Abstract
Small molecule microarray (SMM) technology has become a powerful tool used in high-throughput screening for target-based drug discovery. One area in which SMMs have found use is the identification of small molecule ligands for RNA. RNAs with unique secondary or tertiary three-dimensional structures are considered to be attractive targets for small molecules. Complex RNA structures can form hydrophobic pockets suitable for small molecule binding, representing an opportunity for developing novel therapeutics. Our lab has previously taken a target-based approach, screening a single target against many small molecules on an SMM platform. Here, we report a screening protocol for SMMs to investigate multiple RNAs simultaneously using multi-color imaging. By introducing a mixture containing different fluorophore-labeled RNAs, the fluorescence signal of each binding event can be observed simultaneously. Thus, the specificity of a hit compound binding to one RNA target over other highly abundant RNAs (such as tRNA or rRNA) can be easily evaluated.
Basic Protocol: RNA screening on SMM by multi-color imaging
Support Protocol 1: preparation of SMM slides
Support Protocol 2: fluorophore labeling of RNA through maleimide chemistry
Keywords: small molecule microarray, RNA target, multi-color imaging, high throughput screening
INTRODUCTION
This protocol describes a general procedure for RNA-targeted small molecule screening and multi-color imaging on small molecule microarrays. In recent years, RNAs with complex 3-dimensional structures have become attractive as pharmacological targets of small molecules due to increased understanding of their roles in disease biology and gene expression (Connelly, Abulwerdi, & Schneekloth, 2017; Cooper, Wan, & Dreyfuss, 2009; Matsui & Corey, 2017; Morgan, Forte, Culver, Zhang, & Hargrove, 2017; Warner, Hajdin, & Weeks, 2018). Strategies for the rapid discovery of RNA-binding small molecules are increasingly important for developing RNA-targeted probe molecules or potential therapeutics for RNA-driven diseases such as cancer (Childs-Disney, Wu, Pushechnikov, Aminova, & Disney, 2007; Garcia-Lopez et al., 2018; Haniff, Knerr, Chen, Disney, & Lightfoot, 2020; Lorenz, Vander Roest, Larsen, & Garner, 2017; Noreen F. Rizvi et al., 2018; N. F. Rizvi & Nickbarg, 2019; Noreen F. Rizvi et al., 2019; Tran & Disney, 2010). Among the developed high-throughput screening methods, small molecule microarray (SMM) technology stands out as a powerful tool with advantages including low sample cost, high sensitivity, reproducibility, and sample purity tolerance (Abulwerdi et al., 2016; Connelly et al., 2019; Vegas, Fuller, & Koehler, 2008). The fabrication of SMM including surface chemical modification and microarray printing has been well described previously (Connelly et al., 2017; Vegas et al., 2008). Further, the use of small molecule microarray screening to identify ligands for diverse targets of interest has been reviewed elsewhere (Hong, Neel, Wassaf, Caballero, & Koehler, 2014). Briefly, in order to manufacture SMMs, compounds are spatially arrayed by a robotic printer and covalently linked to glass slides. After incubating the modified slides with fluorescently labeled RNA, the slides are washed and dried for fluorescent imaging. Spots of specific compounds in the array that bind to RNA targets are detected with increased fluorescence (as brighter spots), compared with those on buffer slides. The quantification of fluorescence intensity and statistical analysis of the fluorescence data facilitate the discovery of RNA-binding small molecules.
Similar to other screening strategies for RNA, SMM focuses on a single target of interest. However, in cells, RNAs exist in a complex environment with other biomolecules such as proteins and other highly abundant RNAs (Gerstberger, Hafner, & Tuschl, 2014; Halvorsen, Martin, Broadaway, & Laederach, 2010; Khalil & Rinn, 2011; Lukong, Chang, Khandjian, & Richard, 2008). The ability to screen multiple targets simultaneously would facilitate the discovery of selective RNA-small molecule interactions. 2-dimensional combinatorial screening is one approach that has taken advantage of this potential, though this technique relies on a sequencing readout rather than fluorescence imaging (Tran & Disney, 2010). To date, efforts toward developing multi-color systems for microarrays have been reported, and have been successfully employed with DNA microarrays, lectin microarrays and antibody microarrays (Lee, Reddy, & Kodadek, 2010; Ribeiro & Mahal, 2013; Schröder et al., 2010; Shalon, Smith, & Brown, 1996; Zhu et al., 2010). Applications of such an approach for RNA include screening mutant/wild type pairs or investigating abundant cellular RNAs such as tRNA or rRNA in addition to a target RNA. Here, we describe an SMM-based strategy for screening multiple RNA targets on a single slide. We showcase this strategy by investigating the NRAS RNA G-quadruplex (rG4) and two highly abundant RNAs, tRNA, and 18S rRNA h16 hairpin (Kumari, Bugaut, Huppert, & Balasubramanian, 2007; Pisarev, Kolupaeva, Yusupov, Hellen, & Pestova, 2008).
STRATEGIC PLANNING
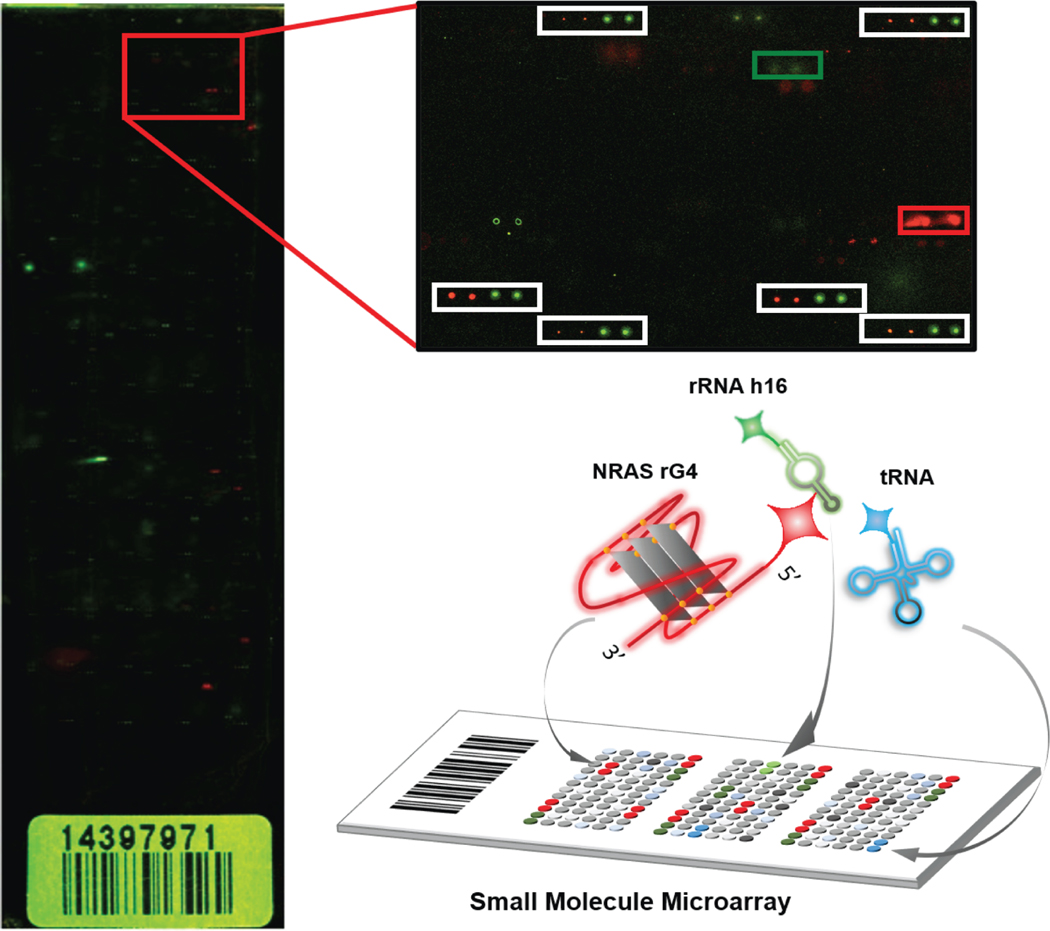
This protocol is compatible with SMMs manufactured on glass slides with different kinds of surface chemical modifications. Typically, our SMM design consists of 48 sub-arrays printed by 48 steel pins simultaneously. Each of the sub-arrays contains 196 (14 ×14) to 256 (16 ×16) individual spots, including library compounds as well as control samples such as dyes (AlexaFluor 647 and 488) or negative controls (DMSO or inactive compounds). Fluorescent dyes are printed at the top-left and bottom-right parts of each sub-array to aid in grid alignment when quantifying the image. For RNA sample screening, the labeled RNA of interest is prepared in an appropriate RNAse-free buffer and annealed under appropriate conditions to generate a folded structure. An SMM slide is incubated with the structured RNA sample for a desired time (usually one hour), washed extensively, and then imaged by fluorescent scanning. In this protocol, a mixture of RNAs labeled with fluorophores that emit at different wavelengths is utilized instead of a single RNA sample. For example, here we report the incubation of the SMM with a mixture of an RNA target of interest together with fluorescently labeled tRNA and rRNA, which are highly abundant in cells and therefore may be common targets for nonspecific binding. NRAS rG4, rRNA h16, and tRNA were labeled with AlexaFluor 647, AlexaFluor 532, and fluorescein (which has an excitation wavelength of 475~490 nm), respectively. By scanning the microarray using different lasers, fluorescence data is generated for red, green and blue wavelengths for each spot (Figure 1). This not only provides the hit rate against each RNA sample, but also reports the quantified binding selectivity for all three RNAs. More importantly, all binding events occur on a single slide in parallel, the complicated binding events (such as competitive and synergistic binding) could be characterized in complex.
Figure 1.
Schematic diagram of SMM screening based on multi-color imaging. SMMs are prepared according to a previously reported protocol. A solution including AlexaFluor 647-labeled NRAS rG4, AlexaFluor 532-labeled rRNA helix h16, and fluorescein-labeled yeast tRNA was incubated with SMM. The fluorescence image of the treated slide is based on confocal scanning using 3 different channels simultaneously. A representative slide is displayed, with the 647 and 532 nm channels displayed together. Inset: hits for NRAS rG4 (red box) and h16 rRNA hairpin (green box) are indicated, along with beacon dyes (white boxes).
BASIC PROTOCOL: RNA SCREENING ON SMM BY MULTI-COLOR IMAGING
Materials
SMM incubation with RNAs of interest
Glass slides with printed small molecule microarrays (Support Protocol 1)
LifterSlips™ Cover Slip (from ThermoFisher Scientific, catalog number 25X60I24789001LS)
RNaseZAP Decontamination Solution
5′ end labeled RNA targets of interest (labeled with AlexaFluor 647, AlexaFluor 532, or fluorescein). In principle, any dye or labeling method can be used as long as it is compatible with the scanner and does not disrupt the structure of the RNA. In this protocol, fluorescence labeled NRAS G4 (5′AlexaFluor 647-UGU GGG AGG GGC GGG UCU GGG-3′) and h16 rRNA (5′AlexaFluor 532-UAC AGG ACU CUU UCG AGG CCC UGU A-3′) were purchased from Integrated DNA Technology. For tRNA preparation and labeling, see Support Protocol 2.
10 × PBS buffer pH7.4 (Invitrogen, AM9624)
Tween 20 (Sigma-Aldrich, P9416)
RNase-free Tris buffer (1 M), pH7.0 (Invitrogen, AM9850G)
Potassium Chloride, BioXtra, ≥99.0% (Sigma-Aldrich, P9333)
Magnesium Chloride, BioReagent, ≥97.0% (Sigma-Aldrich, M4880)
Nuclease-free (DEPC treated) water
Parafilm
4-well rectangular polystyrene dishes (Nunc, catalog number 267061)
50 mL Falcon tubes
Kimwipes
Tweezers
Benchtop centrifuge
InnoScan 1100 AL Fluorescent Scanner (Innopsys) or equivalent
Softwares for data analysis
Microarray data analysis software (Innopsys Mapix version 8.1.1, GenePix Pro 7 or another suitable array imaging software)
JMP Software, or another suitable statistical software package
Methods
SMM incubation with RNAs of interest (Cover Slip method)
-
1
AlexaFluor-647-labeled NRAS rG4 and AlexaFluor-532-labeled rRNA h16 were purchased and delivered as lyophilized powders. In parallel, tRNA was labeled with fluorescein maleimide (Support Protocol 2). Prepare stock solutions by dissolving each RNA in nuclease free water at a concentration of 100 μM. Aliquot the RNA samples and store in −80 °C freezer to prevent degradation. Other buffers can be used if different conditions are required to maintain the RNA structure.
-
2
Dilute the labeled RNAs to 5 μM in annealing buffer (in this case we used 10 mM Tris, pH 7.0, containing 100 mM KCl and 2 mM MgCl2). Anneal the RNA targets for proper folding by heating the RNA at 95°C for 5 min. Allow RNA to cool to room temperature over 3–4 h. Once annealed, store RNA at 4°C overnight (~12 h) if required.
-
3
Clean the bench using RNaseZAP decontamination solution before incubating the slides with RNA. Open a 4-well chamber and fill one of the wells with a piece of wet Kimwipe to maintain humidity during the incubation.
-
4
Dilute the labeled RNA targets to a final concentration of 500 nM for each RNA together in reaction buffer (in this case we used 10 mM Tris, pH7.0, 100 mM KCl and 2 mM MgCl2, pH 7.4, 0.005% Tween 20).
-
5
Place a freshly cleaned LifterSlips™ Cover Slip on a piece of Parafilm (side of the cover slip with lifter slip bars should be facing upwards).
-
6
Transfer 100 μL of the sample solution containing the multiple RNAs to the edge of the cover slip. Carefully place the modified side of the SMM slide on the cover slip, ensuring that buffer makes contact with the array.
-
7
Place the slide and coverslip in an incubation chamber in the 4-well plate. In parallel, prepare a buffer-incubated slide (with no RNA present) as a negative control. Place the lid on the incubation chamber and wrap it with Parafilm to prevent evaporation.
-
8
Allow the SMM slide to incubate with RNA for 2 h at room temperature.
-
9
After incubation, remove the Cover Slipe carefully and put the SMM slide into a 50 mL Falcon tube which contains PBST buffer (1 × PBS, 0.005% Tween 20). Gentally wash the tube by shaking for 5 min. Then, quickly wash the slide with ultra-pure water by 3 times, in order to remove the salts on the surface.
-
10
Place some dry Kimwipe tissue at the bottom of a new Falcon tube and slowly insert the slide. After capping, centrifuge the tube at 1,700 g for 2 min to dry the slide. Then, take the slide out of the tube and store it in a slide box.
Microarray scanning
-
11
Turn on InnoScan 1100 AL Fluorescence Scanner (Innopsys) and wait for 20 min to warm up the light source before scanning.
-
12
Create a new file for scanning. Open the door of the slide holder and insert the slides into the machine face up (up to 24 slides can be scanned each batch). Close the door and click the “loading position” button to check the slide positions.
-
13
Adjust the scanning resolution to 5 μm resolution. Activate all three laser channels (488 nm, 532 nm and 647 nm) and set the gain to 40.
-
14
Start the scanning and name the files. Scan both the buffer slide and the slide incubated with RNA samples.
Data analysis
-
15
Data analysis of SMM image is adapted from previously reported procedure (Connelly et al., 2017). Use Mapix software to align GAL file with the array on the slide image (a GAL file is generated by the robotic arrayer after SMM printing and contains locations of each compound/control spot on the array). The GAL file allows compound identification based on the compound’s location on the slide. Properly align the GAL file using the control dye beacons (observed in the 532 and 647 nm channels) to ensure that labels agree with arrayed spots. Once blocks within the GAL file are properly aligned, further alignment of specific individual spots may be required to accurately capture potential hits.
-
16
Quantify the degree of fluorescence for each spot on the slide image using the Photometric Calculations icon in Mapix. This quantification will produce a .txt file with compound ID, location, and signal-to-noise ratio (SNR) for each measured active channel of the instrument (in this study 488, 532, and 647 nm), among other data that are not necessary for this analysis.
-
17
Import the compound IDs and SNR 488, SNR 532, and SNR 647 for each spot for both the buffer treated and RNA treated slides into a statistical software package. Mask or remove SNR data for control spots (e.g. AlexaFluor488, AlexaFluor647, DMSO, and empty spots) before statistical analysis of the screening library data.
-
18
Calculate the mean SNR (μ) and coefficient of variation (CV) for each pair of replicate spots for each compound for each wavelength using JMP or another appropriate software package. Remove data for outliers with unusually high SNR and/or CV. SNR values above 10 and CV values above 130% tend to be false positives such as dust particles. A visual inspection on the image of the corresponding spots is strongly recommended. It is important to remove these points as they may mask authentic hits in the standardization process. Calculate μ and the standard deviation (σ) of the SNR values for the entire library for each wavelength.
-
19Determine the composite Z-score for each compound using equation (1) below, where μcompound is the mean for the SNR of replicate compound spots, μlibrary is the mean for the SNR of the entire library, and σlibrary is the standard deviation of the entire library, for each wavelength. Perform the same analysis for the buffer-incubated negative control slide. In most cases, Z-scores of the compounds are within a range of −2~20.
(1) -
20Identify hit compounds according to the following criteria:
- CV of the two compounds spots should be below 130 % (to avoid false positives that show signal in only one of the duplicate spots).
- Hit compounds should have a Z-score above three in the presence of RNA targets (Z-scoreRNA > 3).
- The difference between the Z-score of compounds in the presence of RNA targets and to that in the presence of buffer should be above three (Z-scoreRNA – Z-scorebuffer > 3).
- The hit rate of the screening can be expected less than 1%, depending on the concentration of the target RNA during incubation. A higher or lower concentration of RNA solution can be used if the hit rate is too low or too high.
-
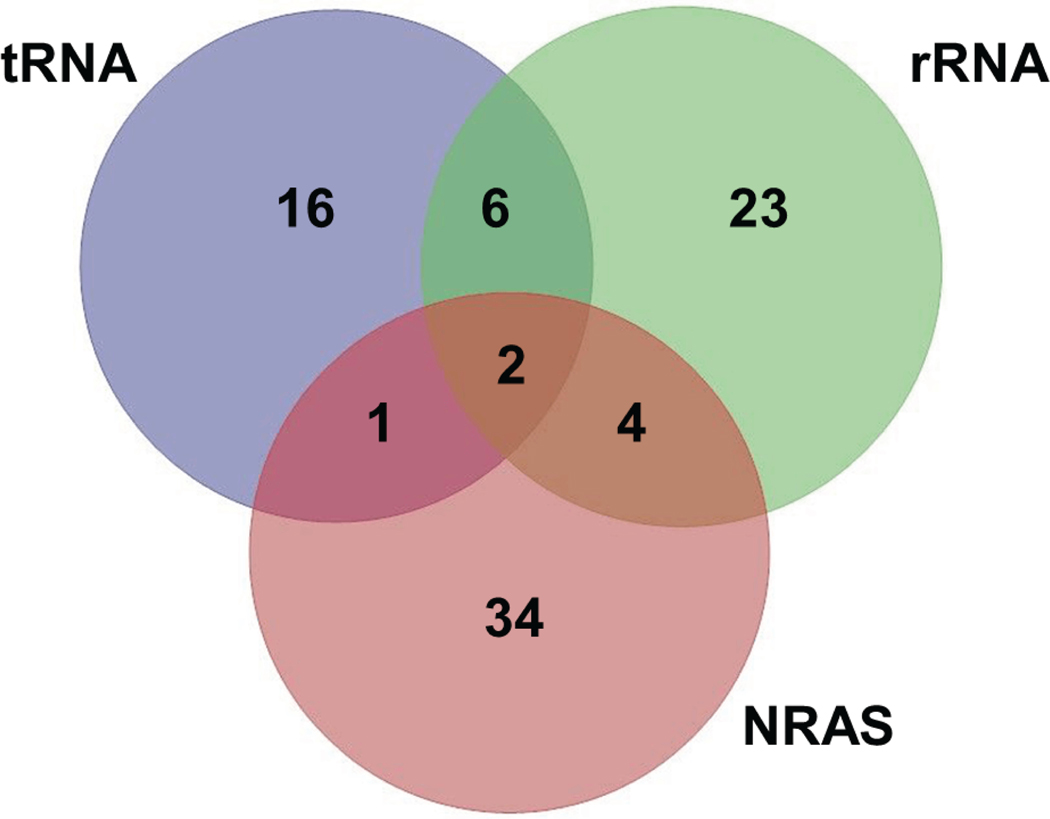
21
Analyze selectivity by generating a Venn diagram of the hits. In this example, we screened two SMM slides containing a total of 11,522 compounds against three RNA targets simultaneously. We identified 34 compounds that bound to the NRAS rG4, 16 compounds that bound to tRNA, and 23 compounds that bound to rRNA h16 selectively (Figure 2). Several compounds also bound to other (or all) RNAs screened. Compounds are considered selective if they score as “hits” for the RNA of interest but not for other RNAs.
-
22
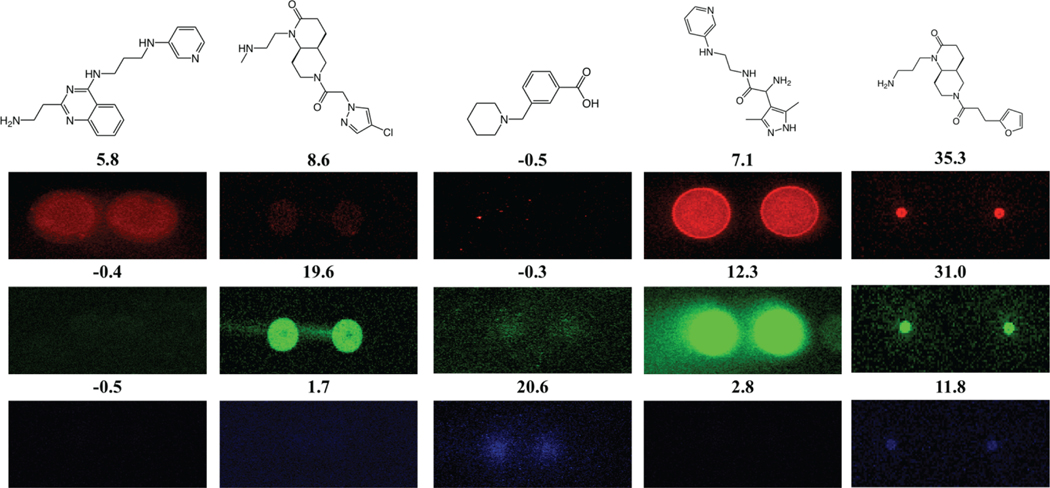
Visually inspect spot morphology of the hit compounds on the slide image. Authentic hit compounds should clearly exhibit fluorescence in both replicate spots in the presence of labeled RNA targets (and display no fluorescence in the presence of buffer only). Representative examples of selective and non-selective RNA binders identified using this approach are illustrated in Figure 3.
Figure 2.
Venn diagram of SMM screening hits against 3 different RNA structures. Compunds were observed to bind selectively to each RNA and promiscuiously to different sets of RNAs.
Figure 3.
Representative images of selective and non-selective compounds identified in the multi-color screen. Z-scores for each of the compounds are included above their respective images.
SUPPORT PROTOCOL 1
PREPARATION OF SMM SLIDES
In this protocol, we use an isocynate-based immobilization strategy to prepare small molecule microarrays, which has been previously reported (Bradner et al., 2006; Connelly et al., 2017). Briefly, glass slides are first cleaned and chemically modified with properly designed linkers with isocyanate groups. In parallel, 10 mM DMSO solutions of compounds that contain nucleophilic groups (such as -OH, -NH2) are prepared in 384-well plates. Within 48 h after the surface modification, the glass slides are placed in a robotic printer (e.g. Arrayit Nanoprint™). All the compounds as well as control dyes are printed as previously reported. After printing, the slides with SMMs are dried under pyridine vapor in a vacuum desiccator to promote covalent attachment to the slide. Next, the slides are blocked with ethylene glycol in order to deactivate the unreacted isocyanate groups. Finally, the slides are rinsed, dried by centrifugation, and stored in a desiccator before further use.
SUPPORT PROTOCOL 2
FLUOROPHORE LABELING OF RNA THROUGH MALEIMIDE CHEMISTRY
If RNAs are ~70 nt or smaller, they can be chemically synthesized with a fluorescent label and purchased commercially from vendors such as Integrated DNA Technology. However, for larger or more complex/structured RNAs, the researcher can prepare the RNA target by in vitro transcription and chemically label RNA as described below for tRNA.
Materials
5′ end labeling kit for nucleic acids (from Vector Labs, catalog number MB-9001)
UltraPure™ Phenol: Chloroform: Isoamyl Alchohol (25:24:1,v/v) (from Invitrogen, catalog number 15593031)
10 mg/mL yeast total RNA (from Invitrogen, catalog number AM7119)
Nuclease-free (DEPC treated) water
Fluorescein maleimide (from Vector Labs, catalog number SP-1502)
5′ end labeling of RNA (Based on protocol from Vector Labs, catalog number: MB-9001)
Prepare sample solution by combining 2 μL of universal reaction buffer, 2 μL of alkaline phosphatase, 1.7 μL of stock solution tRNAyeast, and 4.3 μL of nuclease free water. Lightly mix, vortex, and centrifuge the sample. Incubate the sample at 37 °C for 1 h.
Add 4 μL of universal reaction buffer, 2 μL of ATPγS and 4 μL of T4 polynucleotide kinase to the sample. Lightly mix, vortex, and centrifuge the sample. Incubate the sample at 37 °C for 1 h.
Add 10 μL of fluorescein maleimide. Lightly mix, vortex, and centrifuge the sample. Incubate at room temperature for 2 h.
Add 70 μL of nuclease free water and 100 μL of phenol:chloroform:isoamyl alchohol (resulting in a 1:1 ratio of labeled RNA solution to phenol:choloroform:isoamyl alchohol). Lightly mix, vortex, and centrifuge the sample. Remove the aqueous fraction (top fraction) from the mixture.
To the organic phase of the mixture, add 270 μL of cold 100% ethanol and 5 μL of the precipitant mixture. Lightly mix, vortex, and centrifuge the sample at 4 °C and 13,000 RPM for 30 min. Store the sample at −20 °C overnight.
Carefully, remove most of the 100% ethanol from sample. Wash pelleted RNA with cold 70% ethanol once. Lightly mix, vortex, and centrifuge the sample at 4 °C for 30 min at 13,000 RPM.
Carefully remove most of the 70% ethanol from sample (leaving ~20 μL). Set sample on a SpeedVac for 2–3 min. Reconstitute the pelleted RNA in nuclease free water. Measure the concentration of [RNA]labeled in the sample using a NanoDrop™ OneC or other appropriate instrument.
To calculate labeling efficiency, measure the absorbance of the sample in a 96 or 384-well plate using a Synergy Mx Microplate Reader (BioTek) or an equivalent instrument. Record the corrected absorbance of the sample at 260 nm (for total RNA) and at 494 nm for fluorescein. Determine the molar extinction coefficient for the labeled RNA in nuclease free water using Beer’s Law from plots of absorption vs. concentration. Use the following equation (2) to calculate the labeling efficiency:
| (2) |
where A and ε are the absorption and extinction coefficient, respectively.
COMMENTARY
Background Information
Small molecule microarray screening is a high-throughput strategy for discovering binding interactions between targets of interest and small molecules, and has great potential in drug discovery. Once an SMM is incubated with a fluorescently-labeled target, binding events can be profiled efficiently and quantitively via measurement of fluorescence intensities (Connelly et al., 2017). For RNA targeting, a key issue for the construction of a small molecule microarray is designing a diverse collection of small molecules to populate the array. In this case we used a large-scale library of commercially available compounds with meant to broadly represent “druglike” chemical space. In addition, functional groups such as -OH, -COOH, or -NH2 are normally required for surface attachment.
Statistical analysis of the SNR for each compound provides a Z-score used to distinguish candidate hit compounds for further evaluation. Both the number of compounds that validate and false positive rate for a screen are highly specific to the individual RNA target being studied. With the increasing need to understand the binding selectivity/promiscuity of drug candidates, multi-color imaging technology has been introduced to SMMs. By employing this approach, we demonstrate that it is possible to simultaneously screen multiple RNAs of interest, providing important information about selectivity. For example, one can investigate binding to a target and binding to abundant cellular RNAs, or evaluate similar structures such as mutant/wild type pairs in a single experiment. Once “hit” molecules have been identified in the screen, they are validated using biophysical affinity measurement techniques. Compounds that exhibit good binding may also be assessed in functional assays (biochemical or cell-based).
Critical Parameters
Sample purity/homogeneity
Maximizing the purity of the RNA target is important as it directly affects the degree of labeling, the signal produced for this target during the screening process, and the screening data in general.
Choice of fluorophore and labeling site
It is imperative the fluorophore is linked to a site on the RNA target that causes minimal perturbation of the native 3D structure of the target. Common sites for RNA labeling are either the 5′ or 3′ end, though internal sites of labeling can also be used (especially in the case of multimers) (Bou-Nader & Zhang, 2020; Kolesnikova, Hubálek, Bednárová, Cvacka, & Curtis, 2017).
Incubation
To prevent evaporation during the incubation period,incubation should be performed in a closed and sealed chamber. In case temperature control is not accessible, adding a wet Kim wipe can help maintain constant humidity during incubation.
Time Considerations
Labeling of an RNA target takes 1–2 days and the screening takes one full day. Evaluation of different conditions to impact signal and background binding for specific RNA targets may be considered during the screening process, which may take an additional 1 or 2 days.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (1ZIABC011585–06).
Literature Cited
- Abulwerdi FA, Shortridge MD, Sztuba-Solinska J, Wilson R, Le Grice SFJ, Varani G, & Schneekloth JS (2016). Development of Small Molecules with a Noncanonical Binding Mode to HIV-1 Trans Activation Response (TAR) RNA. Journal of Medicinal Chemistry, 59(24), 11148–11160. doi: 10.1021/acs.jmedchem.6b01450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Nader C, & Zhang J. (2020). Structural Insights into RNA Dimerization: Motifs, Interfaces and Functions. Molecules, 25(12). doi: 10.3390/molecules25122881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner JE, McPherson OM, Mazitschek R, Barnes-Seeman D, Shen JP, Dhaliwal J, . . . Koehler AN (2006). A robust small-molecule microarray platform for screening cell lysates. Chem Biol, 13(5), 493–504. doi: 10.1016/j.chembiol.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Childs-Disney JL, Wu M, Pushechnikov A, Aminova O, & Disney MD (2007). A small molecule microarray platform to select RNA internal loop-ligand interactions. ACS Chem Biol, 2(11), 745–754. doi: 10.1021/cb700174r [DOI] [PubMed] [Google Scholar]
- Connelly CM, Abulwerdi FA, & Schneekloth JS (2017). Discovery of RNA Binding Small Molecules Using Small Molecule Microarrays. In Uttamchandani M. & Yao SQ (Eds.), Small Molecule Microarrays: Methods and Protocols (pp. 157–175). New York, NY: Springer New York. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CM, Numata T, Boer RE, Moon MH, Sinniah RS, Barchi JJ, . . . Schneekloth JS (2019). Synthetic ligands for PreQ1 riboswitches provide structural and mechanistic insights into targeting RNA tertiary structure. Nature Communications, 10(1), 1501. doi: 10.1038/s41467-019-09493-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TA, Wan L, & Dreyfuss G. (2009). RNA and disease. Cell, 136(4), 777–793. doi: 10.1016/j.cell.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lopez A, Tessaro F, Jonker HRA, Wacker A, Richter C, Comte A, . . . Scapozza L. (2018). Targeting RNA structure in SMN2 reverses spinal muscular atrophy molecular phenotypes. Nature Communications, 9(1), 2032-2032. doi: 10.1038/s41467-018-04110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstberger S, Hafner M, & Tuschl T. (2014). A census of human RNA-binding proteins. Nature Reviews Genetics, 15(12), 829–845. doi: 10.1038/nrg3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen M, Martin JS, Broadaway S, & Laederach A. (2010). Disease-Associated Mutations That Alter the RNA Structural Ensemble. PLOS Genetics, 6(8), e1001074. doi: 10.1371/journal.pgen.1001074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniff HS, Knerr L, Chen JL, Disney MD, & Lightfoot HL (2020). Target-Directed Approaches for Screening Small Molecules against RNA Targets. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 2472555220922802. doi: 10.1177/2472555220922802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JA, Neel DV, Wassaf D, Caballero F, & Koehler AN (2014). Recent discoveries and applications involving small-molecule microarrays. Current opinion in chemical biology, 18, 21–28. doi: 10.1016/j.cbpa.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, & Rinn JL (2011). RNA-protein interactions in human health and disease. Seminars in cell & developmental biology, 22(4), 359–365. doi: 10.1016/j.semcdb.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikova S, Hubálek M, Bednárová L, Cvacka J, & Curtis EA (2017). Multimerization rules for G-quadruplexes. Nucleic acids research, 45(15), 8684–8696. doi: 10.1093/nar/gkx637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Bugaut A, Huppert JL, & Balasubramanian S. (2007). An RNA G-quadruplex in the 5’ UTR of the NRAS proto-oncogene modulates translation. Nat Chem Biol, 3(4), 218–221. doi: 10.1038/nchembio864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Reddy MM, & Kodadek T. (2010). Discovery Of An Orexin Receptor Positive Potentiator. Chemical science, 1(1), 10.1039/C1030SC00197J. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz DA, Vander Roest S, Larsen MJ, & Garner AL (2017). Development and Implementation of an HTS-Compatible Assay for the Discovery of Selective Small-Molecule Ligands for Pre-microRNAs. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 23(1), 47–54. doi: 10.1177/2472555217717944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukong KE, Chang KW, Khandjian EW, & Richard S. (2008). RNA-binding proteins in human genetic disease. Trends Genet, 24(8), 416–425. doi: 10.1016/j.tig.2008.05.004 [DOI] [PubMed] [Google Scholar]
- Matsui M, & Corey DR (2017). Non-coding RNAs as drug targets. Nature Reviews Drug Discovery, 16(3), 167–179. doi: 10.1038/nrd.2016.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BS, Forte JE, Culver RN, Zhang Y, & Hargrove AE (2017). Discovery of Key Physicochemical, Structural, and Spatial Properties of RNA-Targeted Bioactive Ligands. Angewandte Chemie International Edition, 56(43), 13498–13502. doi: 10.1002/anie.201707641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev AV, Kolupaeva VG, Yusupov MM, Hellen CUT, & Pestova TV (2008). Ribosomal position and contacts of mRNA in eukaryotic translation initiation complexes. The EMBO journal, 27(11), 1609–1621. doi: 10.1038/emboj.2008.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JP, & Mahal LK (2013). Dot by dot: analyzing the glycome using lectin microarrays. Current opinion in chemical biology, 17(5), 827–831. doi: 10.1016/j.cbpa.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NF, Howe JA, Nahvi A, Klein DJ, Fischmann TO, Kim H-Y, . . . Nickbarg EB (2018). Discovery of Selective RNA-Binding Small Molecules by Affinity-Selection Mass Spectrometry. ACS Chemical Biology, 13(3), 820–831. doi: 10.1021/acschembio.7b01013 [DOI] [PubMed] [Google Scholar]
- Rizvi NF, & Nickbarg EB (2019). RNA-ALIS: Methodology for screening soluble RNAs as small molecule targets using ALIS affinity-selection mass spectrometry. Methods, 167, 28–38. doi: 10.1016/j.ymeth.2019.04.024 [DOI] [PubMed] [Google Scholar]
- Rizvi NF, Santa Maria JP, Nahvi A, Klappenbach J, Klein DJ, Curran PJ, . . . Nickbarg EB (2019). Targeting RNA with Small Molecules: Identification of Selective, RNA-Binding Small Molecules Occupying Drug-Like Chemical Space. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 25(4), 384–396. doi: 10.1177/2472555219885373 [DOI] [PubMed] [Google Scholar]
- Schröder C, Jacob A, Tonack S, Radon TP, Sill M, Zucknick M, . . . Hoheisel JD (2010). Dual-color Proteomic Profiling of Complex Samples with a Microarray of 810 Cancer-related Antibodies. Molecular & Cellular Proteomics, 9(6), 1271–1280. doi: 10.1074/mcp.M900419-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalon D, Smith SJ, & Brown PO (1996). A DNA microarray system for analyzing complex DNA samples using two-color fluorescent probe hybridization. Genome Res, 6(7), 639–645. doi: 10.1101/gr.6.7.639 [DOI] [PubMed] [Google Scholar]
- Tran T, & Disney MD (2010). Two-dimensional combinatorial screening of a bacterial rRNA A-site-like motif library: defining privileged asymmetric internal loops that bind aminoglycosides. Biochemistry, 49(9), 1833–1842. doi: 10.1021/bi901998m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas AJ, Fuller JH, & Koehler AN (2008). Small-molecule microarrays as tools in ligand discovery. Chemical Society reviews, 37(7), 1385–1394. doi: 10.1039/b703568n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KD, Hajdin CE, & Weeks KM (2018). Principles for targeting RNA with drug-like small molecules. Nature Reviews Drug Discovery, 17(8), 547–558. doi: 10.1038/nrd.2018.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lu Y, Deng C, Huang G, Chen S, Xu S, . . . Cheng J. (2010). Assessment of fluorescence resonance energy transfer for two-color DNA microarray platforms. Anal Chem, 82(12), 5304–5312. doi: 10.1021/ac100804p [DOI] [PubMed] [Google Scholar]