In the past three decades, the incidence of Achilles tendinopathy has risen as a result of greater participation in recreational and competitive sporting activities.1,2 The rate of Achilles tendon injuries in runners is about ten times that in age-matched controls. Achilles tendinopathy is also common among athletes participating in racquet sports, track and field, volleyball and soccer. However, the condition is by no means confined to athletes: in one series of 58 patients, nearly one-third did not participate in vigorous physical activity.3
ANATOMY
The Achilles tendon is a confluence of the gastrocnemius and soleus muscles. The soleus muscle lies deep to the gastrocnemius muscle, arising from the posterior surface of the upper tibia. The tendon is inserted on the posterior surface of the calcaneus distal to the posterior-superior calcaneal tuberosity. The Achilles tendon is not encased in a true synovial sheath but is surrounded by paratendon composed of a single layer of cells. This tissue is richly vascularized and is responsible for much of the blood supply,4 which reaches the tendon through a series of transverse vincula that function as passageways for the vessels. The Achilles tendon also receives blood from vessels originating at the musculotendinous and osteotendinous junctions.
At about 12–15 cm proximal to its insertion, rotation of the tendon begins, becoming more marked in the distalmost 5–6 cm. The tendon spirals approximately 90°, with the medial fibres rotating posteriorly and the posterior fibres rotating laterally. Angiographic injection techniques have demonstrated a zone of hypovascularity 2–7 cm proximal to the tendon insertion. Additionally, the number of intratendinous vessels and the relative area occupied by these vessels is lowest 4 cm from the calcaneal insertion.4
Healthy tendons are brilliant white, with a fibroelastic texture. Within the extracellular matrix network, tenoblasts and tenocytes constitute 90–95% of the cellular elements of tendons. The remaining 5–10% consists of fibrochondrocytes, synovial cells of the tendon sheath, endothelial cells and smooth muscle cells. Collagen type 1 accounts for 65–80% and elastin accounts for about 2% of the dry mass of tendons. Tenocytes and tenoblasts lie between the collagen fibres along the long axis of the tendon.5
Tendon innervation originates from three main sources—cutaneous, muscular and peritendinous nerve trunks. At the musculotendinous junction, nerve fibres cross and enter the endotenon septa. Nerve fibres from rich plexuses in the paratenon penetrate the epitenon. Most nerve fibres do not actually enter the main body of the tendon but terminate as nerve endings on its surface. Nerve endings of myelinated fibres function as specialized mechanoreceptors to detect changes in pressure or tension. Unmyelinated nerve endings act as nociceptors, sensing and transmitting pain. Both sympathetic and parasympathetic fibres have been identified.6 Autonomic peptides such as neuropeptide Y and vasoactive intestinal peptide, which regulate vasoactivity, have been demonstrated in tendons.6,7
BIOMECHANICS
Tendons transmit force generated by muscle to bone. Additionally, they act as a buffer by absorbing external forces to limit muscle damage—a function that demands mechanical strength, flexibility and elasticity.5 As collagen fibres deform, they respond linearly to increasing tendon loads.8 The configuration is initially lost when the stretch exceeds 2% but is regained if the strain placed on the tendon remains at less than 4%; if strain exceeds 8% macroscopic rupture will occur.9,10 The tensile strength of tendons is related to thickness and collagen content, and a tendon with a cross-sectional area of 1 sq cm is capable of supporting 500–1000 kg. Loading of the Achilles tendon reaches up to 9 kN during running (corresponding to 12.5 times the body weight), 2.6 kN during slow walking, and less than 1 kN during cycling.11
AETIOLOGY AND PATHOPHYSIOLOGY
Tendon injuries can be acute or chronic. Clearly, in acute trauma extrinsic factors predominate, whereas in chronic disorders intrinsic and extrinsic factors commonly interact.12,13 Examples of intrinsic factors are tendon vascularity, gastrocnemius–soleus dysfunction, age, gender, body weight and height, pes cavus, and lateral ankle instability. Excessive motion of the hindfoot in the frontal plane, especially a lateral heel strike with compensatory pronation, is thought to cause a ‘whipping action’ on the Achilles tendon and predispose it to tendinopathy. Also, forefoot varus is frequent in patients with Achilles tendinopathy. Extrinsic factors that may predispose to Achilles tendinopathy in athletes are changes in training pattern, poor technique, previous injuries, footwear and training on hard, slippery or slanting surfaces.2,9 Excessive loading of tendons during vigorous physical training is regarded as the main pathological stimulus for degeneration.2 Tendons respond to repetitive overload beyond physiological threshold by inflammation of their sheath, degeneration of their body, or a combination of the two.14 Whether different stresses induce different responses remains unclear. Active repair of fatigue damage must occur, or tendons would weaken and eventually rupture. The repair mechanism is probably mediated by resident tenocytes that continually monitor the extracellular matrix. Failure to adapt to recurrent excessive loads results in the release of cytokines, leading to further modulation of cell activity.15 Tendon damage may even result from stresses within physiological limits, since frequent microtrauma may not allow enough time for repair.2 Microtrauma can also result from non-uniform stress within tendons, producing abnormal load concentrations and frictional forces between the fibrils, with localized fibre damage.15
The aetiology of tendinopathy remains uncertain and many factors have been implicated,1 including free radical damage occurring on reperfusion after ischaemia, hypoxia, hyperthermia and impaired tenocyte apoptosis.16 In animals, tendinopathy can be induced by local administration of cytokines and prostaglandins.17 Fluoroquinolones have also been implicated: ciprofloxacin enhances interleukin-1 β mediated release of matrix metalloproteinase (MMP3) release, inhibits tenocyte proliferation and reduces collagen and matrix synthesis.18 Changes in the expression of genes regulating cell–cell and cell–matrix interactions have been reported, with down-regulation of MMP3 mRNA in tendinopathic Achilles tendon samples.19 Type I and type III collagen mRNAs have been found at higher levels in tendinopathic samples than in normal samples.19 In tendinopathic Achilles tendons, upregulation of MMP2 and vascular endothelial growth factor has been described, whilst MMP3 was downregulated.20 Imbalance in MMP activity in response to repeated injury or mechanical strain may result in tendon degeneration.
The main symptom of Achilles tendinopathy is pain, but again the underlying mechanism is not fully understood. In the past it was assumed to arise through inflammation or via collagen fibre separation or degeneration,21,22 but chronically painful Achilles tendons show no evidence of inflammation, and some that show clear intratendinous defects on MRI or ultrasound are not painful.21–24 Since tendinopathies are degenerative rather than inflammatory conditions, pain may originate from a combination of mechanical and biochemical factors.23 Microdialysis sampling revealed twofold higher lactate levels in tendinopathic tendons than in controls. High concentrations of the neurotransmitter glutamate, but no increase in the proinflammatory prostaglandin PGE2, have been found in Achilles and patellar tendinopathy.24
Several studies have confirmed the occurrence of sensory neuropeptides in both animal and human tendons, and substance P has been found in tendinopathic Achilles tendons.7,25 Endogenous opioids provide a peripheral antinociceptive system, and morphine inhibits the release of substance P from peripheral sensory nerve endings.26 Under normal conditions, a balance probably exists between nociceptive and antinociceptive peptides.
HISTOPATHOLOGY
The pathological label ‘tendinosis’ is used for the disorganized healing response, but most clinicians still use the term tendinitis (or tendonitis), with its implication that the condition is essentially inflammatory. We recommend use of the term ‘tendinopathy’ as a generic descriptor of the clinical conditions in and around tendons arising from overuse, with tendinosis and tendinitis being applied only after histopathological examination.27
Histologically, tendinopathy is characterized by an absence of inflammatory cells and a poor healing response, with non-inflammatory intratendinous collagen degeneration, fibre disorientation and thinning, hypercellularity, scattered vascular ingrowth, and increased interfibrillar glycosaminoglycans.8,12,15 Frank inflammatory lesions and granulation tissue, when they occur, are associated mainly with tendon ruptures.28 Various types of degeneration may be seen in tendons, but in the Achilles tendon the usual types are mucoid and lipoid.28 In mucoid degeneration (i.e. proteoglycan/glycosaminoglycan accumulation in the tendon) light microscopy reveals large mucoid patches and vacuoles between fibres. Lipoid degeneration is characterized by abnormal intratendinous accumulation of lipid, with disruption of collagen fibre structure.8
Paratendinopathy may occur alone or in combination with degeneration of the tendon body.29 Histologically, mucoid degeneration, fibrosis and vascular proliferation with a slight inflammatory infiltrate have been reported.13,30,31 Clinically, oedema and hyperaemia of the paratenon are seen. A fibrinous exudate accumulates within the tendon sheath.23
CLINICAL PRESENTATION
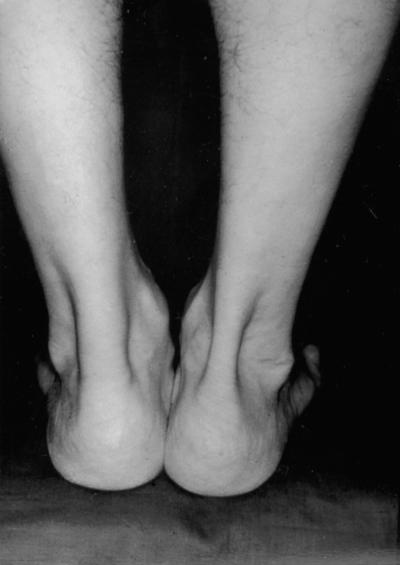
The cardinal symptom of Achilles tendinopathy is pain. Generally it occurs at the beginning and end of a training session, with a period of diminished discomfort in between. As the pathological process progresses, pain may occur during exercise, and, in severe cases, it can interfere with activities of daily living. In the acute phase, the tendon is diffusely swollen and oedematous, and on palpation tenderness is usually greatest 2–6 cm proximal to the tendon insertion (Figure 1). Sometimes, fibrin precipitated from the fibrinogen-rich fluid around the tendon can result in palpable crepitation.1,8,10 In chronic cases, exercise-induced pain is still the cardinal symptom, while crepitations and effusions diminish.8 A tender, nodular swelling is usually present in chronic cases and is believed to signify tendinosis10.
Figure 1.
Left sided Achilles tendinopathy resulting in pronounced swelling
The diagnosis of Achilles tendinopathy is based mainly on history and detailed clinical examination. However, diagnostic imaging may be required to verify a clinical suspicion or, occasionally, to exclude other musculoskeletal disorders.8
IMAGING METHODS
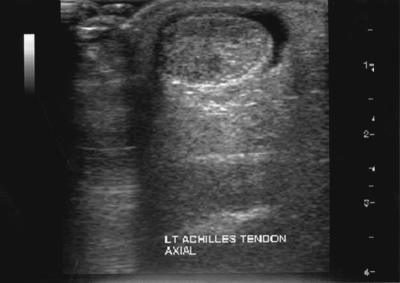
Ultrasonography is commonly employed to examine tendon disorders, being readily available, quick, safe and inexpensive. However, it is operator-dependent, offers limited soft-tissue contrast and is less sensitive than MRI.8,32 In acute cases, ultrasound reveals fluid accumulation around the tendon (Figure 2). In chronic cases, peritendinous adhesions may be shown by thickening of the hypoechoic paratenon with poorly defined borders. A simple grading system has been devised for tendinopathy: grade 1 represents a normal tendon; grade 2 an enlarged tendon; and grade 3 a tendon containing a hypoechoic area.33 Hypoechoic areas can be nodular, diffuse or multifocal, and correlate well with macroscopic findings at surgery. MRI provides extensive information on the internal morphology of tendon and the surrounding structures, and is useful to evaluate various stages of chronic degeneration and for differentiation between peritendinitis and tendinosis. An excellent correlation has been reported between MRI and pathological findings at surgery.34
Figure 2.
Axial ultrasound image demonstrating swelling of the Achilles tendon with surrounding fluid accumulation
A longitudinal ultrasound study has indicated mild-to-moderate changes in both involved and uninvolved Achilles tendons, but the occurrence of these changes was not clearly related to the patients’ symptoms.35 In view of the high sensitivity of these imaging modalities, an abnormality should be interpreted with caution and related to the patient’s symptoms before any recommendations are made on management.12
MANAGEMENT
In the early phase of Achilles tendinopathy, conservative treatments are customary.13,35–37 Individuals who seek early advice may have the best outcomes, since treatment of the chronic condition is more complex and uncertain.18,31 Surgical management is recommended for patients who do not adequately respond to a conservative treatment programme over three to six months.13,30,34,36
The initial conservative programme is directed towards presumed aetiological factors or towards relieving symptoms.39 The strategies include abstention from the activities that caused the symptoms and correction of training errors, foot malalignments and muscle weakness.39
Decreasing the intensity, frequency and duration of the activity that caused the injury, or modification of that activity, may be the only action necessary to control symptoms in the acute phase. Since collagen repair and remodelling is stimulated by tendon loading, complete rest of an injured tendon can be detrimental. Modified rest, reducing activity at the injured site but allowing normal activity elsewhere, has been recommended.8,39 Cryotherapy has been regarded as a useful intervention in the acute phase of Achilles tendinopathy: it has an analgesic effect, reduces the metabolic rate of the tendon and decreases extravasation of blood and protein from the new capillaries found in tendon injuries.8 Therapeutic ultrasound may reduce the swelling in the acute inflammatory phase and improve tendon healing.40 Ultrasound stimulates collagen synthesis in tendon fibroblasts and stimulates cell division during periods of rapid cell proliferation.41
Deep friction massage has been advocated for tendinopathy and paratendinopathy. In chronic cases, this should be accompanied by stretching to restore tissue elasticity and reduce the strain in the muscle–tendon unit with joint motion. ‘Augmented soft tissue mobilization’ is a new non-invasive technique that has been used with success in chronic tendinopathy, probably through controlled infliction of microtrauma with resultant fibroblast proliferation.42 Stretching and strengthening of the triceps surae muscle and Achilles tendon are important to preserve function of the musculotendinous unit, restore normal ankle joint mobility and decrease the strain on the Achilles tendon with normal motion. Eccentric muscle training is superior to concentric training in decreasing pain in chronic Achilles tendinopathy, and promising results have been obtained with an intensive heavy-load training regimen.43
If foot alignment is abnormal, orthoses that place the hindfoot in neutral may prove beneficial. A heel lift of 12–15 mm is typically used as an adjunct to management of Achilles tendon pain.37 Orthotics correction can alter the biomechanics of the foot and ankle and relieve heel pain. In runners orthotics have been used with up to 75% success.44
Several drugs including low-dose heparin, Wydase (hyaluronidase) and aprotinin have been used in the management of peritendinous and intratendinous disease,45,46 but evidence of their long-term effectiveness is still lacking. Peritendinous injections of corticosteroids are controversial and there are no good scientific reasons to support their use.47 Intratendinous injections of corticosteroids are likewise to be avoided47.
In 24–45.5% of patients with Achilles tendinopathy, conservative management is unsuccessful and surgery has to be considered. The objective is to excise fibrotic adhesions, remove degenerate nodules and make longitudinal incisions in the tendon so as to detect intratendinous lesions and restore vascularity (and possibly stimulate the remaining viable cells to initiate cell matrix response and healing).3,14,31 Multiple longitudinal tenotomies have been shown to trigger neoangiogenesis at the Achilles tendon, with increased blood flow.48 This should improve nutrition and provide a more favourable environment for healing. Patients are encouraged to weight-bear as soon as possible after surgery.
Researchers report excellent or good results in up to 85% of cases but success rates are not always so high in routine non-specialized clinical practice.38,49 It is difficult to compare the results of studies as most workers do not report their assessment procedure.49 Also, no prospective randomized studies comparing operative and conservative treatment of Achilles tendinopathy have been published, and most of our knowledge on treatment efficacy comes from clinical experience and descriptive studies.
CONCLUSIONS
Although Achilles tendinopathy has been extensively studied, there is much to be learned about its aetiology, pathology and optimal management. Most patients respond to conservative measures if the condition is recognized early; continuation of the offending activities leads to chronic changes that are more resistant to non-operative management. For many patients, control of the symptoms is a more realistic aim than complete cure.
References
- 1.Kvist M. Achilles Tendon Overuse Injuries. [PhD Thesis]. Turku: University of Turku, Finland, 1991
- 2.Selvanetti ACM, Puddu G. Overuse tendon injuries: basic science and classification. Operative Techniques Sports Med 1997;5: 110–17 [Google Scholar]
- 3.Rolf CMT. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int 1997;18: 565–9 [DOI] [PubMed] [Google Scholar]
- 4.Carr AJ, Norris SH. The blood supply of the calcaneal tendon. Bone Joint Surg 1989;71: 100–1 [DOI] [PubMed] [Google Scholar]
- 5.Kirkendall DT, Garrett WE. Function and biomechanics of tendons. Scand J Med Sports 1997;7: 62–6 [DOI] [PubMed] [Google Scholar]
- 6.Ackermann PW, Jian L, Finn A, Ahmed M, Kreicbergs A. Autonomic innervation of tendons, ligaments and joint capsules: a morphologic and quantitative study in the rat. J Orthopaed Res 2001;19: 372–8 [DOI] [PubMed] [Google Scholar]
- 7.Ljung BO, Forsgren S, Friden J. Sympathetic and sensory innervations are heterogeneously distributed in relation to the blood vessels at the extensor carpi radialis brevis muscle origin of man. Cells Tiss Org 1999;165: 45–554 [DOI] [PubMed] [Google Scholar]
- 8.Jozsa LKP. Human Tendon: Anatomy, Physiology and Pathology. Champaign: Human Kinetics, 1997
- 9.Kvist M. Achilles tendon injuries in athletes. Ann Chir Gynaecol 1991;80: 188–201 [PubMed] [Google Scholar]
- 10.Leppilahti JOS, Karpakka J, et al. Overuse injuries of the Achilles tendon. Ann Chir Gynaecol 1991;80: 202–7 [PubMed] [Google Scholar]
- 11.Komi PV, Fukashiro S, Jarvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med 1992;11: 521–31 [PubMed] [Google Scholar]
- 12.Khan KM, Maffulli N. Tendinopathy: an Achilles’ heel for athletes and clinicians. Clin J Sport Med 1998;8: 151–4 [PubMed] [Google Scholar]
- 13.Williams JG. Achilles tendon lesions in sport. Sports Med 1986;3: 114–35 [DOI] [PubMed] [Google Scholar]
- 14.Benazzo FMN. An operative approach to Achilles tendinopathy. Sports Med Arthrosc Rev 2000;8: 96–1001 [Google Scholar]
- 15.Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med 1992;11: 533–78 [PubMed] [Google Scholar]
- 16.Bestwick CSMN. Reactive oxygen species and tendon problems: review and hypothesis. Sports Med Arthrosc Rev 2000;8: 6–16 [Google Scholar]
- 17.Sullo A, Maffulli N, Capasso G, Testa V. The effects of prolonged peritendinous administration of PGE1 to the rat Achilles tendon: a possible animal model of chronic Achilles tendinopathy. J Orthopaed Sci 2001;6: 349–57 [DOI] [PubMed] [Google Scholar]
- 18.Corps AN, Curry VA, Harrall RI, Dutt D, Hazleman BL, Riley GP. Ciprofloxacin reduces the stimulation of prostaglandin E(2) output by interleukin-1beta in human tendon-derived cells. Rheumatology (Oxford) 2003;42: 1306–10 [DOI] [PubMed] [Google Scholar]
- 19.Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol 2001;20: 159–69 [DOI] [PubMed] [Google Scholar]
- 20.Alfredson H, Lorentzon M, Backman S, Backman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthopaed Res 2003;21: 970–5 [DOI] [PubMed] [Google Scholar]
- 21.Khan KM, Cook J, Maffulli N, Kannus P. Where is the pain coming from in tendinopathy? It may be biochemical, not only structural, in origin. Br J Sports Med 2000;34: 81–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan KM, Cook J. Overuse tendon injuries: where does the pain come from? Sports Med Arthrosc Rev 2000;8: 17–31 [Google Scholar]
- 23.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med 1999;27: 393–408 [DOI] [PubMed] [Google Scholar]
- 24.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthroscopy 1999;7: 378–81 [DOI] [PubMed] [Google Scholar]
- 25.Ackermann WPR. Sensory neuropeptides in achilles tendinosis. Int Soc Arthroscopy Knee Surg Orthopaed Sports Med 2001;17: 516 [Google Scholar]
- 26.Yaksh TL. Substance P release from knee joint afferent terminals: modulation by opioids. Brain Res 1988;458: 319–24 [DOI] [PubMed] [Google Scholar]
- 27.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions. Time to change a confusing terminology. Arthroscopy 1998;14: 840–3 [DOI] [PubMed] [Google Scholar]
- 28.Maffulli N, Barrass V, Ewen SW. Light microscopic histology of achilles tendon ruptures. A comparison with unruptured tendons. Sports Med 2000;28: 857–63 [DOI] [PubMed] [Google Scholar]
- 29.Almekinders LC, Temple JD. Etiology, diagnosis, and treatment of tendonitis: an analysis of the literature. Medicine Sci Sports Exercise 1998;30: 1183–90 [DOI] [PubMed] [Google Scholar]
- 30.Nelen G, Burssens A. Surgical treatment of chronic Achilles tendinitis. Am J Sports Med 1989;17: 754–9 [DOI] [PubMed] [Google Scholar]
- 31.Clancy WGJ, Brand RL. Achilles tendonitis in runners: a report of five cases. Am J Sports Med 1976;4: 46–57 [DOI] [PubMed] [Google Scholar]
- 32.Maffulli N, Regine R, Angelillo M, Capasso G, Filice S. Ultrasound diagnosis of Achilles tendon pathology in runners. Br J Sports Med 1987;21: 158–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archambault JM, Wiley JP, Bray RC et al. Can sonography predict the outcome in patients with achillodynia? J Clin Ultrasound 1998;26: 335–9 [DOI] [PubMed] [Google Scholar]
- 34.Schepsis AA, Leach RE. Surgical management of Achilles tendon overuse injuries: a long-term follow-up study. Am J Sports Med 1994;22: 611–19 [DOI] [PubMed] [Google Scholar]
- 35.Paavola MKP, Paakkala T, Pasanen M, Järvinen M. Long-term prognosis of patients with Achilles tendinopathy. An observational 8-year follow-up study. Am J Sports Med 2000;28: 634–42 [DOI] [PubMed] [Google Scholar]
- 36.Kvist H, Kvist M. The operative treatment of chronic calcaneal paratenonitis. J Bone Joint Surg 1980;62B: 353–7 [DOI] [PubMed] [Google Scholar]
- 37.Clement DB, Taunton J, Smart GW. Achilles tendinitis and peritendinitis: etiology and treatment. Am J Sports Med 1984;12: 179–84 [DOI] [PubMed] [Google Scholar]
- 38.Maffulli N, Binfield P, Moore D, et al. Surgical decompression of chronic central core lesions of the Achilles tendon. Am J Sports Med 1999;27: 747–52 [DOI] [PubMed] [Google Scholar]
- 39.Alfredson H, Lorentzon R. Chronic Achilles tendinosis. Recommendations for treatment and prevention. Sports Med 2000;29: 135–46 [DOI] [PubMed] [Google Scholar]
- 40.Jackson BA, Schwane JA, Starcher BC. Effect of ultrasound therapy on the repair of Achilles tendon injuries in rats. Med Sci Sports Exercise 1991;23: 171–6 [PubMed] [Google Scholar]
- 41.Ramirez A, Schwane JA, McFarland C, Starcher B. The effect of ultrasound on collagen synthesis and fibroblast proliferation in vitro. Med Sci Sports Exercise 1997;29: 326–32 [DOI] [PubMed] [Google Scholar]
- 42.Gehlsen GM, Ganion LR, Helfst R. Fibroblast responses to variation in soft tissue mobilization pressure. Med Sci Sports Exercise 1999;31: 531–5 [DOI] [PubMed] [Google Scholar]
- 43.Silbernagel KG, Thomee R, Thomee P, Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain—a randomized controlled study with reliable testing of the evaluating methods. Scand J Sports Med 2001;11: 197–206 [DOI] [PubMed] [Google Scholar]
- 44.Gross ML, Davlin L, Evanski PM. Effectiveness of orthotic shoe inserts in the long-distance runner. Am J Sports Med 1991;19: 409–12 [DOI] [PubMed] [Google Scholar]
- 45.Capasso G, Maffulli N, Testa V, Sgambato A. Preliminary results with peritendinous protease inhibitor injections in the management of Achilles tendinitis. J Sports Traumatol Rel Res 1993;15: 37–43 [Google Scholar]
- 46.Sundqvist H, Forsskahl B, Kvist M. A promising novel therapy for Achilles peritendinitis: double-blind comparison of glycosaminoglycan polysulfate and high-dose indomethacin. Int J Sports Med 1987;8: 298–303 [DOI] [PubMed] [Google Scholar]
- 47.Speed CA. Corticosteroid injections in tendon lesions. BMJ 2001;323: 82–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedrich T, Schmidt W, Jungmichel D, Horn LC, Josten C. Histopathology in rabbit Achilles tendon after operative tenolysis (longitudinal fiber incisions). Scand J Med Sci Sports 2001;11: 4–8 [DOI] [PubMed] [Google Scholar]
- 49.Tallon C, Coleman BD, Khan KM, Maffuli N. Outcome of surgery for chronic Achilles tendinopathy: a critical review. Am J Sports Med 2001;29: 315–20 [DOI] [PubMed] [Google Scholar]