Abstract
Purpose:
To discuss the safety and efficacy of various forms of ab-interno trabeculotomy procedures.
Methods:
A comprehensive search in PubMed and Google Scholar was done using the keywords “glaucoma”, “microinvasive glaucoma surgery”, “complications”, “goniotomy”, and “trabeculotomy”. Publications discussing ab-interno trabeculotomy procedures were selected; furthermore, the relevant references in these articles were gathered and the search was updated during the article preparation. Since gonioscopy-assisted transluminal trabeculotomy was first introduced in 2014, we had no time restriction.
Results:
Ab-interno trabeculotomy procedures, as a type of minimally invasive glaucoma surgeries, facilitate the natural trabecular outflow and lower the intraocular pressure (IOP) while preserving the conjunctiva for possible future glaucoma surgeries. It can be done alone or in combination with cataract surgery and effectively lowers the IOP and the number of antiglaucoma medications in various forms of glaucoma.
Conclusion:
By appropriate patient selection, ab-interno trabeculotomy could be selected as a safe and effective procedure in the management of various forms of glaucoma either as an isolated procedure or in combination with cataract extraction.
Keywords: Glaucoma, Intraocular pressure, Microinvasive surgery, Trabeculotomy
INTRODUCTION
Glaucoma is one of the most common causes of irreversible visual loss worldwide.1 It affects about 76 million people around the world in 2020, which has been estimated to increase to 111.8 million by 2040.2 Intraocular pressure (IOP) is the only modifiable risk factor in glaucoma management.3,4 Medical, laser, and surgical interventions can be used to lower the IOP by different mechanisms. Minimally invasive glaucoma surgeries (MIGS) mainly increase the natural outflow that consists of trabecular and uveal outflows. Trabecular outflow consists of proximal (trabecular meshwork [TM]) and distal (Schlemm’s canal, collector channels, mid-scleral plexus veins, and episcleral veins) parts.5 It has been stated that major resistance to aqueous outflow occurs at the TM.6,7,8
Trabeculotomy is a blebless IOP-lowering surgery that increases trabecular outflow.9,10,11 It has been estimated that 360° trabeculotomy reduces 50% and 75% of outflow resistance in the eyes with IOPs of around 7 and 25 mmHg, respectively. Considering that trabecular outflow is pressure dependent, this implies that a greater portion of IOP resistance resides in the distal part of trabecular outflow in eyes with lower IOP, which decreases upon IOP elevation.12
Gonioscopy-assisted transluminal trabeculotomy (GATT) is a kind of trabecular-targeted MIGS introduced by Grover in 2014.13 GATT is an ab-interno, conjunctiva sparing, sutureless, and blebless IOP-lowering surgical approach based on the disruption of the TM and the inner wall of Schlemm’s canal aiming at the improvement of the outflow facility.5 This review aims to evaluate the safety and efficacy of ab-interno trabeculotomy procedures.
METHODS
The PubMed and Google Scholar databases were searched using the keywords “glaucoma”, “microinvasive glaucoma surgery”, “complications”, “goniotomy”, and “trabeculotomy” without language or time restriction. Since GATT was first introduced in 2014, we had no time restriction. The reference list of relevant studies was explored for additional resources. The titles and the abstracts of 205 articles were meticulously reviewed and eligible sources with available full texts were gathered and checked for duplication. The search was updated during article preparation. A comprehensive literature review was performed on 66 articles.
RESULTS
GATT is done as a standalone procedure or in combination with cataract extraction (CE) without compromising the efficacy.13,14 In addition, it may be performed before or after the cataract surgery.14,15,16 In doing so, a main temporal incision and a paracentesis tract at inferonasal or supranasal limbus are made depending on the laterality of the eye and the surgeon’s dominant hand. Then, an intracameral miotic agent can be injected, especially in phakic eyes to protect the lens.17 The anterior chamber is then filled with viscoelastic agents, preferably cohesive to limit blood influx into the anterior chamber,18 and by tilting the patient’s head and microscope, the surgeon can have a clear view of the nasal angle structures by using a direct goniolens.
Once the nasal angle structures are identified, 1–2 mm goniotomy is performed with either a 25G needle19 or a microvitreoretinal blade.20 Besides, a trabectome can used to initiate GATT by performing a 90° ablation of nasal TM and proceeding to 360° ab-interno trabeculotomy (trabectome-initiated GATT). As the initial TM incision may cause bleeding and obscure the view of the surgeon, performing TM ablation can provide a clear view of the lumen and the outer wall of Schlemm’s canal, and the possibility of wound reapproximation following tissue ablation is minimized in the trabectome-treated part of the angle.17 Jared et al. started with a Kahook dual blade (KDB) to excise 2 clock hours of the TM and then proceeded with GATT in a case of uveitis and the subsequent steroid-induced glaucoma.21
After performing goniotomy, a suture or microcatheter is introduced into the Schlemm’s canal. When the suture or microcatheter passes 360° of the canal, which can be roughly achieved by 20 passes of 2 mm when threading TM in eyes with normal corneal diameter, the tip emerges through the site of goniotomy. Then, both proximal and distal ends can be grasped and the suture or microcatheter can be externalized through the incision to perform a 360° trabeculotomy. This can be done by externalizing the tip and making a traction on the other end to complete the procedure. Finally, anterior chamber is irrigated and partially (15%–40%) filled with a viscoelastic agent to prevent further blood influx due to postoperative hypotonia.
A surgeon can use a thermally blunted 5-0 or 6-0 prolene or nylon22 suture to avoid Schlemm’s canal trauma and misdirection. The blunted tip 5-0 nylon used in ab-externo trabeculotomy had a more rounded and thinner tip compared to 6-0 prolene23 [Figure 1a and b].
Figure 1.

Blunted tip 6-0 prolene (a) had a thicker and angulated head compared to the 5-0 nylon (b), which had a rounded edge, matchstick like head with less friction
Grover et al. preferred the 4.0 nylon due to higher flexibility and lower ocular structure damage compared to prolene. In addition, integrating the surgical marker ink into the suture by melting made it more visible even through the ocular surface in some cases24 [Figure 2a-d].
Figure 2.
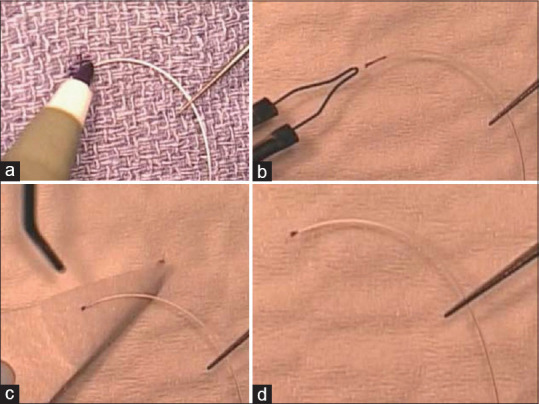
The tip of a clear 4.0 nylon suture is marked with a surgical marking pen (a). By applying low-temperature cautery, the ink is incorporated into the nylon while blunting the tip (b). Excess ink is removed by balanced saline solution and sponge (c). The final picture of the suture (d)
Flexible illuminated microcatheters such as iTrack (Ellex, California, USA) and Glaucolight (DORC International, Zuidland, The Netherlands) have the benefit of continuously tracking the catheter and decrease the chance of subretinal or suprachoroidal misdirection, which is a possible serious complication.18,25 In cases where the catheter is stopped in the canal or is directed into the collector channels, it helps find the precise location and retrieve it through cut-down.26 Glaucolight is a single-use, flexible, blunt-ended microcatheter with a diameter of 150 μm (40G) that has an integrated sterile LED light for illumination. It is not equipped with a lumen for viscoelastic injection and is not connected to an external light source.27 The iTrack has a greater diameter compared to Glaucolight (200 vs. 150 μm). Besides, it has the benefit of a 70-μm diameter lumen, through which the viscoelastic material is injected into the Schlemm’s canal and provides the opportunity to perform ab-interno canaloplasty during the procedure.28 However, the concern about microcatheters is the cost compared to sutures despite the lack of difference in the final results of the surgery.24 Liang et al. noticed that prolene suture had greater pliability compared to the iTrack microcatheter. Pliability was measured through an experiment, assessing the maximum force (m-force) by reading the maximum power that vertically held sutures or catheters of various lengths could apply over a balance with microgram sensitivity. Based on the results, the m-force of the 6.0 prolene suture was one-third of the 5.0 prolene and the iTrack had a 50-time greater m-force compared to the prolene suture. Since the m-force was greater in smaller lengths, the Schlemm’s canal encountered the maximum trauma on inserting the suture or microcatheter and the iTrack traumatized the canal more than others.29
In some cases, it is not possible to perform the 360° trabeculotomy and the procedure may stop at around 180°–270°. In this case, the surgeon can perform an ab-interno cut-down over the stop point by retrieving the externalized end and perform 180°–270° trabeculotomy. By passing the suture or microcatheter in an opposite direction once again, it would be possible to complete the 360° trabeculotomy. Grover et al. performed GATT on 198 patients, and a complete 360° trabeculotomy could be performed in 89.4% of the eyes. In that study, 5.5% and 4.5% of the eyes were treated by 300°–360° and 180°–280°, respectively.16 In another study, 4 out of the 66 eyes could not have the 360° pass and the catheter stopped at about 270°, which was possibly due to a previous cataract surgery scar.20 Furthermore, Fontana et al. reported that about 20 procedures were necessary before becoming a master in performing 360° trabeculotomy.30
After removing the suture or microcatheter, anterior chamber is filled with balanced saline solution (BSS) by placing the irrigation handpiece in the vicinity of the angle structures to flush away the collector channels. This is visible as blanching of the perilimbal vessels of that area, known as episcleral venous fluid wave (EVFW).
EVFW was first described by Fellman et al. in eyes that underwent trabectome (NeoMedix Corp., Tustin, CA). The extent of EVFW was significantly associated with the success of the operation. In fact, EVFW indicated the patency of the collector channels and the integrity of the distal part of the conventional outflow and was shown as blanching of the episcleral, conjunctival, and aqueous veins during the irrigation phase, presenting the surge of the BSS into the collector channels31,32 [Figure 3a and b]. Aktas et al. assessed the EVFW in patients who had GATT. They came to the conclusion that the extent of EVFW was positively correlated to the success of the surgery and a lower postoperation IOP. In addition, the EVFW of <4.5 h was associated with an increased need for restarting antiglaucoma medications after the operation.33 Furthermore, the amount of the viscoelastic agent left in the anterior chamber by the end of the surgery can be adjusted based on the amount of blood influx and the extent of EVFW.13,16
Figure 3.
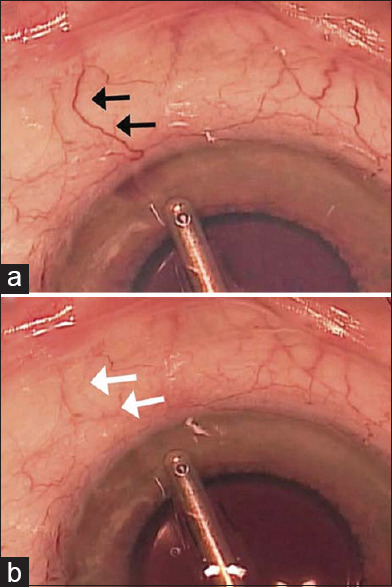
Episcleral venous fluid wave. During hypotony, blood influxes into the anterior chamber. A conjunctival vein is marked with black arrow (a). The balanced saline solution fluid surge into the marked conjunctival vein led to blanching (b)
GATT can be performed in eyes with congenital, juvenile, primary, or secondary open-angle glaucoma (SOAG) and identifiable angle landmarks. Since GATT is accompanied by a moderate hemorrhagic risk, antithrombotic therapy is recommended to be stopped at a proper time prior to surgery.34
A summary of important studies with at least 50 participants and 6-month follow-up period, evaluating the efficacy and safety of GATT in various forms of glaucoma, is presented in Table 1.
Table 1.
The clinical effectiveness of gonioscopy-assisted transluminal trabeculotomy in various studies
| Author (year) | Study design | Eyes/participant | Follow-up (months) | Primary disease | Surgery type | Number of cases with 360° treated angle | Mean IOP reduction (mmHg [%) | Reduction in the number of IOP-lowering agents |
|---|---|---|---|---|---|---|---|---|
| Grover et al. (2014)13 | RCS | 85 (85) | 12 | 57 POAG 28 SOAG | 58 GATT 27 GATT + CE | N/A | 11.1 (39.8) POAG 19.9 (56.8) SOAG | 1.1 POAG 1.9 SOAG |
| Rahmatnejad et al. (2017)20 | RCS | 66 (66) | 12 | 48 POAG 18 SOAG | 56 GATT 10 GATT + CE | 62 | 9.3 (38) POAG 18.6 (61.2) SOAG | 1.7 POAG 1.7 SOAG |
| Grover et al. (2018)16 | RCS | 198 (198) | 24 | 119 POAG 79 SOAG | 137 GATT 61 GATT + CE | 177 | 9.2 (37.3) POAG 14.1 (49.8) SOAG | 1.4 POAG 2 SOAG |
| Sato et al. (2018)37 | PCS | 64 (64) | 24 | 28 POAG 20 NTG 16 PEXG | 50 GATT + CE 14 GATT | N/A | 5 (27.2) | 0.5 |
| Aktas et al. (2019)35 | Comparative RCS | 104 (104) | 18 | 65 POAG 39 SOAG | 81 GATT 23 GATT + CE | N/A | 9.1 (36.4) | 2.2 |
| Wang et al. (2020)52 | RCS | 59 (48) | 18 | JOAG | GATT | N/A | 12.4 (47.9) | 3.3 |
| Sato et al. (2020)61 | Prospective 3-arm randomized trial | 99 (99) | 12 | 51 POAG 22 NTG 26 PEXG | 360° incision group (n=34), upper-180° incision group (n=34), lower-180° incision group (n=31) | N/A | 4.9 (20.8) | 1.7 |
| Salimi et al. (2020)18 | RCS | 56 (47) | 12 | 34 POAG and JOAG 17 SOAG 5 OHTN | 50 GATT 6 GATT + CE | 51 | 13.7 (49) | 1.9 |
| Sharkawi et al. (2020)43 | PCS | 103 (84) | 24 | PEXG | 53 GATT 50 GATT + CE | 97 | 13.4 (51) | 1.8 |
| Bozkurt et al. (2020)96 | RCS | 108 (108) | 12 | 42 POAG 66 PEXG | 40 GATT 68 GATT + CE | 86 | 10.3 (40.9) | 2.5 |
| Shi et al. (2021)51 | PCS | 70 (70) | 12 | JOAG | 70 GATT | 67 | 15.5 (44.3) | 2.4 |
| Fontana et al. (2021)30 | RCS | 110 (110) | 18 | POAG PEXG Uveitic glaucoma | 61 GATT 49 Trabx | 46 | 11.6 (42) GATT 17 (56) Trabx | 2.3 Trabx 2.1 GATT |
| Faria et al. (2021)39 | RCS | 100 (89) | 24 | 69 POAG 26 SOAG 5 congenital glaucoma | 51 GATT 49 GATT + CE | 77 | 12.2 (49.2) | 2.6 |
| Aktas et al. (2022)97 | Comparative RCS | 202 | 36 | 91 POAG 111 PEXG | 153 GATT 49 GATT + CE | 179 | 8.8 (34.4) POAG 12.8 (44.6) PEXG | 2 POAG 2.3 PEXG |
| Wan et al. (2023)40 | PCS | 124 | 24 | POAG | 66 GATT 58 GATT + CE | N/A | 41.5 | 2.54 |
|
| ||||||||
| Author (year) | Definition of success/failure | Success/failure rate | Factors associated with failure | GATT complications | ||||
|
| ||||||||
| Grover et al. (2014) | Failure: Reoperation to control pressure; a postoperative IOP not lowered by at least 20%; IOP of >21 mmHg; a single high-pressure refractory to glaucoma medications | 9% - failure | N/A | 30% hyphema 9.4% IOP spike 1.2% CME 1.2% choroidal folds | ||||
| Rahmatnejad et al. (2017) | Success: IOP reduction >20% from baseline or IOP between 5 and 21 mmHg; no need for further glaucoma surgeries | 63% - success rate | Postoperative IOP spike (defined as IOP >30 mmHg) African-American race | 38% hyphema 24.7% IOP spike | ||||
| Grover et al. (2018) | Failure: Reoperation to control pressure; a postoperative IOP not lowered by at least 20%; IOP of >21 mmHg; a single high-pressure refractory to glaucoma medications | 0.18–0.48 - cumulative proportion of failure | Pseudophakic POAG preoperative MD of −15.0 or worse in POAG eyes | 31.3% hyphema 1.5% iridodialysis 1% Descemet detachment 0.5% IOP spike 0.5% hypotony maculopathy and choroidal effusion | ||||
| Sato et al. (2018) | Success: Postoperative IOP ≤15 mmHg and IOP reduction>20% (criterion A) or IOP ≤12 mmHg and IOP reduction >30% (criterion B) | 49.2% - criterion A and 16.0% - criterion B | Nothing found | 50% hyphema 28% IOP spike | ||||
| Aktas et al. (2019) | Success: Both IOP <21 and <18 mmHg and ≥20% reduction from baseline without further glaucoma surgeries | 83.7% for IOP <21 and 65.4% for IOP <18 | N/A | 28.3% hyphema 15.4% IOP spike 0.9% iridodialysis 0.9% TASS 0.9% suprachoroidal hemorrhage | ||||
| Wang et al. (2020) | Success: IOP ≤18 mmHg and a reduction of IOP by 20% or more from baseline with (qualified success) or without (complete success) glaucoma medications | 58.6% - complete success 81.2% - qualified success | IOP spike | 53.6% macrohyphema 49.1% microhyphema 48.3% IOP spike | ||||
| Sato et al. (2020) | Success: IOP ≤21 mmHg and ≥20% IOP reduction (criterion A) or IOP ≤15 mmHg and ≥20% IOP reduction (criterion B) | Criterion A: 36.7% in the 360° group, 26.5% in the upper-180° group, and 25.5% in the lower-180° group Criterion B: 28.9% in the 360° group, 20.9% in the upper-180° group, and 20.6% in the lower-180° group | N/A | 34.3% IOP spike 14.1% hyphema 5% hypotony | ||||
| Salimi et al. (2020) | Success: IOP between 6 and 21 mmHg (criterion-A) or 6 and 18 mmHg (criterion-B) and a relative IOP reduction of ≥20% compared to baseline | 84% - success rate for both criteria | Older age at the time of glaucoma diagnosis | 71% hyphema 46% IOP spike 16% microcystic corneal edema 5% lens-related changes | ||||
| Sharkawi et al. (2020) | Success: IOP reduction ≥20% from baseline or IOP between 6 and 21 mmHg, without further glaucoma surgeries | 89.2% - success rate | N/A | 1% hyphema persisting >2 weeks 24.2% IOP spike | ||||
| Bozkurt et al. (2020) | Success: IOP reduction >20% from baseline; IOP between 5 and 21 mmHg; if surgery was done for intolerance to medications, preoperative <21 mmHg with medications, postoperatively <21 mmHg without medications; no need for further glaucoma surgery | 87.5% - GATT 83.8% - GATT with CE group | N/A | N/A | ||||
| Shi et al. (2021) | Postoperative IOP of ≤21 mmHg with at least a 20% reduction from preoperative IOP with or without the use of antiglaucoma medications (qualified and complete success, respectively) at each postoperative visit | 91.4% - qualified success 74.3% - complete success | Older age Longer duration of postoperative IOP spike | 74% IOP spike | ||||
| Fontana et al. (2021) | Complete success: IOP decrease ≥30% from baseline and an absolute IOP ≤18 mmHg with (qualified) or without (complete) the use of glaucoma medications | Complete success: 59% Trabx, 46% GATT Qualified success: 27% GATT, 31% Trabx | N/A | 11.5% hyphema 11.5% IOP spike 1.6% vitreous hemorrhage | ||||
| Faria et al. (2021) | Failure: Eyes that required further surgical interventions for IOP control | 26% - failure | Previous vitreoretinal surgery and scleral buckling | 50% hyphema | ||||
| Aktas et al. (2022) | Success: IOP reduction≥20% from baseline and IOP between 6 and 21 mmHg without further glaucoma surgery | 73.3% - POAG 79.4% - PEXG | N/A | 32% hyphema 13.4% IOP spike 1% iridodialysis 0.5% cyclodialysis 0.5% suprachoroidal hemorrhage 0.5% transient myopia | ||||
| Wan et al. (2023) | Success: (1) follow-up for at least 3 months, an IOP ≤18 mmHg, and a reduction of IOP by 20% or more from baseline with (qualified success) or without (complete success) glaucoma medications; or for eyes with preoperative IOP of <21 mmHg on three or four glaucoma medications, postoperative IOP of ≤18 mmHg without any glaucoma medications; (2) no loss of light perception vision; and (3) no need for additional surgical procedures | 68.42% - complete success 84.81% - qualified success | N/A | 39.5% microhyphema 40.3% macrohyphema 37.1% IOP spike 33.1% Supraciliary effusion | ||||
RCS: Retrospective case series, PCS: Prospective case series, POAG: Primary open-angle glaucoma, SOAG: Secondary open-angle glaucoma, NTG: Normal tension glaucoma, PEXG: Pseudoexfoliative glaucoma, JOAG: Juvenile open-angle glaucoma, OHTN: Ocular hypertension, GATT: Gonioscopy-assisted transluminal trabeculotomy, CE: Cataract extraction, N/A: Not available, IOP: Intraocular pressure, MD: Mean deviation, CME: Cystoid macular edema, Trabx: Trabeculectomy, TASS: Toxic anterior segment syndrome
Grover et al. reported a 24-month follow-up of 198 eyes of 198 patients with various forms of glaucoma. In that study, the patients were categorized based on primary open-angle glaucoma (POAG) or non-POAG, phakic, or pseudophakic status as well as solo or combined procedures. Among the patients with POAG, 46 phakic eyes and 37 pseudophakic eyes had GATT and 36 eyes had a combined procedure, with IOP reductions of 10.4, 8.4, and 8.9 mmHg, respectively. In addition, IOP and medication reduced by 37.3% and 1.43, respectively, in the patients with POAG. Moreover, pseudophakic eyes with POAG that underwent GATT had the highest rates of failure and reoperation among the patients, which was possibly due to the older age, worse preoperation mean deviation (MD), and a lower target pressure in these patients. Preoperation visual field MD of −15 or worse was also associated with a greater failure rate in the POAG patients, which could be associated with a more sclerotic distal outflow pathway. The cumulative proportions of failure and reoperation rate were 0.18–0.48 and 0.09–0.43, respectively, and were lowest among the patients with mild glaucoma compared to those with advanced glaucoma.16 Similarly, Rahmatnejad et al. categorized patients into six subgroups and reported the outcomes of 66 eyes undergoing GATT or combined GATT and cataract surgery during a mean follow-up of 11.9 months. Based on the results, 48 eyes had POAG and the remaining had other causes of SOAG including steroid induced, pigment dispersion glaucoma (PDG), uveitic, and pseudoexfoliation glaucoma (PEXG). The mean preoperation IOP was 26.1 ± 9.9 mmHg, which was reduced to 14.6 ± 4.7 mmHg in the 12th month, with an overall success rate of 63%. The patients with POAG had a mean IOP reduction of 10.4 ± 9.4 and 9.3 ± 10.8 mmHg, respectively, in the 6th and 9th months postoperatively. Moreover, there was no significant difference between phakic eyes (n = 27) with isolated GATT or combined procedure (n = 8) regarding the decrease in IOP or medications. However, there was a limited number of patients in the combined group.20 These results were in concordance with those of the research carried out by Aktas et al. on 104 eyes (65 – POAG and 39 – SOAG), which showed a mean IOP reduction of 41.34 ± 17.54%, 38.51 ± 20.72%, and 36.52 ± 17.22% in eyes with isolated GATT, combined procedure, and prior CE, respectively, during 19.4 ± 8.1 months, and the difference was not statistically significant. In addition, the results revealed no significant correlation between the visual field MD and IOP reduction, and only 3.9% of the patients needed further filtering surgeries.35
Fontana et al. compared the 18-month efficacy of GATT ± CE (n = 61) and trabeculectomy (n = 49) in 110 eyes with POAG, PEXG, and uveitic glaucoma. The percentage of IOP reduction was greater for trabeculectomy, which was statistically significant since the first postoperation week. At the 18th month postoperation, the mean reduction of IOP was 11.64 ± 5.34 (42.04 ± 15.56%) and 16.98 ± 8.13 mmHg (56.05 ± 17.72%) for GATT and trabeculectomy, respectively. The results indicated that combined cataract surgery and GATT (n = 12) had a 2 mmHg greater IOP-lowering effect compared to GATT alone in phakic (n = 13) and pseudophakic eyes (n = 36). These results were in contrast to those of the study performed by Rahmatnejad et al. and Grover et al., which demonstrated that there was no significant difference between isolated GATT and combined GATT and cataract surgery in terms of IOP reduction.13,20,30 In another study on 32 eyes, mostly diagnosed as POAG (84.3%), the mean IOP reduction following combined GATT and phacoemulsification was 18% and 21% in the 6th and 12th months, respectively. These values were lower compared to the aforementioned studies, which could be attributed to the lower baseline IOP or incomplete goniotomy (270° or less) in a quarter of patients.36 In a 2-year follow-up study of combined GATT and phacoemulsification, the amount of IOP reduction and success rate were greater than the values obtained among the patients who had GATT alone (28.5% vs. 23.6% for IOP reduction; 56.4% vs. 31.6% for success rate).37
Olgun et al. compared the efficacy of XEN gel (Allergan plc, Dublin, Ireland) implantation in 114 and GATT in 107 eyes with open-angle glaucoma. The most common diagnosis in both groups was POAG (142 eyes) followed by PEXG (63 eyes). The mean IOP-lowering effect was greater in the XEN gel group (57.9% vs. 37.1%) in the 24th month postoperation. Transient hyphema was the most common complication in both groups, and 31.5% of the eyes undergoing XEN gel implantation needed needling during the follow-up course. Despite the possible bleb-associated issues, the authors recommended XEN gel implantation as a better option in patients with lower target IOP.38
Faria et al. studied the efficacy of isolated GATT (51 eyes) and combined GATT and CE (49 eyes) in 89 patients with POAG (69 eyes) and SOAG (31 eyes). The mean IOP was decreased from 24.85 ± 9.00 mmHg on 3.61 ± 0.75 medication to 12.58 ± 1.24 mmHg on 0.96 ± 1.04 medications in the 24th postoperation month. The success rate was similar in both isolated GATT and combined GATT and CE groups, and the highest failure rate was observed in SOAG eyes with previous vitreoretinal surgery (12 eyes). More than 65% of these eyes needed additional IOP-lowering surgery which was possibly attributed to the presence of scleral buckle in half of them, compromising the efficacy of GATT.39
Recently, Wan et al. evaluated the efficacy of performing GATT (66 eyes) versus combined GATT and CE (58 eyes) in eyes with different severities of POAG, and they found that 24 months’ IOP-lowering effect was similar in both procedures. The mean IOP was reduced from 26.40 ± 6.37 mmHg on 3.12 ± 0.80 medications and 27.54 ± 8.09 mmHg on 3.35 ± 0.64 medications preoperatively to 16.08 ± 2.38 mmHg on 0.45 ± 0.96 medications and 15.50 ± 3.40 mmHg on 0.95 ± 1.50 medications at 24 months in combined surgery and isolated GATT surgery, respectively. Furthermore, it was noticed that the surgical success in patients with advanced POAG was worse than in patients with mild-to-moderate POAG at all follow-up visits in both combined and isolated GATT surgeries; however, it was not statistically significant. The complete success rate of isolated GATT and GATT+CE in patients with advanced POAG was 61.95% and 76.67% respectively, and it was 68.48% and 75.00% in cases with mild-to-moderate POAG.40
Grover et al. evaluated the efficacy of GATT in 79 eyes with SOAG including traumatic, steroid-induced, PDG, PEXG, and mixed mechanism glaucoma in 24 months. They showed that the means of IOP and medication reduction were 49.8% and 2.0, respectively.16 Rahmatnejad et al. studied a group of patients including 18 eyes with SOAG (steroid-induced, PDG, uveitic, and PEXG), among which 9 phakic eyes and 7 pseudophakic eyes had isolated GATT and two eyes underwent the combined surgery. The mean amount of IOP reduction was 16.6 ± 15.8 mmHg (54.6%) after 6 months and 18.6 ± 10.7 mmHg (61.2%) after 12 months. The amount of IOP reduction was similar in patients who underwent isolated GATT or combined procedure. However, the number of patients was limited.20 Grover et al. and Rahmatnejad et al. found a greater IOP-lowering effect for GATT in the eyes with SOAG compared to those with POAG (49.8% vs. 37.3% in 24 months and 61.2% vs. 38% in 12 months).16,20
A study on 104 eyes (62.5% – POAG, 24% – PEXG, and 13.5% – PDG/uveitic/steroid-induced glaucoma) revealed a greater IOP reduction in patients with POAG compared to SOAG eyes (40.1% vs. 27.6% at 18 months). However, they could achieve single-digit IOP in PEXG eyes. These contradictory results might be due to the heterogeneous composition as well as the lower number of SOAG eyes.35
GATT has been proved to be an effective option in the treatment of patients with steroid-induced glaucoma. In one study on 13 eyes with steroid-induced glaucoma, the mean IOP decreased from 25.5 ± 7.5 mmHg in the preoperation visit to 10.5 ± 1.0 mmHg in the 24th postoperative month, which accounted for a 63% decline in the IOP. Moreover, the number of antiglaucoma medications decreased from 3.1 to <1 during postoperation visits.19 There are also reports of successful IOP reduction following GATT in a pediatric case of steroid-induced glaucoma secondary to vernal keratoconjunctivitis as well as combined GATT and cataract surgery in a case of postpenetrating keratoplasty (PKP) steroid-induced glaucoma.41,42 Similarly, Sharkawi et al. evaluated patients with PEXG undergoing GATT (n = 53) or combined GATT and CE (n = 50) for 24 months. The results indicated no significant difference between the two groups regarding the reduction in the IOP or medication reduction. By the end of 24 months, the mean IOP reduced from 27.1 to 13 mmHg and the number of glaucoma medications decreased from 2.9 to 1.1. In addition, the overall success rate was 89.2%.43
GATT was successfully used in eight cases with SOAG following vitreoretinal surgery, five of which were silicone oil filled during the GATT and had no concurrent silicone oil removal. All the patients experienced at least 50% IOP reduction in the last follow-up (1–25 months) compared to baseline, which was a great achievement, especially in eyes with previous scleral buckle and scarred conjunctiva, that compromised the success of ab-externo procedures.44
Smith et al. performed GATT in 39 eyes with previous corneal transplant surgeries (59% – PKP, 35.9% – Descemet’s stripping endothelial automated keratoplasty [DSEAK], 2.6% – Descemet’s membrane endothelial keratoplasty [DMEK], and 2.6% – deep anterior lamellar keratoplasty). The preoperation IOP was 30.9 ± 11.5 mmHg on 4.2 ± 1.0 medications, which decreased to 13.9 ± 4.7 mmHg on 0.6 ± 1.0 medications after 24 months (56.2% IOP reduction). Seven eyes required reoperation for uncontrolled IOP at a median of 8.5 months, and four eyes underwent repeated corneal surgeries; one had undergone tube shunt surgery after GATT, one eye with previous DSEAK had preoperation corneal edema that was not resolved after IOP control and required repeated DSEAK, and the remaining two eyes were stable for over 3 years before developing corneal edema.45 Another study also disclosed the management of three eyes with previous PKP and one eye with previous DMEK by performing GATT. Based on the findings, two eyes with previous PKP became medication free and had controlled IOP for at least 4 years after a 360° GATT. However, the other two eyes with incomplete GATT (270° and 180°) needed further glaucoma surgeries. None of the patients showed graft rejection during the follow-up period. Regarding the conjunctival-sparing nature of GATT, it can be a suitable alternative in patients with previous PKP who may need rigid gas-permeable or scleral contact lenses.46 Furthermore, GATT has been reported to be safe and effective in the management of SOAG in a case of multiple endocrine neoplasia type 2B47 and in two patients with scleromalacia secondary to scleritis.48
Grover et al. used GATT as a safe and effective IOP-lowering procedure in four eyes with primary congenital glaucoma (PCG) and ten eyes with juvenile open-angle glaucoma (JOAG). They reported a mean IOP and medication reduction of 12.5 mmHg and 1.8, respectively, during a mean follow-up period of 20.4 months. Nevertheless, it was not possible to pass the microcatheter for 360° in one eye due to angle dysgenesis.49 Another research revealed the successful management of PCG with GATT, even in eyes with a history of prior goniotomy. The results demonstrated several challenges such as cloudy cornea, lack of TM pigmentation, and translucent uveal meshwork during GATT performance in neonates with PCG. In addition, unlike older children, there was no visible blood influx into the TM when IOP was reduced intraoperatively.50
In a prospective study on 70 eyes with JOAG, the mean IOP decreased from 31.3 ± 9.5 mmHg preoperatively to 15.8 ± 2.7 after 12 months (44.3 ± 18.7% IOP-lowering effect). Among the studied eyes, 52 (74%) experienced a greater IOP spike compared to the values reported in the previous studies.20 The longer duration of the IOP spike was associated with the greater IOP in the 1st month after the operation as well as in the last visit. Furthermore, the results revealed supraciliary effusion on the swept-source anterior segment optical coherence tomography (OCT). The higher grade of supraciliary effusion was associated with the lower IOP on the 1st day postoperation day, and the effusion was resolved by the time of IOP spike onset. The visual field MD was −17.4 ± 10.6 dB, and 66% of the eyes had severe glaucoma. Nonetheless, 50 eyes experienced >40% IOP reduction, which signified the efficacy of GATT in eyes with JOAG, even in severe diseases, which might be due to the underlying angle dysgenesis.51
Wang et al. studied the efficacy of isolated GATT in 48 eyes of 59 patients with JOAG during a mean follow-up period of 14.9 months. The mean age of the patients was 27.4 years. Among the eyes under investigation, 26 had prior glaucoma surgeries including trabeculectomy (n = 20), Ex-PRESS shunt (n = 3), trabectome (n = 2), and canaloplasty (n = 1). The mean IOP decreased from 26.5 ± 9.0 mmHg on 3.7 ± 0.9 medications preoperatively to 14.1 ± 2.3 mmHg on 0.4 ± 0.8 medications at 18 months postoperatively (average reduction in IOP = 47.9%). The IOP-lowering effect of GATT was comparable in patients with and without previous glaucoma surgeries. Besides, efficacy was similar in mild-to-moderate and severe cases, which was possibly due to the fact that the main location of resistance in eyes with JOAG was the proximal part of the TM that was addressed by ab-interno trabeculotomy and only a small portion of the TM and Schlemm’s canal was involved in eyes with previous filtering surgeries.52 Another study on 56 eyes including five eyes with ocular hypertension (OHTN), 34 eyes with POAG or JOAG, and 17 eyes with SOAG (PDG, uveitic, and angle recesion glaucoma) showed an average IOP reduction of 49% (from 27.70 ± 10.30 mmHg preoperatively to 14.04 ± 3.75 mmHg at 12 months), with 90% of the eyes achieving IOP <18 mmHg. The results indicated that lower age at the time of OHTN or glaucoma diagnosis was the single predictor of success rate. In that study, no significant difference was observed between the efficacy of isolated GATT (n = 50) and combined GATT and CE (n = 6).18
Shi et al. compared the efficacy of ab-interno and ab-externo microcatheter-assisted trabeculotomy in 115 eyes with clear cornea PCG during a 1-year follow-up. The two groups were similar in terms of IOP reduction and success rate. Complete 360° trabeculotomy could be performed for 40 eyes in the ab-interno trabeculotomy group (69%) and 37 eyes in the ab-externo trabeculotomy group (65%). The rate was even lower in eyes with prior glaucoma surgeries compared to those without previous glaucoma surgeries (40% vs. 84%), which might be due to the angle dysgenesis and surgical destruction of Schlemm’s canal. It should be noted that previous glaucoma surgeries were correlated to worse surgical outcomes and eyes with partial trabeculotomy had less IOP reduction compared to those with 360° trabeculotomy (44.3% vs. 56.3%).53
Qiao et al. compared the efficacy of GATT (36 eyes) and KDB goniotomy (13 eyes) in patients with JOAG. The average treated angle was 340 ± 36.1° in the GATT group and 111.9 ± 16.2° in the KDB group. Besides, the mean number of preoperation antiglaucoma medications was lower in the KDB group (3.08 ± 0.86 vs. 3.71 ± 0.46). Moreover, the mean preoperation IOP was 31.5 ± 14.5 mmHg on 3.7 ± 0.5 medications in the GATT group, which decreased to 15.9 ± 4.9 mmHg on 1.1 ± 1.4 antiglaucoma medications by the end of the 6th month postoperation (37% IOP reduction). In the KDB group, the mean IOP reduction was 14% at 3 months (26.1 ± 12.5 mmHg at baseline to 20 ± 10.8 mmHg) and the mean number of antiglaucoma medications was reduced from 3.1 ± 0.9 preoperatively to 2.3 ± 1.3 after 3 months. In conclusion, the success rate and median survival time were greater after GATT compared to KDB goniotomy, and there was a greater need for additional glaucoma surgeries after KDB goniotomy.54
Grover et al. studied the efficacy of GATT in 35 eyes with various types of open-angle glaucoma and at least one prior glaucoma surgery (trabeculectomy in 19 eyes, glaucoma drainage device (GDD) implantation in 13 eyes, trabectome in 4 eyes, and endoscopic cyclophotocoagulation in 5 eyes). On occasions that the tube or sclerostomy intruded the Schlemm’s canal, ab-interno cut-down was performed and it was tried from the opposite side to complete the 360° trabeculotomy. All the eyes were treated by at least 270°. The mean preoperation IOP was 25.7 mmHg on 3.2 antiglaucoma medications, which was decreased to 15.4 mmHg on 2 medications at 24 months. In subgroup analysis, the cumulative proportion of failure over time was slightly worse in the trabeculectomy group compared to the GDD group, but the difference was not statistically significant. In addition, seven eyes with previous trabeculectomy and two eyes with previous GDD implantation required further glaucoma surgeries. Despite the lower efficacy of GATT in eyes with previous glaucoma surgeries compared to virgin eyes, it can restore the aqueous outflow through the conventional pathway collapsed due to hypoperfusion, thus preventing a second trabeculectomy or GDD implantation in these eyes.55 Cubuk et al. evaluated the effectiveness of GATT after failed trabeculectomy in 12 eyes with POAG and 14 eyes with PEXG. The mean interval between the previous trabeculectomy and the GATT surgery was 42.5 ± 4.7 months. Except for five eyes with 270° trabeculotomy, complete circumferential trabeculotomy could be done in all the patients. The results revealed the greater IOP-lowering effect of GATT in the eyes with PEXG (46.8%) compared to the POAG eyes (32.1%) at 12 months. The success rate was also greater in the PEXG group (64.3% vs. 25%). Thus, the authors stated that GATT could be an appropriate second IOP-lowering procedure in eyes with PEXG with similar efficacy to virgin eyes due to the deposition of pseudoexfoliative materials and pigments in the proximal TM, which could be addressed by trabeculotomy, while distal outflow was involved in POAG eyes.56,57
Wang et al. evaluated the efficacy of GATT in 35 eyes with one previous incisional glaucoma surgery (28 – trabeculectomy, 6 – Ex-PRESS shunt implantation, and 1 – canaloplasty), and 9 eyes with two previous incisional glaucoma surgeries (6 with repeat trabeculectomy, 1 with trabeculectomy and Express mini-shunt implantation, 1 with trabeculectomy and Ahmed valve implantation, and 1 with trabeculectomy and endoscopic cyclophotocoagulation). The study included 21 patients with JOAG and 14 patients with POAG, and the mean IOP was decreased from 27.4 ± 8.8 mmHg on 3.6 ± 0.7 medications preoperatively to 15.3 ± 2.7 mmHg on 0.5 ± 0.9 medications on the 24th months after surgery.58
Up to now, a few absolute contraindications have been reported for GATT including unstable intraocular lenses (IOLs), bleeding diathesis, inability to stop anticoagulant medications, and any condition that precludes the potential to identify angle structures such as endothelial decompensation and extensive peripheral anterior synechia. Routine GATT is also difficult to perform in patients with corneal opacity and those with severe limitation of cervical spine mobility. However, this can be surpassed by using an ophthalmic endoscopic technique.59 Furthermore, it has been recommended to reconsider GATT performance in patients who cannot tolerate head elevation during the first 2 weeks of surgery until the resolution of the possible postoperative hyphema.16
Hyphema is the most common postoperative complication of GATT (occurrence rate = 36%–91.3%),56,60 which occurs because of blood reflux through the collector channels due to hypotonia. However, it is usually resolved by the end of the 1st postoperative month.20 By leaving a modest amount of viscoelastic agents at the end of the procedure, the incidence of postoperation hypotony and the resultant hyphema can be lessened at the expense of a possible early postoperative IOP spike. In cases with layered macroscopic hyphema persisting for 10 days or causing IOP spikes, anterior chamber irrigation is suggested.16,22 In a prior research, the incidence of postoperation hyphema was significantly decreased in patients who had hemi-GATT (180° treated) while preserving the IOP-lowering efficacy compared to the 360° treated TM. Hence, performing hemi-GATT might be considered in patients with active lifestyles.18,61 In another study, hyphema and IOP spike were reported following pharmacologically induced mydriasis in the late follow-up of a case who had undergone uncomplicated cataract surgery and GATT.62 The blood clot may be entrapped between the IOL and the posterior capsule, and it can take up to 4 months to resolve.56 It may also need interventions such as laser capsulotomy. In two patients with PEXG, capsular hematoma, anterior capsular phimosis, and IOL subluxation were detected after combined GATT and phacoemulsification, which were treated by IOL and bag removal and secondary IOL implantation [Figure 4].63
Figure 4.

Intracapsular hematoma, a rare complication of gonioscopy-assisted transluminal trabeculotomy surgery
Postoperation IOP spike is relatively common, occurring in 24.7%–74% of eyes. It usually occurs during the 1st postoperation week most likely due to retained viscoelastic agents and red blood cell clogging the outflow pathway. It is usually treated by antiglaucoma medications and aqueous release from the paracentesis site. Yet, it may need anterior chamber irrigation in cases with massive hyphema. IOP spikes can also rarely happen as a steroid response during the 1st month postoperation.18,51,52,56 It has been shown that an IOP spike increases the surgical failure rate. Although the underlying mechanism is not fully understood, it indicates a poor reserve in the posttrabecular outflow system and lower capability of patients’ drainage systems in occasions with increased flow resistance such as bleeding.20,51,52,64 Higher preoperation IOP can be associated with a greater incidence of IOP spike, while older age and suture misdirection into the anterior chamber or supraciliary space may have a protective role.54
Sato et al. showed a mean endothelial cell loss of 4.9 ± 7.6% during a 2-year follow-up of patients undergoing GATT or combined GATT and CE.37 However, another study evaluating the short-term endothelial cell loss in these patients found GATT as a safe procedure for endothelium during a 1-month follow-up.65
Supraciliary effusion may happen after uneventful GATT and may result in a transient myopic shift and a shallow anterior chamber.66 In an anterior segment OCT study on patients with JOAG, supraciliary effusion was detected in 57% of the cases on the 1st day postoperation, which was resolved in all the cases by the end of the 1st month.51 Akagi et al. reported a prevalence of 42% for ciliochoroidal detachment on the 3rd day after ab-interno trabeculotomy with trabectome in patients with POAG. They attributed the ciliochoroidal detachment mainly to the increased uveoscleral outflow through the site of trabeculotomy, which was the reason for the unexpected low postoperation IOP.67 Other reported complications included partial stripping of Schlemm’s canal, TM, and Descemet membrane,68 microcystic corneal edema, transient worsening in visual acuity, cyclodialysis cleft, hypothony,18 Descemet detachment, hemorrhagic Descemet detachment, iridodialysis,16 and closure of the trabecular shelf with peripheral anterior synechiae or reattachment of trabecular cleft in areas with previous blood clots.51
Aktas et al. reported an unusual case of panscleritis and exudative retinal detachment following GATT in a case of chronic anterior uveitis and SOAG. The patient was treated by prescribing systemic and topical steroid and oral cyclosporine. By the end of the 1st postoperation month, scleritis was resolved, but shallow exudative retinal detachment persisted. Hence, it was advised to prescribe perioperative systemic steroids and control the inflammation for at least 3 months prior to performing GATT in uveitic patients.69
Suture misdirection is an important potential complication of suture trabeculotomy procedures whose incidence can be decreased by using illuminated microcatheters. Subretinal misdirection can result in serous retinal detachment and focal photoreceptor outer segment defect.25,70 Cystoid macular edema, choroidal effusion, hypotony maculopathy, and vitreous hemorrhage are among the other posterior segment complications of GATT.16,18
Several factors have been reported to be associated with an increased failure rate. Rahmatnejad et al. found that IOP spike (IOP >30 mmHg) and African-American ancestry elevated the incidence of failure, with the role of race being due to increased inflammatory response in blacks. Besides, the visual field MD was lower in the surgical failure group compared to the surgical success group, but the difference was not statistically significant.20 Pseudophakic patients with POAG and visual field MD <−15 also showed an increased failure rate.49
In the research carried out by Wang et al. on eyes with JOAG, Humphrey visual field defect severity, prior glaucoma surgery, age, and preoperative IOP and medication had no effects on the surgical failure rate and the only effective factor was postoperative IOP spike.52 Another study on patients with JOAG showed that IOP spike and history of glaucoma surgery were the major risk factors for failure. Moreover, complete trabeculotomy was associated with a better surgical outcome.51 Sato et al. disclosed that the larger number of antiglaucoma medications (≥2) and performing isolated GATT compared to the combined GATT and CE were associated with a higher failure rate.37 A recent study emphasized that postoperative management with topical nonsteroidal anti-inflammatory drugs lowered the incidence of postoperative IOP spike and failure. The results also indicated that eyes with incomplete trabeculotomy had a greater failure rate compared to those with 360° trabeculotomy.64 In contrast, some other studies revealed no significant correlation between the extent and location of trabeculotomy and the IOP-lowering effect of GATT.18,61,71
Salimi et al. found that younger age at the time of diagnosis was the single most determining factor in surgical success and other factors such as prior selective laser trabeculoplasty (SLT), extent of trabeculotomy, prior antiglaucoma medications, glaucoma type and severity, and combination with cataract surgery had no effects on the success rate. This was possibly due to the localization of obstruction in a more proximal part of trabecular outflow in younger patients and the shorter duration of antiglaucoma medication use, which could result in ocular surface problems and distal outflow atrophy.18
Another study on patients with JOAG demonstrated that the duration of postoperative IOP spike and age at the time of surgery had the greatest correlations with surgical success. In addition, the only risk factor for prolonged IOP spikes was the severity of the disease based on Humphrey visual field MD. In that study, the mean open trabecular shelf on the anterior segment OCT was 298.9 ± 76.6°, and there was no significant correlation between the extent of open TM and surgical success. They reported that trabecular shelf closure was usually associated with blood clot contraction and postulated that extensive early postoperative hyphema might have a devastating effect on the final results.51 Another study showed that lower preoperative deep vessel density assessed using anterior segment OCT angiography, especially in the fornix area, was associated with a greater success rate and IOP-lowering effect in eyes undergoing TM-targeted MIGS including suture trabeculotomy. This finding was attributed to a healthy trabecular outflow pathway and normal episcleral venous pressure.72
One of the most important points of suture GATT is the cost-effectiveness, which has turned this operation to the choice of MIGS in developing countries.73 This characteristic is similar to a recently evolved MIGS procedure named bent needle ab-interno goniectomy (BANG) which uses a single 25-gauge needle, bent toward the bevel, to perform ab-interno goniectomy. In a study on 39 eyes with mild-to-severe open-angle glaucoma, BANG procedure could reduce the IOP from 17.4 ± 4.1 mmHg on 1.1 ± 1.4 topical glaucoma medications to 13.3 ± 2.5 mmHg on 0.5 ± 0.8 topical glaucoma medications at the 6th postoperation month and 73% of patients had 20% or greater IOP reduction.74 Furthermore, there is a case report on evolving new aqueous outflow channels in temporal quadrant in aqueous angiography following BANG in temporal angle.75
Voykov et al. introduced microinvasive suture trabeculotomy (MIST), which is a conjunctival sparing ab-interno approach to perform 360° trabeculotomy by grasping and retrieving the 10.0 prolene suture in patients undergoing previous canaloplasty. Similar to other trabeculotomy procedures, it acts by cleavage of the inner wall of the Schlemm’s canal and has a comparable IOP-lowering effect, as observed in the above-mentioned study.76 In long-term follow-up, the procedure showed an IOP-lowering effect of 37.5% in the 3rd month and 41.2% in the 12th month postoperation. The most common complication was postoperative hyphema due to blood influx.77 Baumgarten et al. stated that the eyes that needed early (<3 months) 360° trabeculotomy after canaloplasty had a greater preoperation and lower postoperation (2nd month) IOP compared to the eyes undergoing 360° trabeculotomy later than 3 months after canaloplasty. Overall, they introduced MIST as the first-line treatment in eyes with failed canaloplasty.78
Trab 360 (Sight Sciences, Menlo Park, CA, USA) was introduced in 2015 for the first time. This MIGS device is composed of a beveled access cannula made of stainless steel and a blunted edged, soft, and flexible injection tube from 4 to 0 blue nylon filament (200 um) with a 290-um bulbous tip, which is advanced to or retracted from the Schlemm’s canal using a control wheel on the handle of the device. The cannula pierces the TM and the tube is introduced into the Schlemm’s canal. It can be used through a clear corneal incision to perform either 180° or 360° ab-interno trabeculotomy.28,79
Areaux et al. used Trab 360 in 46 eyes of children with different classes of childhood glaucoma (21 eyes with PCG, 6 with JOAG, 8 with glaucoma associated with a nonacquired systemic disease, 2 with ectropion uvea, 4 with uveitis, and 5 with glaucoma following cataract surgery). Six eyes had previous glaucoma surgeries: micropulse cyclophotocoagulation in 1, goniotomy in 2, and GDD implantation in 3. The mean amount of goniotomy was 290°. Surgical success was defined as the postoperation IOP of ≤24 mmHg with or without medications and no additional surgeries. The overall success rate was 67.4%, and it was greater in patients with PCG as well as in the eyes that had Trab 360 as the first glaucoma surgery. The mean IOP reduction in successful eyes was 13.65 mmHg during 15.3 months, and the only reported complication was cyclodialysis cleft in two eyes.80
Sarkisian et al. evaluated the efficacy of 360° ab-interno trabeculotomy with Trab 360 in 81 eyes with different types of refractory open-angle glaucoma with the predominance of POAG (n = 67). They revealed a mean IOP and medication reduction of 7.3 ± 6.7 mmHg and 0.6, respectively, at 12 months. In that study, 67% of the patients had previous glaucoma procedures, with SLT accounting for 51.9% of the procedures. During 1 year, 18 eyes needed IOP-lowering surgery, 2 eyes underwent cataract surgery (primary reason) with CyPass, and 1 eye underwent goniosynechialysis, which were considered failure.81
Hirabayashi et al. compared the efficacy of CE in combination with KDB goniotomy (74 eyes) to 360° trabeculotomy (19 eyes with Trab 360 and 8 eyes with GATT) for 6 months. The majority of the cases had POAG in both groups. The means of IOP-lowering effect (2.9 ± 5.4 in KDB eyes and 2.9 ± 6.8 in Trab 360/GATT eyes) and antiglaucoma medication reduction (1.1 ± 1.3 in KDB eyes and 1.2 ± 1.1 in Trab 360/GATT eyes) were similar in the two groups despite the lesser extent of the TM procedure in the KDB group (approximately 120° vs. 360°). This might be due to the lower baseline IOP and smaller number of eyes undergoing 360° trabeculotomy in that study.82
Trab 360 has been now replaced by OMNI™ (Sight Sciences, Menlo Park, CA, USA), which has the potential to inject the viscoelastic agent through a hollow cannula upon retraction.
OMNI received the Food and Drug Administration’s approval in 2017. OMNI provides the ground for performing ab-interno canaloplasty followed by trabeculotomy through a single corneal incision, which addresses both proximal and distal resistances of the conventional aqueous outflow pathway.83 GEMINI study evaluated the efficacy of cataract surgery, 360° canaloplasty, and 180° trabeculotomy by OMNI in 137 patients with open-angle glaucoma (93% – POAG, 6% – PEXG, and 1% – PDG). By the end of the 6th month postoperation, the mean IOP reduction was 9 mmHg (38%) and the number of antiglaucoma medications was decreased from 1.8 ± 0.9 to 0.6 ± 1. In addition, 104 out of the 134 eyes became medication free by the end of the 6th month, 86% of which had IOP of 6–18 mmHg.84 In ROMEO study, sequential canaloplasty and trabeculotomy were performed on 48 pseudophakic eyes with mild-to-moderate open-angle glaucoma (46 – POAG, 1– PEXG, and 1 – PDG) and patients were categorized based on their preoperation IOP (>18 or ≤18 mmHg). Based on the results, 21 eyes had canaloplasty in both hemispheres and the rest had canaloplasty in one hemisphere. Trabeculotomy was also performed for 180° in 45 eyes and 360° in three eyes. During the 1-year follow-up, IOP was reduced in patients with baseline IOP >18 mmHg (21.8 to 15.6 mmHg) and remained controlled in eyes with baseline IOP ≤18 mmHg. In addition, the medication burden decreased from 1.7 ± 1.3 to 1.2 ± 1.3 and from 2.0 ± 1.3 to 1.3 ± 1.3, respectively. Five eyes required a second IOP-lowering intervention (2 – SLT, 1 – GDD, 1 – trabeculectomy, and 1 – Express mini-shunt) during days 136–343 postoperation. In this group, the mean IOP was 26.4 ± 3.8 mmHg on 2.4 ± 1.5 medications.85 A similar study was conducted on 81 patients with mild-to-moderate open-angle glaucoma (76 – POAG and 5 – PEXG) undergoing combined cataract surgery, canaloplasty, and trabeculotomy by OMNI. In that study, 24 eyes had baseline IOP >18 mmHg (Group 1) and 57 had baseline IOP ≤18 mmHg (Group 2). During a 12-month follow-up, Group 1 had a 30.8 ± 14.3% IOP reduction and the mean number of medications decreased from 2.0 ± 1.3 preoperatively to 1.1 ± 1.1. Furthermore, 87.5% of the eyes had IOP ≥6 mmHg and ≤18 mmHg. Group 2 showed no significant IOP changes at 12 months, but the number of medications decreased significantly from 1.6 at baseline to 0.9 and 49.1% of the patients were medication free. The results revealed no significant correlation between the extent of canaloplasty and postoperative IOP.86 After a 24-month follow-up of 38 eyes of 27 patients with uncontrolled mild-to-moderate open-angle glaucoma (27 – POAG and 11 – PEXG) who underwent canaloplasty and trabeculotomy by OMNI, the means of IOP and medication reduction were 10 mmHg (39.6%) and 1.4, respectively. Moreover, 88.5% (23/26) of the eyes had IOP <18 mmHg, with 14 (61%) being without medications. Overall, a similar efficacy was observed for the eyes with PEXG and POAG.87
Grabska-Liberek et al. performed canaloplasty and 360° trabeculotomy with OMNI surgical system on 17 eyes of 15 patients. Most eyes had POAG (88.2%), nine had standalone surgery, and the remaining had the operation in combination with cataract surgery. The means of IOP and medication reduction were 7.7 mmHg (36%) and 1.95, respectively, at 12 months. The mean baseline IOP was higher in the standalone group compared to the combined group (22.11 vs. 18.5 mmHg). Similar results were also obtained with regard to IOP reduction (7.9–8.9 vs. 4.6–6.3 mmHg).88
There is a report of the successful management of an infant with congenital glaucoma associated with Sturge–Weber using the OMNI surgical system.89 Pyfer et al. also showed that the OMNI surgical system could reduce diurnal IOP fluctuations. In that study, 128 eyes enrolled in GEMINI study that were mostly diagnosed with POAG were treated via the OMNI surgical system and the mean IOP decreased from 23.7 ± 3.1 to 15.6 ± 4.2 mmHg at the 12th month. Moreover, the peak IOP decreased by over 8 mmHg and 95% of the patients had diminished peak IOP after the operation. Furthermore, the mean IOP fluctuations decreased from 2.8 to 1.8 mmHg at 12 months postoperation.90
Overall, there are few publications on MIST, Trab 360, and OMNI device system. Nonetheless, as they act similarly to GATT, they have similar safety profiles and possible complications.
DISCUSSION
Pressure-dependent or conventional (trabecular) outflow pathway is responsible for 80%–90% of aqueous outflow, through which aqueous passes the TM, enters the Schlemm’s canal, and is drained into the collector channels, aqueous veins, and episcleral vessels.91,92 It has been reported that the juxtacanalicular tissue and the inner wall of the Schlemm’s canal contribute to the main resistance in the proximal part93 and 25%–50% of the resistance in the conventional pathway resides in the distal part.92 By performing ab-interno trabeculotomy, the barrier effect of TM and the inner wall of the Schlemm’s canal can be eliminated. However, resistance in the distal part that can be responsible for as high as 50% of resistance in the conventional pathway has not been addressed.94 Increased distal resistance can result from many factors such as aging, cauterization of sclera during surgery and collapse, and stasis of the Schlemm’s canal and collector channels or occlusion of collector channels ostium by TM herniation in an elevated IOP setting.92 In a healthy distal pathway, 360° ab-interno trabeculotomy is expected to bring IOP to as low as episcleral venous pressure. In a diseased distal pathway, however, this target IOP is not achieved. Thus, TM-targeted MIGS have a golden time and the health of the distal part should be determined before proceeding with the surgery.5
Up to now, several preoperation methods have been recommended to estimate the patency and efficacy of collector channels such as response to SLT or pilocarpine, blood reflux into the Schlemm’s canal during provocative gonioscopy, and observation of pulsatile flow within aqueous veins.5 However, no study has been done on the relationship between the IOP response to pilocarpine and MIGS outcome, and the impact of successful SLT on trabecular MIGS results has not been proven.95 There are also some intraoperative techniques such as observing the EVFW during irrigation and using trypan blue, fluorescein, or indocyanine green canalography.92
In the absence of a healthy distal outflow pathway or elevated episcleral venous pressure, other glaucoma surgeries targeting suprachoroidal or diverting the aqueous into the subconjunctival space are recommended. By inventing new devices such as OMNI, surgeons can benefit from the potential to perform ab-interno canaloplasty alongside ab-interno trabeculotomy and address both proximal and distal trabecular outflow pathways.85 Nevertheless, no study has compared OMNI and GATT in terms of the IOP-lowering effect. Ab-interno trabeculotomy procedures are relatively safe, blebless, and efficient glaucoma surgeries that can be performed in a low-cost setting. These procedures can be performed either isolated or in combination with CE, without compromising the efficacy.
Considering the absence of bleb-related complications such as blebitis, leakage, hypotony, and bleb-related endophthalmitis, this procedure is recommended, especially in cases with mild-to-moderate open glaucoma with or without cataract surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Ms. A. Keivanshekouh at the Research Consultation Center of Shiraz University of Medical Sciences for her invaluable assistance in editing the manuscript.
REFERENCES
- 1.World Health Organization. Global Data on Visual Impairments 2010. Geneva: World Health Organization; 2012. pp. 1–5. [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Sommer A. Glaucoma risk factors observed in the Baltimore Eye Survey. Curr Opin Ophthalmol. 1996;7:93–8. doi: 10.1097/00055735-199604000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Coleman AL, Kodjebacheva G. Risk factors for glaucoma needing more attention. Open Ophthalmol J. 2009;3:38–42. doi: 10.2174/1874364100903010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrew NH, Akkach S, Casson RJ. A review of aqueous outflow resistance and its relevance to microinvasive glaucoma surgery. Surv Ophthalmol. 2020;65:18–31. doi: 10.1016/j.survophthal.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: A review. Open Ophthalmol J. 2010;4:52–9. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1958;60:523–33. doi: 10.1001/archopht.1958.00940080541001. [DOI] [PubMed] [Google Scholar]
- 8.Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm –An attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol (Copenh) 1972;50:295–320. doi: 10.1111/j.1755-3768.1972.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 9.McPherson SD, Jr, McFarland D. External trabeculotomy for developmental glaucoma. Ophthalmology. 1980;87:302–5. doi: 10.1016/s0161-6420(80)35233-0. [DOI] [PubMed] [Google Scholar]
- 10.Smith R. A new technique for opening the canal of Schlemm. Preliminary report. Br J Ophthalmol. 1960;44:370–3. doi: 10.1136/bjo.44.6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harms H, Dannheim R. Epicritical consideration of 300 cases of trabeculotomy 'ab externo'. Trans Ophthalmol Soc U K (1962) 1970;89:491–9. [PubMed] [Google Scholar]
- 12.Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989;8:1233–40. doi: 10.3109/02713688909013902. [DOI] [PubMed] [Google Scholar]
- 13.Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology. 2014;121:855–61. doi: 10.1016/j.ophtha.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Baykara M, Poroy C, Erseven C. Surgical outcomes of combined gonioscopy-assisted transluminal trabeculotomy and cataract surgery. Indian J Ophthalmol. 2019;67:505–8. doi: 10.4103/ijo.IJO_1007_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Habash A, Alrushoud M, Al Abdulsalam O, Al Somali AI, Aljindan M, Al Ahmadi AS. Combined gonioscopy-assisted transluminal trabeculotomy (GATT) with Ab Interno Canaloplasty (ABiC) in conjunction with phacoemulsification: 12-month outcomes. Clin Ophthalmol. 2020;14:2491–6. doi: 10.2147/OPTH.S267303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grover DS, Smith O, Fellman RL, Godfrey DG, Gupta A, Montes de Oca I, et al. Gonioscopy-assisted transluminal trabeculotomy: An Ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma. 2018;27:393–401. doi: 10.1097/IJG.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 17.Smith BL, Ellyson AC, Kim WI. Trabectome-initiated gonioscopy-assisted transluminal trabeculotomy. Mil Med. 2018;183:146–9. doi: 10.1093/milmed/usx174. [DOI] [PubMed] [Google Scholar]
- 18.Salimi A, Nithianandan H, Al Farsi H, Harasymowycz P, Saheb H. Gonioscopy-assisted transluminal trabeculotomy in younger to middle-aged adults: One-year outcomes. Ophthalmol Glaucoma. 2021;4:162–72. doi: 10.1016/j.ogla.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Boese EA, Shah M. Gonioscopy-assisted transluminal trabeculotomy (GATT) is an effective procedure for steroid-induced glaucoma. J Glaucoma. 2019;28:803–7. doi: 10.1097/IJG.0000000000001317. [DOI] [PubMed] [Google Scholar]
- 20.Rahmatnejad K, Pruzan NL, Amanullah S, Shaukat BA, Resende AF, Waisbourd M, et al. Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma. 2017;26:1137–43. doi: 10.1097/IJG.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 21.Widder JR, Schmitz JW. Combining Ab interno kahook trabeculectomy with gonioscopy-assisted transluminal trabeculotomy reduces intraocular pressure. Mil Med. 2019;184:934–6. doi: 10.1093/milmed/usz067. [DOI] [PubMed] [Google Scholar]
- 22.Cubuk MO, Unsal E. One-year results of gonioscopy-assisted transluminal trabeculotomy: Evaluation of prognostic factors. Eur J Ophthalmol. 2021;31:460–8. doi: 10.1177/1120672120908716. [DOI] [PubMed] [Google Scholar]
- 23.Chin S, Nitta T, Shinmei Y, Aoyagi M, Nitta A, Ohno S, et al. Reduction of intraocular pressure using a modified 360-degree suture trabeculotomy technique in primary and secondary open-angle glaucoma: A pilot study. J Glaucoma. 2012;21:401–7. doi: 10.1097/IJG.0b013e318218240c. [DOI] [PubMed] [Google Scholar]
- 24.Grover DS, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy (GATT): Thermal suture modification with a dye-stained rounded tip. J Glaucoma. 2016;25:501–4. doi: 10.1097/IJG.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 25.Verner-Cole EA, Ortiz S, Bell NP, Feldman RM. Subretinal suture misdirection during 360 degrees suture trabeculotomy. Am J Ophthalmol. 2006;141:391–2. doi: 10.1016/j.ajo.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Francis BA, Akil H, Bert BB. Glaucoma Surgery. Switzerland: Karger, Basel; 2017. Ab interno Schlemm's canal surgery; pp. 127–46. [DOI] [PubMed] [Google Scholar]
- 27.Byszewska A, Konopińska J, Kicińska AK, Mariak Z, Rękas M. Canaloplasty in the treatment of primary open-angle glaucoma: Patient selection and perspectives. Clin Ophthalmol. 2019;13:2617–29. doi: 10.2147/OPTH.S155057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elhusseiny AM, El Sayed YM, El Sheikh RH, Gawdat GI, Elhilali HM. Circumferential Schlemm's canal surgery in adult and pediatric glaucoma. Curr Eye Res. 2019;44:1281–90. doi: 10.1080/02713683.2019.1659975. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y, Yu Q, Ji F, Sun H, Yuan Z. Viscocanalostomy combined with nearly 360-degree suture trabeculotomy for the treatment of primary congenital glaucoma: A preliminary report of a novel technique for trabeculotomy. Graefes Arch Clin Exp Ophthalmol. 2020;258:379–86. doi: 10.1007/s00417-019-04537-2. [DOI] [PubMed] [Google Scholar]
- 30.Fontana L, De Maria M, Caristia A, Mastrofilippo V, Braglia L, Iannetta D, et al. Comparison of gonioscopy-assisted transluminal trabeculotomy versus trabeculectomy with mitomycin c in patients with open-angle glaucoma. J Glaucoma. 2021;30:101–8. doi: 10.1097/IJG.0000000000001696. [DOI] [PubMed] [Google Scholar]
- 31.Fellman RL, Feuer WJ, Grover DS. Episcleral venous fluid wave correlates with trabectome outcomes: Intraoperative evaluation of the trabecular outflow pathway. Ophthalmology. 2015;122:2385–91.e1. doi: 10.1016/j.ophtha.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Fellman RL, Grover DS. Episcleral venous fluid wave: Intraoperative evidence for patency of the conventional outflow system. J Glaucoma. 2014;23:347–50. doi: 10.1097/IJG.0b013e31827a06d8. [DOI] [PubMed] [Google Scholar]
- 33.Aktas Z, Ozmen MC, Atalay HT, Ucgul AY. Evaluation of episcleral venous fluid wave during gonioscopy assisted transluminal trabeculotomy in patients with advanced glaucoma. Eye (Lond) 2019;33:668–73. doi: 10.1038/s41433-018-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukla AG, Ramulu PY. Management of anticoagulation and antiplatelet therapy in glaucoma surgery. J Glaucoma. 2020;29:732–41. doi: 10.1097/IJG.0000000000001594. [DOI] [PubMed] [Google Scholar]
- 35.Aktas Z, Ucgul AY, Bektas C, Sahin Karamert S. Surgical outcomes of prolene gonioscopy-assisted transluminal trabeculotomy in patients with moderate to advanced open-angle glaucoma. J Glaucoma. 2019;28:884–8. doi: 10.1097/IJG.0000000000001331. [DOI] [PubMed] [Google Scholar]
- 36.Loayza-Gamboa W, Martel-Ramirez V, Inga-Condezo V, Valderrama-Albino V, Alvarado-Villacorta R, Valera-Cornejo D. Outcomes of combined prolene gonioscopy assisted transluminal trabeculotomy with phacoemulsification in open-angle glaucoma. Clin Ophthalmol. 2020;14:3009–16. doi: 10.2147/OPTH.S272298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T, Kawaji T, Hirata A, Mizoguchi T. 360-degree suture trabeculotomy ab interno to treat open-angle glaucoma: 2-year outcomes. Clin Ophthalmol. 2018;12:915–23. doi: 10.2147/OPTH.S161238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olgun A, Aktas Z, Ucgul AY. XEN gel implant versus gonioscopy-assisted transluminal trabeculotomy for the treatment of open-angle glaucoma. Int Ophthalmol. 2020;40:1085–93. doi: 10.1007/s10792-019-01271-w. [DOI] [PubMed] [Google Scholar]
- 39.Faria BM, Daga FB, Rebouças-Santos V, Araújo RB, Matos Neto C, Jacobina JS, et al. Gonioscopy-assisted transluminal trabeculotomy (GATT) outcomes in eyes with open-angle glaucoma resistant to maximum treatment. Arq Bras Oftalmol. 2021;84:587–93. doi: 10.5935/0004-2749.20210083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan Y, Cao K, Wang J, Sun Y, Du R, Wang Z, et al. Gonioscopy-assisted transluminal trabeculotomy (GATT) combined phacoemulsification surgery: Outcomes at a 2-year follow-up. Eye (Lond) 2023;37:1258–63. doi: 10.1038/s41433-022-02087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopen ML, Gallardo MJ, Grover D. Gonioscopy-assisted transluminal trabeculotomy in a pediatric patient with steroid-induced glaucoma. J Glaucoma. 2019;28:e156–8. doi: 10.1097/IJG.0000000000001326. [DOI] [PubMed] [Google Scholar]
- 42.Nazarali S, Cote SL, Gooi P. Gonioscopy-assisted transluminal trabeculotomy (GATT) in postpenetrating keratoplasty steroid-induced glaucoma: A case report. J Glaucoma. 2018;27:e162–4. doi: 10.1097/IJG.0000000000001034. [DOI] [PubMed] [Google Scholar]
- 43.Sharkawi E, Lindegger DJ, Artes PH, Lehmann-Clarke L, El Wardani M, Misteli M, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. Br J Ophthalmol. 2021;105:977–82. doi: 10.1136/bjophthalmol-2020-315954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan AV, Yannuzzi NA, Chen J, Wang YE, Townsend JH, Chang TC. Gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with secondary open-angle glaucoma following vitreoretinal surgery. J Glaucoma. 2020;29:e23–5. doi: 10.1097/IJG.0000000000001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith OU, Butler MR, Grover DS, Kornmann HL, Emanuel ME, Godfrey DG, et al. Twenty-four-month outcome of gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior corneal transplant surgery. J Glaucoma. 2022;31:54–9. doi: 10.1097/IJG.0000000000001949. [DOI] [PubMed] [Google Scholar]
- 46.Loewen D, Al-Ani A, Gooi P. Gonioscopy-assisted transluminal trabeculotomy in post-corneal transplant glaucoma: A case series. Clin Res Ophthalmol. 2021;4:1–6. [Google Scholar]
- 47.Chang TC, Okafor KC, Cavuoto KM, Dubovy SR, Karp CL. Pediatric multiple endocrine neoplasia type 2B: Clinicopathological correlation of perilimbal mucosal neuromas and treatment of secondary open-angle glaucoma. Ocul Oncol Pathol. 2018;4:196–8. doi: 10.1159/000484053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manabe S, Sawaguchi S, Hayashi K. Suture trabeculotomy Ab interno for secondary glaucoma combined with scleromalacia. J Glaucoma. 2016;25:e718–20. doi: 10.1097/IJG.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 49.Grover DS, Smith O, Fellman RL, Godfrey DG, Butler MR, Montes de Oca I, et al. Gonioscopy assisted transluminal trabeculotomy: An ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol. 2015;99:1092–6. doi: 10.1136/bjophthalmol-2014-306269. [DOI] [PubMed] [Google Scholar]
- 50.Lehmann-Clarke L, Sadeghi Y, Guarnieri A, Sharkawi E. Gonioscopy-assisted transluminal trabeculotomy using an illuminated catheter for infantile primary congenital glaucoma. Case series. Am J Ophthalmol Case Rep. 2020;19:100733. doi: 10.1016/j.ajoc.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Wang H, Oatts JT, Xin C, Yin P, Zhang L, et al. Aprospective study of intraocular pressure spike and failure after gonioscopy-assisted transluminal trabeculotomy in juvenile open-angle glaucoma: A prospective study of GATT in JOAG. Am J Ophthalmol. 2022;236:79–88. doi: 10.1016/j.ajo.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Wang H, Han Y, Shi Y, Xin C, Yin P, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy in juvenile-onset primary open-angle glaucoma. Eye (Lond) 2021;35:2848–54. doi: 10.1038/s41433-020-01320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, Wang H, Oatts J, Cao K, Xin C, Liang X, et al. Ab interno versus ab externo microcatheter-assisted trabeculotomy for primary congenital glaucoma with clear cornea. Clin Exp Ophthalmol. 2020;48:1201–9. doi: 10.1111/ceo.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiao Y, Tan C, Chen X, Sun X, Chen J. Gonioscopy-assisted transluminal trabeculotomy versus goniotomy with Kahook dual blade in patients with uncontrolled juvenile open-angle glaucoma: A retrospective study. BMC Ophthalmol. 2021;21:395. doi: 10.1186/s12886-021-02159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grover DS, Godfrey DG, Smith O, Shi W, Feuer WJ, Fellman RL. Outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior incisional glaucoma surgery. J Glaucoma. 2017;26:41–5. doi: 10.1097/IJG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 56.Cubuk MO, Ucgul AY, Unsal E. Gonioscopy-assisted transluminal trabeculotomy as an option after failed trabeculectomy. Int Ophthalmol. 2020;40:1923–30. doi: 10.1007/s10792-020-01364-x. [DOI] [PubMed] [Google Scholar]
- 57.Nazarali S, Damji F, Damji KF. What have we learned about exfoliation syndrome since its discovery by John Lindberg 100 years ago? Br J Ophthalmol. 2018;102:1342–50. doi: 10.1136/bjophthalmol-2017-311321. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Zhang W, Xin C, Sang J, Sun Y, Wang H. Gonioscopy-assisted transluminal trabeculotomy for open-angle glaucoma with failed incisional glaucoma surgery: Two-year results. BMC Ophthalmol. 2023;23:89. doi: 10.1186/s12886-023-02830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakao I, Mine T, Sakaguchi M, Enaida H. Observation of Schlemm's canal and transluminal trabeculotomy using an ophthalmic endoscope: A case report. J Med Case Rep. 2019;13:249. doi: 10.1186/s13256-019-2186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo CY, Qi XH, Qi JM. Systematic review and meta-analysis of treating open angle glaucoma with gonioscopy-assisted transluminal trabeculotomy. Int J Ophthalmol. 2020;13:317–24. doi: 10.18240/ijo.2020.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato T, Kawaji T. 12-month randomised trial of 360°and 180°Schlemm's canal incisions in suture trabeculotomy ab interno for open-angle glaucoma. Br J Ophthalmol. 2021;105:1094–8. doi: 10.1136/bjophthalmol-2020-316624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Espinoza G, Rodriguez-Una I, Pedraza-Concha A. A case of bilateral delayed-onset hyphema following pupil dilation after gonioscopy-assisted transluminal trabeculotomy. J Curr Glaucoma Pract. 2020;14:72–5. doi: 10.5005/jp-journals-10078-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yalinbas D, Aktas Z, Hepsen İ, Dilekmen N. An unusual complication of combined gonioscopy-assisted transluminal trabeculotomy and phacoemulsification: Vision loss due to intracapsular hematoma. Int Ophthalmol. 2018;38:2223–6. doi: 10.1007/s10792-017-0710-4. [DOI] [PubMed] [Google Scholar]
- 64.Chen J, Wang YE, Quan A, Grajewski A, Hodapp E, Vanner EA, et al. Risk factors for complications and failure after gonioscopy-assisted transluminal trabeculotomy in a young cohort. Ophthalmol Glaucoma. 2020;3:190–5. doi: 10.1016/j.ogla.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Faria BM, Melillo GHL, Daga F, Kanadani FN, Prata TS. Short-term endothelial cell density changes after gonioscopy-assisted transluminal trabeculotomy. Arq Bras Oftalmol. 2021;85:344–50. doi: 10.5935/0004-2749.20220052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aktas Z, Ucgul AY, Segawa A. Transient myopia secondary to supraciliary effusion: Unusual complication after an uneventful prolene gonioscopy-assisted transluminal trabeculotomy. J Glaucoma. 2020;29:e60–3. doi: 10.1097/IJG.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 67.Akagi T, Nakano E, Nakanishi H, Uji A, Yoshimura N. Transient ciliochoroidal detachment after Ab interno trabeculotomy for open-angle glaucoma: A prospective anterior-segment optical coherence tomography study. JAMA Ophthalmol. 2016;134:304–11. doi: 10.1001/jamaophthalmol.2015.5765. [DOI] [PubMed] [Google Scholar]
- 68.Dönmez Gün R, Kuğu S, Erkan M, Şimşek Ş. Partial Schlemm canal, trabecular meshwork, and descemet membrane separation during gonioscopy-assisted transluminal trabeculotomy: A case report. J Glaucoma. 2020;29:e1–2. doi: 10.1097/IJG.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 69.Aktas Z, Bektas C, Hasanreisoglu M. Panscleritis as an unusual complication of gonioscopy-assisted transluminal trabeculotomy. J Glaucoma. 2019;28:e21–3. doi: 10.1097/IJG.0000000000001100. [DOI] [PubMed] [Google Scholar]
- 70.Wise J, Toygar O, Corrêa ZM, Miller DM, Sisk RA. Subretinal cannulation as a complication of suture trabeculotomy surgery in a pediatric patient. Retin Cases Brief Rep. 2017;11:79–82. doi: 10.1097/ICB.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 71.Manabe SI, Sawaguchi S, Hayashi K. The effect of the extent of the incision in the Schlemm canal on the surgical outcomes of suture trabeculotomy for open-angle glaucoma. Jpn J Ophthalmol. 2017;61:99–104. doi: 10.1007/s10384-016-0487-4. [DOI] [PubMed] [Google Scholar]
- 72.Okamoto Y, Akagi T, Kameda T, Suda K, Miyake M, Ikeda HO, et al. Prediction of trabecular meshwork-targeted micro-invasive glaucoma surgery outcomes using anterior segment OCT angiography. Sci Rep. 2021;11:17850. doi: 10.1038/s41598-021-97290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramesh PV, Ray P, Senthil Kumar NK, Ramesh SV, Devadas AK. Commentary: Minimally invasive glaucoma surgery for a surgical take diversion: An economic perspective. Indian J Ophthalmol. 2023;71:566–8. doi: 10.4103/ijo.IJO_2264_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shute T, Green W, Liu J, Sheybani A. An alternate technique for goniotomy: Description of procedure and preliminary results. J Ophthalmic Vis Res. 2022;17:170–5. doi: 10.18502/jovr.v17i2.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dada T, Bukke AN, Huang AS, Sharma N, Verma S. Recruitment of temporal aqueous outflow channels after bent needle ab-interno goniectomy demonstrated by aqueous angiography. J Glaucoma. 2023;32:e15–8. doi: 10.1097/IJG.0000000000002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voykov B, Szurman P, Dimopoulos S, Ziemssen F, Alnahrawy O. Micro-invasive suture trabeculotomy after canaloplasty: Preliminary results. Clin Exp Ophthalmol. 2015;43:409–14. doi: 10.1111/ceo.12482. [DOI] [PubMed] [Google Scholar]
- 77.Seuthe AM, Januschowski K, Szurman P. Micro-invasive 360-degree suture trabeculotomy after successful canaloplasty –One year results. Graefes Arch Clin Exp Ophthalmol. 2016;254:155–9. doi: 10.1007/s00417-015-3192-y. [DOI] [PubMed] [Google Scholar]
- 78.Baumgarten S, Kürten D, Lohmann T, Schellhase H, Plange N, Walter P, et al. Outcomes of 360°suture trabeculotomy after unsuccessful canaloplasty. Graefes Arch Clin Exp Ophthalmol. 2020;258:387–93. doi: 10.1007/s00417-019-04545-2. [DOI] [PubMed] [Google Scholar]
- 79.Ianchulev T, Ahmed II, Stamper RL, Chang DF, Samuelson TW, Lindstrom RL. Innovative alternatives in the surgical management of glaucoma with cataract surgery. Expert Rev Ophthalmol. 2017;12:403–19. [Google Scholar]
- 80.Areaux RG, Jr, Grajewski AL, Balasubramaniam S, Brandt JD, Jun A, Edmunds B, et al. Trabeculotomy Ab interno with the Trab360 device for childhood glaucomas. Am J Ophthalmol. 2020;209:178–86. doi: 10.1016/j.ajo.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 81.Sarkisian SR, Mathews B, Ding K, Patel A, Nicek Z. 360°ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol. 2019;13:161–8. doi: 10.2147/OPTH.S189260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirabayashi MT, Lee D, King JT, Thomsen S, An JA. Comparison of surgical outcomes of 360°circumferential trabeculotomy versus sectoral excisional goniotomy with the Kahook dual blade at 6 months. Clin Ophthalmol. 2019;13:2017–24. doi: 10.2147/OPTH.S208468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dickerson JE, Jr, Brown RH. Circumferential canal surgery: A brief history. Curr Opin Ophthalmol. 2020;31:139–46. doi: 10.1097/ICU.0000000000000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallardo MJ, Sarkisian SR, Jr, Vold SD, Singh IP, Flowers BE, Campbell A, et al. Canaloplasty and trabeculotomy combined with phacoemulsification in open-angle glaucoma: Interim results from the GEMINI study. Clin Ophthalmol. 2021;15:481–9. doi: 10.2147/OPTH.S296740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vold SD, Williamson BK, Hirsch L, Aminlari AE, Cho AS, Nelson C, et al. Canaloplasty and trabeculotomy with the OMNI system in pseudophakic patients with open-angle glaucoma: The ROMEO study. Ophthalmol Glaucoma. 2021;4:173–81. doi: 10.1016/j.ogla.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Hirsch L, Cotliar J, Vold S, Selvadurai D, Campbell A, Ferreira G, et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J Cataract Refract Surg. 2021;47:907–15. doi: 10.1097/j.jcrs.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 87.Klabe K, Kaymak H. Standalone trabeculotomy and viscodilation of Schlemm's canal and collector channels in open-angle glaucoma using the OMNI surgical system: 24-month outcomes. Clin Ophthalmol. 2021;15:3121–9. doi: 10.2147/OPTH.S325394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grabska-Liberek I, Duda P, Rogowska M, Majszyk-Ionescu J, Skowyra A, Koziorowska A, et al. 12-month interim results of a prospective study of patients with mild to moderate open-angle glaucoma undergoing combined viscodilation of Schlemm's canal and collector channels and 360°trabeculotomy as a standalone procedure or combined with cataract surgery. Eur J Ophthalmol. 2022;32:309–15. doi: 10.1177/1120672121998234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porsia L, Nicoletti M. Combined viscodilation of schlemm's canal and collector channels and 360°Ab-interno trabeculotomy for congenital glaucoma associated with sturge-weber syndrome. Int Med Case Rep J. 2020;13:217–20. doi: 10.2147/IMCRJ.S252725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pyfer MF, Gallardo M, Campbell A, Flowers BE, Dickerson JE, Jr, Talla A, et al. Suppression of diurnal (9AM-4PM) IOP fluctuations with minimally invasive glaucoma surgery: An analysis of data from the prospective, multicenter, single-arm GEMINI study. Clin Ophthalmol. 2021;15:3931–8. doi: 10.2147/OPTH.S335486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnstone M, Knepper P, Samples J. 3. Intraocular pressure control through linked trabecular meshwork and collector channel motion. Glaucoma Res Clin Adv, Kugler, Amesterdam, Netherland. 2016:41. [Google Scholar]
- 92.Elhusseiny AM, Jamerson EC, Menshawey R, Tam EK, El Sayed YM. Collector channels: Role and evaluation in Schlemm's canal surgery. Curr Eye Res. 2020;45:1181–7. doi: 10.1080/02713683.2020.1773866. [DOI] [PubMed] [Google Scholar]
- 93.Carreon T, van der Merwe E, Fellman RL, Johnstone M, Bhattacharya SK. Aqueous outflow – A continuum from trabecular meshwork to episcleral veins. Prog Retin Eye Res. 2017;57:108–33. doi: 10.1016/j.preteyeres.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dada T, Mahalingam K, Bhartiya S. Minimally invasive glaucoma surgery-to remove or preserve the trabecular meshwork: That is the question? J Curr Glaucoma Pract. 2021;15:47–51. doi: 10.5005/jp-journals-10078-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flamendorf JS, Huang D, Moster MR, Pro MJ, Myers JS, Lee D, editors. Investigating Pre-Operative Factors That Predict Trabecular Meshwork Bypass Stent Outcomes. 2020 ASCRS Annual Meeting. ASCRS. 2020 [Google Scholar]
- 96.Bozkurt E, Yenihayat F, Olgun A, Yazıcı AT, Şahbaz İ. The efficacy of gonioscopy-assisted transluminal trabeculectomy combined with phacoemulsification. Int Ophthalmol. 2021;41:35–43. doi: 10.1007/s10792-020-01550-x. [DOI] [PubMed] [Google Scholar]
- 97.Aktas Z, Zeydanli EO, Uysal BS, Yigiter A. Outcomes of prolene gonioscopy assisted transluminal trabeculotomy in primary open angle glaucoma and pseudoexfoliation glaucoma: a comparative study. J Glaucoma. 2022;31:751–6. doi: 10.1097/IJG.0000000000002063. [DOI] [PubMed] [Google Scholar]


