Right at the outset of an outbreak that was later declared a Public Health Emergency of International Concern (PHEIC), we hoped there would be a wakeup call for the international community to pay attention to mpox [1]. It did, but not quite enough. Despite some 91,000 cases of clade IIb mpox virus registered worldwide to-date [2], little progress has been made into gathering essential evidence as to what works or does not in preventing and treating mpox, with stark inequalities in access to therapy and vaccination between high-income and low- and middle-income countries, including the African countries with recurring outbreaks of clade I and clade IIa mpox virus [3].
While the PHEIC has been successfully curbed, we see again the challenges of mounting an effective and timely clinical research response within the timeframe of relatively short-lived, geographically dispersed outbreaks. The search for safe and effective treatments is a clear example of this. Ahead of the 2022 clade IIb outbreak there were 2 main candidate therapeutics: tecovirimat and brincidofovir [4]. They have shown activity in vitro and in animal models, but their clinical effectiveness in humans is uncertain. Tecovirimat is currently registered as a treatment for mpox in the European Union (EU) and the United Kindgom (UK), based on “exceptional circumstances.” In the United States (US), tecovirimat is approved only for smallpox, under the “animal rule” exception for treatments for which human efficacy studies are not ethical or feasible and can be obtained for mpox through the Centers for Diseases Control and Prevention’s Expanded Access Investigational New Drug “compassionate use” protocol [5,6]. No similar mechanisms appear to exist at present for brincidofovir. As for clade I endemic countries, tecovirimat can be accessed under expanded access programme [7] in the Central African Republic (CAR) [8] where the treatment is not registered. Additionally, cidofovir is also being used off-label.
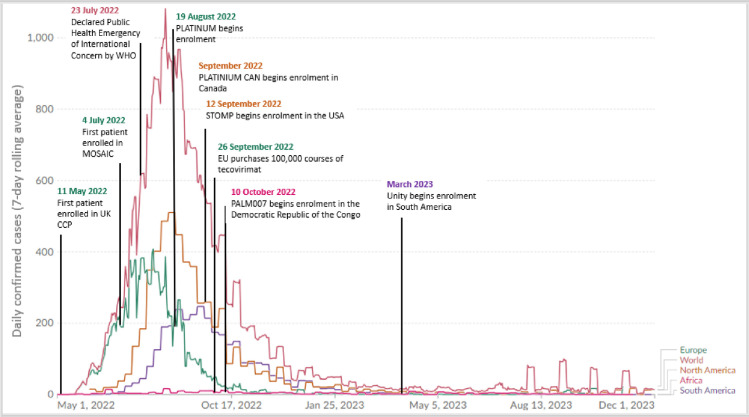
The current research landscape for mpox therapeutics—made up of both interventional and observational studies as well as Expanded Use Protocols (EAP)—is summarised in Table 1. While the situation is evolving, it is clear that, bar a major rebound of cases, this has been a missed opportunity for gathering evidence on the management of clade IIb cases (Fig 1).
Table 1. Summary of mpox research studies and programmes taking place as of December 2023.
| Study type | Study title | Sponsor | Location | Clade primarily studied | Target sample size | Status | Trial registration ID |
|---|---|---|---|---|---|---|---|
| RCT | PLATINUM-UK | University of Oxford | United Kingdom | Clade IIb | 450 | Stopped recruiting | ISRCTN17461766 |
| RCT | PLATINUM-CAN | McGill University | Canada | Clade IIb | 120 | Not yet recruiting | NCT05534165 |
| RCT | STOMP | NIAID/DAIDS | Multiple countries | Clade IIb | 530 | Actively recruiting | NCT05534984 |
| RCT | UNITY | Hospital University Geneva (HUG) | Brazil, Argentina, Switzerland | Clade IIb | 150 | Actively recruiting | NCT05597735 |
| RCT | EPOXI | UMC Utrecht ECRAID | Multiple countries (Europe) | Clade IIb | 644 | In set-up | NK |
| RCT | MOSA | PANTHER | Multiple countries (Africa) | Clade I and IIa | 500 | In set-up | NK |
| RCT | PALM007 | NIAID | Democratic Republic of Congo | Clade I | 450 | Actively recruiting | NCT05559099 |
| EAP | - | US Army Medical Research and Development Command | United States | Clade IIa | NA | Actively recruiting | NCT02080767 |
| EAP | Tecovirimat for mpox in CAR | University of Oxford | Central African Republic | Clade I | NA | Actively recruiting | ISRCTN43307947 |
| MEURI | - | WHO | Global | All clades | NA | Actively recruiting | NA |
| Observational/LICT | MOSAIC | University of Oxford | Multiple countries (Europe) | Clade IIb | NA | Stopped recruiting | 2022-501132-42-00 |
| Observational | UK CCP | University of Oxford | United Kingdom | All clades | NA | Actively recruiting (stopped recruiting clade IIb) | NA |
| Observational | NETPOX | Hospital Israelita Albert Einstein | Brazil | Clade IIb | 80 | In set-up | NCT05784038 |
CAR, Central African Republic; CTIS, Clinical Trials Information System; EAP, Expanded Use Protocol; EMA, European Medicine Agency; EU, European Union; LICT, Low Interventional Clinical Trial; PHEIC, Public Health Emergency of International Concern; UK, United Kingdom; US, United States.
Fig 1. Timeline of the events during the 2022 multicountry mpox outbreak and daily confirmed cases (7-day rolling average).
Image credit: Our World in Data: mpox [9].
While recruitment to one study, MOSAIC, started in July 2022, no more than 2 months after the outbreak commenced, most active trials began recruiting after the peak of the epidemic had passed and cases were already in a rapid decline (Fig 1), and as of October 2023, the rest are not recruiting. The EAP taking place in the US has to-date included almost 7,000 patients; while so far longitudinal data are publicly available from only on a minority of treated patients [9], it is hoped that more data will be released soon [10].
For clade I, the ongoing randomised controlled trial (RCT)—PALM 007—in the Democratic Republic of the Congo will hopefully provide a definitive answer to the effectiveness of tecovirimat for this clade. Meanwhile, the EAP taking place in CAR has treated so far 25 confirmed cases of mpox and collected data on clinical outcomes over at least 28 days.
While study protocols for clade IIb could be developed by adapting available clade I protocols, hurdles were faced in mounting a timely clinical research response to the clade IIb outbreaks, the reasons for which are both systemic and disease specific.
The systemic issues are in the inordinate amount of time that it takes after a protocol is developed to get through regulatory processes before enrolment can start. The lessons we learned from COVID-19 have not been used to make the system more efficient. The MOSAIC study, for example, which collects data on clinical and virological outcomes of patients with laboratory-confirmed mpox, has been the victim of a convoluted regulatory landscape that led to the study being treated as both an observational study in the UK and Switzerland and a Low Interventional Clinical Trial (LICT) in the EU—the latter requiring submission and review via the European Medicine Agency’s (EMA) Clinical Trials Information System (CTIS) platform and compliance with the EU Clinical Trial Regulation (despite no treatment being administered or altered under the MOSAIC protocol). Despite the declaration of a PHEIC, the median approval time for the study in the EU was 46.5 days, compared to 14 days in the UK and 20 days in Switzerland [11]. As a result, some of the prospective participating countries “missed the boat” as they could not start recruitment due to delays in contract negotiation. The true burden of these delays becomes evident when these prospective studies are compared with one of the success stories of mpox—the rapid generation of clinical characterisation and outcome data from retrospective case-data (such as SHARE-NET). These collaborations were often quick to produce novel clinical insights (such as cohorts of women [12] and people living with HIV/AIDS [13], or descriptions of re-infection following vaccination [13]). They demonstrate a strong desire from clinical researchers to commit to international collaboration and rapid evidence generation—and a clear dividend of doing so when not encumbered by regulatory barriers. A key frustration is that there is no to negligible difference in the level of risk to patients between these case series and observational cohorts such as MOSAIC.
More generally, due to the moving nature of clade IIb outbreaks (see Fig 1), current studies may not actually cover all areas of ongoing transmission. The present clinical research ecosystem is not designed to favour international collaborations establishing pre-positioned protocols and “dormant” studies which could be activated where and when cases occur.
There are also complications that are more disease specific. Differences in transmission routes [14], virology [15], and other unknown factors cause the variation in clinical presentation between clade IIb (also referred to as clade III) [16] and the known clade IIa and clade I patterns. Decisions on methodological issues lacked the benefit of consistently recorded clinical characterisation data for clade I (and the early cases for clade IIb), leading to uncertainty in trial protocols that could have impacts on sample size and efficiency. While most interventional and observational studies now share broadly consistent criteria and outcomes, uncertainties remain about study endpoints [17] and about reliability and agreement among clinicians in clade IIb lesions assessment [18].
These considerations are not unique to mpox. Most high-consequence pathogens cause outbreaks that are short-lived, geographically confined or sometimes dispersed, often occurring in difficult environments in low-resource countries where they affect marginalised populations. These diseases are neglected because of “market failure,” as they do not represent an appealing “business case” for profit-driven pharmaceutical companies. Yet, investing in collaborative research into these diseases would be crucial both to ensure equitable access to healthcare for marginalised populations, and also to be better prepared for potential further onward transmission and spread to wider geographical areas, including high-resource countries.
While it will never be possible to be entirely prepared for new outbreaks, we know the essential ingredients of an effective clinical research response.
First, a coherent strategy making the best use of observational studies—to give information on risk factors, clinical characterisation and expected outcomes—together with interventional clinical trials—to generate robust safety and efficacy data on vaccines and treatments. A case in point in the recent mpox outbreak being the speed at which cohort studies and case series were able to generate a substantial amount of data characterizing hundreds of patients across a diverse population [12,13,19,20].
Second, international collaborations to reach larger numbers of participants and greater geographic diversity, leading to results that are more generalizable, based on agreed-upon standardized or, at least, compatible, methodologies. Such has been the case for mpox, although the ongoing clade IIa studies may not reach the planned sample sizes. To offset these shortcomings, at least in part, a meta-analysis of individual patient data that is currently under discussion among investigators of placebo-controlled trials of tecovirimat. Creative collaborations and research framework are essential to provide rapid answers to health emergencies.
Third, a regulatory and political environment enabling processes and systems to support research, from prompt, adequate funding to rapid reviews of clinical trial applications so that research can take place where and when cases occur and have a chance to reach the planned sample size and generate robust evidence.
Fourth, investing into strengthening clinical research capacity in outbreak-prone areas beyond frontiers is not only an essential integral part of the preparedness strategy, but also a way to help low-resource countries build the agency to deal with locally relevant priority health issues.
Lastly, upfront commitments to equitable, affordable access to medical interventions found to be safe and effective in studies [17].
Lessons should have been learnt from COVID-19, but they were not acted upon for mpox. All actors in the research community—from individual research teams to regulators, to political and public health decision-makers to drug developers to legal departments at research sites—must step up and address pressing issues to provide an enabling environment for essential clinical research in outbreak response.
Funding Statement
This work was supported by the UK Foreign, Commonwealth and Development Office and Wellcome (215091/Z/18/Z) to P.H, the Bill & Melinda Gates Foundation [OPP1209135] to P.H and EU MPX Response to Y.Y, F.X.L and B.G. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nakoune E, Olliaro P. Waking up to monkeypox. BMJ. 2022;377:o1321. Epub 20220525. doi: 10.1136/bmj.o1321 . [DOI] [PubMed] [Google Scholar]
- 2.Mathieu E, Spooner F, Dattani S, Ritchie H, Roser M. Mpox (monkeypox): Our World in Data 2022. [05/12/2023]. Available from: https://ourworldindata.org/monkeypox. [Google Scholar]
- 3.The LM. Vaccines need equity to really work. Lancet Microbe. 2022;3(12):e888. doi: 10.1016/S2666-5247(22)00334-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox T, Gould S, Princy N, Rowland T, Lutje V, Kuehn R. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;(3). doi: 10.1002/14651858.CD015769 CD015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services FaDA. Product Development Under the Animal Rule. 2015.
- 6.United States Centers for Disease Control and Prevention. Guidance for Tecovirimat Use. 2023.
- 7.United States Food & Drug Administration. Expanded Access. 2022.
- 8.Mbrenga F, Nakouné E, Malaka C, Bourner J, Dunning J, Vernet G, et al. Tecovirimat for Monkeypox in Central African Republic under Expanded Access. N Engl J Med. 2022;387(24):2294–2295. Epub 20221130. doi: 10.1056/NEJMc2210015 ; PubMed Central PMCID: PMC10117058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Laughlin K, Tobolowsky FA, Elmor R, Overton R, O’Connor SM, Damon IK, et al. Clinical Use of Tecovirimat (Tpoxx) for Treatment of Monkeypox Under an Investigational New Drug Protocol ‐ United States, May-August 2022. MMWR Morb Mortal Wkly Rep. 2022;71(37):1190–1195. Epub 20220916. doi: 10.15585/mmwr.mm7137e1 ; PubMed Central PMCID: PMC9484807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Y, Peterson B. Personal communication. 2023. [Google Scholar]
- 11.Patrick-Brown TDJH, Bourner J, Kali S, Trøseid M, Yazdanpanah Y, Olliaro P, et al. Experiences and challenges with the new European Clinical Trials Regulation. Research Square. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornhill JP, Palich R, Ghosn J, Walmsley S, Moschese D, Cortes CP, et al. Human monkeypox virus infection in women and non-binary individuals during the 2022 outbreaks: a global case series. Lancet. 2022;400(10367):1953–1965. doi: 10.1016/S0140-6736(22)02187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitjà O, Alemany A, Marks M, Lezama Mora JI, Rodríguez-Aldama JC, Torres Silva MS, et al. Mpox in people with advanced HIV infection: a global case series. Lancet. 2023;401(10380):939–949. Epub 20230221. doi: 10.1016/S0140-6736(23)00273-8 . [DOI] [PubMed] [Google Scholar]
- 14.Angelo KM, Smith T, Camprubí-Ferrer D, Balerdi-Sarasola L, Díaz Menéndez M, Servera-Negre G, et al. Epidemiological and clinical characteristics of patients with monkeypox in the GeoSentinel Network: a cross-sectional study. Lancet Infect Dis. 2023;23(2):196–206. Epub 20221007. doi: 10.1016/S1473-3099(22)00651-X ; PubMed Central PMCID: PMC9546520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120(8):e2220415120. Epub 20230214. doi: 10.1073/pnas.2220415120 ; PubMed Central PMCID: PMC9974501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med. 2022;387(19):1783–1793. Epub 20221026. doi: 10.1056/NEJMra2208860 . [DOI] [PubMed] [Google Scholar]
- 17.Rojek A, Dunning J, Olliaro P. Monkeypox: how will we know if the treatments work? Lancet Infect Dis. 2022;22(9):1269–1270. Epub 20220802. doi: 10.1016/S1473-3099(22)00514-X ; PubMed Central PMCID: PMC9629677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones B, Paterson A, AlKhoury N, Bourner J, Dunning J, Olliaro P, et al. Variability in clinical assessment of clade IIb mpox lesions. Int J Infect Dis. 2023;137:60–62. doi: 10.1016/j.ijid.2023.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Fink DL, Callaby H, Luintel A, Beynon W, Bond H, Lim EY, et al. Clinical features and management of individuals admitted to hospital with monkeypox and associated complications across the UK: a retrospective cohort study. Lancet Infect Dis. 2023;23(5):589–597. Epub 20221222. doi: 10.1016/S1473-3099(22)00806-4 . [DOI] [PubMed] [Google Scholar]
- 20.Silva MST, Coutinho C, Torres TS, Peixoto E, Ismério R, Lessa F, et al. Ambulatory and hospitalized patients with suspected and confirmed mpox: an observational cohort study from Brazil. Lancet Reg Health Am. 2023;17:100406. Epub 20221213. doi: 10.1016/j.lana.2022.100406 ; PubMed Central PMCID: PMC9904017. [DOI] [PMC free article] [PubMed] [Google Scholar]