Abstract
Amyotrophic Lateral Sclerosis (ALS) is a devastating neurodegenerative disease which is strongly associated with age. The incidence of ALS increases from the age of 40 and peaks between the ages of 65 and 70. Most patients die of respiratory muscle paralysis or lung infections within three to five years of the appearance of symptoms, dealing a huge blow to patients and their families. With aging populations, improved diagnostic methods and changes in reporting criteria, the incidence of ALS is likely to show an upward trend in the coming decades. Despite extensive researches have been done, the cause and pathogenesis of ALS remains unclear. In recent decades, large quantities of studies focusing on gut microbiota have shown that gut microbiota and its metabolites seem to change the evolvement of ALS through the brain-gut-microbiota axis, and in turn, the progression of ALS will exacerbate the imbalance of gut microbiota, thereby forming a vicious cycle. This suggests that further exploration and identification of the function of gut microbiota in ALS may be crucial to break the bottleneck in the diagnosis and treatment of this disease. Hence, the current review summarizes and discusses the latest research advancement and future directions of ALS and brain-gut-microbiota axis, so as to help relevant researchers gain correlative information instantly.
Keywords: Amyotrophic lateral sclerosis, brain-gut-microbiota axis, gut microbiota, potential treatment, short-chain fatty acid, microglia
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized primarily by progressive and painless muscle weakness for the death of upper and lower motor neurons [1, 2]. The clinical manifestations of ALS can be divided into two categories: non-motor symptoms and motor symptoms. Non-motor symptoms mainly include frontotemporal dementia and cognitive impairment, while motor symptoms include dysphagia, dyspnea, and upper and lower limbs muscle weakness [1, 3].
Age and region are closely associated with the incidence of ALS. Studies show that the incidence of ALS increases gradually from the age of 40, peaks at 65 to 70, and then declines sharply [4]. Accordingly, age may play a significant role in ALS development. Furthermore, ALS was considered a relatively rare condition previously, but given factors such as the progress of aging population and the improvement of diagnostic methods, the incidence of ALS may show an increasing trend in the coming decades [3, 5]. There are also obvious regional differences in the incidence of ALS. According to statistics, the incidence in Europe was 2.2/1000000, higher than that in East Asia (0.89/10000) and South Asia (0.79/1000000) [6]. When latitude is taken as the reference, researchers found that the incidence in the northern population is higher than that in the southern [7]. The most common mutation of ALS is the C9orf72 gene, and some scholars believe that the mutation first occurred in ScandiNavia peninsula and spread outward from there [8]. Therefore, the incidence of ALS varies by region and may be the result of a combination of gene mutations and population migration. In addition, the onset of ALS may also closely relate to exercise. Several researches have demonstrated that most ALS patients have a high level of exercise intensity before the onset of the disease [9]. To be more specifically, the incidence of ALS may relate to the sports selected by the patients [10]. Scarmeas and his colleagues proposed that ALS is more prevalent among football players than among other athletes, and the risk of death is about 6.5 times that of other ALS athletes [11].
According to statistics, most patients die within 3-5 years of onset [12, 13]. However, there are also patients who live longer. A review showed that up to 10% of ALS patients survived for more than eight years after the onset [14]. Turner et al. found that the survival rate for patients over ten years was 4% [12]. There are many factors that can affect the survival time of patients, such as the location of lesion, gender, age, diet and environment [6, 15, 16]. Interestingly, the progression of ALS may also be strongly influenced by nutritional factors. Patients with ALS generally suffer from malnutrition, which is mainly caused by insufficient nutrition intake and exuberant metabolism [17, 18]. Malnutrition negatively affects neuromuscular status, which in turn is exacerbated by insufficient nutrition intake due to poor muscle status in ALS patients. In addition, we also summarized other potential positive and negative factors that may affect the occurrence and progression of ALS (Fig. 1) [9, 19-23]. It should be noted that there is still a lack of consensus on the role of certain factors in ALS. Therefore, the potential positive and negative factors of ALS are also one of the topics that need to be further explored in the future.
Figure 1.
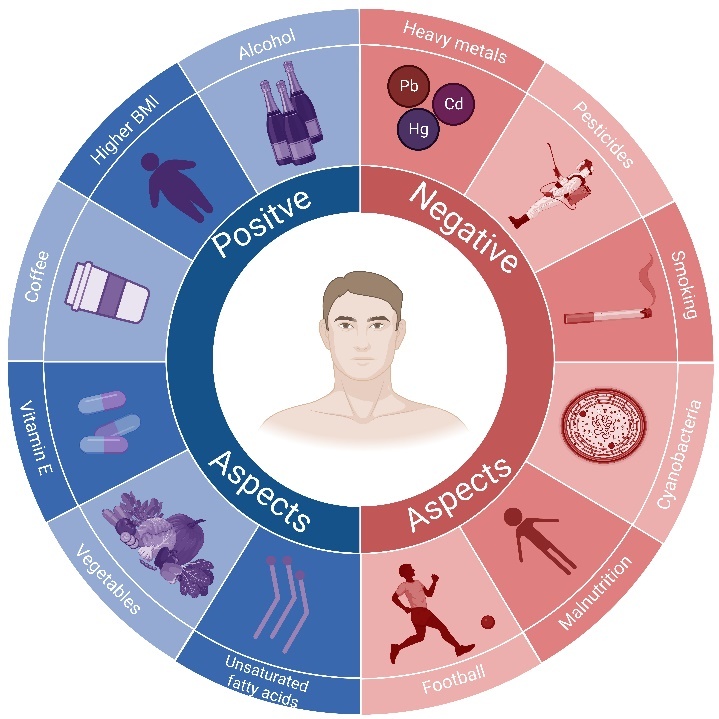
Potential positive and negative aspects for the onset and progression of ALS. Many factors are thought to influence the onset and progression of ALS. Among them, positive aspects such as higher alcohol consumption, higher BMI and active intake of Vitamin E are believed to delay the progression of the disease and are associated with longer survival. In contrast, negative aspects such as exposure to heavy metals and pesticides, smoking and playing football are thought to accelerate the progression of the disease and are closely related to poor prognosis.
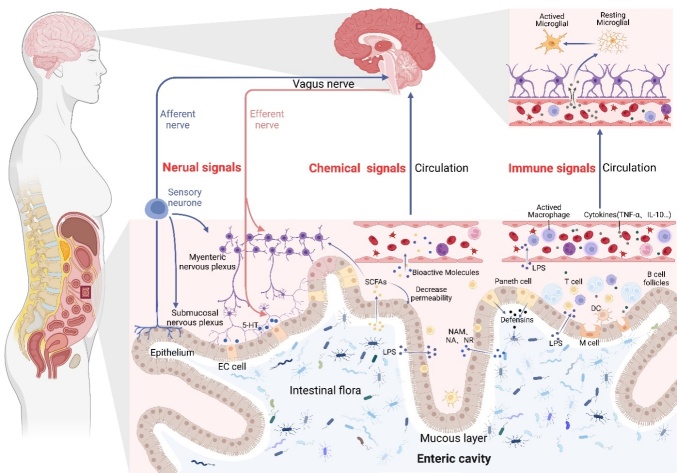
The dysbiosis of gut microbiota and the disorder of brain-gut-microbiota axis are thought to play a significant role in many neurodegenerative diseases [24-26]. Firmicutes and Bacteroides make up the majority of the gut microbiota, which help in maintaining intestinal function [27]. Gut microbiota is also involved in absorbing and storing nutrients, converting metabolites, maintaining intestinal barrier integrity and participating in immune responses [28]. The concept of brain-gut-microbiota axis was put forward and gradually improved in the 1840s. It mainly describes the information interaction process between the gut microbiota and the brain from three aspects: nerves, immunity and endocrine [29, 30]. Despite extensive researches have been done, the cause and pathogenesis of ALS remains unclear. However, many research results in recent years show that dysbiosis of gut microbiota may contribute to the occurrence and development of ALS. Through the brain-gut-microbiota axis, signals from the brain of patients with ALS can shape the gut microbiota (Fig. 2). Similarly, brain function can also be affected by gut microbial information [24, 31]. Therefore, improving the dysbiosis of gut microbiota may delay the progression of ALS and prolong the survival time of patients.
Figure 2.
Potential role of the brain-gut-microbiota axis in ALS. There is a two-way interaction between the brain-gut-microbiota axis. It acts in many ways, including immune, nervous, and endocrine mechanisms. In the immune mechanism, the gut microbiota can affect the CNS through two links of peripheral inflammation and central inflammation, in which LPS plays an important role. LPS can activate monocytes and macrophages in peripheral blood to induce the release of a large number of pro-inflammatory cytokines. In addition, TNF-α and IL-10 also play an important role in the immune mechanism. In the neural mechanism, gut microbiota signals can communicate bidirectionally with the CNS through ANS and ENS, and the mainly functional parts include vagus nerve, Paneth cell, and ENS. As for endocrine mechanism, small molecular substances from various bacteria, including SCFAs, NAM, NA, NR and others, play an important role in the development of ALS disease. They can enter the circulatory system through the blood and affect CNS. Any abnormality in any part of any one mechanism may cause the disorder of the whole brain-gut-microbiota axial circuit.
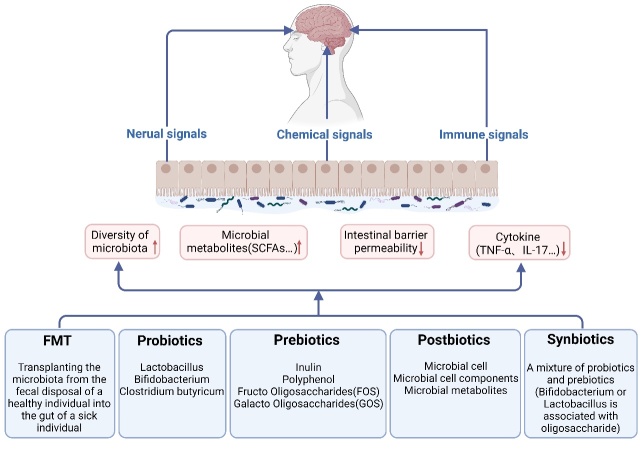
According to the research results of the brain-gut-microbiota axis, scientists have proposed many therapeutic strategies for gut microbiota, such as fecal microbial transplantation (FMT), supplementing probiotics, prebiotics, synbiotics and postbiotics. Current review aims to summarize the latest research results related to ALS, gut microbiota and brain-gut-microbiota axis, clarifying the mechanism of action between gut microbiota and brain. We hope that this review can help scholars in related fields to quickly grasp the research progress related to the disease, find new research ideas and promote the development of ALS treatment.
2. Dysbiosis of gut microbiota in ALS
With the problem of population aging becoming more and more prominent, research on aging is also deepening. In the latest research, the dysbiosis of gut microbiota is considered one of the twelve characteristics of aging, revealing the close correlation between aging and gut microbiota [32]. The composition and diversity of gut microbiota such as the ratio of Firmicutes/Bacteroides will change with age increased [33, 34]. Dysbiosis of gut microbiota was also observed in two mouse models of progeria [35].At the meantime, dysbiosis of gut microbiota will also affect aging process. After transplanting the fecal microbiota of the aged mice into the young mice, the quantity of pro-inflammatory cytokines and activated microglia in the young mice were increased, and aging conditions such as age-related neuroinflammation also occurred in the young mice[36]. On the contrary, after the fecal microbiota of young mice were transplanted into old mice, the aging condition of old mice was significantly improved [36]. ALS, as an age-related neurodegenerative disease, is also closely related to the gut microbiota. In a study of SOD1G93A mice, it was found that the progression of ALS can change the gut microbiota, thus influencing the life span of mice [37]. Therefore, studying the gut microbiota of patients with ALS may help us to better understand ALS.
More and more studies show that ALS is related to the dysbiosis of gut microbiota, and there are many differences between the ALS patients and healthy individuals in gut microbiota (Table 1). In the gastrointestinal tract, Firmicutes mainly produces butyrate, while Bacteroides mainly produces propionate [38], the ratio between them is closely related to human health [39]. The ratio of Firmicutes to Bacteroidetes commonly show a decline in ALS patients [40-42]. However, some studies have also pointed out that the ratio of Firmicutes to Bacteroides in ALS patients shows an increasing trend [43]. Studies have also shown that the decrease of Fusicatenibacter and Catenibacterium and the increase of Lachnospira in patients may associate with the higher risk of ALS [44]. Moreover, the increase in the relative abundance of OTU4607 Soutella and Lactobacillales order in ALS patients was also correlated with the genetically predicted increased susceptibility to ALS [45]. The bacteria such as Bacteroidetes, Odoribacter, Kineothrix, Sporobacter, Parabacteroides and unclassified Porphyromonadaceae are significantly increased in the intestinal tract of ALS patients [40]. Interestingly, a research found that the richness and uniformity of gut microbiota in ALS patients were obviously higher than their spouses, except for Prevotella spp and P.timonensis [46]. On the animal model, an experiment on SOD1G93A mice found that the quantity of Akkermansia, Coriobacteriaceae and Adlercreutzia in the intestine was low, indicating that the gut microbiota of ALS animals will change as well [37].
Table 1.
Dysbiosis of gut microbiota in ALS.
| Experimental subject
|
Conclusion | Ref. | ||
|---|---|---|---|---|
| Animal model | Human volunteer | |||
| Ning et al | _ | 20,806 cases with ALS and 59,804 controls (GWAS summary statistics from IALSC); 18,340 participants (GWAS summary statistics from the MiBioGen); 7824 participants (GWAS summary statistics from TwinsUK and KORA) |
Decreased abundance of Fusicatenibacter and Catenibacterium; increased abundance of Lachnospira; these changes may be related to the higher risk of ALS | [44] |
| Zhang et al | _ | 20,806 cases with ALS and 59,804 controls (GWAS summary statistics from IALSC); 1812 sanples (GWAS summary statistics); 7824 adult individuals (GWAS summary statistics from 2 European cohorts) |
Increased abundance of OTU4607 Soutella and Lactobacillales order; this change may be correlated with the genetically predicted increased susceptibility to ALS | [45] |
| Zeng et al | _ | 8 females and 12 females diagnosed with ALS; 8 females and 12 females matched controls |
Up-regulation of Bacteroidetes, Kineothrix, Parabacteroides, Odoribacter, Sporobacter, and unclassified Porphyromonadaceae | [40] |
| Hertzberg et al | _ | 10 patients diagnosed with ALS (3 females and 7 males) and their opposite sex spouses (7 females and 3 males); 10 opposite sex couples' healthy controls |
Compared with their spouses, down-regulation of Prevotella spp and P.timonensis; up-regulation of species richness and uniformity of gut microbiota. | [46] |
| Figueroa-Romero et al | SOD1(G93A) mice | _ | Down-regulation of Akkermansia, Coriobacteriaceae and Adlercreutzia | [37] |
| Nicholson et al | _ | 26 females and 40 males diagnosed with ALS; 36 females and 25 males' healthy controls; 7 females and 5 males' Neurodegenerative controls |
Decreased abundance of Roseburia intestinalis and Eubacterium rectale (two major butyrate-producing bacteria); decreased abundance of eight major butyrate-producing bacteria | [47] |
| Fang et al | _ | 6 patients diagnosed with ALS; 5 healthy controls |
Decreased abundance of Anaerostipes, Lachnospiraceae (two butyrate-producing bacteria) and Oscillabacter; Increased abundance of Dorea | [41] |
| Zhai et al | _ | 4 females and 4 males diagnosed with ALS; 4 females and 4 males' healthy controls |
Decreased abundance of Faecalibacterium (one butyrate-producing bacteria) | [43] |
| Zhang et al | SOD1(G93A) mice | _ | Decreased abundance of Lachnospiraceae (one butyrate-producing bacteria) occurred at the early stage of ALS disease | [48] |
| Wu et al | SOD1(G93A) mice | _ | Decreased abundance of Butyrivibrio Fibrisolvens (one butyrate-producing bacteria) |
[49] |
Of note, butyrate-producing bacteria showed reduced abundance in both ALS patients and animal models. In ALS patients, the abundance of Roseburia intestinalis and Eubacterium rectale was significantly reduced, which are two major butyrate-producing bacteria. Besides, the total relative abundance of eight main butyrate-producing bacteria was also obviously reduced [47]. Moreover, a high-throughput sequencing study showed that the relative abundance levels of two butyrate-producing bacteria, Anaerostipes and Lachnospiraceae, and genus Oscillabacter in the intestinal tract of ALS patients were significantly lower than those in the healthy control group, while the relative abundance of the harmful bacterium, Dorea, was significantly increased [41]. Markedly decreased of Faecalibacterium (a butyrate-producing bacterium) was also observed in patients with ALS [43]. The decrease of butyrate-producing bacteria was also observed in ALS animal model research. The butyrate-producing bacteria such as Lachnospiraceae in the intestinal tract of SOD1G93A mice were also significantly reduced, and the change of gut microbiota occurred at the early stage of ALS, even earlier than the deterioration of locomotor ability [48]. Similarly, the decrease of Butyrivibrio Fibrisolvens was also found in another group of SOD1G93A mice in the early phase of symptoms [49].
The experimental results in many animal models of ALS indicate that the dysbiosis of gut microbiota will further worsen ALS diseases. Compared with the SOD1G93A mice not treated with antibiotics, the SOD1G93A mice treated with antibiotics showed poorer locomotor ability and more severe ALS disease progression [50]. Not only that, researchers inoculated 11 microorganisms which were altered in ALS patients into SOD1G93A mice, and claimed that the disease progression in SOD1G93A mice was aggravated after P. dasonis and R.torques were transplanted [50]. Meanwhile, the dysbiosis of gut microbiota is closely concerned with the increase of intestinal microbial translocation. Plasma lipopoly-saccharide-binding protein (LBP) levels can reveal whether microbiota have entered the blood. And the obvious increase of LBP level in sALS patients indicates that dysbiosis of gut microbiota might promote intestinal microbial translocation in patients with sALS and thus affect the progression of sALS disease [51]. It is worth mentioning that at present, most experimental studies on antibiotic treatment of ALS animal models are conducted using SOD1G93A mice. And treatment of SOD1G93A mice with antibiotics often worsened the disease. On the contrary, antibiotic treatment for C9orf72 mutant mice can delay the progression of ALS disease [52].
3. Barrier dysfunction in ALS
There are many important "barriers" in the human body to maintain the microenvironment balance in different regions of the body, which are crucial for the health of the body. At present, many animal experiments and patient data of ALS have shown that the intestinal barrier, blood-brain barrier and blood-spinal cord barrier have different degrees of dysfunction, and the destruction of barrier function occurs before motor neuron degeneration and inflammatory reaction (Table 2).
Table 2.
Barrier Dysfunction in ALS.
| Experimental subject
|
Conclusion | Ref. | ||
|---|---|---|---|---|
| Animal model | Human volunteer | |||
| Intestinal barrier | ||||
| Wu et al | SOD1(G93A) mice | _ | Decreased expression of ZO-1,E-Cadherin-1 and lysozyme 1; increased expression of haptoglobin and abnormal Paneth cells | [49] |
| Zhang et al | _ | 7 females and 16 males diagnosed with sALS (mean age 59.2 ± 8.7 years); 6 females and 12 males' healthy donors (mean age 54.5 ± 8.4 years); 11 females and 7 males diagnosed with AD (mean age 78.9 ± 8.3 years) |
High expression of plasma LPS, increasing with the aggravation of clinical symptoms | [53] |
| Blood-spinal cord barrier | ||||
| Garbuzova-Davis et al | SOD1(G93A) mice | _ | Intracellular and extracellular edema of blood vessels; high degree of vacuolation and degenerative damage of endothelial cells | [55] |
| Nicaise et al | SOD1(G93A) mice | _ | Swelling and detachment of astrocyte terminal podoid from the endothelium; reduction of basement membrane component; capillary leakage; downregulation of tight junction proteins | [56] |
| Garbuzova-Davis et al | SOD1(G93A) mice | _ | Microbleeds | [57] |
| Nicaise et al | SOD1(G93A) mice | _ | Increased expression of AQP-4 | [58] |
| Zhong et al | SOD1(G93A) mice SOD1(G37R) mice SOD1(G85R) mice |
_ | Decreased expression of ZO-1, Occudin, Claudin-5, Glut-1, total length of capillaries, blood flow to the spinal cord; microbleeds and hemosiderin deposits in the spinal cord parenchyma | [60] |
| Garbuzova-Davis et al | _ | 12 males and 13 females diagnosed with ALS (mean age 64.8 ± 1.90 years); 13 males and 5 females’ controls (59.3 ± 3.78 years) |
Degeneration of pericytes around the blood-spinal cord barrier; dilation of type IV collagen in the perivascular basement membrane; ultrastructural abnormalities of white matter capillaries | [61] |
| Blood-brain barrier | ||||
| Garbuzova-Davis et al | SOD1(G93A) mice | _ | High degree of vacuolation and degeneration of endothelial cells | [55] |
| Nicaise et al | SOD1(G93A) mice | _ | Astrocytic degeneration; intracellular and extracellular edema of vascular endothelial cells; decreased levels of tight junction proteins; capillary leakage | [56] |
| Garbuzova-Davis et al | _ | 13 ALS patients (11 patients diagnosed with sALS, 2 patients diagnosed with fALS); 6 healthy controls |
Decreased level of circulating endothelial cells in the peripheral blood | [67] |
| Boston-Howes et al | SOD1(G93A) mice SOD1(B6SJL) mice |
_ | Increased expression of P-glycoprotein in spinal cord | [73] |
| Chan et al | SOD1(G93A) mice | _ | With the progression of ALS, the transporter activity of P-glycoprotein is increasing, and the expression level is also increasing | [72] |
| Garbuzova-Davis et al | _ | _ | The composition of the basement membrane is changed; the basement membrane metalloproteases MMP-2 and MMP-9 are activated | [68] |
3.1. Intestinal barrier
The intestinal barrier consists of mucus layer, epithelial barrier and intestinal vascular barrier. It can effectively regulate and control the nutrient substance in the intestinal tract to enter the blood, and simultaneously prevent the harmful substance from entering. It also plays a good barrier function by participating in the regulation of gut microbiota and intestinal secretion.
Currently, many experimental results have reported that the integrity of intestinal barrier is broken in ALS animal models and patients. For instance, decreased expression of ZO-1, E-Cadherin and increased haptoglobin were observed in the SOD1G93A mice, showing impaired tight junction and adhesive junction [49]. Besides, abnormal Paneth cells increased and lysozyme 1 decreased in SOD1G93A mice, which may induce autophagy dysfunction [49]. The decreased function of the body to eliminate misfolded proteins will further lead to intestinal dysfunction. The same study also proved that the intestinal uptake of FITC dextran was increased in ALS model mice, which means the increased intestinal permeability. At the same time, it was also proved that patients with sALS have a high level of Lipopolysaccharide (LPS) [53]. Physiological concentration of LPS can lead to impaired intestinal tight junction and increased intestinal permeability through a TLR4-dependent process[54]. In addition to increasing intestinal permeability, high level of LPS can also induce intestinal inflammation through the signal transduction axes of TLR4, FAK and MyD88, further destroying the intestinal barrier [54].
3.2. Blood-spinal cord barrier
The blood-spinal cord barrier is a unique barrier existing between the blood and spinal cord which is made up of capillary endothelial cells, endothelial basal cells, pericytes and astrocytes. It can effectively maintain the homeostasis around the spinal cord and ensure the normal physiological environment of spinal neurons.
At present, obvious damage to the integrity of the blood-spinal cord barrier can be observed in animals and patients with ALS. Ultrastructurally, scientists observed extracellular edema, endothelial cell edema and high vacuolation of capillaries [55]. In addition, pathological changes such as astrocyte foot process swelling, reduced basement membrane components, downregulation of tight junction protein and microbleeding have also been reported [56, 57]. Extensive vascular endothelial cell edema may be associated with elevated AQP-4 [58]. Moreover, the change of AQP-4 may weaken the capacity of astrocytes to preserve water and K+ homeostasis in the central nervous system (CNS), resulting in swelling of astrocyte foot process [59]. Before the onset of ALS in animal models, scientists have observed a 10-15% reduction in total capillary length and a 30-45% reduction in spinal cord blood flow [60]. In addition, microbleeds and hemosiderin deposits as well as decreased levels of ZO-1, Occudin, Claudin-5 and Glut-1 in the spinal cord parenchyma were also observed [60], all of which indicated that the damage of the blood-spinal cord barrier occurred before the onset of the disease.
It is worth mentioning that peripheral cell degeneration, type Ⅳ collagen dilatation of basement membrane and abnormal ultrastructure of white matter capillaries of spinal cord also occurs in patients with ALS [61]. But none of these changes were observed in animal models. After the integrity of the blood-spinal cord barrier is damaged and spinal cord injury occurs, reactive astrocytes express type IV collagen and promote the formation of glial scars [62]. In vitro experiments of the same study, IL-1β and TGFβ-1 cytokines were proved having the ability to induce the expression of type IV collagen in astrocytes [62]. The proliferation of reactive astrocytes and the secretion of various cytokines such as TNF-α and TGFβ-1 are also important factors for inflammation and neuronal degeneration in ALS[63, 64]. After reactive astrocyte activating factors such as TNF-α, IL-1α and C1q were knocked out, the survival time of SOD1G93A mice was significantly prolonged [65].
3.3. Blood-brain barrier
The blood-brain barrier exists not only between brain cells and plasma, but also between cerebrospinal fluid and plasma. It consists of brain microvascular endothelial cells, endothelial basement membrane, pericytes, and astrocyte perivascular foot. Similarly, it can effectively prevent macromolecular substances, harmful molecules and cells from entering the brain, regulate the beneficial substances into the brain, so as to maintain the steady state of the internal environment in the brain. At present, the integrity of the blood-brain barrier can be damaged in some animals and patients with ALS. At the ultrastructural level, the destruction of the blood-brain barrier was similar to that of the blood-spinal cord barrier. High degree of vacuolation and degeneration of endothelial cells occurs in SOD1G93A mice [55]. The increase of circulating endothelial cells is a signal of endothelial injury [66]. However, the quantity of circulating endothelial cells may show a downward trend in ALS patients as a whole because the increased endothelial cells in circulation attach to the damaged cells and form multi-layered endothelial structures [67]. In addition, the structure of the basement membrane was also disrupted. Studies showed that the basement membrane metalloproteases MMP-2 and MMP-9 which are able to degrade laminin, fibronectin, and proteoglycan were activated, thus leading to basement membrane damage [68]. SOD1G93A mice will survive longer without the MMP-9 [69]. Furthermore, astrocytic degeneration, intracellular and extracellular edema of vascular endothelial cells, decreased levels of tight junction proteins and capillary leakage also indicates that the integrity of the blood-brain barrier is damaged [56, 68].
Due to increased permeability, immune cells can cross the blood-brain barrier and gather in the CNS, inducing neuroinflammation or promoting neuronal apoptosis [70]. Moreover, microglia can secrete a variety of cytokines when activated by oxidative stress or inflammatory cytokines from the blood. Some cytokines are protective for nerve cells, while others are harmful. At the same time, blood-derived harmful substances can also reside in the CNS and cause toxic effects [70]. The lack of gut microbiota is related to the increase of the blood-brain barrier permeability. A study showed that the blood-brain barrier permeability was significantly higher in the sterile mice. Since then, scientists transplanted SCFAs-producing bacteria into the sterile mice and eventually found that the permeability was decreased [71].
P-glycoprotein, as an ABC transporter, is expressed in the microcapillary endothelial cells and has a wide range of substrate specificity. It is able to transport substrates from the blood-brain barrier and the blood-spinal cord barrier back to the blood, so it is the main obstacle for drug delivery to the brain and spinal cord [72]. As a drug used for treating ALS, the drug concentration of Riluzole in CNS is closely affected by P-glycoprotein. P-glycoprotein which expresses in spinal cord was increased in the SOD1 transgenic mice model [73]. Notably, recent studies found that with the deterioration of ALS animal, the expression level of P-glycoprotein in the blood-spinal cord barrier and blood-brain barrier increase, as well as its transport activity [72]. However, the absorption of riluzole in brain was significantly increased after the SOD1G93A mice were injected the P-glycoprotein inhibitor Elacridar [74]. It was also found that Riluzole uptake in the brain of mice was also obviously increased when Riluzole was used in combination with P-glycoprotein inhibitor Minocycline [75]. Therefore, multiple generations of P-glycoprotein inhibitors have been developed for P-glycoprotein [76].
4. Brain-gut-microbiota axis
4.1. Neural pathway of brain-gut-microbiota axis
Changes in gut microbiota can affect CNS and enteric nervous system (ENS) [77]. Neurodevelopment is consistently affected by gut microbiota [78]. In 1-month-old SOD1G93A mice, there was already an observable gut microbial disturbance in the absence of neuromuscular symptoms [48]. Although this phenomenon has not been demonstrated in human ALS patients, the results observed in animal models provide us with a new possibility that the gut microbiota may silently change and affect the neurodevelopment before the onset of ALS in susceptible individuals. Intestinal microbial signals can communicate bidirectionally with the CNS through the autonomic nervous system (ANS) and ENS [30]. ANS consists of sympathetic nervous system and parasympathetic nervous system. ENS is a neural network that exists at the interface between host and gut microbiota which consists of myenteric plexus and submucosal plexus. Although the ENS can operate completely independently of neural inputs from the spinal cord and brain, in actual physiological processes, ENS, sympathetic nervous system and parasympathetic nervous systems often cooperate to complete the information interaction between the CNS and the gut [79].
Enterochromaffin cells and vagus nerve may play an essential role in the process of transmitting intestinal microbial signals to the CNS in ALS patients. Enterochromaffin cells are bidirectional information converters between gut microbiota and enteric nerves. The intestinal lumen side of enterochromaffin cells can detect the stimulation from gut microbiota and food ingested by the host. Subsequently, the vagal afferent nerve endings located in the lamina propria can be activated by substances such as 5-HT and histamine secreted by chromaffin cells [80-82]. The vagus nerve consists of 80% afferent fibers and 20% efferent fibers, which is an important part of the ANS [30]. In a mouse experiment, researchers found that local infection of Campylobacter jejuni in the intestine can activate the sensory neurons of the vagus nerve, thus activating nucleus tractus solitarius (nTS) and areas related to the main visceral sensory pathways in the brain [83]. Ronchi et al. observed reduced activation of hippocampal microglia on the 15th day after injury to vagal afferents in mice [84]. This suggests that the vagus nerve may start providing warning signals to the brains of ALS susceptible persons as soon as small changes occur in the gut microbiota. And these warning signals may have long-term effects on the brain by activating chronic neuroinflammation.
Constipation caused by decreased intestinal motility is one of the common clinical manifestations of ALS [85]. Intestinal motility is mainly regulated by ANS and ENS [79]. Intrinsic sensory neurons such as Dogiel type I and type II neurons in the gastrointestinal tract provide a wide range of action sites for metabolites [79]. Metabolites such as SCFAs, chemotactic peptides and tryptamine derived from microbiota are believed to act on the ENS and then affect the intestinal transport rate [81, 86]. Therefore, we suppose that constipation symptoms in ALS patients may relate to the altered gut microbiota metabolites which can act on the ENS. In addition, ENS is very similar to the CNS in terms of structure and neurochemistry [87], but Kulkarni and his colleagues found substantial neuronal transformations and neurogenesis in adult intestinal neurons [88]. The gut microbiota of the human body is constantly changing dynamically. If the above conclusion is correct, the gut microbiota may also directly affect ENS neurons in the development stage, thereby changing the intestinal function of patients with ALS.
Not only is the function of CNS affected by the gut microbiota, but the gut microbiota is also affected by the neuromuscular dysfunction caused by ALS itself. ALS is a progressive degenerative disease of motor neurons. The chewing, swallowing and intestinal motor function of ALS patients will deteriorate to varying degrees over the course of progression. Therefore, with the progression of ALS, the gut microbiota not only needs to face external environmental pressure, but also is affected by the disease symptoms themselves. There are many factors affecting the gut microbiota. First of all, the slow food intake rate of patients with ALS and even the changed food intake mode (gastrostomy can be selected for patients with ALS due to dysphagia) may affect the type, quantity and quality of food intake [89], affecting the composition of gut microbiota. Secondly, reduced intestinal transport capacity will lead to the excessive reproduction of some gut microbiota [90]. In the condition of limited living resources, the original balance of the gut microbiota will be broken. In addition, the intestinal mucus layer is one of the main living areas of gut microbiota, and the dysfunction in secreting mucus, bicarbonate and water will also change the living environment of intestinal microorganisms and affect the composition of the microbiota. Furthermore, Macfarlane and his colleagues also proposed the possibility that ANS could directly regulate the response of gut immune cells to the gut microbiota [91].
4.2. Immune pathway of brain-gut-microbiota axis
The progression of ALS and the survival time of ALS patients may greatly be influenced by immune inflammatory response induced by gut microbiota. A study on C9orf72 mice showed that broad-spectrum antibiotics could effectively interfere with the inflammation and autoimmune phenotype in ALS mice before and after the onset of disease. Subsequent studies further confirmed that the therapeutic effect of antibiotics was actually achieved by changing the gut microbiota [92].
The biological signals emitted by the intestinal microbiota can finally affect the central neurons through peripheral inflammation and central inflammation. LPS seems to play an essential role in peripheral inflammation of ALS patients. The results of serum analysis showed that plasma LPS levels in sALS patients were significantly higher than healthy individuals, but none of the ALS patients participating in the study had any active infection during the trial [53]. Therefore, we speculate that the high plasma LPS level in patients with ALS is likely to come from the gut microbiota. This conjecture is also indirectly supported by the impaired intestinal barrier of SOD1G93A mice [49]. An intact mucosal barrier is the first line of defense against pathogenic bacteria in the organism, and the increase of intestinal permeability is not conducive to the host's maintenance of internal balance. In addition, Qin and his colleagues found that after a single intraperitoneal injection of LPS, the microglial cells in wild-type mice will be chronic, voluntary, and progressive activated [93]. Nguyen et al. further found that a single acute intraperitoneal injection of LPS did not make a significant difference between SOD1G93A and wild-type mice [94]. Only the chronic non-toxic dose of LPS injection resulted in accelerated motor axon loss and shortened median survival time in genetically susceptible mice, and none of these effects seen in wild-type mice. These experimental results provide us with a new idea that chronic LPS derived from gut microbiota is very likely an essential reason for the progression of ALS.
In a healthy body, the blood-brain barrier has very low permeability, and rarely can the peripheral LPS directly enter the brain. Therefore, LPS is more likely to have an indirect effect on neuroinflammation[95]. The research by Burberry et al. found that chemical analogs of microbial components could stimulate bone marrow-derived macrophages of C9orf72 mice [92], indicating that there might be other links between peripheral LPS and central system inflammation. Not only in animal models, Zhang R et al. also observed activated monocytes/macrophages in plasma of sALS patients [96], and further reported that the activation level of monocytes in sALS patients was directly related to plasma LPS levels four years later [53]. In addition, many other teams have repeatedly mentioned in their research results that there are proinflammatory cytokines released by macrophages such as TNF-α and IL-6 in the cerebrospinal fluid and serum of ALS patients [97, 98]. In summary, we speculated that products of gut microbiota represented by LPS could continuously and slowly enter the bodies of ALS patients, and the damage of intestinal barrier facilitated this process. LPS can activate monocytes and macrophages in peripheral blood to induce the release of a large quantity of pro-inflammatory cytokines, thereby affecting the CNS. In addition to increased proinflammatory cytokines, decreased secretion of anti-inflammatory cytokines such as IL-10 and inflammatory regulatory products such as butyric acid may also promote the progression of ALS [53, 99].
TNF-α may be a bridge between peripheral inflammation and central inflammatory response. The level of TNF-α in cerebrospinal fluid of ALS patients is increased[100], and animal experiments show that the increase of TNF-α in brain lags behind that of liver and serum [93]. Therefore, the production of TNF-α in the brain may be initiated after peripheral TNF-α enters the CNS through blood circulation. TNF-α entering the CNS may promote microglia-mediated neuronal death. Microglia is a type of glial cells, which is equivalent to macrophages in the brain and spinal cord. Microglial cell-centered neuroinflammation can cause neuronal death directly or indirectly and aggravate the symptoms of the disease. The role of TLR2-mediated signaling pathway in neuronal death has begun to receive more attention. It is found that chronic LPS stimulation can activate TNF-α gene and continuously induce the expression of TLR2 in microglia [94]. TLR2 can enhance the inflammatory response of microglia in the early stage while inducing microglia apoptosis in the late stage [101, 102]. In addition, motor neurons death mediated by FasR which belongs to TNF receptor superfamily, may also be one of the mechanisms of motor neuron death in ALS patients. Fas-induced neuronal death is realized through Fas-Daxx-ASK1-p38-NO-nNOS-peroxynitrite pathway.
Interestingly, the study also found that the SOD1G93A mutant is highly sensitive to the activation of NO downstream pathway induced by Fas[103], which may explain the role of heredity in the development of ALS. As stated above, the neuronal damage mediated by TLR2 and FasR also supports the key bridging role of TNF-α. In addition, the dysfunction of DNA damage response (DDR) in microglia is also closely related to neurodegeneration. Normally, TDP-43 positive inclusions only appeared in the nucleus. However, TDP-43 positive inclusion was found in the cytoplasm of microglia and MDMi (microglia-like cells induced by PBMCs from peripheral blood) of ALS patients, and all inclusion were abnormally phosphorylated [104]. Furthermore, DNA damage accumulation and impaired phagocytic function have been found in MDMi cells of patients with ALS [104]. Therefore, we speculate that TDP-43 in ALS patients cannot be recruited in time to the foci of DNA damage in the nucleus. Dysfunctional DDR results in the accumulation of DNA mutations in neurons or microglia, ultimately causing abnormal neuroprotein synthesis and cell death.
NLRP3 inflammasome is an essential part of the innate immunity of the body after tissue damage [105]. Recent research suggests that NLRP3 inflammasome may also promote neuronal death in ALS patients. It has been found that NLRP3 and IL-1β could already be detected in SOD1G93A mice in the pre-symptom stage, and this phenomenon was more obvious in 14-week-old animals [106]. Behavioral and cognitive abnormalities in ALS patients are thought to be associated with neurodegeneration in the subcortical region. NLRP3 expression was increased in both dorsal thalamic nucleus and neurons of SOD1G93A mice [107]. It was also found that down-regulating the steroid hormone 17β-estradiol activated by inflammatory bodies can reduced motor neuron death in SOD1G93A mice [108]. Increased levels of NLRP3, ASC, IL-18 and caspase-1 have been observed not only in animal models, but also in human patients with ALS [106, 109, 110].Therefore, NLRP3 inflammasome may become a potential target for intervention of neuronal death. The activation of NLRP3 inflammasome can be divided into two stages: initiation and activation [111]. Risk signals such as oxidative stress, lysosomal damage and Ca2+ mobilization can be identified through the damage-related molecular pattern (DAMPs) or the pathogen-related molecular pattern (PAMPs), thereby initiating the assembly of NLRP3 inflammasome [112]. Activated caspase-1 can generate cell membrane pores and initiate cell apoptosis [111]. Trimethylamine oxide (TMAO) and short-chain fatty acids (SCFAs) are important metabolites of intestinal microorganisms. By promoting the expression of TLR4, TMAO can activate the NLRP3 inflammasome, which increases the level of inflammatory factors in the CNS [113]. SCFAs, on the other hand, could inhibit the over-expressions of ASC, NLRP3, IL-18, IL-1β and caspase-1, showing the opposite effect to that of TMAO [114]. Activation of NLRP3 inflammasome can regulate caspase-1-dependent pathways and the release of pro-inflammatory cytokines such as IL-1β to respond to cell stress and infection. However, persistent neuroinflammation and brain injury will occur when NLRP3 inflammasome is over-activated [85], therefore aggravate the symptoms of the disease.
It has been widely recognized that the innate immune response is involved in the process of ALS, but whether adaptive immunity participates in the degeneration and death of motor neurons is still controversial. Burberry et al. reported neutrophils and T-lymphocyte infiltration in the spinal cord of C9orf72 mice [92], while Nguyen's team failed to detect CD8+ and CD4+ cells after immunohistochemistry of the spinal cord and brain of SOD1G93A mice [94]. However, due to the differences between the two groups of experimental animal models, the interference of genetic factors in the experimental results cannot be excluded.
Through immune pathways, the CNS can be influenced by the gut microbiota, and the gut microbiota can also be affected by the CNS. The intestinal mucosal immune system is composed of intestinal mucosal epithelial tissues, mucosa-associated lymphoid tissues, immune cells and their secretions and symbiotic microbiota on the mucosa. It is an essential part of the immune system and the main place for local specific immune response. The Paneth cell, located at the base of the small intestinal crypt, is an intestinal epithelial cell that secretes defensins, which can penetrate the bacterial cell membrane to lyse bacteria and regulate the gut microbiota. Therefore, the dysfunction of Paneth cell will affect the steady state of gut microbiota. A study reported that the number of abnormal Paneth cells in SOD1G93A mice increased while the production of defensin 5α decreased. This result implies a diminished role for Paneth cells in maintaining intestinal microbial homeostasis [49]. In addition, studies have shown that the ratio of Sclerotinia to Bacteroides of mice with the caspase-1 gene knockout is significantly reduced [115], which means that activated NLRP3 inflammasomes may aggravate the imbalance of gut microbiota by regulating its composition. Furthermore, a study evaluating gastrointestinal health and fecal microbiota of patients with ALS found that the concentrations of inflammatory markers such as sIgA, calmodulin and eosinophils in the feces of patients increased [116], which indicated that adaptive immune response activated in ALS patients may also pose challenges to the survival of some gut microbiota.
4.3. Endocrine pathway of brain- gut-microbiota axis
SCFAs, NAD+ precursors, amino acid derivatives and bile acids are all small molecules derived from gut microbiota [117]. In the following sections, we will focus on the recent findings of SCFAs and NAD+ precursors in ALS.
4.3.1. SCFAs
SCFAs mainly include propionate, acetate and butyrate. Most SCFAs come from fermentation of undigested fiber by colonic and cecal microbiota, with a small proportion are produced through amino acid metabolism [118]. After entering the bloodstream through uptake of intestinal epithelial cells, SCFAs can even enter the CNS through the blood-brain barrier [118].SCFAs can enter cells through passive diffusion, the MCT1 transporter, the SMCT1 transporter and the bicarbonate co-transport pathway [119]. After entering cells, SCFAs can act as histone deacetylase (HDAC) inhibitors or participate in cellular energy metabolism to regulate cellular functions. SCFAs can also induces a series of intracellular reactions by binding to cell surface receptors GPR41(FFAR3), GPR43(FFAR2) and GPR109A(NIACR1) [120, 121].
SCFAs can reduce intestinal barrier permeability by affecting the mucus layer and cell layer. SOD1G93A mice showed increased intestinal barrier permeability and decreased intestinal butyric acid-producing bacteria. However, after butyrate treatment, scientists found that increased expression of the tight junction protein in the mice delayed the occurrence of ALS [49, 99]. Multiple studies have demonstrated that butyrate helps maintain the intestinal barrier's stability. Butyrate stimulates the expression of MUC-2 in goblet cells by triggering the release of prostaglandin E1/E2 from subepithelial myofibroblasts [122]. MUC-2, as an important glycoprotein in the mucus layer, isolates pathogenic bacteria and controls inflammation [123]. MUC-2 can also form the receptor complex of galectin-3-Dectin-1-FCGR2B and activate β-catenin to inhibit nuclear factor kB (NF-kB), thereby inhibiting the immune response of inflammatory dendritic cells (DC) [124]. For the cell layer, butyrate can promote the intestinal epithelial cells to consume oxygen, produce the hypoxia-inducible factor (HIF) and induce the expression of claudin-1 [125, 126]. In addition, butyrate can upregulate the actin-binding protein synaptophysin (SYNPO) in epithelial cells by inhibiting HDAC. Located at the tight junction of the intestinal epithelium and within F- actin fibers, SYNPO helps maintain the integrity of the intestinal barrier and cell movement. SYNPO-deficient mice have increased gut permeability and are vulnerable to colitis, and butyrate supplementation contributes to symptomatic improvement [127].
SCFAs regulate the body's inflammatory response through multiple pathways, the most important of which is through regulatory T cells (Tregs) and microglia. Tregs can inhibit pro-inflammatory T cells, activated macrophages and microglia, thus effectively controlling the peripheral and central inflammatory response [128]. It is proved that the quantity of Tregs in patients with faster progression of ALS is decreased, with a decrease in the expression of FOXP3 which is an important transcription factor for Tregs to function [128, 129]. A study by Furusawa et al. showed that butyrate derived from the microbiota could inhibit HDAC and up-regulate the expression of FoxP3, thereby inducing the differentiation of Tregs [130]. In addition, the binding of butyric acid to GPR 109 A (which is also a nicotinic acid receptor) can also induce the differentiation of Tregs [131]. Central inflammation caused by abnormal activation of microglia is an important pathogenesis of ALS [52]. Inhibition of HDAC in microglia by SCFAs reduced the release of pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) and increased the release of anti-inflammatory cytokines (TGF-β and IL-4), thereby inhibiting inflammation [132, 133]. Ionized calcium-binding adaptor molecule 1 (IBA1) is a calcium-binding protein specifically expressed by macrophages and microglia. IBA1 is upregulated when microglia are activated. Butyrate supplementation has been shown to negatively regulate the serum IL-17 and LPS levels of SOD1G93A mice and inhibit the expression of IBA1 in the spinal cord [134]. This study further sheds light on the mechanism by which butyrate improves ALS disease progression by inhibiting neuroinflammation.
SCFAs can act directly on the ENS as well as the ANS. GPR41/43 is an SCFAs receptor which is expressed in the myenteric plexus, the nodose ganglia and the dorsal root ganglia. When SCFAs act on the above receptors, the discharge of vague afferent nerves will be increased, the spinal cord will be excited, and eventually the intestinal activity will be enhanced [135, 136]. SCFAs can also act on the sympathetic nervous system. Muller and his colleagues found the regulatory effect of SCFAs on sympathetic ganglia and its neural circuit for promoting intestinal motility [137]. In addition, by increasing the proportion of cholinergic neurons in ENS, butyrate can also enhance the locomotor function of the colon [138]. The potential association between reduced SCFA-producing bacteria and disturbed intestinal motility in ALS patients may be associated with the above mechanisms. Unfortunately, the improvement effect of SCFAs on intestinal motility was mostly reflected in animal experiments, and no human trial has yet shown that SCFAs supplementation can significantly improve human intestinal motility [139, 140].
In conclusion, SCFAs can affect ALS in many ways. Drug design and research for SCFAs targets will have a broad prospect [141]. Recent clinical trials have shown that SCFA supplementation or high SCFA diet has a certain effect on improving ALS-related symptoms [142, 143]. However, the effect of SCFAs on improving the core symptoms of ALS needs further study.
4.3.2. NAD+ precursor
NAD+ precursors include nicotinamide nucleoside (NR), nicotinic acid (NA) and nicotinamide (NAM). NAD+ is an essential coenzyme for cellular energy transduction and antioxidant function. It is synthesized in the human body mainly through three pathways: de novo synthesis using dietary tryptophan (kynurenine pathway), de novo synthesis using dietary NA (Preiss-Handler pathway), and salvage synthesis using NAM [144]. Metabolic disorder and decreased level of NAD+ is closely related to ALS [144, 145]. ALS patients were found to have high levels of tryptophan and low levels of NAM in serum and cerebrospinal fluid, which indicated that there may be injuries in both the kynurenine pathway and salvage synthesis pathway, resulting in NAD+ deficiency and weakened antioxidant capacity [50, 144]. The main reason for the decrease of NAD+ content may be the deficiency of salvage synthesis [146]. Restoration of NAD+ level can ameliorate ALS from multiple perspectives, such as reducing the toxicity of astrocytes to neurons, promoting neurogenesis, enhancing muscle function and regulating gene expression [145]. In recent years, it has been found that the gut microbiota is conducive to maintaining the level of NAD+ [147]. Colonization of A. muciniphila of SOD1G93A mice can increase NAM in the plasma and cerebrospinal fluid, resulting in improved ALS symptoms and prolonged survival [50]. The same experiment also showed that supplementing NAM can down-regulate the expression of ALS-related genes in the spinal cord of mice and improve the symptoms [50]. In a controlled trial, bacterial genes associated with NAM metabolism were reduced in stool and NAM levels in cerebrospinal fluid and serum were significantly reduced in ALS patients [89]. It was deduced from the above results that NAM derived from the microbiota could enter the CNS and affect the progression of ALS.
NAD+ precursor can mediate bidirectional communication between the host and gut microbiota. The intestinal microbiota can synthesize NA using NAM in the host's blood circulation, and NA can then return to the blood circulation for the host to synthesize NAD+. In germ-free mice, elevated levels of NAM in the intestinal lumen were accompanied by a decrease in NA levels. Furthermore, with intact gut microbiota, NA levels in blood circulation remained stable even when mice did not consume NA from the diet [148]. Therefore, the imbalance of gut microbiota may lead to the imbalance of NAD+ metabolism. In addition, gut microbiota may promote NR transformation. NR (vitamin B3) is a common NAD nutritional supplement with a stronger ability to produce NAD+ than NAM and NA [114].Compared to antibiotic-treated mice, mice with intact gut microbiota have a greater capacity to generate tissue NAD+ using NR [148]. Treatment of SOD1G93A mice with NR can clear the mistakenly accumulated hSOD1 protein in mitochondria and induce neurogenesis [146]. Interestingly, in this experiment, NR treatment improved the locomotor ability of the mice but fail to delay the occurrence of ALS and extend the survival time, which is different from the results of the above-mentioned study supplemented with the NAM-producing strain A. muciniphila. Whether the microbiota imbalance in SOD1G93A mice drives this outcome requires further investigation.
5. Potential treatment
Currently, only three drugs have been approved by the FDA for clinical treatment of ALS, namely, Riluzole, Edaravone, and Relyvrio [149, 150]. With increasing research on the relationship between gut microbiota and CNS diseases, many researchers hope to treat ALS by regulating the gut microbiota. Currently proposed potential therapeutic strategies mainly include FMT, probiotics, prebiotics, synbiotics and postbiotics (Fig. 3, Table 3). However, most of the research has been done in animal models. Few experimental data are available on patients. Whether the same conclusions can be switched between animal models and patients still needs to be further investigated.
Figure 3.
Potential therapeutic strategies based on brain-gut-microbiota axis for ALS. Potential therapeutic strategies for ALS include supplementation with probiotics, prebiotics, synbiotics, postbiotics and FMT. Based on the association of the brain-gut-microbiota axis with ALS, regulating the intestinal microbial composition or interfering with the microbiota metabolites can improve the pathology of ALS and delay disease progression by regulating the neural, immune and endocrine pathways. To date, most relevant studies have been conducted on animal models. The conversion between the research results on animal models and the treatment effect of human patients still needs to be further investigated.
Table 3.
Potential therapeutic strategies based on brain-gut-microbiota axis for ALS.
| Experimental subject
|
Conclusion | Ref. | ||
|---|---|---|---|---|
| Animal model | Human volunteer | |||
| FMT | ||||
| Mandrioli et al | _ | 42 patients diagnosed with ALS | No research results | [152] |
| Probiotics | ||||
| Blacher E et al Gotkine et al |
SOD1(G93A) mice | _ | Improved movement ability and survival rate after oral administration of Akkermansia muciniphila; Increased content of NAM | [50][89] |
| Di Gioia et al | _ | 28 males and 22 females diagnosed with ALS (mean age 60.24 years); 28 males and 22 females' matched controls (mean age 53.60 years) |
After patients with ALS were treated with a probiotic formula consisting of five lactic acids, the diversity of the gut microbiota of the patients was regulated | [155] |
| Prebiotics | ||||
| Etxeberria et al | Wistar rats | _ | Increased abundance of Akkermansia muciniphila in the intestinal tract after intake of quercetin for six weeks | [157] |
| Jeong et al | C57/BL6 mice | _ | Increased abundance of Akkermansia muciniphila in the intestinal tract after a period of green tea intake | [158] |
| Song et al | SOD1(G93A) mice | _ | Increased life span; decreased loss of motor neurons; inhibited expression of inflammatory factors TNF-α and iNOS after intake of GOS and prebiotic yogurt rich in GOS | [161] |
| Postbiotics | ||||
| Zhang et al | SOD1(G93A) mice | _ | Significant recovery of the intestinal barrier function; delay in the progress of ALS disease; increased life span after butyrate treatment | [99] |
| Ogbu et al | SOD1(G93A) mice | _ | Obviously decreased of inflammatory factors such as IL-17 and LPS after butyrate treatment | [134] |
| Kaji R et al | _ | 260 patients diagnosed with ALS | Prolonged survival and improved the symptoms of ALS patients (diagnosed within 1 year of symptom onset) after treatment with ultra-high doses of mecobalamin | [166] |
| Oki R et al | _ | 126 patients diagnosed with ALS (completed the observation period and double-blind stage) | Prolonged survival and improved the symptoms of ALS patients (diagnosed within 1 year of symptom onset) after treatment with ultra-high doses of mecobalamin | [167] |
5.1. FMT
FMT refers to the directional transplantation of microbiota from fecal of healthy individuals into the intestines of diseased individuals, reestablishing the intestinal microecology of patients and treating diseases [151]. Based on the function of gut microbiota in neurodegenerative diseases, FMT has shown promising therapeutic effects in the treatment of various central nervous system diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) [24]. FMT is a promising research direction for ALS treatment. However, there are not many clinical trials to verify the efficacy of FMT for ALS disease. A team conducted a multi-center randomized double-blind clinical trial of FMT for ALS in 2019 [152], but no trial result is available yet.
Interestingly, experimental results comparing the effects of fresh and frozen fecal transplantation indicated that the ability of FMT to restore intestinal microecology in mice decreased with the extension of freezing time [153]. The same experiment also found that freezing reduced the intestinal colonization ability of some microorganisms [153]. Therefore, the influence of fecal storage conditions on the therapeutic effect of FMT is also worthy of attention.
5.2. Probiotics
Probiotics are active microorganisms which can bring health to the host [154]. Probiotics improve the immune, nervous, and endocrine functions of the host through their metabolites. In addition, supplying the probiotics also helps to maintain the intestinal microecological balance of the host. Therefore, probiotics are a relatively safe and potential treatment for ALS and other CNS diseases. Common probiotics include Lactobacillus, Bifidobacterium, Clostridium butyricum.
It is demonstrated that NAM in cerebrospinal fluid of SOD1G93A mice was significantly decreased. After oral administration of Akkermansia muciniphila, the content of NAM in cerebrospinal fluid is effectively increased and the movement ability of SOD1G93A mice is effectively improved [89]. At the same time, another study also proved that colonization of Akkermansia muciniphila in SOD1G93A mice can increase the NAM level in cerebrospinal fluid and improve the locomotor ability and survival rate of mice [50]. In addition, after patients with ALS were treated with a probiotic formula consisting of Lactobacillus salivarius, Lactobacillus plantarum, Lactobacillus delbrueckii subsp. delbrueckii, Lactobacillus fermentum and Streptococcus thermophilus, the diversity of gut microbiota increased [155].
5.3. Prebiotics
Prebiotics are non-digestible food ingredient which are beneficial to the host through selective modulation of microbiota [156]. It is often served as an alternative to probiotics or as an additional supplement. Administration of appropriate prebiotics can regulate the microecological balance of the host. At present, the common prebiotics include inulin, polyphenol, oligofructose and galactooligosaccharides.
Polyphenols are prebiotics widely studied. Many substances belong to polyphenol category, such as quercetin, catechins, naringin, hesperidin, genistein, proanthocyanidin, baicalin, gallic acid, caffeic acid, resveratrol, pterostilbene and curcumin. After intake of quercetin for six weeks, the abundance of Akkermansia muciniphila in rat model was obviously increased [157]. The same result has also been reported in a mouse after ingestion of green tea, and it was later demonstrated that the increase in Akkermansia muciniphila level was mainly affected by the polyphenol substance EGCG in green tea [158]. At the same time, EGCG can prolong the life of SOD1G93A mice and alleviate the symptoms of ALS disease [159]. EGCG can also selectively promote the growth of Lactobacillus and Bifidobacterium, and significantly increase the level of SCFAs [160].
Galactooligosaccharide (GOS) is a common prebiotic. After the SOD1G93A mice were ingested with GOS and prebiotic yogurt rich in GOS, the life span of the mice was significantly prolonged, the loss of motor neurons was reduced, and the expression of inflammatory factors TNF-α and iNOS was inhibited [161].
5.4. Synbiotics
Synbiotic is the mixture of probiotics and prebiotics. Synbiotics supplementation is conducive to the interaction of probiotics and prebiotics, which can better regulate the microecology of gut microbiota and bring benefits to the host [162]. To some extent, synbiotics supplementation should provide better results than supplementation with either probiotics or prebiotics alone. The most common synbiotics are mainly composed of Bifidobacterium or Lactobacillus paired with oligofructose. There have been few studies investigating the effect of synbiotics on ALS patients or animal models. However, there have been many studies related to synbiotics in other neurodegenerative diseases. Overall, synbiotics have beneficial effects on neurodegenerative diseases. Synbiotic treatment of AD mice model improves the cognitive symptom of mice and significantly reduces the level of inflammatory factor TNF-α [163].
5.5. Postbiotics
Postbiotics are bioactive compounds produced by food-grade microorganisms, including inanimate microbial cells, microbial cell components and metabolites [164, 165]. The common metabolites in postbiotics include vitamins, SCFAs, lipids and proteins. The common microbial cell components include peptidoglycan and teichoic-acid. After butyrate treatment of SOD1G93A mice, the integrity of intestinal barrier was significantly improved, the progress of ALS was delayed, and their life span was prolonged [99]. Similarly, after butyrate treatment of SOD1G93A mice, the inflammatory factors such as IL-17 and LPS in the mice serum were obviously reduced [134].
Vitamin B12, as a kind of postbiotic, can be converted into methylcobalamin in the body. A Japanese clinical study of ultra-high dose mecobalamin in ALS patients found that mecobalamin at an ultra-high dose did not show significant efficacy in the entire study population [166]. However, this experiment also showed that in patients diagnosed within one year of symptom onset, treatment with ultra-high doses of mecobalamin has shown to prolong survival and improve the symptoms of ALS [166]. Moreover, Oki and his colleagues further validated the efficacy of ultra-high dose mecobalamin in patients with early ALS and reached the same conclusion [167].
6. Future directions
As for the regularity of gut microbiota changes in ALS patients, no unified conclusion has been reached by various teams. We hope that more scientists will participate in the research of this issue. Such an investigation may lead to successful localization of one or several microbes that are specifically altered in ALS patients. Thus, combined analysis of the changes of ALS-related microbiota through fecal microbial detection may be able to predict the risk of ALS, diagnose or evaluate ALS patients. In addition, after clarifying the effects of various microbial changes on ALS, we can supplement certain beneficial bacteria through fecal transplantation and other means, restore the gut microbiota in ALS patients and alleviate the progression of ALS. In addition, we found that the differences in gut microbiota of ALS patients of different ages are currently in the preliminary stage of research. More efforts are needed to explore the characteristics of gut microbiota in ALS patients at different ages.
The effect of antibiotics on different genotypes in animal models is also of great interest. SOD1G93A and C9orf72 mice showed distinct responses to broad-spectrum antibiotics [50, 52]. However, because the broad-spectrum antibiotics and animal models used by the two teams in animal experiments are different, it is not clear what factors lead to the opposite effects. This would be a very interesting study, because the results of this experiment could in part reveal the function of genetic factors in regulating the gut microbiota. If genetic factors can indeed affect the composition of gut microbiota in ALS patients to a large extent, we may need to pay more attention to the pathogenic genes of ALS patients in the future and use specific drugs according to the patient's genotype.
Current studies have found that the intestinal, blood-spinal cord and blood-brain barrier function are damaged in ALS patients and animal models. Furthermore, the results of animal experiments show that barrier function damage happens before the occurrence of ALS [60]. Broken barrier function may play a key role in ALS. Therefore, it is significant to clarify whether the impairment of barrier function is the cause or the pathogenesis of ALS, and what factors lead to the impairment of barrier function.
Unlike neurons in the CNS, neurogenesis occurs in adult enteric neurons [88]. To what extent can the altered gut microbiota affect the function and structure of enteric neurons in ALS patients? Moreover, to what extent do enteric neuronal abnormalities affect intestinal motor function in ALS patients? These are questions that both deserve further exploration. In addition, whether adaptive immunity is involved in neuronal degeneration and death is also a worthy research direction. For the answer to this question, the experimental results of different experimental teams give different answers, and there is no pathological study report related to human ALS patients. Our review summarizes potential therapeutic strategies for ALS. Most of these therapies have only been tested in animal models of ALS and have not yet entered the clinical trial stage. In the future, we look forward to more scientists participating in to further clarify the therapeutic effect of gut microbiota intervention on ALS animal models and promote the development of ALS treatment strategies.
Finally, we can find that most of the current research results on ALS are experimental conclusions obtained on animal models. The key to benefit ALS patients is to further explore the advantages and disadvantages of various animal models of ALS and to strengthen the translation between experimental results of animal models and human diseases.
Conclusion
Brain-gut-microbiota axis is a sophisticated interaction system, including neural, immune, and endocrine networks. Accumulating evidence suggests that the gut microbiota plays an essential role in ALS. Therefore, the brain-gut-microbiota axis provides a new direction to elucidate the pathogenesis of ALS. This paper summarizes the dysbiosis of gut microbiota found in ALS animal model and patients recently and tries to clarify the relationship between gut microbiota and pathological changes of ALS. However, the exact mechanism of the role of the brain-gut-microbiota axis in ALS is still unclear. Further studies are needed to clarify the role of gut microbiota in the progression of ALS in the future. In addition, strengthening the transformation between the results of animal models and human diseases is also a very critical work.
Further understanding of the relevance between gut microbiota and ALS may help the diagnosis and therapy of ALS, ultimately benefiting patients. Although therapeutic methods such as FMT, prebiotic supplements and probiotics are still at the primary stage of research, these therapeutic strategies are likely to bring new hope to ALS patients based on the therapeutic mechanism of restoring intestinal microbial homeostasis.
Acknowledgments
This research was funded by Guangdong Basic and Applied Basic Research Foundation and Presidential Foundation of Zhujiang Hospital, Southern Medical University, grant number 2023A1515030045 and yzjj2022ms4.
Funding Statement
This research was funded by Guangdong Basic and Applied Basic Research Foundation and Presidential Foundation of Zhujiang Hospital, Southern Medical University, grant number 2023A1515030045 and yzjj2022ms4.
Footnotes
Statements and Declarations
The authors have no competing interests to declare relating to the content of this article.
References
- [1].Goutman SA, Hardiman O, Al-Chalabi A, Chió A, Savelieff MG, Kiernan MC, et al. (2022). Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol, 21:480-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kirmani BF, Shapiro LA, Shetty AK (2021). Neurological and Neurodegenerative Disorders: Novel Concepts and Treatment. Aging Dis, 12:950-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. (2022). Amyotrophic lateral sclerosis. Lancet, 400:1363-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marin B, Fontana A, Arcuti S, Copetti M, Boumédiene F, Couratier P, et al. (2018). Age-specific ALS incidence: a dose-response meta-analysis. Eur J Epidemiol, 33:621-634. [DOI] [PubMed] [Google Scholar]
- [5].Arthur KC, Calvo A, Price TR, Geiger JT, Chiò A, Traynor BJ (2016). Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun, 7:12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marin B, Boumédiene F, Logroscino G, Couratier P, Babron MC, Leutenegger AL, et al. (2017). Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis. Int J Epidemiol, 46:57-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chiò A, Cucatto A, Calvo A, Terreni AA, Magnani C, Schiffer D (1999). Amyotrophic lateral sclerosis among the migrant population to Piemonte, northwestern Italy. J Neurol, 246:175-180. [DOI] [PubMed] [Google Scholar]
- [8].Harms MB, Cady J, Zaidman C, Cooper P, Bali T, Allred P, et al. (2013). Lack of C9ORF72 coding mutations supports a gain of function for repeat expansions in amyotrophic lateral sclerosis. Neurobiol Aging, 34:2234.e2213-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Julian TH, Glascow N, Barry ADF, Moll T, Harvey C, Klimentidis YC, et al. (2021). Physical exercise is a risk factor for amyotrophic lateral sclerosis: Convergent evidence from Mendelian randomisation, transcriptomics and risk genotypes. EBioMedicine, 68:103397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Al-Chalabi A, Hardiman O (2013). The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol, 9:617-628. [DOI] [PubMed] [Google Scholar]
- [11].Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP (2002). Premorbid weight, body mass, and varsity athletics in ALS. Neurology, 59:773-775. [DOI] [PubMed] [Google Scholar]
- [12].Turner MR, Parton MJ, Shaw CE, Leigh PN, Al-Chalabi A (2003). Prolonged survival in motor neuron disease: a descriptive study of the King's database 1990-2002. J Neurol Neurosurg Psychiatry, 74:995-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Masrori P, Van Damme P (2020). Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol, 27:1918-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hardiman O, van den Berg LH, Kiernan MC (2011). Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol, 7:639-649. [DOI] [PubMed] [Google Scholar]
- [15].McCombe PA, Henderson RD (2010). Effects of gender in amyotrophic lateral sclerosis. Gend Med, 7:557-570. [DOI] [PubMed] [Google Scholar]
- [16].Burkhardt C, Neuwirth C, Sommacal A, Andersen PM, Weber M (2017). Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLoS One, 12:e0177555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Norris SP, Likanje MN, Andrews JA (2020). Amyotrophic lateral sclerosis: update on clinical management. Curr Opin Neurol, 33:641-648. [DOI] [PubMed] [Google Scholar]
- [18].Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P (2000). Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord, 1:91-96. [DOI] [PubMed] [Google Scholar]
- [19].Figueroa-Romero C, Mikhail KA, Gennings C, Curtin P, Bello GA, Botero TM, et al. (2020). Early life metal dysregulation in amyotrophic lateral sclerosis. Ann Clin Transl Neurol, 7:872-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goutman SA, Boss J, Patterson A, Mukherjee B, Batterman S, Feldman EL (2019). High plasma concentrations of organic pollutants negatively impact survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry, 90:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Westeneng HJ, van Veenhuijzen K, van der Spek RA, Peters S, Visser AE, van Rheenen W, et al. (2021). Associations between lifestyle and amyotrophic lateral sclerosis stratified by C9orf72 genotype: a longitudinal, population-based, case-control study. Lancet Neurol, 20:373-384. [DOI] [PubMed] [Google Scholar]
- [22].Wang MD, Little J, Gomes J, Cashman NR, Krewski D (2017). Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology, 61:101-130. [DOI] [PubMed] [Google Scholar]
- [23].Peters S, Broberg K, Gallo V, Levi M, Kippler M, Vineis P, et al. (2021). Blood Metal Levels and Amyotrophic Lateral Sclerosis Risk: A Prospective Cohort. Ann Neurol, 89:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S (2022). A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin Microbiol Rev, 35:e0033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].De la Fuente M (2021). The Role of the Microbiota-Gut-Brain Axis in the Health and Illness Condition: A Focus on Alzheimer's Disease. J Alzheimers Dis, 81:1345-1360. [DOI] [PubMed] [Google Scholar]
- [26].Hashim HM, Makpol S (2022). A review of the preclinical and clinical studies on the role of the gut microbiome in aging and neurodegenerative diseases and its modulation. Front Cell Neurosci, 16:1007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, et al. (2015). The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis, 26:26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cryan JF, Dinan TG (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci, 13:701-712. [DOI] [PubMed] [Google Scholar]
- [29].Margolis KG, Cryan JF, Mayer EA (2021). The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology, 160:1486-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. (2019). The Microbiota-Gut-Brain Axis. Physiol Rev, 99:1877-2013. [DOI] [PubMed] [Google Scholar]
- [31].Jing Y, Bai F, Yu Y (2021). Spinal cord injury and gut microbiota: A review. Life Sci, 266:118865. [DOI] [PubMed] [Google Scholar]
- [32].López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2023). Hallmarks of aging: An expanding universe. Cell, 186:243-278. [DOI] [PubMed] [Google Scholar]
- [33].Adav SS, Wang Y (2021). Metabolomics Signatures of Aging: Recent Advances. Aging Dis, 12:646-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ling Z, Liu X, Cheng Y, Yan X, Wu S (2022). Gut microbiota and aging. Crit Rev Food Sci Nutr, 62:3509-3534. [DOI] [PubMed] [Google Scholar]
- [35].Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, et al. (2019). Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med, 25:1234-1242. [DOI] [PubMed] [Google Scholar]
- [36].Parker A, Romano S, Ansorge R, Aboelnour A, Le Gall G, Savva GM, et al. (2022). Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome, 10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Figueroa-Romero C, Guo K, Murdock BJ, Paez-Colasante X, Bassis CM, Mikhail KA, et al. (2019). Temporal evolution of the microbiome, immune system and epigenome with disease progression in ALS mice. Dis Model Mech, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Louis P, Flint HJ (2017). Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol, 19:29-41. [DOI] [PubMed] [Google Scholar]
- [39].Di Pierro F (2021). Gut Microbiota Parameters Potentially Useful in Clinical Perspective. Microorganisms, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zeng Q, Shen J, Chen K, Zhou J, Liao Q, Lu K, et al. (2020). The alteration of gut microbiome and metabolism in amyotrophic lateral sclerosis patients. Sci Rep, 10:12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fang X, Wang X, Yang S, Meng F, Wang X, Wei H, et al. (2016). Evaluation of the Microbial Diversity in Amyotrophic Lateral Sclerosis Using High-Throughput Sequencing. Front Microbiol, 7:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Niccolai E, Di Pilato V, Nannini G, Baldi S, Russo E, Zucchi E, et al. (2021). The Gut Microbiota-Immunity Axis in ALS: A Role in Deciphering Disease Heterogeneity? Biomedicines, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhai CD, Zheng JJ, An BC, Huang HF, Tan ZC (2019). Intestinal microbiota composition in patients with amyotrophic lateral sclerosis: establishment of bacterial and archaeal communities analyses. Chin Med J (Engl), 132:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ning J, Huang SY, Chen SD, Zhang YR, Huang YY, Yu JT (2022). Investigating Casual Associations Among Gut Microbiota, Metabolites, and Neurodegenerative Diseases: A Mendelian Randomization Study. J Alzheimers Dis, 87:211-222. [DOI] [PubMed] [Google Scholar]
- [45].Zhang L, Zhuang Z, Zhang G, Huang T, Fan D (2022). Assessment of bidirectional relationships between 98 genera of the human gut microbiota and amyotrophic lateral sclerosis: a 2-sample Mendelian randomization study. BMC Neurol, 22:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hertzberg VS, Singh H, Fournier CN, Moustafa A, Polak M, Kuelbs CA, et al. (2022). Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph Lateral Scler Frontotemporal Degener, 23:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nicholson K, Bjornevik K, Abu-Ali G, Chan J, Cortese M, Dedi B, et al. (2021). The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener, 22:186-194. [DOI] [PubMed] [Google Scholar]
- [48].Zhang Y, Ogbu D, Garrett S, Xia Y, Sun J (2021). Aberrant enteric neuromuscular system and dysbiosis in amyotrophic lateral sclerosis. Gut Microbes, 13:1996848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu S, Yi J, Zhang YG, Zhou J, Sun J (2015). Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature, 572:474-480. [DOI] [PubMed] [Google Scholar]
- [51].Kim HS, Son J, Lee D, Tsai J, Wang D, Chocron ES, et al. (2022). Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC Neurol, 22:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cox LM, Calcagno N, Gauthier C, Madore C, Butovsky O, Weiner HL (2022). The microbiota restrains neurodegenerative microglia in a model of amyotrophic lateral sclerosis. Microbiome, 10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, et al. (2009). Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol, 206:121-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guo S, Nighot M, Al-Sadi R, Alhmoud T, Nighot P, Ma TY (2015). Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J Immunol, 195:4999-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR (2007). Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res, 1157:126-137. [DOI] [PubMed] [Google Scholar]
- [56].Nicaise C, Mitrecic D, Demetter P, De Decker R, Authelet M, Boom A, et al. (2009). Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res, 1301:152-162. [DOI] [PubMed] [Google Scholar]
- [57].Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, et al. (2007). Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS One, 2:e1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nicaise C, Soyfoo MS, Authelet M, De Decker R, Bataveljic D, Delporte C, et al. (2009). Aquaporin-4 overexpression in rat ALS model. Anat Rec (Hoboken), 292:207-213. [DOI] [PubMed] [Google Scholar]
- [59].Zou S, Lan YL, Wang H, Zhang B, Sun YG (2019). The potential roles of aquaporin 4 in amyotrophic lateral sclerosis. Neurol Sci, 40:1541-1549. [DOI] [PubMed] [Google Scholar]
- [60].Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O'Banion MK, et al. (2008). ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci, 11:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MC, Haller E, Frisina-Deyo A, Mirtyl S, et al. (2012). Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res, 1469:114-128. [DOI] [PubMed] [Google Scholar]
- [62].Liesi P, Kauppila T (2002). Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in formation of the glial scar. Exp Neurol, 173:31-45. [DOI] [PubMed] [Google Scholar]
- [63].Guidotti G, Scarlata C, Brambilla L, Rossi D (2021). Tumor Necrosis Factor Alpha in Amyotrophic Lateral Sclerosis: Friend or Foe? Cells, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tripathi P, Rodriguez-Muela N, Klim JR, de Boer AS, Agrawal S, Sandoe J, et al. (2017). Reactive Astrocytes Promote ALS-like Degeneration and Intracellular Protein Aggregation in Human Motor Neurons by Disrupting Autophagy through TGF-β1. Stem Cell Reports, 9:667-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Guttenplan KA, Weigel MK, Adler DI, Couthouis J, Liddelow SA, Gitler AD, et al. (2020). Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat Commun, 11:3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Erdbruegger U, Haubitz M, Woywodt A (2006). Circulating endothelial cells: a novel marker of endothelial damage. Clin Chim Acta, 373:17-26. [DOI] [PubMed] [Google Scholar]
- [67].Garbuzova-Davis S, Woods RL 3rd, Louis MK, Zesiewicz TA, Kuzmin-Nichols N, Sullivan KL, et al. (2010). Reduction of circulating endothelial cells in peripheral blood of ALS patients. PLoS One, 5:e10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Garbuzova-Davis S, Rodrigues MC, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, et al. (2011). Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res, 1398:113-125. [DOI] [PubMed] [Google Scholar]
- [69].Kiaei M, Kipiani K, Calingasan NY, Wille E, Chen J, Heissig B, et al. (2007). Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol, 205:74-81. [DOI] [PubMed] [Google Scholar]
- [70].Tang W, Zhu H, Feng Y, Guo R, Wan D (2020). The Impact of Gut Microbiota Disorders on the Blood-Brain Barrier. Infect Drug Resist, 13:3351-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med, 6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chan GN, Evans RA, Banks DB, Mesev EV, Miller DS, Cannon RE (2017). Selective induction of P-glycoprotein at the CNS barriers during symptomatic stage of an ALS animal model. Neurosci Lett, 639:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Boston-Howes W, Williams EO, Bogush A, Scolere M, Pasinelli P, Trotti D (2008). Nordihydroguaiaretic acid increases glutamate uptake in vitro and in vivo: therapeutic implications for amyotrophic lateral sclerosis. Exp Neurol, 213:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jablonski MR, Markandaiah SS, Jacob D, Meng NJ, Li K, Gennaro V, et al. (2014). Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann Clin Transl Neurol, 1:996-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Milane A, Fernandez C, Vautier S, Bensimon G, Meininger V, Farinotti R (2007). Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood-brain barrier. J Neurochem, 103:164-173. [DOI] [PubMed] [Google Scholar]
- [76].Mohamed LA, Markandaiah S, Bonanno S, Pasinelli P, Trotti D (2017). Blood-Brain Barrier Driven Pharmacoresistance in Amyotrophic Lateral Sclerosis and Challenges for Effective Drug Therapies. Aaps j, 19:1600-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Niesler B, Kuerten S, Demir IE, Schäfer KH (2021). Disorders of the enteric nervous system - a holistic view. Nat Rev Gastroenterol Hepatol, 18:393-410. [DOI] [PubMed] [Google Scholar]
- [78].Hughes HK, Rose D, Ashwood P (2018). The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr Neurol Neurosci Rep, 18:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Spencer NJ, Hu H (2020). Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol, 17:338-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O'Donnell TA, et al. (2017). Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell, 170:185-198.e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rhee SH, Pothoulakis C, Mayer EA (2009). Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol, 6:306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].O'Hara JR, Ho W, Linden DR, Mawe GM, Sharkey KA (2004). Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol, 287:G998-1007. [DOI] [PubMed] [Google Scholar]
- [83].Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M (2005). Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun, 19:334-344. [DOI] [PubMed] [Google Scholar]
- [84].Ronchi G, Ryu V, Fornaro M, Czaja K (2012). Hippocampal plasticity after a vagus nerve injury in the rat. Neural Regen Res, 7:1055-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen D, Sui L, Chen C, Liu S, Sun X, Guan J (2022). Atorvastatin suppresses NLRP3 inflammasome activation in intracerebral hemorrhage via TLR4- and MyD88-dependent pathways. Aging (Albany NY), 14:462-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD (1985). Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience, 16:223-240. [DOI] [PubMed] [Google Scholar]
- [87].Rao M, Gershon MD (2016). The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol, 13:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, et al. (2017). Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A, 114:E3709-e3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gotkine M, Kviatcovsky D, Elinav E (2020). Amyotrophic lateral sclerosis and intestinal microbiota-toward establishing cause and effect. Gut Microbes, 11:1833-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Szalay C, Abrahám I, Papp S, Takács G, Lukáts B, Gáti A, et al. (2010). Taste reactivity deficit in anorexia nervosa. Psychiatry Clin Neurosci, 64:403-407. [DOI] [PubMed] [Google Scholar]
- [91].Macfarlane S, Dillon JF (2007). Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol, 102:1187-1196. [DOI] [PubMed] [Google Scholar]
- [92].Burberry A, Wells MF, Limone F, Couto A, Smith KS, Keaney J, et al. (2020). C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature, 582:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. (2007). Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia, 55:453-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nguyen MD, D'Aigle T, Gowing G, Julien JP, Rivest S (2004). Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci, 24:1340-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nadeau S, Rivest S (1999). Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience, 93:1449-1464. [DOI] [PubMed] [Google Scholar]
- [96].Zhang R, Gascon R, Miller RG, Gelinas DF, Mass J, Hadlock K, et al. (2005). Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol, 159:215-224. [DOI] [PubMed] [Google Scholar]
- [97].Babu GN, Kumar A, Chandra R, Puri SK, Kalita J, Misra UK (2008). Elevated inflammatory markers in a group of amyotrophic lateral sclerosis patients from northern India. Neurochem Res, 33:1145-1149. [DOI] [PubMed] [Google Scholar]
- [98].Ono S, Hu J, Shimizu N, Imai T, Nakagawa H (2001). Increased interleukin-6 of skin and serum in amyotrophic lateral sclerosis. J Neurol Sci, 187:27-34. [DOI] [PubMed] [Google Scholar]
- [99].Zhang YG, Wu S, Yi J, Xia Y, Jin D, Zhou J, et al. (2017). Target Intestinal Microbiota to Alleviate Disease Progression in Amyotrophic Lateral Sclerosis. Clin Ther, 39:322-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, Francolini G, et al. (2000). Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Lett, 287:211-214. [DOI] [PubMed] [Google Scholar]
- [101].Akira S, Takeda K, Kaisho T (2001). Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol, 2:675-680. [DOI] [PubMed] [Google Scholar]
- [102].Laflamme N, Soucy G, Rivest S (2001). Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J Neurochem, 79:648-657. [DOI] [PubMed] [Google Scholar]
- [103].Raoul C, Estévez AG, Nishimune H, Cleveland DW, deLapeyrière O, Henderson CE, et al. (2002). Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron, 35:1067-1083. [DOI] [PubMed] [Google Scholar]
- [104].Quek H, Cuní-López C, Stewart R, Colletti T, Notaro A, Nguyen TH, et al. (2022). ALS monocyte-derived microglia-like cells reveal cytoplasmic TDP-43 accumulation, DNA damage, and cell-specific impairment of phagocytosis associated with disease progression. J Neuroinflammation, 19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bai Y, Mu Q, Bao X, Zuo J, Fang X, Hua J, et al. (2021). Targeting NLRP3 Inflammasome in the Treatment Of Diabetes and Diabetic Complications: Role of Natural Compounds from Herbal Medicine. Aging Dis, 12:1587-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Johann S, Heitzer M, Kanagaratnam M, Goswami A, Rizo T, Weis J, et al. (2015). NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia, 63:2260-2273. [DOI] [PubMed] [Google Scholar]
- [107].Debye B, Schmülling L, Zhou L, Rune G, Beyer C, Johann S (2018). Neurodegeneration and NLRP3 inflammasome expression in the anterior thalamus of SOD1(G93A) ALS mice. Brain Pathol, 28:14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Heitzer M, Kaiser S, Kanagaratnam M, Zendedel A, Hartmann P, Beyer C, et al. (2017). Administration of 17β-Estradiol Improves Motoneuron Survival and Down-regulates Inflammasome Activation in Male SOD1(G93A) ALS Mice. Mol Neurobiol, 54:8429-8443. [DOI] [PubMed] [Google Scholar]
- [109].Italiani P, Carlesi C, Giungato P, Puxeddu I, Borroni B, Bossù P, et al. (2014). Evaluating the levels of interleukin-1 family cytokines in sporadic amyotrophic lateral sclerosis. J Neuroinflammation, 11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kadhim H, Deltenre P, Martin JJ, Sébire G (2016). In-situ expression of Interleukin-18 and associated mediators in the human brain of sALS patients: Hypothesis for a role for immune-inflammatory mechanisms. Med Hypotheses, 86:14-17. [DOI] [PubMed] [Google Scholar]
- [111].Zhang Y, Yu W, Flynn C, Chang W, Zhang L, Wang M, et al. (2022). Interplay between Gut Microbiota and NLRP3 Inflammasome in Intracerebral Hemorrhage. Nutrients, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhang Y, Zhang S, Li B, Luo Y, Gong Y, Jin X, et al. (2022). Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc Res, 118:785-797. [DOI] [PubMed] [Google Scholar]
- [113].Li X, Geng J, Zhao J, Ni Q, Zhao C, Zheng Y, et al. (2019). Trimethylamine N-Oxide Exacerbates Cardiac Fibrosis via Activating the NLRP3 Inflammasome. Front Physiol, 10:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. (2012). The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab, 15:838-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chen L, Qing W, Yi Z, Lin G, Peng Q, Zhou F (2021). NU9056, a KAT 5 Inhibitor, Treatment Alleviates Brain Dysfunction by Inhibiting NLRP3 Inflammasome Activation, Affecting Gut Microbiota, and Derived Metabolites in LPS-Treated Mice. Front Nutr, 8:701760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rowin J, Xia Y, Jung B, Sun J (2017). Gut inflammation and dysbiosis in human motor neuron disease. Physiol Rep, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Liu J, Tan Y, Cheng H, Zhang D, Feng W, Peng C (2022). Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis, 13:1106-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Silva YP, Bernardi A, Frozza RL (2020). The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne), 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Erber AC, Cetin H, Berry D, Schernhammer ES (2020). The role of gut microbiota, butyrate and proton pump inhibitors in amyotrophic lateral sclerosis: a systematic review. Int J Neurosci, 130:727-735. [DOI] [PubMed] [Google Scholar]
- [120].Kim CH, Park J, Kim M (2014). Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw, 14:277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D (2008). Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem, 19:587-593. [DOI] [PubMed] [Google Scholar]
- [122].Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA (2003). Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut, 52:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Pellegrini C, Fornai M, D'Antongiovanni V, Antonioli L, Bernardini N, Derkinderen P (2023). The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol Hepatol, 8:66-80. [DOI] [PubMed] [Google Scholar]
- [124].Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. (2013). Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science, 342:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. (2015). Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe, 17:662-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, et al. (2015). HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell, 26:2252-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang RX, Lee JS, Campbell EL, Colgan SP (2020). Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci U S A, 117:11648-11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Beers DR, Appel SH (2019). Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies. Lancet Neurol, 18:211-220. [DOI] [PubMed] [Google Scholar]
- [129].Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, et al. (2013). Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med, 5:64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 504:446-450. [DOI] [PubMed] [Google Scholar]
- [131].Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. (2014). Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity, 40:128-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wang P, Zhang Y, Gong Y, Yang R, Chen Z, Hu W, et al. (2018). Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol Dis, 111:12-25. [DOI] [PubMed] [Google Scholar]
- [133].Boddy SL, Giovannelli I, Sassani M, Cooper-Knock J, Snyder MP, Segal E, et al. (2021). The gut microbiome: a key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med, 19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ogbu D, Zhang Y, Claud K, Xia Y, Sun J (2022). Target Metabolites to Slow Down Progression of Amyotrophic Lateral Sclerosis in Mice. Metabolites, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Barki N, Bolognini D, Börjesson U, Jenkins L, Riddell J, Hughes DI, et al. (2022). Chemogenetics defines a short-chain fatty acid receptor gut-brain axis. Elife, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. (2013). GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology, 154:3552-3564. [DOI] [PubMed] [Google Scholar]
- [137].Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, et al. (2020). Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature, 583:441-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. (2010). Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology, 138:1772-1782. [DOI] [PubMed] [Google Scholar]
- [139].Cherbut C, Aubé AC, Blottière HM, Galmiche JP (1997). Effects of short-chain fatty acids on gastrointestinal motility. Scand J Gastroenterol Suppl, 222:58-61. [DOI] [PubMed] [Google Scholar]
- [140].Kamath PS, Phillips SF, O'Connor MK, Brown ML, Zinsmeister AR (1990). Colonic capacitance and transit in man: modulation by luminal contents and drugs. Gut, 31:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sun Y, Bedlack R, Armon C, Beauchamp M, Bertorini T, Bowser R, et al. (2022). ALSUntangled #64: butyrates. Amyotroph Lateral Scler Frontotemporal Degener, 23:638-643. [DOI] [PubMed] [Google Scholar]
- [142].Seethaler B, Nguyen NK, Basrai M, Kiechle M, Walter J, Delzenne NM, et al. (2022). Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am J Clin Nutr, 116:928-942. [DOI] [PubMed] [Google Scholar]
- [143].Gill PA, Muir JG, Gibson PR, van Zelm MC (2022). A Randomized Dietary Intervention to Increase Colonic and Peripheral Blood Short-Chain Fatty Acids Modulates the Blood B- and T-cell Compartments in Healthy Humans. Am J Clin Nutr, 116:1354-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, et al. (2020). NAD(+) metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther, 5:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Carrera-Juliá S, Moreno ML, Barrios C, de la Rubia Ortí JE, Drehmer E (2020). Antioxidant Alternatives in the Treatment of Amyotrophic Lateral Sclerosis: A Comprehensive Review. Front Physiol, 11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zhou Q, Zhu L, Qiu W, Liu Y, Yang F, Chen W, et al. (2022). Erratum: Nicotinamide Riboside Enhances Mitochondrial Proteostasis and Adult Neurogenesis through Activation of Mitochondrial Unfolded Protein Response Signaling in the Brain of ALS SOD1(G93A) Mice: Erratum. Int J Biol Sci, 18:2181-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Shats I, Williams JG, Liu J, Makarov MV, Wu X, Lih FB, et al. (2020). Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab, 31:564-579.e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Chellappa K, McReynolds MR, Lu W, Zeng X, Makarov M, Hayat F, et al. (2022). NAD precursors cycle between host tissues and the gut microbiome. Cell Metab, 34:1947-1959.e1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Jaiswal MK (2019). Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med Res Rev, 39:733-748. [DOI] [PubMed] [Google Scholar]
- [150].Aschenbrenner DS (2023). New Drug Approved For ALS. Am J Nurs, 123:22-23. [DOI] [PubMed] [Google Scholar]
- [151].Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. (2019). Fecal microbiota transplantation: Review and update. J Formos Med Assoc, 118 Suppl 1:S23-s31. [DOI] [PubMed] [Google Scholar]
- [152].Mandrioli J, Amedei A, Cammarota G, Niccolai E, Zucchi E, D'Amico R, et al. (2019). FETR-ALS Study Protocol: A Randomized Clinical Trial of Fecal Microbiota Transplantation in Amyotrophic Lateral Sclerosis. Front Neurol, 10:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Takahashi M, Ishikawa D, Sasaki T, Lu YJ, Kuwahara-Arai K, Kamei M, et al. (2019). Faecal freezing preservation period influences colonization ability for faecal microbiota transplantation. J Appl Microbiol, 126:973-984. [DOI] [PubMed] [Google Scholar]
- [154].Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol, 11:506-514. [DOI] [PubMed] [Google Scholar]
- [155].Di Gioia D, Bozzi Cionci N, Baffoni L, Amoruso A, Pane M, Mogna L, et al. (2020). A prospective longitudinal study on the microbiota composition in amyotrophic lateral sclerosis. BMC Med, 18:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Markowiak P, Śliżewska K (2017). Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Etxeberria U, Arias N, Boqué N, Macarulla MT, Portillo MP, Martínez JA, et al. (2015). Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem, 26:651-660. [DOI] [PubMed] [Google Scholar]
- [158].Jeong HW, Kim JK, Kim AY, Cho D, Lee JH, Choi JK, et al. (2020). Green Tea Encourages Growth of Akkermansia muciniphila. J Med Food, 23:841-851. [DOI] [PubMed] [Google Scholar]
- [159].Koh SH, Lee SM, Kim HY, Lee KY, Lee YJ, Kim HT, et al. (2006). The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci Lett, 395:103-107. [DOI] [PubMed] [Google Scholar]
- [160].Zhang X, Zhu XL, Sun YK, Hu B, Sun Y, Jabbar S, et al. (2013). -Fermentation in vitro of EGCG, GCG and EGCG3 '' Me isolated from Oolong tea by human intestinal microbiota. - 54:- 1595. [Google Scholar]
- [161].Song L, Gao Y, Zhang X, Le W (2013). Galactooligosaccharide improves the animal survival and alleviates motor neuron death in SOD1G93A mouse model of amyotrophic lateral sclerosis. Neuroscience, 246:281-290. [DOI] [PubMed] [Google Scholar]
- [162].Gibson GR, Roberfroid MB (1995). Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr, 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- [163].Deng SM, Chen CJ, Lin HL, Cheng IH (2022). The beneficial effect of synbiotics consumption on Alzheimer's disease mouse model via reducing local and systemic inflammation. IUBMB Life, 74:748-753. [DOI] [PubMed] [Google Scholar]
- [164].Wegh CAM, Geerlings SY, Knol J, Roeselers G, Belzer C (2019). Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol, 18:649-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Kaji R, Imai T, Iwasaki Y, Okamoto K, Nakagawa M, Ohashi Y, et al. (2019). Ultra-high-dose methylcobalamin in amyotrophic lateral sclerosis: a long-term phase II/III randomised controlled study. J Neurol Neurosurg Psychiatry, 90:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Oki R, Izumi Y, Fujita K, Miyamoto R, Nodera H, Sato Y, et al. (2022). Efficacy and Safety of Ultrahigh-Dose Methylcobalamin in Early-Stage Amyotrophic Lateral Sclerosis: A Randomized Clinical Trial. JAMA Neurol, 79:575-583. [DOI] [PMC free article] [PubMed] [Google Scholar]