Abstract
Sortase A is a thiol transpeptidase expressed by Gram-positive bacteria. This enzyme is capable of site-specifically ligating peptides containing the C-terminal recognition motif LPXTG to peptides containing an N-terminal polyglycine sequence, forming a native peptide bond. Here, we describe the preparation and application of sortase A to the ligation of two individually folded disulfide-rich animal venom peptides in order to form a heterodimeric double-knotted peptide with a native peptide linker. This method is mild enough to preserve the structures and disulfide connectivities of the peptides during ligation. We employed a highly efficient sortase A pentamutant (SrtA5°), which brings the reaction to completion within 15 min with a ~50–80% yield of ligated peptide.
Keywords: Sortase A, Enzymatic ligation, Disulfide-rich peptide, Double-knotted peptide, Transpeptidase, Site-specific ligation
1. Introduction
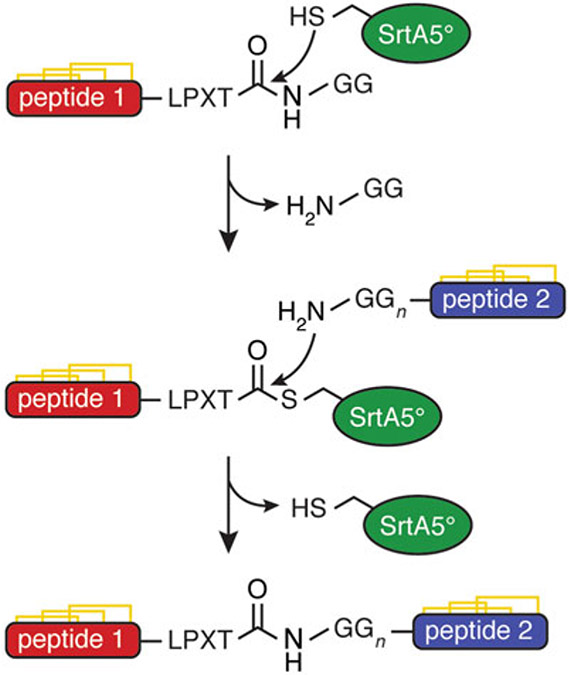
The synthetic formation of native peptide bonds can be greatly facilitated by the use of transpeptidases such as sortase A (SrtA). SrtA is a thiol transpeptidase expressed by Gram-positive bacteria, which recognizes the LPXTG motif (where X is any amino acid) in the C-terminus of surface proteins and ligates them to N-terminal polyglycine sequences in the cell wall [1]. This enzyme can, therefore, be used for site-specific C- to N-terminal ligations of peptides designed to contain the appropriate recognition motifs, resulting in a continuous native peptide backbone (Fig. 1).
Fig. 1.
Mechanism of sortase A ligation of two disulfide-rich peptides
Animal venom peptides often contain multiple disulfide bonds, arranged in a structurally rigid knot, which are important for stabilizing the secondary structure and maintaining the activity of the peptides [2-5]. Bivalent animal venom peptides with interesting pharmacological properties are a relatively recent discovery, creating a new structural class of toxins [6-9]. These peptides consist of a single continuous peptide backbone forming two distinct disulfide-rich domains, with a flexible portion of the peptide chain connecting the two domains. Bivalent peptides derived from spider venoms have been shown to have exceptionally high affinity for their target channels and are more potent than when separated into single-domain peptides [6, 7]. Interest in these properties has led to the desire to be able to synthesize these novel bivalent peptides through the ligation of naturally monovalent peptides [10-12].
Because bivalent peptides generally contain more amino acid residues and disulfides than monovalent peptides, and must form two distinct domains, these peptides are not practical to synthesize as a single continuous peptide with traditional solid-phase peptide synthesis and fold using thermodynamic oxidation, as is typically the process when producing monovalent disulfide-rich peptides. Instead, the monovalent peptides are synthesized and folded individually, then ligated to form the desired bivalent product [10-12]. Since many currently reported chemical ligation methods are carried out under reducing conditions [13, 14], enzymatic ligation with SrtA is particularly appealing for the synthesis of bivalent peptides because of its mild, nonreducing conditions, high yield, and excellent efficiency [11, 12]. This ligation strategy results in peptides that have a continuous peptide backbone, two correctly folded domains, and a flexible peptide linker containing the LPXTG recognition motif [11, 12]. This protocol uses a SrtA pentamutant (SrtA5°) with a higher catalytic efficiency than the wild-type enzyme [15], achieving the desired peptide in good yield (~50–80%) within 15 min [11, 12, 16].
2. Materials
2.1. Solid-Phase Peptide Synthesis
Coupling solutions: 0.25 M ethyl cyano(hydroxyimino)acetate (Oxyma Pure) in dimethylformamide (DMF) and 2 M N,N-′-diisopropylcarbodiimide (DIC) in DMF.
Amino acid solutions: 0.5 M each Fmoc-protected, side chain-protected amino acid in DMF (see Note 1).
Fmoc deprotection solution: 25% (v/v) pyrrolidine in DMF.
Cleavage solution: 95% (v/v) trifluoroacetic acid (TFA), 2.5% (v/v) triisopropylsilane (TIPS), and 2.5% (v/v) water.
Peptide isolation: diethyl ether, HPLC solvent A/B (see Subheading 2.4).
2.2. Oxidative Folding
Oxidative folding buffer: alkaline buffer salt (~pH 8), denaturing agent (urea or guanidinium chloride), oxidized glutathione (GSSG), reduced glutathione (GSH), organic solvent (if peptide is hydrophobic), and water (see Note 2).
Quenching: TFA and water.
2.3. Ligation
10× concentrated sortagging buffer: 0.5 M tris (hydroxymethyl)aminomethane (Tris), 1.5 M NaCl, and 0.1 M CaCl2 in water, adjusted to pH 8 with 1 M HCl or NaOH.
Quenching: 1% (v/v) TFA in water.
Sortase stock solution: SrtA5° and water.
2.4. Peptide Purification and Analysis
HPLC solvent A: 0.05% (v/v) TFA and water.
HPLC solvent B: 0.05% (v/v) TFA, 90% (v/v) acetonitrile (ACN), and water.
HPLC solvent A/B: 0.05% (v/v) TFA, 45% (v/v) ACN, and water (or 50% (v/v) HPLC solvent A and 50% (v/v) HPLC solvent B).
LC/MS solvent A: 0.05% (v/v) formic acid (FA) and water.
LC/MS solvent B: 0.05% (v/v) FA, 90% (v/v) ACN, and water.
2.5. Additional Equipment Required
Automated peptide synthesizer.
Column fitted with frit.
Vacuum flask.
Centrifuge.
Lyophilizer.
Sonicator.
HPLC instrument.
LC/MS instrument.
pH meter.
pH indicator paper.
NMR instrument.
Nanodrop.
Incubator with shaker.
3. Methods
Prepare all aqueous solutions with ultrapure water (deionized water purified to a sensitivity of 18.2 MΩ-cm). Handle all reagents carefully according to safety data sheets and dispose of all waste according to regulations.
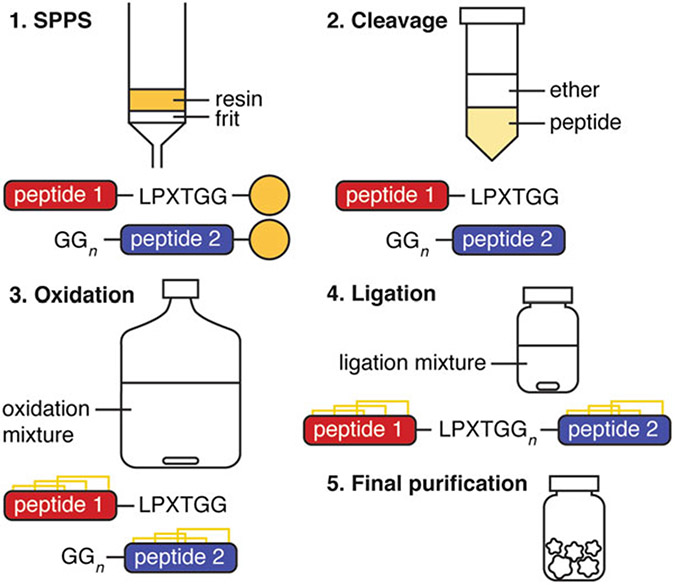
The full protocol is summarized in Fig. 2.
Fig. 2.
General procedure for the production of a bivalent peptide with sortase A
3.1. Peptide Synthesis
Peptides were assembled on a CEM Liberty Prime automated microwave peptide synthesizer using Fmoc chemistry and an appropriate resin as directed by the synthesizer’s manufacturer guidelines. However, other automated peptide synthesizers or manual synthesis will suffice. A column fitted with a frit and attached to a vacuum flask is required for drying the resin following peptide assembly, prior to cleavage.
Synthesize the N-terminal peptide with the sortase recognition motif, LPXTG, in the C-terminus (see Note 3). Synthesize the C-terminal peptide with an N-terminal polyglycine (see Note 4). Remove final Fmoc protecting group of both peptides.
Weigh out the appropriate resins according to loading capacity and desired scale.
Prepare coupling solutions, necessary amino acid solutions, and Fmoc deprotection solution according to required amounts for desired peptides (Subheading 2.1).
Assemble peptides using automated peptide synthesizer. After peptide assembly, drain the solvent from the resin in a fritted column attached to a vacuum flask. Rinse resin well with 50% dichloromethane (DCM)/50% methanol (MeOH). Drain and dry resin completely under vacuum and N2 flow.
Cleave peptide from resin and remove side chain protecting groups by suspending in 20 mL cleavage solution (Subheading 2.1, item 4) per gram of resin. Stir for 2 h at room temperature, then evaporate excess TFA to 25% volume (see Note 5).
Precipitate peptide with 20 mL ice-cold diethyl ether. Agitate to mix well, then centrifuge at 12500 × g for 5 min, and remove supernatant. Repeat this step twice (for a total of three iterations) (see Note 6).
Dissolve pellet in HPLC solvent A/B, freeze, and lyophilize (see Note 7).
Purify peptide by RP-HPLC to isolate desired linear peptide (see Note 8).
3.2. Peptide Oxidation
Peptide oxidation protocols differ for each peptide. All steps listed are a general protocol.
Dissolve peptide at <0.1 mg/mL concentration in appropriate oxidative folding buffer (see Note 9). Stir at room temperature or 4 °C for 2–72 h.
Quench oxidation reaction by acidifying to <pH 4 with neat TFA and dilute reaction mixture with water to achieve <1% TFA and < 5% organic solvent.
Purify reaction mixture by RP-HPLC to isolate correctly folded oxidized peptide.
Dissolve ~1 mg peptide in 10% D2O/90% H2O and verify correct peptide folding by one-dimensional (1D) and two-dimensional (2D) TOCSY and NOESY high-field NMR experiments (see Note 10).
3.3. Ligation
Perform a trial ligation reaction on a smaller scale prior to scaling up in order to determine optimal reaction time to achieve the highest yield of the desired ligated product. Take samples every 5 min, quench with 1% TFA, and monitor the reaction progress by LC/MS (see Notes 11 and 12).
SrtA5° can be purchased from commercial sources or expressed recombinantly as previously described [16]. Prepare a concentrated stock solution of the enzyme in water (0.1–0.5 mM) and store at −20 °C, or − 80 °C for long-term storage.
Prepare solutions of folded N-terminal and C-terminal peptides in water and quantify accurately by Nanodrop measurement at 280 nm (see Note 13).
Combine N-terminal and C-terminal peptides in a 1:3 molar ratio for desired micromolar concentrations (i.e., 90 μM and 270 μM final concentrations) in an appropriately sized reaction vessel (see Note 14). Add appropriate amount of 10× concentrated sortagging buffer (Subheading 2.3, item 1) and dilute to 1 × concentration with water.
Add sortase in a 1:3 molar ratio to the N-terminal peptide (i.e., 30 μM) directly before incubating at 37 °C on a shaker. Take time points at appropriate intervals.
Once reaction is complete, quench the reaction by cooling the reaction vessel on ice and adding ice-cold 1% TFA in water until acidified to <pH 4.
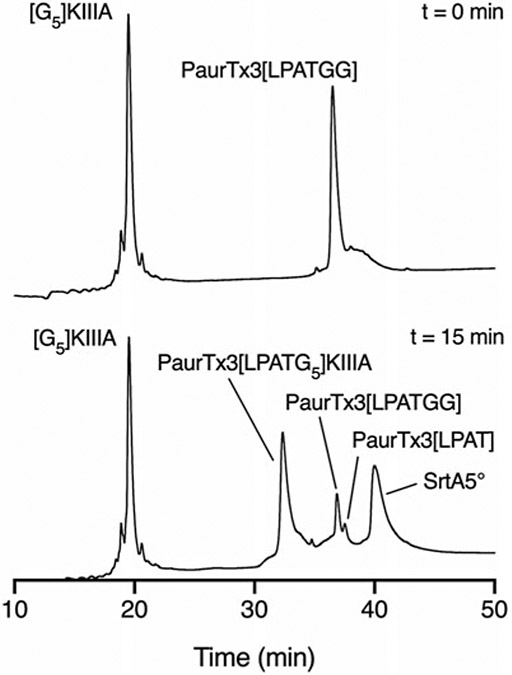
Purify the ligated peptide by RP-HPLC (Fig. 3) (see Note 15). Confirm successful ligation by LC/MS and NMR (see Note 16).
Fig. 3.
LC/MS traces showing the SrtA5°-mediated ligation of [G5]KIIIA and PaurTx3[LPATGG] after 15 min. (Adapted from Tran et al. [12])
3.4. Peptide Purification and Analysis
Filter all HPLC and LC/MS solvents through a 0.45 μm filter and store at room temperature.
Dissolve peptides in minimum amount of HPLC solvent A/B. Dilute with HPLC solvent A for a final organic solvent concentration of <10% (see Note 17).
Filter peptide solution through a 0.45 μm filter before loading onto HPLC column. Select column size (preparative, semipreparative, analytical) based on the quantity of peptide being purified.
Purify peptides by RP-HPLC using a 0.25–1%/min HPLC solvent A to B gradient.
Use LC/MS to identify correct products and analyze purity.
4. Notes
Fully dissolve amino acids in DMF with agitation. Sonicate if necessary.
Optimal oxidative folding protocols are specific to each peptide. The materials listed are general components commonly found in oxidative folding buffers.
Include an additional amino acid at the end of the sortase recognition motif to increase enzyme binding (i.e., LPXTGG) [17].
Although one glycine residue in the N-terminus has been reported to be sufficient [16], include at least two glycine residues in the N-terminal polyglycine for increased ligation efficiency [18]. The number of glycine residues can be increased as needed to increase linker length.
Peptide may begin to precipitate during this step.
Following extraction with ice-cold di-ethyl ether, keep the organic layer until correct peptide mass has been detected in the aqueous layer, especially for hydrophobic peptides which may not precipitate when adding diethyl ether.
Verify that the correct peptide was assembled by running a sample of the dissolved pellet using LC/MS.
Each peptide will display an individual HPLC profile depending on the hydrophobicity of the peptide. In order to maximize the preparative HPLC, run an analytical sample on a 0–60% gradient over 60 min in order to assess the retention time of each individual peptide.
Hydrophobic linear peptides may need to be dissolved with some organic solvent eg. acetonitrile.
Use 1D and 2D 1H NMR experiments to ensure that the individual peptides are folded correctly.
Hydrolysis of the N-terminal peptide in the sortase recognition sequence between Thr and Gly is a side product which consumes the starting material and the ligated product. This product cannot be reused for sortase ligation. Determination of the optimal reaction time is important for reducing the amount of the hydrolyzed peptide that is formed, thereby maximizing the yield of ligated peptide.
Quench samples with ice-cold 1% TFA in water prior to loading onto LC/MS. Reactions with SrtA5° are often complete after ~15 min [11, 12, 16].
Extinction coefficients are calculated as follows: εTrp = 5690 M−1 cm−1, εTyr = 1280 M−1 cm−1, εcystine = 120 M−1 cm−1 [19].
The 1:3 ratio of N-terminal:C-terminal peptide ensures that there is an excess of the nucleophile to drive the reaction forward [20].
Excess starting materials can be isolated and reused.
Use 1D and 2D NMR experiments to ensure no disulfide bond shuffling has occurred during ligation.
Hydrophobic peptides may require higher concentrations of organic solvent to prevent precipitation. Dilute the organic solvent to ~50% of the amount required to elute the peptide from the HPLC column.
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council (NHMRC) through a Project Grant (APP1080405), and an Australian Research Council (ARC) Future Fellowship (FT160100055) to C.I.S. P.T is supported by a University of Queensland Research Training Scholarship. We thank Prof. David Liu at Howard Hughes Medical School at Harvard University for providing the SrtA5° plasmid, Mr. Alan Zhang at the University of Queensland Centre for Advanced Imaging for assistance with SrtA5° expression, and Ms. Hue N.T. Tran for assistance with peptide synthesis.
References
- 1.Schneewind O, Model P, Fischetti VA (1992) Sorting of protein A to the staphylococcal cell wall. Cell 70(2):267–281 [DOI] [PubMed] [Google Scholar]
- 2.Ojeda PG, Chan LY, Poth AG, Wang CK, Craik DJ (2014) The role of disulfide bonds in structure and activity of chlorotoxin. Future Med Chem 6(15):1617–1628 [DOI] [PubMed] [Google Scholar]
- 3.Herzig V, King GF (2015) The cystine knot is responsible for the exceptional stability of the insecticidal spider toxin omega-hexatoxin-Hv1a. Toxins (Basel) 7(10):4366–4380. 10.3390/toxins7104366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kikuchi K, Sugiura M, Kimura T (2015) High proteolytic resistance of spider-derived inhibitor cystine knots. Int J Pept 2015:537508. 10.1155/2015/537508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agwa AJ, Huang YH, Craik DJ, Henriques ST, Schroeder CI (2017) Lengths of the C-terminus and interconnecting loops impact stability of spider-derived gating modifier toxins. Toxins 9(8):248–262. 10.3390/toxins9080248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D (2010) A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141 (5):834–845. 10.1016/j.cell.2010.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chassagnon IR, McCarthy CA, Chin YK, Pineda SS, Keramidas A, Mobli M, Pham V, De Silva TM, Lynch JW, Widdop RE, Rash LD, King GF (2017) Potent neuroprotection after stroke afforded by a double-knot spidervenom peptide that inhibits acid-sensing ion channel 1a. Proc Natl Acad Sci U S A 114 (14):3750–3755. 10.1073/pnas.1614728114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassilevski AA, Fedorova IM, Maleeva EE, Korolkova YV, Efimova SS, Samsonova OV, Schagina LV, Feofanov AV, Magazanik LG, Grishin EV (2010) Novel class of spider toxin: active principle from the yellow sac spider Cheiracanthium punctorium venom is a unique two-domain polypeptide. J Biol Chem 285(42):32293–32302. 10.1074/jbc.M110.104265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell M, Undheim EAB, Mobli M (2018) Secreted cysteine-rich repeat proteins “SCREPs”: a novel multi-domain architecture. Front Pharmacol 9:1333. 10.3389/fphar.2018.01333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray JK, Biswas K, Holder JR, Zou A, Ligutti J, Liu D, Poppe L, Andrews KL, Lin FF, Meng SY, Moyer BD, McDonough SI, Miranda LP (2015) Sustained inhibition of the NaV1.7 sodium channel by engineered dimers of the domain II binding peptide GpTx-1. Bioorg Med Chem Lett 25 (21):4866–4871. 10.1016/j.bmcl.2015.06.033 [DOI] [PubMed] [Google Scholar]
- 11.Agwa AJ, Blomster LV, Craik DJ, King GF, Schroeder CI (2018) Efficient enzymatic ligation of inhibitor cystine knot spider venom peptides: using sortase A to form double-knottins that probe voltage-gated sodium channel NaV1.7. Bioconjug Chem 29 (10):3309–3319. 10.1021/acs.bioconjchem.8b00505 [DOI] [PubMed] [Google Scholar]
- 12.Tran HNT, Tran P, Deuis JR, Agwa AJ, Zhang AH, Vetter I, Schroeder CI (2020) Enzymatic ligation of a pore blocker toxin and a gating modifier toxin: creating double-knotted peptides with improved sodium channel NaV1.7 inhibition. Bioconjug Chem 31(1):64–73. 10.1021/acs.bioconjchem.9b00744 [DOI] [PubMed] [Google Scholar]
- 13.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH (1994) Synthesis of proteins by native chemical ligation. Science 266(5186):776–779 [DOI] [PubMed] [Google Scholar]
- 14.Cistrone PA, Bird MJ, Flood DT, Silvestri AP, Hintzen JCJ, Thompson DA, Dawson PE (2019) Native chemical ligation of peptides and proteins. Curr Protoc Chem Biol 11(1): e6. 10.1002/cpch.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen I, Dorr BM, Liu DR (2011) A general strategy for the evolution of bond-forming enzymes using yeast display. Proc Natl Acad Sci U S A 108(28):11399–11404. 10.1073/pnas.1101046108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agwa AJ, Craik DJ, Schroeder CI (2019) Cyclizing disulfide-rich peptides using sortase a. In: Nuijens T, Schmidt M (eds) Enzyme-mediated ligation methods, Methods in molecular biology, vol 2012. Humana, New York, NY, pp 29–41 [DOI] [PubMed] [Google Scholar]
- 17.Popp MW, Antos JM, Ploegh HL (2009) Site-specific protein labeling via sortase-mediated transpeptidation. Curr Protoc Protein Sci Chapter 15:Unit 15.13. 10.1002/0471140864.ps1503s56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao H, Hart SA, Schink A, Pollok BA (2004) Sortase-mediated protein ligation: a new method for protein engineering. J Am Chem Soc 126(9):2670–2671 [DOI] [PubMed] [Google Scholar]
- 19.Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182(2):319–326 [DOI] [PubMed] [Google Scholar]
- 20.Williamson DJ, Fascione MA, Webb ME, Turnbull WB (2012) Efficient N-terminal labeling of proteins by use of sortase. Angew Chem Int Ed Engl 51(37):9377–9380. 10.1002/anie.201204538 [DOI] [PubMed] [Google Scholar]
- 21.Peschel A, Cardoso FC, Walker AA, Durek T, Stone MRL, Emidio NB, Dawson PE, Muttenthaler M, King GF (2020) Two for the price of one: Heterobivalent ligand design targeting two binding sites on voltage-gated sodium channels slows ligand dissociation and enhances potency. J Med Chem 63 (21):12773–12785. 10.1021/acs.jmedchem.0c01107 [DOI] [PMC free article] [PubMed] [Google Scholar]