Abstract
Context
Glycogen storage disease type Ia (GSDIa) is an inborn metabolic disorder characterized by impaired endogenous glucose production (EGP). Monitoring of patients with GSDIa is prioritized because of ongoing treatment developments. Stable isotope tracers may enable reliable EGP monitoring.
Objective
The aim of this study was to prospectively assess the rate of appearance of endogenous glucose into the bloodstream (Ra) in patients with GSDIa after a single oral D-[6,6-2H2]-glucose dose.
Methods
Ten adult patients with GSDIa and 10 age-, sex-, and body mass index–matched healthy volunteers (HVs) were enrolled. For each participant, 3 oral glucose tracer tests were performed: (1) preprandial/fasted, (2) postprandial, and (3) randomly fed states. Dried blood spots were collected before D-[6,6-2H2]-glucose administration and 10, 20, 30, 40, 50, 60, 75, 90, and 120 minutes thereafter.
Results
Glucose Ra in fasted HVs was consistent with previously reported data. The time-averaged glucose Ra was significantly higher in (1) preprandial/fasted patients with GSDIa than HV and (2) postprandial HV compared with fasted HV(P < .05). A progressive decrease in glucose Ra was observed in preprandial/fasted patients with GSDIa; the change in glucose Ra time-course was directly correlated with the change in capillary glucose (P < .05).
Conclusion
This is the first study to quantify glucose Ra in patients with GSDIa using oral D-[6,6-2H2] glucose. The test can reliably estimate EGP under conditions in which fasting tolerance is unaffected but does not discriminate between relative contributions of EGP (eg, liver, kidney) and exogenous sources (eg, dietary cornstarch). Future application is warranted for longitudinal monitoring after novel genome based treatments in patients with GSDIa in whom nocturnal dietary management can be discontinued.
Keywords: glycogen storage disease type Ia, stable isotopes, diet, monitoring, precision medicine
Glycogen storage disease type Ia (GSDIa) is an inborn disorder of carbohydrate metabolism characterized by severe fasting hypoglycemia due to impaired endogenous glucose production (EGP). GSDIa is caused by glucose 6-phosphatase-α (G6Pase-α) deficiency due to biallelic G6PC1 gene variants, resulting in defective glycogenolysis and gluconeogenesis, which in turn impairs EGP. Strict medically prescribed nutrition therapy is the cornerstone of the treatment (1). Although controversies still exist (2), the medically prescribed dietary regimens include frequent daytime feedings which are restricted in fructose, sucrose and (ga)lactose, as a form of substrate reduction. In addition, uncooked cornstarch (UCCS), extended-release cornstarch (Glycosade) or continuous nocturnal gastric drip-feeding (CNGDF) serve as product supplementation (3, 4).The general aims of these strict diets is to achieve normoglycemia, to normalize secondary metabolic perturbations, and to prevent long-term, chronic complications as much as possible. Liver transplantation is an increasingly recognized alternative treatment option; its advantages and disadvantages need to be weighed individually (5). Novel treatment approaches for GSDIa are currently being investigated, including adeno-associated virus (AAV) serotype 8 (AAV8)–mediated gene therapy (NCT05139316) and mRNA-mediated therapy (NCT05095727). In vivo genome editing is in clinical development following promising results in GSDIa mice (6). Ideally, because of these novel treatments, EGP would be restored, allowing patients with GSDIa no longer being dependent on strict medically prescribed diets and safely sleep throughout the night, without hypoglycemia risks.
The recent multistakeholder international priority setting partnership for liver GSDs has emphasized the need for new, low burdensome methods for monitoring metabolic control (7). Appropriate monitoring of patients with GSDIa is crucial to reliably assess the acute and long-term efficacy of any treatment. Among the primary outcome parameters used in the aforementioned clinical trials are changes in (1) the percentage time-in-range as measured by continuous glucose monitoring (CGM) (8), and (2) time to hypoglycemia assessed by in-hospital controlled fasting challenges.
In theory, stable isotope methods have the potential to monitor EGP in patients with GSDIa in a reliable, longitudinal, minimally invasive, safe fashion (9). At least 2 major methodological aspects challenge the use of stable isotopes in patients with GSDIa. First, most approaches require intravenous or nasogastric tube administration of stable isotope tracers using a primed continuous infusion and repeated venous blood sampling during the test. Second, patients with GSDIa depend on dietary treatment to maintain euglycemia and cannot be studied safely under fasted conditions. Consequently, any stable isotope approach is likely to result in EGP overestimation due to the contribution of unlabeled exogenous dietary glucose, which increases the total glucose rate of appearance (Ra; ie, the rate of endogenous glucose production by liver, kidney and intestine, plus any glucose originating from dietary sources).
In recent years, intraperitoneal or oral stable isotope-labeled glucose administration followed by serial dried blood spot (DBS) sampling has proven a valid approach to assess glucose Ra and EGP in small laboratory animals (10-13). Since these animals were fasted, glucose Ra was equal to EGP (10-13). These studies suggest that oral administration of glucose tracers may provide a minimally invasive approach to estimate glucose turnover in humans. The aim of the current study was to overcome the limitations of traditional stable isotope approaches by developing a minimally invasive method enabling Ra quantification in patients with GSDIa which can be translated in regular care and clinical studies. Here we report our prospective, investigator-initiated human pilot study in 10 adult patients with GSDIa and 10 age-, sex-, and body mass index (BMI)–matched healthy volunteers (HVs) receiving a single oral D-[6,6-2H2]-glucose dose. For each participant, glucose Ra was estimated under 3 conditions: overnight fasted before breakfast, postprandially after lunch, and at a random time.
Subjects and Methods
Study Approval
The Medical Ethics Committee of the University Medical Center Groningen (UMCG), The Netherlands approved the study protocol (ref. no. METc 2020/342). The study was conducted according to the principles of the Helsinki Declaration of 1975 as revised in 2013. All participants provided written informed consent prior to inclusion in the study.
Study Design
This was a prospective, investigator-initiated human pilot study (ENGLUPRO GSDIa, NCT04311307). Study days 1 and 2 were scheduled at the UMCG after written informed consent was signed by the participants. Information on participants’ demographic, genotype (for patients with GSDIa), and diet was collected. The procedures on study days 1 and 2 were performed under the supervision of a research nurse and a physician. The procedures on study day 3 were performed at home (or in hotel, in case of participants’ preferences) by the participants without supervision, after participants were provided with detailed instructions and written information during study days 1 and 2. Participants stayed at the hotel (or at home, in case of participants’ preferences) during the night between day 1 and 2. All participants were asked to return the study material to the study site after completion of all study procedures.
Study Participants
The trial was conducted at the UMCG between October 2020 and July 2021. Patients with GSDIa and an equal number of age-, gender- and BMI–matched HVs were recruited at the UMCG. Inclusion criteria were (1) age >16 years, (2) stable medical condition before the start of the test procedures and for patients with GSDIa, and (3) confirmation of GSDIa with enzyme assay and/or G6PC1 variation analysis. Exclusion criteria included (1) pregnancy, (2) recent (<1 month) history of hospitalization due to hypoglycemia, (3) intercurrent illness (defined as a combination of decreased dietary intake, vomiting, diarrhea, and fever (>38.5 °C) in the week prior to the study visit), and for HVs also (4) confirmed diagnosis or history suggestive of diabetes mellitus, (5) first-grade family member with a confirmed diagnosis associated with fasting intolerance, and (6) symptoms or signs by suggestive of fasting intolerance, metabolic instability, fever or gastrointestinal complaints.
Based on their G6PC1 genotype (or clinical features in case the genotype was not available), patients with GSDIa were classified as “severe” (2 nonsense or active site variants or clinical ascertainment before the age of 2 years) or “attenuated” (2 missense variants not in the active site or 1 nonsense or active site variant and 1 missense variant not in the active site or clinical ascertainment after the age of 2 years). Variants were reported according to ClinVar, or based on published literature in case a mutation was not published/deposited on ClinVar. Each participant was assigned a code which was used to label the study materials (ie, data collection forms, biological material, cloud data).
Study Procedures
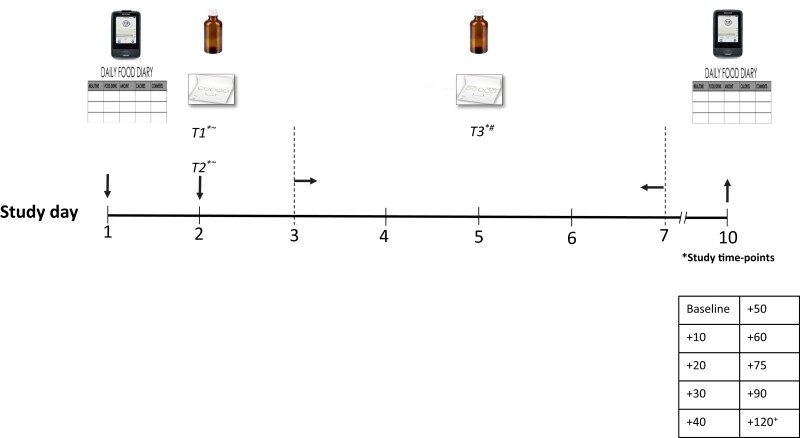
The study protocol is presented in Fig. 1. For each participant, 3 glucose stable isotope blood load tests (referred to as “glucose-SIB tests”) were performed. The first 2 glucose-SIB tests were performed at the study site under supervised conditions, during which 1.25 g of D-[6,6-2H2]-glucose dissolved in water (referred to as the “study drink”) were either orally administered before breakfast (glucose-SIB test 1) or after lunch (glucose-SIB test 2). The glucose-SIB test 3 was performed in an outpatient setting (at home or hotel) without supervision at a random time, after careful instruction of the study participant.
Figure 1.
Study protocol. T1, glucose-SIB test 1 (before breakfast); T2, glucose-SIB test 2 (after lunch); T3, glucose-SIB test 3 (random time); ∼supervised; #unsupervised; +1 additional sample at +180 was collected in a subset of participants. Light grey arrows, start; black arrows, end.
For each glucose-SIB test, data were collected at multiple time points, namely at baseline just before taking the study drink, and subsequently at 10, 20, 30, 40, 50, 60, 75, 90, 120 (and 180 if available) minutes after taking the study drink. For each time point the following data were collected: 2 DBS samples, capillary blood glucose (CBG) concentrations (capillary blood was obtained by fingerprick), CGM values, and information on any symptoms or signs. The glucose-SIB test was stopped in case (1) the CBG dropped to <3.6 mmol/L and the participant showed other signs or symptoms of hypoglycemia or (2) the participant requested to discontinue the experiment.
On day 1, a CGM device was placed (Dexcom G6) in either the upper arm or the abdomen. Instructions on how to appropriately use the CGM device were provided by an experienced research nurse. Additionally, all participants (patients and volunteers) were given the same type of CBG device (Freestyle freedom Lite) to be used for the entire study. Participants were also instructed on how to fill a food diary during the entire study.
On day 2, glucose-SIB test 1 was started between 05:00 and 06:00 Am and before breakfast, according to each participant's feeding pattern. For patients with GSDIa, the timing of the last meal prior to glucose-SIB test 1 was accommodated to their usual fasting tolerance. HVs were asked to fast for at least 8 hours. For 3 patients with GSDIa, proper fasting was not possible due to CNGDF. In these patients, glucose-SIB test 1 was performed during the last 2 hours of CNGDF. Glucose-SIB test 2 was started between 12:00 and 02:00 Pm on day 2, after taking lunch according to each participant's regular (prescribed) nutrition regimen. For glucose-SIB test 1 and 2, detailed information on the last (or concurrent) meal was recorded for each participant. On day 2 all participants were provided with instructions for glucose-SIB test 3, CGM, and shipping of the study material back to the site.
In order to minimize the risk of CGM malfunction and/or disconnection during glucose-SIB test 3, participants were asked to perform this test between day 3 and 7 and to send the study material back to the site on study day 10.
Noninvestigational Medical Product: The “Study Drink”
The D-[6,6-2H2]-glucose was purchased from Cambridge Isotope Laboratories, Inc. (Cambridge MA, USA). The study drink was prepared by Apotheek A15, and purity was confirmed to be 97%. The study drink was stored at the UMCG pharmacy at the study site according to good manufacturing practice. For each participant, 3 vials (ie, 1 vial for each glucose-SIB test) were distributed to the investigators 1 day before study day 1. Each study vial contained 1.25 g of D-[6,6-2H2]-glucose powder (each glucose molecule carried 2 hydrogen atoms as deuterium with a molar mass of 182.17) and was labeled with the participant's study code. This dose was based on pilot studies previously performed in healthy subjects (T. H. van Dijk, unpublished observations). The D-[6,6-2H2]-glucose powder was dissolved in water in 2 steps (total volume of 200 mL) by the investigators for glucose-SIB tests 1 and 2, and by the participants for glucose-SIB test 3 after instruction.
Outcome Measures
The primary study endpoint was assessing glucose Ra. Calculated glucose Ra was compared between (1) patients with GSDIa vs matched HVs; (2) severe and attenuated patients with GSDIa; (3) the preprandial and the fed state (ie, glucose-SIB test 1 and glucose-SIB test 2) in subjects with GSDIa; (4) the preprandial and fed state (ie, glucose-SIB test 1 and glucose-SIB test 2) in HVs; (5) the controlled hospital setting and at home setting (ie, glucose- SIB test 1 and glucose-SIB test 3) in patients with GSDIa; (6) the controlled hospital setting vs at home setting (ie, glucose-SIB test 1 and glucose-SIB test 3) in HVs. CBG and CGM data were collected and stored as exploratory endpoints and these results have been published previously (14).
Analytical Procedures
Glucose derivatization and gas chromatography mass spectrometry measurements
Collected DBSs were air dried horizontally for at least 3 hours at room temperature avoiding direct sunlight. Sample preparation for gas chromatography mass spectrometry (GCMS) analysis to determine the fractional distribution of glucose isotopomers was performed according to Van Dijk et al (15). Briefly, a 6.5-mm disk was punched out from each DBS, wetted with 50 µL of water for 15 minutes followed by the addition of 500 µL of ethanol, and incubated overnight at room temperature. After centrifuging, 400 µL of the supernatant was transferred to a Teflon-capped reaction vial and dried at 60 °C under a stream of N2. Glucose was subsequently converted to its pentaacetate derivative by adding 300 µL of pyridine/acetic anhydride (1:2) to the residue and incubating for 30 minutes at 60 °C (or overnight at room temperature). After drying at 60 °C under a stream of N2, the residue was dissolved in 200 µL of ethylacetate and transferred into an injection vial for GCMS analysis [(GC, Agilent 7890A; MS, Agilent 5975C inert Mass Selective Detector (MSD)]; Agilent Technologies, Amstelveen, The Netherlands). Derivatives were separated on a Zebron ZB-1701 30 m × 0.25 mm ID (0.25 µM film thickness) capillary column (Phenomenex, Utrecht, The Netherlands). Mass spectrometric analyses were performed by positive chemical ionization with ammonia. Out of the ions monitored, namely, m/z 408 to 412 (m0-m4), the fractional contribution of D-[6,6-2H2]-glucose in blood glucose is represented by M2, which was used for calculations.
Correction for naturally occurring isotopes and assessment of isotopomer data quality
The isotopologue distributions obtained by GCMS analysis were corrected for naturally occurring isotopes as previously described (15). The correction was based on the measured average isotopologue distribution in the baseline samples of all glucose-SIB test (ie, samples collected just before the study drink was taken). In most baseline samples the measured M2 fraction deviated less than 0.3% from the theoretical prediction by ChemCalc (https://www.chemcalc.org/) (16). Only baseline samples from participant 004_glucose-SIB test 3, participant 008_ glucose-SIB test 2, and participant 018_glucose-SIB test 2 contained 0.45, 0.96, and 0.39% excess M2 compared with the theoretical prediction, respectively. To account for the possibility that certain foods consumed by these participants affected the basal isotopomer abundances (17), the data from these 3 tests were corrected for the participant's own baseline sample of the respective glucose-SIB test.
Model description
To derive the Ra from the tracer data, we used the 2-compartment model and parameter fitting procedure recently described by Vieira-Lara et al (13). The difference with the latter study design was that our subjects received only 2H2-labeled glucose in the study drink, whereas they supplied a mixture of labeled and unlabeled glucose. This was taken into account in the modeling of the data.
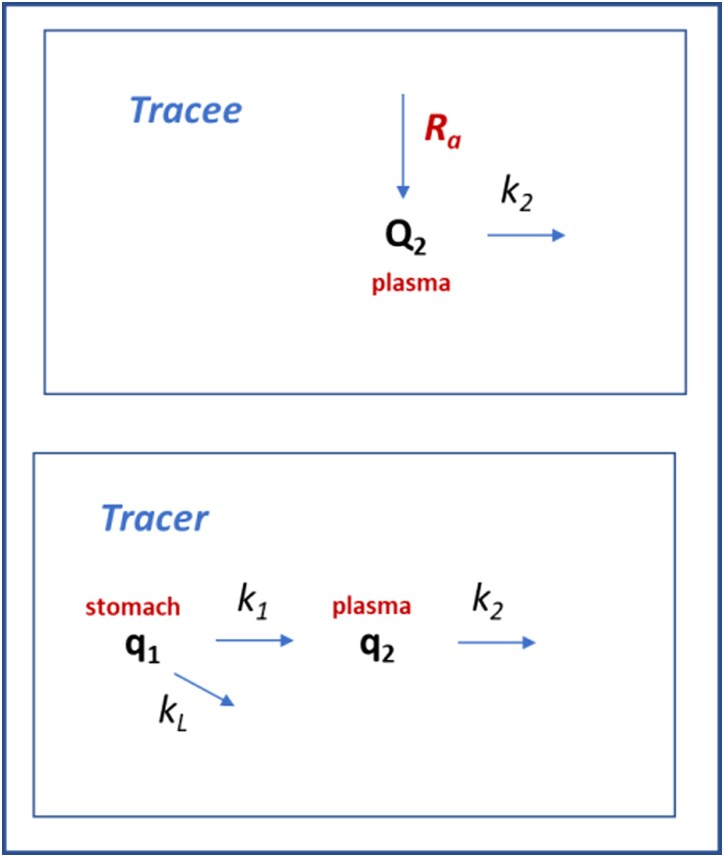
In brief, we considered 2 compartments, the external (stomach) compartment (compartment 1) and the blood plasma (compartment 2). The pool sizes (q) and concentrations (c) of labeled glucose were denoted by q1 and q2, c1, and c2 respectively, and those of unlabeled glucose in plasma analogously by Q2 and C2. Pool sizes Q and q were expressed in µmol/kg (referring to kg body weight), and concentrations C and c in mmol/L.
The tracer model consisted of 3 rates v (Fig. 2): import of glucose into the blood plasma compartment (v1), elimination from the plasma into the tissues (v2); and metabolism or storage of glucose before it enters into the blood (vL, with subscript L denoting loss). All rates v were expressed in µmol/kg/minute and described by first-order kinetics, with rate constants k per minute with the following rate equations for the tracer:
Figure 2.
Two-compartment model of tracer kinetics. The aim is to compute the rate of appearance (Ra) of unlabeled glucose. Ra represents the sum of endogenous glucose production (mainly by the liver) and other sources of unlabeled glucose (eg, in the nonfasted tests including intestinal glucose uptake). Q2 is the pool of unlabeled glucose in the plasma compartment (the tracee), q1 the pool of labeled glucose (tracer) administered orally, and q2 the pool of labeled glucose tracer observed in the plasma. Reaction rates v depend on rate constants k, which are assumed to be identical for tracer and tracee, since these are biochemically indistinguishable.
The conversion factor Vol in mL kg−1 was the distribution volume of the plasma compartment and served as a conversion factor between concentration and pool size. Analogously, the elimination rate of the unlabeled glucose was defined by:
In contrast to the model of Vieira-Lara (13), current model lacked the equations for uptake and loss of unlabeled glucose since the bolus did not contain unlabeled glucose in the present study. Finally, the rate of appearance of unlabeled glucose was quantified as the Ra−* in mM/minute or Ra in in µmol/kg/minute.
This then led to the following set of Ordinary Differential Equations (ODEs):
By implication time t was expressed in minutes.
Calculation of Ra from the tracer time courses
The analytical solution of the above-described model as derived before (13) reads:
with:
The c2 time courses were obtained by multiplication of the measured total glucose concentrations and the measured M2 enrichment at each time point. Based on the data of all subjects and tests in this study, and following the procedure outlined by Vieira-Lara (13), C was estimated from the data at 0.34 mM and ka was constrained between 0.028 and 0.33 minute−1. Subsequently, ka and k2 were fitted to each individual tracer time course, yielding their values for each individual test subject and test. It can be derived (13) that:
in which q1(0) is the amount of tracer administered and F (dimensionless) the bioavailability of the tracer (ie, the fraction of the tracer that reaches the sampled plasma pool):
We assumed a constant bioavailability F (equal for all subjects) of 0.89 based on triple-tracer tests with 88 healthy subjects (18). This then allowed the distribution volume Vol to be computed:
We note that an independent assessment of Vol and F would have required intravenous (IV) administration of the label.
First, the time-averaged Ra was computed, based on the fitted k2. To this end, the time-averaged tracee concentration (C2,avg) was computed by integrating the concentration of unlabeled glucose over time (area under the curve) and dividing by the duration of the test (tend – t0). A steady state was assumed for the tracee concentration. At steady state the rate of glucose elimination from the plasma pool equals the rate of appearance, and thus:
Second, only for glucose-SIB test 1 (ie, preprandial/fasted conditions) the Ra was computed as a function of time. The time course of unlabeled glucose was fitted to a linear function:
Hence:
At the same time (above):
Setting the 2 expressions equal to each other gives:
To convert Ra in µmol/kg/minute, was multiplied by the distribution volume Vol. Note that the estimated Ra in µmol/kg/minute is based on an assumed bioavailability F of 0.89 (18) whereas the estimated in mM is independent of F.
In 3 tests, k2 could not be calculated, since there was a less than 25% decrease between the peak concentration of D-[6,6-2H2]-glucose and the concentration at the last time point. This concerned participant 008 (HV) glucose-SIB test 1 (25% decrease), participant 019 (HV) glucose-SIB test 2 (2% decrease), and participant 020 (GSDIa attenuated) glucose-SIB test 2 (6% decrease). These tests were excluded from further computational analysis (file 1 (19)).
Software and Statistical Analysis
Correction for natural abundance of isotopes was performed in Excel 2019. All other calculations on glucose kinetics were done in Python, using Jupyter Notebook 6.1.4. Statistical analysis was performed using IBM SPSS Statistics 23. The comparisons between numerical variables were performed by Student's t-test corrected for Fisher's exact test. The normality of the distribution was checked by the Shapiro–Wilk test. Comparison between CBG curves generated in patients with GSDIa and HVs both during glucose-SIB test 1 and glucose-SIB test 2 was performed by 2-way analysis of variance analysis with the Geisser–Greenhouse correction. CBG and CGM values collected during glucose-SIB test 3 were excluded from the analysis of variance analysis in order to minimize possible bias due to data collected under nonsupervised conditions. Correlation study was performed by Spearman's rank correlation. Statistical significance was set at P < .05. For results on (1) CBG values, (2) comparison between CBG and CGM, and (3) correlation between change in glucose Ra and change in CBG, patients with GSDIa were analyzed as a group (n = 10). For results on (1) glucose Ra (both time-averaged and time courses) and (2) M1 fraction patients with GSDIa with an attenuated (n = 6) and a severe (n = 4) phenotype were analyzed separately, unless stated otherwise.
Results
Study Participants
Ten patients with GSDIa (5 females, 5 males) with a median age of 22.2 years (range 17.8-53.1) and a median BMI of 26.1 kg/m2 (range 22.4-29.8) were enrolled. Ten age-, gender-, and BMI-matched HVs were also enrolled. Table 1 presents the clinical and genetic characterization of the patients with GSDIa, and the details of their nutrition diary are summarized in Table 2.
Table 1.
Clinical and molecular characteristics of the study participants
| Participant | Age (years) | Gender | BMI (kg/m2) | Genotype (G6PC1 variants) | Clinical ascertainment years) | Phenotype | Dietary management | Nocturnal carbohydrate intake (g/kg/hour) | |
|---|---|---|---|---|---|---|---|---|---|
| Nucleotide change | Protein change | ||||||||
| 001 | 44.1 | F | 25.3 | c.809G>T c.1039C>T |
p.Gly270Val p.Gln347Ter |
<0.1 (diagnosed before symptoms onset following her elder sibling) | attenuated | Frequent feedings Glycosade (3.2 g/kg/day) |
0.38 |
| 002 | 21.6 | M | 29.8 | c.189G>A c.189G>A |
p.Trp63Ter p.Trp63Ter |
0.7 | Severe | Frequent feedings Glycosade (2.2 g/kg/day) |
0.35 |
| 004 | 17.8 | F | 22.4 | c.1039C>T c.1039C>T |
p.Gln347Ter p.Gln347Ter |
1.8 | Severe | Frequent feedings Glycosade (1.6 g/kg/day) |
0.30 |
| 006 | 53.1 | F | 27.3 | c.1039C>T c.247C>T |
p.Gln347Ter p.Arg83Cys |
N.A. | Severe | Frequent feedings Glycosade (2.4 g/kg/day) |
0.38 |
| 007 | 22.7 | M | 29.5 | c.1039C>T c.247C>T |
p.Gln347Ter p.Arg83Cys |
3.5 | Severe | Frequent feedings Glycosade (2.1 g/kg/day) CNGDF |
0.28 |
| 009 | 18.0 | F | 24.5 | c.562G>A c.508C>T |
p.Gly188Arg p.Arg170Ter |
<0.1 | Attenuated | Frequent feedings CNGDF |
0.06 |
| 014 | 26.9 | F | 25.6 | c.247C>T c.187 T>C |
p.Arg83Cys p.Trp63Arg |
0.5 | Attenuated | Frequent feedings Glycosade (3.2 g/kg/day) |
0.36 |
| 015 | 19.3 | M | 23.0 | c.247C>T c.187T>C |
p.Arg83Cys p.Trp63Arg |
<0.1 (diagnosed before symptoms onset following his elder sibling) | Attenuated | Frequent feedings Glycosade (3.1 g/kg/day) |
0.29 |
| 017 | 18.3 | M | 26.6 | c.247C>T c.866G>A |
p.Arg83Cys p.Ser289Asn |
N.A. | Attenuated | Frequent feedings UCCS (1.9 g/kg/day) CNGDF |
0.25 |
| 020 | 48.3 | M | 26.9 | c.809G>T c.1039C>T |
p.Gly270Val p.Gln347Ter |
0.4 | Attenuated | Frequent feedings UCCS + Glycosade (2.3 g/kg/day) |
0.24 |
| Healthy volunteers | 22.4 (17.1-50.8)a | 5 M 5 F |
23.2 (19.8-28.9)a | — | — | — | — | — | |
Abbreviations: BMI, body mass index; CNGDF, continuous nocturnal gastric drip-feeding; N.A., not available; UCCS, uncooked cornstarch.
a Median and range are shown.
Table 2.
Participants’ nutritional details during each of the 3 glucose-SIB tests
| Patient with GSDIa | Fasting interval before glucose-SIB test 1 (hours) | CH intake before glucose-SIB test 2 (g/kg) | Feeding status during glucose-SIB test 3 | Matched HV | Fasting interval before glucose-SIB test 1 (hours) | CH intake before glucose-SIB test 2 (g/kg) | Feeding status during glucose-SIB test 3 |
|---|---|---|---|---|---|---|---|
| 001 | 5 | 0.8 | Fed | 003 | 9 | 0.8 | Fasted |
| 002 | 2 | 0.4 | Fed | 008 | 10 | 0.4 | Fed |
| 004 | 6 | 0.8 | Fed | 010 | 9 | 0.3 | Fasted |
| 006 | 1.5 | 0.5 | Fasted | 005 | 10 | 0.5 | Fasted |
| 007 | 0a | 1.4 | Feda | 012 | 9.5 | 0.5 | Fasted |
| 009 | 0a | 0.2 | Feda | 011 | 9 | 0.6 | Fed |
| 014 | 3 | 0.6 | Fed | 013 | 8 | 0.6 | Fasted |
| 015 | 3.5 | 0.6 | Fed | 016 | 8 | 0.5 | Fasted |
| 017 | 0a | 0.5 | Feda | 019 | 9.5 | 0.4 | Fasted |
| 020 | 4.5 | 1 | Fasted | 018 | 10 | 1 | Fasted |
Abbreviations: CH, carbohydrate; glucose-SIB test, glucose stable isotopes blood load test performed before breakfast (test 1), after lunch (test 2), or at a random time (test 3); GSDIa, glycogen storage disease type Ia; HV, healthy volunteer.
a Continuous nocturnal gastric drip-feeding.
Safety
No serious adverse events were recorded during the glucose-SIB tests. Three patients with GSDIa showed asymptomatic CBG <3.6 mmol/L between 90 and 120 minutes during glucose-SIB test 1. These events were deemed unrelated to the study drink and most likely related to GSDIa.
Glucose Concentrations
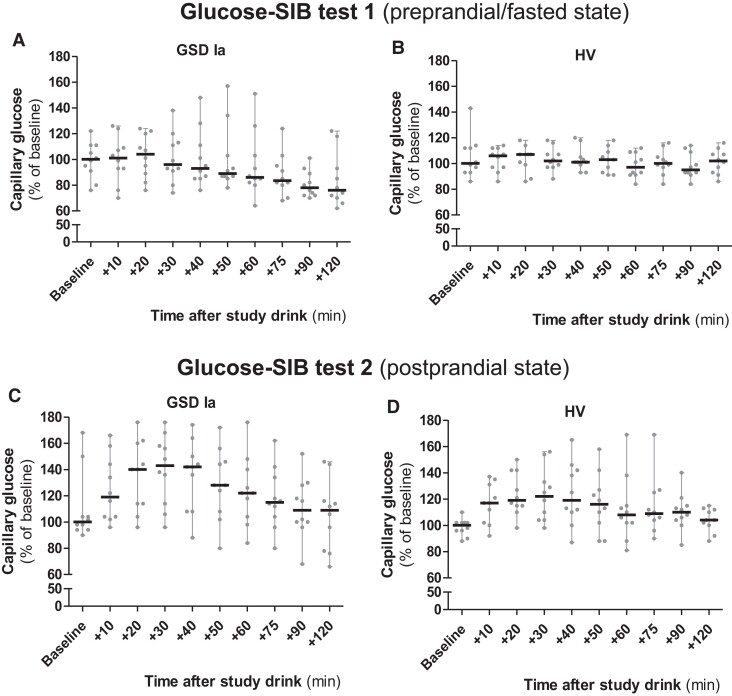
CBG data collected under supervised conditions (ie, glucose-SIB tests 1 and 2) are presented in Fig. 3 and elsewhere (file 2 (19)). During glucose-SIB test 1 (ie, supervised preprandial/fasted condition), no major increases in glucose concentrations were observed after ingestion of the study drink in any of the participants. As expected, based on the metabolic defect, a significant progressive decrease in glucose concentrations during glucose-SIB test 1 was observed in patients with GSDIa compared with HVs (P < .01) (Fig. 3A and 3B). A major increase in glucose concentrations was observed during glucose-SIB test 2 (supervised postprandial condition) in both patients with GSDIa and HVs. No difference between the groups was observed (P > .05) (Fig. 3C and 3D). A comparison of the CBG and CGM measurements was previously reported elsewhere (14).
Figure 3.
Capillary and CGM glucose concentrations during glucose-SIB test 1 and glucose-SIB test 2. Capillary glucose concentrations (CBG) during glucose-SIB test 1 and 2 in patients with GSDIa (n = 100 [ie, 10 time points × 10 participants] per each glucose-SIB test) and HVs (HVs, n = 100 [ie, 10 time points × 10 participants] per each glucose-SIB test). Results are calculated compared with median baseline values calculated in each subgroup (ie, GSDIa and HVs, respectively) for each glucose-SIB test (100% = median of baseline values in each subgroup) and presented as median with range (grey circles show single participants’ values). 20% = 1 mmol/L glucose.
Glucose Ra
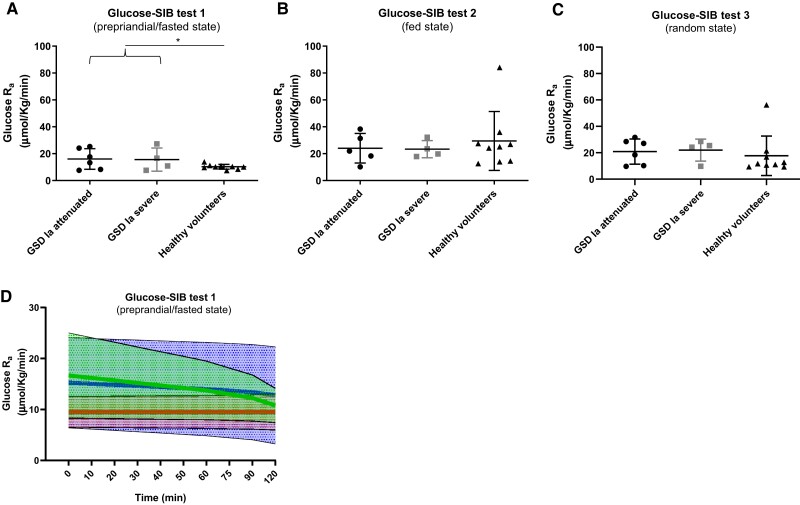
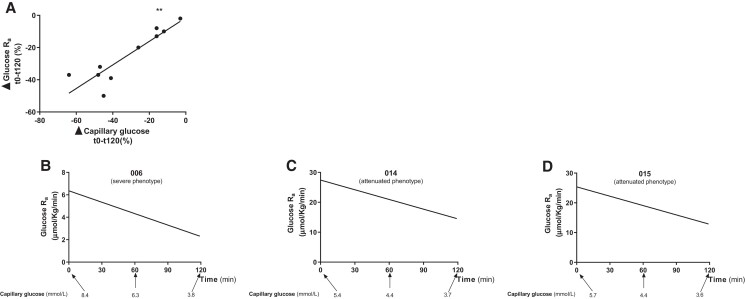
Fifty-nine M2 glucose curves (glucose-SIB test 1, n = 20 [10 GSDIa and 10 HVs]; glucose-SIB test 2, n = 20 [10 GSDIa and 10 HVs]; glucose-SIB test 3, n = 19 [10 GSDIa and 9 HVs]) were generated. For participant 012, CBG was not collected during glucose-SIB test 3 and therefore an M2 curve could not be generated. Data from the 56 approved tests were subsequently used for computational modeling. Kinetic constants and other model parameters are presented elsewhere (file 3 (19)). Time-averaged glucose Ra and glucose Ra time courses are presented in Table 3 and Fig. 4. The time-averaged glucose Ra was significantly higher in patients with GSDIa than in HVs (P < .05) (for both Ra in µmol/kg/minute and mM/minute) in glucose-SIB test 1 (ie, supervised preprandial/fasted condition) (Fig. 4A). No significant differences in the time-averaged glucose Ra between patients with GSDIa and HVs were observed in glucose-SIB test 2 (ie, supervised postprandial condition) and glucose-SIB test 3 (ie, unsupervised random fed condition) for both glucose Ra in µmol/kg/minute and mM/minute (Fig. 4B and 4C). Mean glucose Ra in HV was significantly higher in glucose-SIB test 2 than glucose-SIB test 1 (P < .05) for both Ra in µmol/kg/minute and mM/minute) but did not differ significantly between glucose-SIB tests 1 and 2 in patients with GSDIa (Fig. 4A and 4B). No significant correlation between the time-averaged glucose Ra and (1) fasting time before glucose-SIB test 1, (2) carbohydrate intake before glucose-SIB test 2, (3) daily UCCS/Glycosade intake, or (4) overnight UCCS/Glycosade intake was found in patients with GSDIa. Time courses of glucose Ra during glucose-SIB test 1 showed a stable trend in HVs and a progressive decrease in patients with GSDIa (Fig. 4D). The median decrease in glucose Ra during glucose-SIB test 1 was 28.5% in patients with GSDIa with an attenuated phenotype and 14% in patients with GSDIa with a severe phenotype. No significant correlation between glucose Ra calculated either at baseline or at the end (+120 minutes) of glucose-SIB test 1 and fasting time prior to glucose-SIB test 1 was found in patients with GSDIa. Among patients with GSDIa, the change in glucose Ra (ie, the difference between the glucose Ra at the end of the test and the glucose Ra at baseline) was directly correlated with the change in CBG (ie, the difference between the CBG concentration at the end of the test and the CBG at baseline) during glucose-SIB test 1 (Fig. 5).
Table 3.
Time-averaged glucose Ra in patients with GSDIa and healthy volunteers
| Glucose Ra | Glucose-SIB test 1 (preprandial/fasted state) | Glucose-SIB test 2 (fed state) | Glucose-SIB test 3 (random fed state) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| µmol/kg/min | mg/kg/min | mM/min | µmol/kg/min | mg/kg/min | mM/min | µmol/kg/min | mg/kg/min | mM/min | |
| GSDIa attenuated | 7.6-24.1 | 1.4-4.3 | 0.06-0.11 | 10.2-38.3 | 1.8-6.9 | 0.04-0.13 | 9.7-31.7 | 1.7-5.7 | 0.06-0.16 |
| GSDIa severe | 7.6-27.3 | 1.4-4.9 | 0.04-0.17 | 17.8-32.2 | 3.2-5.8 | 0.10-0.14 | 9.8-28.6 | 1.7-5.1 | 0.04-0.17 |
| Healthy volunteers | 7.5-13.9 | 1.4-2.5 | 0.03-0.08 | 13.9-84.1 | 2.5-15.1 | 0.02-0.34 | 9.4-56.3 | 1.7-10.1 | 0.04-0.16 |
Abbreviations: GSDIa, glycogen storage disease type Ia; SIB, stable isotope blood.
For each glucose-SIB test the range of measured glucose Ra is expressed in µmol/kg/minute, mg/kg/minute and mM/min, parameters that are commonly used in preclinical and clinical settings, respectively
Figure 4.
Glucose Ra in the study participants. (A-C) Time-averaged glucose Ra calculated with fixed F and constrained C and Ka. Mean and standard deviation are shown. (D) Glucose Ra time course calculated with fixed F and constrained C and Ka. A line connecting the mean value calculated at each time point (thick line) and SD (shaded area) are shown (green, GSDIa attenuated; blue, GSDIa severe; red, HVs). *P < .05.
Figure 5.
(A) Correlation between the change in glucose Ra and capillary blood glucose concentrations (CBG) during glucose-SIB test 1 in patients with GSDIa (r = 0.79, **P < .01). Δ was calculated as . The baseline values were considered as the value start test; values at +120 (or +180) were considered as the value end test. (B-D) Relationship between calculated glucose Ra and CBG during glucose-SIB test 1 in patients with GSDI with severe (B) and attenuated (C, D) phenotypes who developed hypoglycemia at the end of glucose-SIB test 1.
Discussion
This is the first study in patients with GSDIa aiming to quantify glucose Ra using a single oral D-[6,6-2H2]-glucose dose. We demonstrated that oral administration of D-[6,6-2H2]-glucose combined with DBS sampling allows estimation of glucose Ra both in patients with GSDIa and HVs. Moreover, we showed that calculated glucose Ra is influenced by the subject feeding status (eg, frequent feedings and UCSS/Glycosade). As such, glucose Ra likely exceeded EGP in patients with GSDIa and fed HVs due to the contribution of unlabeled dietary glucose.
Assessing EGP in patients with GSDIa has been a key research challenge for decades. In theory, EGP represents an ideal biomarker for monitoring patients with GSDIa. Firstly, it is directly influenced by defective G6Pase-α activity. Secondly, it is significantly lower in patients with GSDIa than in the healthy population. Thirdly, it can be monitored over time. EGP can be estimated by (stable isotope labeled) tracer studies (20). Hence, proper background knowledge on physiology and stable isotope technique is essential to adequately interpret the data. EGP represents the amount of glucose that is physiologically produced by the body to avoid hypoglycemia. The liver ensures up to ∼80% of EGP, with the remainder largely accounted for by the kidneys (21). Biochemically, 2 main processes contribute to EGP, namely gluconeogenesis and glycogenolysis (22). When stable isotope tracers are employed, the glucose Ra refers to the sum of unlabeled glucose produced endogenously by the organs (ie, EGP), plus that from exogenous sources (eg, diet, IV glucose) appearing per unit of time in the bloodstream (23).
Intriguingly, previous stable isotope studies have shown that patients with GSDIa display considerable residual EGP despite (often almost complete) G6Pase-α deficiency (Table 4) (24-31). In those studies, EGP was derived from the glucose Ra. Since many adult patients with GSDIa require frequent meals and UCCS every 4 to 6 hours (or alternatively IV glucose infusion or carbohydrates administration via nasogastric tube) to maintain euglycemia, the EGP was likely overestimated. This limitation applies to any approach aiming at quantifying EGP in patients with GSDIa. Importantly, different tracers, administration routes and feeding conditions were used in previous studies, potentially contributing to the variability observed among patients with GSDIa (24, 31-33).
Table 4.
Previous studies assessing endogenous glucose production (EGP) in GSDI patients using stable isotope tracers.
| Author | Year | Population (no. of patients with GSDI) | Tracer | Feeding condition | Glucose Ra | |
|---|---|---|---|---|---|---|
| µmol/kg/min | mg/kg/min | |||||
| Taslikian (24) | 1984 | 5 (1 GSDIa, 4 GSDIb) | 6,6-2H2-glucose IV | Fasted (0.75 hours after IV glucose infusion discontinuation) | 16.7-26.7 | 3.0-4.8 |
| Schwenk (25) | 1986 | 6 (2 GSDIa, 4 GSDIb) | 6,6-2H2-glucose via NG tube | IV glucose infusiona | 0.0-16.1 | 0.0-2.9 |
| Kalderon (26) | 1988 | 3 (2 GSDIa, 1 GSDIb) | 13C-glucose IV | IV glucose infusiona | 10.0-19.4 | 1.8-3.5 |
| Kalderon (27) | 1989 | 5 (4 GSDIa, 1 GSDIb) | 13C-glucose via NG tube | IV glucose infusiona | 6.1-19.4 | 1.1-3.5 |
| Collins (28) | 1990 | 6 (5 GSDIa, 1 GSDIb) | 3-3H-glucose/6,6-2H2-glucose IV | Variableb | 4.4-30.5 | 0.8-5.5 |
| Rother (29) | 1995 | 6 (2 GSDIa, 4 GSDIb) | 6,6-2H2-glucose/13C-galactose via NG tube | N.G. tube glucose infusionc | 2.2 ± 0.6 | 0.4 ± 0.1d |
| Weghuber (30) | 2007 | 4 | 6,6-2H2-glucose + MRS | IV glucose infusione | 2.8-3.3 | 0.5-0.6 |
| Huidekoper (31) | 2010 | 1 (GSDIa) | 6,6-2H2-glucose IV | Fasted (2.0-2.6 hours after drip-feeding discontinuation) | 3.9-5.0f | 0.7-0.9 |
Due to the various feeding conditions in the different studies, results are presented as glucose rate of appearance (Ra). For each study Ra is presented both as µmol/kg/minute and mg/kg/minute, parameters which are commonly used in preclinical and clinical settings, respectively.
Abbreviations: GSDI, glycogen storage disease type I; IV, intravenous; MRS, magnetic resonance spectroscopy; NG, nasogastric.
a Unlabeled glucose IV infusion at variable rate (3-12 mg/kg/minute).
b Some subjects were assessed in a fasted (5-12 hours) state and some subjects during unlabeled glucose IV infusion at variable rate (1.8-9.9 mg/kg/minute).
c Unlabeled glucose NG tube infusion at variable rate (5.8-8.7 mg/kg/minute).
d Only cumulative data available.
e Subjects were assessed during unlabeled glucose IV infusion (1.4 mg/kg/minute) before and after glucagon challenge.
f Depending on the fasting time.
Based on previous encouraging results obtained in non-GSDIa mice (10, 15) and taking into account the above-mentioned considerations, we tested the feasibility of glucose Ra and EGP quantification in adult patients with GSDIa after a single oral D-[6,6-2H2]-glucose dose. Collected data showed that this method allows to quantify glucose Ra in adult patients with GSDIa and HVs. It is unlikely, however, that the estimated Ra exclusively represents EGP in patients with GSDIa. The contribution of UCCS may explain why the glucose Ra in test 1 was higher in patients than in HVs, since the HVs had fasted for at least 8 hours.
Time-averaged glucose Ra in preprandial patients was 7.7 to 27.0 µmol/kg/minute (1.4-4.9 mg/kg/minute). Our results are in line with previous studies (Table 4), confirming the reported variability among patients with GSDIa. In this study that included a larger number of patients with GSDIa, glucose Ra was significantly higher in preprandial patients with GSDIa compared to HVs while no major differences were observed between patients and HVs in (random) fed states. Although no correlation between glucose Ra and fasting time prior to glucose-SIB test 1 and carbohydrate intake prior to glucose-SIB test 2 or daily UCCS intake was found in patients with GSDIa, results support the role of exogenous sources (ie, diet) to the estimated glucose Ra in patients. The slowly digested UCCS likely represented a major contributor to the estimated glucose Ra. Cornstarch is relatively highly enriched in 13C (17, 34). Yet, the baseline M1 fraction in blood glucose observed in patients with GSDIa (0-0.2%) was found to be negligible, suggesting that the UCCS used by the patients in this study was not overenriched in 13C compared with the diet of HVs.
Insulin resistance is observed in GSDIa (35) and UCCS requirements decrease in aging patients with GSDIa (36). Although none of the patients with GSDIa included in this study displayed increased HbA1c levels during regular care visits, these potential contributing factors were not formally assessed in the present study and may have affected glucose Ra in a postprandial state (37).
Time-averaged glucose Ra was 7.5 to 13.9 µmol/kg/minute (1.4-2.5 mg/kg/minute) in fasted HVs. After a physiological overnight fast, glucose metabolism is in steady state, meaning that the rate of glucose entering the bloodstream (Ra) equals the flow rate of glucose leaving the bloodstream (rate of disappearance; Rd). In this situation there is no contribution of exogenous sources to the glucose Ra, such as it occurs in healthy who do not require UCCS. Therefore, it can be assumed that the estimated glucose Ra equals the EGP in fasted HVs. Indeed, estimated glucose Ra in fasted HVs is in line with data on EGP previously published upon continuous glucose tracer infusion, supporting the reliability of our method (20).
The time course analysis performed on the preprandial/fasted tests revealed a stable glucose Ra in HVs while a progressive decline was observed in patients with GSDIa. The degree of glucose Ra decline directly correlated with the decline in CBG. Moreover, the decline in glucose Ra was sharper in patients with an attenuated phenotype than in those with a severe phenotype. Again, the progressive depletion of the UCCS may have contributed to this finding. The different interval between subsequent UCCS doses (likely broader in patients with GSDIa with an attenuated phenotype than in severely affected patients) may contribute to the difference observed between the 2 patient subgroups. Indeed, mean fasting time prior to glucose-SIB test 1 was shorter in patients with a severe vs attenuated GSDIa phenotypes (2 hours and 24 minutes vs 2 hours and 54 minutes). Whether this difference was statistically significant could not be ascertained in this relatively small study.
For data analysis, conversion of the Ra from mM/minute to µmol/kg/minute required an estimation of the distribution volume of the tracer. To this end it was assumed that the bioavailability of the tracer was equal for all study participants. This did not affect the differences observed between the study groups (either when expressing Ra in mM/minute or µmol/kg/minute). However a potential contribution of residual intestinal and/or hepatic G6Pase activity to the labeled glucose bioavailability cannot be ruled out. Interpatient differences in residual G6Pase activities may affect intestinal uptake and/or hepatic retention, resulting in differences in glucose tracer bioavailability, hence contributing to the observed variability among patients with GSDIa (38, 39). The relatively low number of patients included in the present study reflects the challenge of patient recruitment when studying rare disorders. It is unknown whether the current sample size adequately reflects the large clinical and biochemical heterogeneity observed in GSDIa (40).
Despite such limitations in patients with GSDIa, our test can be used to adequately estimate EGP in fasted HVs and presumably under conditions in which fasting tolerance is not impaired, such as diabetes and chronic kidney disease. Theoretically, it may develop into a baseline assessment method in patients in whom glycogenolysis or gluconeogenesis is decreased (eg, ketotic GSD subtypes, fructose-1,6-bisphosphatase deficiency) and as a confirmation tool to clarify the impact of genetic variants of unknown significance. Importantly, as this approach does not require intravenous infusion or collection of multiple venous blood samples it can be performed in outpatient settings. As such, this method overcomes organizational, financial, and psychological burden related to hospital admission, frequent venous sampling, and specific sample storing and processing.
Interestingly, in several of the 15 currently registered clinical trials for patients with GSDI, outcome parameters for efficacy have been selected that are counterintuitive or even dangerous, such as controlled fasting challenges. Novel techniques are urgently needed to capture alterations in metabolic flux homeostasis that may better reflect therapeutic changes in pharmacodynamics and pharmacokinetics (41), such as CGM (7, 14), and stable isotope methods (9, 42). As glucose Ra is directly linked to the underlying enzyme defect in GSDIa, it has the potential to develop into a primary disease activity biomarker as opposed to current biomarkers (43). The case–control application of the oral D-[6,6-2H2]-glucose method may be develop into a tool to longitudinally monitor patients with GSDIa who discontinued nocturnal dietary management after a so-called single shot innovative treatment (such as gene transfer or gene editing approach). In these individuals the contribution of the diet to the estimated glucose Ra is expected to decrease because of less intense dietary management. Because current mRNA treatments require repeated in-hospital intravenous administrations, a modified approach by making use of short-term multitracer IV solution of [U-13C]-glucose, [2-13C]-glycerol, [1-2H]-galactose, and acetaminophen may allow one to reliably estimate EGP and separately quantify contributions of gluconeogenesis and glycogenolysis (44, 45).
Acknowledgments
The authors would like to thank all the patients and HVs for their participation in the study. We are grateful to Giancarlo Parenti, Rebecca Riba-Wolman, and Folkert Kuipers for fruitful discussion on the study protocol and manuscript. We appreciate the assistance of Emmalie A. Jager and Candelas Gross Valle. We thank Margreet Steinfort and Petra Haarsma for practical support and precious help with the study procedures. All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest.
Abbreviations
- BMI
body mass index
- CBG
capillary blood glucose
- CGM
continuous glucose monitoring
- CNGDF
continuous nocturnal gastric drip feeding
- DBS
dried blood spot
- EGP
endogenous glucose production
- GCMS
gas chromatography mass spectrometry
- GSDIa
glycogen storage disease type Ia
- HV
healthy volunteer
- Ra
rate of appearance
- SIB
stable isotope blood
- UCCS
uncooked corn starch
Contributor Information
Alessandro Rossi, Department of Pediatrics, Section of Metabolic Diseases, Beatrix Children's Hospital, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands; Department of Translational Medicine, Section of Pediatrics, University of Naples “Federico II”, 80131 Naples, Italy.
Maaike H Oosterveer, Department of Pediatrics, Laboratory of Pediatrics, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands; Department of Laboratory Medicine, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands.
Theo H van Dijk, Department of Laboratory Medicine, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands.
Aycha Bleeker, Department of Pediatrics, Laboratory of Pediatrics, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands.
Martijn Koehorst, Department of Laboratory Medicine, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands.
David A Weinstein, Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA.
Barbara M Bakker, Department of Pediatrics, Laboratory of Pediatrics, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands.
Terry G J Derks, Department of Pediatrics, Section of Metabolic Diseases, Beatrix Children's Hospital, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands.
Funding
The “Endogenous Glucose Production in patients with Glycogen Storage Disease Type Ia” (ENGLUPRO GSDIa; NCT04311307) study funded by Ultragenyx Pharmaceutical Inc. (protocol number UX007-IST225 to T.G.J.D. and M.H.O.), and Associazione Italiana Glicogenosi (grant n. 01/2020 to A.R. and T.G.J.D.). M.H.O. holds a Rosalind Franklin Fellowship from the University of Groningen.
Disclosures
The authors have no competing interests to disclose concerning the content of this manuscript. There are confidentiality agreements between T.G.J.D. and with third parties. In the past 36 months, there have been consultation agreements (with Danone, Ultragenyx Pharmaceutical Inc, ModernaTX Inc, and Beam Therapeutics), contracts for financial research support for investigator-initiated research (NCT04311307) and sponsor-initiated research (NCT03517085, NCT03970278, NCT05139316, and NCT05196165), honoraria for lectures or presentations (by MEDTalks, Prelum, and Danone), and participations in a Data Safety Monitoring Board (NCT05095727) and Advisory Boards (Ultragenyx Pharmaceutical Inc, ModernaTX Inc, and Beam Therapeutics). For all private–public relationships, all contracts are via UMCG Contract Research Desk and all payments are to UMCG. A.R. received honoraria for lectures and/or advisory services from Ultragenyx Pharmaceutical Inc. and Vitaflo Italia.
Data Availability
Data analyzed during the current study are available from the corresponding author on reasonable request.
Clinical Trial Information
NCT04311307 (registered March 17, 2020).
References
- 1. Derks TGJ, Rodriguez-Buritica DF, Ahmad A, et al. Fischinger moura de Souza C, riba-wolman R, rossi A, saavedra H, gupta RN, valayannopoulos V, mitchell J. Glycogen storage disease type ia: current management options, burden and unmet needs. Nutrients. 2021;13(11):3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ross KM, Ferrecchia IA, Dahlberg KR, Dambska M, Ryan PT, Weinstein DA. Dietary management of the glycogen storage diseases: evolution of treatment and ongoing controversies. Adv Nutr. 2020;11(2):439‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European study on glycogen storage disease type I (ESGSD I). Eur J Pediatr. 2002;161(1):S20‐S34. [DOI] [PubMed] [Google Scholar]
- 4. Kishnani PS, Austin SL, Abdenur JE, et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American college of medical genetics and genomics. Genet Med. 2014;16(11):e1. [DOI] [PubMed] [Google Scholar]
- 5. Walter JH, Labrune P, Laforêt P. The glycogen storage diseases and related disorders. In: Saudubray JM, Baumgartner MR, García-Cazorla Á, Walter J, eds. Inborn Metabolic Diseases. Springer; 2022:179-200. [Google Scholar]
- 6. Arnaoutova I, Zhang L, Chen HD, Mansfield BC, Chou JY. Correction of metabolic abnormalities in a mouse model of glycogen storage disease type ia by CRISPR/cas9-based gene editing. Mol Ther. 2021;29(4):1602‐1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peeks F, Boonstra WF, de Baere L, et al. Research priorities for liver glycogen storage disease: an international priority setting partnership with the james lind alliance. J Inherit Metab Dis. 2020;43(2):279‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peeks F, Hoogeveen IJ, Feldbrugge RL, et al. A retrospective in-depth analysis of continuous glucose monitoring datasets for patients with hepatic glycogen storage disease: recommended outcome parameters for glucose management. J Inherit Metab Dis. 2021;44(5):1136‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rossi A, Rutten MGS, van Dijk TH, et al. Dynamic methods for childhood hypoglycemia phenotyping: A narrative review. Front Endocrinol (Lausanne). 2022a;13:858832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Dijk TH, Laskewitz AJ, Grefhorst A, et al. A novel approach to monitor glucose metabolism using stable isotopically labelled glucose in longitudinal studies in mice. Lab Anim. 2013;47(2):79‐88. [DOI] [PubMed] [Google Scholar]
- 11. Dommerholt MB, Blankestijn M, Vieira-Lara MA, et al. Short-term protein restriction at advanced age stimulates FGF21 signalling, energy expenditure and browning of white adipose tissue. FEBS J. 2021;288(7):2257‐2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huerta Guevara AP, McGowan SJ, Kazantzis M, et al. Increased insulin sensitivity and diminished pancreatic beta-cell function in DNA repair deficient Ercc1d/- mice. Metab Clin Exp. 2021;117:154711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vieira-Lara MA, Reijne AC, Koshian S, et al. Age and diet modulate the insulin-sensitizing effects of exercise: a tracer-based oral glucose tolerance test. Diabetes. 2023;72(7):872‐883. [DOI] [PubMed] [Google Scholar]
- 14. Rossi A, Venema A, Haarsma P, et al. A prospective study on continuous glucose monitoring in glycogen storage disease Type Ia: towards glycemic targets. J Clin Endocrinol Metab. 2022;107(9):e3612‐e3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Dijk TH, Boer TS, Havinga R, Stellaard F, Kuipers F, Reijngoud DJ. Quantification of hepatic carbohydrate metabolism in conscious mice using serial blood and urine spots. Anal Biochem. 2003;322(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 16. Patiny L, Borel A. Chemcalc: a building block for tomorrow's Chemical infrastructure. J Chem Inf Model. 2013;53(5):1223‐1228. [DOI] [PubMed] [Google Scholar]
- 17. Osborne CP, Beerling DJ. Nature's green revolution: the remarkable evolutionary rise of C4 plants. Philos Trans R Soc Lond B Biol Sci. 2006;361(1465):173‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287(4):E637‐E643. [DOI] [PubMed] [Google Scholar]
- 19. Rossi A, Oosterveer MH, van Dijk TH, et al. Supplemental files from Endogenous glucose production in patients with glycogen storage disease type Ia estimated by oral D [6,6–2H2]-glucose. figshare. 2023. Dataset. 10.6084/m9.figshare.23786520. [DOI]
- 20. Bier DM, Leake RD, Haymond MW, et al. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977;26(11):1016‐1023. [DOI] [PubMed] [Google Scholar]
- 21. Sharabi K, Tavares CD, Rines AK, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med. 2015;46:21‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickson JL, Hewett JN, Gunn CA, Lynn A, Shaw GM, Chase JG. On the problem of patient-specific endogenous glucose production in neonates on stochastic targeted glycemic control. J Diabetes Sci Technol. 2013;7(4):913‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feller DD, Strisower EH, Chaikoff IL. Turnover and oxidation of body glucose in normal and alloxan-diabetic rats. J Biol Chem. 1950;187(2):571‐588. [PubMed] [Google Scholar]
- 24. Tsalikian E, Simmons P, Gerich JE, Howard C, Haymond MW. Glucose production and utilization in children with glycogen storage disease type I. Am J Physiol. 1984;247(4 Pt 1):E513‐E519. [DOI] [PubMed] [Google Scholar]
- 25. Schwenk WF, Haymond MW. Optimal rate of enteral glucose administration in children with glycogen storage disease type I. N Engl J Med. 1986;314(11):682‐685. [DOI] [PubMed] [Google Scholar]
- 26. Kalderon B, Lapidot A, Korman SH, Gutman A. Glucose recycling and production in children with glycogen storage disease type I, studied by gas chromatography/mass spectrometry and (U-13C)glucose. Biomed Environ Mass Spectrom. 1988;16(1-12):305‐308. [DOI] [PubMed] [Google Scholar]
- 27. Kalderon B, Korman SH, Gutman A, Lapidot A. Estimation of glucose carbon recycling in children with glycogen storage disease: A 13C NMR study using [U-13C]glucose. Proc Natl Acad Sci U S A. 1989;86(12):4690‐4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins JE, Bartlett K, Leonard JV, Aynsley-Green A. Glucose production rates in type 1 glycogen storage disease. J Inherit Metab Dis. 1990;13(2):195‐206. [DOI] [PubMed] [Google Scholar]
- 29. Rother KI, Schwenk WF. Glucose production in glycogen storage disease I is not associated with increased cycling through hepatic glycogen. Am J Physiol. 1995;269(4 Pt 1):774. [DOI] [PubMed] [Google Scholar]
- 30. Weghuber D, Mandl M, Krssák M, et al. Characterization of hepatic and brain metabolism in young adults with glycogen storage disease type 1: a magnetic resonance spectroscopy study. Am J Physiol Endocrinol Metab. 2007;293(5):E1378‐E1384. [DOI] [PubMed] [Google Scholar]
- 31. Huidekoper HH, Visser G, Ackermans MT, Sauerwein HP, Wijburg FA. A potential role for muscle in glucose homeostasis: in vivo kinetic studies in glycogen storage disease type 1a and fructose-1,6-bisphosphatase deficiency. J Inherit Metab Dis. 2010;33(1):25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shieh JJ, Pan CJ, Mansfield BC, Chou JY. A potential new role for muscle in blood glucose homeostasis. J Biol Chem. 2004;279(25):26215‐26219. [DOI] [PubMed] [Google Scholar]
- 33. Hijmans BS, Boss A, van Dijk TH, et al. Hepatocytes contribute to residual glucose production in a mouse model for glycogen storage disease type Ia. Hepatology. 2017;66(6):2042‐2054. [DOI] [PubMed] [Google Scholar]
- 34. Schoeller DA, Klein PD, Watkins JB, Heim T, MacLean WC Jr. 13C Abundances of nutrients and the effect of variations in 13C isotopic abundances of test meals formulated for 13CO2 breath tests. Am J Clin Nutr. 1980;33(11):2375‐2385. [DOI] [PubMed] [Google Scholar]
- 35. Rossi A, Ruoppolo M, Formisano P, et al. Insulin-resistance in glycogen storage disease type ia: linking carbohydrates and mitochondria? J Inherit Metab Dis. 2018;41(6):985‐995. [DOI] [PubMed] [Google Scholar]
- 36. Dahlberg KR, Ferrecchia IA, Dambska-Williams M, et al. Cornstarch requirements of the adult glycogen storage disease Ia population: A retrospective review. J Inherit Metab Dis. 2020;43(2):269‐278. [DOI] [PubMed] [Google Scholar]
- 37. Boers HM, Alssema M, Mela DJ, Peters HPF, Vonk RJ, Priebe MG. The rate of glucose appearance is related to postprandial glucose and insulin responses in adults: A systematic review and meta-analysis of stable isotope studies. J Nutr. 2019;149(11):1896‐1903. [DOI] [PubMed] [Google Scholar]
- 38. Stümpel F, Burcelin R, Jungermann K, Thorens B. Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2001;98(20):11330‐11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Dijk TH, Grefhorst A, Oosterveer MH, et al. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr−/− mice. J Biol Chem. 2009;284(16):10315‐10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peeks F, Steunenberg TAH, de Boer F, et al. Clinical and biochemical heterogeneity between patients with glycogen storage disease type IA: the added value of CUSUM for metabolic control. J Inherit Metab Dis. 2017;40(5):695‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Derks TGJ, Oosterveer MH, De Souza CF. Next-generation glycogen storage diseases. J Inherit Metab Dis. 2018;41(6):911‐912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reijngoud DJ. Flux analysis of inborn errors of metabolism. J Inherit Metab Dis. 2018;41(3):309‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kakkis ED. The transformation of drug development for the 21st century: time for a change. Mol Genet Metab. 2022;137(1-2):107‐113. [DOI] [PubMed] [Google Scholar]
- 44. Hellerstein MK, Neese RA, Linfoot P, Christiansen M, Turner S, Letscher A. Hepatic gluconeogenic fluxes and glycogen turnover during fasting in humans. A stable isotope study. J Clin Invest. 1997;100(5):1305‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Dijk TH, van der Sluijs FH, Wiegman CH, et al. Acute inhibition of hepatic glucose-6-phosphatase does not affect gluconeogenesis but directs gluconeogenic flux toward glycogen in fasted rats. A pharmacological study with the chlorogenic acid derivative S4048. J Biol Chem. 2001;276(28):25727‐25735. doi: 10.1074/jbc.M101223200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed during the current study are available from the corresponding author on reasonable request.