Abstract
ESAT-6 is an important T-cell antigen recognized by protective T cells in animal models of infection with Mycobacterium tuberculosis. In an enzyme-linked immunosorbent assay (ELISA) with overlapping peptides spanning the sequence of ESAT-6, monoclonal antibody HYB76-8 reacted with two peptides in the N-terminal region of the molecule. Assays with synthetic truncated peptides allowed a precise mapping of the epitope to the residues EQQWNFAGIEAAA at positions 3 to 15. Hydrophilicity plots revealed one hydrophilic area at the N terminus and two additional areas further along the polypeptide chain. Antipeptide antibodies were generated by immunization with synthetic 8-mer peptides corresponding to these two regions coupled to keyhole limpet hemocyanin. Prolonged immunization with a 23-mer peptide (positions 40 to 62) resulted in the formation of antibodies reacting with the peptide as well as native ESAT-6. A double-antibody ELISA was then developed with monoclonal antibody HYB76-8 as a capture antibody, antigen for testing in the second layer, and antipeptide antibody in the third layer. The assay was suitable for quantification of ESAT-6 in M. tuberculosis antigen preparations, showing no reactivity with M. bovis BCG Tokyo culture fluid, used as a negative control, or with MPT64 or antigen 85B, previously shown to cross-react with HYB76-8. This capture ELISA permitted the identification of ESAT-6 expression from vaccinia virus constructs containing the esat-6 gene; this expression could not be identified by standard immunoblotting.
A key question in the development of new vaccines against tuberculosis is whether particular antigens of Mycobacterium tuberculosis are of major importance in the development of protective immunity.
Various techniques are used for the identification of protective antigens. In particular, we have studied the specificity of T-cell responses in a mouse model of memory immunity after infection with M. tuberculosis (1, 5). This system involves M. tuberculosis infection of C57BL/6J mice for 1 month, treatment with isoniazid and rifampin to clear the infection, and then resting for a longer period prior to reinfection. Following M. tuberculosis reinfection, these memory immune mice develop a rapid and intense T-cell response which controls the infection. By testing individual fractions of a short-term culture filtrate (ST-CF) enriched in proteins actively secreted by M. tuberculosis (4), the protective T cells were found to exhibit a very restricted specificity. Two fractions (3 to 10 and 25 to 31 kDa) were strongly recognized and induced both marked proliferative responses and high levels of gamma interferon (IFN-γ) release (5).
In the fraction containing 25- to 31-kDa proteins, the components of the antigen 85 complex are major constituents (34, 37). Among these, 85B has been demonstrated to induce proliferation and high levels of IFN-γ release in T-cell cultures from memory immune mice (1, 2). The 85A protein has previously been shown to induce IFN-γ production in cultures of spleen cells from mice recently infected with M. tuberculosis (9). In agreement with this finding, antigen 85A has recently been shown to be protective in DNA vaccination (17).
In the second fraction, containing proteins ranging in molecular mass from 3 to 10 kDa, a protein with an apparent molecular mass of 6 kDa designated ESAT-6 (6-kDa early secretory antigenic target) was shown to possess the major activity (1). Overlapping peptides spanning the sequence of ESAT-6 have been used to map two T-cell epitopes on this molecule in mice. One epitope, recognized in the context of H-2b,d, was located in the N-terminal part of the molecule; the other epitope, recognized in the context of H-2a,k, covered amino acids 51 to 60 (7). The esat-6 gene, which lacks a signal sequence (1, 30), is present in M. tuberculosis and virulent Mycobacterium bovis but absent in the M. bovis BCG vaccine strain (16).
One possible avenue toward improved vaccines against tuberculosis would therefore be recombinant live vaccine carriers such as BCG or attenuated vaccinia virus expressing ESAT-6 (13, 25). Specific quantification of ESAT-6 expression in sonicates and culture fluids of recombinant microorganisms in which the esat-6 gene has been introduced is essential as part of the selection of candidate recombinant microorganisms for further vaccination experiments. The purpose of the present work was to characterize B-cell epitopes on the ESAT-6 molecule as a basis for the development of an enzyme-linked immunosorbent assay (ELISA) for its quantification.
MATERIALS AND METHODS
Bacterial cultures and antigen preparations.
M. bovis BCG substrain Danish 1331 was obtained from Statens Seruminstitut, Copenhagen, Denmark, and substrain Tokyo 172 was obtained from the National Institute of Health, Tokyo, Japan, and grown on Sauton medium. ST-CF, enriched in proteins actively secreted by M. tuberculosis H37Rv, was produced as described previously (3). Culture fluids, 3 to 5 weeks old from stationary cultures of M. tuberculosis H37Rv containing secreted proteins and only small amounts of cytosolic proteins released by bacterial lysis were prepared as described previously (27, 35).
Clinical isolates of M. tuberculosis were identified by standard diagnostic methods at the Mycobacteria Department, Statens Seruminstitut, grown on Ogawa slants or Löwenstein-Jensen medium, and then transferred to liquid Sauton medium for further cultivation.
Isolation of proteins.
Proteins were isolated as described in detail previously, and then tested for homogeneity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), protein staining, and immunological techniques. MPT59 (85B) and MPT64 were purified from M. tuberculosis culture filtrate (27). The term MPB was introduced by Nagai et al. (26) for the designation of a protein purified from M. bovis BCG, with a number denoting the relative mobility in PAGE (7.7% polyacrylamide) at a running pH of 9.5. The corresponding term MPT is used to denote a protein isolated from M. tuberculosis. ESAT-6 was purified from 3- to 5-week-old culture filtrate of M. tuberculosis H37Rv (30).
Preparation and purification of recombinant ESAT-6.
A synthetic DNA sequence (5′-G CGA CAT CAC CAT CAC CAT CAC CAT CAC ATC GAG GGC A3′ plus protruding 5′ GATC overhangs), coding for a stretch of eight His residues followed by a factor Xa cleavage site, was inserted in the unique BglII recognition site of the expression plasmid pGH433 (31). This generated plasmid pMCT6, in which the BglII site downstream of the insert was maintained. The DNA sequence coding for the full-length ESAT-6 protein was PCR amplified from cloned M. tuberculosis genomic DNA with the primers 5′-GAAGATCTATGACAGAGCAGCAGTGG (nucleotides 1 to 18 of the coding sequence with a BglII site added upstream) and 5′-CCGCCATGGTAAACACGAGAAAGGGCG (nucleotides 67 to 84 after the stop codon, with a NcoI site added downstream) and inserted between the BglII and NcoI sites of pMCT6. The recombinant protein was produced in Escherichia coli XL1 blue and purified by metal ion affinity chromatography on a Ni+ column esentially as described previously (32) but with phosphate buffers containing 8 M urea, which was removed after the purification.
Hydrophilicity plots.
Hydrophilicity plots were prepared by the method of Kyte and Doolittle (21).
Synthesis of ESAT-6 peptides and generation of antibodies.
Monoclonal antibody (MAb) HYB76-8, reacting with ESAT-6, was obtained from purified protein derivative-immunized CF1 × BALB/c F1 mice (20). The MAb (batch 0.5C8.025B3) was purified by affinity chromatography on protein A-Sepharose CL-4B as specified by the manufacturer (Pharmacia Biotech, Uppsala, Sweden).
Eight overlapping 20- to 24-mer peptides covering the amino acid sequence of ESAT-6 and a series of truncated peptides in the residue 1 to 20 region from the N-terminal end were synthesized as described in detail previously (7). To produce antibodies to peptides from regions within the ESAT-6 molecule presumed to contain B-cell epitopes, two 9-mer peptides (DEGKQSLTK, positions 30 to 38 [p30–38], and YQGVQQKWD, positions 51 to 59 [p51–59]) were obtained from MedProbe A/S, Oslo, Norway, as peptide amides, blocking the C-terminal carboxyl group of the peptide to more closely mimic the charge environment of the native protein. A cysteine residue was added at the N terminus, using a part of the peptide for coupling to keyhole limpet hemocyanin (KLH) as the carrier protein for immunization. The heterobifunctional reagent m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS) was used for coupling by a two-step procedure, first activating the carrier and then coupling the peptide (12, 19, 22). The rabbits were immunized by repeated subcutaneous injections of peptide coupled to KLH in an emulsion with incomplete Freund’s adjuvant at multiple sites by standard procedures (14). The same technique was used to generate antibodies against the 23-mer peptide AAAWGGSGSEAYQGVQQKWDATA (p40–62). The rabbits were bled on the day before the first immunization, immediately before subsequent immunizations, or separately as indicated.
To obtain anti-p30–38 and anti-p51–59, 180 μg of coupled protein was used to immunize each rabbit on day 1 and 90 μg was used for each of the three subsequent immunizations 3, 5, and 9 weeks later. The rabbits were bled on day 1 and then 3, 7, 9, and 11 weeks later.
To obtain anti-p40–62, 830 μg of protein was used to immunize each rabbit on day 1 and then 2, 9, and 13 weeks later. These animals were bled on day 1 and then 3, 9, 11, 13, 15, and 16 weeks later.
Polyvalent rabbit anti-BCG immunoglobulin was kindly provided by DAKO Immunoglobulins, Copenhagen, Denmark. Generation of polyvalent anti-M. tuberculosis culture fluid and monospecific polyclonal rabbit antibodies to isolated mycobacterial proteins has been described previously (35).
SDS-PAGE with immunoblotting.
SDS-PAGE was performed with the Pharmacia system for horizontal electrophoresis in a Multifor II electrophoresis unit 2117 (Pharmacia LKB Biotechnology AB, Uppsala, Sweden) with precast polyacrylamide gels, ExcelGel SDS gradient 8–18 (Pharmacia). Samples (10 μl) of the various culture filtrates containing 10 μg of total protein were applied in each lane. Semidry Western blotting was performed with the model 2117-250 Novablot electrophoretic transfer kit (LKB, Bromma, Sweden) onto 0.2-μm-pore-size nitrocellulose membranes (Schleicher and Schüll, Dassel, Germany). After electrophoretic transfer, antigen bands were detected with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin (Amersham International plc, Little Chalfont, United Kingdom), with diaminobenzidine in 0.1 M sodium acetate buffer (pH 4.0) as the substrate.
ELISA technology.
To test for antibody reactivity with synthetic peptides, the plates were coated with individual peptides by applying 1 μg of peptide per well in 100 μl of phosphate-buffered saline (PBS) (pH 7.4), blocking with 5 mg of bovine serum albumin per ml in PBS. For reactivity with MAb HYB76-8, detection was carried out with HRP-labelled sheep anti-mouse immunoglobulin (Amersham); for reactivity with polyclonal rabbit anti-peptide antibodies, detection was carried out with HRP-labelled donkey anti-rabbit immunoglobulin (Amersham).
Based on initial titer determination experiments outlined below, a double-antibody ELISA was set up according to the principle described in detail previously (16, 36). Briefly, Immunoplate MaxiSorp 96-well plates (no. 442404; NUNC, Roskilde, Denmark) were coated with 100 μl of purified MAb HYB76-8 (500 μg/ml) diluted 1:200. Blocking was carried out with 5 mg of bovine serum albumin per ml in PBS. The second layer contained serial twofold dilutions of the antigen to be tested (culture fluids, sonicates, or purified proteins) in the concentrations indicated. Dilutions of culture fluids of M. tuberculosis H37Rv and BCG Tokyo were included as positive and negative controls, respectively. The third layer contained polyclonal rabbit anti-p40–62 peptide at 1:200. The indicator system consisted of HRP-conjugated donkey anti-rabbit immunoglobulin (Amersham) at 1:1,000. The substrate was 2,2′-azino-di-[3-ethylbenzthiazolinesulfonate (16)] (ABTS). The samples were washed four times between each step with PBS containing 0.1% Tween 20. All reaction mixtures were set up in triplicate, and the median values were used for recording and calculations. The results were read on an MR 7000 ELISA reader (Dynatech Laboratories Inc., Chantilly, Va.).
rVV expressing ESAT-6.
Two recombinant vaccinia virus (rVV)–ESAT-6 sequences were constructed with the following foreign coding sequences; (i) esat-6 alone or (ii) esat-6 fused downstream from a heterologous eukaryotic signal sequence, tpa, belonging to tissue plasminogen activator (tPA) (28). These were inserted into the nonessential thymidine kinase locus of wild-type VV (strain WR) with the transfer plasmids p1108 and pSC11. These transfer plasmids included a cloning site for foreign sequence insertion under the vaccinia early/late p7.5 promoter, flanking viral thymidine kinase sequence which permits homologous recombination and a selectable marker under a second VV promoter, and the E. coli genes, gpt for p1108 and lacZ for pSC11 (23). For rVV–tPA–ESAT-6, p1108-tPA was constructed by cloning synthetic sense and antisense oligonucleotide strands (MWG Biotech, Ebersberg, Germany) encoding the 21-amino-acid leader sequence of tPA and a multiple-cloning site containing NheI, BamHI, SmaI, and EcoRI (24). An esat-6 coding sequence was inserted into both p1108 and p1108-tPA on a BamHI-EcoRI PCR-generated fragment with restriction sites incorporated within the primers. Following confirmation of plasmid sequence fidelity, homologous recombination into VV was carried out as described elsewhere (23). Briefly, a near-confluent monolayer of TK-143 cells was infected with wild-type VV at a multiplicity of infection of 1:10, followed 1 h later by transfection with p1108–ESAT-6 or p1108–tPA–ESAT-6 with the cationic lipid transfection reagent N-[1-(2,3-dioleoyloxy)propyl]-N,N,N- trimethylammonium methylsulfate (DOTAP; Boehringer, Mannheim, Germany). Viral recombinants were selected with mycophenolic acid (23), and three rounds of plaque purification were performed. rVV–ESAT-6 and rVV–tPA–ESAT-6 were verified with PCR primers both flanking the coding region and within it (tpa and esat-6). A similar procedure was used with the pSC11 transfer plasmid, but in this case, recombinants were color selected with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Sigma Chemical Co., St. Louis, Mo.). Protein cell lysates were obtained from rVV-infected TK-143 cells (multiplicity of infection, 30:1) 18 h postinfection. The cells were lysed in 1 ml of 1% Nonidet-P-40 in 50 mM Tris (pH 8.0)–150 mM NaCl, representing 8 × 105 cells/ml. The cell lysates were assayed for their protein concentration with the bicinchoninic acid protein assay reagent (Pierce Chemical Co., Rockford, Ill.) as specified by the manufacturer and stored at −70°C prior to use.
Southern blotting, RT-PCR, and Western blotting of rVV–ESAT-6.
Southern blotting of rVV-ESAT-6 genomic DNA was performed with a digoxigenin-labelled whole-gene probe. Cell monolayers were infected with rVV-ESAT-6 and rVV–β-galactosidase (negative control), and genomic DNA was extracted by standard methods. Genomic and plasmid (pSC11–ESAT-6 and pSC11 alone) DNA were digested with EcoRI, resulting in a predicted 700-bp fragment incorporating esat-6. These digests were subjected to standard Southern blotting, and the signal was detected by a chemiluminescence method (Boehringer Mannheim) with an anti-digoxigenin alkaline phosphate-conjugate antibody, Lumigen purified protein derivative substrate, and a digoxigenin-UTP-labelled esat-6 whole-gene probe as specified by the manufacturer.
Reverse transcription-PCR (RT-PCR) was performed for recombinant esat-6 transcripts. mRNA was purified from infected-cell lysates and reverse transcribed with SuperScript (Life Technologies, Paisley, United Kingdom). esat-6-specific PCR primers were used to amplify a predicted 300-bp product. Given that the mRNA preparation would be contaminated with viral genomic DNA (VV is a cytoplasmic virus), the samples were treated with DNase (Life Technologies) before the RT step. Relevant controls included no RT step (both with and without DNase treatment), no DNase treatment, a plasmid incorporating esat-6 as a positive control, and cDNA from rVV lacking esat-6 as a negative control.
Western blotting was performed on rVV-infected cell lysates. Samples were run on SDS-PAGE under both reducing and nonreducing conditions and with both large- and small-scale gel systems. Following protein transfer, the nitrocellulose membrane was probed with HYB76-8 followed by HRP-conjugated secondary antibody (P260; Dako). The blotting procedure and signal detection were performed as specified for the enhanced chemiluminescence Western blotting detection system (Amersham).
RESULTS
Reactivity of MAb HYB76-8 with ESAT-6.
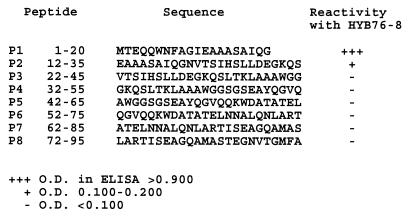
MAb HYB76-8 defines the ESAT-6 antigen (1, 16, 30). To precisely locate the epitope recognized by HYB76-8, a series of overlapping peptides covering the sequence was tested in an ELISA. Figure 1 shows the reactivity of eight synthetic 20- to 24-mer peptides on the solid phase with MAb HYB76-8 diluted 1:100. Reactivity is shown by +++, +, and − signs as indicators of the signal strength based on the optical densities (ODs) obtained on an ELISA plate in which the recorded peptides were tested simultaneously. Strong reactivity was observed with the peptide corresponding to residues 1 to 20 from the N-terminal end. Weaker but significant reactivity was observed with the peptide corresponding to residues 12 to 35, while the six remaining peptides showed no reactivity with HYB76-8.
FIG. 1.
Reactivity of overlapping synthetic peptides derived from the sequence of ESAT-6 in ELISA.
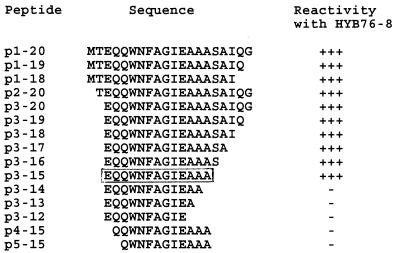
For more precise localization of the reactive epitope, truncated peptides were synthesized. The reactivities of 15 peptides are shown in Fig. 2. The peptide designation is indicated according to the residues present.
FIG. 2.
Mapping of the HYB76-8-reactive epitope in ELISA through reaction with synthetic peptides. The core peptide is boxed.
Strong reactivity was observed with 10 of the peptides, with a striking decrease in ODs from peptide p3–15 to p3–14 (0.735 and 0.062, respectively). The corresponding ODs for p3–15, p4–15, and p5–15 were 0.735, 0.049, and 0.039 respectively. These findings thus indicate a requirement of the 13 EQQWNFAGIEAAA residues for full reactivity with MAb HYB76-8. This conclusion was supported by testing of eight additional peptides (p3–11, p3–10, p4–14, p5–20, p5–14, p6–14, p7–14, and p8–14), which gave negative reactions with ODs of <0.050 in plates in which a positive control with peptide p1–20 gave ODs of >0.850.
Reactivity of antipeptide antibodies with ESAT-6.
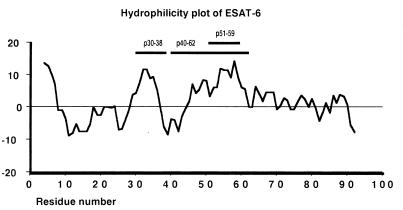
Having established the location of the HYB76-8-reactive epitope, we continued our work to develop antibodies to other parts of the molecule. Figure 3 shows a hydrophilicity plot of ESAT-6. In addition to the N-terminal region, there are two other main hydrophilic areas which would be expected to contain surface-exposed areas of the polypeptide chain with B-cell epitopes. Consequently, two peptides, corresponding to positions 30 to 38 and 51 to 59, were synthesized with a cysteine residue added at the N-terminal end for coupling to KLH as the carrier molecule for immunization. In three rabbits immunized with one of the peptides coupled to KLH, antibodies were rapidly formed and reacted with the corresponding free peptide bound on a solid phase in an ELISA; however, no reactivity was observed with the other peptide. Two weeks after the second immunization, the titer in an ELISA was >1:1,000 with ODs at a 1:100 dilution of >0.850 with the relevant peptide in five rabbits and <0.035 with the other peptide.
FIG. 3.
Hydrophilicity plot of ESAT-6. The hydrophilicity value at each point is the sum of the results for seven consecutive amino acids blotted at the middle residue. The horizontal bars indicate the positions of the synthetic peptides used for immunization.
The anti-p30–38 antibody reacted significantly with three of the overlapping peptides of ESAT-6; the strongest reaction was with peptide P3 containing residues 22 to 45. The anti-p51–59 antibody reacted with the P4 and P5 peptides; the strongest reaction was observed with P4 containing residues 32 to 55, as shown in Table 1. However, these antibodies did not react with native ESAT-6 in Western blotting or in capture ELISA with HYB76-8 at the solid phase, M. tuberculosis culture fluid or rESAT-6 in the second layer, the antipeptide antibody in the third layer, and the standard detection system for binding of rabbit immunoglobulin.
TABLE 1.
Reactivity of antibodies to synthetic peptides of ESAT-6 in ELISA
| Peptide | OD values
|
||
|---|---|---|---|
| Anti-p30–38a | Anti-p51–59 | Anti-p40–62 | |
| P1 (1–20) | 0.049b | 0.040 | 0.059 |
| P2 (12–35) | 0.136 | 0.050 | 0.066 |
| P3 (22–45) | 0.499 | 0.040 | 0.043 |
| P4 (32–55) | 0.141 | 0.877 | 0.350 |
| P5 (42–65) | 0.049 | 0.688 | 1.147 |
| P6 (52–75) | 0.046 | 0.066 | 0.681 |
| P7 (62–85) | 0.029 | 0.030 | 0.049 |
| P8 (72–95) | 0.033 | 0.030 | 0.047 |
| rESAT-6 | 0.147 | 0.106 | 0.394 |
Each antiserum shown is representative for three rabbits immunized with each peptide.
The OD value recorded is for the second bleeding of each rabbit.
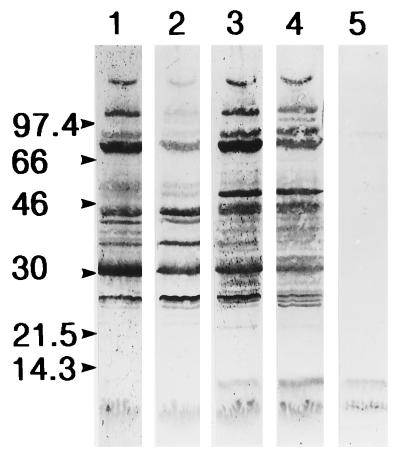
We therefore continued by immunizing three rabbits with a longer peptide corresponding to the extended hydrophilic region furthest from the HYB76-8 reactive epitope. A 23-mer peptide containing residues 40 to 62, containing the main hydrophilic region with neutral and hydrophobic residues on both sides, was selected. In ELISA with the peptide at the solid phase, significant antibody activity was demonstrated in serum taken 1 week after the second immunization. Reactivity in the ELISA with the overlapping peptides of ESAT-6 is shown for a representative antiserum (K618) in Table 1, with maximal activity as would be expected against peptide P5. Sera from early bleedings were again without reactivity with native ESAT-6 in Western blotting experiments or the capture ELISA. The immunization was therefore continued, and in late bleedings the antibodies also reacted with native ESAT-6 in Western blotting experiments, as shown in Fig. 4. In ELISA with rESAT-6 from E. coli at the solid phase, reactivity was low initially and markedly higher from the third bleeding onward; this is similar to the findings in Western blotting experiments. Figure 4 also illustrates the reactivity of rabbit serum obtained prior to immunization with several components of M. tuberculosis culture fluid, including the antigen 85 complex.
FIG. 4.
Reactivity of the anti-p40–62 antipeptide antibody with M. tuberculosis culture fluid in Western blotting. Lanes: 1, serum obtained before immunization; 2 to 4, serum obtained 3, 11, and 15 weeks, respectively, after immunization; 5, reaction of MAb HYB76-8 with ESAT-6 for comparison. The positions of molecular mass markers are indicated at the left. The strong band at 30 kDa represents the antigen 85 complex.
Double-antibody ELISA for quantification of ESAT-6.
Having obtained antibodies reacting with two different epitopes on ESAT-6, titer determinations were performed to obtain the optimal conditions for an ELISA. For MAb HYB76-8, we selected a dilution (1:100) ensuring its presence in antibody excess in a reaction with M. tuberculosis culture fluids. Antigen preparations in the second layer were tested in twofold serial dilutions. Culture fluids and sonicates started at 4 μg of total protein/well, isolated proteins tested for cross-reactivity in this system started at 4 μg of protein/well, and purified rESAT-6 started at 0.1 μg/well. The anti-p40–62 antiserum was used as a 1:200 dilution to ensure a sufficient strength of this antibody while avoiding the effects of coexisting antibodies to proteins of the antigen 85 complex in normal rabbit sera (Fig. 4), which tend to give rise to unwanted signals when more concentrated serum samples are used. Typical results are shown in Fig. 5.
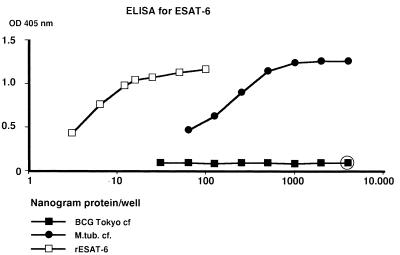
FIG. 5.
Reactivity of M. tuberculosis culture fluid (M.tub. cf.) and purified recombinant ESAT-6 in the double-antibody ELISA, compared with BCG Tokyo culture fluid as negative control. The open circle indicates that corresponding ODs were obtained with the isolated proteins MPT64 and MPT85B (MPT59).
M. tuberculosis H37Rv culture fluid gave a curve with a plateau at the three initial dilutions with ODs of about 1.20 and then decreasing. The curve of rESAT-6 isolated from E. coli initially decreased slowly; this was followed by a portion of the curve with an angle similar to that of the M. tuberculosis culture fluid curve. BCG Tokyo culture fluid gave no OD signals above 0.100 at any dilution, in agreement with the previous demonstration of the lack of this antigen in BCG. MAb HYB76-8 has previously (16) been demonstrated to cross-react with MPT64 and the antigen 85 complex. Purified MPT64 gave an OD of 0.093 at 4 μg/well, and MPT59 (85B) gave a value of 0.084, both with entirely flat curves upon further dilution in the ELISA. The cross-reactivity of HYB76-8 thus had no significant influence on the assay system developed.
Application of double-antibody ELISA to recombinant microorganisms.
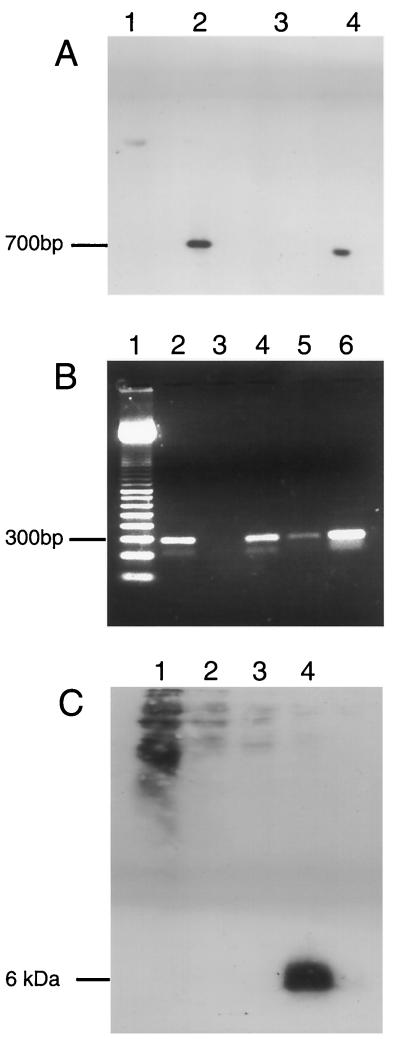
Three rVV-ESAT-6 constructs were made with pSC11 initially and then p1108. p1108 was used to create rVV both with and without tPA. The two rVV constructs without tPA were ostensibly identical and included the same protein-coding sequence and promoter. The latter construct was included as a control for the p1108-derived rVV–tPA–ESAT-6. Successful incorporation of esat-6 into VV was confirmed by plasmid sequencing and PCR (data not shown) or Southern blotting (Fig. 6A). This figure shows signals indicating esat-6 in both digested pSC11–ESAT-6 and rVV-ESAT-6 and no signal for empty vector constructs. ESAT-6 transcription was also readily identified by RT-PCR (Fig. 6B). In this experiment, relevant controls excluded the possibility of contamination from the viral genome acting as the template. Despite confirming sequence fidelity and the presence of mRNA, protein expression in immunoblotting could not be identified by Western blotting (Fig. 6C). This absence of signal was true for the three separate rVV constructs with MAb HYB76-8 at a wide range of dilutions, blotting in the presence or absence of various blocking reagents, and the use of “preparatory” large-scale SDS-PAGE. ST-CF from M. tuberculosis was used as a positive control, and ESAT-6 was readily identified (Fig. 6C) in ST-CF diluted 1:100 containing approximately 10 to 100 ng of ESAT-6. Of note, nonspecific background was particularly apparent in lane 1, which has a high protein content. However, the background was limited to the higher-molecular-mass proteins and would not have interfered with a signal of 6 kDa.
FIG. 6.
(A) Southern blot of EcoRI-digested plasmid and purified viral DNA. Lanes: 1, nonrecombinant insertion plasmid, pSC11; 2, pSC11-ESAT-6; 3, rVV–β-galactosidase; 4, rVV–ESAT-6. (B) RT-PCR of virally encoded ESAT-6 transcripts. Lanes: 1, 100-bp pair molecular mass marker; 2, DNase-treated, RT step included; 3: DNase treated, no RT step; 4, no DNase, no RT step (contaminating viral DNA); 5, no DNase, no RT step; 6, plasmid containing esat-6 gene (positive control). (C) Western blot of rVV-infected cell lysates in a large-gel system with 285 μl of cell lysate/well. Lanes: 1, neat cell lysate; 2, diluted 1:2; 3, 1:4; 4, M. tuberculosis culture filtrate (positive control).
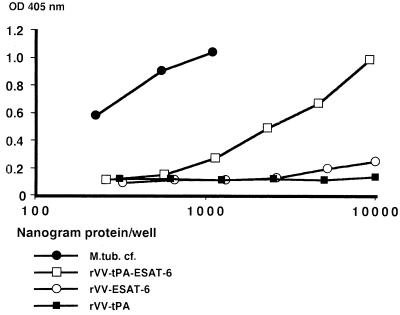
Total-cell lysates (containing viral and cellular proteins) from rVV-infected cells were assessed by the capture ELISA; twofold serially diluted samples were tested as illustrated in Fig. 7. The three rVV constructs illustrated were from monolayers with similar confluence and rVV titers, and the highest concentration corresponded to lysates of 4 × 103 cells. The lysate of cells containing rVV–tPA–ESAT-6 gave a strong signal. Upon dilution, the curve showed an angle similar to that for M. tuberculosis culture fluid, requiring about a 20-fold-higher total-protein content to give an ESAT-6 signal of similar strength. Lack of tPA in the rVV–ESAT-6 construct did not affect viral replication, in that PFU counts postinfection were similar for the two constructs. The construct containing esat-6 alone, rVV–ESAT-6, gave a considerably weaker signal, still considered positive, while the lysate of cells with empty vector gave a flat curve.
FIG. 7.
Reactivity of double-antibody ELISA for ESAT-6 with rVV. M.tub. cf., M. tuberculosis culture fluid.
DISCUSSION
In the experiments involving ELISA with overlapping peptides of the ESAT-6 sequence at the solid phase, the HYB76-8 reactive epitope was localized to the N-terminal part of the polypeptide chain (Fig. 1), with further localization to a core area with the EQQWNFAGIEAAA residues at positions 3 to 15 by the use of synthetic truncated peptides from this region, as shown in Fig. 2.
Comparing the reactivity illustrated in these figures, a striking feature emerges: The C-terminal border of the epitope is revealed by the striking difference in HYB76-8 reactivity with peptides p3–15 and p3–14, giving ODs in the ELISA of 0.735 and 0.062, respectively. In assays with the overlapping peptides, the ODs for peptides P1, P2, and P3 were 0.918, 0.186, and 0.009, respectively, thus showing a positive reaction with peptide P2 containing only four residues of the core epitope, EAAA. This indicates a striking influence of further flanking residues in the P2 peptide for maintenance of reactivity with MAb HYB76-8.
The mapping of the HYB76-8-reactive epitope to residues 3 to 15 corresponds exactly to the previous mapping of T-cell-reactive epitopes on this molecule in mice by Brandt et al. (7). Two T-cell epitopes, recognized in the context of different H-2 types, were defined. In BALB/c (H-2d) and C57BL/6j (H-2b) mice, only the N-terminal peptide 1 of nine overlapping peptides covering the ESAT-6 sequence reacted with T cells recovered during recall of immunity to M. tuberculosis. Mapping with truncated synthetic peptides in C57BL/6j mice showed a slight variation in different test systems, defining the epitope to a 13-amino-acid core sequence corresponding to residues 3 to 15 in tests of IFN-γ release upon stimulation of T cells from memory immune mice.
This localization of reactivity was observed in only two of the six inbred strains tested, and we note that MAb HYB76-8 was generated in CF1 × BALB/c F1 mice (20) expressing H-2d. Following infection with M. tuberculosis (8) or M. bovis BCG (18), formation of antibody to components of mycobacterial sonicates and culture fluids varies markedly, depending on the genes in the major histocompatibility complex. Additional work is needed to see whether B-cell epitope localization on a single protein, like ESAT-6, will differ in different inbred mouse strains.
While B-cell epitopes in general correspond to surface-exposed areas of the polypeptide chain and T-cell epitopes depend on antigen processing and presentation by the major histocompatibility complex, it is well established that areas of polypeptide chains may overlap with regard to the presence of B- and T-cell epitopes. Regarding mycobacterial proteins, this feature has been demonstrated in several instances. In the M. tuberculosis 16-kDa antigen, two linear B-cell epitopes at p31–40 and p61–70 were demonstrated in mice (33), while three T-cell epitopes, one of them at p21–40, were demonstrated in human T-cell responses (11). A significant overlap of B- and T-cell epitopes has been demonstrated in the 19-kDa lipoprotein (6). Overlapping B-cell and T-cell epitopes were demonstrated regarding reactivity with the 65-kDa heat shock protein in cells obtained from patients with Behcet’s disease (10). Pollock et al. (29) have demonstrated two T-cell epitopes, at positions 118 to 135 and 174 to 194 with numbering starting at Met 1 of the signal peptide, on the secreted MPB70 protein using cells from cattle infected with M. bovis. One of these, at the C-terminal end of the polypeptide chain, overlaps the B-cell epitope at position 174 to 190, reacting with the monoclonal antibody 1-1D (38). Billman-Jacobe et al. (6a) demonstrated another T-cell epitope on MPB70, at positions 103 to 113, overlapping the MAb 1-5C reactive epitope at positions 109 to 118 (38).
Reaction of our initial antipeptide antibodies with the peptide used for immunization without reactivity with the native ESAT-6 molecule is a well-known feature of antipeptide antibodies. When a longer peptide corresponding to the extended hydrophilic region of ESAT-6 was used, the fine specificity of the antipeptide antibodies changed upon extended immunization, and reactivity with native ESAT-6 was obtained, permitting the establishment of a double-antibody ELISA and giving a strong signal with M. tuberculosis H37Rv culture fluid. BCG Tokyo culture fluid was considered a particularly suitable negative control, containing proteins of the antigen 85 complex as well as large amounts of MPB64 (15), which was previously demonstrated to cross-react with HYB76-8 (16). Its signal was sufficiently low, giving a flat curve as illustrated in Fig. 5, thus demonstrating a marked difference in reactivity with respect to M. tuberculosis and BCG.
Observing the strong signal obtained in the double-antibody ELISA with lysates of cells containing rVV–tPA–ESAT-6 (Fig. 7), it was surprising to obtain no signal in Western blotting with MAb HYB76-8 on the same lysates, as shown in Fig. 6C. This observation is probably explained by the tendency of MAbs to depend strongly on the three-dimensional structure of the antigen and its reactive epitope while polyclonal antibodies are less sensitive in this regard.
A variety of M. tuberculosis preparations are being tested. ST-CF (3) gives a good signal, albeit somewhat weaker than that of culture fluids obtained from longer-lasting stationary-phase cultures of M. tuberculosis like the 4-week culture shown in Fig. 5. Culture fluids from 10 clinical isolates of M. tuberculosis gave strong signals, with all but one being stronger than those of the ST-CF preparations. In M. tuberculosis sonicates, a positive signal was obtained although it varied in strength. This corresponds to the demonstration of ESAT-6 in culture fluid as well as cytosol of M. tuberculosis by Sørensen et al. (30). A thorough investigation of culture fluids and sonicates of washed M. tuberculosis cells obtained from individual cultures is needed for further characterization of the release of the ESAT-6 protein from the mycobacterial cell (35).
Furthermore, the assay will be valuable for the demonstration and quantification of ESAT-6 produced by various recombinant organisms like BCG, Mycobacterium smegmatis, and VV to select candidate vaccines for protection studies. Information on ESAT-6 expression in different constructs of the same carrier is readily obtained, as illustrated in Fig. 7. Quantitative information comparing the expression in different carriers is more uncertain, since the sensitivity of the assay may vary for recombinant ESAT-6 in different carriers and in relation to native ESAT-6 in M. tuberculosis.
ACKNOWLEDGMENTS
This work was supported by grants from the Anders Jahre Fund for the Promotion of Science, the World Health Organization Programme for Vaccine Development (IMMYC project V25/181/124), and the European Community (project TS3*/CT94/0313). Adam Malin is an MRC (United Kingdom) Clinical Training Fellow. The vaccinia construction was partially funded by the Mason Medical Research Foundation.
We thank Suzanne Garman-Vik for work on the manuscript.
REFERENCES
- 1.Andersen P, Andersen A B, Sørensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 2.Andersen P, Askgaard D, Gottschau A, Bennedsen J, Nagai S, Heron I. Identification of immunodominant antigens during infection with Mycobacterium tuberculosis. Scand J Immunol. 1992;36:823–831. doi: 10.1111/j.1365-3083.1992.tb03144.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Askgaard D, Ljungqvist L, Bentzon M W, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991;59:1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen P, Heron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect Immun. 1993;61:844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashbridge K R, Backstrom B T, Liu H X, Vikerfors T, Englebretsen D R, Harding D R, Watson J D. Mapping of T helper cell epitopes by using peptides spanning the 19-kDa protein of Mycobacterium tuberculosis. Evidence for unique and shared epitopes in the stimulation of antibody and delayed-type hypersensitivity responses. J Immunol. 1992;148:2248–2255. [PubMed] [Google Scholar]
- 6a.Billman-Jacobe H, Radford A J, Rothel J S, Wood P R. Mapping of the T and B cell epitopes of the Mycobacterium bovis protein MPB70. Immunol Cell Biol. 1990;68:359–365. doi: 10.1038/icb.1990.49. [DOI] [PubMed] [Google Scholar]
- 7.Brandt L, Oettinger T, Holm A, Andersen A B, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 8.Brett S J, Ivanyi J. Genetic influences on the immune repertoire following tuberculous infection in mice. Immunology. 1990;71:113–119. [PMC free article] [PubMed] [Google Scholar]
- 9.De Bruyn J, Huygen K, Bosmans R, Fauville M, Lippens R, Van Vooren J P, Falmagne P, Weckx M, Wiker H G, Harboe M, Turneer M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987;2:351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- 10.Direskeneli H, Hasan A, Shinnick T, Mizushima R, van der Zee R, Fortune F, Stanford M R, Lehner T. Recognition of B-cell epitopes of the 65 kDa HSP in Behcet’s disease. Scand J Immunol. 1996;43:464–471. doi: 10.1046/j.1365-3083.1996.d01-53.x. [DOI] [PubMed] [Google Scholar]
- 11.Friscia G, Vordermeier H M, Pasvol G, Harris D P, Moreno C, Ivanyi J. Human T cell responses to peptide epitopes of the 16-kD antigen in tuberculosis. Clin Exp Immunol. 1995;102:53–57. doi: 10.1111/j.1365-2249.1995.tb06635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green N, Alexander H, Olson A, Alexander S, Shinnick T M, Sutcliffe J G, Lerner R A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982;28:477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- 13.Harboe M, Andersen P, Colston M J, Gicquel B, Hermans P W, Ivanyi J, Kaufman S H. Vaccines against tuberculosis. Vaccine. 1996;14:701–716. doi: 10.1016/s0264-410x(96)90051-1. [DOI] [PubMed] [Google Scholar]
- 14.Harboe M, Closs O, Svindahl K, Deverill J. Production and assay of antibodies against one antigenic component of Mycobacterium bovis BCG. Infect Immun. 1977;16:662–672. doi: 10.1128/iai.16.2.662-672.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harboe M, Nagai S, Patarroyo M E, Torres M L, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:857–859. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 18.Huygen K, Drowart A, Harboe M, ten Berg R, Cogniaux J, Van Vooren J P. Influence of genes from the major histocompatibility complex on the antibody repertoire against culture filtrate antigens in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1993;61:2687–2693. doi: 10.1128/iai.61.6.2687-2693.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitagawa T, Aikawa T. Enzyme coupled immunoassay of insulin using a novel coupling reagent. J Biochem (Tokyo) 1976;79:233–236. doi: 10.1093/oxfordjournals.jbchem.a131053. [DOI] [PubMed] [Google Scholar]
- 20.Klausen J, Magnusson M, Andersen A B, Koch C. Characterization of purified protein derivative of tuberculin by use of monoclonal antibodies: isolation of a delayed-type hypersensitivity reactive component from M. tuberculosis culture filtrate. Scand J Immunol. 1994;40:345–349. doi: 10.1111/j.1365-3083.1994.tb03471.x. [DOI] [PubMed] [Google Scholar]
- 21.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu F T, Zinnecker M, Hamaoka T, Katz D H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of d-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979;18:690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- 23.Mackett M. Construction and characterization of vaccinia virus recombinants. In: Glover D M, Hames B D, editors. DNA cloning, 4. Mammalian systems. A practical approach. Oxford, United Kingdom: IRL Press; 1995. pp. 43–83. [Google Scholar]
- 24.Malin, A. S., K. Huygen, J. Content, M. Mackett, L. Brandt, P. Andersen, and H. M. Dockrell. Vaccinia expression of Mycobacterium tuberculosis antigen 85 and ESAT-6 secreted proteins: tissue plasminogen activator signal sequence enhances expression and immunogenicity. Submitted for publication. [DOI] [PubMed]
- 25.Malin A S, Young D B. Designing a vaccine for tuberculosis. Br Med J. 1996;312:1495. doi: 10.1136/bmj.312.7045.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai S, Matsumoto J, Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981;31:1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ny T, Elgh F, Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci USA. 1984;81:5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollock J M, Douglas A J, Mackie D P, Neill S D. Identification of bovine T-cell epitopes for three Mycobacterium bovis antigens: MPB70, 19,000 MW and MPB57. Immunology. 1994;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 30.Sørensen A L, Nagai S, Houen G, Andersen P, Andersen A B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theisen M, Potter A A. Cloning, sequencing, expression, and functional studies of a 15,000-molecular-weight Haemophilus somnus antigen similar to Escherichia coli ribosomal protein S9. J Bacteriol. 1992;174:17–23. doi: 10.1128/jb.174.1.17-23.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theisen M, Vuust J, Gottschau A, Jepsen S, Hogh B. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin Diagn Lab Immunol. 1995;2:30–34. doi: 10.1128/cdli.2.1.30-34.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verbon A, Hartskeerl R A, Moreno C, Kolk A H. Characterization of B cell epitopes on the 16K antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 1992;89:395–401. doi: 10.1111/j.1365-2249.1992.tb06969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiker H G, Harboe M, Nagai S. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J Gen Microbiol. 1991;137:875–884. doi: 10.1099/00221287-137-4-875. [DOI] [PubMed] [Google Scholar]
- 36.Wiker H G, Harboe M, Nagai S, Bennedsen J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am Rev Respir Dis. 1990;141:830–838. doi: 10.1164/ajrccm/141.4_Pt_1.830. [DOI] [PubMed] [Google Scholar]
- 37.Wiker H G, Harboe M, Nagai S, Patarroyo M E, Ramirez C, Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81:307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- 38.Wiker, H. G., K. P. Lyashchenko, A. M. Aksoy, K. A. Lightbody, J. M. Pollock, S. V. Komissarenko, S. O. Bobrovnik, I. N. Kolesnikova, L. O. Mykhalsky, M. L. Gennaro, and M. Harboe. Immunochemical characterization of the MPB70/80 and MPB83 proteins of Mycobacterium bovis. Submitted for publication. [DOI] [PMC free article] [PubMed]