Abstract
Previous reports have shown that Treponema denticola causes rearrangement of filamentous actin (F-actin) in human gingival fibroblasts (HGF). The purpose of this investigation was to determine the effect of T. denticola on the generation of inositol phosphates (IPs) in relation to a time course for F-actin disruption in HGF. Cultured HGF were exposed to washed cells of T. denticola ATCC 35405 for 140 min. Changes in the fluorescence intensity of rhodamine-phalloidin-labeled F-actin in serial optical sections of single HGF were quantified by confocal microscopy image analysis. The percentage of cells with stress fiber disruption was also determined by fluorescence microscopy. Challenge with T. denticola caused a significant reduction in F-actin within the first hour, especially at the expense of F-actin in the ventral third of the cells, and a significant increase in the percentage of HGF with altered stress fiber patterns. Significant concentration-dependent disruption of stress fibers was also caused by HGF exposure to a Triton X-100 extract of T. denticola outer membrane (OM). IPs were measured by a radiotracer assay based on the incorporation of myo-[3H]inositol into IPs in HGF incubated with LiCl to inhibit endogenous phosphatases. HGF challenge with several strains of T. denticola and the OM extract of T. denticola ATCC 35405 resulted in a diminished accumulation of radiolabeled IPs relative to both 15 and 1% fetal bovine serum, which served as strongly positive and background control agonists, respectively. The significantly diminished IP response to T. denticola ATCC 35405 occurred within 60 min, concomitant with significant reduction of total F-actin and disruption of stress fibers. Pretreatment with the proteinase inhibitor phenylmethylsulfonyl fluoride, which had previously been found to block T. denticola’s degradation of endogenous fibronectin and detachment of HGF from the extracellular matrix, had little effect on F-actin stress fiber disruption and the IP response. Therefore, in addition to its major surface chymotrypsin-like properties, T. denticola expresses cytopathogenic activities that diminish the generation of IPs during the time course associated with significant cytoskeletal disruption in fibroblasts.
Treponemes are usually among the most prevalent bacteria in the microbiota of periodontal pockets adjacent to inflamed and progressively deteriorating tissues. Treponema denticola, which is the most thoroughly characterized oral spirochete, produces several types of factors that may contribute to its virulence, including outer sheath-associated peptidases, chymotrypsin-like and trypsin-like proteinases, hemolytic and hemagglutinating activity, adhesins that bind to a variety of matrix proteins and host cells, and an outer sheath protein with pore-forming activity (6, 8, 13, 19, 20, 21, 27, 28, 29, 30, 45). Within the subgingival periodontal environment, T. denticola and other treponemes are most often concentrated among motile species at the mucosal interface. They have access for direct contact with sulcular and junctional epithelium, and they have been documented to penetrate the gingival connective tissue in acute disease (26, 34). Thus, the treponemes, their metabolites, and the constituents of their shedding outer sheath have the opportunity for direct interactions with cellular membranes of stromal cells as well as inflammatory cells in the gingival tissues.
We and others have documented cytopathic effects of T. denticola on the cytoskeleton of cultured gingival fibroblasts and epithelial cells (2, 11, 14, 43). Although T. denticola rarely invades intracellularly and is not immediately cytotoxic, its adhesion to host cells perturbs filamentous actin (F-actin), causing its rearrangement and the downstream effects of cellular rounding, membrane blebbing, and subsequent detachment from the substratum (2, 44). These profound effects on the cytoskeleton of gingival fibroblasts would be expected to alter their locomotion and phagocytosis of collagen, which are actin-dependent functions important for physiological tissue remodelling and periodontal wound repair. Cytopathic activities of T. denticola are also associated with perturbation of cytoskeletal functions such as volume regulation, maintenance of cell-cell junctional integrity, and barrier function of cultured oral epithelial cells (11, 43). As physiological actin turnover depends on complex transmembrane and intracellular communication pathways, our laboratory set out to determine the upstream signalling events crucial for the cytopathological perturbation of F-actin by T. denticola.
Homeostasis of actin in mammalian cells is known to depend on the local concentrations of polyphosphoinositides and cytosolic calcium (23, 41). The relationship of Ca2+ transients to phosphoinositides is linked further in that metabolites of the inositol phosphate (IP) pathway of phospholipase C-catalyzed phosphatidylinositol-4,5-bisphosphate metabolism, especially inositol-1,4,5-trisphosphate (IP3), stimulate release of Ca2+ from intracellular stores (4, 10, 33). Some pathogenic bacteria which parasitize humans have evolved virulence factors to exploit these host cell signalling pathways (16, 39). For example, enteroinvasive and diarrheagenic pathogens have been shown to stimulate increases in intracellular IPs and Ca2+ concomitant with accumulation of F-actin and associated cytoskeletal proteins adjacent to their point of adhesion to the host cell membrane or surrounding bacteria which are internalized (3, 12, 17, 18, 25, 35, 38). In contrast, contact with the periodontal pathogen T. denticola apparently leads to a decrease of F-actin in cultured fibroblasts and epithelial cells, and neither the adherent bacteria nor the infrequent bacteria which have been detected in an intracellular position have been found in conjunction with localized accumulations of specific host cell cytoskeletal proteins (11, 14, 43). The purpose of this investigation was to determine the effect of T. denticola on cellular accumulation of IPs, the calcium-mobilizing arm of the cytoskeleton-regulating phosphoinositide signalling pathway, in human gingival fibroblasts (HGF). We have also conducted a more precisely timed determination of localized F-actin depolymerization than we reported previously (2), so that changes in IPs and other second messengers may be related temporally to T. denticola perturbation of actin.
MATERIALS AND METHODS
Treponemal cultures and cultural conditions.
Stock cultures of T. denticola ATCC 35405 (type strain), originally provided by W. J. Loesche, University of Michigan, and ATCC 35404, ATCC 33520, e, and e′, provided by E. C. S. Chan, McGill University, were maintained and grown for experiments in a complex spirochete broth medium containing brain heart infusion, tryptic peptone, yeast extract, and volatile fatty acids and supplemented with 2.0% rabbit serum as previously described (7). Strains ATCC 35405, e, and e′ have been shown to induce actin rearrangement and detachment of cultured HGF from the substratum (2). Treponema vincentii ATCC 35580 and Treponema socranskii ATCC 35536 were obtained from the American Type Culture Collection and maintained similarly. Cells were grown at 37°C and subcultured weekly in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, Mich.) in an atmosphere of 80% N2, 10% CO2, and 10% H2 (Canox, Toronto, Ontario, Canada). For experiments in which bacterial suspensions were used to challenge gingival fibroblasts, 3-day, stationary-phase cultures were harvested by centrifugation at 12,000 × g for 6 min and washed twice in 0.01 M phosphate-buffered saline at pH 7.2 (PBS) prior to resuspension in a CO2-independent tissue culture medium.
T. denticola OM preparation.
Spirochete medium was inoculated with a 3-day culture of T. denticola ATCC 35405 at a ratio of 30:1 (fresh medium to inoculum) and incubated at 37°C for 4 days. Bacteria were harvested by centrifugation at 12,000 × g for 15 min at 4°C and washed twice in PBS. The pellet was weighed, dispersed uniformly, and resuspended at 1.0 g (wet weight) per 10 ml of PBS containing 10 mM MgCl2. A modification of the detergent extraction method of Penn et al. (5, 31) was used for the initial extraction. Triton X-100 (Surfact-Amps X-100; Pierce, Rockford, Ill.) was added to a final concentration of 0.2% (vol/vol). The suspension was incubated with constant mixing at 37°C for 30 min and then repeatedly centrifuged at 12,000 × g for 6 min until no visible pellet remained. The clear supernatant was dialyzed (molecular mass cutoff, 50 kDa; Spectra/Por, Spectrum, Houston, Tex.) against deionized H2O at 4°C for several days until precipitates formed. The contents of the dialysis tubing were centrifuged at 25,000 × g for 45 min. The pellet was resuspended in deionized H2O to the predialysis volume and stored at −70°C. The dry weight of a lyophilized aliquot of the outer membrane (OM) extract was determined so that the actin-perturbing activity of the extract could be compared with that of whole T. denticola cells on a dry weight basis. Aliquots were also run by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12% acrylamide gel and silver stain to compare the migration of OM polypeptides qualitatively with migration patterns of polypeptides from whole cells that had been boiled in SDS for 10 min.
T. denticola cell suspensions and the OM extract were tested for peptidase activity by using the chromogenic peptides N-succinyl-l-alanyl-l-alanyl-l-prolyl-l-phenylalanine-p-nitroanilide (SAAPNA) and N-benzoyl-dl-arginine-p-nitroanilide (BAPNA; Sigma) (15). Whole T. denticola cells at the concentration used for the assays described below had the equivalent SAAPNA-degrading activity of 0.2 μg of chymotrypsin per ml and BAPNA-degrading activity of 0.04 μg of trypsin per ml. The undiluted OM extract contained SAAPNA-degrading activity equivalent to 4.0 μg of chymotrypsin per ml and no detectable BAPNA-degrading activity.
Gingival fibroblast cultures.
HGF cell cultures were established from primary human tissue explants as described previously (1). The cells were cultured in alpha minimal essential medium (αMEM) containing 400 U of penicillin G per ml and 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; BioWhittaker, Walkersville, Md.) and maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. The fibroblasts were subcultured weekly and were used for experiments between passages 14 and 25.
Bacterial perturbation of F-actin in HGF.
Two assays were used in time course experiments to measure the degree of actin perturbation in HGF challenged by type strain ATCC 35405 whole cell or OM extract suspensions.
(i) Stress fiber assay.
HGF in confluent monolayers were trypsinized, washed in PBS, and adjusted to a density of 105 cells/ml in αMEM containing penicillin G and FBS. The cell suspensions were distributed at 1.0 ml/well in 24-well dishes (Corning Glass Works), each containing one sterilized, circular glass coverslip (no. 1, 12-mm diameter; Fisher Scientific). The HGF were incubated at 37°C in a 5% CO2 atmosphere for 3 h to allow spreading, and they were nonconfluent for the bacterial challenge experiments. Prior to the addition of bacteria, each well was washed once with CO2-independent medium (CIM; Gibco). The treponemes were washed, resuspended in serum-free CIM to a cell density of 2 × 109 bacteria/ml, and added to the wells containing the coverslips at 1.0 ml/well in duplicate. Control wells received CIM without bacteria. The dishes were incubated at 37°C. In a time course experiment of 140 min, coverslips were selected at 20-min intervals and the HGF were fixed in 1.0 ml of 3.75% (vol/vol) formaldehyde in PBS. The coverslips were then washed with PBS, and the HGF were permeabilized and labeled for F-actin.
The permeabilizing and labeling solution contained rhodamine-phalloidin (Molecular Probes, Eugene, Oreg.), 0.6 U/ml in 0.1% (vol/vol) Nonidet P-40 (Sigma) in PBS. Five hundred microliters was added per well, and the dishes were incubated at room temperature for 30 min. The coverslips were washed twice in deionized water and mounted on glass slides with an antifade mounting medium containing 0.1% (wt/vol) p-phenylenediamine, 10% (vol/vol) 0.01 M PBS (pH 7.4), and 90% (vol/vol) glycerol, adjusted to pH 8.0 with 0.5 M carbonate-bicarbonate buffer. Single HGF were examined by fluorescence microscopy at a magnification of ×400, using a Leitz Dialux microscope equipped with a Leitz numerical-aperture 1.30, 160/0.17 objective lens, and epifluorescence with an N2 filter block (excitation filter, 530 to 560 nm; suppression filter, 580 nm) for red fluorescence. Two hundred HGF on duplicate coverslips were scored dichotomously according to preset criteria for presenting either a normal or altered stress fiber pattern, and the outcome was expressed as the percentage of cells with altered stress fibers. HGF scored as normal had stress fibers that were abundant in quantity, evenly and brightly fluorescent, long, mostly straight, and detected in well-spread cells. In contrast, HGF scored as altered had stress fibers that were reduced in quantity or absent entirely, fragmented and unevenly fluorescent, and found in cells with retraction of cytoplasmic processes. The samples were coded to obscure their identity. Statistical significance between experimental and control groups at each time point and within-group differences between time points was determined by chi-square analysis.
(ii) Confocal microscopy.
The degree of fluorescence in optical sections of control and T. denticola ATCC 35405-challenged HGF was measured by confocal microscopy. The HGF were prepared as for the stress fiber assay. The confocal laser scanning microscope (Leica Lasertechnik GmbH, Heidelberg, Germany) was set with an argon ion laser of 488/514-nm emission, BP568 laser filter, RSP 488/568 beam splitter, pinhole setting at 40, and offset at −30. Fluorescence intensity of rhodamine-phalloidin-labeled F-actin was recorded in serial 1-μm optical sections in the x-y plane at a magnification of ×400 from the dorsal to the ventral (substratum) surface. Thirty cells per group per time point were examined, and fluorescence intensity per section was recorded. The total fluorescence intensity in an individual cell was calculated as the sum of fluorescence in all its optical sections, and the mean fluorescence per cell was derived for the 30 cells counted per time period per experimental group. As exposure to T. denticola caused many of the HGF to undergo progressive changes in shape, including cellular rounding and increased height, the number of optical sections for complete coverage of each single cell was determined, and the sections were categorized into thirds by cell height. The mean fluorescence per ventral, middle, and dorsal third for the 30 cells per group was calculated. For statistical analysis, significant differences in mean fluorescence between experimental groups at each time period and within-group differences at various time periods were determined by Student’s t test.
A comparison of whole T. denticola cells and OM extract for actin-perturbing activity was determined in 90-min assays using the stress fiber assay. The effect of inhibiting the proteolytic activity of the bacteria and OM extracts was also tested. They were pretreated with phenylmethylsulfonyl fluoride (PMSF) at 170 μg/ml, which we had shown previously to inhibit T. denticola’s fibronectin-degrading activity and detachment of HGF from the extracellular matrix (15).
IP assay.
IPs generated during the experimental time course were measured by a modification of the radiotracer assays of Ruschkowski et al. (38) and Dean and Beaven (9). HGF in confluent monolayers were harvested by trypsinization and resuspended in αMEM with 10% FBS to a density of 2 × 105 cells/ml. The cell suspensions were dispensed (2.5 ml in 60- by 15-mm petri dishes [Falcon]) and incubated at 37°C to allow spreading. After 2 h, each petri dish was washed twice with warm PBS, and 2.0 ml of αMEM containing penicillin G and 1% (vol/vol) FBS was added. One hundred microliters of 1/10-concentrated myo-[3H]inositol (1.0 mCi/ml; Amersham, Arlington Heights, Ill.) in 10% (vol/vol) ethanol was added to each dish. The dishes were incubated for at least 18 h, the culture fluids were removed, and the dishes were washed once with warm serum-free CIM. Two milliliters of CIM containing 1.0% (vol/vol) FBS and 10 mM LiCl was added, and the dishes were incubated for 15 min at 37°C in air. The LiCl inhibits endogenous phosphatases and thereby allows the accumulation of 3H-labeled intermediates in the IP pathway.
Three-day cultures of treponemes were harvested by centrifugation, washed once in CIM, and resuspended in CIM containing 1.0% (vol/vol) FBS and 10 mM LiCl to a density of 2 × 109 bacteria/ml. Three milliliters of the bacterial suspension was added to each culture dish in the experimental groups. Positive control dishes, for which substantial increases in accumulated 3H-labeled IP intermediates would be anticipated received 3.0 ml of CIM containing 15% (vol/vol) FBS and LiCl, and the background control dishes received 3.0 ml of CIM containing 1.0% (vol/vol) FBS and LiCl as specified by Ruschkowski et al. (38). In some experiments ATP, 100 μM in CIM containing 1.0% FBS, was used as the positive agonist.
Duplicate dishes per treatment group were analyzed for total IPs immediately and following a 60-min period. The reactions were terminated by removal of the medium and one wash with ice-cold PBS. Cells in each dish were harvested into 1.0 ml of ice-cold PBS and collected into screw-capped polypropylene tubes. Four milliliters methanol-chloroform (2:1) solution was added to each tube, mixed, and allowed to react at ambient temperature for at least 30 min. Then a mixture of 0.125 ml of 0.5 M EDTA (pH 8.0), 1.2 ml of chloroform, and 1.0 ml of deionized H2O was added. The mixtures were vortexed and then centrifuged at 500 × g for 8 min. The aqueous phase, which contained the water-soluble IPs, was collected and passed through a Dowex AG 1-X8 anion-exchange resin column (formate form resin and Poly-Prep chromatography columns; Bio-Rad) followed by a wash of 5.0 ml of deionized H2O. The IPs were eluted with a 5.0-ml solution containing 0.1 M formic acid and 1.0 M ammonium formate. A 300-μl aliquot of each sample was added to 5.0 ml of scintillation fluid (Ecolume; ICN, Costa Mesa, Calif.), and the radioactivity (counts/minute) was measured in the tritium channel (RackBeta scintillation counter; LKB-Wallac).
Procedural controls included (i) omission of the LiCl, which would be expected to reduce the amount of accumulated IPs, and (ii) testing the effects of 0.05% ethanol, the solvent for the myo-inositol, in the IP assay. HGF incubated as usual with either 1.0 or 15% FBS and with 10.0 mM LiCl led to an increase in radiolabeled IPs of 125 and 240%, respectively, over 60 min. In contrast, parallel samples for which LiCl was deleted yielded negligible radiolabeled IPs at both the beginning of the assay (10 and 14% of the 1.0% FBS–LiCl and 15% FBS–LiCl controls, respectively) and at 60 min (6 and 7% of the controls). These results verified that incubation of HGF with LiCl indeed allowed the accumulation of radiolabeled inositols. Ethanol at its assay concentration led to an insignificant change in accumulated IPs over 60 min.
IP assays were also run with T. denticola cells pretreated with either PMSF or N-α-p-tosyl-l-lysine chloromethyl ketone (TLCK; Sigma) at 150 μg/ml, as described previously (2). At least two independent experiments were conducted for each condition tested.
For IP assays in which OM extract of T. denticola ATCC 35405 was substituted for the whole bacteria, the OM extract was serially diluted 1/4 to 1/16 in CIM containing 1.0% FBS, and 3.0 ml of the suspension was added to each dish. The effect of pretreatment of the OM extract with PMSF was also determined.
RESULTS
Time course for F-actin depolymerization.
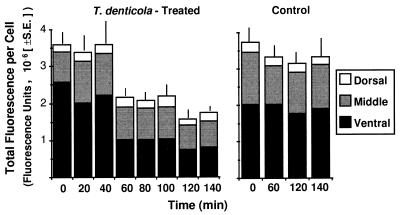
Rhodamine-phalloidin fluorescence intensity was used as a measure of F-actin concentration in optical sections analyzed by confocal microscopy. The mean fluorescence per cell was significantly less for T. denticola ATCC 35405-challenged HGF than for control HGF by 60 min and all subsequent time periods ([2.15 ± 0.24] × 106 [mean ± standard error of mean] for T. denticola and [3.32 ± 0.34] × 106 for control at 60 min; P < 0.01 [Fig. 1]). To analyze changes in rhodamine-phalloidin fluorescence in optical sections by confocal microscopy, the HGF were arbitrarily divided into thirds from their ventral to their dorsal surface. The mean fluorescence intensity, representing the mean concentration of F-actin, was greatest in the ventral third of the cells (Fig. 1), where the cytoskeleton interfaces via integral membrane proteins with the extracellular substratum. At the beginning of the experiment, a mean of 71.6% of the total cellular fluorescence was attributable to the ventral, 22.7% was attributable to the middle, and 5.7% was attributable to the dorsal one-third of optical sections for the T. denticola-challenged HGF (Fig. 1). Over the time course of exposure to T. denticola ATCC 35405, total cellular fluorescence decreased by 52% (P < 0.001), and the greatest proportional change came at the expense of F-actin in the ventral one-third, where the fluorescence intensity decreased by 34% from the initial level. In contrast, neither the mean total fluorescence intensity per cell nor the proportional distribution of fluorescence among grouped optical sections of control HGF changed significantly during the time course.
FIG. 1.
Histogram of fluorescence intensity of rhodamine-phalloidin-labeled F-actin in HGF during a 140-min time course, analyzed by confocal microscopy. There was significant depolymerization of F-actin in the T. denticola ATCC 35405-challenged HGF (left graph) in comparison with the control HGF (right graph). The degree of diminished fluorescence in T. denticola-challenged cells was significant at 60 min (P < 0.01). Much of the decrease was proportionately at the expense of F-actin in the ventral third of the cells (black).
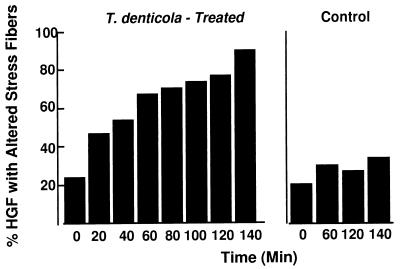
Changes in whole-cell F-actin determined by the frequency of HGF with altered stress fiber patterns and by fluorescence intensity measured in optical sections by confocal microscopy were in close agreement. Normal stress fiber pattern and distribution were increasingly altered with time in T. denticola ATCC 35405-challenged HGF (Fig. 2). By 140 min, 90% of the HGF were found to have an altered stress fiber pattern, compared with 35% for the control HGF. A significant difference between the frequency of altered stress fibers in the T. denticola-challenged and control HGF was reached by 60 min (P < 0.001). Regression analysis was used to determine the correlation between data sets when changes in actin were determined by calculating the mean fluorescence per cell detected by confocal microscopy and by calculating the mean percentage of cells with disrupted stress fibers by the dichotomous fluorescence microscopy assay at six points in the time course. The correlation coefficient for the two outcomes (labeled F-actin per cell versus percentage of cells with abnormal stress fibers) was −0.89 (P < 0.01).
FIG. 2.
Histogram illustrating disruption of F-actin in T. denticola-treated HGF expressed as the percentage of HGF with an altered stress fiber pattern (left graph) compared with the stable stress fiber pattern in control HGF (right graph). The difference between T. denticola-challenged and control HGF was significant at 60 min (P < 0.001). These data were from the same set of 30 cells analyzed by confocal microscopy in Fig. 1, illustrating the inverse relationship of total F-actin measured by confocal microscopy and percentage of HGF with altered stress fibers. Replicate experiments with 200 HGF per group yielded almost identical distribution of data (not shown).
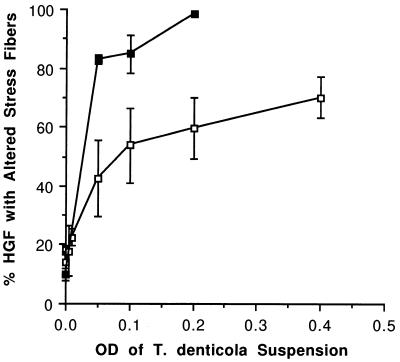
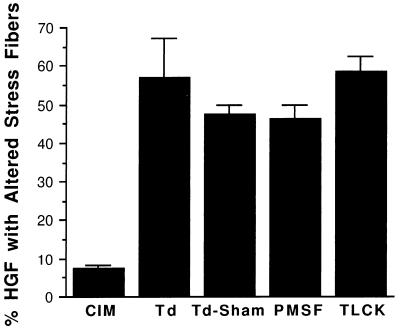
The dichotomous stress fiber assay was also used to compare the degree of cytoskeletal disruption by whole cells and OM extract of T. denticola ATCC 35405. The percentage of HGF with altered stress fibers increased with increasing concentration of both bacterial cells and OM extract (Fig. 3). The effect of the OM extract was greater on a dry weight basis. T. denticola cells were pretreated with proteinase inhibitors PMSF and TLCK to determine whether cell-associated proteinases could account for stress fiber disruption. Neither inhibitor reduced the percentage of HGF with altered stress fibers compared with untreated control and sham-treated (centrifuged) T. denticola cells (Fig. 4).
FIG. 3.
Comparison of filamentous actin disruption (mean ± standard deviation) in HGF challenged with different concentrations of whole cells of T. denticola ATCC 35405 (□) and OM extract (▪). The dry weight for the OM and the dry weight for the T. denticola cells were equivalent at each optical density (OD) point shown on the x axis.
FIG. 4.
Histogram of data (mean ± standard deviation) examining the effects of proteinase inhibitors on the disruption of stress fibers by T. denticola. Disruption of filamentous actin in T. denticola-treated HGF was not affected by pretreatment with PMSF or TLCK. CIM, HGF challenged with bacterium-free CIM; Td, HGF challenged with T. denticola ATCC 35405; Td-Sham, HGF challenged with T. denticola cells incubated in proteinase inhibitor-free buffer and centrifuged identically to samples containing PMSF and TLCK, which is the appropriate control for the inhibitor-containing samples.
IPs in HGF challenged with T. denticola cells.
The effect of T. denticola ATCC 35405 cells on IPs recovered in the extracts of HGF was measured over 60 min relative to the effect of a background (1.0% FBS) and a strongly positive control (15% FBS). Any manipulation of LiCl-treated HGF, even just by washing them, caused an increase in accumulated IPs. Values for IPs recovered from HGF at the first experimental sample varied, and IPs from HGF incubated with the 1.0% FBS background control increased with time. Therefore, following the procedure of Ruschkowski et al. (38), we adopted the 1.0% FBS control for the standard comparison. Considering data from independent experiments which included T. denticola-treated, 1.0% FBS standard, and 15% FBS positive control groups (n = 11), the increase in radiolabeled IP for the 1.0% FBS background control was 212.3% ± 14.4% (mean ± standard error of mean; range, 107.8 to 287.6%; P < 0.01 comparing the 60-min sample with the initial sample). Challenge by T. denticola diminished IP accumulation as evidenced by the consistently lower and insignificant percent increase in concentration of radiolabeled IPs in T. denticola-treated HGF in the presence of 1.0% FBS (mean, 143.1% ± 13.9%; range, 64.7 to 210.4%; P > 0.05 comparing 60-min and initial samples) (Tables 1 and 2). The increase in 3H-labeled IPs for 15% FBS was usually severalfold greater on each assay occasion (mean, 416.3% ± 49.1%; range, 245.5 to 837.9%; P < 0.001 comparing 60-min and initial samples). By 60 min, the mean IP concentrations for the 15 and 1.0% FBS controls were significantly different from one another (P < 0.01), showing that the IP pathway of the HGF would indeed respond maximally to a strongly positive agonist. Although initially similar (P > 0.10), the mean IP concentration in the T. denticola-treated HGF was significantly lower than that for both FBS controls by 60 min (P < 0.02).
TABLE 1.
IP response to T. denticola ATCC 35405 and OM extract
| Assay sample | Relative cpm in [3H]IPsa
|
% Change, 0–60 min | |
|---|---|---|---|
| 0 min | 60 min | ||
| 1% FBS (bacterium-free control) | 100 | 254 | +154 |
| +Whole 35405 | 94 | 169 | +79 |
| +Whole 35405/PMSFc | 120 | 170 | +42 |
| +Whole 35405/TLCKc | 100 | 177 | +77 |
| 15% FBS (bacterium-free control) | 123 | 459 | +273 |
| 1% FBS + ATP (bacterium-free control)b | 100 | 382 | +282 |
| +Whole 35405 | 132 | 347 | +163 |
| 1% FBS (OM-free control) | 100 | 161 | +61 |
| +1/4 OMd | 117 | 119 | +2 |
| +1/4 OM/PMSFc | 81 | 92 | +11 |
| +1/8 OM | 96 | 128 | +33 |
| +1/8 OM/PMSF | 75 | 94 | +25 |
| +1/16 OM | 113 | 209 | +85 |
| +1/16 OM/PMSF | 94 | 207 | +120 |
| 15% FBS (OM-free control) | 100 | 307 | +207 |
Value relative to 1.0% FBS–LiCl background control at beginning of experiment, time 0 min (100%); average of duplicates.
HGF were pretreated with T. denticola or untreated (control) for 30 min prior to addition of ATP. Data are for 0 and 60 min after addition of ATP.
T. denticola cells or OM extract were pretreated with PMSF at either 170 μg/ml to inhibit chymotrypsin-like or TLCK at 150 μg/ml to inhibit trypsin-like proteinase activity.
1/4 dilution of T. denticola ATCC 35405 OM extract.
TABLE 2.
Effect of various strains of treponemes on inositol phosphates in HGF
| Assay sample | Relative cpm in [3H]IPsa
|
% Change, 0–60 min | |
|---|---|---|---|
| 0 min | 60 min | ||
| 1% FBS (bacterium-free control) | 100 | 290 | +190 |
| +T. denticola | |||
| ATCC 35405 | 140 | 200 | +43 |
| ATCC 35404 | 115 | 182 | +58 |
| ATCC 33520 | 85 | 108 | +27 |
| e′ | 121 | 196 | +62 |
| e | 108 | 272 | +152 |
| +T. vincentii 35580 | 130 | 174 | +34 |
| +T. socranskii 35536 | 126 | 223 | +77 |
| 15% FBS (bacterium-free control) | 128 | 901 | +690 |
Value relative to 1.0% FBS–LiCl control at beginning of experiment, time 0 min (100%); average of duplicates. For example, the mean value of the 1.0% FBS control at 0 min, 589 cpm, rose to 1,707 cpm at 60 min. The 15% FBS control value at 0 min, 753 cpm, rose much more, to 5,307 cpm at 60 min. For HGF treated with T. denticola 35405, the value at 0 min, 826 cpm, rose less to 1,178 cpm at 60 min.
Attempts to determine whether T. denticola could suppress the great increase in IPs in response to 15% FBS yielded inconsistent results. As a high concentration of serum contains a complex of potentially confounding agonists for stimulating the IP pathway, we tested the effect of T. denticola ATCC 35405 on the response of HGF to ATP in 1.0% FBS. ATP is known to cause increased IP reactions, and our concurrent study found that T. denticola OM decreased Ca2+ responses of HGF to exogenous ATP (24). ATP stimulated a 320% increase in radiolabeled IPs over 60 min (average of duplicates). When T. denticola and ATP were added simultaneously, there was a small reduction seen as a 236% increase in the accumulation of IPs (data not shown). Preincubation of HGF with T. denticola for 30 min prior to addition of ATP (90-min experiment) led to a 60-min increase in IPs of 163%, compared with 282% for ATP alone (Table 1; average of quadruplicates), an approximately 40% reduction in IP response.
IPs in HGF challenged with OM extract.
The OM extract prepared from T. denticola ATCC 35405 contained several polypeptides comparable in size to those of a boiled extract of the whole bacteria (Fig. 5). The OM extract yielded a concentration-dependent reduction in labeled IPs relative to the 1.0% FBS background control (Table 1). Only at 1/16 dilution was the low level increase in radiolabeled IPs for the OM extract plus 1.0% FBS comparable to that for the 1.0% FBS alone. Whole cells of T. denticola ATCC 35405 and the OM extract were pretreated with PMSF or TLCK prior to the assay to determine their effects on diminished IP generation. Neither proteinase inhibitor had an effect on the reduction in the yield of radiolabeled IPs when HGF were incubated with 1.0% FBS and whole cells of T. denticola ATCC 35405 (Table 1). Likewise, there was little difference in the relative concentrations of radiolabeled IPs between HGF exposed to 1.0% FBS and PMSF-pretreated or untreated OM extract (Table 1).
FIG. 5.
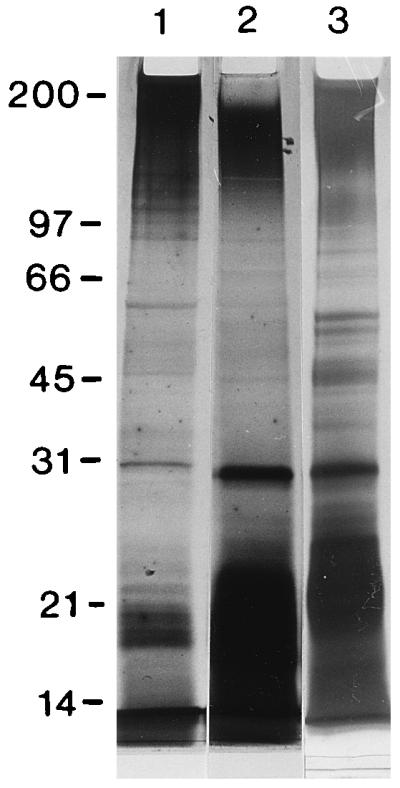
Qualitative comparison by SDS-PAGE of T. denticola ATCC 35405 whole cells (lane 1), OM extract run at the same time (lane 2), and repeat of the OM extract run in a different gel to resolve mid-range bands more clearly (lane 3). The positions of molecular size standards (broad-range SDS-PAGE standards; Bio-Rad) are shown in kilodaltons at the left. Conditions were as follows: Washed T. denticola suspensions (optical density of 1.2 at 550 nm) from 3-day cultures or undiluted OM extract were mixed 1:1 with sample buffer (0.25 M Tris-HCl [pH 6.8], 20% glycerol, 4.6% SDS, 0.02% bromophenol blue, 10% β-mercaptoethanol) and boiled for 10 min; 25 μl was added to each lane of a 1-mm-thick Ready Gel (Bio-Rad) with a 4% stacking gel and 12% resolving gel. Electrophoresis was run in a Mini-Protean II cell (Bio-Rad). Whole cells and OM extract had several bands in common.
IPs in HGF challenged with additional strains of T. denticola and other treponemes.
Additional strains of T. denticola and two strains of other oral treponemes were tested to determine whether the IP results could be generalized beyond ATCC 35405. The percent increase in 3H-labeled IPs over the 60-min incubation period was lower for all samples containing either T. denticola, T. vincentii, or T. socranskii cells and 1.0% FBS than that found for the bacterium-free 1.0% FBS control (Table 2). At 60 min, the total pool of radiolabeled IPs was approximately three- to fourfold higher in HGF treated with the 15% FBS control in these experiments, showing that different strains of T. denticola are clearly unable to stimulate the IP pathway and that the diminished IP response is not limited to this species.
DISCUSSION
Upon exposure to T. denticola strains, cultured HGF undergo profound actin rearrangement and perturbation of some related signalling pathways prior to and concomitant with the downstream effects of cell rounding and detachment from the extracellular matrix. By establishing a time course for stress fiber disruption and localization of F-actin depolymerization, this investigation provides the opportunity to relate fluctuations in some second messengers to actin rearrangement, at least temporally. The use of confocal microscopy to quantify fluorescence intensity in optical sections of individual HGF detected a significant decrease in whole cell F-actin and a temporally related proportional shift in F-actin from the ventral third to the middle, presumably perinuclear third, of the cells. The decrease in ventral third actin is consistent with our finding perturbed stress fibers in nearly all the HGF by the end of the assay period, and it probably accounts for the strong agreement between the two assays which we have used to quantify outcomes of actin integrity. The quantitative method used in a previous study from our laboratory had actually characterized the actin rearrangement as an initial increase in total fluorescence (2). By using microscopic spectrofluorimetry to measure emissions in a fixed area of an HGF monolayer rather than in optical sections of single cells, the substantial diminution of fluorescence intensity in the cell periphery was probably masked by the more intense emissions of bright fluorescence that condensed around the nuclei of cells in the selected window (2). In the present study, diminished IP yield, as reflected in the inability of T. denticola-challenged HGF to respond even to the level of the background 1.0% FBS-stimulated HGF, was apparent concomitant with the detection of significant differences in total F-actin and stress fiber integrity between control and T. denticola-treated cells. Both actin and IPs were affected significantly within 60 min of challenge. Thus, some of the inhibitory effects of T. denticola on the IP response probably preceded the more downstream rearrangement of cytoskeletal proteins that normally maintain cellular structure, and they certainly preceded significant detachment of HGF from the substratum, which we had found to occur after the first hour (2).
IPs, calcium, and actin assembly.
Phosphoinositides and their products of catalysis by phospholipase C, the IPs, are significant for maintaining homeostasis of cortical F-actin near the plasma membrane of fibroblasts and other mammalian cells (23, 41). A locally elevated concentration of phosphatidylinositol-4,5-bisphosphate, the precursor of IP3, is thought to promote the uncapping of the barbed ends of actin filaments and the release of actin monomers from actin-sequestering proteins, which would favor F-actin assembly (41). Exposure of HGF to T. denticola caused depolymerization of the F-actin network and disruption of stress fibers. Thus, the concomitant, diminished activity in the IP pathway that we observed may be a reflection of either (i) bacterial inhibition of the normal recycling and phosphorylation of polyphosphoinositides, leading to impaired F-actin polymerization or its actual depolymerization, (ii) lack of or altered agonists for phospholipase C receptor activation, or (iii) bacterial inhibition of phospholipase C activity. Although this investigation did not address these mechanisms, our finding of low IP yields within 1 h and even more rapid effects on calcium transients (24) suggests that T. denticola OM components act directly at the external surface of the HGF plasma membrane. Since T. denticola ATCC 35405, ATCC 35404, and ATCC 33520 are known to produce their own phospholipase C (40), it is evident from our data that their potential exogenous catalysis of membrane polyphosphoinositides is not realized under conditions of treponemal challenge in intact HGF.
Cytoskeletal disruption and a weak IP response to T. denticola are activities apparently opposite those reported for several pathogenic enteric species. Some enteropathogens stimulate F-actin polymerization as a critical step in signalling their own uptake into host cells, and others stimulate the accumulation of F-actin immediately adjacent to the area of intimate adhesion to membrane receptors on host cells (12, 16, 17, 35). Increases in both total IPs (18, 38) and specifically IP3 (12) follow exposure of epithelial cells to enteric pathogens. The method used by us to quantify IPs in comparison to a 1.0% FBS background control was the same as that used to demonstrate increased IP fluxes associated with host cell invasion by Salmonella typhimurium (38). Therefore, our affirmation that T. denticola either suppressed or at least did not stimulate increases in IP concentrations in the period leading up to F-actin disruption was not unexpected. Our finding a reduced but still rather robust IP response in ATP-stimulated HGF that had been pretreated with T. denticola suggests that T. denticola does not totally block crucial steps in the pathway.
Elevated intracellular calcium ion concentration [Ca2+]i generally opposes the effects of polyphosphoinositides on actin assembly by promoting the capping of actin’s barbed ends and activating actin-severing proteins, which would tend to promote actin depolymerization (23, 41). We have recently found that T. denticola OM causes an immediate but short-lived increase in the frequency of spontaneous calcium oscillations (24), corresponding to a time early in the period when IP yields are apparently low. This combination of early effects would be consistent with the concept that a bacterially induced imbalance in second messengers established a permissive intracellular environment for the disruption of F-actin in stress fibers and the generalized decrease in phalloidin-stained F-actin in the cell. The complete blockade of calcium oscillations, which occurred later in the time course (24), would be consistent with the rearrangement of F-actin into a brightly staining perinuclear array. We stress, however, that the possible relationships between IP concentration, [Ca2+]i fluctuations, and F-actin location and integrity are limited to interpretation of temporal, not mechanistic, data. Moreover, actin assembly is thought to be sensitive to fluctuations in the concentration of signalling messengers in discrete microenvironments, and our assays for IPs and [Ca2+]i analyzed either whole cells or pools of cells.
The IP and calcium signalling pathways are interrelated. One of the major activities of IPs is to mobilize calcium from intracellular stores, leading to a transient increase in [Ca2+]i (10, 33). This relationship has been cited as an explanation for the significantly increased [Ca2+]i in host cells exposed to enteric pathogens (12, 16, 22). In contrast, exposure of HGF to OM extracts of T. denticola causes depletion of intracellular calcium, possibly by affecting calcium release-activated channels, which serve as a source of calcium replenishment for HGF (24). The OM extract significantly reduced [Ca2+]i transients in response to both thapsigargin and ATP, two reagents that mobilize calcium from internal stores (24). In addition to these effects on resting cells, OM extract greatly reduced membrane stretch-activated [Ca2+]i transients, which rely in part on calcium-activated internal calcium release in HGF (1).
Partial characterization of treponemal IP-suppressive activity.
Several components of the outer sheath of T. denticola have the potential to initiate perturbation of actin. For example, we have shown that the chymotrypsin-like activity of T. denticola degrades endogenous fibronectin on the surface of HGF and that PMSF blocks this activity as well as the downstream effect of HGF detachment from the substratum (2, 15). This enzyme also contributes to cytoskeletal rearrangement, disruption of tight junctions, and loss of barrier function in oral epithelial cells (43). Therefore, there is considerable evidence supporting the hypothesis that the major outer sheath chymotrypsin-like proteinase, originally isolated and characterized by Uitto et al. (42), may be significant for disrupting physiologic signalling pathways involving the extracellular matrix.
This study of IP responses and our concurrent investigation of calcium fluctuations in HGF (24) do not support a direct relationship of these second messengers to the chymotrypsin-like activity of treponemes. PMSF inhibited neither stress fiber disruption, the IP pathway effects (this study), nor the inhibition of mechanosensitive calcium flux by T. denticola (24). Moreover, OM extract diluted 1/16 failed to diminish the yield of IPs even though its SAAPNA-degrading activity was higher than that of T. denticola cells at the concentration which caused actin perturbation and a diminished IP response. Therefore, it is unlikely that the diminished IP response in this study was due to proteolysis of serum-containing agonists or receptors. To the contrary, these findings suggest that the immediate, direct effects on intracellular signalling pathways may be triggered by T. denticola outer sheath components independent of the proteolytic activities which contribute to degradation of extracellular matrix and cellular detachment from the substratum. Despite its inhibition of plasma membrane fibronectin degradation, PMSF did not diminish the adhesion of T. denticola cells to the HGF (15).
Perhaps one or several of T. denticola’s adhesins which are distinct from the catalytic site of the chymotrypsin-like proteinase will be found to block the generation of second messengers which regulate actin or other cytoskeletal proteins. For example, the well-studied pathogens in the genus Yersinia express a functionally conserved, outer membrane and secretory protein (Yop) cascade which is activated by contact with host cells (32, 37). YopE is a cytotoxin which blocks actin assembly upon translocation into target cells (36), and other Yops probably perturb actin by dephosphorylating proteins required for physiologic signalling (16, 32). Following this analogy, it is feasible that T. denticola proteins which impact on actin assembly in HGF include more than the OM proteins which obviously have direct actin-perturbing activity in our studies. Therefore, future studies should seek to identify individual T. denticola surface and secretory proteins which contribute to host cell contact linked with actin perturbation and to determine the specific mechanisms by which they affect actin-regulating intracellular messengers, including IPs.
ACKNOWLEDGMENTS
This study was supported by grant MT-5619, a maintenance grant for confocal microscopy, and a dental fellowship to P.F.Y. from the Medical Research Council of Canada.
We thank C. A. G. McCulloch for generous use of his laboratory facilities and for valuable discussions.
REFERENCES
- 1.Arora P D, Bibby K J, McCulloch C A G. Slow oscillations of free intracellular calcium ion concentration in human fibroblasts responding to mechanical stretch. J Cell Physiol. 1994;161:187–200. doi: 10.1002/jcp.1041610202. [DOI] [PubMed] [Google Scholar]
- 2.Baehni P, Song M, McCulloch C A G, Ellen R P. Treponema denticola induces actin rearrangement and detachment of human gingival fibroblasts. Infect Immun. 1992;60:3360–3368. doi: 10.1128/iai.60.8.3360-3368.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge M J. Inositol trisphosphates and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 5.Chan, E. C. S. Personal communication.
- 6.Chu L, Burgum A, Kolodrubetz D, Holt S C. The 46-kilodalton-hemolysin gene from Treponema denticola encodes a novel hemolysin homologous to aminotransferases. Infect Immun. 1995;63:4448–4455. doi: 10.1128/iai.63.11.4448-4455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson J R, Ellen R P. Tip-oriented binding of Treponema denticola to fibronectin. Infect Immun. 1990;58:3924–3928. doi: 10.1128/iai.58.12.3924-3928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson J R, Ellen R P. Clustering of fibronectin adhesins towards Treponema denticola tips upon contact with immobilized fibronectin. Infect Immun. 1994;62:2214–2221. doi: 10.1128/iai.62.6.2214-2221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean N M, Beaven M A. Methods for the analysis of inositol phosphates. Anal Biochem. 1989;183:199–209. doi: 10.1016/0003-2697(89)90468-5. [DOI] [PubMed] [Google Scholar]
- 10.De Camilli P, Emr S D, McPherson P S, Novick P. Phosphoinositides as regulators of membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 11.DeFilippo A, Ellen R P, McCulloch C A G. Induction of cytoskeletal rearrangements and loss of volume regulation in epithelial cells by Treponema denticola. Arch Oral Biol. 1995;40:199–207. doi: 10.1016/0003-9969(95)98809-d. [DOI] [PubMed] [Google Scholar]
- 12.Dytoc M T, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 13.Egli C, Leung W K, Müller K-H, Hancock R E W, McBride B C. Pore-forming properties of the major 53-kilodalton surface antigen from the outer sheath of Treponema denticola. Infect Immun. 1993;61:1694–1699. doi: 10.1128/iai.61.5.1694-1699.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellen R P, Dawson J R, Yang P F. Treponema denticola as a model for polar adhesion and cytopathogenicity of spirochetes. Trends Microbiol. 1994;2:114–119. doi: 10.1016/0966-842x(94)90597-5. [DOI] [PubMed] [Google Scholar]
- 15.Ellen R P, Song M, McCulloch C A G. Degradation of endogenous plasma membrane fibronectin concomitant with Treponema denticola 35405 adhesion to gingival fibroblasts. Infect Immun. 1994;62:3033–3037. doi: 10.1128/iai.62.7.3033-3037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 17.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1992;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 18.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grenier D. Characteristics of hemolytic and hemagglutinating activities of Treponema denticola. Oral Microbiol Immunol. 1991;6:246–249. doi: 10.1111/j.1399-302x.1991.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 20.Grenier D, Uitto V-J, McBride B C. Cellular location of a Treponema denticola chymotrypsin-like protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990;58:347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haapasalo M, Müller K-H, Uitto V-J, Leung W K, McBride B C. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect Immun. 1992;60:2058–2065. doi: 10.1128/iai.60.5.2058-2065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janmey P A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;55:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- 24.Ko K S-C, Glogauer M, McCulloch C A G, Ellen R P. Treponema denticola outer membrane inhibits calcium flux in gingival fibroblasts. Infect Immun. 1998;66:703–709. doi: 10.1128/iai.66.2.703-709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Listgarten M A, Lewis D W. The distribution of spirochetes in the lesion of acute necrotizing ulcerative gingivitis: an electron microscopic and statistical survey. J Periodontol. 1967;38:379–386. [Google Scholar]
- 27.Mäkinen K K, Syed S A, Mäkinen P, Loesche W J. Benzoylarginine peptidase and iminopeptidase profiles of Treponema denticola strains isolated from the human periodontal pocket. Curr Microbiol. 1986;14:85–89. [Google Scholar]
- 28.Mathers D A, Leung W K, Fenno J C, Hong Y, McBride B C. The major surface protein complex of Treponema denticola depolarizes and induces ion channels in HeLa cell membranes. Infect Immun. 1996;64:2904–2910. doi: 10.1128/iai.64.8.2904-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikx F H M, Jacobs F, Satumalay C. Cell-bound peptidase activities of Treponema denticola ATCC 33520 in continuous culture. J Gen Microbiol. 1992;138:1837–1842. doi: 10.1099/00221287-138-9-1837. [DOI] [PubMed] [Google Scholar]
- 30.Mikx F H M, Keulers R A C. Hemagglutination activity of Treponema denticola grown in serum-free medium in continuous culture. Infect Immun. 1992;60:1761–1766. doi: 10.1128/iai.60.5.1761-1766.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penn C W, Cockayne A, Bailey M J. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol. 1985;131:2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- 32.Pettersson J, Nordfelth T, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 33.Putney J W, Jr, St G, Bird J. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocrine Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- 34.Riviere G R, Weisz K S, Adams D F, Thomas D D. Pathogen-related spirochetes from dental plaque are invasive. Infect Immun. 1991;59:3357–3380. doi: 10.1128/iai.59.10.3377-3380.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 36.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yop E cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosqvist R, Håkansson S, Forsberg A, Wolf-Watz H. Functional conservation of the secretion and translocation machinery for virulence proteins of yersiniae, salmonellae, and shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruschkowski S, Rosenshine I, Finlay B B. Salmonella typhimurium induces an inositol phosphate flux in infected epithelial cells. FEMS Lett. 1992;95:121–126. doi: 10.1016/0378-1097(92)90416-l. [DOI] [PubMed] [Google Scholar]
- 39.Sansonetti P J. Genetic and molecular basis of epithelial cell invasion by Shigella species. Rev Infect Dis. 1991;13:285–292. doi: 10.1093/clinids/13.supplement_4.s285. [DOI] [PubMed] [Google Scholar]
- 40.Siboo R, Al-Joburi W, Gornitsky M, Chan E C S. Synthesis and secretion of phospholipase C by oral spirochetes. J Clin Microbiol. 1989;27:568–570. doi: 10.1128/jcm.27.3.568-570.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stossel T P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989;264:18261–18264. [PubMed] [Google Scholar]
- 42.Uitto V-J, Grenier D, Chan E C S, McBride B C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988;56:2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uitto V-J, Pan Y-M, Leung W K, Larjava H, Ellen R P, Finlay B B, McBride B C. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect Immun. 1995;63:3401–3410. doi: 10.1128/iai.63.9.3401-3410.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberg A, Holt S C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990;58:1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg A, Holt S C. Chemical and biological activities of a 64-kilodalton outer sheath protein from Treponema denticola strains. J Bacteriol. 1991;173:6935–6947. doi: 10.1128/jb.173.21.6935-6947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]