Abstract
Brain metastases and related complications are one of the major fatal factors in cancer. Patients with breast cancer, lung cancer, and melanoma are at a high risk of developing brain metastases. However, the mechanisms underlying the brain metastatic cascade remain poorly understood. Microglia, one of the major resident macrophages in the brain parenchyma, are involved in multiple processes associated with brain metastasis, including inflammation, angiogenesis, and immune modulation. They also closely interact with metastatic cancer cells, astrocytes, and other immune cells. Current therapeutic approaches against metastatic brain cancers, including small-molecule drugs, antibody-coupled drugs (ADCs), and immune-checkpoint inhibitors (ICIs), have compromised efficacy owing to the impermeability of the blood-brain barrier (BBB) and complex brain microenvironment. Targeting microglia is one of the strategies for treating metastatic brain cancer. In this review, we summarize the multifaceted roles of microglia in brain metastases and highlight them as potential targets for future therapeutic interventions.
Keywords: brain metastases, microglia, inflammation, microenvironment, immunotherapy, drugs, delivery system
1. Introduction
Brain metastasis is one of the leading causes of cancer-related deaths worldwide. It may cause many complications, including cognitive impairment, hydrocephalus, cerebral hernia, and encephalomeningitis, resulting in a poor quality of life and short life span. Lung cancer, breast cancer, and melanoma have been reported to have a higher risk of brain metastases than other types of cancers [1-3]. Brain metastases develop in 30%-50% of patients with non-small cell lung cancer (NSCLC), which severely affect their overall survival [4]. An increasing number of patients with breast cancer develop brain metastases, especially those with HER2-positive or triple-negative tumors [5]. Brain metastases from melanoma are also common and have a poor prognosis, leading to the death of 60%-70% of patients with melanoma [6]. Currently, effective therapies for metastatic brain cancer are particularly limited because of the impermeability of the blood-brain barrier (BBB) and the immunosuppressive state of the brain. Currently, the following five treatment options are available for brain metastases: chemotherapy, local or whole-brain radiotherapy, surgery, molecularly targeted agents, and immune checkpoint inhibitors (ICIs). However, none of these have achieved satisfactory efficacy with tolerable side effects, and the five-year survival rates remain low [7, 8]. Therefore, new strategies are needed to effectively treat brain metastases.
The BBB maintains the brain microenvironment under a tight control by regulating fluctuations in the chemical composition, transport of immune cells, and entry of pathogens and toxins [9, 10]. The central nervous system (CNS) has also been considered to be immune privileged, a concept that involves keeping adaptive immunity and inflammation under tight control. Microglia, which are resident macrophages of the brain parenchyma, are key elements in brain metastases, participating in immune responses and maintaining CNS homeostasis [11, 12]. Not only the different phenotypes of microglia themselves, but also their crosstalk with the surrounding cells, affect the colonization, proliferation, and migration of tumor cells [13]. Activated microglia are classically divided into two distinct phenotypes: the classic M1 state with anti-tumor effects and M2 state with pro-tumor effects [14, 15]. Crosstalk between microglia-astrocytes, microglia-other immune cells, and microglia-tumor cells can help circulating tumor cells (CTCs) to colonize the brain parenchyma [16]. Targeting microglia can potentially aid in treating brain metastases [17, 18].
In this review, we summarize the microglia-mediated reprogramming of brain microenvironment in cancer in terms of inflammation, angiogenesis, and immune modulation. We will also discuss the interactions between microglia and other cell types in the brain microenvironment as well as current drugs targeting microglia for the treatment of metastatic brain cancers.
2. Microglia-mediated reprogramming of brain microenvironment in cancer
Microglia control the fates of neural progenitors, astrocyte activation, neuronal homeostasis, and synaptogenesis [19]. They may undergo distinct morphological, molecular, and functional changes that create distinct biological states associated with the onset and progression of various diseases, including autism, brain tumors, depression, and neurodegenerative diseases [20-22].
Successful intracerebral colonization requires that CTCs in the bloodstream be able to arrest and extravasate into brain capillaries, and then survive in the brain parenchyma. The BBB separates peripheral blood circulation from the tightly regulated CNS environment, and penetration of the BBB is the first step for CTCs to colonize the brain [23]. In addition to endothelial cells, astrocytes and pericytes are important components of the BBB [24]. Disruption of the BBB and the recruitment of peripheral immune cells are associated with CNS inflammation. Mechanistically, breast cancer cells pass through the BBB by overexpressing COX-2, HBEGF, and ST6GALNAC5. ST6GALNAC5 enhances the attachment of breast cancer cells to brain endothelial cells [25]. In addition to breast cancer, an antagonist of the HBEGF receptor was observed to reduce brain metastasis in NSCLC [26]. CXCL12-CXCR4 also promotes the invasion of breast cancer cells by increasing vascular permeability [27].
2.1. Inflammatory responses
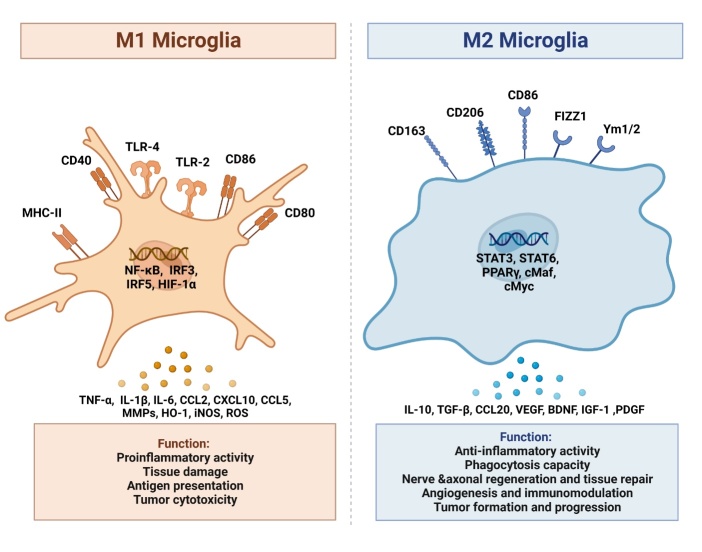
Primary tumors release a range of inflammatory factors, including tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and interleukin 1-beta (IL-1β), which disrupt the BBB and cause inflammation in the CNS [28]. Under these conditions, microglia sense and respond to pro-inflammatory cytokines and modulate the responses of neighboring cells throughout the CNS [29]. Different stimuli in the microenvironment affect the differentiation of microglia into different subtypes [30]. The M1 phenotype (inducible nitric oxide synthase (iNOS) and CD86 markers) is pro-inflammatory, is mainly induced by lipopolysaccharide (LPS) or interferon-γ (IFN-γ), and secretes a large number of pro-inflammatory cytokines, such as IFN-γ, TNF-α, IL-1β, chemokines, such as CCL2, CXCL9, and CXCL10, protein hydrolases (heme oxygenase-1 (HO-1)), iNOS, and reactive oxygen species (ROS). M1 microglia play a key role in killing cancer cells [31]. He et al. have reported that LPS-activated M1 microglia induced apoptosis of metastatic lung cancer cells in the brain in a dose- and time-dependent manner in vitro [32]. In contrast, M2 microglia (CD206 and Arg-1 markers) can promote tumor growth by releasing various anti-inflammatory and immunosuppressive factors such as transforming growth factor-beta (TGF-β), IL-10, and CCL20 [33]. The M2 phenotype is induced by IL-4/IL-3 and is subdivided into M2a, M2b, and M2c phenotypes. A few surface markers, including CD206, CD163, CD86, FIZZ1, YM1/2, and arginase-1(Arg-1), can distinguish M2 from M1 microglia [34]. Consistently, patients with glioma with increased M2 microglial levels have a poor prognosis [35]. Massive infiltration of M2 microglia has also been reported in brain metastases of premenopausal patients with breast cancer [36]. The functional differences between M1 and M2 microglia are shown in Figure 1.
Figure 1.
Different functions of M1/M2 microglial phenotypes in brain microenvironment. The figure illustrates that the resting microglia can be activated into M1/M2 phenotype in response to different stimuli. Microglia of M1 phenotype play roles in promoting inflammation, damaging the tissues, antigen presentation, and killing tumor cells. Microglia of M2 phenotype play roles in anti-inflammation, repairing of the tissues, promoting angiogenesis, suppressing immunity, and promoting tumor progression.
One mechanism of M1/M2 polarization is that the JAK2/STAT3 axis skews microglia/macrophage toward M2 polarization during cerebral ischemia/reperfusion injury [37]. In addition, STAT3 is a key checkpoint for suppressing anti-tumor immune responses [38]. IL-6 in brain metastatic NSCLC cells was observed to induce anti-inflammatory microglia via JAK2/STAT3 signaling, which in turn promoted the colonization of NSCLC cells [39]. Therefore, targeting the M1/M2 phenotypic polarization is one of the strategy to combat cancers with brain metastases.
Microglia can be divided into subpopulations based on their gene expression profiles. The naïve microglial population is characterized by high expression of microglial homeostasis genes (Cx3cr1, Hexb, and Jun). Microglial cluster in a primed state is marked by a high expression of mitochondrial genes and a slight decrease in the expression of microglial homeostasis genes. Microglial clusters in different inflammatory states are characterized by the upregulation of pro-inflammatory genes (S100a4, S100a6, and S100a10), anti-inflammatory genes (Lgals1, Lgals3, and Ifitm3), and migratory genes (Vim and Anxa), and downregulation of microglia homeostasis genes (Cx3cr1, P2ry12, Hexb, and Cst3) in myeloid cells in the CNS [40]. The classification of microglia based on their transcriptional status and their relationship with disease progression needs to be confirmed in future.
2.2. Immune modulation
Microglia are involved in both the innate and acquired immune responses. Phagocytosis is an important process by which microglia eradicate tumor cells. Amyloid beta (Aβ) secreted by melanoma cells suppresses microglial activation and prevents their phagocytosis [41]. Microglia of the M1 phenotype also function as antigen presenting cells that present antigens (MHC-II) to Th1 cells once activated, inducing T cell-mediated cell lysis and CD8+ T cell proliferation [42]. M2 microglia can help tumor cells establish a tumor-immunosuppressive environment and promote tumor progression [43].
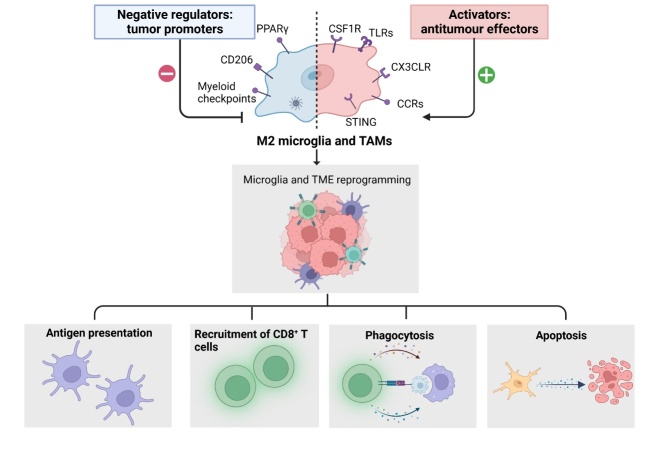
Nicotine promotes tumor progression by skewing the polarity of microglia toward the M2 phenotype and suppressing their anti-tumor innate immunity [44]. Mechanistically, nicotine polarizes microglia via the nAch receptor-STAT3 pathway and restricts their phagocytic ability through the overexpression of signal regulatory protein alpha (SIRPα) in microglia [44]. Native myeloid cells in the CNS include microglia and border-associated macrophages (BAMs). Guldner et al. have reported that Cx3cr1 knockout in CNS myeloid cells upregulated CXCL10 expression, which promoted immunosuppression and brain metastases of cancer cells originating from the lung, breast, colon, stomach, esophagus, pancreas, kidney, ovary, bladder, skin, and thyroid gland [40]. In brain metastases, indoleamine 2,3-dioxgenase-1 (IDO) appears to be a key regulator of autoimmunity, inhibiting T cell responses to apoptotic cell-associated antigens and controlling T cell activity at the site of graft-versus-host disease [45]. A recent study has reported that in melanoma brain metastases, comparative tissue analysis revealed microglia and tumor-associated macrophages as the primary sources of IDO expression, thus limiting T cell function in the brain parenchyma [46]. Similarly, intertumoral variations in IDO expression levels may mediate the surface expression of PD-L1 on T cells. Owing to the importance of IDO in tumor-mediated immunosuppression, small-molecule IDO inhibitors are currently in late-stage clinical trials as a strategy to enhance anti-tumor immunity [47]. In vitro co-culture has shown that collaboration between T cells and microglia leads to significant expression of immunosuppressive molecules, including IL-10, PD-1, and TGF-β [48, 49]. IL-10 increases the expression of TIM-3 on T cells [49]. Factors secreted by tumor cells, such as MIC-1, CSF-1, TGF-β, and IL-33, impair the anti-tumor functions of microglia. In addition, “don’t eat me” signals, such as CD47 and CD24, on glioma cells, which are potential ligands for SIRPα and Siglec-10 receptors on microglia, respectively, can impair the microglial phagocytosis [50]. We have schematically described microglial reprogramming and its roles in innate and adaptive immune responses in Figure 2.
Figure 2.
Reprogramming of microglia and activation of innate and adaptive immune responses. By inhibiting CSF1R, TLRs, CX3CLR, CCRs, and myeloid checkpoints, pro-inflammatory microglia can be activated to modulate innate immune responses, thereby enhancing antigen presentation, recruitment of CD8+T cells, and phagocytosis.
Exosomes are 30-100 nm membrane vesicles that contain functional genetic material and proteins. Exosome-mediated intercellular communication greatly affects the brain microenvironment, contributing to the proliferation, survival, and colonization of cancer cells in the brain [51]. Exosomes are released by most cell types, including tumor cells and macroglia [52, 53]. Circulating tumor-derived exosomes not only predict metastatic tendencies, but also identify organ sites of future metastasis [54]. The dysregulation of miRNAs and proteins in breast cancer/melanoma cell line-derived exosomes promotes the adhesion and invasive properties of cancer cells in the brain [51]. Among them, upregulated miR-210, downregulated miR-19a and miR-29c, and elevated PDK1, 14-3-3ε, or receptors, such as AR, Erα, HER3, cyclin D1, and MAPK, are thought to promote brain metastases [51]. Deletion of XIST in breast cancer cells enhances the secretion of exosomal miRNA-503, which triggers the M1-M2 polarization switch in microglia, thereby upregulating immunosuppressive cytokines in microglia and suppressing T-cell proliferation [55]. Lung cancer cell-derived exosomes induce the release of endogenous Dkk-1 from brain endothelial cells, resulting in an absolute decrease in M1 microglia and a relative increase in M2 microglia, which acquire a pro-tumorigenic profile in the premetastatic niche [56]. The miR196a-5p released from the extracellular vesicles of nasopharyngeal carcinoma is transferred to microglia, which then regulates the structure and function of microglia by downregulating ROCK1 expression; promoting microglial proliferation, phagocytic activity, and inflammatory cytokine secretion; and inhibiting invasion of the brain parenchyma [57].
In addition to tumor-derived exosomes, exosomes from other cell types are substantially involved in brain metastasis. Astrocyte-derived exosomes mediate the intercellular transfer of PTEN-targeted miRNAs to metastatic tumor cells. Furthermore, the adaptive deletion of PTEN in brain metastatic tumor cells leads to increased secretion of chemokine CCL2, which recruits microglia expressing CCL2 receptor (CCR2) to mutually promote the growth of brain metastatic tumor cells by enhancing proliferation and reducing apoptosis [58].
2.3. Angiogenesis
Resident microglia are an alternative source of proangiogenic growth factors and cytokines and play a central role in the regulation of vascular homeostasis and angiogenesis in brain tumors [59]. Switching microglia from the M1 to the M2 phenotype is the first step in promoting angiogenesis. Peroxisome proliferator activated receptor-gamma (PPARγ) has been reported to be responsible for this conversion, and PPARγ agonists improve microglial polarization to the M2 phenotype [60-63]. After cerebral ischemia/reperfusion, M2 microglia promote neurogenesis and angiogenesis by secreting IGF-1, VEGF, and BDNF [64, 65]. Inhibition of angiopoietin-2 (Ang2)/VEGF reprograms pro-tumor M2 microglia toward an anti-tumor M1 phenotype [43].
In cancer research, tumor angiogenesis occurs at a late stage of metastasis after successful invasion and proliferation of cancer cells. M2 microglial polarization is positively correlated with microvessel density in patients with glioblastoma. M2 microglia express potent angiogenic factors, such as VEGF and CXCL2, to promote tumor growth. Blocking the CXCL2-CXCR2 signaling pathway is known to result in a significant reduction in glioma sizes [59]. Glioblastoma-associated M2 microglia promote the angiogenesis in glioblastoma via transporting the exosomal circKIF18A into human brain microvessel endothelial cells (hBMECs). Mechanistically, circKIF18A binds to and maintains FOXC2 stability and nuclear translocation in hBMECs [66]. Microglia also produce HGF/SF and express c-Met, which promotes angiogenesis by stimulating endothelial cell migration and proliferation [67-70]. Tumor-secreted S100 calcium-binding protein B (S100B) activates the receptor for advanced glycation end products (RAGE) on microglia, which induces STAT3 activation and suppresses the function of M1 microglia. Another study confirmed that activated RAGE signal in microglia maintains an M2-like phenotype and promotes glioma angiogenesis [71]. However, the role of microglia in promoting angiogenesis in extracerebral brain tumors remains largely unknown.
3. The crosstalk between microglia, astrocytes, and tumor cells promotes brain metastases
The ‘seed and soil’ hypothesis, first proposed by Stephen Paget, emphasizes the importance of reciprocal correspondence between tumor cells and host organs during the formation of metastatic lesions [72]. Metastasis to distant organs depends on the pathological crosstalk between tumor cells and various tissue-specific stromal components. Astrocytes and microglia are the two primary types of stromal cells in the brain [73]. Astrocytes and microglia reduce the number of metastatic brain cancer cells. Astrocytes are a part of the BBB because the end feet of astrocytes enclose the blood vessels [74]. Astrocytes and microglia can also be very hostile in terms of destroying metastatic cells. Most metastatic cancer cells do not survive because of the action of astrocytes, which produce plasmin from neuron-derived plasminogen and promote the release of membrane-bound astrocyte FasL. Another target of active plasmin, L1CAM, is an adhesion molecule that blocks the interactions between cancer cells and capillaries [75]. However, there is growing evidence that astrocytes and microglia may be “highjacked” by tumor cells for their settlement and development [76]. Reactive astrocytes have been shown to enhance tumor cell proliferation, survival, invasion, and resistance to chemotherapy [77, 78]. Microglia can be polarized into tumor-supportive and immunosuppressive cells by certain tumor-derived soluble factors, thus promoting tumor maintenance and progression.
The interaction between astrocytes and tumor cells is closely associated with brain metastases. Mechanistically, C-C motif chemokine ligand 2 (CCL2), produced mainly by astrocytes, promotes cancer cell chemotaxis through CCR2 [79]. Melanoma cells were found to alter the astrocyte secretion and evoke CCL2 expression and secretion, which in turn induced CCR2 expression in melanoma cells and enhanced their in vitro tumorigenic properties such as proliferation and metastasis [80]. Other proteins can also be released by astrocytes and tumor cells to establish brain metastases niche. Astrocytes also secrete neurotrophic factors such as the nerve growth factor (NGF) family of neurotrophins. Neurotrophin-3 (NT-3), a member of this family, promotes the growth of metastatic breast cancer cells in the brain by promoting their re-epithelialization and reducing the cytotoxic response of microglia [81]. Connexin43 (Cx43) protein, a major component of intercellular channels in astrocytes, promotes glioma cell migration and anti-apoptosis [82]. Metastatic tumor cells were also found to take advantage of Cx43 secretion to exchange nutrients and metabolites, thereby establishing brain metastases niche [76].
In addition to inflammatory, angiogenic, and immunomodulatory processes, interactions between cancer cells and microglia are directly involved in the proliferation and migration of cancer cells. Melanoma cells remodel microglia and upregulate matrix metalloproteinase-2 (MMP2) secretion to enhance cell proliferation and migration [83]. ANXA1 was found to be secreted by metastatic 4T1 mammary cancer cells and promoted microglial migration, which, in turn, promoted tumor cell migration. Silencing ANXA1 or inhibiting FPR1/FPR2 in 4T1 cells inhibited microglial migration and reduced STAT3 activation [84].
Furthermore, astrocytes and microglia cooperate with cancer cells at the same time to promote brain metastasis. miR-19a-containing exosomes are secreted by astrocytes and taken up by cancer cells to promote CCL2 expression. In turn, increased CCL2 on tumor cells recruits microglia to stimulate proliferation and inhibit apoptosis of metastatic brain cancer cells [85]. In addition, astrocytes in the brain are capable of promoting the metastatic transformation of circulating breast cancer cells and localizing them to the brain through secretion of chemokine CXCL12 [78]. Consistently, high expression of CCL2 or CXCL12 has been observed in patients with advanced (metastatic) breast cancer [86]. In addition, interactions between astrocytes and other immune cells, (e.g., granulocytes), promote brain metastasis [87]. The upregulated functional molecules secreted by microglia, astrocytes, and tumor cells that promote brain metastases are listed in Table 1.
Table 1.
Upregulated functional molecules secreted by microglia, astrocytes, and tumor cells promote brain metastases.
| Molecules | Cellular Source | Function | Reference |
|---|---|---|---|
| MMP2 | M1 Microglia | Damage BBB and degrade the tight junctions between capillary endothelial cells | [97] |
| IL-1β | Damage BBB and intrigue CNS inflammation | [98, 99] | |
| IL-6 | [28] | ||
| COX-2 | [97, 100] | ||
| CXCL10 | Recruit PD-L1+ CNS-native myeloid cells and suppress T cell function | [40, 44] | |
| IDO | M2 Microglia | Suppress T cell responses to apoptotic cell-associated antigens | [46] |
| CXCL2 | Promote tumor angiogenesis | [59] | |
| VEGF | [43, 59] | ||
| TGF-β | Promote tumor growth and induce immunosuppressive niche | [39] | |
| IL-10 | [40] | ||
| CCL20 | Promote tumor progression and stemness | [38] | |
| IGF-1 | [44] | ||
| miR-19a containing Exosomes | Astrocytes | Increase CCL2 secretion by tumor cells | [51] |
| Connexin43 | Promote metastatic cancer cell migration and resistance to apoptosis | [76, 82] | |
| CCL2 | Recruit microglia and inhibit cancer cell apoptosis | [79, 80, 85] | |
| ANXA1 | Breast cancer cells | Promote microglial migration and enhance tumor cell migration | [84] |
| miRNA-503 | Trigger M1-M2 polarization conversion of microglia, upregulate immune suppressive cytokines, and suppress T cell proliferation | [55] |
4. Heterogeneous microenvironment of lung cancer and its reciprocal crosstalk with various stroma, immune cells, and extracellular matrix
Lung cancer is a highly heterogeneous disease. Cancer cells and cells within the tumor microenvironment (TME), including blood vessels, cancer-associated fibroblasts (CAFs), the extracellular matrix (ECM), and infiltrating immune cells, together determine the disease progression and response to therapy.
Immunosuppression is a major feature of the TME in lung cancer and is mediated by various immune cells. Lung cancer cells produce inhibitory molecules, including COX2, PGE2, PDL1, and IDO, which impair the activity of CD8+ tumor-infiltrating lymphocytes [88]. Dendritic cells (DCs) in patients with NSCLC upregulate the co-suppressor molecule B7-H3, and therefore fail to stimulate T cells [89]. Myeloid-derived suppressor cells (MDSCs), a heterogeneous cell population, inhibit T cell proliferation and cytokine production [90]. Neutrophil-infiltrating mouse tumors support tumor-associated inflammation, angiogenesis, and metastasis [91]. CAFs secrete IL-6, which stimulates JAK2-STAT3 signaling in human lung cancer cells and increases their metastatic potential [92]. Another study has reported that Gas6 from CAFs promotes the migration of Axl-expressing lung cancer cells during chemotherapy [93]. In addition, CAFs regulate immune responses. CAFs isolated from a subpopulation of human NSCLC cells expressing ligands of the PD1 receptor, namely PDL1 and PDL2, were shown to suppress T cell function [94]. The ECM, which is composed of collagens, proteoglycans, and glycosaminoglycans, is a major component of the TME and mediates the interactions between cancer and stromal cells to promote cancer progression. The receptor for the glycosaminoglycan hyaluronan, known as hyaluronan-mediated motility receptor (HMMR), enhances ECM-mediated signaling and facilitates the outgrowth of micrometastases [95]. Excessive collagen deposition in Lkb1-deficient lung tumors was found to lead to enhanced cancer cell proliferation and invasiveness through the activation of β1 integrin signaling [96].
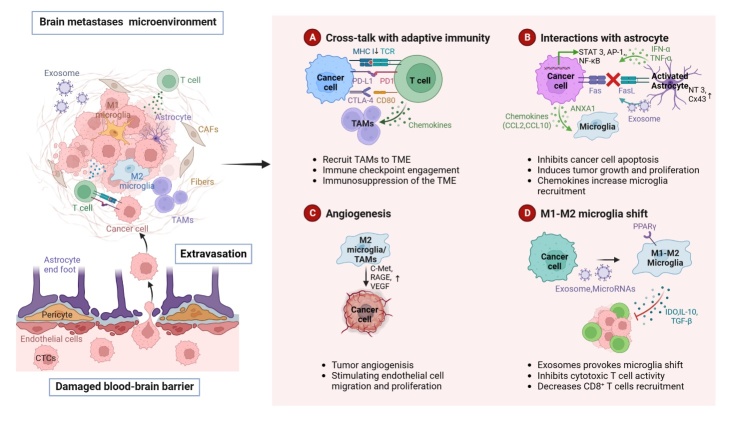
We have schematically depicted the abovementioned main mechanisms underlying brain metastasis in Figure 3.
Figure 3.
Major mechanisms underlying brain metastasis. Circulating tumor cells (CTCs) cross the blood-brain barrier (BBB) via extravasation after BBB disruption. Subsequently, the tumor microenvironment is reestablished through angiogenesis and reprogrammed immunity. Metastatic cancer cells produce molecules, such as miRNAs and immunosuppressive factors, that help them communicate and adapt to the brain environment. (A) Metastatic cancer cells reprogram adaptive immunity through the PD1/PD-l1 and CTLA4/CD80 immune checkpoints, leading to immune evasion and immunosuppression. (B) Astrocytes, cancer cells, and microglia interact with each other through the secretion of chemokines and cytokines. Activated STAT3, NF-κB, AKT-MAPK pathways in cancer cells lead to tumor proliferation and growth. CCL2 and CCL10, which are secreted by metastatic cancer cells, recruit more immunosuppressive microglia. (C) c-Met, RAGE, and VEGF overproduced by M2 microglia promote tumor angiogenesis. (D) Interactions between metastatic cancer cells, microglia, and T cells promote immune escape. Exosomal miRNAs contribute to microglial M2 polarization, which promotes cancer cell colonization and inhibits the cytotoxic effects of T-cells through the secretion of immunosuppressive cytokines.
5. Microglia are a promising target for the treatment of metastatic brain cancers
5.1. Targeting anti-inflammatory microglia
Anti-inflammation in the CNS of the brain parenchyma helps in establishing an appropriate environment for tumor cell survival and colonization, and the induction of inflammation may inhibit brain metastasis [55, 58]. As mentioned above, microglia, as resident macrophages in the CNS parenchyma, play an important role in the onset and termination of inflammation, depending on their subtype [101].
The survival of resident microglia is dependent on CSF-1R signaling. PLX3397, a CSF1R inhibitor, blocked M2 polarization of microglia, which significantly suppressed nicotine-related brain metastases from lung cancer and prolonged brain metastasis-free survival [44]. Another CSF1R inhibitor, BLZ945, in combination with a STAT5 inhibitor, AC4-130, sustained tumor control and normalized microglial activation state [102]. PI3K signaling is a master regulator of metastasis-promoting microglia during CNS colonization. Buparlisib (BKM120), a pan-PI3K Class I inhibitor, re-educated microglia-induced invasion of breast cancer cells into the brain parenchyma [103]. In another study, exosomes were used to encapsulate curcumin (Exo-cur) or the signal transducer and STAT3 inhibitor JSI124 (Exo-JSI124). Intranasal administration of Exo-cur or Exo-JSI124 led to the rapid delivery of the exosome-encapsulated drug to the brain, selective uptake by microglia, and subsequent induction of microglial apoptosis. This strategy may provide a novel noninvasive therapeutic approach for the treatment of inflammation-related diseases in the brain [104].
5.2. Targeting brain immune metastatic niche and biomarkers of response to immune-checkpoint inhibitors
Toll-like receptors (TLRs) recognize a conserved set of molecular structures, known as pathogen-associated molecular patterns, which enable them to sense and initiate innate and adaptive immune responses. TLRs are expressed on microglia, neurons, astrocytes, and endothelial cells. Therefore, TLR agonists have received much attention as therapeutic agents against primary tumors and metastases [105, 106]. CpG-oligodeoxynucleotides (ODNs), which are TLR-9 agonists, enhanced antigen presentation by microglia and promoted apoptosis in glioma [107]. Another TLR-9 agonist, CpG-C, activated microglia to phagocytose tumor cells, thereby reducing brain metastasis from lung cancer and melanoma [108]. CpG oligodeoxynucleotides conjugated with carbon nanotubes (CNT-CpG) were found to be more potent than free CpGs. Intracranial melanomas were infiltrated by TLR-9 positive microglia, causing inflammatory responses and anti-tumor cytotoxicity against brain melanoma [109].
As mentioned above, melanoma-secreted Aβ impairs microglial phagocytosis. LY2886721, a BACE-i that blocks Aβ production, reduced the burden of brain metastases [41]. Parthenolide inhibited brain metastasis from lung cancer by blocking M2 polarization [44]. Elevated TGF-β secretion by microglia was detected in mice with intracerebral melanomas. However, blocking TGFβ signaling with small molecule inhibitors or monoclonal antibodies, namely 1D11 or LY2157299, did not improve their survival. In contrast, tumor antigen-specific vaccination combined with focal radiotherapy reversed the tolerance and improved survival. This regimen was found to be associated with enhanced CD8+ T cell polyfunctionality, increased ratio of T effector to T regulatory cells, and decreased microglial TGF-β release [110].
ICIs may be an effective treatment for brain metastases originating from melanoma; however, their clinical role in brain metastases from other solid malignancies remains uncertain [111]. Brain metastases are the most common type of brain tumors, harboring an immune microenvironment that can be targeted by immunotherapy [38]. ICIs enhance the immune recognition of tumors by interfering with CTLA-4, PD-1, LAG-3, Gal-9/TIM-3, and other pathways. Over the last decade, these agents have significantly improved the prognosis of patients with metastatic cancers [112]. Ipilimumab, with or without in combination with nivolumab and pembrolizumab, showed improved overall survival in patients with melanoma and brain metastases [3, 113, 114]. Another study has confirmed that the efficacy of these anti-PD-1 and anti-CTLA-4 inhibitors depends on the presence of extracranial disease and enhanced trafficking of CD8+ T cells into the brain [115]. The immunotherapeutic agents currently in clinical trials for the treatment of brain metastases are listed in Table 2.
Table 2.
Ongoing clinical trials for immunotherapeutic agents for the treatment of brain metastases.
| Interventions | NCT Identifier | Clinical Trial Phase | Cancer Types | Case Number | Primary End Point | Status | Ref. |
|---|---|---|---|---|---|---|---|
| Sintilimab plus SRS | NCT04180501 | II | NSCLC | 25 | PFS | Not yet recruiting | [119] |
| Camrelizumab plus WBR | NCT04291092 | II | NSCLC | 63 | PFS | Recruiting | [120] |
| ICIs plus SRS | NCT05522660 | III | NSCLC, Melanoma | 190 | CNS-specific PFS | Recruiting | [121] |
| Pembrolizumab plus SRS | NCT02858869 | II | NSCLC, Melanoma | 27 | Dose limiting toxicities | Not yet Recruiting | [122] |
| Nivolumab and ipilimumab plus chemotherapy | NCT05012254 | II | NSCLC | 71 | PFS, ORR | Recruiting | [123] |
| Pembrolizumab plus chemotherapy | NCT04333004 | I/II | NSCLC | 40 | iORR, iPFS, PFS | Unknown | [124] |
| Tislelizumab plus carboplatin and pemetrexed | NCT04507217 | II | NSCLC | 78 | OS, PFS | Not yet recruiting | [125] |
| Sintilimab plus bevacizumab | NCT04213170 | II | NSCLC | 60 | iORR | Unknown | [126] |
| Camrelizumab plus pemetrexed and carboplatin | NCT04211090 | II | NSCLC | 45 | iORR | Active, not recruiting | [127] |
| Pembrolizumab plus bevacizumab | NCT02681549 | II | NSCLC, melanoma | 53 | ORR | Recruiting | [128] |
| Nivolumab plus SRS | NCT02978404 | II | NSCLC, SCLC | 26 | PFS | Active, not recruiting | [129] |
| Durvalumab plus SRS | NCT04889066 | II | NSCLC | 46 | iDCR | Recruiting | [130] |
| Nivolumab and radiotherapy with/without ipilimumab | NCT02696993 | I/II | NSCLC | 88 | PFS | Recruiting | [131] |
| atezolizumab/tiragolumab/durvalumab | NCT04513925 | III | NSCLC | 800 | PFS | Recruiting | [132] |
The most widely studied predictive biomarkers for immunotherapy are PD-L1, microsatellite instability/ defective mismatch repair (MSI/dMMR), and tumor mutational burden (TMB). MSI/dMMR has been approved for clinical use irrespective of the tumor type, whereas PD-L1 has only been approved for certain cancer types (e.g., for predicting the response to first-line NSCLC pembrolizumab monotherapy). TMB can predict the responses to several immunotherapies in multiple cancer types; however, there is a lack of standardized assay system. Few studies have shown that tumor-infiltrating CD8+ lymphocytes, specific genetic signatures, and IDO1 and JAK mutations have the potential to predict immunotherapy responses [116-118]. New, efficient biomarkers with standardized assays and the role of microglia in predicting immunotherapy responses need to be investigated further.
5.3. Targeting the re-programming of M1/M2 microglia
Microglia play a “friend or foe” role in brain metastasis, depending on the M1/M2 subtype upon activation. Therefore, reprogramming a pro-tumor/ immunosuppressive/anti-inflammatory M2 phenotype to an anti-tumor/immunostimulatory/inflammatory M1 phenotype is a strategy for combating tumor brain metastasis. Pharmacological inhibition of anti-inflammatory microglial phenotypes significantly reduced the tumor burden of breast cancer brain metastases [133]. Pretreatment with a phyto-glyceroglycolipid, 1,2-di-O-α-linolenoyl-3-O-β-galactopyranosyl-sn-glycerol (dLGG), drove the polarization of M2-like microglia to M1-like microglia and reduced melanoma brain metastases [134]. Tamoxifen treatment suppressed brain metastasis originating from breast cancer by blocking the polarization of M2 microglia and increasing their anti-tumor phagocytosis [36]. IL-6/JAK2/STAT3 signaling is involved in M2 microglial polarization. Tocilizumab, a monoclonal anti-IL6R neutralizing antibody, and fedratinib, a JAK2 inhibitor, were found to reduce the incidence of NSCLC brain metastasis [39]. We have described the promising microglial reprogramming targets in innate and adaptive immune responses in Figure 2.
5.4. Drug delivery systems
The poor prognosis of brain metastases is partially attributable to the BBB preventing most anticancer drugs from entering the brain [135]. Small molecules with improved BBB permeability are promising candidates in this regard [136]. Table 3 lists such inhibitors under clinical trials for the treatment of cancers with brain metastases. In addition, some large molecule monoclonal antibodies (mAbs) or antibody-coupled drugs (ADCs) have intracranial efficacy when the integrity of the BBB is compromised by the tumor itself, by radiotherapy, by low-intensity focused ultrasound (FUS) pulses, or with the assistance of delivery vehicles [137]. Studies pertaining to drugs with intracranial efficacy or adjuvant by delivery vehicles are essential for the treatment of brain tumors.
Table 3.
Ongoing clinical trials for small molecular inhibitors for the treatment of brain metastases.
| Compound | Target | NCT Identifier | Clinical Trial Phase | Cancer Type | Combination Partners | Ref. |
|---|---|---|---|---|---|---|
| Osimertinib | EGFR | NCT02296125 | III | NSCLC with an EGFR mutation | None | [152] |
| Aumolertinib | EGFR | NCT03849768 | III | NSCLC with EGFR Exon 19 deletion or L858R mutations | None | [153] |
| Furmonertinib | EGFR | NCT03787992 | III | NSCLC with EGFRm | None | [154] |
| MTI-31 (LXI-15029) | mTOR | NCT03125746 | I | Advanced malignant solid tumors | Exemestane | [140] |
| T7-DSNPs/9291 | EGFR | NCT03257124 | II | NSCLC with EGFR T790M | None | [150] |
| Bevacizumab | VEGF | NCT01004172 | II | Breast cancer | carboplatin | [155] |
| Trastuzumab | HER2 | NCT03529110 | III | Breast cancer | None | [156] |
| Trastuzumab | HER2 | NCT04752059 | II | Breast cancer | None | [157] |
| Tucatinib | HER2 | NCT02614794 | III | Breast cancer | Capecitabine /Trastuzumab | [158] |
Trifluoperazine (TFP), an antipsychotic agent, was found to suppress brain metastases from breast cancer and was highly bioavailable in the brain [138]. TFP has also been reported as a new adjuvant drug for treating patients with melanoma with brain, lung, and bone metastases, by disrupting the autophagic flux of melanoma [139]. MTI-31 (LXI-15029) is a novel mTORC1/mTORC2 inhibitor that is currently under clinical trials and has been found to reduce the recruitment of microglia in the TME [140].
Gold nanoparticles, superparamagnetic iron oxide nanoparticles, dual-targeting liposomes, and carbon dots have been suggested as delivery vehicles for treating brain metastases [141-144]. A microenvironment-tailored micelle (T-M/siRNA), co-delivering therapeutic siRNA and paclitaxel (PTX), could penetrate the BBB and target the immunosuppressive activation of microglia, thus significantly enhancing anti-tumor effects [145]. Mucic acid-based targeted nanoparticles, carrying camptothecin (CPT)/herceptin, significantly improved the therapeutic outcomes of brain metastases originating from breast cancer [146]. Hyaluronic-doxorubicin (hDOX), assembled using dual-targeting nanoparticles (NPs), showed the ability to reciprocally target the BBB and metastatic breast cancer cells by enzyme-recovered DNA insertion, thereby significantly prolonging the median survival time of mice [147]. Dual-targeting liposomal co-delivery systems provide another promising strategy for the treatment of patients with advanced EGFRT790M NSCLC and brain metastases, reducing the rate of drug resistance [148]. Lapatinib-loaded hiPSC platelets delivered to brain metastatic breast cancer cells inhibited the tumor growth and prolonged survival in tumor-bearing mice [149]. T7 peptide with osimertinib (AZD9291)-loaded intracellular glutathione (GSH)-responsive doxorubicin prodrug self-assembly nanocarriers (T7-DSNPs/9291) was designed as a potential targeted co-delivery system and exhibited anti-NSCLC brain metastasis effects [150]. CTLA-4 aldehyde modification was used as a CAR-targeting group to precisely target the central M1 microglia through aldehyde/hydroxylamine condensation on the surface of macrophages (CAR-M-UZPM) [151]. Notably, drugs targeting microglia, with or without delivery carriers, remain largely unexplored.
Concluding remarks
Long-distance tumor-derived factors and local immune interactions are the main reasons for the establishment of a pre-metastatic niche in the brain. Different stimuli in the microenvironment determine the differentiation of microglia into an anti-tumor M1 phenotype or a pro-tumor M2 phenotype. Interactions between microglia and tumor cells, astrocytes, and other immune cells provide an appropriate environment for the survival and proliferation of metastatic tumor cells. In this review, we discussed the roles of microglia in inflammation, angiogenesis, and immune modulation. Therefore, targeting microglia is a potential therapeutic approach for treating metastatic brain tumors.
The attraction of ICIs lies in their ability to achieve long-term or even complete responses. However, most patients do not benefit from these treatments because of following biological and technical challenges, especially in the treatment of brain metastases:1) BBB impermeability, which hampers the entry of most molecules; 2) Tumor heterogeneity, which limits the efficacy of a single agent; 3) Expression of various immune checkpoints; 4) Lack of useful predictive biomarkers; And 5) Immune-related side effects. Therefore, it is important to study the small molecular inhibitors with intracranial permeability, the specific role of cellular components and their interactions in brain microenvironment. In addition, the combination of systemic therapy and stereotactic radiosurgery, or that of targeted therapy (anti-proliferating, pro-apoptotic, anti-angiogenic inhibitors) and ICIs, or dual ICIs (targeting different immune checkpoints) are potential strategies to treat brain metastases [3, 113, 114, 159, 160].
Recent studies have shown that metabolic regulation is closely associated with brain metastases. Brain-derived metabolism may provide a propensity for brain metastasis [161]. Further, Microglia polarization is associated with lactate metabolism and oxidative stress [162-164]. More studies pertaining to microglia-mediated metabolic re-reprogramming are needed. In addition, the role of microglia in the response to therapies remains unclear.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (82122075, 82074232, 82030118, 81830120), Shanghai Frontier Research Base of Disease and Syndrome Biology of Inflammatory Cancer Transformation (2021KJ03-12), “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (21SG43), Three-year Plan Project of Shanghai Traditional Chinese Medicine(ZY(2021-2023)-0208), Clinical Research Plan of SHDC (SHDC2020CR4043) and Shanghai Youth Talent Support Program. Figures were created with BioRender.com.
Funding Statement
This work was funded by the National Natural Science Foundation of China (82122075, 82074232, 82030118, 81830120), Shanghai Frontier Research Base of Disease and Syndrome Biology of Inflammatory Cancer Transformation (2021KJ03-12), “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (21SG43), Three-year Plan Project of Shanghai Traditional Chinese Medicine(ZY(2021-2023)-0208), Clinical Research Plan of SHDC (SHDC2020CR4043) and Shanghai Youth Talent Support Program. Figures were created with BioRender.com.
Footnotes
Author contribution
YF and X H conceptualized and wrote the manuscript. YF and YR Z edited the critical revisions. YF, YR Z carried out the painting of graphics. YW provided supervision of the entire manuscript. All authors approved the final version of the manuscript for submission.
Conflict of interests
The authors declare no competing interests.
References
- [1].Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S, Du L, et al. (2018). Non-small-cell Lung Cancer with Brain Metastasis at Presentation. Clin Lung Cancer, 19:e373-e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Corti C, Antonarelli G, Criscitiello C, Lin NU, Carey LA, Cortés J, et al. (2022). Targeting brain metastases in breast cancer. Cancer Treat Rev, 103:102324. [DOI] [PubMed] [Google Scholar]
- [3].Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. (2018). Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol, 19:672-681. [DOI] [PubMed] [Google Scholar]
- [4].Wang Q, Li J, Liang X, Zhan Q (2022). Improved Survival With Surgical Treatment of Primary Lung Lesions in Non-Small Cell Lung Cancer With Brain Metastases: A Propensity-Matched Analysis of Surveillance, Epidemiology, and End Results Database. Front Oncol, 12:888999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hosonaga M, Saya H, Arima Y (2020). Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev, 39:711-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gutzmer R, Vordermark D, Hassel JC, Krex D, Wendl C, Schadendorf D, et al. (2020). Melanoma brain metastases - Interdisciplinary management recommendations 2020. Cancer Treat Rev, 89:102083. [DOI] [PubMed] [Google Scholar]
- [7].Petrelli F, Ghidini M, Lonati V, Tomasello G, Borgonovo K, Ghilardi M, et al. (2017). The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur J Cancer, 84:141-148. [DOI] [PubMed] [Google Scholar]
- [8].Lu H, Chen T, Wang Y, He Y, Pang Z, Wang Y (2022). Dual targeting micelles loaded with paclitaxel and lapatinib for combinational therapy of brain metastases from breast cancer. Sci Rep, 12:2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Linville RM, DeStefano JG, Sklar MB, Xu Z, Farrell AM, Bogorad MI, et al. (2019). Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials, 190-191:24-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mo F, Pellerino A, Soffietti R, Rudà R (2021). Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int J Mol Sci, 22:12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gibson EM, Monje M (2021). Microglia in Cancer Therapy-Related Cognitive Impairment. Trends Neurosci, 44:441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wright-Jin EC, Gutmann DH (2019). Microglia as Dynamic Cellular Mediators of Brain Function. Trends in Molecular Medicine, 25:967-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. (2019). Brain metastases. Nat Rev Dis Primers, 5:5. [DOI] [PubMed] [Google Scholar]
- [14].Orihuela R, McPherson CA, Harry GJ (2016). Microglial M1/M2 polarization and metabolic states. Br J Pharmacol, 173:649-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kadomoto S, Izumi K, Mizokami A (2021). Macrophage Polarity and Disease Control. Int J Mol Sci, 23:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].You H, Baluszek S, Kaminska B (2019). Immune Microenvironment of Brain Metastases-Are Microglia and Other Brain Macrophages Little Helpers? Front Immunol, 10:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu Y, Wang A, Zhang S, Kim J, Xia J, Zhang F, et al. (2022). Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J Adv Res, S2090-1232(22)00209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cannarile MA, Weisser M, Jacob W, Jegg A-M, Ries CH, Rüttinger D (2017). Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer, 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vandenbark AA, Offner H, Matejuk S, Matejuk A (2021). Microglia and astrocyte involvement in neurodegeneration and brain cancer. J Neuroinflammation, 18:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang Q, Cheng S, Wang Y, Wang M, Lu Y, Wen Z, et al. (2021). Interrogation of the microenvironmental landscape in spinal ependymomas reveals dual functions of tumor-associated macrophages. Nat Commun, 12:6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sevenich L (2018). Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front Immunol, 9:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pasqualini C, Kozaki T, Bruschi M, Nguyen THH, Minard-Colin V, Castel D, et al. (2020). Modeling the Interaction between the Microenvironment and Tumor Cells in Brain Tumors. Neuron, 108:1025-1044. [DOI] [PubMed] [Google Scholar]
- [23].Andersen BM, Faust Akl C, Wheeler MA, Chiocca EA, Reardon DA, Quintana FJ (2021). Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat Rev Cancer, 21:786-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kadry H, Noorani B, Cucullo L (2020). A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS, 17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bos PD, Zhang XH-F, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. (2009). Genes that mediate breast cancer metastasis to the brain. Nature, 459:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rolland Y, Demeule M, Fenart L, Béliveau R (2009). Inhibition of melanoma brain metastasis by targeting melanotransferrin at the cell surface. Pigment Cell Melanoma Res, 22:86-98. [DOI] [PubMed] [Google Scholar]
- [27].Lee B-C, Lee T-H, Avraham S, Avraham HK (2004). Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res, 2:327-338. [PubMed] [Google Scholar]
- [28].He C, Cai P, Li J, Zhang T, Lin L, Abbasi AZ, et al. (2017). Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J Control Release, 246:98-109. [DOI] [PubMed] [Google Scholar]
- [29].Simon A, Yang M, Marrison JL, James AD, Hunt MJ, O’Toole PJ, et al. (2020). Metastatic breast cancer cells induce altered microglial morphology and electrical excitability in vivo. J Neuroinflammation, 17:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Qiao S, Qian Y, Xu G, Luo Q, Zhang Z (2019). Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J Neuroinflammation, 16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nishida M, Yamashita N, Ogawa T, Koseki K, Warabi E, Ohue T, et al. (2021). Mitochondrial reactive oxygen species trigger metformin-dependent antitumor immunity via activation of Nrf2/mTORC1/p62 axis in tumor-infiltrating CD8T lymphocytes. J Immunother Cancer, 9:e002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].He BP, Wang JJ, Zhang X, Wu Y, Wang M, Bay B-H, et al. (2006). Differential reactions of microglia to brain metastasis of lung cancer. Mol Med, 12:161-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schulz M, Sevenich L (2021). TAMs in Brain Metastasis: Molecular Signatures in Mouse and Man. Front Immunol, 12:716504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li B, Yang W, Ge T, Wang Y, Cui R (2022). Stress induced microglial activation contributes to depression. Pharmacological Research, 179:106145. [DOI] [PubMed] [Google Scholar]
- [35].Tanaka S, Ohgidani M, Hata N, Inamine S, Sagata N, Shirouzu N, et al. (2021). CD206 Expression in Induced Microglia-Like Cells From Peripheral Blood as a Surrogate Biomarker for the Specific Immune Microenvironment of Neurosurgical Diseases Including Glioma. Front Immunol, 12:670131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu S-Y, Sharma S, Wu K, Tyagi A, Zhao D, Deshpande RP, et al. (2021). Tamoxifen suppresses brain metastasis of estrogen receptor-deficient breast cancer by skewing microglia polarization and enhancing their immune functions. Breast Cancer Res, 23:35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [37].Zhong Y, Gu L, Ye Y, Zhu H, Pu B, Wang J, et al. (2022). JAK2/STAT3 Axis Intermediates Microglia/Macrophage Polarization During Cerebral Ischemia/Reperfusion Injury. Neuroscience, 496:119-128. [DOI] [PubMed] [Google Scholar]
- [38].Fares J, Ulasov I, Timashev P, Lesniak MS (2021). Emerging principles of brain immunology and immune checkpoint blockade in brain metastases. Brain, 144:1046-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin Y, Kang Y, Wang M, Wu B, Su B, Yin H, et al. (2022). Targeting polarized phenotype of microglia via IL6/JAK2/STAT3 signaling to reduce NSCLC brain metastasis. Signal Transduct Target Ther, 7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guldner IH, Wang Q, Yang L, Golomb SM, Zhao Z, Lopez JA, et al. (2020). CNS-Native Myeloid Cells Drive Immune Suppression in the Brain Metastatic Niche through Cxcl10. Cell, 183:1234-1248.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kleffman K, Levinson G, Rose IVL, Blumenberg LM, Shadaloey SAA, Dhabaria A, et al. (2022). Melanoma-Secreted Amyloid Beta Suppresses Neuroinflammation and Promotes Brain Metastasis. Cancer Discov, 12:1314-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kwon HS, Koh S-H (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener, 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, et al. (2016). Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A, 113:4476-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wu S-Y, Xing F, Sharma S, Wu K, Tyagi A, Liu Y, et al. (2020). Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. Journal of Experimental Medicine, 217:e20191131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao Fei, Xiao C, Evans KS, Theivanthiran T, DeVito N, Holtzhausen A, et al. (2018). Paracrine Wnt5a-β-catenin Signaling Triggers a Metabolic Program That Drives Dendritic Cell Tolerization. Immunity, 48:147-160.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Herrera-Rios D, Mughal SS, Teuber-Hanselmann S, Pierscianek D, Sucker A, Jansen P, et al. (2020). Macrophages/Microglia Represent the Major Source of Indolamine 2,3-Dioxygenase Expression in Melanoma Metastases of the Brain. Front Immunol, 11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Beatty GL, O’Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle PA, et al. (2017). First-in-Human Phase 1 Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients With Advanced Solid Malignancies. Clin Cancer Res, 23:3269-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chabot S, Williams G, Hamilton M, Sutherland G, Yong VW (1999). Mechanisms of IL-10 production in human microglia-T cell interaction. J Immunol, 162:6819-6828. [PubMed] [Google Scholar]
- [49].Mirzaei R, Yong VW (2022). Microglia-T cell conversations in brain cancer progression. Trends Mol Med, 28:951-963. [DOI] [PubMed] [Google Scholar]
- [50].Zhang H, Wang C, Fan J, Zhu Q, Feng Y, Pan J, et al. (2022). CD47 promotes the proliferation and migration of adamantinomatous craniopharyngioma cells by activating the MAPK/ERK pathway, and CD47 blockade facilitates microglia-mediated phagocytosis. Neuropathol Appl Neurobiol, 48:e12795. [DOI] [PubMed] [Google Scholar]
- [51].Camacho L, Guerrero P, Marchetti D (2013). MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PLoS One, 8:e73790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, et al. (2020). Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain, 143:1476-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang S, Xu M, Li X, Su X, Xiao X, Keating A, et al. (2018). Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol, 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xing F, Liu Y, Wu S-Y, Wu K, Sharma S, Mo Y-Y, et al. (2018). Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res, 78:4316-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gan D-X, Wang Y-B, He M-Y, Chen Z-Y, Qin X-X, Miao Z-W, et al. (2020). Lung Cancer Cells-Controlled Dkk-1 Production in Brain Metastatic Cascade Drive Microglia to Acquire a Pro-tumorigenic Phenotype. Front Cell Dev Biol, 8:591405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen P, Liu R, Yu Z, Cui G, Zong W, Wang M, et al. (2022). MiR196a-5p in extracellular vesicles released from human nasopharyngeal carcinoma enhance the phagocytosis and secretion of microglia by targeting ROCK1. Exp Cell Res, 411:112988. [DOI] [PubMed] [Google Scholar]
- [58].Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, et al. (2015). Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature, 527:100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Brandenburg S, Müller A, Turkowski K, Radev YT, Rot S, Schmidt C, et al. (2016). Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol, 131:365-378. [DOI] [PubMed] [Google Scholar]
- [60].Yuan J, Ge H, Liu W, Zhu H, Chen Y, Zhang X, et al. (2017). M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARγ signaling pathway. Oncotarget, 8:19855-19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wen L, You W, Wang H, Meng Y, Feng J, Yang X (2018). Polarization of Microglia to the M2 Phenotype in a Peroxisome Proliferator-Activated Receptor Gamma-Dependent Manner Attenuates Axonal Injury Induced by Traumatic Brain Injury in Mice. J Neurotrauma, 35:2330-2340. [DOI] [PubMed] [Google Scholar]
- [62].Kinouchi T, Kitazato KT, Shimada K, Yagi K, Tada Y, Matsushita N, et al. (2018). Treatment with the PPARγ Agonist Pioglitazone in the Early Post-ischemia Phase Inhibits Pro-inflammatory Responses and Promotes Neurogenesis Via the Activation of Innate- and Bone Marrow-Derived Stem Cells in Rats. Transl Stroke Res, 9:306-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chu K, Lee S-T, Koo J-S, Jung K-H, Kim E-H, Sinn D-I, et al. (2006). Peroxisome proliferator-activated receptor-gamma-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res, 1093:208-218. [DOI] [PubMed] [Google Scholar]
- [64].Xiong X-Y, Liu L, Yang Q-W (2016). Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol, 142:23-44. [DOI] [PubMed] [Google Scholar]
- [65].Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013). Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol, 229:176-185. [DOI] [PubMed] [Google Scholar]
- [66].Jiang Y, Zhao J, Xu J, Zhang H, Zhou J, Li H, et al. (2022). Glioblastoma-associated microglia-derived exosomal circKIF18A promotes angiogenesis by targeting FOXC2. Oncogene, 41:3461-3473. [DOI] [PubMed] [Google Scholar]
- [67].Badie B, Schartner J, Klaver J, Vorpahl J (1999). In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth factor/scatter factor. Neurosurgery, 44:1077-1082; discussion 1082-1083. [DOI] [PubMed] [Google Scholar]
- [68].Di Renzo MF, Bertolotto A, Olivero M, Putzolu P, Crepaldi T, Schiffer D, et al. (1993). Selective expression of the Met/HGF receptor in human central nervous system microglia. Oncogene, 8:219-222. [PubMed] [Google Scholar]
- [69].Yamagata T, Muroya K, Mukasa T, Igarashi H, Momoi M, Tsukahara T, et al. (1995). Hepatocyte growth factor specifically expressed in microglia activated Ras in the neurons, similar to the action of neurotrophic factors. Biochem Biophys Res Commun, 210:231-237. [DOI] [PubMed] [Google Scholar]
- [70].Rosen EM, Laterra J, Joseph A, Jin L, Fuchs A, Way D, et al. (1996). Scatter factor expression and regulation in human glial tumors. Int J Cancer, 67:248-255. [DOI] [PubMed] [Google Scholar]
- [71].Chen X, Zhang L, Zhang IY, Liang J, Wang H, Ouyang M, et al. (2014). RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res, 74:7285-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Radin DP, Tsirka SE (2020). Interactions between Tumor Cells, Neurons, and Microglia in the Glioma Microenvironment. Int J Mol Sci, 21:E8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Linnerbauer M, Wheeler MA, Quintana FJ (2020). Astrocyte Crosstalk in CNS Inflammation. Neuron, 108:608-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, et al. (2002). Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A, 99:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH-F, Lee DJ, et al. (2014). Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 156:1002-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Uzu M, Sin WC, Shimizu A, Sato H (2018). Conflicting Roles of Connexin43 in Tumor Invasion and Growth in the Central Nervous System. Int J Mol Sci, 19:E1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jandial R, Choy C, Levy DM, Chen MY, Ansari KI (2017). Astrocyte-induced Reelin expression drives proliferation of Her2+ breast cancer metastases. Clin Exp Metastasis, 34:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kaverina N, Borovjagin AV, Kadagidze Z, Baryshnikov A, Baryshnikova M, Malin D, et al. (2017). Astrocytes promote progression of breast cancer metastases to the brain via a KISS1-mediated autophagy. Autophagy, 13:1905-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hajal C, Shin Y, Li L, Serrano JC, Jacks T, Kamm RD (2021). The CCL2-CCR2 astrocyte-cancer cell axis in tumor extravasation at the brain. Sci Adv, 7:eabg8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pozzi S, Scomparin A, Ben-Shushan D, Yeini E, Ofek P, Nahmad AD, et al. (2022). MCP-1/CCR2 axis inhibition sensitizes the brain microenvironment against melanoma brain metastasis progression. JCI Insight, 7:e154804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Louie E, Chen XF, Coomes A, Ji K, Tsirka S, Chen EI (2013). Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene, 32:4064-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Fumagalli A, Heuninck J, Pizzoccaro A, Moutin E, Koenen J, Séveno M, et al. (2020). The atypical chemokine receptor 3 interacts with Connexin 43 inhibiting astrocytic gap junctional intercellular communication. Nat Commun, 11:4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Izraely S, Ben-Menachem S, Sagi-Assif O, Telerman A, Zubrilov I, Ashkenazi O, et al. (2019). The metastatic microenvironment: Melanoma-microglia cross-talk promotes the malignant phenotype of melanoma cells. Int J Cancer, 144:802-817. [DOI] [PubMed] [Google Scholar]
- [84].Foo SL, Sachaphibulkij K, Lee CLY, Yap GLR, Cui J, Arumugam T, et al. (2022). Breast cancer metastasis to brain results in recruitment and activation of microglia through annexin-A1/formyl peptide receptor signaling. Breast Cancer Res, 24:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Alečković M, Kang Y (2015). Welcoming Treat: Astrocyte-Derived Exosomes Induce PTEN Suppression to Foster Brain Metastasis. Cancer Cell, 28:554-556. [DOI] [PubMed] [Google Scholar]
- [86].Lu X, Kang Y (2009). Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem, 284:29087-29096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Adler O, Zait Y, Cohen N, Blazquez R, Doron H, Monteran L, et al. (2023). Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2. Nat Cancer, 4:401-418. [DOI] [PubMed] [Google Scholar]
- [88].Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. (2019). The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer, 19:9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Schneider T, Hoffmann H, Dienemann H, Schnabel PA, Enk AH, Ring S, et al. (2011). Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol, 6:1162-1168. [DOI] [PubMed] [Google Scholar]
- [90].Veglia F, Perego M, Gabrilovich D (2018). Myeloid-derived suppressor cells coming of age. Nat Immunol, 19:108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sionov RV, Fridlender ZG, Granot Z (2015). The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron, 8:125-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang L, Cao L, Wang H, Liu B, Zhang Q, Meng Z, et al. (2017). Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget, 8:76116-76128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kanzaki R, Naito H, Kise K, Takara K, Eino D, Minami M, et al. (2017). Gas6 derived from cancer-associated fibroblasts promotes migration of Axl-expressing lung cancer cells during chemotherapy. Sci Rep, 7:10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Yokota SJ, Bankert RB (2007). Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol, 178:5552-5562. [DOI] [PubMed] [Google Scholar]
- [95].Stevens LE, Cheung WKC, Adua SJ, Arnal-Estapé A, Zhao M, Liu Z, et al. (2017). Extracellular Matrix Receptor Expression in Subtypes of Lung Adenocarcinoma Potentiates Outgrowth of Micrometastases. Cancer Res, 77:1905-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gao Y, Xiao Q, Ma H, Li L, Liu J, Feng Y, et al. (2010). LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci U S A, 107:18892-18897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wilhelm I, Fazakas C, Molnár K, Végh AG, Haskó J, Krizbai IA (2018). Foe or friend? Janus-faces of the neurovascular unit in the formation of brain metastases. J Cereb Blood Flow Metab, 38:563-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Huang X, Hussain B, Chang J (2021). Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther, 27:36-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G (2018). Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol, 135:311-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Obermeier B, Daneman R, Ransohoff RM (2013). Development, maintenance and disruption of the blood-brain barrier. Nat Med, 19:1584-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Prinz M, Priller J (2014). Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci, 15:300-312. [DOI] [PubMed] [Google Scholar]
- [102].Klemm F, Möckl A, Salamero-Boix A, Alekseeva T, Schäffer A, Schulz M, et al. (2021). Compensatory CSF2-driven macrophage activation promotes adaptive resistance to CSF1R inhibition in breast-to-brain metastasis. Nat Cancer, 2:1086-1101. [DOI] [PubMed] [Google Scholar]
- [103].Blazquez R, Wlochowitz D, Wolff A, Seitz S, Wachter A, Perera-Bel J, et al. (2018). PI3K: A master regulator of brain metastasis-promoting macrophages/microglia. Glia, 66:2438-2455. [DOI] [PubMed] [Google Scholar]
- [104].Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. (2011). Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther, 19:1769-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Engelhardt B, Vajkoczy P, Weller RO (2017). The movers and shapers in immune privilege of the CNS. Nat Immunol, 18:123-131. [DOI] [PubMed] [Google Scholar]
- [106].Volovitz I, Marmor Y, Azulay M, Machlenkin A, Goldberger O, Mor F, et al. (2011). Split immunity: immune inhibition of rat gliomas by subcutaneous exposure to unmodified live tumor cells. J Immunol, 187:5452-5462. [DOI] [PubMed] [Google Scholar]
- [107].El Andaloussi A, Sonabend AM, Han Y, Lesniak MS (2006). Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia, 54:526-535. [DOI] [PubMed] [Google Scholar]
- [108].Benbenishty A, Gadrich M, Cottarelli A, Lubart A, Kain D, Amer M, et al. (2019). Prophylactic TLR9 stimulation reduces brain metastasis through microglia activation. PLoS Biol, 17:e2006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Fan H, Zhang I, Chen X, Zhang L, Wang H, Da Fonseca A, et al. (2012). Intracerebral CpG immunotherapy with carbon nanotubes abrogates growth of subcutaneous melanomas in mice. Clin Cancer Res, 18:5628-5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jackson CM, Kochel CM, Nirschl CJ, Durham NM, Ruzevick J, Alme A, et al. (2016). Systemic Tolerance Mediated by Melanoma Brain Tumors Is Reversible by Radiotherapy and Vaccination. Clin Cancer Res, 22:1161-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Nunno VD, Nuvola G, Mosca M, Maggio I, Gatto L, Tosoni A, et al. (2021). Clinical efficacy of immune checkpoint inhibitors in patients with brain metastases. Immunotherapy, 13:419-432. [DOI] [PubMed] [Google Scholar]
- [112].Aquilanti E, Brastianos PK (2020). Immune Checkpoint Inhibitors for Brain Metastases: A Primer for Neurosurgeons. Neurosurgery, 87:E281-E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med, 363:711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Schachter J, Ribas A, Long GV, Arance A, Grob J-J, Mortier L, et al. (2017). Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet, 390:1853-1862. [DOI] [PubMed] [Google Scholar]
- [115].Taggart D, Andreou T, Scott KJ, Williams J, Rippaus N, Brownlie RJ, et al. (2018). Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc Natl Acad Sci U S A, 115:E1540-E1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Duffy MJ, Crown J (2019). Biomarkers for Predicting Response to Immunotherapy with Immune Checkpoint Inhibitors in Cancer Patients. Clin Chem, 65:1228-1238. [DOI] [PubMed] [Google Scholar]
- [117].Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. (2019). Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol, 30:44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, Plaen ED, et al. (2017). Constitutive IDO1 Expression in Human Tumors Is Driven by Cyclooxygenase-2 and Mediates Intrinsic Immune Resistance. Cancer Immunol Res, 5:695-709. [DOI] [PubMed] [Google Scholar]
- [119].Dong X (2019). A Phase II Study on the Treatment of Advanced Non-small Cell Lung Cancer With Brain Metastasis by SRS Sequential Sintilimab. clinicaltrials.gov; 2019.
- [120].Lei Gong (2021). Camrelizumab Combined With Chemotherapy and Local Treatment in Non-small Cell Lung Cancer Patients With Brain Metastasis, a Single-arm, Multi-center, Open-labeled Phase II Clinical Trial. clinicaltrials.gov; 2021.
- [121].ETOP IBCSG Partners Foundation (2023). A Multicentre Randomised Open-label Phase III Study of Stereotactic Radiosurgery, in Addition to Standard Systemic Therapy for Patients With Metastatic Melanoma or Newly Diagnosed Metastatic NSCLC and Asymptomatic or Oligo-symptomatic Brain Metastases. clinicaltrials.gov; 2023.
- [122].Khan MK (2022). Pilot Study of Pembrolizumab and Stereotactic Radio-Surgery (SRS) for Patients With Melanoma or Non-Small Cell Lung Cancer (NSCLC) Brain Metastases (BM). clinicaltrials.gov; 2022.
- [123].Fundación GECP (2022). Nivolumab Plus Ipilimumab Plus Two Cycles of Platinum-based Chemotherapy as First Line Treatment for Stage IV/Recurrent Non-small Cell Lung Cancer (NSCLC) Patients With Synchronous Brain Metastases. clinicaltrials.gov; 2022.
- [124].Dong X (2020). Analysis of Gut Microbiota in Patients With Brain Metastasis of Non-small Cell Lung Cancer Treated by Pembrolizumab Combined With Chemotherapy. clinicaltrials.gov; 2020.
- [125].MD LZ (2020). A Phase II, Open-Label, Multicenter, Prospective Clinical Study to Investigate the Efficacy and Safety of Tislelizumab Combined With Pemetrexed/ Carboplatin in Patients With Brain Metastases of Non-squamous Non-small Cell Lung Cancer. clinicaltrials.gov; 2020. [Google Scholar]
- [126].Chen L (2019). Prospective Phase II Clinical Study of Sintilimab Combined With Bevacizumab for Driving Gene-negative, Asymptomatic Brain Metastases From Non-small Cell Lung Cancer. clinicaltrials.gov; 2019.
- [127].Chen L (2022). A Phase II Study to Evaluate Camrelizumab With Pemetrexed / Carboplatin in Patients With Brain Metastases of Driven Gene-negative, Non-squamous Non-small Cell Lung Cancer. clinicaltrials.gov; 2022.
- [128].Kluger H (2022). A Phase 2 Trial of Pembrolizumab Plus Bevacizumab in Patients With Metastatic Melanoma or Non-small Cell Lung Cancer With Untreated Brain Metastases. clinicaltrials.gov; 2022.
- [129].Centre hospitalier de l’Université de Montréal (CHUM) (2023). A Phase II, Multi-centre Study, of Combining Radiosurgery and Nivolumab in the Treatment of Brain Metastases From Non-small Cell Lung Cancer and Renal Cell Cancer. clinicaltrials.gov; 2023.
- [130].Kumar K (2022). A Phase II Clinical Trial of Durvalumab (MEDI4736) and Fractionated Stereotactic Radiotherapy (fSRT) vs. Personalized Ultra-Fractionated Stereotactic Adaptive Radiotherapy (PULSAR) for the Treatment of Brain Metastases From Non-Small Cell Lung Cancer (NSCLC). clinicaltrials.gov; 2022.
- [131].M.D. Anderson Cancer Center (2022). Phase I/II Trial of Nivolumab With Radiation or Nivolumab and Ipilimumab With Radiation for the Treatment of Intracranial Metastases From Non-Small Cell Lung Cancer. clinicaltrials.gov; 2022.
- [132].Hoffmann-La Roche (2023). A Phase III, Open-Label, Randomized Study of Atezolizumab and Tiragolumab Compared With Durvalumab in Patients With Locally Advanced, Unresectable Stage III Non-Small Cell Lung Cancer Who Have Not Progressed After Concurrent Platinum-Based Chemoradiation. clinicaltrials.gov; 2023.
- [133].Andreou KE, Soto MS, Allen D, Economopoulos V, de Bernardi A, Larkin JR, et al. (2017). Anti-inflammatory Microglia/Macrophages As a Potential Therapeutic Target in Brain Metastasis. Front Oncol, 7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Yang C-C, Chang M-T, Chang C-K, Shyur L-F (2021). Phytogalactolipid dLGG Inhibits Mouse Melanoma Brain Metastasis through Regulating Oxylipin Activity and Re-Programming Macrophage Polarity in the Tumor Microenvironment. Cancers (Basel), 13:4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bernatz S, Ilina EI, Devraj K, Harter PN, Mueller K, Kleber S, et al. (2019). Impact of Docetaxel on blood-brain barrier function and formation of breast cancer brain metastases. J Exp Clin Cancer Res, 38:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Page S, Milner-Watts C, Perna M, Janzic U, Vidal N, Kaudeer N, et al. (2020). Systemic treatment of brain metastases in non-small cell lung cancer. Eur J Cancer, 132:187-198. [DOI] [PubMed] [Google Scholar]
- [137].Kunte S, Abraham J, Montero AJ (2020). Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer, 126:4278-4288. [DOI] [PubMed] [Google Scholar]
- [138].Feng Z, Xia Y, Gao T, Xu F, Lei Q, Peng C, et al. (2018). The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Dis, 9:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Xia Y, Xu F, Xiong M, Yang H, Lin W, Xie Y, et al. (2021). Repurposing of antipsychotic trifluoperazine for treating brain metastasis, lung metastasis and bone metastasis of melanoma by disrupting autophagy flux. Pharmacol Res, 163:105295. [DOI] [PubMed] [Google Scholar]
- [140].Zhang Q, Zhang Y, Chen Y, Qian J, Zhang X, Yu K (2019). A Novel mTORC1/2 Inhibitor (MTI-31) Inhibits Tumor Growth, Epithelial-Mesenchymal Transition, Metastases, and Improves Antitumor Immunity in Preclinical Models of Lung Cancer. Clin Cancer Res, 25:3630-3642. [DOI] [PubMed] [Google Scholar]
- [141].Du J, Shao Y, Hu Y, Chen Y, Cang J, Chen X, et al. (2021). Multifunctional Liposomes Enable Active Targeting and Twinfilin 1 Silencing to Reverse Paclitaxel Resistance in Brain Metastatic Breast Cancer. ACS Appl Mater Interfaces, 13:23396-23409. [DOI] [PubMed] [Google Scholar]
- [142].Feng Q, Xu X, Wei C, Li Y, Wang M, Lv C, et al. (2021). The Dynamic Interactions between Nanoparticles and Macrophages Impact Their Fate in Brain Tumors. Small, 17:e2103600. [DOI] [PubMed] [Google Scholar]
- [143].Sun C, Ding Y, Zhou L, Shi D, Sun L, Webster TJ, et al. (2017). Noninvasive nanoparticle strategies for brain tumor targeting. Nanomedicine, 13:2605-2621. [DOI] [PubMed] [Google Scholar]
- [144].Calabrese G, De Luca G, Nocito G, Rizzo MG, Lombardo SP, Chisari G, et al. (2021). Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int J Mol Sci, 22:11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zhao Z, Zhang Y, Li C, Li X, Chu Y, Guo Q, et al. (2022). Microenvironment-tailored micelles restrain carcinoma-astrocyte crosstalk for brain metastasis. J Control Release, 349:520-532. [DOI] [PubMed] [Google Scholar]
- [146].Wyatt EA, Davis ME (2020). Nanoparticles Containing a Combination of a Drug and an Antibody for the Treatment of Breast Cancer Brain Metastases. Mol Pharm, 17:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Ju X, Chen H, Miao T, Ni J, Han L (2021). Prodrug Delivery Using Dual-Targeting Nanoparticles To Treat Breast Cancer Brain Metastases. Mol Pharm, 18:2694-2702. [DOI] [PubMed] [Google Scholar]
- [148].Yin W, Zhao Y, Kang X, Zhao P, Fu X, Mo X, et al. (2020). BBB-penetrating codelivery liposomes treat brain metastasis of non-small cell lung cancer with EGFRT790M mutation. Theranostics, 10:6122-6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bhan A, Ansari K, Chen MY, Jandial R (2021). Human induced pluripotent stem cell-derived platelets loaded with lapatinib effectively target HER2+ breast cancer metastasis to the brain. Sci Rep, 11:16866. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [150].Wang X, Mao W, Wang Z, Li X, Xiong Y, Lu H, et al. (2020). Enhanced Anti-Brain Metastasis from Non-Small Cell Lung Cancer of Osimertinib and Doxorubicin Co-Delivery Targeted Nanocarrier. Int J Nanomedicine, 15:5491-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Liu Y, Hu P, Zheng Z, Zhong D, Xie W, Tang Z, et al. (2022). Photoresponsive Vaccine-Like CAR-M System with High-Efficiency Central Immune Regulation for Inflammation-Related Depression. Adv Mater, 34:e2108525. [DOI] [PubMed] [Google Scholar]
- [152].Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. (2020). Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med, 382:41-50. [DOI] [PubMed] [Google Scholar]
- [153].Lu S, Dong X, Jian H, Chen J, Chen G, Sun Y, et al. (2022). AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol, 40:3162-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, et al. (2022). Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med, 10:1019-1028. [DOI] [PubMed] [Google Scholar]
- [155].Leone JP, Emblem KE, Weitz M, Gelman RS, Schneider BP, Freedman RA, et al. (2020). Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res, 22:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, et al. (2022). Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med, 386:1143-1154. [DOI] [PubMed] [Google Scholar]
- [157].Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. (2022). Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med, 28:1840-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. (2020). Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med, 382:597-609. [DOI] [PubMed] [Google Scholar]
- [159].Rishi A, Yu H-HM (2020). Current Treatment of Melanoma Brain Metastasis. Curr Treat Options Oncol, 21:45. [DOI] [PubMed] [Google Scholar]
- [160].Lee H-W (2021). Multidiscipline Immunotherapy-Based Rational Combinations for Robust and Durable Efficacy in Brain Metastases from Renal Cell Carcinoma. Int J Mol Sci, 22:6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Kalita-de Croft P, Straube J, Lim M, Al-Ejeh F, Lakhani SR, Saunus JM (2019). Proteomic Analysis of the Breast Cancer Brain Metastasis Microenvironment. Int J Mol Sci, 20:E2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Longhitano L, Vicario N, Forte S, Giallongo C, Broggi G, Caltabiano R, et al. (2023). Lactate modulates microglia polarization via IGFBP6 expression and remodels tumor microenvironment in glioblastoma. Cancer Immunol Immunother, 72:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhang S, Hu L, Jiang J, Li H, Wu Q, Ooi K, et al. (2020). HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J Neuroinflammation, 17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, et al. (2019). Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: Role of renin-angiotensin system. Redox Biol, 26:101295. [DOI] [PMC free article] [PubMed] [Google Scholar]