Abstract
Rapid advances in the potency, safety, and availability of modern HIV antiretroviral therapy (ART) have yielded a near-normal life expectancy for most people living with HIV (PLWH). Ironically, considering the history of HIV/AIDS (initially called “slim disease” because of associated weight loss), the latest dilemma faced by many people starting HIV therapy is weight gain and obesity, particularly Black people, women, and those who commenced treatment with advanced immunodeficiency. We review the pathophysiology and implications of weight gain among PLWH on ART and discuss why this phenomenon was recognized only recently, despite the availability of effective therapy for nearly 30 years. We comprehensively explore the theories of the causes, from initial speculation that weight gain was simply a return to health for people recovering from wasting to comparative effects of newer regimens vs prior toxic agents, to direct effects of agents on mitochondrial function. We then discuss the implications of weight gain on modern ART, particularly concomitant effects on lipids, glucose metabolism, and inflammatory markers. Finally, we discuss intervention options for PLWH and obesity, from the limitations of switching ART regimens or specific agents within regimens, weight-gain mitigation strategies, and potential hope in access to emerging antiobesity agents, which are yet to be evaluated in this population.
Keywords: weight gain, obesity, antiretroviral treatment, HIV, metabolic consequences
Modern antiretroviral therapy (ART) has been one of the most remarkable and rapid medical evolutions of the last 30 years. People living with HIV (PLWH) enjoy a life expectancy approaching normal because of marked improvements in the potency and ease of use of ART, where HIV routinely caused progressive morbidity and eventual death from AIDS in the vast majority of people prior to the discovery of effective combination therapy (1, 2). Current first-line therapy involves a combination of 3 (or occasionally 2) antiretrovirals (ARVs) in a single, well-tolerated coformulated tablet that routinely completely suppresses viral replication in adherent patients, usually in a matter of weeks (3-5). ARV resistance to newer agents, a common problem with even slight lapses in adherence to older regimens, is now very unusual. Current ART has largely achieved its intended goals of providing highly tolerable, easily administered drugs that quickly and persistently suppress the HIV virus and allow a rapid return to health for PLWH.
Increasing attention is now being paid to chronic complications observed among people taking lifelong ART. Chief among them is excessive weight gain and resulting obesity (6). Notably, the degree of weight gain that has been demonstrated for a high proportion of PLWH on modern ART regimens seems out of proportion to but enhanced by a concomitant worldwide obesity epidemic (7-9). Although the mechanisms that cause weight gain in this population are not fully resolved, we discuss the current leading hypotheses, including an expected “return to health” where the inflammation-induced metabolic demand is reversed with virological control of HIV, medication-specific weight gain–mitigating or enhancing effects, and immunologic and microbiome-mediated weight changes caused by HIV infection itself (2-4). We also briefly review the development of ART regimens, which therapies are most tightly linked to weight gain, and discuss risk factors for weight gain among PLWH on ART, including female sex, Black race, advanced HIV disease, and older age, as well as their implications for the global HIV epidemic.
We explore the implications of weight gain among PLWH on ART. Although obesity is associated with a myriad of well-documented long-term medical complications, there are limited data yet supporting these same associations among PLWH having treatment-emergent weight gain, mainly as the association has been recognized only recently and follow-up has been limited. Despite these limitations, recent studies have begun demonstrating increased risk of type 2 diabetes (10). We conclude with a discussion of proposed strategies to combat weight gain for PLWH on ART and discuss research priorities to address the gaps. We searched PubMed for clinical trials, cohort studies, case reports, and relevant conference abstracts investigating weight gain, using our own knowledge of the area, having been involved in one of the most intensely characterized modern ARV studies demonstrating weight gain on current ART.
Weight Changes on Antiretroviral Therapy, and Mechanisms
Immune Dysregulation, Weight Loss, and AIDS
Historically, weight loss was a hallmark of HIV disease progression in the pre-ART era; it was known locally as “slim disease” in Uganda as it was such a characteristic finding of HIV infection (11). The syndrome often signified an inexorable and rapid decline in immunological and clinical reserve that heralded AIDS and death. Weight was strongly correlated with prognosis, and weight loss was included both in World Health Organization and US Centers for Disease Control and Prevention staging definitions of AIDS (12).
HIV replication and the ensuing inflammatory response causes a catabolic form of weight loss. During acute infection, viremia causes significant and permanent lymphoid damage and immunologic dysfunction, which is followed by immune-mediated virologic control. Ongoing viral replication, microbial translocation from the gut, inflammation, cellular activation, proliferation, and exhaustion, lead to immune depletion and progressive loss of virologic control. The persistently high turnover of T cells and removal of billions of virions daily, with associated ongoing inflammation, significantly elevates basal metabolic rate, with increased energy expenditure (13). Persistent inflammation has an effect on appetite, and one of the early reported benefits of ART initiation is increased hunger, presumably due to rapid control of viremia and inflammation (14).
This damage to the gastrointestinal immunological tissue facilitates ongoing inflammation within the gut wall, substantial changes to the gut microbiome, and consequent malabsorption of the many components of food, documented in many studies in PLWH (8, 15-18). This is further complicated by the development of opportunistic infections and neoplasms as immune deficiency develops, where the gastrointestinal system, along with the lung, bears a major brunt of the HIV-related disease load, again with effect on appetite, malabsorption, dysphagia, decreased energy intake, and subsequent weight loss.
This panoply of weight-loss–inducing mechanisms led to the stereotypical, stigmatized portrayal of the emaciated AIDS patient at the end of life. It led to the common use of the “Lazarus-like response” description of people with advanced HIV and AIDS who gained access to ART beginning in the mid-1990s, as people rapidly recovered, often from a hospice-level placement, in a matter of weeks or months (1).
Why is this immune deficiency catabolic state and associated weight loss important in the pathophysiology of weight gain? One of the major predictors of weight gain on successful modern ART is prior severe immune deficiency, which is associated with weight loss, at the initiation of ART—even years before—when switching across to newer drugs (9, 19, 20). In addition, those who have lost weight previously do seem to have the most rapid rebound in weight, although it is unclear how much of a return to health, or whether the trajectory is sustained after health is restored (19). Hypotheses concerning starvation states being associated with subsequent weight gain, once ART is started, are discussed later, but these may be applicable to patients with late-stage AIDS.
History of Antiretroviral Therapy Drug Evolution
In both the early and modern ART era, many HIV-positive people gained weight following ART initiation during initial immunological recovery (9, 21-23). This initial weight gain occurred by control of HIV viremia, immune reconstitution, and resolution of opportunistic infections. Viremia resolves often in just a few weeks, and inflammation subsides, appetite returns, and opportunistic infections are brought under control, with suppressive ART (13, 24).
However, weight loss and aberrant fat redistribution due to mitochondrial toxicity, termed lipodystrophy, and lipoatrophy, were a feature of prolonged use of older ART therapies, rather than routine weight gain (25). Lipoatrophy, a toxicity related to mitochondrial depletion that led to differential fat depletion in the limbs and face, a disfiguring and often permanent feature of certain nucleoside reverse transcriptase inhibitors (NRTIs), commonly used drugs like stavudine and zidovudine that have largely disappeared from clinical use in the last decade. Other classes, notably the protease inhibitors (PIs), were associated with considerable gastrointestinal side effects ranging from nausea to diarrhea, and again could lead to weight loss, and again, are far less prescribed than before as alternate ARVs have become available (24, 26, 27).
Widespread use of the PIs and associated abdominal fat accumulation, the “protease paunch,” were assigned causation. However, retrospective examination of registration data sets later revealed that this paunch, as well as reports of breast enlargement among women, was actually a combination of weight gain similar to what we are seeing with current regimens, coupled with loss of surrounding fatty tissue due to lipoatrophy after concomitant NRTI thymidine analogue administration, largely stavudine and zidovudine, and that this ongoing weight gain over-accentuated abdominal and breast tissue enlargement even further, rather than the PIs causing abdominal enlargement (26).
Advent of Modern Antiretroviral Therapy
Adherence improved throughout the 2000s as legacy drugs were replaced with less toxic ARVs that had less mitochondrial effect and better tolerability. These efforts have been remarkably successful, with most of the older agents no longer in use, as tenofovir disoproxil fumarate (TDF) and abacavir replaced stavudine and zidovudine. Later TDF and abacavir's role in weight loss were interrogated, as a new tenofovir prodrug, tenofovir alafenamide (TAF), appeared on the market in 2016 (28).
Similarly, widespread use of efavirenz, a nonnucleoside reverse transcriptase inhibitor (NNRTI) had come into use, replacing older, more toxic agents in the class, nevirapine, and the PIs, which had substantial gastrointestinal and metabolic side effects and a large pill burden. Efavirenz has a low resistance barrier and considerable side effects, including an adverse effect on glucose and lipids. The clinical step forward from nevirapine and PIs was significant in terms of tolerability, with most patients tolerating the drug well, and clinical use shifted quickly (24).
For perspective, by 2012, most of the world's tens of millions of PLWH globally were simultaneously on a TDF and efavirenz-containing single-pill compound, in combination with a cytosine analogue, either lamivudine or emtricitabine. It was during the advent of this new era of once-daily, better tolerated ART regimens that weight gain first came to attention. A large study in North America, with 14 000 people from 17 cohorts and conducted prior to the widespread introduction of dolutegravir, bictegravir, and TAF-containing regimens, found that approximately 20% changed from a normal body mass index to overweight after 3 years of treatment, and a similar proportion (18%) went from being overweight to obese in the same time frame (22). Although it was perhaps underappreciated at the time, this was one of the first signals that the better ART drugs might be associated with unexpected increases in body weight.
Risk Factors for Weight Gain With Modern Agents
Further concerns about weight gain emerged with the entry of TAF on the market, and the large-scale use of the second-generation integrase strand transfer inhibitors (INSTIs), that rapidly began replacing the NNRTIs, flagged initially in 2017, as patients began switching from TDF to TAF, and some patients gained weight, with greater gains reported on INSTI-based regimens compared to other regimens (7, 28). Most PLWH in low- and middle-income countries are receiving TDF, lamivudine, and dolutegravir, while in high-income countries, patients receive variations of that combination, including as a dual-therapy dolutegravir and lamivudine combination, or a combination of TAF, emtricitabine, and bictegravir. All these combinations have equivalent potency and are very well tolerated (3-5). Dolutegravir and bictegravir, as well as lamivudine and emtricitabine, have minor differences in terms of biochemistry and side effects. TDF and TAF have different metabolic and weight trajectory effects, discussed later, with more data available on TDF and dolutegravir and weight gain; both have been available longer and are used far more widely.
Further data highlighted the differential effect of different classes of ARVs on weight, but also the effect of race and sex, with INSTIs having the biggest effect on weight gain, and Black patients and women being most affected by weight gain. In 2019, the first prospective randomized evidence on weight gain emerged from 2 major, large, independent studies both using INSTIs and one using TAF, demonstrating significantly greater weight gain using these agents when compared to an efavirenz-containing comparator arm widely used across the world at the time, in PLHIV initiating ART (29, 30). Notably, weight mass gains were higher among women than men. In the study conducted in South Africa, dual-energy x-ray absorptiometry data were collected and demonstrated both lean and fat mass increased in the limbs and trunk, although increase in fat mass accounted for more of the weight gain. Women had a mean weight gain of more than 8 kg after 96 weeks, with subsequent longer-term data presented in 2022, demonstrated ongoing weight gain (31). A meta-analysis of registration data for the bictegravir studies, which used a range of comparator ARV agents in the multiple studies that were required for registration of the TAF/emtricitabine/bictegravir combination in use throughout North America and Europe, demonstrated very similar results (9).
Tenofovir Prodrugs and Effect on Weight Trajectories
Two NRTIs formed the backbone of almost every ART regimen for decades, until very recently, with the rise of dual therapy and injectable ARVs (32). Currently, with older drugs implicated in lipoatrophy steadily falling out of use, only 2 cytosine analogues, lamivudine and emtricitabine, widely regarded as being indistinguishable from each other and having minimal effect on weight, and 2 tenofovir prodrugs TDF and TAF, and abacavir, are still advocated in guidelines for first-line therapy.
The 2 tenofovir prodrugs are unique in that they have been used in studies in HIV-negative populations as preexposure prophylaxis in combination with the cytosine analogues, both in placebo-controlled studies as well as in head-to-head studies against each other. This allows for comparison without the confounding effects of HIV and associated disease-specific weight loss (28, 33). In these studies, TDF has a weight-mitigating effect, with HIV-negative participants in registration studies comparing TDF and placebo, in combination with emtricitabine, where an approximate 1-kg weight difference was seen between blinded arms after a year. Other randomized and observational studies supported this weight-mitigation role of TDF, both in prophylaxis and HIV treatment studies (33). A recent switch study, from TAF to TDF, in patients treated on a TAF-containing regimen for 192 weeks and then switched, showed a 1.2-kg weight loss after 48 weeks, similar to a Finnish and German cohort (7, 34).
It is unclear as to the mechanisms of weight mitigation seen with TDF. The drug decreases fat mitochondrial DNA in excision biopsy studies when measured against abacavir and affects fat distribution differently from abacavir and TAF (35, 36). A popular hypothesis, based on trial data and clinical practice, is that TDF may cause low-level loss of appetite or nausea, sufficient to offset food intake, although rarely affecting adherence, although this does not explain the aforementioned excision biopsy data (33).
The alternative prodrug TAF was developed after TDF and quickly replaced TDF in high-income countries based on benefits in reduced renal and bone mineral density toxicities that are less prevalent with TAF. However, concern about its effect on weight began to mount, as weight gain data became available in patients switching from TDF to TAF (4, 37-45). A 1-kg weight difference was seen in TAF preexposure prophylaxis registration data, between TAF and TDF, in HIV-negative men and transgender women in head-to-head comparison studies (28, 33). Initiation of regimens in ART-naive populations has shown significant weight differences, out to 192 weeks of treatment in prospective independent studies, of 3 kg vs TDF (4.4 kg in women) and was confirmed in retrospective review of registration data (9, 31). It is unclear whether differences in weight trajectories between these two drugs are because TDF is mitigating against weight gain, whether TAF directly causes weight gain, or some combination of both (28). However, a recent retrospective study comparing different doses of TAF found a higher dose appeared to be associated with greater weight gain, albeit with significant risk of bias (46). Studies comparing TAF-based regimens to new long-acting classes of medications are eagerly awaited in 2023, and should they demonstrate similar weight changes, would suggest TAF is weight-neutral, that is, it does not affect the weight trajectory.
Abacavir, which has been used in the last 20 years primarily as an alternative to TDF due to a better renal toxicity profile, has recently been reexamined in light of this concern regarding weight gain with TDF and TAF (28, 47). Abacavir's use is limited by concerns around increased cardiovascular events in high-risk patients, and an expensive complex genetic screen recommended before initiation in White patients for a potentially severe hypersensitivity side effect. Data are far more limited regarding weight gain or possible mechanisms, but it appears that abacavir does cause weight mitigation relative to TAF, although not as much as TDF (47).
In the aggregate, it appears that TDF and abacavir have a weight-mitigating effect over TAF. The jury is still out on TAF's individual effect on weight gain, or whether it is weight-neutral, but registration data with new agents from completely different classes, where TAF is commonly within the comparator regimen, will shed light on this in 2023. There have been almost no current data on whether these 3 agents have a further direct effect on hormonal or cognitive pathways that may affect appetite or metabolic pathways that may govern energy expenditure.
Integrase Inhibitors and Effect on Weight Trajectories
With clear signals from large observational trials, followed then by large randomized clinical trials (RCTs) and pooled retrospective analyses of registration RCT data, a large amount of research has consistently accumulated demonstrating that INSTIs, except for the newest INSTI, cabotegravir (importantly, not tested with TDF or TAF), are associated with weight gain (47). In AFRICOS, a prospective observational cohort from 4 African countries, PLWH on dolutegravir-based ART were twice as likely to have a high body mass index of 25 or more compared to those on non-INSTI regimens (48).
Data suggesting that INSTIs are implicated in weight gain initially came from large observational studies, showing a greater role for this class than PIs or NNRTIs (7, 28, 45, 49-51). Prospective data came from the ADVANCE and NAMSAL studies, RCTs conducted in South Africa and Cameroon, respectively, in which ART-naive adults were randomly assigned to dolutegravir-containing drug combinations and compared against efavirenz (36, 52). Dolutegravir-based regimens resulted in significant weight gain, with differences observed as early as 4 weeks post ART initiation and persisting through the latest visit at week 192, especially when combined with TAF in the case of ADVANCE, and were greatest among women in both trials. At 192 weeks, ADVANCE demonstrated a 2.7-kg difference between the dolutegravir and efavirenz arms, when NRTI backbones were kept the same (31). This pattern of weight gain seen in these 2 studies and the relatively continuous trajectory has been confirmed across other studies in which INSTIs-based regimens were used as first-line or switch options (9, 20, 43).
Overall, it appears that dolutegravir and bictegravir, the 2 agents used largely for treatment and postexposure prophylaxis regimens, are associated with the most weight gain among the INSTI class, followed by 2 older INSTIs that have largely fallen into disuse, raltegravir (currently only used in pediatrics) and elvitegravir (47). The one exception was cabotegravir, which was not associated with weight gain in either treatment or prevention studies, probably as it was used in combination with rilpivirine, not TDF, TAF, or efavirenz, which may have had more weight-change effects (47, 53).
Mechanistic pathways have been proposed for INSTI-related weight gain, although current data are unconvincing (3, 12). Limited qualitative and subjective data suggest that dolutegravir increases feelings of hunger (32), and in macaques, decreases levels of leptin (33). Dolutegravir could also drive increased capacity for energy harvest (34). Important in glucose and presumably appetite regulation, in patients switched to dolutegravir from PIs (54). Controversial studies have addressed human recombinant melanocortin-4 receptor, implicated in appetite regulation, and where dolutegravir has been found to affect binding ability (55). Several studies have shown profibrotic and other cellular effects on adipocytes by different INSTIs, both in HIV-positive and -negative patients (56, 57). One study demonstrated mitochondrial dysfunction with dolutegravir or elvitegravir compared with PIs or NRTIs (58).
The most influential study refuting that INSTIs are causative in weight gain trajectories came from ADVANCE (59, 60). Prior work has demonstrated that efavirenz is metabolized via the cytochrome P450 2B6 enzyme (CYP2B6) pathways, and that slow metabolizers have associated significant side effects closely associated with elevated drug levels, including metabolic changes (lipid and glucose levels), as well as bone, central nervous system, and hepatic toxicity. Subsequently, researchers demonstrated that slow metabolizers of efavirenz switched to INSTI-containing regimens had the most weight gain. ADVANCE researchers demonstrated that medium and fast metabolizers of efavirenz had the same weight trajectory as the dolutegravir arm once the slow metabolizers were removed from the analysis, and a subsequent study revealed that increasing efavirenz levels in the cohort was associated with greater weight loss. The practical implications of this are 2-fold: Switching from an INSTI to efavirenz would not be anticipated to lead to weight loss in people who are medium or fast metabolizers. Second, and important, patients in whom it did work would be those most prone to efavirenz’s worst side effects, including a range of effects that add significantly to cardiovascular risk.
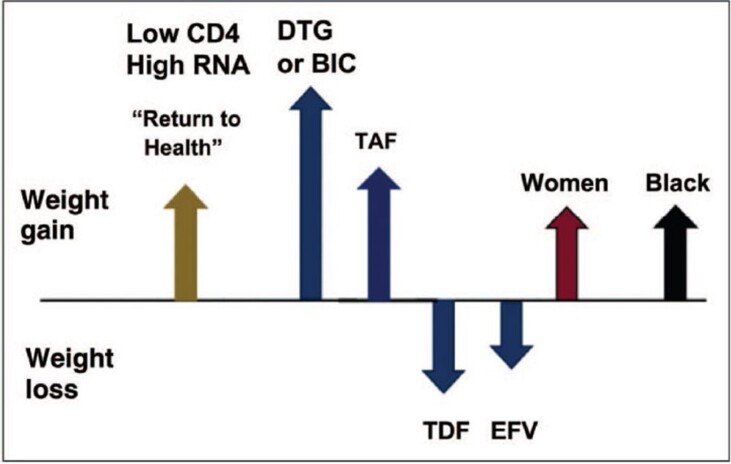
Fig. 1 summarizes factors driving or mitigating weight changes for PLWH on ART.
Figure 1.
Summary of factors driving or mitigating weight changes for people living with HIV on antiretroviral therapy (not to scale). Source: Hill A. Are new antiretroviral treatments increasing the risks of obesity? [conference presentation]. In: 17th European AIDS conference, Basel, Switzerland; 2019. Used with permission ref Shah, S. and A. Hill, Risks of metabolic syndrome and diabetes with integrase inhibitor-based therapy. Curr Opin Infect Dis, 2021. 34(1): p. 16-24.
Modern Antiretroviral Therapy and Weight Gain in Pediatric Populations
Although substantial gains have been made in vertical transmission of HIV, pediatric HIV infections continue to occur. Side effects of many antiretrovirals, especially mitochondrial toxicity, tend to be delayed in children, but do follow a similar pattern with time. However, very few data are yet available on weight gain or cardiometabolic complications in children and adolescents with the new agents, with a recent systematic review showing no concerns with raltegravir or dolutegravir use (61).
Antiretroviral Therapy Pipeline
Many long-acting therapeutic agents are under investigation, partly as the current daily oral offerings are remarkably safe and effective, offering little room for improvement, and partly as patient preference studies have indicated strong preference for long-acting agents over conventional daily dosing (32, 62). The first long-acting treatment offering, a dual injection of rilpivirine and cabotegravir given every 2 months as 2 separate injections, appears to be safe, effective, and weight-neutral when compared against a standard daily offering, and was recently registered by the US Food and Drug Administration based on noninferiority studies.
Other offerings that are in phase 2 and 3 include islatravir and lenacapavir, both with recent setbacks regarding toxicity but with development now recommenced, in combination with each other, and with existing and novel investigational ARVs. The comparison gold-standard arm in these arms is largely TAF/emtricitabine and bictegravir, the major commercially successful combination in North America and Europe, with weight analysis part of the evaluation, and data keenly anticipated. Lenacapavir is also being explored as preexposure prophylaxis, again with weight measurements, but the lymphocyte toxicity signal seen with higher doses of islatravir has meant that the oral and implant prophylaxis studies have been terminated. Favorable early efficacy and toxicity data with these 2 agents, using lower islatravir oral weekly dosing, suggests they may form part of the treatment landscape soon, meaning close attention on their effect on weight (32, 62). While these early offerings have already had challenges during development, it is likely that long-acting ARVs will steadily become a major alternative, if not altogether replace, daily therapy in the future, with weight gain a major factor in agent selection.
Alternative HIV-specific Mechanisms That May Effect Weight Gain
Could there be other mechanisms to explain the greater-than-expected weight gain trajectory, especially in high-risk groups? Theories have been advanced that the effect on gut lymphoid tissue may affect food reabsorption, although this seems unlikely, as nutrient uptake is already very efficient. More plausible is that absorption is altered somehow in a way that affects hunger hormones that regulate appetite (8).
An alternative, perhaps allied hypothesis, is that PLWH have undergone a starvation-type state, not unlike that seen with dieting and other starvation states, that exert strong compensatory forces on the neuroendocrine axis to reverse and often even have an over-rebound effect on weight thresholds. Whether this applies to underweight PLWH is unknown, but fetuses, children, adolescents, and adults exposed to famine conditions, on being returned to normal feeding conditions, have a greater incidence of obesity (63, 64).
Weight gain associated with medication use, as an unwanted side effect, is now well recognized but often underappreciated by clinicians (65). HIV status is associated with comorbid mental health conditions in many communities, and the psychiatric medication used to treat many of these conditions are among the most potent causes of weight gain. Many of the RCTs and observational trials described earlier controlled for this, as well for other common medications associated with weight gain, such as contraception and steroid use, and did not find an association. Other mental health issues, specifically depression, associated with HIV and other chronic conditions, may further influence eating patterns and weight gain on ART.
Finally, sleep disturbances, specifically sleep apnea as a feature of HIV infection, are a characteristic of HIV, and similarly have complex interplay in the pathophysiology of obesity (66, 67). Sleep, specifically insomnia, altered dreams, and decreased quality of sleep has long been described as a side effect of specific ARVs, most recently of second-generation INSTIs as discussed earlier, but little is understood about the causal relationship between weight gain HIV, ARVs, and sleep alteration.
Implications of Weight Gain Among People Living With HIV on Antiretroviral Therapy
The long-term changes and cardiovascular consequences of ART-associated weight gain are still unknown. Weight gain associated with the newer agents has been associated with surprisingly few short-term changes in cardiovascular risk factors such as blood pressure, lipids, and glucose, considering the large number of studies conducted and the magnitude of weight gain in many of the studies, and the fact that these data are often routinely collected in registration and other studies (20, 36). Reported changes so far have been the expected metabolic changes associated with TDF, which has a beneficial effect on lipid profiles, and with efavirenz, which has a detrimental effect both on lipids and glucose (34, 68). Type 2 diabetes has been reported from observational studies, but again, considering the magnitude of the INSTI roll-out across the world, it is difficult to separate this from normal incidence. Some studies do suggest an association with elevated blood pressure, and more recently associations with elevated glucose and type 2 diabetes (10, 69-77).
However, several modeling studies suggest the weight gain alone will have a substantial effect on cardiovascular risk, as well as on pregnancy maternal and fetal outcomes (78-81). Time will tell how much clinical effect weight gain will have in this population, and there are several HIV cohorts that are well positioned to supply these data, although prior and even current associations with cardiovascular risk and different ART drugs and drug classes have been frustratingly difficult to untangle regarding association and causation (10, 68, 71, 82-85).
What Needs to Be Done?
Little is known about what might prevent or help alleviate weight gain in PLWH. The immediate priority is studies to both understand the underlying mechanisms of weight gain, as well as optimal approaches to address obesity (6).
At an individual level, several approaches may need to be tested. As a revolution in approaches to obesity management enter the medical world, spanning new understandings of the physiology of weight gain, evidence-based understandings of the role and limitations of lifestyle advice, the rise of safer bariatric surgery techniques, but especially the new availability of weight loss pharmaceuticals, we need to ensure effective interventions are available to PLWH, and whether these advances are suitable for this group that may have a relatively unique obesity physiology (86, 87).
First, identifying less-obesogenic ART regimens, especially in those at risk of weight gain, may help patients and providers concerned about weight gain. This may differ for PLWH initiating ART vs those on established treatment and may vary for those with established obesity vs those on a trajectory at risk of obesity. The studies listed earlier already give us some understanding as to risk factors, and which medications are most associated with weight gain. However, in many studies, weight gain was lowest on medications like efavirenz, but only when associated with a high risk of significant toxicity, suggesting that some weight gain may be inevitable. Some studies have been proposed that have replaced TAF and INSTIs with alternatives, with weight outcomes as a primary outcome. It may be that the new long-acting formulations do not affect weight gain trajectories, and that other interventions are needed.
Second, focus on healthy living deserves emphasis, as it does in the general population. In HIV-negative populations, exercise and caloric-restrictive diets have a very limited effect in the medium term on weight loss in those with established obesity (88, 89). It is possible that the pathophysiology of weight gain in HIV-positive people is sufficiently different that there may be more success in this population, but this would need to be tested. Irrespective, small studies have demonstrated metabolic success, if not significant weight reduction, with diet and exercise interventions, in PLWH (90-95).
Finally, the rise of highly effective weight-loss medication, with results rivaling bariatric surgery, has created options for patients and providers in the general community frustrated at the lack of success of traditional medical “eat less and move more” advice. Older oral combination medications were realizing average weight reductions over 5%, traditionally the benchmark for a clinically relevant effect on other risk factors, and some were able to approach 10% after just over a year. Initial studies using repurposed diabetes medications such as subcutaneous glucagon-like peptide-1 daily injectables had a modest effect on weight loss, but semaglutide 2.4 mg, a weekly offering, recently showed results approaching 15% at 72 weeks, and a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, suggested results beyond 20%, with many other agents under development (88, 89, 96, 97), summarized in Table 1. The safety profiles of these agents suggest that they could be used far more confidently and broadly than older, more toxic weight-loss regimens.
Table 1.
Comparison of antiobesity medications showing treatment effect from randomized controlled trials, highest and lowest available national prices, and estimated minimum price per course
| Drug (route) (course duration) | Average weight loss on treatment vs placebo, % (kg) (study duration) | Highest national price | Lowest national price | Estimated minimum price |
|---|---|---|---|---|
| Oral treatments | ||||
| Orlistat (120 mg 3×/d for 30 d) | Treatment −8.8% (−8.7 kg) vs placebo −5.7% (−5.8 kg) (after 52 wk) | $100 (US VETS) | $1 (Vietnam) | $7 |
| Naltrexone-bupropion (8 mg/90 mg 4×/d for 30 d) | Treatment −6.4% (−6.2 kg) vs placebo −1.9% (−1.3 kg) (after 56 wk) | $326 (US PHARM) | $56 (South Africa) | $54 |
| Topiramate-phentermine (92/15 mg/d for 30 d) | Treatment −9.8% (−10.2 kg) vs placebo −1.2% (−1.4 kg) (after 56 wk) | $199 (US PHARM) | $1.3 (Kenya) | $1.4–$5 |
| Semaglutide (14 mg/d for 30 d) | Treatment −5.3% (−5.0 kg) vs placebo −1.3% (−1.2 kg) (after 20 mg 1×/d for 26 wk, in patients with T2DM) | $578 (US VETS) | $65 (India) | NA |
| S/C treatments | ||||
| Semaglutide (2.4 mg/wk, price calculated for 10.25 mg per 30 d) | Treatment −14.9% (−15.3 kg) vs placebo −2.4% (−2.6 kg) (after 68 wk) | $804 (US PHARM) | $95 (Turkey) | $40 |
| Liraglutide (3 mg 1×/d for 30 d) | Treatment −8.0% (−8.4 kg) vs placebo −2.8% (−2.8 kg) (after 3 mg 1×/d for 56 wk) | $1418 (US PHARM) | $252 (Norway) | $50 |
| Tirzepatide (15 mg once weekly, price calculated for 12.67 mg per 30 d) | Treatment −20.9% (−21.4 kg) vs placebo −3.1% (−3.2 kg) (after 72 wk) | $1100.70 (US PHARM) | $715.56 (US VETS) | NA |
Used with permission ref Levi J, Wang J, Venter F, Hill A. Estimated minimum prices and lowest available national prices for antiobesity medications: Improving affordability and access to treatment. Obesity (Silver Spring). 2023; 1-10. doi:10.1002/oby.23725.
Abbreviations: NA, not available; S/C, subcutaneous; T2DM, type 2 diabetes mellitus; US PHARM, US Drug Online Pharmaceutical Drug Price Database; US VETS, US Department of Veterans Affairs Medical Insurance Drug Price Database.
It is possible that both the therapeutic and structural interventions will be no different for PLWH, when compared to people without HIV, but the pathophysiology of weight gain after HIV infection is sufficiently different to suggest that specific clinical trials and interventions be considered for this group, and the safety and tolerability be studied given the potential for interactions with ART regimens, and to ensure comparator HIV-negative populations are included.
Structural population-wide interventions (eg, sugar beverage taxes, food labeling, and legislation addressing processed food) as part of the ongoing public health crisis, especially in countries with existing and developing obesity issues, need to be addressed, as for HIV-negative populations (98-100).
Conclusion
In summary, excess weight gain on newer ART has emerged as a major clinical concern, as increasingly safe and potent ART agents are developed for the millions of PLWH globally. The mechanisms underlying greater weight gain in Black and female patients are currently unknown, but the fact that this population is most affected both by the HIV and the obesity epidemics, may represent one of the biggest public health challenges for areas with high HIV prevalence.
Abbreviations
- ART
antiretroviral therapy
- ARV
antiretroviral
- INSTI
integrase strand transfer inhibitor
- NNRTI
nonnucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- PLWH
people living with HIV
- TAF
tenofovir alafenamide
- TDF
tenofovir disoproxil fumarate
Contributor Information
Nomathemba C Chandiwana, Ezintsha, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg 2193, South Africa.
Mark J Siedner, Medical Practice Evaluation Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02115, USA.
Vincent C Marconi, Division of Infectious Diseases and Department of Global Health, Emory University School of Medicine and Rollins School of Public Health, Atlanta, GA 4223, USA.
Andrew Hill, Department of Pharmacology and Therapeutics, University of Liverpool, Liverpool L69 7BE, UK.
Mohammed K Ali, Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA 4223, USA; Department of Family and Preventive Medicine, School of Medicine, Emory University, Atlanta, GA 30322, USA.
Rachel L Batterham, Department of Medicine, University College London, London WC1E 6BT, UK.
Willem Daniel Francois Venter, Ezintsha, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg 2193, South Africa; Department of Public Health Medicine, Faculty of Health Sciences, School of Health Systems and Public Health, University of Pretoria, Pretoria 0028, South Africa.
Funding
This work was supported by the National Institutes of Health (NIH) (K24 HL166024) and the HLB-SIMPLe Alliance, sponsored by the National Heart, Lung and Blood Institute and funded under grant numbers UG3HL156388 with the US Department of Health and Human Services, NIH, National Heart, Lung and Blood Institute (NIH/NHLBI). The contents are solely the responsibility of the authors and do not necessarily represent the views of the Department of Health and Human Services or NIH/NHLBI. In addition, we acknowledge support from Unitaid and the South African Medical Research Council.
Author Contributions
First draft conceived by N.C., M.S., and W.D.F.V. All authors provided major editing, provided critical review, and approved the final version for submission.
Disclosures
N.C.C. declares receiving grants and nonfinancial support from Shin Poong Pharm Co Ltd, ViiV Healthcare, Gilead Sciences, Merck, and Johnson & Johnson grants, as well as personal fees and nonfinancial support from Cipla, Frontiers Biotech, and Novo Nordisk. M.J.S. acknowledges receiving funding from the NIH (K24 HL166024). V.C.M. reports receiving grants from the NIH during the study and grants from the NIH, Veterans Affairs, and Centers for Disease Control and Prevention, as well as grants, personal fees, nonfinancial support, and other from Lilly and Gilead. Furthermore, V.C.M. receives grants and personal fees from ViiV, and nonfinancial support from Bayer outside the submitted work. M.K.A. discloses receiving a grant to Emory University from Merck and Co, and consulting fees from Eli Lilly and Bayer, all beyond the scope of this manuscript's content. R.L.B. receives research grants and honoraria from Novo Nordisk, International Medical Press, ViiV, Pfizer, Gila Therapeutics, Epitomee Medical Ltd, and Eli Lilly. A.H. declares no conflicts of interest. W.D.F.V. acknowledges research funding from the Bill and Melinda Gates Foundation, SA Medical Research Council, National Institutes for Health, Unitaid, Foundation for Innovative New Diagnostics (FIND), the Children's Investment Fund Foundation (CIFF), and previous funding from USAID. Additionally, W.D.F.V. receives drug donations from ViiV Healthcare, Merck, Johnson & Johnson, and Gilead Sciences. W.D.F.V. receives honoraria for educational talks and advisory board membership for Gilead, ViiV, Mylan/Viatris, Merck, Adcock-Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, and Virology Education. These potential conflicts of interest have been disclosed in accordance with the relevant policies and guidelines. They have been provided for transparency and to ensure that the integrity and objectivity of the reported research and its findings are upheld.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Fauci AS, Lane HC. Four decades of HIV/AIDS—much accomplished, much to do. N Engl J Med. 2020;383(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 2. Trickey A, Sabin CA, Burkholder G, et al. . Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. 2023;10(5):e295‐e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nel J, Dlamini S, Meintjes G, et al. . Southern African HIV Clinicians Society guidelines for antiretroviral therapy in adults: 2020 update. South Afr J HIV Med. 2020;21(1):1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi RT, Bedimo R, Hoy JF, et al. . Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society–USA panel. JAMA. 2023;329(1):63‐84. [DOI] [PubMed] [Google Scholar]
- 5. Ryom L, De Miguel R, Cotter AG, et al. . Major revision version 11.0 of the European AIDS Clinical Society guidelines 2021. HIV Med. 2022;23(8):849‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gharakhanian S. Evolution of weight and BMI during the course of HIV infection: historical perspective and challenges for clinical research. AIDS. 2022;36(15):2217‐2219. [DOI] [PubMed] [Google Scholar]
- 7. Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad. 2019;5(1):41‐43. [PMC free article] [PubMed] [Google Scholar]
- 8. Godfrey C, Bremer A, Alba D, et al. . Obesity and fat metabolism in human immunodeficiency virus-infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis. 2019;220(3):420‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sax PE, Erlandson KM, Lake JE, et al. . Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bannister WP, Mast TC, de Wit S, et al. . Changes in body mass index and clinical outcomes after initiation of contemporary antiretroviral regimens. AIDS. 2022;36(15):2107‐2119. [DOI] [PubMed] [Google Scholar]
- 11. Mhiri C, Bélec L, Di Costanzo B, Georges A, Gherardi R. The slim disease in African patients with AIDS. Trans R Soc Trop Med Hyg. 1992;86(3):303‐306. [DOI] [PubMed] [Google Scholar]
- 12. Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther. 2007;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill AL, Rosenbloom DIS, Nowak MA, Siliciano RF. Insight into treatment of HIV infection from viral dynamics models. Immunol Rev. 2018;285(1):9‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rehman AM, Woodd S, Chisenga M, et al. . Appetite testing in HIV-infected African adults recovering from malnutrition and given antiretroviral therapy. Public Health Nutr. 2015;18(4):742‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Costiniuk CT, Angel JB. Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity? Mucosal Immunol. 2012;5(6):596‐604. [DOI] [PubMed] [Google Scholar]
- 16. Shacklett BL. Mucosal immunity in HIV/SIV infection: T cells, B cells and beyond. Curr Immunol Rev. 2019;15(1):63‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lake JE. The fat of the matter: obesity and visceral adiposity in treated HIV infection. Curr HIV/AIDS Rep. 2017;14(6):211‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia JM, Dong Y, Richardson P, et al. . Effect of HIV and antiretroviral therapy use on body weight changes in a cohort of U.S. veterans living with and without HIV. HIV Med. 2023;24(2):180‐190. [DOI] [PubMed] [Google Scholar]
- 20. Erlandson KM, Carter CC, Melbourne K, et al. . Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis. 2021;73(8):1440‐1451. [DOI] [PubMed] [Google Scholar]
- 21. Yuh B, Tate J, Butt AA, et al. . Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koethe JR, Jenkins CA, Lau B, et al. . Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32(1):50‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasse B, Iff M, Ledergerber B, et al. . Obesity trends and body mass index changes after starting antiretroviral treatment: the Swiss HIV Cohort Study. Open Forum Infect Dis. 2014;1(2):ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258‐271. [DOI] [PubMed] [Google Scholar]
- 25. Koethe JR, Lagathu C, Lake JE, et al. . HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers. 2020;6(1):48. [DOI] [PubMed] [Google Scholar]
- 26. de Waal R, Cohen K, Maartens G. Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction. PLoS One. 2013;8(5):e63623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy RA, Sunpath H, Kuritzkes DR, Venter F, Gandhi RT. Antiretroviral therapy-associated toxicities in the resource-poor world: the challenge of a limited formulary. J Infect Dis. 2007;196(Suppl 3):S449‐S456. [DOI] [PubMed] [Google Scholar]
- 28. Wood BR, Huhn GD. Excess weight gain with integrase inhibitors and tenofovir alafenamide: what is the mechanism and does it matter? Open Forum Infect Dis. 2021;8(12):ofab542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Venter WDF, Moorhouse M, Sokhela S, et al. . Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803‐815. [DOI] [PubMed] [Google Scholar]
- 30. NAMSAL ANRS 12313 Study Group; Kouanfack C, Mpoudi-Etame M, et al. . Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816‐826. [DOI] [PubMed] [Google Scholar]
- 31. Venter WDF, Bosch B, Sokhela S, et al. . 24th International AIDS Conference. 2022: Montreal.
- 32. Flexner CW, Kashuba A. Editorial: new drugs for HIV: quo vadis? Curr Opin HIV AIDS. 2022;17(1):1‐3. [DOI] [PubMed] [Google Scholar]
- 33. Shah S, Pilkington V, Hill A. Is tenofovir disoproxil fumarate associated with weight loss? AIDS. 2021;35(Suppl 2):S189‐S195. [DOI] [PubMed] [Google Scholar]
- 34. Bosch B, Akpomiemie G, Chandiwana N, et al. . Weight and metabolic changes after switching from tenofovir alafenamide (TAF)/emtricitabine (FTC)+dolutegravir (DTG), tenofovir disoproxil fumarate (TDF)/FTC+DTG and TDF/FTC/efavirenz (EFV) to TDF/lamivudine (3TC)/DTG. Clin Infect Dis. 2023;76(8):1492‐1495. [DOI] [PubMed] [Google Scholar]
- 35. McComsey GA, Daar ES, O’Riordan M, et al. . Changes in fat mitochondrial DNA and function in subjects randomized to abacavir-lamivudine or tenofovir DF-emtricitabine with atazanavir-ritonavir or efavirenz: AIDS Clinical Trials Group Study A5224s, substudy of A5202. J Infect Dis. 2013;207(4):604‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Venter WDF, Sokhela S, Simmons B, et al. . Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666‐e676. [DOI] [PubMed] [Google Scholar]
- 37. Surial B, Mugglin C, Calmy A, et al. . Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med. 2021;174(6):758‐767. [DOI] [PubMed] [Google Scholar]
- 38. Shah S, Hindley L, Hill A. Are new antiretroviral treatments increasing the risk of weight gain? Drugs. 2021;81(3):299‐315. [DOI] [PubMed] [Google Scholar]
- 39. Ruderman SA, Crane HM, Nance RM, et al. . Brief report: weight gain following ART initiation in ART-naïve people living with HIV in the current treatment era. J Acquir Immune Defic Syndr. 2021;86(3):339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Darnell J, Jain S, Sun X, et al. . Impact of switching to tenofovir alafenamide on weight gain as compared to maintaining a non-tenofovir alafenamide containing regimen. Medicine (Baltimore). 2021;100(34):e27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mallon PW, Brunet L, Hsu RK, et al. . Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J Int AIDS Soc. 2021;24(4):e25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Erlandson KM, Wu K, Lake JE, et al. . Mitochondrial DNA haplogroups and weight gain following switch to integrase strand transfer inhibitor-based antiretroviral therapy. AIDS. 2021;35(3):439‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martínez-Sanz J, Blanco JR, Muriel A, et al. . Weight changes after antiretroviral therapy initiation in CoRIS (Spain): a prospective multicentre cohort study. J Int AIDS Soc. 2021;24(5):e25732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lake JE, Trevillyan J. Impact of integrase inhibitors and tenofovir alafenamide on weight gain in people with HIV. Curr Opin HIV AIDS. 2021;16(3):148‐151. [DOI] [PubMed] [Google Scholar]
- 45. Lahiri CD, Xu Y, Wang K, et al. . Weight and body mass index change after switching to integrase inhibitors or tenofovir alafenamide among women living with HIV. AIDS Res Hum Retroviruses. 2021;37(6):461‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emond B, Rossi C, Rogers R, Lefebvre P, Lafeuille M-H, Donga P. Real-world analysis of weight gain and body mass Index increase among patients with HIV-1 using antiretroviral regimen containing tenofovir alafenamide, tenofovir disoproxil fumarate, or neither in the United States. J Health Econ Outcomes Res. 2022;9(1):39‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanters S, Renaud F, Rangaraj A, et al. . Evidence synthesis evaluating body weight gain among people treating HIV with antiretroviral therapy - a systematic literature review and network meta-analysis. EClinicalMedicine. 2022;48:101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Esber AL, Chang D, Iroezindu M, et al. . Weight gain during the dolutegravir transition in the African Cohort Study. J Int AIDS Soc. 2022;25(4):e25899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lake JE, Wu K, Bares SH, et al. . Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis. 2020;71(9):e471‐e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bourgi K, Rebeiro PF, Turner M, et al. . Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bansi-Matharu L, Phillips A, Oprea C, et al. . Contemporary antiretrovirals and body-mass index: a prospective study of the RESPOND cohort consortium. Lancet HIV. 2021;8(11):e711‐e722. [DOI] [PubMed] [Google Scholar]
- 52. Calmy A, Tovar Sanchez T, Kouanfack C, et al. . Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV. 2020;7(10):e677‐e687. [DOI] [PubMed] [Google Scholar]
- 53. Zhao AV, Crutchley RD, Guduru RC, Ton K, Lam T, Min AC. A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection. Retrovirology. 2022;19(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. González-Cordón A, Assoumou L, Moyle G, et al. . Switching from boosted PIs to dolutegravir decreases soluble CD14 and adiponectin in high cardiovascular risk people living with HIV. J Antimicrob Chemother. 2021;76(9):2380‐2393. [DOI] [PubMed] [Google Scholar]
- 55. Domingo P, Villarroya F, Giralt M, Domingo JC. Potential role of the melanocortin signaling system interference in the excess weight gain associated to some antiretroviral drugs in people living with HIV. Int J Obes (Lond). 2020;44(9):1970‐1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lagathu C, Béréziat V, Gorwood J, et al. . Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin Drug Saf. 2019;18(9):829‐840. [DOI] [PubMed] [Google Scholar]
- 57. Gorwood J, Bourgeois C, Pouchier V, et al. . The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis. 2020;71(10):e549‐e560. [DOI] [PubMed] [Google Scholar]
- 58. Korencak M, Byrne M, Richter E, et al. . Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight. 2019;4(12):e126675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leonard MA, Cindi Z, Bradford Y, et al. . Efavirenz pharmacogenetics and weight gain following switch to integrase inhibitor-containing regimens. Clin Infect Dis. 2021;73(7):e2153‐e2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Griesel R, Maartens G, Chirehwa M, et al. . CYP2B6 genotype and weight gain differences between dolutegravir and efavirenz. Clin Infect Dis. 2021;73(11):e3902‐e3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Townsend CL, O’Rourke J, Milanzi E, et al. . Effectiveness and safety of dolutegravir and raltegravir for treating children and adolescents living with HIV: a systematic review. J Int AIDS Soc. 2022;25(11):e25970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chandiwana NC, Serenata CM, Owen A, et al. . Impact of long-acting therapies on the global HIV epidemic. AIDS. 2021;35(Suppl 2):S137‐S143. [DOI] [PubMed] [Google Scholar]
- 63. Kyle UG, Pichard C. The Dutch Famine of 1944-1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006;9(4):388‐394. [DOI] [PubMed] [Google Scholar]
- 64. Meng R, Lv J, Yu C, et al. . Prenatal famine exposure, adulthood obesity patterns and risk of type 2 diabetes. Int J Epidemiol. 2018;47(2):399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wharton S, Lau DCW, Vallis M, et al. . Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875‐E891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Owens RL, Hicks CB. A wake-up call for human immunodeficiency virus (HIV) providers: obstructive sleep apnea in people living with HIV. Clin Infect Dis. 2018;67(3):472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roche J, Vos AG, Lalla-Edward ST, Venter WDF, Scheuermaier K. Relationship between sleep disorders, HIV status and cardiovascular risk: cross-sectional study of long-haul truck drivers from Southern Africa. Occup Environ Med. 2021;78:393‐399. [DOI] [PubMed] [Google Scholar]
- 68. Martínez-Sanz J, Serrano-Villar S, Muriel A, et al. . Metabolic-related outcomes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in adults with HIV: a multicenter prospective cohort study. Clin Infect Dis. 2023;76(3):e652‐e660. [DOI] [PubMed] [Google Scholar]
- 69. Hsu R, Brunet L, Fusco JS, et al. . Incident type 2 diabetes mellitus after initiation of common HIV antiretroviral drugs. AIDS. 2021;35(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 70. Herrin M, Tate JP, Akgün KM, et al. . Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr. 2016;73(2):228‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Spieler G, Westfall AO, Long DM, et al. . Trends in diabetes incidence and associated risk factors among people with HIV in the current treatment era. AIDS. 2022;36(13):1811‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McComsey GA, Emond B, Shah A, et al. . Association between weight gain and the incidence of cardiometabolic conditions among people living with HIV-1 at high risk of weight gain initiated on antiretroviral therapy. Infect Dis Ther. 2022;11(5):1883‐1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. O’Halloran JA, Sahrmann J, Parra-Rodriguez L, et al. . Integrase strand transfer inhibitors are associated with incident diabetes mellitus in people with human immunodeficiency virus. Clin Infect Dis. 2022;75(12):2060‐2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Asundi A, Olson A, Jiang W, et al. . Integrase inhibitor use associated with weight gain in women and incident diabetes mellitus. AIDS Res Hum Retroviruses. 2022;38(3):208‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tiozzo E, Rodriguez A, Konefal J, Farkas GJ, Maher JL, Lewis JE. The relationship between HIV duration, insulin resistance and diabetes risk. Int J Environ Res Public Health. 2021;18(8):3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Summers NA, Lahiri CD, Angert CD, et al. . Metabolic changes associated with the use of integrase strand transfer inhibitors among virally controlled women. J Acquir Immune Defic Syndr. 2020;85(3):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brennan AT, Nattey C, Kileel EM, et al. . Change in body weight and risk of hypertension after switching from efavirenz to dolutegravir in adults living with HIV: evidence from routine care in Johannesburg, South Africa. EClinicalMedicine. 2023;57:101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baxevanidi EE, Asif S, Qavi A, et al. . Predicted long-term adverse birth and child health outcomes in the ADVANCE trial, in Conference on Retroviruses and Opportunistic Infections. 2021, Virtual.
- 79. Hindley L, Mccan K, Sokhela S, et al. . Predicted 10-year risks of cardiovascular disease and diabetes in the ADVANCE trial, in Conference on Retroviruses and Opportunistic Infections. 2021, Virtual.
- 80. McCann K, Shah S, Hindley L, et al. . Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS. 2021;35(10):1657‐1665. [DOI] [PubMed] [Google Scholar]
- 81. Masyuko SJ, Page ST, Kinuthia J, et al. . Metabolic syndrome and 10-year cardiovascular risk among HIV-positive and HIV-negative adults: a cross-sectional study. Medicine (Baltimore). 2020;99(27):e20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Manne-Goehler J, Baisley K, Vandormael A, et al. . BMI and all-cause mortality in a population-based cohort in rural South Africa. Obesity (Silver Spring). 2020;28(12):2414‐2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chang D, Esber A, Dear N, et al. . Non-communicable diseases in older people living with HIV in four African countries: a cohort study. Lancet HIV. 2022;9(Suppl 1):S5. [DOI] [PubMed] [Google Scholar]
- 84. Neesgaard B, Greenberg L, Miró JM, et al. . Associations between integrase strand-transfer inhibitors and cardiovascular disease in people living with HIV: a multicentre prospective study from the RESPOND cohort consortium. Lancet HIV. 2022;9(7):e474‐e485. [DOI] [PubMed] [Google Scholar]
- 85. Dakum P, Avong YK, Okuma J, et al. . Prevalence and risk factors for obesity among elderly patients living with HIV/AIDS in a low-resource setting. Medicine (Baltimore). 2021;100(15):e25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Firman C, Batterham RL. A new era in gut hormone-based pharmacotherapy for people with obesity. Proc Nutr Soc. 2022;81(3):217‐226. [DOI] [PubMed] [Google Scholar]
- 87. Sharma G, Strong AT, Boules M, et al. . Comparative outcomes of bariatric surgery in patients with and without human immunodeficiency virus. Obes Surg. 2018;28(4):1070‐1079. [DOI] [PubMed] [Google Scholar]
- 88. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):1492. [DOI] [PubMed] [Google Scholar]
- 89. LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172‐1191. [DOI] [PubMed] [Google Scholar]
- 90. Mbayo V, Sookan T. Effects of a resistance training programme in people living with HIV in Zimbabwe. Sport Sci Health. 2020;16(3):551‐560. [Google Scholar]
- 91. Kelly TA, Kim S, Jemmott LS, . Physical activity intervention effects on waist-to-hip ratio in African American men living with HIV. Am J Mens Health. 2022;16(6):15579883221130664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shim MS, Noh D. Effects of physical activity interventions on health outcomes among older adults living with HIV: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(14):8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kalatzi P, Dinas PC, Chryssanthopoulos C, Karatzanos E, Nanas S, Philippou A. Impact of supervised aerobic exercise on clinical physiological and mental parameters of people living with HIV: a systematic review and meta-analyses of randomized controlled trials. HIV Res Clin Pract. 2022;23(1):107‐119. [PubMed] [Google Scholar]
- 94. Panza E, Wing EJ, Wing R. Behavioral weight loss: a promising treatment for obesity in adults with HIV. AIDS Behav. 2020;24(4):1085‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wing RR, Becofsky K, Wing EJ, et al. . Behavioral and cardiovascular effects of a behavioral weight loss program for people living with HIV. AIDS Behav. 2020;24(4):1032‐1041. [DOI] [PubMed] [Google Scholar]
- 96. Jastreboff AM, Aronne LJ, Ahmad NN, et al. . Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205‐216. [DOI] [PubMed] [Google Scholar]
- 97. Wilding JPH, Batterham RL, Calanna S, et al. . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989‐1002. [DOI] [PubMed] [Google Scholar]
- 98. Dicken SJ, Batterham RL. Ultra-processed food: a global problem requiring a global solution. Lancet Diabetes Endocrinol. 2022;10(10):691‐694. [DOI] [PubMed] [Google Scholar]
- 99. Boachie MK, Thsehla E, Immurana M, Kohli-Lynch C, Hofman KJ. Estimating the healthcare cost of overweight and obesity in South Africa. Glob Health Action. 2022;15(1):2045092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lachat C, Otchere S, Roberfroid D, et al. . Diet and physical activity for the prevention of noncommunicable diseases in low- and middle-income countries: a systematic policy review. PLoS Med. 2013;10(6):e1001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.