Abstract
Salmonella typhi is the etiologic agent of human typhoid. During infection, S. typhi adheres to and invades epithelial and M cells that line the distal ileum. To survive in the human host, S. typhi must overcome numerous complex extracellular and intracellular environments. Since relatively little is known about S. typhi pathogenesis, studies were initiated to identify S. typhi genes involved in the early steps of interaction with the host and to evaluate the environmental regulation of these genes. In the present study, TnphoA mutagenesis was used to study these early steps. We isolated 16 Salmonella typhi TnphoA mutants that were defective for both adherence and invasion of the human small intestinal epithelial cell line Int407. Twelve of sixteen mutations were identified in genes homologous to the S. typhimurium invG and prgH genes, which are known to be involved in the type III secretion pathway of virulence proteins. Two additional insertions were identified in genes sharing homology with the cpxA and damX genes from Escherichia coli K-12, and two uncharacterized invasion-deficient mutants were nonmotile. Gene expression of TnphoA fusions was examined in response to environmental stimuli. We found that the cpxA, invG, and prgH genes were induced when grown under conditions of high osmolarity (0.3 M NaCl). Expression of invG and prgH genes was optimal at pH 6.5 and strongly reduced at low pH (5.0). Transcription of both invG and prgH TnphoA gene fusions was initiated during the late logarithmic growth phase and was induced under anaerobic conditions. Finally, we show that both invG and prgH genes appear to be regulated by DNA supercoiling, a mechanism influenced by environmental factors. These results are the first to demonstrate that in S. typhi, (i) the prgH and cpxA genes are osmoregulated, (ii) the invG gene is induced under low oxygen conditions, (iii) the invG gene is pH regulated and growth phase dependent, and (iv) the prgH gene appears to be regulated by DNA supercoiling. Since our experimental conditions were designed to mimic the in vivo environmental milieu, our results suggest that specific environmental conditions act as signals to induce the expression of S. typhi invasion genes.
Typhoid fever is a human disease caused by the facultative, intracellular pathogen Salmonella enterica serovar typhi (S. typhi). Since 30 million cases occur annually, this disease is responsible for significant morbidity and mortality worldwide (20, 79). Indeed, this disease remains a major public health concern and continues to be a priority of the World Health Organization (20). According to the seminal studies of Hornick et al. (43, 44), after S. typhi is ingested in contaminated food and/or water, the organisms migrate to the distal ileum. Presumably, S. typhi then adheres to and invades the intestinal epithelium (56, 99), migrates to the underlying Peyer’s patches, and disseminates throughout the reticuloendothelial system (43, 44). However, relatively little is known about the bacterium host interactions that occur at each of these steps in the pathogenesis of S. typhi.
S. typhimurium has been widely used as a model to study S. typhi pathogenesis since this organism causes a typhoid-like disease in mice, and no effective animal model currently exists for S. typhi (11, 76, 77, 83). Over the past 5 years, in studies using both in vivo and in vitro models, numerous S. typhimurium genes that are involved in the initial, essential step of bacterial-host interaction, the adherence to and invasion of intestinal epithelial cells, have been identified. These genes include hilA (6), inv (4, 13, 21, 32, 35, 38, 52, 53), prg (10), spa (41), ssp (sip) (54, 55), and lpf and pef (9).
In 1989, Elsinghorst et al. (22) identified the first S. typhi genes involved in invasion of intestinal epithelial cells. These investigators cloned from S. typhi a 33-kb chromosomal DNA fragment that conferred the ability to enter Int407 intestinal epithelial cells on the noninvasive Escherichia coli strain HB101. Since homologous sequences from S. typhimurium failed to confer invasiveness on this E. coli strain, these data suggested the existence of distinct invasion loci between S. typhi and S. typhimurium. However, Lee (58) suggested that the S. typhi invasion genes identified by Elsinghorst et al. (22) may not be involved in Salmonella invasion but instead may have indirectly altered the ability of E. coli to enter cells in vitro. More recently, DNA fragments containing the S. typhimurium invasion genes invA, invE, invH, and orgA have been identified on the S. typhi chromosome (4, 34, 38, 51). Miras et al. (71) have also identified two S. typhi genes, iagA and iagB, that appear to be involved in invasion of HeLa cells by S. typhi. The iagA gene was shown to be identical to the S. typhimurium hilA gene that encodes an activator of invasion gene expression (6). Finally, Hermant et al. (42) characterized additional invasion genes in S. typhi, sipEBCDA. These genes were shown to be homologous to the S. typhimurium genes spaT/sicA (54) and sspBCDA/sipBCDA (46, 54, 55) and to the Shigella genes ipgC and ipaBCDA (8, 88, 93, 94), which encode virulence factors.
Although the S. typhi invasion genes identified to date are homologous to the S. typhimurium genes, distinct invasion genes may remain unidentified, given that these two Salmonella species cause distinctly different diseases in humans; S. typhi causes the disseminated disease, typhoid fever, whereas S. typhimurium causes a relatively localized gastroenteritis. Moreover, recent data suggest that S. typhi and S. typhimurium differentially adhere to, invade, and stimulate intestinal epithelial cells to secrete soluble factors in vitro (95). Taken together, these findings imply that S. typhi may express distinct genes or may use different mechanisms of regulation for invasion of small intestinal epithelial cells than other Salmonella pathogens. One set of variables that could regulate invasion gene expression is environmental signals stimulated by the host. Thus, prior to entry of S. typhi into intestinal epithelial cells, the bacteria are exposed to a variety of host environmental conditions that may influence their functional capacity. Such environmental cues include temperature upshift after ingestion, acidity of the stomach, high osmolarity, and decreased oxygen tension within the intestine. Studies of S. typhimurium have shown that modulation of gene expression is required for bacteria to survive and replicate within these environments. For instance, concentrations of Ca2+ (74) and Mg2+ (36), nutrient availability (27), osmolarity (33), oxygen tension (24, 30, 52, 59, 89), pH (3, 10, 29), and other complex environments (reviewed in reference 66) have been shown to control virulence or virulence gene expression. However, little is known about environmental regulation of virulence genes in S. typhi. In this study, we report the identification and molecular characterization of 14 independent S. typhi invasion-defective TnphoA mutants and their regulation by environmental stimuli.
(A preliminary account of this work has been presented elsewhere [57].)
MATERIALS AND METHODS
Bacterial strains, bacteriophage, plasmids, and growth conditions.
S. typhi ISP1820 (clinical isolate, gift of David Hone, Medical Biotechnology Center, University of Maryland Biotechnology Institute, Baltimore, Md.) and its derivatives were routinely grown at 37°C in Luria-Bertani (LB) medium supplemented with 0.3 M NaCl unless otherwise specified. E. coli XL1-Blue MRF′ (Stratagene, Inc., La Jolla, Calif.) was used as the host strain for cloning experiments. Antibiotics were used at the following concentrations: kanamycin, 50 μg/ml; tetracycline, 12 μg/ml; ampicillin, 85 μg/ml; gentamicin, 100 μg/ml; and novobiocin, 2 to 10 μg/ml. 5-Bromo-4-chloro-3-indolyl phosphate (XP; Sigma) was used at a concentration of 40 μg/ml as the chromogenic substrate for detecting the alkaline phosphatase activity on LB agar plates. p-Nitrophenyl phosphate (Sigma) was used as the substrate for quantitative measurement of alkaline phosphatase activity. Plasmid pJM703.1 (gift from D. Hone), is a derivative of the suicide plasmid pGP704 that carries TnphoA and confers both ampicillin and kanamycin resistance. Bacteriophage P22 HTint was propagated and used in transduction experiments as described previously (15).
TnphoA mutagenesis.
To eliminate the background activity of nonspecific periplasmic acid phosphatase encoded by the phoN gene, an S. typhi 1820 phoN51::Tn10d-Tetr mutant was constructed. The phoN51::Tn10d-Tetr mutation was introduced in S. typhi 1820 by P22 HTint-mediated transduction from S. typhimurium LT2 strain TT13216 (nadB499::MudJ phoN51::Tn10d-Tet) obtained from K. E. Sanderson (Salmonella Genetic Stock Center, Calgary, Canada). One transductant, DC27, that showed wild-type levels of cell-associated and invasion of Int407 cells was chosen for TnphoA mutagenesis. Plasmid pJM703.1 was introduced in DC27 by electroporation, using a modification of the procedure described by O’Callaghan and Charbit (78). Briefly, the bacteria were grown in LB broth to an A600 of ≈0.6, chilled on ice, and washed three times with ice-cold distilled water. The bacterial pellet was suspended in 1/600 of the initial volume in ice-cold distilled water. Forty microliters of this suspension was used for the electroporation procedure. TnphoA mutants were selected on LB agar plates supplemented with kanamycin, tetracycline, and the chromogenic substrate XP.
Invasion and adherence assays.
Human small intestinal epithelial Int407 cells (Int407; ATCC CCL6; American Type Culture Collection, Rockville, Md.) were maintained and prepared for adherence and invasion assays as described elsewhere (96). The qualitative and quantitative invasion assays were conducted essentially as described by Tartera and Metcalf (91) and Weinstein et al. (96). Briefly, S. typhi strains were grown in a shaking water bath in LB medium supplemented with 0.3 M NaCl to mid/late logarithmic growth phase (A600 of ≈0.6). One milliliter of each culture was centrifuged and resuspended in an equal volume of saline. Twenty-five microliters of this suspension was added to 2.5 × 105 Int407 cells (multiplicity of infection of ∼20). High osmolarity of the growth media has been shown to be optimal for S. typhi cell-associated and invasion of human small intestinal epithelial cells (91). The bacteria were centrifuged onto the monolayers to allow intimate contact. Bacteria and Int407 cells were incubated at 37°C in a 5% CO2 atmosphere for 90 min and washed three times with Earle’s balanced salt solution (EBSS; Gibco BRL). The monolayers were then incubated for 90 min in tissue culture medium containing 100 μg of gentamicin per ml to kill extracellular bacteria. Subsequently, the monolayers were washed three times with the EBSS and lysed with 0.2 ml of 1% Triton X-100 (Sigma) to release intracellular bacteria. Samples were vigorously mixed, and 0.8 ml of saline was added. In the qualitative invasion assays, 100 μl of this suspension were plated on LB agar plates. Under these conditions, the parental strain, DC27, gave confluent growth whereas invasion-defective mutants gave individual colonies. For quantitative invasion assays, the suspension was diluted and viable counts were quantified on LB plates. Percent invasion = (CFU released by 1% Triton X-100/CFU in the initial inoculum of bacteria added to each well) × 100.
For adherence assays, bacteria were allowed to attach for 90 min at 37°C in 5% CO2 atmosphere and then washed six times with EBSS. The cell-associated bacteria were released by treatment of monolayers with 0.2 ml of 1% Triton X-100 in saline for 10 min, followed by addition of 0.8 ml of saline. CFU were quantified by plating appropriate dilutions on LB agar plates. Percent adherence = (CFU of cell-associated bacteria released by 1% Triton X-100/CFU in the initial inoculum of bacteria added to each well) × 100.
Phenotypic characterization of TnphoA mutants.
The presence of the capsule (Vi) and the lipopolysaccharide (LPS) O:9 antigen was determined by slide agglutination. Briefly, freshly grown, isolated bacterial colonies of S. typhi parental and mutant strains were suspended in either LPS O:9 Salmonella antiserum (Difco, Detroit, Mich.) or Vi antiserum (Difco), and agglutination was assessed. Motility of all bacterial strains was assessed by the ability of the bacteria to swarm on a semisolid (0.25%) LB agar plate. TnphoA mutants were tested for nutritional requirements by their ability to grow on M9 minimal medium (86) supplemented with cysteine (20 μg/ml) and tryptophan (20 μg/ml).
Restriction enzyme, cloning, and Southern blot analysis.
Restriction enzyme digestion was performed as recommended by the manufacturer (Gibco BRL). Plasmid DNA was purified by using Qiagen plasmid kits and QIAprep spin plasmid kits procedures as described by the manufacturer (Qiagen, Inc., Chatsworth, Calif.). S. typhi chromosomal DNA was purified by the procedure of Mekalanos (67), using proteinase K digestion for 18 h. DNA manipulations and Southern blot hybridization were carried out as described by Sambrook et al. (86). The oligonucleotide probes Km (5′-GTGGAGAGGCTATTCGGCTATGAC-3′) and phoA (5′-CAGAGCGGCAGTCTGATCAC-3′), complementary to the kanamycin and phoA genes, respectively, were labeled and detected by using the ECL 3′ oligolabeling system as described by the manufacturer (Amersham Life Science, Inc., Arlington Heights, Ill.). Chromosomal DNA from the TnphoA invasion-defective mutants was digested to completion with SalI or EcoRI, transferred onto a nylon membrane, and hybridized with the kanamycin or phoA oligonucleotide. EcoRI and SalI cleave within the TnphoA to generate fragments that yield bands representing the downstream and upstream sequences of the TnphoA insertion, respectively.
For the cloning of DNA fragments containing a portion of the TnphoA sequence, chromosomal DNA from invasion-defective mutants was digested to completion with EcoRI or SalI, cloned into Bluescript plasmids (Stratagene, Inc.), and then transformed into E. coli XL1-Blue MRF′. Recombinants were selected for kanamycin resistance encoded by the TnphoA transposon. The presence of the kanamycin resistance gene was confirmed by Southern hybridization analysis with the labeled oligonucleotide Km probe as described above.
DNA sequence analysis.
The nucleotide sequence of the chromosome-TnphoA junction was carried out on double-stranded plasmid DNA templates, using the dideoxy-chain termination method of Sanger et al. (87) and Sequenase (U.S. Biochemical, Cleveland, Ohio) as instructed by the manufacturer. The phoA reverse primer (5′-CAGAGCGGCAGTCTGATCAC-3′) and IS50R primer (5′-TAGGAGGTCACATGGAAGTCAGAT-3′), complementary to the phoA and IS50R sequences, respectively, were used to generate nucleotide sequences from upstream and downstream regions of the transposon insertion. Nucleotide and deduced amino acid sequences data were analyzed with the Genetics Computer Group software package, version 8.0 (16), and the BLAST program at the server of the National Center for Biotechnology Information at the National Library of Medicine (5).
Environmental regulation.
To determine the osmoregulation of selected TnphoA gene fusions, each mutant was grown to mid-logarithmic phase (A600 of ≈0.6) in 10.0 ml of LB with 0.3 or 0.06 M NaCl containing tetracycline and kanamycin. Cells were washed twice with phosphate-buffered saline and resuspended in 1 ml of 100 mM NaCl–10 mM Tris (pH 8.0). Bacterial cells were disrupted by sonication in a model 550 sonic dismembrator (Fisher Scientific, Pittsburgh, Pa.), and cell debris was removed by centrifugation at 12,000 rpm for 5 min. Protein concentrations were determined by using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.). Samples containing 50 μg of protein were assayed to measure the alkaline phosphatase activity. Specific activity units were calculated and expressed as described by Galán and Curtiss (33), using the following formula: units = {optical density at 420 nm [OD420]/[time (minutes) × protein (milligrams)]} × 1,000.
To analyze the effect of the pH on the expression of the TnphoA gene fusions, strains were grown to mid-logarithmic phase (A600 of ≈0.6) in LB buffered with 0.1 M MES (2-[N-morpholino]ethanesulfonic acid) (69) at pH 5.0 or 6.5, supplemented with the appropriate antibiotics from overnight cultures grown under the same conditions. Alkaline phosphatase activity was measured per milligram of protein as described above for the osmoregulation analysis.
For analysis of the effect of oxygen tension on the expression of the TnphoA gene fusions, S. typhi strains were grown aerobically in 500-ml Erlenmeyer flasks with 15 ml of LB medium containing either 0.06 or 0.3 M NaCl. Bacteria were incubated at 37°C with strong agitation in a rotary shaker until the cultures reached an A600 of 0.6. Anaerobiosis was achieved by growing the bacteria in Brewer GasPak jars under an atmosphere of 4 to 10% CO2 generated by the GasPak Plus system (Becton Dickenson, Cockeysville, Md.). The cultures were incubated at 37°C with agitation, and samples processed after the A600 of each culture in LB medium supplemented with either 0.06 or 0.3 M NaCl was about 0.3. Alkaline phosphatase activity was measured per milligram of protein as described above for the osmoregulation analysis.
To assay growth phase-related expression of TnphoA gene fusions, bacterial strains were grown in LB with 0.3 M NaCl containing tetracycline and kanamycin. Three 1.0-ml samples were removed every 30 min, and the A600 was measured. In these experiments, the cells were treated with 0.1% CHCl3 and 0.1% sodium dodecyl sulfate and directly assayed for alkaline phosphatase activity, expressed in Miller units (69). Units were calculated by the following formula: units = {[OD420 − (1.75 × OD550)]/[time (minutes) × volume × OD600]} × 1,000. For strains containing either an invG or prgH TnphoA insertion, the alkaline phosphatase activity was also assayed per milligram of protein as described above.
RESULTS
Isolation of TnphoA mutants that are defective in invasion of Int407 small intestinal epithelial cells.
To identify S. typhi genes involved in the invasion of human small intestinal epithelial cells, S. typhi DC27 was mutagenized with the TnphoA transposon. DC27 is a derivative of wild-type S. typhi ISP1820 from which the periplasmic nonspecific acid phosphatase was eliminated by the introduction of the phoN51::Tn10 mutation. The suicide plasmid pJM703.1, containing TnphoA, was electroporated into DC27, and PhoA+ fusions were identified on LB agar plates containing tetracycline, kanamycin, and the chromogenic substrate XP. From 15 independent electroporations, yielding approximately 27,000 TnphoA mutants, 600 blue colonies expressing the alkaline phosphatase were purified and frozen for further analysis; 437 PhoA+ cells were tested qualitatively for the ability to invade the small intestinal epithelial Int407 cell line when grown in LB with 0.3 M NaCl. Seventeen noninvasive mutants, which showed decreased invasiveness compared to the wild-type strain DC27, were identified. Examples of each mutant type are shown in Table 1. All of the mutants and the wild-type strain DC27 had similar growth characteristics on M9 supplemented minimal medium and growth rates in LB medium. All mutants expressed both the LPS O:9 and Vi antigens, and two mutants, A19 and H1021, were nonmotile (data not shown). Since mutations affecting chemotaxis or motility have been reported to confer a noninvasive phenotype on S. typhi (63), A19 and H1021 were not characterized further at this time.
TABLE 1.
Adherence, invasion, and characterization of S. typhi invasion-defective TnphoA mutants
| Strain | % Adherencea | % Invasionb | Locusc/amino acid position of TnphoA insertion |
|---|---|---|---|
| HB101 | 6.5 | 0.003 | NAd |
| DC27 | 66.7 | 90.9 | NA |
| A1e | 61.6 | 80.0 | NA |
| K14 | 1.4 | 0.010 | invG/53 |
| H5 | 0.4 | 0.016 | prgH/184 |
| I25 | 1.9 | 2.1 | cpxA/89 |
| L5 | 12.9 | 14.9 | damX/222 |
Percentage of cell-associated bacteria after 90 min of incubation with respect to the initial inoculum. Values are representative of two independent experiments.
Percentage of bacteria resistant to gentamicin treatment after 90 min with respect to the initial inoculum. Values are representative of two similar experiments.
Deduced amino acids from partial DNA sequences were determined and used to search the GenBank database. The invG and prgH genes are from S. typhimurium; the cpxA and damX genes are from E. coli K-12.
NA, not applicable.
Mutant A1 represents a randomly selected TnphoA insertion with wild-type levels of adherence and invasion and serves as a positive control.
To determine the number of the TnphoA insertions in each mutant and to obtain an estimate of the number of different chromosomal loci involved in the invasion-defective phenotype observed, the TnphoA mutants were analyzed by Southern blot hybridization (data not shown). Chromosomal DNA was digested with EcoRI and SalI and probed with labeled oligonucleotides generated from the kanamycin or phoA sequences present on TnphoA. No signal was observed with the parental strain DC27 chromosomal DNA. Sixteen of the mutants had a single insertion, while one mutant, H1026, contained a double TnphoA insertion and therefore was not characterized further. At least four different band patterns were readily identified. Results obtained from the EcoRI digestions hybridized with the phoA probe and the SalI digestions hybridized with the Km probe allowed the identification of four distinct classes of mutants (data not shown).
The percentages of both adherence and invasion of small intestinal epithelial cells were determined for the 14 mutants. Examples of the mutant classes are shown in Table 1. One randomly selected TnphoA insertion, mutant A1, showed wild-type levels of adherence and invasion and was used as a positive control. As expected, E. coli HB101 modestly adhered to but did not invade Int407 cells. The majority of the mutants were 5,000- to 10,000-fold less invasive than the parental strain DC27. In contrast, TnphoA mutants I25 and L5 were only 43- and 6-fold, respectively, less invasive than DC27. Interestingly, none of the mutants was able to adhere to the Int407 monolayers as well as the wild type did, which suggests that the defects in invasion could be due to the inability of the mutants to attach to the Int407 monolayers. Decreases in cell-associated bacteria ranged from 35- to 166-fold compared to the parental strain DC27, except for the cell association of mutant L5, which was decreased approximately 5-fold. A similar cell association-defective phenotype was observed with S. typhi SB130 (invA::Km) and H553 (invE::Km), which suggests that adherence and invasion may be tightly linked in S. typhi (95).
Identification and characterization of S. typhi TnphoA gene insertions.
To characterize the 14 TnphoA invasion-defective mutants, EcoRI and SalI chromosomal DNA fragments containing a portion of the TnphoA insertion were cloned into the pBluescript plasmid. Recombinant plasmids were identified by restriction enzyme analysis, and the presence of the kanamycin gene in the inserts was confirmed by Southern hybridization analysis using a Km oligonucleotide as a probe (data not shown). Nucleotide sequences were determined from the junction of the TnphoA insertion by using the IS50R or phoA reverse primers which generated sequences from the downstream or upstream flanking region of the insertion, respectively. The DNA sequence data were compiled and analyzed with the Genetics Computer Group software program (16). Deduced amino acid sequences were used to search the GenBank database sequences. Six independent mutations, A25, F106, L106, K14, N16, and O1012, were identified in a gene sharing homology with the invG invasion gene of S. typhimurium (53). One example is shown in Table 1. Ginocchio et al. (39) demonstrated that the invG gene product is required for the formation of bacterial organelles expressed at the surface of S. typhimurium and is involved in entry into cultured epithelial cells. Kaniga et al. (53) showed that InvG is homologous to the PulD family of protein translocases and suggested that the gene encoding this protein could be involved in the secretion apparatus or assist in the translocation of target proteins through the outer membrane. It has been shown that the invG gene product is involved in the secretion of InvJ, which is required for S. typhimurium invasion (13). InvG shares homology with the Shigella sp. MxiD (1), Yersinia enterocolitica YscC (68), E. coli SepD (48), Pseudomonas solanacearum HrpA (40), Pseudomonas syringae HrpH (45), and Xanthomonas campestris HrpA1 (97). The InvG, MxiD, YscC, SepD, and HrpA proteins are all involved in a type III secretion pathway of virulence factors (reviewed in reference 31).
The deduced amino acid sequences from K14, L106, and O1012 TnphoA insertions ranged between 82 and 85% identity over the 44 to 60 amino acids analyzed compared to S. typhimurium InvG. These data suggest that the InvG amino-terminal domain is highly conserved between S. typhi and S. typhimurium. In contrast, the amino acid sequence deduced from mutants N16 and F106, in which the TnphoA insertions were located in the carboxyl-terminal region of InvG, showed homology of only 63% and 68% amino acid identity. These data suggest that the carboxyl-terminal domain may be less conserved between S. typhi and S. typhimurium.
Six additional independent TnphoA mutations, G3, H5, I17, M9, and M29, were identified in a gene that shares extensive homology to the S. typhimurium PhoP-repressed gene, prgH, a part of the prgHIJK operon (10, 80). Table 1 shows one example of this mutant class. Pegues et al. (80) have suggested that products of some genes encoded by the prgHIJK operon are necessary for synthesis or secretion of Ssp (Sip) proteins required for bacterial entry into epithelial cells. PrgH is homologous to MxiG from Shigella flexneri, a protein that is involved in the secretion of Ipa proteins (2). Since no TnphoA insertions were obtained in the amino-terminal domain of PrgH, we speculate that the amino-terminal domain of PrgH is located in the cytoplasm. This notion is supported by the conjecture that the amino terminus of MxiG is also located in the cytoplasm (2). The deduced amino acid sequences obtained from the regions downstream of the TnphoA insertions of mutant G3 showed 83% identity over 47 amino acids analyzed with the S. typhimurium prgI located downstream from the prgH gene (80). This observation suggests that the prgHIJK operon may be organized in the same manner for both S. typhi and S. typhimurium.
The insertion in mutant I25 was shown to be in a gene sharing homology to the cpxA gene from E. coli K-12. The deduced amino acid sequence of mutant I25 revealed 91% identity over 105 amino acids with CpxA (17). The cpxA gene encodes a sensor kinase that is a part of an operon also encoding the transcriptional regulator, cpxR (17, 85); CpxA and CpxR belong to a family of two-component regulatory systems. Danese et al. (14) suggested that the activated CpxA/CpxR signal transduction pathway is capable of relieving certain envelope-associate stresses and may play a role in directing protein trafficking functions within the cell envelope by inducing the production of multiple extracytoplasmic factors such as the DegP (HtrA) protease.
Finally, the mutant L5 has an insertion in a gene sharing homology with the damX (urf-74.3) gene from E. coli K-12 (51). Although the function of the damX gene is unknown, it has been suggested that this gene may be involved in cell cycle regulation because overexpression induced cell filamentation (65). The damX gene is a part of a superoperon containing genes of unrelated function such as aromatic amino acid biosynthesis (aroK, aroB, and trpS), cell cycle regulation (damX), DNA adenine methyltransferase (dam), and carbohydrate metabolism (gph and rpe) (65).
Environmental signals that regulate S. typhi cpxA, damX, invG, and prgH gene expression. (i) Effect of medium osmolarity.
Osmolarity has been identified as an environmental signal that controls virulence gene expression in several organisms, including the expression of the invasion genes invA, invF, prgH, prgK, orgA, sspA, and sspC of S. typhimurium (7, 33) and the tcp pili of Vibrio cholerae (70). In S. typhi, osmolarity and growth phase overlap in the regulation of adherence to and invasion of human intestinal epithelial cells (91). However, no S. typhi invasion genes have been shown to be regulated by these environmental signals. To evaluate the osmoregulation of the cpxA, damX, invG, and prgH genes, each of the 14 TnphoA gene fusions was analyzed. Whole-cell extracts of mid-logarithmic-phase cultures grown in LB medium with either 0.06 NaCl or 0.3 M NaCl were assayed for alkaline phosphatase (PhoA) activity. The results are shown in Table 2. As previously reported, expression of invA::TnphoA gene fusion from S. typhimurium χ3642 was osmoregulated (33). The random TnphoA insertion mutant, strain A1, showed no significant difference in the level of PhoA activity when grown in either high- or low-osmolarity medium. In the invG::TnphoA and prgH::TnphoA gene fusions, the phosphatase activity was induced in high-osmolarity LB medium. The invG gene expression was induced 5- to 12-fold, whereas prgH gene expression was induced 9- to 20-fold in high-osmolarity medium. In addition, the phosphatase activity in the cpxA::TnphoA gene fusion was induced fourfold in high-osmolarity medium. Conversely, no induction or repression of the PhoA activity was observed with the damX::TnphoA gene fusion under the same conditions. These results indicate that invG, prgH, and to a lesser extent cpxA are osmoregulated genes that may be involved in the entry of S. typhi into intestinal epithelial cells.
TABLE 2.
Effect of osmolarity on the expression of invasion-defective TnphoA gene fusions
| Straina | Locus/amino acid position | Mean PhoA activity (Miller units)b ± SEM
|
Ratioc | |
|---|---|---|---|---|
| LB–0.3 M NaCl | LB–0.06 M NaCl | |||
| χ3642d | invA | 129 ± 2.3 | 34 ± 1.1 | 3.8 |
| A1 | Z::TnphoA | 190 ± 53 | 329 ± 52 | 0.6 |
| K14 | invG/53 | 216 ± 18 | 17 ± 7 | 12.7 |
| H5 | prgH/184 | 1278 ± 422 | 110 ± 5 | 11.6 |
| I25 | cpxA/89 | 166 ± 11 | 38 ± 11 | 4.4 |
| L5 | damX/222 | 156 ± 6 | 192 ± 15 | 0.8 |
Strains were grown to mid-logarithmic phase (OD600 of ≈0.6) in LB supplemented with 0.3 or 0.06 M NaCl and with kanamycin and tetracycline. Cell extracts were prepared by sonication, and the amount (milligrams) of protein was determined by the Bio-Rad assay.
Alkaline phosphatase activity was measured as described in Materials and Methods. The data represent means of duplicate samples from three independent experiments.
Ratio of PhoA activity LB–0.3 M NaCl/LB–0.06 M NaCl.
invA::TnphoA mutant of S. typhimurium.
(ii) Effect of gyrase inhibitor on cpxA, invG, and prgH gene expression.
Previous studies have shown that DNA supercoiling can modulate gene expression in response to high osmolarity, anaerobiosis, and growth phase (19, 73). Galán and Curtiss (33) reported that the osmoinduction of invA was independent of the ompB (ompR/envZ) regulon but affected by the degree of DNA superhelicity. The level of DNA supercoiling in vivo can be perturbed by using novobiocin, which specifically inhibits the β subunit of DNA gyrase and results in relaxation of intracellular DNA (37). To determine whether the cpxA, invG, and prgH genes were affected by DNA supercoiling, the effects of novobiocin were examined. TnphoA mutants A1, K14, H5, and I25 were grown overnight in LB medium with 0.3 M NaCl and increasing amounts of novobiocin and then subcultured into fresh medium of the same characteristics. In contrast to S. typhimurium, 10 to 20 μg of novobiocin per ml significantly inhibited the growth of S. typhi (data not shown). More importantly, when the S. typhi mutants were grown in LB supplemented with 2 μg of novobiocin per ml, expression of invG::TnphoA and prgH::TnphoA fusion products was inhibited 9- and 17-fold, respectively (Table 3). In contrast, the PhoA expression from the cpxA::TnphoA or the random Z::TnphoA insertion fusion was not affected by changes in DNA supercoiling. Collectively, these data suggest that transcription from chromosomal invG and prgH promoters is sensitive to the degree of DNA supercoiling in response to different environmental stimuli such as osmolarity.
TABLE 3.
Effect of novobiocin on expression of cpxA, invG, and prgH
| Straina | Locus | Novobiocin concn (μg/ml) | Mean PhoA activity (Miller units)b ± SEM |
|---|---|---|---|
| A1 | Z::TnphoA | 0 | 124 ± 10 |
| 2 | 160 ± 5 | ||
| 5 | 38 ± 6 | ||
| 10 | 109 ± 6 | ||
| K14 | invG | 0 | 376 ± 47 |
| 2 | 42 ± 0.2 | ||
| 5 | 39 ± 1 | ||
| 10 | 46 ± 3 | ||
| H5 | prgH | 0 | 928 ± 367 |
| 2 | 53 ± 4 | ||
| 5 | 27 ± 1 | ||
| 10 | 29 ± 1 | ||
| I25 | cpxA | 0 | 127 ± 2 |
| 2 | 177 ± 6 | ||
| 5 | 169 ± 7 | ||
| 10 | 119 ± 4 |
Strains were grown to mid-logarithmic phase (OD600 of ≈0.6) in LB–0.3 M NaCl with or without various concentrations of novobiocin. Cell extracts were prepared by sonication, and the amount (milligrams) of protein was determined by the Bio-Rad assay.
Alkaline phosphatase activity was measured as described in Materials and Methods. The data represent means from two independent experiments.
(iii) Effect of pH.
Acid adaptation is likely to be an important variable in Salmonella pathogenicity, since pH has been identified as a regulator of virulence gene expression in both human and plant pathogens. These genes include the invF, pagC, prgH, prgK, orgA, sspA, and sspC genes of S. typhimurium (3, 7, 10), the inv gene of Y. enterocolitica (81), the hrp genes from P. syringae pv. phaseolicola (84), and the vir gene of Agrobacterium tumefaciens (98). To determine whether S. typhi invasion genes are regulated by pH, expression of the TnphoA gene fusions was analyzed at pH 5.0 and 6.5. Bacterial strains were grown to mid-logarithmic phase in LB medium buffered with 0.1 M MES at pH 5.0 and 6.5 from overnight cultures grown under the same conditions. Cell extracts were prepared, and alkaline phosphatase activity was assessed. The growth curves of the mutants in the different conditions tested were similar (data not shown). As shown in Table 4, the invG::TnphoA and prgH::TnphoA gene fusion products were highly reduced at pH 5.0. In contrast, expression of neither cpxA::TnphoA nor damX::TnphoA gene fusion products was altered by pH. These results indicate that invG and prgH genes are repressed at low pH. A low-pH environment is encountered in the stomach by the pathogens during passage to the intestine or intracellularly within endosomes after invasion of intestinal epithelial cells or macrophages (90).
TABLE 4.
Effect of pH on the expression of invasion-defective TnphoA gene fusions
| Bacterial straina | Locus/amino acid position | Mean PhoA activity (Miller units)b ± SEM
|
Ratioc | |
|---|---|---|---|---|
| LB, pH 5.0 | LB, pH 6.5 | |||
| A1 | Z::TnphoA | 662 ± 229 | 187 ± 34 | 0.3 |
| K14 | invG/53 | 22 ± 11 | 497 ± 98 | 22.5 |
| H5 | prgH/184 | 171 ± 70 | 1,027 ± 145 | 6.0 |
| I25 | cpxA/89 | 165 ± 23 | 128 ± 4 | 0.8 |
| L5 | damX/222 | 120 ± 39 | 125 ± 10 | 1.0 |
Strains were grown to mid-logarithmic phase (OD600 of ≈0.6) in LB buffered with 0.1 M MES at pH 5.0 or 6.5 with kanamycin and tetracycline. Cell extracts were prepared by sonication, and the amount (milligrams) of protein was determined by the Bio-Rad assay.
Alkaline phosphatase activity was measured as described in Materials and Methods. The data represent means of duplicate samples from four independent experiments.
Ratio of PhoA activity LB at pH 6.5/LB at pH 5.0.
(iv) Effect of oxygen tension.
Another variable that S. typhi may encounter when entering a host is the reduced oxygen tension of the distal ileum (90). Independent studies have shown that reduced oxygen tension during growth of bacteria enhanced attachment and invasiveness of S. cholerasuis and S. typhimurium in vitro, which suggested that invasion genes may be induced under anaerobic conditions (24, 30, 52, 59, 60, 89). For instance, the invF, prgH, prgK, orgA, sspA, and sspC genes, which are involved in the virulence of S. typhimurium, have been shown to be induced under anaerobic conditions (7, 52). However, Behlau and Miller (10) showed that the S. typhimurium prgH invasion locus was repressed under anaerobic conditions. To determine the effect of oxygen tension on cpxA, damX, invG, and prgH gene expression in S. typhi, TnphoA mutants A1, I25, K14, H5, and L5 were grown anaerobically and cell extracts were assayed for alkaline phosphatase activity (Table 5). In comparison to growth in media on a rotary shaker in air, anaerobiosis decreased the S. typhi growth rate (data not shown). Nonetheless, as shown in Table 5, low oxygen tension had a strong effect on the expression of the invG and prgH genes. The alkaline phosphatase activity of the invG TnphoA mutant was 14-fold higher under anaerobic conditions and was enhanced similarly in both high- and low-osmolarity media. The prgH gene was also strongly regulated under low-oxygen conditions when bacteria were grown in LB medium supplemented with 0.06 M NaCl (low osmolarity). In the cultures, the alkaline phosphatase activity of the gene fusion was 29-fold higher when bacteria were grown under anaerobic conditions compared to the aerobic cultures. In contrast, the osmoinduction of cpxA was not affected by oxygen tension, while invG and prgH genes were strongly affected. The activity of the cpxA, damX (data not shown), and control Z::TnphoA fusions was not affected by oxygen tension in either high- or low-osmolarity conditions. These results suggest that osmolarity and oxygen tension appear to be the major environmental signals controlling the expression of invG and prgH genes.
TABLE 5.
Effect of oxygen tension on the expression of invasion-defective TnphoA gene fusions
| Strain, TnphoA fusion | Medium | Mean PhoA activity (Miller units)a ± SEM
|
Ratiod | |
|---|---|---|---|---|
| Aerobicb | Anaerobicc | |||
| A1, Z::TnphoA | LB–0.06 M NaCl | 182 ± 72 | 133 ± 25 | 0.7 |
| LB–0.3 M NaCl | 127 ± 29 | 194 ± 21 | 1.5 | |
| I25, cpxA | LB–0.06 M NaCl | 21 ± 2 | 19 ± 2 | 0.9 |
| LB–0.3 M NaCl | 64 ± 1 | 80 ± 20 | 1.3 | |
| K14, invG | LB–0.06 M NaCl | 10 ± 3 | 105 ± 39 | 10.5 |
| LB–0.3 M NaCl | 28 ± 6 | 377 ± 78 | 13.5 | |
| H5, prgH | LB–0.06 M NaCl | 11 ± 2 | 314 ± 79 | 28.6 |
| LB–0.3 M NaCl | 96 ± 1 | 370 ± 42 | 3.8 | |
Alkaline phosphatase activity was measured as described in Materials and Methods. The data represent means of duplicate samples from two independent experiments.
Strains were grown with aeration and agitation to mid-logarithmic phase (OD600 of ≈0.6) in LB–0.06 M NaCl or LB–0.3 M NaCl containing kanamycin and tetracycline. Cell extracts were prepared by sonication, and the amount (milligrams) of protein determined by the Bio-Rad assay.
Strains were grown under an anaerobic atmosphere of 4 to 10% CO2 with agitation to mid-logarithmic phase (OD600 of ≈0.3) in LB–0.06 M NaCl or LB–0.3 M NaCl with kanamycin and tetracycline. Cell extracts were prepared by sonication, and the amount (milligrams) of protein was determined by the Bio-Rad assay.
Ratio of PhoA activity, anaerobic/aerobic.
(v) Effect of growth phase.
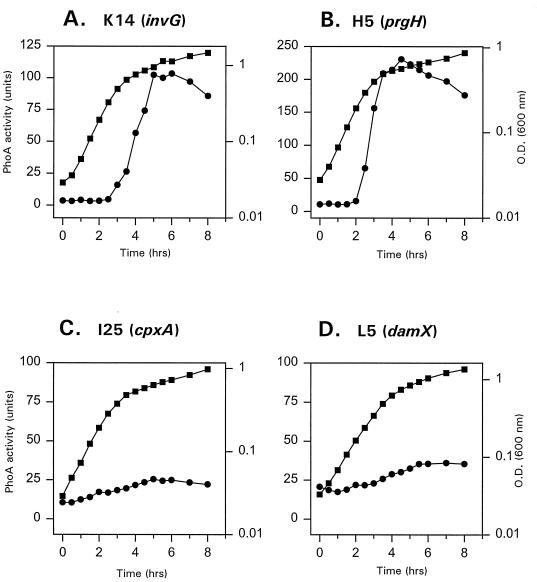
Previous studies from our laboratory demonstrated that high osmolarity and growth phase conditions overlap for optimal induction of adherence of S. typhi to intestinal epithelial cells (91). These studies also showed that optimal adherence occurred during the mid- to late log growth phase. Lee and Falkow (59) have also shown that the capacity of S. cholerasuis to enter mammalian cells was a function of the bacterial growth state. Moreover, Behlau and Miller (10) demonstrated that expression of all S. typhimurium prg loci was maximal in late logarithmic growth phase. Collectively, these data suggested that S. typhi invasion genes may be also temporally regulated by the growth phase. To test whether expression of S. typhi cpxA, damX, invG, and prgH genes is growth phase dependent, the PhoA activity of the TnphoA gene fusions was analyzed at different phases of growth. Bacterial strains were grown in optimal conditions using LB medium with 0.3 M NaCl; samples were removed periodically and assayed for alkaline phosphatase activity. The results presented in Fig. 1 show that the expression of prgH and invG genes was induced in late logarithmic phase or early stationary phase, indicating that expression of both genes was growth phase dependent. Similar results were obtained when PhoA activity was assayed per milligram of protein (data not shown). In addition, the expression of the invG and prgH genes was reduced approximately 50% in late stationary growth phase (data not shown). In contrast, the cpxA and damX genes were not regulated by growth phase. The timing of invG and prgH gene expression correlates with the timing of maximal expression of all S. typhimurium prg loci and secretion of the Ssp (Sip) proteins (10, 80).
FIG. 1.
Temporal expression of invG, prgH, cpxA, and damX::TnphoA gene fusions. Strains were grown in LB–0.3 M NaCl containing kanamycin and tetracycline. Samples were removed at 30-min intervals, and cell density (▪) and PhoA activity (•) were measured. Alkaline phosphatase activity is expressed in Miller units as described in Materials and Methods. Data are representative of two independent experiments done in duplicate.
DISCUSSION
To persist in the human host, S. typhi, as well as other facultative intracellular pathogens, must encounter and survive numerous complex extracellular (stomach, intestine, lymphatic system, and bloodstream) and intracellular (phagocytic and nonphagocytic cells) environments. Previous studies have suggested that some of these environments modulate the expression of virulence factors (reviewed in reference 66). However, relatively little is known about the effects of these environmental signals on the expression of S. typhi virulence genes. Because no physiologically relevant animal model exists for S. typhi, investigators have analyzed the response to S. typhimurium, which causes a typhoid-like disease in mice, in an attempt to understand the molecular mechanisms of S. typhi pathogenesis. In this report, we have identified and characterized 14 S. typhi TnphoA mutants that are involved in the initial interactions of S. typhi with the host and analyzed gene expression in response to environmental stimuli. The results demonstrate that 12 of 16 mutations were in genes that shared homologies with S. typhimurium genes known to be involved in the type III secretion pathway of virulence proteins. Six independent mutations in the invasion gene, invG, and six in the PhoP repressed gene, prgH, were found. Two additional insertions were identified in genes sharing homology with the cpxA and damX genes of E. coli K-12. Finally, two invasion-deficient mutants (A19 and H1021) were found to be nonmotile.
Initially, our TnphoA mutants were screened only for defects in invasion of intestinal epithelial cells. However, our results demonstrated that TnphoA insertions in invG, prgH, cpxA, and damX genes also rendered S. typhi defective in adherence to, as well as invasion of, the small intestinal epithelial cell line Int407. The possibility that the TnphoA insertion has exerted a polar effect on other adherence/invasion genes located downstream cannot be excluded at this time. Nonetheless, this linkage between adherence and invasion has also been observed in S. typhi mutants SB130 (invA::Km) and H553 (invE::Km) (95). However, in S. typhimurium, the results are different. S. typhimurium invA and invE, as well as invC, invF, invG, invI, invJ, and prgH, invasion mutants are able to adhere to intestinal epithelial cell monolayers at levels similar to wild-type levels (10, 13, 21, 35, 38, 53). These observations suggest that adherence and invasion mechanisms of the two organisms may be distinct. Differences between S. typhimurium and S. choleraesuis have also been reported. TnphoA mutants of S. choleraesuis that were unable to pass through (transcytose) polarized MDCK epithelial monolayers were also unable to adhere to or invade MDCK cells (28). An S. choleraesuis invH mutant was shown to be deficient in both attachment and invasion of cultured epithelial cells (4). Taken together, our results and those of Altmeyer et al. (4) suggest that host-adapted pathogen adherence/invasion genes have acquired or modified their function for optimal adherence and invasion of intestinal epithelial cells. It is also possible that adherence and invasion phenotypes are mediated by the same protein(s) in S. typhi as previously shown for the inv gene of Yersinia spp. (47). Mutations in the inv genes of Y. enterocolitica and Y. pseudotuberculosis altered both the ability to enter and to adhere to eukaryotic cells (47). Alternatively, the adherence/invasion defect phenotype that we observed in S. typhi could be caused by the nonspecific toxic effect exhibited by an active PhoA+ fusion that could block the export pathway of other proteins needed for invasion. In this case, it is possible that TnphoA mutations in the cpxA and damX genes indirectly affect the adherence/invasion mechanism of S. typhi.
In E. coli, the CpxA/CpxR signal transduction pathway regulates the expression of the DegP (HtrA) protease under certain envelope stresses (14). Thus, the function of the CpxA/CpxR regulon in bacterial pathogenesis may be related to the role of the DegP (HtrA) protease. In S. typhimurium, mutations in the htrA gene were shown to be avirulent or highly attenuated when given orally to mice (12, 25, 49). Interestingly, Everest et al. (25) showed that expression of the htrA gene was significantly increased when the Salmonella entered eukaryotic cells. The HtrA protease has also been shown to be involved in the virulence of Y. enterocolitica and Brucella abortus (23, 62). Recently, Nakayama and Watanabe (72) reported that cpxA could be involved in a pH-dependent regulation of the expression of Shigella sonnei virF gene, which positively regulates ipaBCD gene expression. In contrast, our results shown that the expression of the S. typhi cpxA gene is pH independent but osmoregulated, indicating that Shigella and Salmonella might sense different environmental signals to control the expression of virulence factors. Taken together, these data suggest that the CpxA/CpxR regulon may play a role in S. typhi virulence as well as other pathogens, by modulating the expression of the HtrA protease.
Recently, Hermant et al. (42) identified 15 noninvasive TnphoA mutants from S. typhi Ty2 that were unable to enter HeLa cells. Two mapped within the iagAB locus, and six others mapped in the sipEBCDA locus. It is interesting that none of our 16 TnphoA insertions were in the sipEBCDA locus but were found primarily within the invG and prgH genes. It is possible that the conditions used for our invasion assay favored the identification of some osmoregulated invasion genes such as the invG and prgH instead of the sip genes. In our standard invasion assay, the bacteria are grown in LB with 0.3 M NaCl, the optimal growth conditions for S. typhi adherence to and invasion of intestinal epithelial cells (91). Our results suggest that a more substantial decrease in invasion is observed when a subset of osmoregulated invasion mutants are compared to the wild type under conditions of high osmolarity.
Our studies demonstrate that osmolarity is one of the major environmental signals controlling the expression of S. typhi virulence genes. It is interesting that osmolarity not only controls the expression of S. typhi invG and prgH invasion genes but also appears to regulate the level of Vi capsule antigen synthesis (82). This notion is consistent with our published and preliminary data that show optimal adherence occurs at high osmolarity when the capsule is the smallest (91, 92, 95). Our results also show that low oxygen tension is an important factor that regulates the expression of genes involved in the invasive phenotype of S. typhi and are in agreement with studies conducted with S. typhimurium (7, 52). However, our results are the first to show that anaerobiosis affects the expression of the invG gene under both high- and low-osmolarity conditions. The prgH gene is also induced under low oxygen tension, but the effect is stronger under low-osmolarity conditions. It is possible that the effects of osmolarity, pH, and anaerobiosis are exerted on the posttranslational modification of the gene products. However, since other investigators have reported direct regulation on related genes (7, 33), we have assumed that the effects observed in our studies are at the transcriptional level. Discrepancies between our results and data from other investigators could be explained by differences in the growth conditions of the bacterial cultures used.
Expression of the S. typhi invG and prgH genes was also shown to be growth phase dependent. Transcription of invG and prgH genes was initiated in the late log/early stationary phase (Fig. 1). This observation suggests that these two genes may be regulated by the RNA polymerase subunit ςS (RpoS) that is required for expression of stationary phase as well as osmotically regulated genes in E. coli (reviewed in reference 64). In S. typhimurium, the rpoS gene mediates the expression of the spv plasmid virulence genes during bacterial starvation (26, 75). Interestingly, the S. typhi invG and prgH genes were repressed under starvation conditions (data not shown). In addition, it has been shown that ςS is required for a sustained acid tolerance response in virulent S. typhimurium (61). A sustained acid tolerance response also provided cross-protection to a variety of other environmental stresses such as heat, H2O2, and osmolarity (61). Taken together, our findings suggest that S. typhi invG and prgH invasion genes can be regulated by the sigma factor ςS (RpoS) in response to environmental signals such as osmolarity, pH, and growth phase.
Gene expression can be modulated by changes in DNA supercoiling in response to high osmolarity, anaerobiosis, and growth phase (19, 73). Increases in DNA superhelicity can either activate expression of some genes or repress others (18). Salmonella invasiveness is affected by environmental conditions that influence the degree of DNA supercoiling (33, 59). For instance, the S. typhimurium invA gene has been shown to be induced by DNA supercoiling (33). Our findings support the view that changes in DNA supercoiling may be a regulatory mechanism for the control of virulence gene expression (18). Expression of S. typhi invG and prgH genes was significantly decreased in the presence of novobiocin, an inhibitor of DNA gyrase (Table 3). The mechanism of gene regulation by DNA topology, in response to environmental signals, is still unknown. However, such a mechanism may require a signal transduction system to sense changes in the environment for controlling gene expression. Studies have shown that in S. typhimurium, invF and prgH genes were activated by HilA or by a mutation that overexpressed the hilA gene (6, 10). Bajaj et al. (6) showed that the hilA gene encodes a transcriptional regulator. Recently, Bajaj et al. (7) reported that S. typhimurium invF, prgH, prgK, orgA, sspA, and sspC genes were coordinately regulated by oxygen, osmolarity, pH, PhoP/Q, and HilA. The expression of the hilA gene was shown to be activated by sirA, a newly identify phosphorylated response regulator gene (50). A homologous hilA gene has been identified in S. typhi (71), which suggests that S. typhi invG and prgH gene can also be regulated by the regulatory cascade SirA-HilA. Consequently, the sirA and hilA genes may be excellent candidates to control invasion gene expression by DNA supercoiling in response to environmental conditions.
In summary, we have attempted to establish in vitro culture conditions that reflect the environmental milieu present in the intestinal lumen of the human host. We have analyzed the effects of these conditions on expression of genes involved in invasion of intestinal epithelial cells by S. typhi. Our studies suggest that specific environmental conditions act as signals to induce the expression of S. typhi invasion genes. These studies further our understanding of the initial steps in the pathogenesis of S. typhi and the interactions of this pathogen with intestinal epithelial cells.
ACKNOWLEDGMENTS
We thank D. Chabot, A. Jerse, G. LeClerc, R. Sandlin, R. Schuch, and D. Weinstein for review of the manuscript and helpful discussions. We thank Barbara O’Neill for outstanding assistance with tissue culture experiments.
This work was supported by NIH grant AI32951 and USUHS grants RO7305 and R07FE.
REFERENCES
- 1.Allaoui A, Sansonetti P J, Parsot C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol Microbiol. 1993;7:59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 2.Allaoui A, Sansonetti P J, Menard R, Barzu S, Mounier J, Phalipon A, Parsot C. MxiG, a membrane protein required for secretion of Shigella ssp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol Microbiol. 1995;17:461–470. doi: 10.1111/j.1365-2958.1995.mmi_17030461.x. [DOI] [PubMed] [Google Scholar]
- 3.Alpuche-Aranda C M, Swanson J A, Loomis W P, Miller S I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altmeyer R M, McNern J K, Bossio J C, Rosenshine I, Finlay B B, Galán J E. Cloning and molecular characterization of a gene involved in Salmonella adherence and invasion of cultured epithelial cells. Mol Microbiol. 1993;7:89–98. doi: 10.1111/j.1365-2958.1993.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 5.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 8.Baudry B, Kaczorek M, Sansonetti P J. Nucleotide sequence of the invasion plasmid antigen B and C genes (ipaB and ipaC) of Shigella flexneri. Microb Pathog. 1988;4:345–357. doi: 10.1016/0882-4010(88)90062-9. [DOI] [PubMed] [Google Scholar]
- 9.Baumler A J, Tsolis R M, Heffron F. Contribution of fimbrial operons to attachment to and invasion of epithelial cell lines by Salmonella typhimurium. Infect Immun. 1996;64:1862–1865. doi: 10.1128/iai.64.5.1862-1865.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter P B, Collins F M. Growth of typhoid and paratyphoid bacilli in intravenously infected mice. Infect Immun. 1974;10:810–822. doi: 10.1128/iai.10.4.816-822.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harboring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 13.Collazo C M, Zierler M K, Galán J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 14.Danese P N, Snyder W B, Cosma C L, Davis L J B, Silhavy T J. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 15.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 16.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong J, Iuchi S, Kwan H-S, Lu Z, Lin E C C. The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transuction system of Escherichia coli. Gene. 1993;136:227–230. doi: 10.1016/0378-1119(93)90469-j. [DOI] [PubMed] [Google Scholar]
- 18.Dorman C J. DNA supercoiling and environmental regulation of gene expression in pathogenic bacteria. Infect Immun. 1991;59:745–749. doi: 10.1128/iai.59.3.745-749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorman C J, Barr G C, Ni Bhriain N, Higgins C F. DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol. 1988;170:2816–2826. doi: 10.1128/jb.170.6.2816-2826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelman R, Levine M M. Summary of an international workshop on typhoid fever. Rev Infect Dis. 1986;8:329–349. doi: 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
- 21.Eichelberg K, Ginocchio C C, Galán J E. Molecular and functional characterization of the Salmonella typhimurium invasion genes invB and invC: homology of invC to the F0F1 ATPase family of proteins. J Bacteriol. 1994;176:4501–4519. doi: 10.1128/jb.176.15.4501-4510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsinghorst E A, Baron L S, Kopecko D J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elzer P H, Phillips R W, Kovach M E, Peterson K M, Roop R M., II Characterization and genetic complementation of a Brucella abortus high-temperature-requirement (htrA) deletion mutant. Infect Immun. 1994;62:4135–4139. doi: 10.1128/iai.62.10.4135-4139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ernst R K, Dombroski D M, Merrick J M. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect Immun. 1990;58:2014–2016. doi: 10.1128/iai.58.6.2014-2016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everest P, Frankel G, Li J, Lund P, Chatfield S, Dougan G. Expression of LacZ from the htrA, nirB and groE promoters in a Salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microbiol Lett. 1995;126:97–102. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 26.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative ς factor KatF (RpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finlay B B, Falkow S. Salmonella as an intracellual parasite. Mol Microbiol. 1989;3:1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 28.Finlay B B, Starnbach M N, Francis C L, Stocker B A D, Chatfield S, Dougan G, Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988;2:757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 29.Foster J W, Hall H K. Adaptative acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990;172:771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis C L, Starnbach M N, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992;6:3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 31.Galán J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 32.Galán J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galán J E, Curtiss R., III Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galán J E, Curtiss R., III Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect Immun. 1991;59:2901–2908. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galán J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of invA to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Vescovi E, Soncini F C, Groisman E A. Mg+2 as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;82:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 37.Gellert M, O’Dea M H, Itoh T, Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci USA. 1976;73:4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginocchio C, Pace J, Galán J E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci USA. 1992;89:5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginocchio C C, Olmsted S B, Wells C L, Galán J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 40.Gough C L, Genin S, Zischek C, Boucher C A. hrp genes of Pseudomonas solanacearum are homologous to pathogenicity determinants of animal pathogenic bacteria and are conserved among plant pathogenic bacteria. Plant-Microbe Interact. 1992;5:384–389. doi: 10.1094/mpmi-5-384. [DOI] [PubMed] [Google Scholar]
- 41.Groisman E A, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermant D, Menard R, Arricau N, Parsot C, Popoff Y. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol Microbiol. 1995;17:781–789. doi: 10.1111/j.1365-2958.1995.mmi_17040781.x. [DOI] [PubMed] [Google Scholar]
- 43.Hornick R B, Greisman S E, Woodward T E, Dupont H L, Dawkins A T, Snyder M J. Typhoid fever: pathogenesis and immunologic control (first two parts) N Engl J Med. 1970;283:739–746. doi: 10.1056/NEJM197010012831406. [DOI] [PubMed] [Google Scholar]
- 44.Hornick R B, Greisman S E, Woodward T E, Dupont H L, Dawkins A T, Snyder M J. Typhoid fever: pathogenesis and immunologic control (second two parts) N Engl J Med. 1970;283:686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 45.Huang H C, He S Y, Bauer D W, Collmer A. The Pseudomonas syringae pv. syringae 61 hrpH product, an envelope protein required for elicitation of the hypersensitive response in plants. J Bacteriol. 1992;174:6878–6885. doi: 10.1128/jb.174.21.6878-6885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 47.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 48.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson K, Charles I, Dougan G, Pickard D, O’Gaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnston C, Pegues D A, Hueck C J, Lee C A, Miller S I. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 51.Jonczyk P, Hines R, Smith D W. The Escherichia coli dam gene is expressed as a distal gene of a new operon. Mol Gen Genet. 1989;217:85–96. doi: 10.1007/BF00330946. [DOI] [PubMed] [Google Scholar]
- 52.Jones B D, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994;62:3745–3752. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaniga K, Bossio J C, Galán J E. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 54.Kaniga K, Trollinger D, Galán J E. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaniga K, Tucker S, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohbata S, Yokoyama H, Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer’s patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol. 1986;30:1225–1237. doi: 10.1111/j.1348-0421.1986.tb03055.x. [DOI] [PubMed] [Google Scholar]
- 57.Leclerc G, Metcalf E S. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Osmoregulated genes of Salmonella typhi involved in the invasion of intestinal epithelial cells, abstr. B-258. [Google Scholar]
- 58.Lee C A. Genetic approaches to understanding Salmonella pathogenicity. In: Miller V L, Kaper J B, Portnoy D A, Isberg R R, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C: American Society for Microbiology; 1994. pp. 215–234. [Google Scholar]
- 59.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee C A, Jones B D, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. The stationary-phase sigma factor ςS (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 62.Li S-R, Dorrell N, Everest P H, Dougan G, Wren B W. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infect Immun. 1996;64:2088–2094. doi: 10.1128/iai.64.6.2088-2094.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu S-L, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56:1967–1973. doi: 10.1128/iai.56.8.1967-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 65.Lyngstadaas A, Lobner-Olesen A, Boye E. Characterization of three genes in the dam-containing operon of Escherichia coli. Mol Gen Genet. 1995;247:546–554. doi: 10.1007/BF00290345. [DOI] [PubMed] [Google Scholar]
- 66.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 68.Michiels T, Vanooteghem J-C, Lambert de Rouvroit C, China B, Gustin A, Boudry P, Cornelis G R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:494–509. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 70.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholera requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miras I, Hermant D, Arricau N, Popoff M Y. Nucleotide sequence of iagA and iagB genes involved in invasion of HeLa cells by Salmonella enterica subsp. enterica ser. typhi. Res Microbiol. 1995;146:17–20. doi: 10.1016/0923-2508(96)80267-1. [DOI] [PubMed] [Google Scholar]
- 72.Nakayama S-I, Watanabe H. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J Bacteriol. 1995;177:5062–5069. doi: 10.1128/jb.177.17.5062-5069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ni Bhriain N, Dorman C J, Higgins C F. An overlap between osmotic and anerobic stress responses: a potential role for DNA supercoiling in the coordinate regulation of gene expression. Mol Microbiol. 1989;3:933–942. doi: 10.1111/j.1365-2958.1989.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 74.Niesel D W, Peterson J W. Calcium enhances Salmonella typhimurium invasion of HeLa cells. FEMS Microbiol Lett. 1987;41:299–304. [Google Scholar]
- 75.Norel F, Robbe-Saule V, Popoff M Y, Coynault C. The putative sigma factor KatF (RpoS) is required for the transcription of the Salmonella typhimurium virulence gene spvB in Escherichia coli. FEMS Microbiol Lett. 1992;99:271–276. doi: 10.1016/0378-1097(92)90039-q. [DOI] [PubMed] [Google Scholar]
- 76.O’Brien A D. Innate resistance of mice to Salmonella typhi infection. Infect Immun. 1982;28:948–952. doi: 10.1128/iai.38.3.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Brien A D, Taylor B A, Metcalf E S. Host genes that influence the pathogenesis of murine typhoid. In: Keusch G, Wadstrom T, editors. Experimental bacterial and parasitic infections. New York, N.Y: Elsevier; 1983. pp. 39–49. [Google Scholar]
- 78.O’Callaghan D, Charbit A. High efficiency transformation of S. typhimurium and S. typhi by electroporation. Mol Gen Genet. 1990;226:156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- 79.Pang T. Preface: typhoid fever—a continuing problem. In: Pang T, Koh C L, Puthucheary S D, editors. Typhoid fever, strategies for the 90’s. Singapore, Republic of Singapore: World Scientific; 1992. pp. 1–2. [Google Scholar]
- 80.Pegues D A, Hanyman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 81.Pepe J C, Badger J L, Miller V L. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol Microbiol. 1994;11:123–135. doi: 10.1111/j.1365-2958.1994.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 82.Pickard D, Li J, Roberts M, Maskell D, Hone D, Levine M, Dougan G, Chatfield S. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun. 1994;62:3984–3993. doi: 10.1128/iai.62.9.3984-3993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Plant J, Glynn A A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976;133:72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- 84.Rahme L G, Mindros M N, Panopoulos N J. Plant and environmental sensory signals control the expression of hrp genes in Pseudomonas syringae pv. phaseolicola. J Bacteriol. 1992;174:3499–3507. doi: 10.1128/jb.174.11.3499-3507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ronson C W, Nixon B T, Ausubel F M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987;49:579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- 86.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 87.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sasakawa C, Adler B, Tobe T, Okada N, Nagai S, Komatsu K, Yoshikawa M. Functional organization and nucleotide sequence of virulence region-2 on the large virulence plasmid of Sh. flexneri 2a. Mol Microbiol. 1989;3:1191–1201. doi: 10.1111/j.1365-2958.1989.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 89.Schiemann D A, Shope S R. Anaerobic growth of Salmonella typhimurium results in increased uptake by Henle 407 epithelial and mouse peritoneal cells in vitro and repression of a major outer membrane protein. Infect Immun. 1991;59:437–440. doi: 10.1128/iai.59.1.437-440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sleisenger M H. Pathophysiology of the gastrointestinal tract. In: Smith L H, Thier S O, editors. Pathophysiology: the biological principles of disease. Philadelphia, Pa: Saunders; 1981. pp. 1526–1537. [Google Scholar]
- 91.Tartera C, Metcalf E S. Osmolarity and growth phase overlap in regulation of S. typhi adherence to and invasion of human intestinal cells. Infect Immun. 1993;61:3084–3089. doi: 10.1128/iai.61.7.3084-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tartera C, van der Sluijs A, Metcalf E S. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Environmental regulation of adherence and invasion of Salmonella typhi, abstr. 1380. [Google Scholar]
- 93.Venkatesan M M, Buysse J M. Nucleotide sequence of invasion plasmid antigen gene ipaA from Shigella flexneri 5. Nucleic Acids Res. 1991;18:1648. doi: 10.1093/nar/18.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Venkatesan M M, Buysse J M, Kopecko D J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci USA. 1988;85:9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weinstein, D. L., B. L. O’Neill, D. E. Hone, and E. S. Metcalf. Differential early interactions between Salmonella typhi and two other pathogenic Salmonellae with intestinal epithelial cells. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 96.Weinstein D L, O’Neill B L, Metcalf E S. Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infect Immun. 1997;65:395–404. doi: 10.1128/iai.65.2.395-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wengelnik K, Marie C, Russel M, Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol. 1996;178:1061–1069. doi: 10.1128/jb.178.4.1061-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winans S C. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J Bacteriol. 1990;172:2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yokoyama H, Ikedo M, Kohbata S, Ezaki T, Yabuuchi W. An ultrastructural study of HeLa cell invasion with Salmonella typhi GIFU 10007. Microbiol Immunol. 1987;31:1–11. doi: 10.1111/j.1348-0421.1987.tb03063.x. [DOI] [PubMed] [Google Scholar]