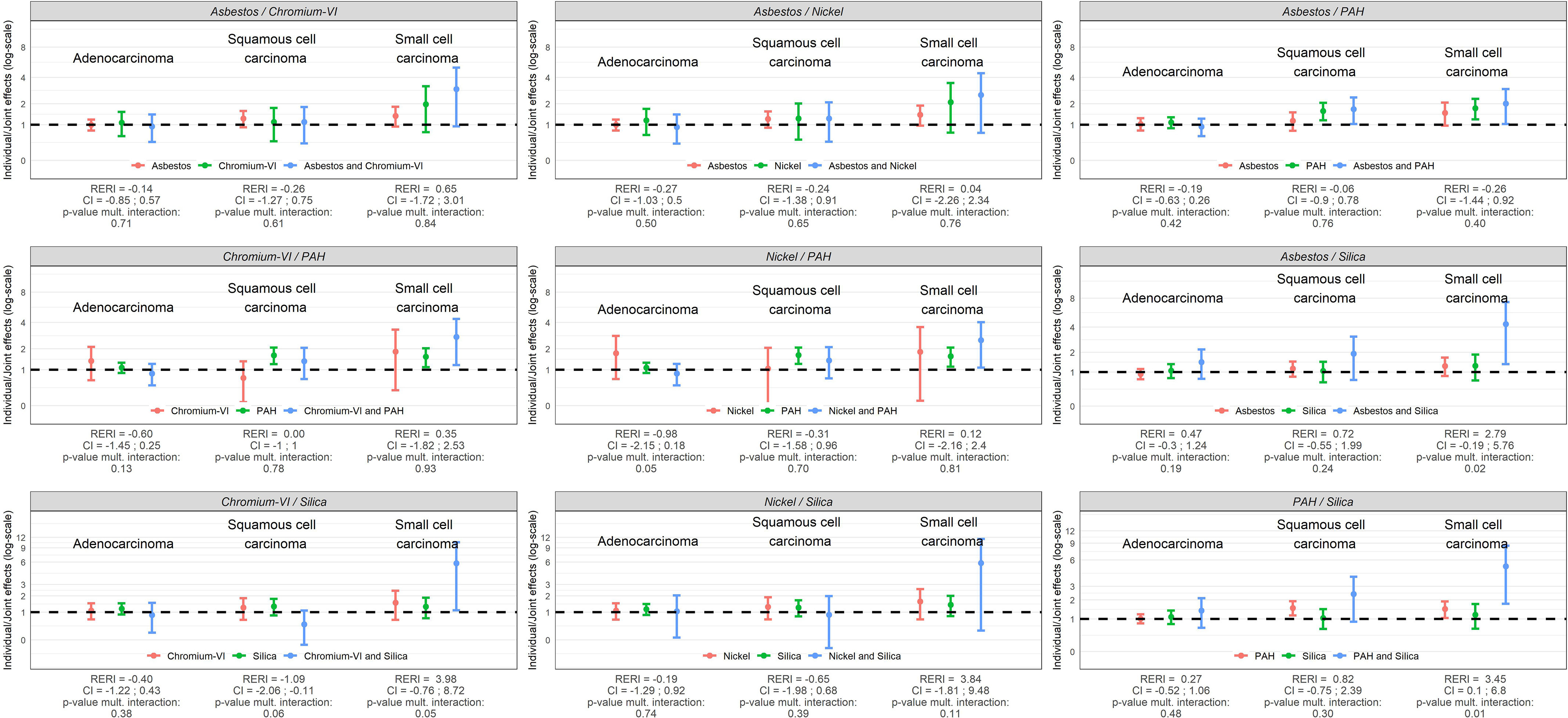
Individual and joint effects (ORs) of occupational exposures, RERIs, and tests for multiplicative interaction (p-values) between occupational exposures in relation to major histological subtypes of lung cancer in SYNERGY for women ( lung cancer cases and control subjects). Note: CI, confidence interval; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; RERI, relative excess risk due to interaction.
Among men, all pairwise joint exposure of occupational lung carcinogens were associated with an increased risk of lung cancer when compared to the never exposed reference group. The OR was generally the highest for Sqc followed by SCC. Most of the investigated pairwise exposures showed no statistically significant departure from neither additivity nor multiplicativity. Solely, asbestos/chromium-VI, asbestos/nickel, chromium-VI/PAH, and nickel/PAH showed statistically significant negative departures on both scales for Sqc and/or lung cancer overall, with upper limits of the confidence intervals of the RERIs below 0; while the joint effect of chromium-VI/silica was slightly more than additive in relation to AC (RERI: 0.24; 95% CI: 0.02, 0.46; = 0.05).
Among women, although joint effects (ORs) had large confidence intervals, several pairwise joint exposures were associated with an increased risk of SCC specifically: asbestos/PAH (for SqC too), asbestos/silica, chromium-VI/PAH, nickel/PAH, chromium-VI/silica, and PAH/silica. The joint effect between PAH and silica resulted in a more than additive effect (RERI: 3.45; 95% CI: 0.10, 6.8; = 0.01), and asbestos/silica (OR = 4.32; 95% CI: 1.35, 7.29; = 0.02) and chromium-VI/silica (OR = 5.80; 95% CI: 1.10, 10.51; = 0.05) in more than multiplicative effects.
Pairwise Interactions between Occupational Exposures and Lung Cancer Risk by Smoking Status
Main and joint effects of occupational exposures between the selected lung carcinogens, RERIs, and tests for multiplicative interaction (p-values) in lung cancer risk among men stratified by smoking status are illustrated in Figure 6 and presented in numbers in Table S3. All ORs were elevated for the different pairs of exposures, although with wide CIs for chromium-VI/PAH, nickel/PAH, nickel/silica, and PAH/silica among never smokers. The ORs were generally higher among never smokers compared to former and current cigarette smokers but with wider confidence intervals. The pair asbestos/chromium-VI showed submultiplicative associations in former smokers ( = 0.04) and current smokers ( = 0.02), as did asbestos/nickel in former smokers ( = 0.02), while departure from additivity was not statistically significant for any of these. The remaining pairs of exposures revealed no deviations, neither on the additive nor on the multiplicative scale.
Figure 6.
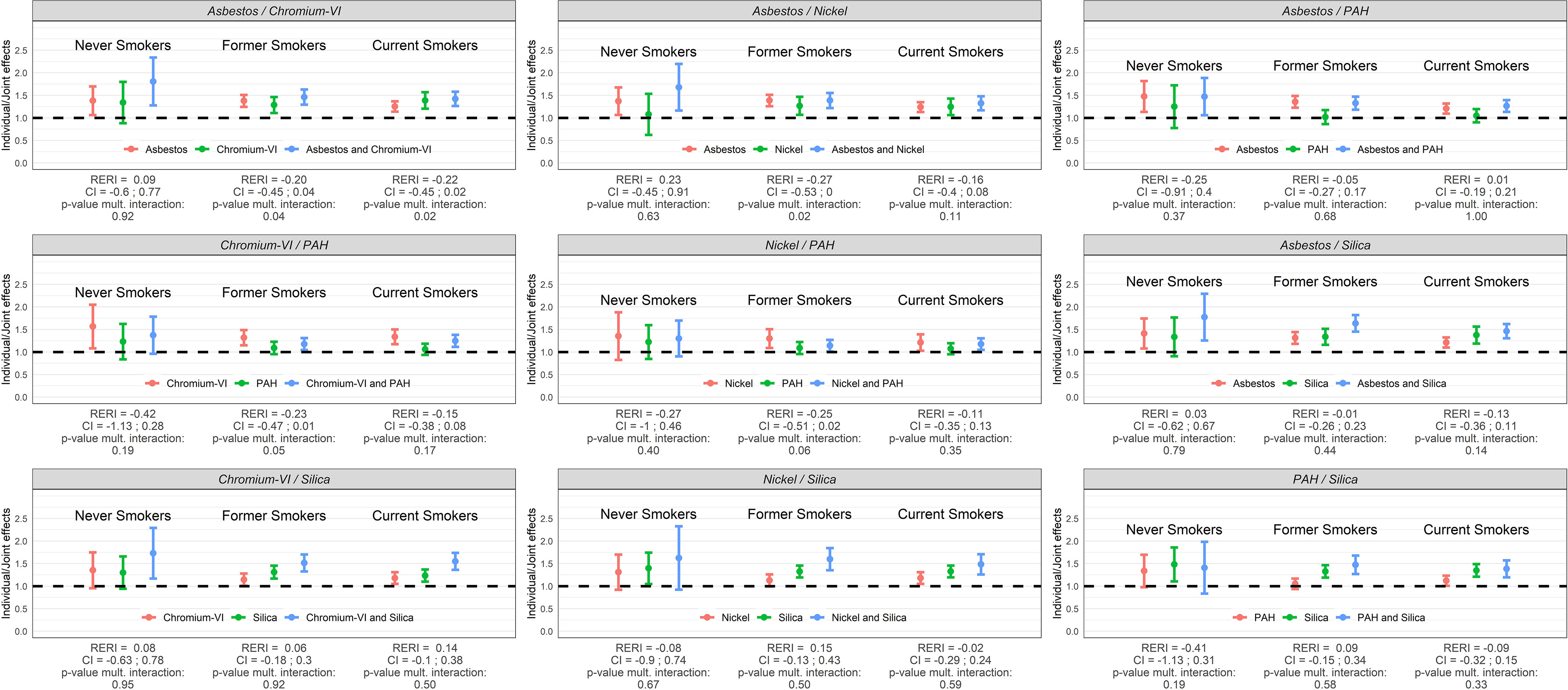
Individual and joint effects (ORs) of occupational exposure to asbestos, chromium-VI, nickel, PAH, and silica, RERIs, and test for multiplicative interaction (p-values) among male workers ( lung cancer cases and control subjects) in SYNERGY by smoking status (never, former, and current cigarette smokers). Note: CI, confidence interval; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; RERI, relative excess risk due to interaction.
Discussion
The present large pooled analysis of lung cancer case–control studies on the pairwise joint effects of five occupational carcinogens (asbestos, chromium-VI, nickel, PAH, and silica) on lung cancer risk—while adjusting for tobacco smoking—showed less than additive effects for asbestos/chromium-VI, asbestos/nickel, chromium-VI/PAH, and nickel/PAH pairs for overall lung cancer in men and more strongly for the subtype SqC. Modest synergistic effect was observed for chromium-VI/silica exposure for adenocarcinoma among men. Among women, strong positive departure from additivity (albeit relatively imprecise) was observed for SCC for silica and joint exposures with either asbestos, PAH, or chromium-VI. Stratified analysis by smoking status and restricted to men showed no statistically significant joint effects either on the additive or on the multiplicative scale, except for asbestos/chromium-VI in former and current smokers and asbestos/nickel in former smokers resulting in submultiplicative associations. ORs and RERI estimates were generally associated with wider CIs among the never smoker subgroup as well as among women compared to men because of smaller sample sizes and lower prevalence of occupational exposures in both subgroups.
To our knowledge, the current results are the first epidemiological analyses of lung cancer in humans to systematically investigate the joint effects of these occupational exposures and the risk of lung cancer while adjusting for tobacco smoking. We note that the prevalence of exposure to at least one carcinogen in SYNERGY (65% in men and 30% in women) is substantially higher than in CAREX Europe (23%) and the recent study in Australia (38%), likely explained by the different denominators (general working population compared to an older population included in a case-control study) and that CAREX and AWES were cross-sectional (current job), while SYNERGY estimated exposures throughout the study participant’s full career.1,2 The average number of carcinogens among the exposed was higher in AWES (3.7 in men and 2.7 in women) compared to in SYNERGY (2.3 in men and 1.5 in women), as well as the proportion of workers exposed to carcinogens (AWES: 15% in men, 3% in women; SYNERGY: 4.2% in men, 0.2% in women), a direct consequence of the number of carcinogens included in the estimation (AWES: ; SYNERGY: ).
The strengths of the SYNERGY study include the large number of subjects allowing us to study combined exposures by important strata such as sex, lung cancer subtype, and smoking status; the complete job histories and detailed data on smoking habits; the large proportion of control subjects derived from the general population (79%) and that most information was obtained from the study participants themselves (). Another strength is the SYN-JEM assigning occupational exposures to the study participants’ job histories based on measurements from different countries covering almost 40 years, albeit in the present analysis exposure is only considered as a binary covariate. The SYN-JEM and the smoking data have been used in previous SYNERGY papers, which corroborated that the selected exposures are lung carcinogens as estimated and assessed in SYNERGY.6,9,10,12
Several weaknesses are also present, such as relatively low participation in a few studies (Table 1), which may have resulted in selection bias. One attempt to assess this phenomenon as well as unmeasured confounding by socioeconomic status has been to restrict the study population to blue collar workers in previous papers. We have observed some attenuated effect estimates but with increased risks still present, so we believe it is not a major concern in SYNERGY.6,9,10,12 Other weaknesses include potential incorrect recall of previous occupations and smoking habits and the conversion of occupational histories from national classification systems to ISCO-68. Self-reported occupational histories are considered more reliable than self-reported occupational exposures.24 The original studies in SYNERGY, except MORGEN, were designed to investigate occupational exposures and therefore committed to collect detailed occupational histories and to code the data correctly. Automated conversion was used for the LUCAS study, from Swedish codes for occupation (NYK 83) to ISCO-68. Unmeasured confounders, e.g., occupational exposure to other lung carcinogens, may have played a role, but we believe that these types of exposures and exposure misclassification would not be differential—between cases and controls—and thereby would not distort our results substantially. Job exposure matrices, including SYN-JEM, assigns exposure to job codes ignoring the exposure variability between workers and that some workers within a job may not be exposed. However, the job or group-based assignment will result in a Berkson-type error, which usually does not bias the risk estimate but will result in a loss of precision of the risk estimate.25 Occupational exposure to nickel and chromium-VI was highly correlated (Cramer’s ), so we cannot with certainty distinguish their effects.
Despite the large data set and detailed information on all jobs and on smoking habits in SYNERGY, our current results must be interpreted with caution. Firstly, we did not consider if the two exposures occurred in the same job or in different jobs one at a time. Secondly, we did not consider cumulative occupational exposure levels or any other continuous or categorical exposure metrics other than ever vs. never, e.g., if the concentration of one of the exposures was very low and the other high. Thirdly, we ignored multiple exposures beyond pairwise combinations because it was beyond the scope of the present analysis and because of small numbers of individuals in each stratum with at least three or more exposure to the five lung carcinogens.
Conclusion
We assessed pairwise interactions of occupational exposure to asbestos, silica, nickel, chromium-VI, and PAH on lung cancer risk, while accounting for cigarette smoking, on an additive and on a multiplicative scale, with numbers and risk estimates shown for single and combined exposures, along with measures of interactions. In this analysis, we observed small or no deviations from additive or multiplicative effects, which means that additive calculation for creating compensation schemes would be pragmatic for these exposures. This is currently an uncommon practice. We show that most co-exposure to the selected lung carcinogens result in higher risk compared to individual exposures that underline the importance to eliminate or reduce and control exposures to carcinogens in workplaces and the general environment.
Supplementary Material
Acknowledgments
Isabelle Stücker will be remembered for her professionalism and generosity regarding the SYNERGY project. We also thank the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands, for their contribution of the MORGEN Study to these analyses.
The SYNERGY project was supported by the German Social Accident Insurance, grant FP 271, between 2007 and 2011. Grant sponsors of the original studies were the Canadian Institutes of Health Research and Guzzo-SRC Chair in Environment and Cancer, the Canadian Cancer Society, the Fondation de France, the German Federal Ministry of Education, Science, Research, and Technology and the Ministry of Labour and Social Affairs, EC’s INCO-COPERNICUS Program, Polish State Committee for Science Research, Roy Castle Foundation, NIH/NCI/DCEG Intramural Research Program, Lombardy Region, INAIL and the European Union Nuclear Fission Safety Program, Italian Association for Cancer Research, Region Piedmont, Compagnia di San Paolo, Europe Against Cancer Program, the Swedish Council for Work Life Research and the Swedish EPA, the University of Oviedo, the European Regional Development Fund and the State Budget of the Czech Republic (RECAMO, CZ.1.05/2.1.00/03.0101), CIBERESP, and FISS-PI060604.
Scientists may contact the corresponding author (olssona@iarc.who.int) to discuss secondary analysis, but a Data Use Agreement (DTA) with the International Agency for Research on Cancer must be established defining the purpose and modalities.
The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the decisions, policy, or views of their affiliated institutes.
References
- 1.Kauppinen T, Toikkanen J, Pedersen D, Young R, Ahrens W, Boffetta P, et al. 2000. Occupational exposure to carcinogens in the European union. Occup Environ Med 57(1):10–18, PMID: , 10.1136/oem.57.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenzie JF, El-Zaemey S, Carey RN. 2021. Prevalence of exposure to multiple occupational carcinogens among exposed workers in Australia. Occup Environ Med 78(3):211–217, 10.1136/oemed-2020-106629. [DOI] [PubMed] [Google Scholar]
- 3.Gustavsson P, Nyberg F, Pershagen G, Schéele P, Jakobsson R, Plato N. 2002. Low-dose exposure to asbestos and lung cancer: dose-response relations and interaction with smoking in a population-based case-referent study in Stockholm, Sweden. Am J Epidemiol 155(11):1016–1022, PMID: , 10.1093/aje/155.11.1016. [DOI] [PubMed] [Google Scholar]
- 4.Lee PN. 2001. Relation between exposure to asbestos and smoking jointly and the risk of lung cancer. Occup Environ Med 58(3):145–153, PMID: , 10.1136/oem.58.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry G, Liddell FDK. 2001. The interaction of asbestos and smoking in lung cancer: a modified measure of effect. Ann Occup Hyg 48(5):459–462, PMID: , 10.1093/annhyg/meh023. [DOI] [PubMed] [Google Scholar]
- 6.Olsson AC, Vermeulen R, Schüz J, Kromhout H, Pesch B, Peters S, et al. 2017. Exposure-response analyses of asbestos and lung cancer subtypes in a pooled analysis of case-control studies. Epidemiology 28(2):288–299, PMID: , 10.1097/EDE.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Zoghbi M, Salameh P, Stücker I, Brochard P, Delva F, Lacourt A. 2017. Absence of multiplicative interactions between occupational lung carcinogens and tobacco smoking: a systematic review involving asbestos, crystalline silica and diesel engine exhaust emissions. BMC Public Health 17(1):156, PMID: , 10.1186/s12889-017-4025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebe S, Leigh J, Henderson DW, Nurminen M. 2019. Asbestos, smoking and lung cancer: an update. Int J Environ Res Public Health 17(1):258, 10.3390/ijerph17010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge C, Peters S, Olsson A, Portengen L, Schüz J, Almansa J, et al. 2020. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks. A pooled analysis of case-control studies. Am J Respir Crit Care Med 202(3):412–421, PMID: , 10.1164/rccm.201910-1926OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson A, Guha N, Bouaoun L, Kromhout H, Peters S, Siemiatycki J, et al. 2022. Occupational exposure to polycyclic aromatic hydrocarbons and lung cancer risk: results from a pooled analysis of case-control studies (SYNERGY). Cancer Epidemiol Biomarkers Prev 31(7):1433–1441, PMID: , 10.1158/1055-9965.EPI-21-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge C, Peters S, Olsson A, Portengen L, Schüz J, Almansa J, et al. 2020. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. A pooled exposure-response analysis of 14 case-control studies. Am J Respir Crit Care Med 202(3):402–411, PMID: , 10.1164/rccm.201911-2101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens T, Ge C, Vermeulen R, Kendzia B, Olsson A, Schüz J, et al. 2023. Occupational exposure to nickel and hexavalent chromium and the risk of lung cancer in a pooled analysis of case-control studies (SYNERGY). Int J Cancer 152(4):645–660, PMID: , 10.1002/ijc.34272. [DOI] [PubMed] [Google Scholar]
- 13.Consonni D, De Matteis S, Pesatori AC, Bertazzi PA, Olsson AC, Kromhout H, et al. 2015. Lung cancer risk among bricklayers in a pooled analysis of case-control studies. Int J Cancer 136(2):360–371, PMID: , 10.1002/ijc.28986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendzia B, Behrens T, Jöckel K-H, Siemiatycki J, Kromhout H, Vermeulen R, et al. 2013. Welding and lung cancer in a pooled analysis of case-control studies. Am J Epidemiol 178(10):1513–1525, PMID: , 10.1093/aje/kwt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guha N, Bouaoun L, Kromhout H, Vermeulen R, Brüning T, Behrens T, et al. 2021. Lung cancer risk in painters: results from the SYNERGY pooled case-control study consortium. Occup Environ Med 78(4):269–278, PMID: , 10.1136/oemed-2020-106770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soziales BfAu. 2007. Scientific rationale for the occupational disease “lung cancer due to the combined exposure to asbestos fibre dust and polycyclic aromatic hydrocarbons” announcement by the BMAS of February 1, 2007 - IV a 4-45222 -. GMBl 23:474–495. [Google Scholar]
- 17.Olsson AC, Gustavsson P, Kromhout H, Peters S, Vermeulen R, Brüske I, et al. 2011. Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am J Respir Crit Care Med 183(7):941–948, PMID: , 10.1164/rccm.201006-0940OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesch B, Kendzia B, Gustavsson P, Jöckel K-H, Johnen G, Pohlabeln H, et al. 2012. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer 131(5):1210–1219, PMID: , 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters S, Vermeulen R, Olsson A, Van Gelder R, Kendzia B, Vincent R, et al. 2012. Development of an exposure measurement database on five lung carcinogens (ExpoSYN) for quantitative retrospective occupational exposure assessment. Ann Occup Hyg 56(1):70–79, PMID: , 10.1093/annhyg/mer081. [DOI] [PubMed] [Google Scholar]
- 20.Peters S, Vermeulen R, Portengen L, Olsson A, Kendzia B, Vincent R, et al. 2016. SYN-JEM: a quantitative job-exposure matrix for five lung carcinogens. Ann Occup Hyg 60(7):795–811, PMID: , 10.1093/annhyg/mew034. [DOI] [PubMed] [Google Scholar]
- 21.Knol MJ, VanderWeele TJ, Groenwold RH, Klungel OH, Rovers MM, Grobbee DE. 2011. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol 26(6):433–438, PMID: , 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosmer DW, Lemeshow S. 1992. Confidence interval estimation of interaction. Epidemiology 3(5):452–456, PMID: , 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Oehlert GW. 1992. A note on the Delta method. American Statistician 46(1):27–29, 10.1080/00031305.1992.10475842. [DOI] [Google Scholar]
- 24.Teschke K, Olshan AF, Daniels JL, De Roos AJ, Parks CG, Schulz M, et al. 2002. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med 59(9):575–593, PMID: , 10.1136/oem.59.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong BG. 1998. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med 55(10):651–656, PMID: , 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


