Abstract
SARM1 is an NAD+ hydrolase and cyclase involved in axonal degeneration. In addition to NAD+ hydrolysis and cyclization, SARM1 catalyzes a base exchange reaction between nicotinic acid (NA) and NADP+ to generate NAADP, which is a potent calcium signaling molecule. Herein, we describe efforts to characterize the hydrolysis, cyclization, and base exchange activities of TIR-1, the C. elegans ortholog of SARM1; TIR-1, also catalyzes NAD(P)+ hydrolysis and/or cyclization and regulates axonal degeneration in worms. We show that the catalytic domain of TIR-1 undergoes a liquid-to-solid phase transition that not only regulates the hydrolysis and cyclization reactions, but also the base exchange reaction. We define the substrate specificities of the reactions, demonstrate that cyclization and base exchange reactions occur within the same pH range, and establish that TIR-1 uses a ternary complex mechanism. Overall, our findings will aid drug discovery efforts and provide insight into the mechanism of recently described inhibitors.
Keywords: axon degeneration, intestinal immunity, kinetic mechanism, catalytic mechanism, SARM1, hydrolysis, cyclization, base exchange
Graphical Abstract

Introduction
Sterile alpha and toll/interleukin receptor (TIR) motif containing protein 1 (SARM1) is the founding member of a novel class of enzymatic TIR domains and is the central executioner of axon degeneration.1, 2 The SARM1 domain architecture consists of an N-terminal mitochondrial localization sequence (MLS), followed by armadillo repeats (ARM domain), tandem sterile alpha motifs (SAM domains), and a TIR domain.3–6 These domains function in autoinhibition, multimerization, and catalysis respectively (Fig. 1A).3, 7, 8 Under normal healthy conditions, the enzyme is held in an autoinhibited state by the ARM domain. Specifically, the TIR domains are separated from each other by intervening ARM domains, which are locked in place by NAD+ binding an allosteric site in the ARM domain.9
Figure 1. Reactions catalyzed by SARM1/TIR-1.
A) Domain architecture of SARM1. B) SARM1 activation in axon degeneration. C) NAD(P)+ hydrolysis reaction by SARM1/TIR-1. NAD(P)+ is cleaved into nicotinamide and (c)ADPR(P). D) TIR-1 role in intestinal immunity. E) Base exchange reaction by SARM1/TIR-1. The nicotinamide moiety on NADP+ is exchanged for nicotinic acid to generate NAADP.
After injury, NAD+ in the allosteric site is exchanged for nicotinamide mononucleotide (NMN).9, 10 Consequently, SARM1 undergoes an NMN-induced conformational change and a concomitant phase transition that facilitates and stabilizes the oligomerization of the TIR domains to generate the active form of the enzyme.6, 8, 10–12 Active SARM1 cleaves NAD+ into a mixture of ADPR and cADPR, which is thought to trigger an influx of calcium into the axon (Fig. 1B–C).2, 6, 13, 14 The calcium activates neuronal calpains which promote the breakdown of microtubules and neurofilaments, leading to axonal fragmentation, which is characteristic of Wallerian-like degeneration.15 In animal models of traumatic brain injury and chemotherapy induced peripheral neuropathy (CIPN), SARM1 knockout protects against degeneration.16–18 As such, SARM1 is an attractive therapeutic target and many efforts are underway to develop pharmacological inhibitors of SARM1.5, 8, 19–24
In addition to axon degeneration, the NAD+ hydrolase activity of SARM1 and TIR-1, the Caenorhabditis elegans ortholog, is important for immune signaling.25, 26 For example, SARM1 was first identified as a negative regulator of Toll-like receptor signaling.25 Moreover, TIR-1 is upstream the p38 PMK-1 MAPK signaling cascade involved in intestinal immunity (Fig. 1D). Activation of this pathway results in the expression of immune response genes. We recently found that a phase transition and the catalytic activity of TIR-1 is required for activation of the p38 PMK-1 MAPK signaling pathway under conditions of low cholesterol or during pathogen infection in C. elegans.26 Under these conditions, TIR-1 forms puncta, indicating that a phase transition is required in vivo for the activation of MAPK signaling through PMK-1.26 Although TIR-1 and its associated phase transition are required for the activation of the PMK-1 pathway, it remains unclear how the pathway is activated downstream of TIR-1 activation. Notably, TIR-1 puncta formation also increases in C. elegans models of axon degeneration indicating that this phase transition is relevant in multiple cellular contexts.12
While cADPR is thought to mediate the downstream effects of SARM1 activation, attempts to directly correlate calcium signaling with cADPR production by SARM1 have been contradictory in the context of axon degeneration. For example, Ko et al. found that ATP loss and mitochondrial dysfunction precede calcium influx after SARM1 activation. Based on these results, others have argued that SARM1 activation triggers an energetic crisis that ultimately causes the neuron to die.27, 28 By contrast, Li et al. found that cADPR production by SARM1 is partially responsible for calcium influx in CIPN by directly gating calcium channels.14, 28 It is also possible that SARM1-mediated calcium signaling could play a larger role in other backgrounds like immune signaling.
In addition to its hydrolase and cyclase activities, SARM1 can also catalyze transglycosidation reactions (hereafter called “base exchange”) in which an alternative nitrogenous base (e.g., nicotinic acid, NA) reacts to form a novel dinucleotide.29, 30 The best characterized SARM1 catalyzed base exchange reaction occurs when nicotinamide adenine dinucleotide phosphate (NADP+) and NA react to form nicotinic acid adenine dinucleotide phosphate (NAADP; Fig. 1E).21, 29, 30 Like cADPR, NAADP is also a calcium mobilizer. Recently, the base exchange reaction mediated by SARM1 was shown to occur in neurons treated with neurotoxins or chemotherapeutics.21, 30 Therefore, an explicit role for SARM1 in promoting calcium signaling via NAADP production cannot be ruled out.
The additional base exchange activity and regulation of pathways downstream of SARM1 pose interesting questions about the relevant metabolites in axon degeneration and other potential mechanisms by which SARM1 (and TIR-1) function in signaling pathways.6 To begin to answer these questions, we describe the first detailed mechanistic characterization of TIR-1. Herein, we show that, in addition to NAD+, TIR-1 cleaves other NAD+ analogs and can use additional substrates in the base exchange reaction. Like the hydrolysis reaction, a liquid-to-solid phase transition potentiates the base exchange activity of the catalytic TIR domain of TIR-1. Further characterization of the base exchange activity reveals that TIR-1 favors the base exchange at mildly acidic conditions and the hydrolysis reaction above neutral pH, even though there is overlap in the pH ranges where these reactions are catalyzed. Finally, we take advantage of the bi-substrate nature of the base exchange reaction to show that both TIR1 and SARM1 follow a ternary complex mechanism. We anticipate that these findings will aid the development of inhibitors targeting SARM1.
Results
The TIR domain of TIR-1 hydrolyzes cADPR and other NAD+ analogs
The substrate specificity of the glycohydrolase and cyclase activities of human SARM1 are well established (Fig. 1A). By contrast, the substrate scope of TIR-1 is less well explored. We initially investigated the substrate specificity of the TIR-1 catalyzed hydrolysis reaction using the isolated TIR domain from TIR-1. The use of the TIR domain allowed us to assess the similarities between the C. elegans and human orthologs of SARM1 and help define parameters for in vivo studies of this enzyme.
First, we examined NAD+ hydrolysis and cyclization over 120 min in the presence of 25% PEG 3350 by HPLC; we previously showed that PEG 3350 induces a liquid-to-solid phase transition that increases the catalytic activity of the TIR domains of SARM1 and TIR-1 by 2,000-fold and 22-fold respectively.12, 26 We found that cADPR production peaks within the first 15 min before decreasing over the remainder of the time course. By contrast, ADPR production is only detectable after 15 min (Fig. 2A). These data indicate that cADPR is the major product of TIR-1 and the delay in ADPR production suggests a stepwise mechanism of ADPR production in which cADPR is first generated and then used as the substrate for ADPR production. Indeed, cADPR is rapidly hydrolyzed to ADPR when incubated with TIR-1 in the presence of PEG 3350 (Fig. 2B). We additionally determined whether the TIR domain could hydrolyze several NAD+-like metabolites to probe the substrate scope of TIR-1 more broadly. Notably, NHD, NHDH, NADP+, thio-NAD+ (sNAD), and 3-acetyl pyridine adenine dinucleotide were all TIR-1 substrates (3apAD, Fig. 2B and S2). SARM1 cleaves a similar repertoire of substrates.12
Figure 2. The TIR domain of TIR-1 hydrolyzes cADPR and other NAD+ analogs in addition to NAD+.
A) NAD+ hydrolysis time course analyzed by HPLC reveals that cADPR is the major product of NAD+ hydrolysis by TIR-1. Top – HPLC chromatograph, 0–5 minutes shown. Bottom – Plot of peak areas for NAD+, cADPR, and ADPR. B) Substrate specificity of NAD+ hydrolysis reaction analyzed by HPLC. C) cADPR is a substrate of TIR-1. Top– 120-min time course of cADPR hydrolysis (0.5–2 minutes of the HPLC chromatogram shown). Bottom – Quantification of peak area from cADPR hydrolysis reaction. D) NADP+ is a substrate of TIR-1. Top left – 120-min time course of NADP+ hydrolysis (3–5 minutes of HPLC chromatogram shown). Bottom left – hydrolysis product peak areas from the 120-min NADP+ hydrolysis reaction. Top Right – 5-min time course of NADP+ hydrolysis (3–5 minutes of HPLC chromatogram shown). Bottom Right – NADP+ and hydrolysis product peak areas from the 5-minute NADP+ hydrolysis reaction.
Next, we determined the kinetics of cADPR and NADP+ hydrolysis in 25% PEG 3350. Time course experiments in 25% PEG 3350 showed that cADPR was nearly completely consumed over a 120 min period at a rate of 0.017 min−1 (Fig. 2C). By contrast, the NADP+ hydrolysis rate was significantly faster as all the NADP+ was consumed in the first 5 min to produce cADPRP (Fig 2D). Thereafter, the cADPRP peak area decreased over the remaining 115 min at a rate of 0.037 min-1. This value is similar to that obtained for the hydrolysis of cADPR, indicating that the hydrolysis of cADPR and cADPRP occurs with similar kinetics. The surprising finding that all the NADP+ was consumed during the first 5 min prompted us to complete a shorter time course. Notably, the reaction reached completion in just 2 min. As expected, all the NADP was consumed at a rate of 1.6 min−1 and cADPRP was produced at a rate of 2.3 min−1 (Fig. 2D). Taken together, these results show that NAD+, cADPR, NADP+, and cADPRP are TIR-1 substrates and suggest that NADP+/cADPRP are better substrates for TIR-1 with respect to NAD+/cADPR
A phase transition is required for the base exchange activity of TIR domain of TIR-1
We recently showed that a phase transition potentiates the hydrolysis and cyclase activities of both SARM1 and TIR-1.12, 26 To characterize this phase transition further, we examined the effects of 25% PEG 3350 and 500 mM sodium citrate on the base exchange activity of the catalytic domain of TIR-1 in a direct fluorescent assay.12, 26 We also tested 100 μM NMN because this compound activates full length SARM1.10, 29, 30 In this assay, the nicotinamide on NAD+ is exchanged for an alternative base called PC6 to generate the fluorogenic product PAD6 (Fig. 3A).21 PEG and citrate activate the base exchange activity of TIR-1 just as they activate the hydrolysis activity of TIR-1. By contrast, NMN had no effect on the catalytic activity of the TIR domain. (Fig. 3B). This finding is unsurprising because we used a truncated version of the enzyme that lacks the ARM domain and allosteric pocket where NMN binds. Overall, our results indicate that a phase transition activates the base exchange reaction in addition to the hydrolysis reaction. Next, we confirmed that the phase transition is required for TIR domain activity in a gel-based assay. For these studies, we incubated the enzyme with increasing concentrations of PEG or citrate, centrifuged the samples, separated the supernatant and pellet fractions, resuspended the pellet, and ran all the fractions on a gel. We found that the protein is dose-dependently present in the pellet fractions with both PEG and citrate. Importantly, we also observed base exchange activity in the pellets at PEG and citrate concentrations ≥ 17.5% or 500 mM, respectively (Fig. 3C and Fig. S3). Taken together, these data show that a liquid-to-solid phase transition activates the base exchange activity of this enzyme.
Figure 3. A phase transition is required for the base exchange activity of the TIR domain of TIR-1.
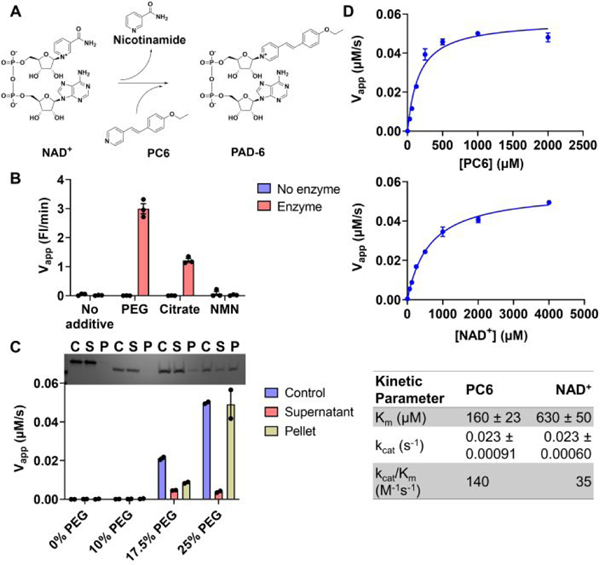
A) Direct fluorescent assay with NAD+ and PC6. The nicotinamide moiety on NAD+ is exchanged for PC6 to generate PAD-6. B) PEG and citrate, but not NMN, activate the base exchange activity of the TIR domain. Velocities of the base exchange reaction with NAD+ and PC6 in the absence or presence of 25% PEG 3350, 500 mM citrate, or 100 μM NMN. C) ceTIR precipitation correlates with base exchange activity. Top – SDS-PAGE of pre-centrifuged control, supernatant, and pellet fractions of the TIR domain incubated with 0–25% PEG 2250. Bottom – Velocities of the base exchange reaction of the pre-centrifuged control, supernatant, and pellet fractions of the TIR domain incubated with 0–25% PEG 3350. D) Initial kinetic characterization of the TIR domain in 25% PEG 3350. Top – Titration of PC6 at a saturating concentration of NAD+. Middle – Titration of NAD+ at a saturating concentration of PC6. bottom – Summary table of kinetic parameters.
Next, we performed kinetic analyses on the TIR domain in the presence of 25% PEG 3350. First, we titrated the PC6 concentration at a saturating concentration of NAD+. For PC6, we found that the is 165 μM, the turnover number () is 0.023 s−1, and the catalytic efficiency () is 140 M−1s−1 (Fig. 3D). Then we titrated NAD+ at a saturating concentration of PC6 and observed that the is 630 μM, is 0.023 s−1, and the catalytic efficiency is 35 M−1s−1 with respect to NAD+ (Fig. 3D). We repeated the kinetics in 500 mM citrate and overall found similar trends. However, with respect to PC6 in citrate, we found that the was 5100 M−1s−1, a 35-fold increase compared to 25% PEG. Given that the PC6 was approximately 4 μM under these conditions, the increase in the catalytic efficiency in citrate is driven by an increase in apparent affinity for the alternative base (Fig. S3). Notably, the catalytic efficiency for the base exchange reaction, at least under some experimental conditions, is greater than that for the hydrolysis reaction, suggesting that the base exchange reaction is physiologically relevant.
Characterization of the TIR-1 base exchange reaction
The TIR-1 base exchange reaction was further characterized by determining whether the TIR domain of TIR-1 could utilize alternative bases in addition to nicotinic acid. For these studies, we incubated TIR-1 with a suite of alternative bases in the presence of 25% PEG 3350 for 30 min. When NAD+ was tested as the substrate, TIR-1 utilized 3-acetyl pyridine (3ap), fluoro-, chloro-, bromo-, and iodoisoquinolines, and PC6 (Figs. 3, 4A, and S4). When NADP+ was tested as the substrate, TIR-1 catalyzed the base exchange reaction with pyridoxine (B6) and nitro-, fluoro-, chloro-, bromo-, and iodoisoquinolines in addition to NA (Fig. 4A). Notably, when NAD+ was the substrate, (c)ADPR production dominated over the base exchange reaction. By contrast, when NADP+ was the substrate, the base exchange reaction was favored. The fact that the isoquinolines are substrates for the TIR-1 catalyzed base exchange reaction is consistent with prior studies showing that isoquinolines are SARM1 substrates and mechanism-based inhibitors.8 6
Figure 4. Initial characterization of the base exchange reaction.
A) Substrate specificity of the base exchange reaction analyzed by HPLC. B) pH profile of the base exchange reaction in the direct fluorescent assay with PC6 in 25% PEG 3350. C) pH profile of the base exchange reaction with endogenous substrates in 25% PEG 3350 analyzed by HPLC. D) Plot of peak areas from the HPLC analysis.
Our finding that NA is not utilized in the base exchange reaction with NAD+ was surprising and prompted us to do a pH profile using the direct fluorescent assay. We tested the base exchange activity over the pH range 4.5 to 9 and found that the pH optimum for the base exchange reaction with NAD+ and PC6 was 7 and no activity was observed below pH 6 (Fig. 4B). These results were surprising given that the base exchange reaction is expected to occur under acidic conditions.29, 31 Therefore, we reasoned that the pH 7 optimum in the direct fluorescent base exchange assay is specific to the substrates used in the reaction and prompted us to repeat the pH profile with the endogenous substrates NA and NADP+. Using these substrates, we observed both NADP+ cyclization to cADPRP and conversion of NADP+ to NAADP in the pH range 5.5–7. Cyclization did not show any apparent pH dependence with the TIR domain of TIR-1, while the base exchange reaction occurred optimally at pH 6. Above pH 7, the cyclization reaction occurred exclusively and neither catalytic activity was observed between pH 4.5–5 (Figs. 4C and 4D). Notably, the dinucleotide substrate concentration in the direct fluorescent assay was relatively high (4 mM), while in the HPLC-based assay, the substrate concentration was relatively low (100 μM). The former provides information about preferred protonation states relevant to (V conditions) and the latter provides information about protonation states important for substrate binding and turnover (V/K conditions). Both pH profiles show that the overall catalytic activity is greatest at approximately pH 7. However, the assay under V/K conditions suggests that the protonation state of the enzyme or substrate influences which reaction (hydrolysis/cyclization or base exchange) occurs.
The SARM1 and TIR-1 catalytic mechanism involves the formation of a stabilized cationic intermediate
The fact that TIR-1 catalyzes both the cyclization and base exchange reactions between pH 5.5–7 suggests that TIR-1 catalyzes the formation of an intermediate common to all TIR-1 reactions. If TIR-1 does exploit a common intermediate, we initially hypothesized that the kinetic mechanism would resemble a double displacement (i.e., ping pong) mechanism wherein NAD(P)+ would first bind the enzyme to form an intermediate species followed by the release of nicotinamide. Nicotinic acid would then bind and react with the intermediate to form NAAD(P) (Fig. 5A). To evaluate this hypothesis, we performed initial velocity experiments with the TIR domain of TIR-1 in the presence of 25% PEG 3350. Specifically, NAD+ was titrated at several fixed concentrations of PC6. The inverse experiments were also performed. If the base exchange follows a double displacement mechanism, the double reciprocal plots should show parallel lines. However, the double reciprocal plots intersected to the left of the y-axis, indicating that the base exchange utilizes a ternary complex mechanism, and not a double displacement mechanism (Fig. 5B). These data indicate that NAD(P)+ and PC6/nicotinic acid bind to the enzyme prior to catalysis (Fig. 5B).
Figure 5. The SARM1 and TIR-1 Catalyzed Reaction Mechanism.
A) Hypothetical kinetic mechanisms for SARM1 and TIR-1 catalysis. Top - Ping pong type mechanism wherein NAD(P)+ would first bind the enzyme to form an intermediate species followed by the release of nicotinamide. Nicotinic acid would then bind and react with the intermediate to form NAAD(P). Bottom – Ternary complex mechanism wherein NAD(P)+ and nicotinic acid bind to the enzyme to form a ternary complex prior to the formation of an intermediate species (either a covalent intermediate or an oxocarbenium ion intermediate) that reacts an incoming nucleophile. B) Double reciprocal plots of initial velocity experiments with the TIR domain of TIR-1 where one substrate is titrated at several fixed concentrations of the other substrate. Left – NAD+ titration. Right – PC6 titration. C) Double reciprocal plots of initial velocity experiments as in B but with SARM1ΔMLS. Left – NAD+ titration. Right – PC6 titration. D) Methanolysis experiments with the TIR domain of TIR-1 (left) or SARM1ΔMLS. The O-methyl-ADPR:ADPR ratio is plotted vs. [MeOH]. The slope of the line is multiplied by 55 to determine the partition ratio.
We performed similar experiments with near full-length human SARM1, which only lacks the N-terminal the mitochondrial localization sequence (SARM1ΔMLS). Analysis of the double reciprocal plots revealed the same pattern of intersecting lines (Fig. 5C). These data indicate that full-length SARM1 also follows a ternary complex mechanism. Overall, these data suggest that both SARM1 and TIR-1 employ a common intermediate that undergoes nucleophilic attack by water or an alternative base.
Initial velocity experiments yield important information about the steps in a reaction but provide limited insight into the mechanisms of catalysis. Methanol is a better nucleophile than water and methanolysis experiments provide insights into the nature of the catalytic intermediate.32, 33 In these experiments, one measures the ratio of O-methyl-ADPR to ADPR and this ratio can be used to calculate a partition ratio. Large partition ratios indicate the presence of a highly electrophilic intermediate.
Methanolysis experiments were performed by incubating the TIR domain of TIR-1 or SARM1ΔMLS with NAD+ or cADPR for 4 h in the presence of 0–2 M methanol. The reactions were then quenched, and the reaction products analyzed by HPLC. O-methyl-ADPR was produced in a dose dependent manner and a plot of the O-methyl-ADPR/ADPR ratio versus methanol concentration showed a linear relationship. Partition ratios for TIR-1 were 79.0 and 88.4 for NAD+ and cADPR, respectively. For SARM1ΔMLS, the NAD+ and cADPR partition ratios were 63.6 and 9.7, respectively (Figs. 5D and S5). Control experiments in the absence of enzyme produced no O-methyl-ADPR and a partition ratio could not be calculated. The partition ratio for a related NAD+ glycohydrolase CD38 is 11.32 These data indicate that the TIR-1 and SARM1 intermediates are highly reactive.
We also evaluated the stereochemistry of the methanolysis reaction by NMR. When NAD+ was used as the substrate and TIR-1 used as the catalyst, only one O-methylADPR peak was present in the HPLC trace. The O-methylADPR generated in this reaction was purified and characterized by LC-MS and 13C NMR. The LC-MS data confirmed that this species is O-methyl-ADPR ([M+H]+ = 574) and the 13C NMR chemical shift is consistent with the preferential generation of the β-O-methyl-ADPR anomer (δ C1” = 108 ppm; Fig. S6).34 Similar experiments were performed with SARM1 and virtually identical results obtained. These demonstrate that the methanolysis reaction results in the preferential generation of the β-anomer for both SARM1 and TIR-1 (Fig. S6).
The large partition ratio observed in the methanolysis reaction is consistent with the formation of an oxocarbenium ion or oxocarbenium-like intermediate. However, the production of O-methyl-ADPR alone cannot completely rule out the formation of a covalent intermediate. To distinguish between the oxocarbenium and covalent intermediates, chemical quenching was used to trap any potential intermediates. TIR domains from TIR-1 or SARM1 were incubated with or without NAD+ for 1 min, quenched with TFA, and analyzed by LMCS. No ADPR adducts were observed (Fig. S7), suggesting that a covalent intermediate does not form during catalysis.
Discussion
The studies described herein demonstrate that the TIR domain of TIR-1 possesses a broad substrate scope and catalyzes cyclization, hydrolysis, and base exchange reactions similarly to its mammalian ortholog SARM1. Notably, these activities are also all regulated by a liquid-to-solid phase transition. The mechanistic studies of the base exchange reaction further highlight that TIR-1 catalysis involves the formation of a ternary complex in which NAD+ and the incoming base are bound to the enzyme prior to catalysis. The large partition ratio observed in the methanolysis experiments indicates that the intermediate generated during catalysis is highly electrophilic and, as we argue below, suggests the presence of an electrophilic oxocarbenium ion or oxocarbenium-like intermediate.
Our kinetic and methanolysis data argue for an Sni-type ternary complex mechanism that entails the formation of a highly reactive cationic intermediate that can undergo reaction with water (hydrolysis), adenine (cyclization), or a nitrogenous base (base exchange) whilst nicotinamide remains bound to the enzyme (Scheme 1). The formation of a ternary complex in which the nucleophile binds (either to the free enzyme or to an enzyme substrate complex) before nicotinamide leaves (Fig. 5A bottom) is supported by our initial velocity experiments which show an intersecting pattern of lines. Note that this line pattern rules out a mechanism in which nicotinamide dissociates from the active site before a nucleophile binds (Fig. 5A top); for such a mechanism a parallel line pattern is expected.
Scheme 1.
Proposed Sni mechanism of SARM1 and TIR-1 catalysis. Nu = water, N1 of adenine, or pyridine base
The initial velocity studies do not, however, report on the nature of the reactive intermediate. In fact, the specific mechanism by which SARM1 catalyzes these various reactions is controversial. For example, some have argued for the existence of a covalent intermediate between ADPR and a key catalytic residue, i.e. E642. This mechanism is supported by mutagenesis studies highlighting the importance of E642 and X-ray crystallography structures of ara-F-NAD+ bound to SARM1, which show that this compound can form a covalent intermediate with E642;8 ara-F-NAD+ is an NAD+ analog that contains a fluorine atom at the 2’ carbon of the nicotinamide-ribosyl moiety with stereochemistry opposite that of the hydroxyl in NAD+. However, we have previously shown that ara-F-NAD+ is neither a substrate nor inhibitor of SARM1.12 These data suggest that that the formation of the covalent adduct is related to the long incubation times required for structural studies.6 Additionally, the fluorine substitution and resultant altered stereochemistry likely prevent ara-F-NAD+ from binding the active site in the same way as NAD+ and promote an alternative glutamate conformer that favors the formation of the covalent intermediate. Similar covalent intermediates have been observed by crystallography with ara-F-NMN+ and the related hydrolase CD38, but the covalent intermediates are not observed for substrates with ribosyl stereochemistry. In other words, the propensity of ara-F-NAD+ to form covalent intermediates is a property of this compound and not a reflection of active site chemistries with endogenous substrates.33 Finally, we failed to detect a covalent intermediate in chemical quenching experiments with either TIR-1 or SARM1.
Our methanolysis experiments support the existence of a highly electrophilic intermediate. The partition ratio for NAD+ and cADPR methanolysis/hydrolysis by the TIR domain of TIR-1 is 79.0 ± 2.0 m 88.4 ± 0.7, respectively and 63.6 ± 0.3 and 9.7 ± 0.4, respectively, for SARM1ΔMLS. These partition coefficients are 6 to 8-fold greater when compared to the related glycohydrolase CD38, which has a partition ratio of 11, and suggests that the intermediate formed during SARM1 or TIR-1 catalysis is more electrophilic than that for CD38. Since CD38 is thought to employ an oxocarbenium-like intermediate,33 35, 36 these data argue in favor of the existence of such an intermediate. Although retention of configuration is expected for a covalent intermediate and we did observe the production of the β-O-methyl-ADPR anomer in our methanolysis experiments, the production of the β-anomer cannot rule out an oxocarbenium-like intermediate since an Sni mechanism involves a “front-side” attack with retention of stereochemistry.37, 38
With both the nucleophile and leaving group in the active site, the two transiently interact to form an intermediate with substantial oxocarbenium-like character. Furthermore, the interaction between the nicotinamide leaving group and the nucleophile forces retention of stereochemistry at the anomeric carbon, which is a key feature of Sni type reactions. In this model, the catalytic glutamate stabilizes the intermediate by electrostatic interactions and/or by hydrogen bonding with the hydroxyls of the nicotinamide-ribosyl moiety to promote nicotinamide leaving. This binding mode is supported by cryo-EM data, where mechanism-based inhibitors are bound in the active site;8, 22 the inhibitor is formed when the free base “pro-drug” participates in the base exchange reaction with NAD+ and remains bound to the enzyme active site. In these structures, the catalytic glutamate hydrogen bonds with the hydroxyls of the core ADPR scaffold common to the inhibitors in these structures.8, 22 Because these inhibitors are derived from NAD+ and are formed in the active site, it is highly likely that the binding mode of these inhibitory ADPR adducts reflects the binding mode of the endogenous substrate NAD+. Notably, these structures were determined by two independent groups.
Our studies also provide insights into the differences between human SARM1 and TIR-1. With respect to TIR-1, cADPR is cleaved to ADPR over a period of 120 min, confirming that cADPR is a bona fide substrate of TIR-1. These data suggest a stepwise model for TIR-1 catalysis in which NAD+ is cyclized to cADPR first and then hydrolyzed to ADPR. Moreover, cADPR(P) is the major product of NAD(P)+ hydrolysis, suggesting that cADPR(P) is the more relevant NAD+ metabolite in vivo in C. elegans. This is distinct from human SARM1, which produces a mixture of ~ 88% ADPR and 10–12% cADPR during catalysis.12, 30 Another distinction between SARM1 and TIR-1 is that 5bromoisoquinoline is a better substrate for the base exchange reaction than 5-iodoisoquinoline (Fig. 4A). When a series of isoquinolines were examined as SARM1 inhibitors, 5-iodoisoquinoline was found to be a more potent inhibitor than 5-bromoisoquinoline.19 In fact, 5iodoisoquinoline is the second most potent inhibitor of SARM1 identified to date.6, 22 Overall, these distinctions may reflect underlying differences in the structure of the active site in each enzyme or could reflect the differences in the experimental conditions (i.e. TIR domain only versus truncated SARM1 with the SAM and TIR domains).
NADP+ is also a TIR-1 substrate. In the hydrolysis reaction, NADP+ is cleaved at a much faster rate than NAD+, indicating that NADP+ is a better substrate than NAD+ for TIR-1. These data suggest that NADP+ hydrolysis and other reactions that utilize NADP+ (e.g., the base exchange reaction) merit further investigation. We used two methods to study the base exchange reaction: the direct fluorescent assay with PC6 and NAD+ and an HPLC based assay with the endogenous substrates NADP+ and NA. In the direct fluorescent assay, we observe an increase in catalytic efficiency relative to that for the hydrolysis reaction, particularly in citrate, which suggests that the base exchange is physiologically relevant. Notably, there is overlap in the product specificity for the reactions between pH 5.5 and 7, in support of oxocarbenium intermediate shared by SARM1/TIR-1 catalyzed hydrolysis, cyclization, and base exchange reactions.
In conclusion, while there are notable differences between TIR-1 and SARM1, key findings are consistent across experimental conditions. The TIR domain of TIR-1 in PEG and SARM1ΔMLS in the absence of crowding agents show similar convergent plots in the initial velocity experiments for example. Additionally, the partition ratio in both conditions is similar. Therefore, even though the TIR domain of TIR-1 requires PEG or citrate for activation, it is unlikely that the active site is grossly different from the physiological state. Therefore, the cyclization, hydrolysis, and base exchange activities are likely mediated by a common oxocarbenium-like intermediate. Intriguingly, TIR-1 preferentially catalyzes the cyclization reaction. By contrast, for SARM1, cADPR production does not precede ADPR production. These studies of the TIR domain of TIR-1 further highlight the broad chemical diversity of TIR domains, which in addition to NAD(P)+ hydrolysis, cyclization, and base exchange reactions, have been shown to catalyze a multitude of reactions including 2’,3’-cAMP/cGMP synthesis.39
Materials and Methods
Expression and purification of the TIR domain of TIR-1
The expression and purification of the TIR domain of TIR-1 was carried out as previously described. Briefly, the TIR domain expression construct contains a Twin-Strep tag, GGGSSGGAS linker, TEV protease site, and the TIR domain of TIR-1 sequence (residues 707–930). This construct will generate a fusion protein with a Twin-Strep tag and TEV protease site at the N-terminus of the TIR domain and a 6x His tag at the C-terminus. This construct was transformed into E. coli BL21(DE3) (NEB) and maintained as a glycerol stock at −80 °C. 5 mL LB cultures supplemented with 50 μg/mL kanamycin were inoculated by scraping the frozen glycerol stock with an inoculation loop. The cultures were grown overnight at 37 °C with agitation. The next day, the cultures were diluted 1:400 in LB media with 50 μg/mL kanamycin and grown at 37 °C with agitation to an OD600 of 0.7–0.8. Once this OD was reached, the cultures were cooled on ice for 30 min, after which expression of the TIR domain of TIR-1 was induced by adding IPTG (50 μM final; ThermoFisher) to the cultures. The incubator temperature was decreased to 16 °C and the cultures were incubated overnight. The following morning, bacteria were collected by centrifugation and the pellets were flash frozen in liquid nitrogen and stored at −80 ۥ°C for future use.
To purify the TIR domain of TIR-1, bacterial pellets from 5 L of culture were thawed on ice and resuspended in 200 mL lysis buffer (100 mM Tris•HCl, pH 7.0; 300 mM NaCl; 10% glycerol [w/v]; and 0.001% Tween 20 [v/v]) supplemented with EDTA-free Pierce Protease Inhibitor Tablets (ThermoFisher). The resuspension was incubated with 100 μg/mL lysozyme (Sigma) on ice for 10 min to aid lysis. To lyse the cells, the resuspension was sonicated in 50 mL batches using a Fisher Scientific Sonic Dismembrator sonicator (FB-705) at an amplitude of 30 for 20 s pulsing on and off for 1 s each. Sonication was repeated a total of 12 times, with a 30 s resting period between each sonication round. The lysate was clarified at 21,000 x g for 30 min at 4 °C and the supernatant was loaded onto 2 mL column volume (CV) Strep-Tactin XT 4Flow resin pre-equilibrated with Strep wash buffer (50 mM Tris•HCl, pH 7.0; 300 mM NaCl). Supernatant was allowed to flow over the column by gravity and then the column was washed with 30 CV wash buffer. Protein was eluted from the Strep-Tactin column with 30 CV Strep elution buffer (wash buffer plus 50 mM biotin). Next, the eluate was loaded onto a 2 mL CV TALON Metal Affinity Resin (Takara Bio) column pre-equilibrated with His wash 1 buffer (50 mM Tris•HCl, pH 7.0; 150 mM NaCl; 5 mM imidazole). The eluate was allowed to flow over the resin by gravity. Subsequently, the column was washed with His wash 1 buffer (15 column volumes) and His wash 2 buffer (as His wash 1 buffer but with 10 mM imidazole, 15 column volumes). Protein was eluted with His elution buffer (His wash 1 buffer with 150 mM imidazole) over 25 CV and dialyzed overnight in dialysis buffer (50 mM Tris•HCl, pH 7.0; 150 mM NaCl) at 4 °C. The following day, dialyzed protein was concentrated in an Amicon Centrifugal Filter Unit MWCO 10,000 at 4 °C and evaluated for purity by SDS-PAGE. Protein concentration was determined by the Bradford assay (BioRad). 25 μL aliquots of the TIR domain of TIR-1 were flash frozen in liquid nitrogen and stored at −80 °C until use.
Expression and purification of SARM1ΔMLS
The sequence for amino acids 28–724 of human SARM1, a PreScission Protease cut site, and two tandem Protein A tags was synthesized and cloned into the pcDNA 3.4 (+) vector with the BspEI and EcoRV restriction sites flanking the sequence (GenScript); this sequence generates a SARM1 construct without the mitochondrial localization sequence and with a cleavable C-terminal Protein A tag. The construct was transformed into XL1Blue E. coli (Agilent). Subsequently, the plasmid was purified from 5 mL LB cultures supplemented with 100 μg/mL ampicillin and 10 μg/mL tetracycline inoculated with single colonies from the transformation (Promega) and the sequence of the purified plasmid was verified by Sanger sequencing (Genewiz). Following sequence verification, the plasmid was maintained as a glycerol stock at −80 °C.
For transfection, the plasmid was purified from 1 L LB cultures supplemented with 100 μg/mL ampicillin and 10 μg/mL tetracycline according to the manufacturer’s protocol from the QIAGEN Plasmid Maxi Kit. A day prior to transfection, Expi293F cells growing in FreeStyle 293 Expression Medium (ThermoFisher) were passaged and seeded at 2.5 × 106 cells/mL and incubated overnight at 37 °C, 8% CO2, < 80% relative humidity, and with agitation at 125 rpm. The next day, cells were diluted to 2.5 × 106 cells/mL. 3 μg of plasmid DNA per mL of culture was diluted in Opti-MEM (ThermoFisher) and 3 μg PEI-MAX per 1 μg DNA was diluted in Opti-MEM. The Opti-MEM solutions were incubated separately at room temperature for 5 min, after which the solutions were mixed 1:1. Following incubation for 30 min at room temperature, the DNA:PEI mixture was added to the cultures, such that the final concentration of plasmid was 3 μg/mL. Transfected cells were incubated for 24 h. The day after transfection, valproic acid (Sigma) was dissolved in FreeStyle media at a final concentration of 4.4 mM. Cells were diluted 1:1 with valproic acid supplemented media, such that the final concentration of valproic acid in the culture was 2.2 mM. Protein was allowed to express for 3 days before harvesting cells at 500 x g for 5 min at room temperature. Pellets were flash frozen in liquid nitrogen and stored at −80 °C until use.
For purification, pellets from 300 mL culture were thawed on ice and resuspended in 10 mL lysis buffer (50 mM HEPES, pH 8.0; 400 mM NaCl; 5% glycerol [w/v]). To lyse the cells, the resuspension was sonicated in 2–5 mL batches with Fisher Scientific Sonic Dismembrator sonicator (FB-705) at amplitude 10 for 10 s pulsing on and off for 1 s each. Sonication was repeated for a total of 10 times, with a 20 s resting period between each sonication round. The crude lysate was clarified by centrifugation at 15,000 x g at 4 °C for 15 min. In the meantime, 5 mL rabbit IgG agarose resin (Sigma) was equilibrated with lysis buffer. Following clarification, the supernatant was added to the equilibrated resin and the protein was bound in batch for 1 h at 4 °C using an end-over-end rocker. Unbound proteins were allowed to flow off the column by gravity. Subsequently, the column was washed two times in series with 10 CV of lysis buffer and 5 CV of wash buffer (25 mM HEPES, pH 7.4; 150 mM NaCl). 250 μL PreScission Protease was diluted in 4.75 mL wash buffer. The protease containing solution was used to resuspend the resin once, and the column was left to incubate with PreScission protease overnight at 4 °C without agitation.
The following day, cleaved protein was allowed to flow off the column by gravity. Next, the column was washed twice in series with 5 CV of wash buffer. Glutathione Sepharose resin (1 mL, Sigma) was equilibrated with wash buffer and the eluate and first wash from the IgG agarose resin was applied to the glutathione Sepharose column. The protein was allowed to flow by gravity. The glutathione resin was washed twice in series with 5 CV of wash buffer. Purity was evaluated by SDS-PAGE and the flow through and first wash from the glutathione column were concentrated in an Amicon Centrifugal Filter Unit MWCO 30,000. Protein concentration was determined by Bradford. 25 μL aliquots were flash frozen in liquid nitrogen and stored at −80 °C.
Expression and purification of the TIR domain of SARM1
The expression of the TIR domain from SARM1 was carried out as previously described. Briefly, 5 mL LB cultures supplemented with 50 μg/mL kanamycin were inoculated by scraping the frozen glycerol stock (C43 transformed with a pET-30a(+) vector containing the sequence for a Twin Strep tag, TEV protease cut site, and the TIR domain from SARM1) with an inoculation loop. The cultures were grown overnight at 37 °C with agitation. The next day, the cultures were diluted 1:400 in LB media with 50 μg/mL kanamycin and grown at 37 °C with agitation to an OD600 of 0.7–0.8. Once this OD was reached, expression of the TIR domain of SARM1 was induced by adding IPTG (500 μM final; ThermoFisher) to the cultures. The incubator temperature was decreased to 16 °C and the cultures were incubated overnight. The following morning, bacteria were collected by centrifugation and the pellets were flash frozen in liquid nitrogen and stored at −80 ۥ°C for future use.
To purify the TIR domain of SARM1, bacterial pellets from 10 L of culture were thawed on ice and resuspended in 200 mL lysis buffer (100 mM HEPES, pH 8.0; 200 mM NaCl; 10% glycerol [w/v]; and 0.01% Tween 20 [v/v]) supplemented with EDTA-free Pierce Protease Inhibitor Tablets (ThermoFisher). The resuspension was incubated with 100 μg/mL lysozyme (Sigma) on ice for 10 min to aid lysis. To lyse the cells, the resuspension was sonicated in 50 mL batches using a Fisher Scientific Sonic Dismembrator sonicator (FB-705) at an amplitude of 30 for 20 s pulsing on and off for 1 s each. Sonication was repeated a total of 12 times, with a 30 s resting period between each sonication round. The lysate was clarified at 15,000 x g for 30 min at 4 °C and the supernatant was loaded onto 3 mL column volume (CV) Strep-Tactin XT 4Flow resin pre-equilibrated with Strep wash buffer (50 mM HEPES, pH 8.0; 500 mM NaCl). Supernatant was allowed to flow over the column by gravity and then the column was washed with 30 CV wash buffer. Protein was eluted from the Strep-Tactin column with 30 CV Strep elution buffer (wash buffer plus 50 mM biotin). Next, the eluate was concentrated in an Amicon Centrifugal Filter Unit MWCO 10,000 at 4 °C and the concentrate was loaded onto a HiLoad 16/600 Superdex 200 pg gel filtration column with 50 mM HEPES, pH 8.0 and 150 mM NaCl. Fractions containing the TIR domain of SARM1 were analyzed for purity by SDS-PAGE and then pooled. Protein was concentrated again, and a Bradford assay was performed to determine protein concentration. 25 μL aliquots of the TIR domain of TIR-1 were flash frozen in liquid nitrogen and stored at −80 °C until use.
NAD+ hydrolysis by the TIR domain of TIR-1
The TIR domain (2.5 μM) was incubated with 100 μM NAD for 0, 5, 15, 30, 60, 90, and 120 min at 25 °C in TIR-1 reaction buffer (50 mM Tris•HCl, pH 8.0; 150 mM NaCl) with 25% PEG 3350 in duplicate. The reactions were stopped at the specified times by adding TFA to a final concentration of 0.25% and then flash frozen in liquid nitrogen. After thawing, the samples were filtered through a 0.22 μm cellulose acetate Costar Spin-X Centrifuge Tube filter. 20 μL of the filtered sample was loaded onto a POROS HQ column (10 μm, 4.6 × 100 mm, 1.7 mL; ThermoScientific) attached to an Agilent 1260 HPLC.
Reaction components were eluted from the column using a gradient of 100% buffer A for 1 min, 0 to 50% buffer B over 8.25 min, 50% to 100% buffer B over 3 min, 100% buffer B to 100% buffer A over 1 min, and 100% buffer A for 3 min. Buffer A consisted of 50 mM Tris pH 8.0 and buffer B consisted of 50 mM Tris pH 8.0 and 1 M NaCl. Absorbance was collected at 254 nm and HPLC traces were plotted in GraphPad Prism. Areas under the curve were calculated using Chem Station (Agilent) and plotted in GraphPad Prism.
Substrate specificity of the TIR domain of TIR-1 hydrolysis reaction
The TIR domain (2.5 μM) was incubated with 100 μM NAD, NADH, NHD, NHDH, cADPR, NMN, NaMN, NADP, NaADP, ATP, GTP, sNAD, 3apAD, or NR for 30 min at 25 °C in TIR-1 reaction buffer (50 mM Tris•HCl, pH 8; 150 mM NaCl) with 25% PEG 3350 in duplicate. After 30 min, sample preparation and HPLC analyses were performed as described above in “NAD hydrolysis by the TIR domain of TIR-1.”. Percent cleavage was calculated by the equation below.
| (Eq.1) |
Evaluation of cADPR as a TIR-1 substrate
This experiment was carried out as above in “NAD hydrolysis by the TIR domain of TIR-1” except with cADPR in place of NAD. The was calculated by fitting the plot of peak areas to a one-phase decay equation in GraphPad Prism.
Evaluation of NADP as a TIR-1 substrate
This experiment was also carried out as described in the “NAD hydrolysis by the TIR domain of TIR-1” section, except with NADP in place of NAD. After analyzing the initial iteration of the experiment, we repeated it using the same concentrations, but stopping the reaction at 0, 30 s, 1, 2, 3, 4, and 5 min. values for both time courses were calculated by fitting the plot of peak areas to a one-phase decay equation in GraphPad Prism.
PC6 synthesis
PC6 was synthesized according to previously described methods.40, 41 Briefly, 4-iodophenol (1 g, 4.55 mmol was combined with potassium carbonate (3.1 g, 22.7 mmol, 5.0 eq) in anhydrous ethanol (20 mL) in a 100 mL round bottom flask at room temperature under N2. The reaction was stirred at room temperature for 15 min. Iodoethane (0.44 mL, 5.46 mmol, 1.2 eq) was added to the mixture though a septum. The reaction was refluxed for 12 h. The next day, the reaction was cooled and quenched with water. Ethyl acetate was used to extract the reaction mixture three times. The ethyl acetate layer was dried over anhydrous Na2SO4, filtered, and concentrated by rotary evaporation. Flash chromatography using ethyl acetate/hexane (1:10) was used to purify the product 1-ethoxy-4-iodobenzene.40 The product was validated by 1H-NMR (Fig. S8A). Yellow oil; 1H NMR (CDCl3, 500 MHz): δ = 7.47 (d, J = 8.95 Hz, 2H, Ar-H), 6.59 (d, J = 8.95, 2H, Ar-H), 3.92 (q, J = 7.00, 7.00, 6.96 Hz, 2H, -CH2-), 1.33 (t, J = 7.00, 7.00 Hz, 3H, -CH3) (Fig. S5A).
Next, Pd(OAc)2 (0.097 g, 0.43 mmol, 0.14 eq) was added quickly to a stirring solution of 1-ethoxy-4-iodobenzene (0.7456 g, 3.01 mmol), 4-vinylpyridine (0.39 mL, 3.61 mmol, 1.2 eq), P(o-tol)3 (0.262 g, 0.86 mmol, 0.29 eq), and triethylamine (2.097 mL, 15.05 mmol, 5.0 eq) in degassed acetonitrile (15 mL) under nitrogen. The reaction was stirred at 100 °C for 12 h under a nitrogen atmosphere. The next day, the reaction was stopped by the addition of 20 mL water and extracted with 15 mL ethyl acetate three times. The organic layer was dried over Na2SO4, filtered, and concentrated by rotary evaporation. PC6 was purified by flash chromatography using ethyl acetate/hexanes (3:1). We validated the product by NMR and LCMS (Fig. S8B–C). Off-white solid; 1H NMR (CDCl3, 500 MHz): δ = 8.47 (d, J = 6.00 Hz, 2H, Ar-H), 7.40 (d, J = 8.7 Hz, 2H, Ar-H), 7.27 (d, J = 6.1 Hz, 2H, Ar-H), 7.19 (m, 1H, Ar-H), 6.81 (m, 3H, Ar-H), 3.99 (q, J = 6.95, 7.00, 7.00, 2H, -CH2-), 1.36 (t, J = 7.00, 6.95, 3H, -CH3); 13C NMR (CDCl3, 100 MHz) δ = 159.7, 149.6, 145.5, 133.2, 128.7, 128.5, 123.4, 120.7, 114.8, 63.6, 14.8. LC-MS (ESI) m/z calculated for C15H15NO [M+H]+ 226.12, observed 226.20.
Direct fluorescent assay with PC6
Initially, we generated a standard curve by incubating 400 μM of PC6 and 2 mM NAD with excess TIR domain of TIR-1 in 50 mM Tris, pH 8.0 and 150 mM NaCl in the presence of 25% PEG 3350 or 500 mM sodium citrate in duplicate. The reaction was monitored for 1.5 h at 25 °C using the excitation and emission wavelengths 390 nm and 520 nm, respectively. The plate reader used was a PerkinElmer EnVision 2104 Multilabel Reader with monochromator functionality and Wallac EnVision Manager software. Reaction progress was monitored every minute until a plateau was reached, at which point, a 1:2 serial dilution was performed with 50 mM Tris, pH 8.0 and 150 mM NaCl in the presence of 25% PEG 3350 or 500 mM sodium citrate. After dilution, one reading was taken and these values were plotted in GraphPad Prism to generate the standard curve, which is the slope of the line.
The effect of NMN or molecular crowding agents on the base exchange activity of the TIR domain of TIR-1 in the direct fluorescent assay
The TIR domain of TIR-1 (0 or 2.5 μM) was incubated for 10 min with 50 μM PC6 in the absence or presence of 25% PEG 3350, 500 mM sodium citrate, or 100 μM NMN in 50 mM Tris, pH 8 and 150 mM NaCl in triplicate. The reaction was initiated with 100 μM NAD and monitored for 1 h every 15 s at 25 °C at. Fluorescence intensities were converted to product (PAD-6) concentrations using the standard curve described above. Reaction velocities were determined by calculating the slope of the fluorescence intensities versus time. Velocities were plotted in GraphPad Prism.
Phase transition of the TIR domain of TIR-1 activates the base exchange activity
The TIR domain of TIR-1 (5 μM) was incubated with 0, 10, 17.5, or 25% PEG 3350 or 0, 125, 250, 500, 750, or 1000 mM sodium citrate in 50 mM Tris, pH 8 and 150 mM NaCl for 15 min at room temperature in duplicate. Samples were removed for controls before centrifuging the remaining sample at 21,000 x g for 10 min at room temperature. Immediately after centrifugation, the supernatant was removed. The pellet was resuspended in buffer with the corresponding additive. Base exchange activity was tested in these samples with 4 mM NAD and 500 μM PC6; fluorescence intensity readings were taken every 15 s for 15 min at 25 °C at. Fluorescence intensities were converted to PAD-6 concentrations using the standard curve. Reaction velocities were determined by calculating the slope of the fluorescence intensities versus time. Velocities were plotted in GraphPad Prism. The presence of protein in the samples was determined by SDS-PAGE. Notably, samples were prepared with 2x loading dye so that the high concentrations of PEG or citrate were diluted; high concentrations of additives impede the entrance of the protein into the gel and account for the apparent decrease in protein concentration in these samples.
Kinetic analysis of the base exchange reaction
For the PC6 titration, 2.5 μM TIR domain of TIR-1 was incubated for 10 min with 0–2000 μM PC6 in the presence of 25% PEG 3350 in 50 mM Tris, pH 8 and 150 mM NaCl in triplicate. The reaction was initiated with 4000 μM NAD and monitored for 15 min every 15 s at 25 °C at. In 500 mM sodium citrate, the experimental conditions are the same except 1 μM enzyme and 0–250 μM PC6 were used.
For the NAD titration, 2.5 μM TIR domain of TIR-1 was incubated for 10 min with 1 mM PC6 in the presence of 25% PEG 3350 in 50 mM Tris, pH 8 and 150 mM NaCl in triplicate. The reaction was initiated with 0–4000 μM NAD and monitored for 15 min every 15 s at 25 °C at. In 500 mM sodium citrate, the experimental conditions are the same except 1 μM enzyme and 250 μM PC6 were used.
For both, fluorescence intensities were converted to product (PAD-6) concentrations using the standard curve described above. Reaction velocities were determined by calculating the slope of the fluorescence intensities versus time. Velocities were plotted in GraphPad Prism and fit to the Michaelis-Menten equation.
Base exchange pH profiles
To generate a pH rate profile using the direct fluorescence assay, a series of buffers were used including sodium acetate (pH 4.5 and 5.0), MES buffer (pH 5.5–6.5), and Tris (pH 7.0–9.0). Specifically, 2.5 μM of the TIR domain of TIR-1 was incubated for 10 min with 500 μM PC6 in the presence of 25% PEG 3350 in 50 mM buffer and 150 mM NaCl in triplicate. The base exchange reaction was initiated with 4 mM NAD. Fluorescence intensity was monitored for 15 min every 15 s at 25 °C at. Fluorescence intensities were converted to PAD-6 concentrations using the standard curve. Velocities were determined by calculating the slope of the line. Log values of the velocities were plotted in GraphPad Prism.
For the HPLC based assay, 2.5 μM of the TIR domain of TIR-1 was incubated for 30 min at 25 °C with 2.5 mM nicotinic acid and 100 μM NADP in the presence of 25% PEG 3350 in 50 mM buffer and 150 mM NaCl in duplicate. For pH 4.5 and 5.0, sodium acetate buffer was used; MES buffer was used for pH 5.5–6.5; and Tris was used for pH 7.0–8.0. The reactions were stopped by the addition of 0.25% TFA (final concentration) and flash freezing in liquid N2. After thawing, the samples were filtered through a 0.22 μm Costar Spin-X cellulose acetate centrifugal filter. 20 μL was loaded onto a POROS HQ column (10 μm, 4.6 × 100 mm, 1.7 mL; ThermoScientific) attached to an Agilent 1260 HPLC. Metabolites were eluted from the column using the following gradient: 100% buffer A for 1 min, 0% to 50% buffer B over 8.25 min, 50% to 100% buffer B over 3 min, 100% buffer B to 100% buffer A over 1 min, and 100% buffer A for 3 min at a 3 mL/min flow rate. Buffer A consisted of 50 mM Tris pH 8.0 and buffer B consisted of 50 mM Tris pH 8.0 and 1 M NaCl. Absorbance was collected at 254 nm and HPLC traces were plotted in GraphPad Prism. Areas under the curve were calculated using Chem Station (Agilent) and plotted in GraphPad Prism.
Substrate specificity of the TIR domain of TIR-1 base exchange reaction
The TIR domain of TIR-1 (2.5 μM) was incubated in 50 mM MES pH 6.0, 150 mM NaCl, and 25% PEG 3350 with 100 μM NAD or NADP and 1 mM alternative substrate in triplicate. Alternative substrates include nicotinic acid, 3-acetyl pyridine, pyridoxine, nicotine, indole, quinoline, isoquinoline, and 5-amino-, 5-nitro, 5-fluoro, 5-chloro-, 5-bromo, and 5-iodoisoquinoline. The reaction was incubated at 25 °C for 30 min, after which the reaction was stopped by the addition 0.25 % TFA and flash freezing in liquid N2. Samples were filtered through a 0.22 μm Costar Spin-X cellulose acetate centrifugal filter. 25 μL was loaded onto a Synergi Fusion-RP (4.6 mm × 150 mm, 4 μm; Phenomenex) with an Agilent 1260 HPLC. Metabolites were eluted from the column using an isocratic method (98.5% 40 mM ammonium acetate, pH 6 and 1.5% methanol) over 12 min; the column was thermostatted at 55 °C. HPLC traces were plotted in GraphPad Prism. Peak areas were calculated using ChemStation (Agilent) and plotted in GraphPad Prism. Percent cleavage was calculated using Equation 1.
Initial velocity experiments to evaluate the kinetic mechanism of SARM1 and TIR-1
For the TIR domain of TIR-1, we first titrated NAD at different concentrations of PC6. 2.5 μM TIR domain of TIR-1 was incubated for 10 min with 50, 150, 2000 μM PC6 in the presence of 25% PEG 3350 in 50 mM Tris pH 8.0 and 150 mM NaCl in triplicate. The reaction was initiated with 0–4000 μM NAD and monitored for 15 min every 15 s at 25 °C at. In 500 mM sodium citrate, the experimental conditions are the same except 1 μM enzyme and 2, 4, 10, 50, 150, or 250 μM PC6 were used. Next, we titrated PC6 at several concentrations of NAD. The TIR domain of TIR-1 (2.5 μM) was incubated for 10 min with 0–2000 μM PC6 in the presence of 25% PEG 3350 in 50 mM Tris pH 8.0 and 150 mM NaCl in triplicate. The reaction was initiated with 200, 600, or 4000 μM NAD and monitored for 15 min every 15 s at 25 °C at. In 500 mM sodium citrate, the experimental conditions are the same except 1 μM enzyme and 0–250 μM PC6 were used.
For SARM1ΔMLS, we repeated the above analysis with SARM1ΔMLS instead of the TIR domain of TIR-1. For these experiments, SARM1ΔMLS (750 nM) was incubated for 10 min with 3.125, 6.25, 12.5, or 25 μM PC6 in 20 mM HEPES, pH 7.5 and 150 mM NaCl in triplicate. The reaction was initiated with 0–1000 μM NAD+ and monitored for 20 min every 15 s at 25 °C at. Next, we completed the inverse experiment wherein 750 nM SARM1ΔMLS was incubated for 10 min with 500 μM PC6 in 20 mM HEPES, pH 7.5 and 150 mM NaCl in triplicate. The reaction was initiated with 50, 100, 200, 400, 800, or 4000 μM NAD and monitored for 20 min every 15 s at 25 °C at.
Fluorescence intensities were converted to product (PAD-6) concentrations using the standard curve described above. Reaction velocities were determined by calculating the slope of the fluorescence intensities versus time. Reciprocal velocities and reciprocal were plotted in GraphPad Prism for TIR-1 and GraFit for SARM1ΔMLS. We elected to fit the data to the Michaelis-Menten equation as opposed to a ternary complex or double displacement equation to allow the pattern of the double reciprocal plots to reveal itself.
Methanolysis of NAD+ and cADPR by TIR-1 and SARM1
The TIR domain of TIR-1 (5 μM) was incubated at 25 °C in 50 mM Tris pH 8.0, 150 mM NaCl, and 25% PEG 3350 with 0–2 M methanol and 500 μM NAD+ or cADPR in triplicate. For SARM1ΔMLS, 1.5 μM enzyme was incubated 25 °C in 20 mM HEPES pH 7.5 and 150 mM NaCl with 0–2 M methanol and 500 μM NAD+ or cADPR in triplicate. The reactions were stopped by the addition of 0.25% TFA after 4 h. Control experiments without enzyme were also run and stopped with 0.25% TFA after 16 h. Samples were filtered through a 0.22 μm Costar Spin-X cellulose acetate centrifugal filter and 25 μL was injected onto a POROS HQ column (10 μm, 4.6 × 100 mm, 1.7 mL; ThermoScientific) attached to an Agilent 1260 HPLC. Metabolites were eluted from the column using the following gradient: 100% buffer A for 1 min, 0% to 50% buffer B over 8.25 min, 50% to 100% buffer B over 3 min, 100% buffer B to 100% buffer A over 1 min, and 100% buffer A for 3 min at a 1 mL/min flow rate. Buffer A consisted of 10 mM ammonium acetate pH 5.0 and buffer B consisted of 1 M ammonium acetate pH 5.0. Areas under the curve were calculated using Chem Station (Agilent), converted to μM by a standard curve, and plotted in GraphPad Prism. The partition ratio was calculated by linear regression and multiplying the slope by the concentration of water (~55 M).
| (Eq. 2) |
To determine the anomeric composition of the O-methyl-ADPR produced during methanolysis, the TIR domain of TIR-1 (5 μM) was incubated in 50 mM Tris pH 8.0, 150 mM NaCl, and 25% PEG 3350 with 2000 μM NAD+. SARM1ΔMLS (1.5 μM) was incubated in 20 mM HEPES pH 7.5 and 150 mM NaCl with 2000 μM NAD+ in parallel. The reactions were stopped after 16–24 h with 0.25% TFA and filtered through a 0.22 μm Costar Spin-X cellulose acetate filter. The O-methyl-ADPR peak was purified by HPLC using the same column and gradient described above and lyophilized. Dried product was reconstituted in water, injected onto a Supelcosil LC-18 column (5 μm, 4.6 × 250 mm; Supelco) attached to an Agilent 1260 HPLC and quadrupole ESI mass spectrometer (Agilent 6130). Metabolites were eluted with a gradient consisting of 10 mM ammonium phosphate pH 5.5 (solvent A) and 1–5% acetonitrile (solvent B. The gradient was 1% solvent B for 2 min, 1% to 5% solvent B for 18 min, 5% to 1% solvent B, and 1% solvent B for 1 min. The stereochemistry of the purified product was determined by 13C NMR.
Chemical quenching to trap potential covalent intermediates
SARM1ΔMLS (0.5 mg/mL) was incubated with 0 or 1000 μM NAD+ in 20 mM HEPES pH 7.5 and 150 mM NaCl for 1 min at 25 °C. In parallel, the TIR domain of TIR-1 (0.5 mg/mL) was incubated with 0 or 1000 μM NAD+ in 50 mM Tris pH 8.0, 150 mM NaCl, and 25% PEG 3350 for 1 min at 25 °C. The reactions were quenched with 0.5% TFA and diluted 1:1 with water. 100 μL of each sample was injected onto an Infinity Lab Poroshell 120 EC-C18 coupled to an Agilent 1260 HPLC and 6130 quadrupole ESI mass spectrometer. Protein was eluted with a gradient of 100% solvent A for 2 min, 0% to 100% solvent B over 18 min, and 100% to 0% solvent B for 2 min at a flow rate of 1 mL/min. Solvent A was water with 0.1% formic acid and Solvent B was acetonitrile with 0.1% formic acid.
Supplementary Material
Proteins of Interest
| Protein | Uniprot ID | Link |
|---|---|---|
| SARM1 | Q6SZW1 | https://www.uniprot.org/uniprot/Q6SZW1 |
| TIR-1A | Q86DA5 | https://www.uniprot.org/uniprot/Q86DA5 |
Funding Sources
This work was supported in part by National Institutes of Health grants R35 GM118112 (PRT), T32 AI132152 (JDI), and F31 NS122423) and the Dan and Diane Riccio Fund for Neuroscience (PRT).
ABBREVIATIONS
- SARM1
sterile alpha and toll-interleukin receptor motif containing protein 1
- TIR
toll/interleukin receptor
- TIR-1
toll and interleukin 1 receptor
- ADPR
ADP ribose
- cADPR
cyclic ADP ribose
- NAD+
nicotinamide adenine dinucleotide
- NADP+
nicotinamide adenine dinucleotide phosphate
- NMN
nicotinamide mononucleotide (NMN)
- NA
nicotinic acid
- NAADP
nicotinic acid adenine dinucleotide phosphate
- ADPRP
ADP ribose phosphate
- cADPRP
cyclic ADPR ribose phosphate
- ENAD
nicotinamide 1,N6-ethenoadenine dinucleotide
- EADPR
etheno-ADPR
- PC6
pyridyl conjugate 6
- PAD6
PC6 adenine dinucleotide
- PEG 3350
poly ethylene glycol 3350
- NHD
NHDH
- 3ap
3-acetyl pyridine
- 3apAD
3-acetyl pyridine adenine dinucleotide
- sNAD
thio-NAD
- B6
pyridoxine
Footnotes
ASSOCIATED CONTENT
Supporting Information
Figures S1–S8 depicting full HPLC chromatographs, structures of alternative substrates, additional phase transition and kinetic experiments, NMR analysis of methanolysis reaction, chemical trapping experiment, and NMR and LCMS validation of PC6 synthesis. The Supporting Information is available free of charge at http://pubs.acs.org
The authors declare no competing conflicts of interest.
REFERENCES
- (1).Essuman K, Summers DW, Sasaki Y, Mao X, Yim AKY, DiAntonio A, and Milbrandt J. (2018) TIR Domain Proteins Are an Ancient Family of NAD(+)Consuming Enzymes, Curr Biol 28, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, and Milbrandt J. (2017) The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD(+) Cleavage Activity that Promotes Pathological Axonal Degeneration, Neuron 93, 13341343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Gerdts J, Summers DW, Sasaki Y, DiAntonio A, and Milbrandt J. (2013) Sarm1-mediated axon degeneration requires both SAM and TIR interactions, J Neurosci 33, 13569–13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH Jr., Conforti L, Coleman M, Tessier-Lavigne M, Züchner S, and Freeman MR (2012) dSarm/Sarm1 is required for activation of an injuryinduced axon death pathway, Science 337, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Loring HS, and Thompson PR (2020) Emergence of SARM1 as a Potential Therapeutic Target for Wallerian-type Diseases, Cell Chem Biol 27, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Icso JD, and Thompson PR (2022) The chemical biology of NAD(+) regulation in axon degeneration, Curr Opin Chem Biol 69, 102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Summers DW, Gibson DA, DiAntonio A, and Milbrandt J. (2016) SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injuryinduced SARM1 activation, Proc Natl Acad Sci U S A 113, E6271–e6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Shi Y, Kerry PS, Nanson JD, Bosanac T, Sasaki Y, Krauss R, Saikot FK, Adams SE, Mosaiab T, Masic V, Mao X, Rose F, Vasquez E, Furrer M, Cunnea K, Brearley A, Gu W, Luo Z, Brillault L, Landsberg MJ, DiAntonio A, Kobe B, Milbrandt J, Hughes RO, and Ve T. (2022) Structural basis of SARM1 activation, substrate recognition, and inhibition by small molecules, Mol Cell 82, 16431659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Jiang Y, Liu T, Lee CH, Chang Q, Yang J, and Zhang Z. (2020) The NAD(+)-mediated self-inhibition mechanism of pro-neurodegenerative SARM1, Nature 588, 658–663. [DOI] [PubMed] [Google Scholar]
- (10).Figley MD, Gu W, Nanson JD, Shi Y, Sasaki Y, Cunnea K, Malde AK, Jia X, Luo Z, Saikot FK, Mosaiab T, Masic V, Holt S, Hartley-Tassell L, McGuinness HY, Manik MK, Bosanac T, Landsberg MJ, Kerry PS, Mobli M, Hughes RO, Milbrandt J, Kobe B, DiAntonio A, and Ve T. (2021) SARM1 is a metabolic sensor activated by an increased NMN/NAD(+) ratio to trigger axon degeneration, Neuron 109, 1118–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bratkowski M, Xie T, Thayer DA, Lad S, Mathur P, Yang YS, Danko G, Burdett TC, Danao J, Cantor A, Kozak JA, Brown SP, Bai X, and Sambashivan S. (2020) Structural and Mechanistic Regulation of the Pro-degenerative NAD Hydrolase SARM1, Cell Rep 32, 107999. [DOI] [PubMed] [Google Scholar]
- (12).Loring HS, Czech VL, Icso JD, O’Connor L, Parelkar SS, Byrne AB, and Thompson PR (2021) A phase transition enhances the catalytic activity of SARM1, an NAD(+) glycohydrolase involved in neurodegeneration, Elife 10, e66694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).George EB, Glass JD, and Griffin JW (1995) Axotomy-Induced Axonal Degeneration Is Mediated by Calcium Influx Through Ion-Specific Channels, J, Neurosci 15, 6445–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Li Y, Pazyra-Murphy MF, Avizonis D, de Sa Tavares Russo M, Tang S, Chen CY, Hsueh YP, Bergholz JS, Jiang T, Zhao JJ, Zhu J, Ko KW, Milbrandt J, DiAntonio A, and Segal RA (2022) Sarm1 activation produces cADPR to increase intra-axonal Ca++ and promote axon degeneration in PIPN, J Cell Biol 221, e202106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, and Tessier-Lavigne M. (2013) Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin, Neuron 80, 1175–1189. [DOI] [PubMed] [Google Scholar]
- (16).Henninger N, Bouley J, Sikoglu EM, An J, Moore CM, King JA, Bowser R, Freeman MR, and Brown RH Jr. (2016) Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1, Brain 139, 10941105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Geisler S, Doan RA, Strickland A, Huang X, Milbrandt J, and DiAntonio A. (2016) Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice, Brain 139, 30923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Turkiew E, Falconer D, Reed N, and Hoke A. (2017) Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy, J Peripher Nerv Syst 22, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hughes RO, Bosanac T, Mao X, Engber TM, DiAntonio A, Milbrandt J, Devraj R, and Krauss R. (2021) Small Molecule SARM1 Inhibitors Recapitulate the SARM1(−/−) Phenotype and Allow Recovery of a Metastable Pool of Axons Fated to Degenerate, Cell Rep 34, 108588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bosanac T, Hughes RO, Engber T, Devraj R, Brearley A, Danker K, Young K, Kopatz J, Hermann M, Berthemy A, Boyce S, Bentley J, and Krauss R. (2021) Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy, Brain 144, 3226–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Li WH, Huang K, Cai Y, Wang QW, Zhu WJ, Hou YN, Wang S, Cao S, Zhao ZY, Xie XJ, Du Y, Lee CS, Lee HC, Zhang H, and Zhao YJ (2021) Permeant fluorescent probes visualize the activation of SARM1 and uncover an anti-neurodegenerative drug candidate, Elife 10, e67381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bratkowski M, Burdett TC, Danao J, Wang X, Mathur P, Gu W, Beckstead JA, Talreja S, Yang YS, Danko G, Park JH, Walton M, Brown SP, Tegley CM, Joseph PRB, Reynolds CH, and Sambashivan S. (2022) Uncompetitive, adductforming SARM1 inhibitors are neuroprotective in preclinical models of nerve injury and disease, Neuron 110, 3711–3726. [DOI] [PubMed] [Google Scholar]
- (23).Feldman HC, Merlini E, Guijas C, DeMeester KE, Njomen E, Kozina EM, Yokoyama M, Vinogradova E, Reardon HT, Melillo B, Schreiber SL, Loreto A, Blankman JL, and Cravatt BF (2022) Selective inhibitors of SARM1 targeting an allosteric cysteine in the autoregulatory ARM domain, Proc Natl Acad Sci U S A 119, e2208457119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Loring HS, Parelkar SS, Mondal S, and Thompson PR (2020) Identification of the first noncompetitive SARM1 inhibitors, Bioorg Med Chem 28, 115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, and Bowie AG (2006) The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling, Nat Immunol 7, 1074–1081. [DOI] [PubMed] [Google Scholar]
- (26).Peterson ND, Icso JD, Salisbury JE, Rodriguez T, Thompson PR, and Pukkila-Worley R. (2022) Pathogen infection and cholesterol deficiency activate the C. elegans p38 immune pathway through a TIR-1/SARM1 phase transition, Elife 11, e74206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Sasaki Y, Engber TM, Hughes RO, Figley MD, Wu T, Bosanac T, Devraj R, Milbrandt J, Krauss R, and DiAntonio A. (2020) cADPR is a gene dosage-sensitive biomarker of SARM1 activity in healthy, compromised, and degenerating axons, Exp Neurol 329, 113252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ko KW, Devault L, Sasaki Y, Milbrandt J, and DiAntonio A. (2021) Live imaging reveals the cellular events downstream of SARM1 activation, Elife 10, 71148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhao ZY, Xie XJ, Li WH, Liu J, Chen Z, Zhang B, Li T, Li SL, Lu JG, Zhang L, Zhang LH, Xu Z, Lee HC, and Zhao YJ (2019) A Cell-Permeant Mimetic of NMN Activates SARM1 to Produce Cyclic ADPRibose and Induce Non-apoptotic Cell Death, iScience 15, 452–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Angeletti C, Amici A, Gilley J, Loreto A, Trapanotto AG, Antoniou C, Merlini E, Coleman MP, and Orsomando G. (2022) SARM1 is a multi-functional NAD(P)ase with prominent base exchange activity, all regulated bymultiple physiologically relevant NAD metabolites, iScience 25, 103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Zhao YJ, He WM, Zhao ZY, Li WH, Wang QW, Hou YN, Tan Y, and Zhang D. (2021) Acidic pH irreversibly activates the signaling enzyme SARM1, FEBS J 288, 6783–6794. [DOI] [PubMed] [Google Scholar]
- (32).Sauve AA, Munshi C, Lee HC, and Schramm VL (1998) The Reaction Mechanism for CD38. A Single Intermediate Is Responsible for Cyclization, Hydrolysis, and Base-Exchange Chemistries †, Biochemistry 37, 13239–13249. [DOI] [PubMed] [Google Scholar]
- (33).Liu Q, Kriksunov IA, Jiang H, Graeff R, Lin H, Lee HC, and Hao Q. (2008) Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38, Chem Biol 15, 1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Fliegert R, Watt JM, Schöbel A, Rozewitz MD, Moreau C, Kirchberger T, Thomas MP, Sick W, Araujo AC, Harneit A, Potter BVL, and Guse AH (2017) Ligand-induced activation of human TRPM2 requires the terminal ribose of ADPR and involves Arg1433 and Tyr1349, Biochem J 474, 2159–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Pascal M, and Schuber F. (1976) The stereochemistry of calf spleen NAD-glycohydrolase-catalyzed NAD methanolysis, FEBS Lett 66, 107–109. [DOI] [PubMed] [Google Scholar]
- (36).Muller-Steffner HM, Augustin A, and Schuber F. (1996) Mechanism of cyclization of pyridine nucleotides by bovine spleen NAD+ glycohydrolase, J Biol Chem 271, 23967–23972. [DOI] [PubMed] [Google Scholar]
- (37).Lee HC, and Zhao YJ (2019) Resolving the topological enigma in Ca(2+) signaling by cyclic ADP-ribose and NAADP, J Biol Chem 294, 19831–19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sinnott ML, and Jencks WP (1980) Solvolysis of D-glucopyranosyl derivatives in mixtures of ethanol and 2,2,2-trifluoroethanol, Journal of the American Chemical Society 102, 2026–2032. [Google Scholar]
- (39).Yu D, Song W, Tan EYJ, Liu L, Cao Y, Jirschitzka J, Li E, Logemann E, Xu C, Huang S, Jia A, Chang X, Han Z, Wu B, Schulze-Lefert P, and Chai J. (2022) TIR domains of plant immune receptors are 2’,3’cAMP/cGMP synthetases mediating cell death, Cell 185, 2370–2386. [DOI] [PubMed] [Google Scholar]
- (40).Kapoor M, Liu D, and Young MC (2018) Carbon Dioxide-Mediated C(sp(3))-H Arylation of Amine Substrates, J Am Chem Soc 140, 6818–6822. [DOI] [PubMed] [Google Scholar]
- (41).Li WH, Huang K, Cai Y, Wang QW, Zhu WJ, Hou YN, Wang S, Cao S, Zhao ZY, Xie XJ, Du Y, Lee C-S, Lee HC, Zhang H, and Zhao YJ (2021) Permeant fluorescent probes visualize the activation of SARM1 and uncover an anti-neurodegenerative drug candidate, eLife 10, e67381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.