Abstract
Heart failure (HF) remains a serious health and socioeconomic problem in the Middle East and Africa (MEA). The age-standardized prevalence rate for HF in the MEA region is higher compared to countries in Eastern Europe, Latin America, and Southeast Asia. Also cardiovascular-related deaths remain high compared to their global counterparts. Moreover, in MEA, 66% of HF readmissions are elicited by potentially preventable factors, including delay in seeking medical attention, nonadherence to HF medication, suboptimal discharge planning, inadequate follow-up, and poor social support. Patient support in the form of activation, counseling, and caregiver education has been shown to improve outcomes in patients with HF. A multidisciplinary meeting with experts from different countries across the MEA region was convened to identify the current gaps and unmet needs for patient support for HF in the region. The panel provided insights into the real-world challenges in HF patient support and contributed strategic recommendations for optimizing HF care.
Keywords: Heart failure, HF patient support, Middle East and Africa
Highlights
Nearly three-fourths of patients with HF are diagnosed with New York Heart Association class III and IV disease, emphasizing the high unmet need for patient support in HF in the MEA.
Delay in seeking medical attention, nonadherence to HF medication, suboptimal discharge planning, inadequate follow-up, and poor social support are the potentially preventable factors for HF readmissions.
The expert panel provided insight on practical challenges and recommendations to overcome the gaps in HF patient support.
Introduction
Heart failure (HF) remains a serious public health problem in the world, affecting 64.3 million individuals, and this number is expected to increase during the next few decades.1 The age-standardized prevalence rate (per 100 000) of HF in the Middle East and Africa (MEA) region is relatively high (972.3) compared to Latin America (709.8-870.7), Eastern Europe (703.8), and Southeast Asia (655.0).1 According to the Gulf CARE registry study, 59% of the patients had reduced ejection fraction (EF), 21% had midrange EF, and 20% had preserved EF.2 In MEA, the increasing prevalence rate of HF is driven by the increase in risk factors such as hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, obesity, smoking, and a sedentary lifestyle.2,3
Despite significant advances in HF prevention and therapeutic armamentarium, mortality rates remain high, with 17% to 45% of deaths occurring within 1 year of diagnosis—the majority of deaths occur within 5 years of admission.4,5 Compared to their global counterparts, cardiovascular-related deaths remain high in MEA (308.9 per 100 000 versus 264.3 per 100 000).6 In the last 30 years in the MEA region, the total number of cardiovascular-related deaths has risen by 48%.7 This reflects gaps in early detection and control of risk factors, alongside the health systems-related challenges for the management of HF.
Similar to developed countries (North America, Latin America, Australia, and Japan) in MEA, particularly in the Middle East, HF accounts for up to 1.31% of all hospitalizations.5,8 Also, recurrent admissions for HF are common in MEA, with nearly 1 in 5 readmitted within 3 months and 2 in 5 within 12 months following admission for acute HF. In Gulf countries (2012), in-hospital mortality was 6.3%, doubled at 3 months following discharge, and reached 20.2% at 1 year following discharge.2 The Gulf CARE study reported hospital readmission rates of 18% and 40% at 3 and 12 months, respectively,2 highlighting an urgent need to address this major public health burden. Moreover, the MEA region has one of the youngest populations of patients with HF across the globe; the age of affected individuals on average is 10 years younger compared to the Western counterparts,2 yet patients have similar mortality rates, hospitalization rates, and rehospitalization rates, highlighting significant deficits in HF care. Literature indicates that up to 66% of HF readmissions are elicited by potentially preventable factors, including nonadherence to HF medication, suboptimal discharge planning, inadequate follow-up, poor social support, delays in seeking medical attention, and a lack of affordability of newer, more effective, but more expensive medications.9
In MEA countries, there are some variations in the use of essential medications for HF.2,10-12 The HEARTS study conducted in Saudi Arabia reported that angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) and beta-blocker usage were high in patients with HF (86% and 94%), and mineralocorticoid receptor antagonist use was modest (42%).11 The Gulf CARE study found that 81% of the patients with HF used ACEIs/ARBs and 57% used beta-blockers.2 However, a multinational study from the Africa region found that 74% of patients with HF used ACEIs/ARBs, 66.5% used beta-blockers, and 48% used mineralocorticoids.13 Similarly, the use of cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillator (ICD) varied greatly among MEA countries, with Saudi Arabia having the highest rate.14 In the Gulf nations, use of ICD and CRT are documented in 10%-20% and 3%-8%, respectively, among eligible patients.14 Whereas in countries like Egypt, ICD/CRT devices were used in less than 1% of patients with HF.15 Heart failure causes a substantial economic burden on the health-care system, including hospitalizations, drug treatment, monitoring systems, CRT, ICD, left ventricular assist devices, emergency visits, and heart transplantation.16 In 2012, the estimated total HF cost in the United States (US) was 30.7 billion US dollars, and projections suggest that by 2030, the total cost of HF will increase to $69.8 billion.17 Hospitalizations account for most HF-associated costs. Very recently published data showed that the total annual national direct and indirect costs of HF are estimated to be $1 billion in 2021 in Türkiye, which is a very important amount for middle-income countries.16
Appropriate patient support in HF seems to reduce the risk of rehospitalization and prolong survival relative to standard care. Hence, to identify the current gaps and unmet needs for patient support for HF in the MEA region, a multidisciplinary meeting with experts from different countries across the region was convened. The panel aimed to gain insights into the real-world challenges in HF patient support and contribute strategic recommendations for optimizing HF care.
Methodology
An expert panel of 14 members with expertise in the management of HF across the MEA region [2 experts from Egypt, 2 from Saudi Arabia, 2 from Türkiye, and 1 each from Iraq, Jordan, Kenya, Kuwait, Lebanon, Morocco, South Africa, and the United Arab Emirates (UAE)] provided insights on practical challenges and recommendations to overcome the gaps in HF patient support. This article is an outcome of the literature review, expert group discussion, and consensus recommendations for bridging gaps in HF patient support in the MEA region.
Patient Journey in Heart Failure
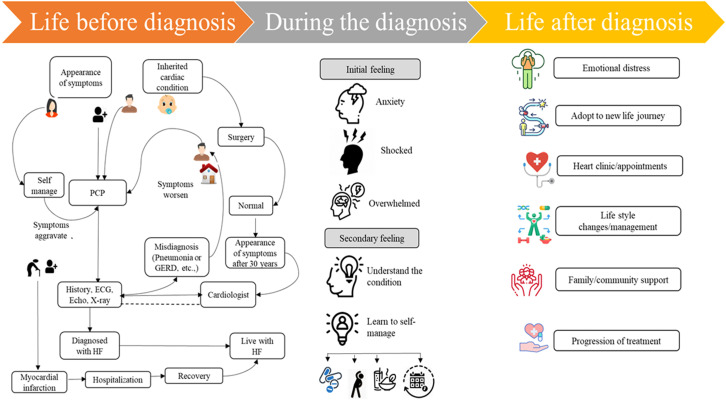
Patient journeys in HF differ from individual to individual, varying from symptoms of HF, mode of onset, etiology, comorbidities, and social/financial factors. Heart failure may present as a gradual functional decline with recurrent episodes of acute deterioration, frequent hospitalizations, recovery, and seemingly unexpected or sudden death.4 Patients are distressed when HF is diagnosed. Figure 1 summarizes the journey that a patient with HF experiences over time.
Figure 1.
Patient journey in heart failure. ECG, electrocardiogram; HF, heart failure; PCP, primary care physician; GERD, gastroesophageal reflux disease.
Despite their younger age, most patients presenting with acute HF from the MEA region have 1 or more comorbid conditions, particularly hypertension, diabetes mellitus, hyperlipidemia, and coronary artery disease.2 At initial presentation, although most patients exhibit classic symptoms and signs of HF, the disease often remains undiagnosed at the primary care physician (PCP) level.2 In most cases, the diagnosis is made during an emergency hospital admission,2 and is associated with prolonged hospital stays18 and subsequent frequent hospitalizations.2,3 In MEA, almost 56%-75% of the patients present with New York Heart Association (NYHA) class III and IV disease.2,3 Timely diagnosis and treatment remain critical for the survival and improved prognosis of patients with HF.
Approximately 22%-52% of patients in MEA receive suboptimal treatment (deviation from guideline-directed medical therapy),19- 21 which limits their quality of life (QOL). According to real-world data from Türkiye, patients with a prior diagnosed HF were admitted; now, with deteriorating HF, guideline-recommended drugs were less likely to be used (<73%) before admission.18
In MEA, most patients lack awareness about the disease, and to overcome low awareness, patients diagnosed with HF need explanations regarding their condition and the methods used to treat it, the lifestyle factors that need attention, a support system, and guidance in preparing them to resume their normal activities.
Role of PCP to Diagnose and Leverage Early Referral to Cardiologists
Most patients with HF will initially present themselves to general physicians (GPs) or PCPs. Therefore, the role of GPs or PCPs is crucial for diagnosing HF and the early referral of patients with HF to the cardiologist.
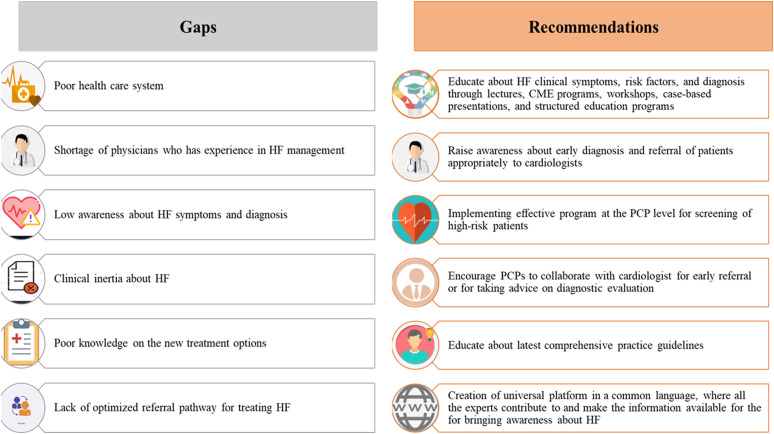
The condition poses a major diagnostic challenge for PCPs22 because, in the early stages of the disease, the symptoms and signs may be less obvious; therefore, they are particularly difficult to diagnose with the limited availability of the necessary investigative modalities, especially in low-middle income countries (LMICs). Accurate and early diagnosis is important since early treatment can delay or reverse disease progression. Lack of awareness among PCPs and GPs regarding the identification of risk factors for HF symptoms, and specialist referral remains a crucial challenge in the MEA. Clinical inertia about HF among PCPs is another big challenge. In the MEA region, non-HF specialists are treating the majority of the patients with HF, and only very few countries have developed structured HF programs with specialized HF clinics run by certified cardiologists and other disciplines.14 Figure 2 presents expert recommendations to bridge gaps in MEA at the PCP level to diagnose and leverage early referral to cardiologists.
Figure 2.
Expert recommendations to bridge gaps at PCP level to diagnose and leverage early referral to cardiologists. CME, continuous medical education; HF, heart failure; PCP, primary care physician.
Intervention Programs for Improving Heart Failure Care
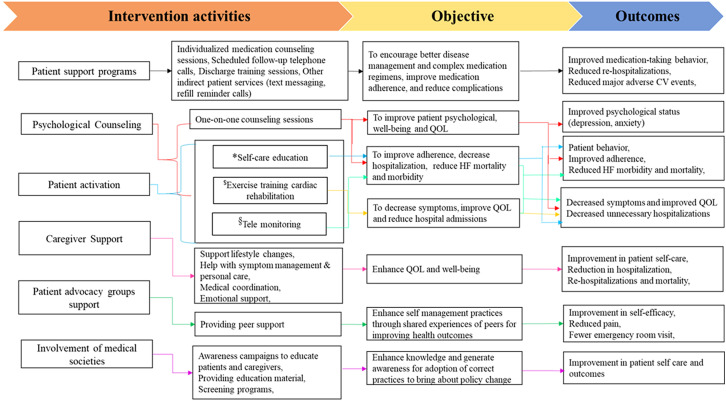
The experts recommended different intervention programs for improving patient care in HF management. Figure 3 demonstrates an intervention framework for these programs.
Figure 3.
Intervention framework for improving HF care. *Self-care education: educating patients about disease, risk factors, complications, medication, exercise regimens, the importance of adherence to diet, implementing lifestyle changes, and daily weight measurement habit. $Cardiac rehabilitation: exercise training and physical activity, health education, disease risk management, and psychological support.§Telemonitoring: patient education and follow-up through telephone call-based remote assessment by a physician or nurse, mobile phone-based monitoring, and videoconferencing. CV, cardiovascular; QOL, quality of life.
Patient Support Programs in Heart Failure Management
Patient support programs (PSPs) are enhanced self-management programs designed for direct patient or patient–caregiver engagement to support patients in managing their disease and complex medication regimens, improve medication adherence, and reduce potential complications and related costs.23,24 Activities included in PSPs are demonstrated in Figure 3.
A meta-analysis revealed that medication adherence, clinical, and humanistic outcomes were positively impacted by PSPs.23 A study by Lorig et al revealed that implementing PSPs can improve communication between patients and their physicians.25 In MEA countries like Lebanon and Saudi Arabia, self-care management is poor in patients with HF.26,27 Besides, approximately 50% of the patients have poor medication adherence,24,27 which could lead to increased complications of the disease, reduced QOL, and increased overall health-care costs related to readmissions. Hence, to improve medication adherence, QOL, and clinical outcomes among patients with HF in the MEA region, several pharmaceutical companies facilitate PSPs, including medication management and counseling, across many health-care facilities free of charge. However, there is a severe lack of awareness about PSPs in MEA countries.24 Given the important role that PSPs play in creating value for patients in terms of health-care follow-up practices, improved adherence habits, and potential cost savings, concerted efforts are imperative from cross-functional entities such as the government, pharmaceutical companies, and health-care organizations to expand PSPs in the MEA.
Psychological Counseling in Patients with Heart Failure
Heart failure is a chronic condition that affects not only the physical health but also the psychological well-being of the patient, because patients with HF often cope with numerous changes, including the consequences of the disease or its treatment on their QOL and functioning. Heart failure decreases the opportunities to participate in social life, leading to a deterioration of social interaction, social isolation, and a possible lack of social support.28 In addition, patients have to deal with adherence to a new lifestyle (exercise, smoking cessation, healthy eating, weight loss, and alcohol cessation).28 Among patients attending a chronic HF clinic, symptoms of anxiety and depression were found in more than 50% of the patients.29 A meta-analysis conducted on patients with HF reported clinically significant depression and anxiety in 21.5% and 28.79% of patients, respectively.30,31 There is considerable evidence that these psychological health conditions are associated with reduced adherence to treatment, poor function, increased hospitalizations, and poor cardiac outcomes.32-35 However, health-care professionals often pay less attention to psychological health. Most people in the world, including in MEA countries, who have mental illnesses such as anxiety, mood, etc., receive no treatment.36
Studies reveal that counseling can improve patients’ psychological condition37 (reduction in anxiety and depression),38 decrease HF-related hospitalizations39,40 morbidity, and mortality.41 Dracup et al emphasized that health-care professionals should have discussions with patients regarding the seriousness of their disease and effective treatment options that are available for HF, and they also emphasized that the majority of patients do very well on guideline-directed medical therapy.39
Experts have also stated that patient counseling is a key component in the HF intervention, as counseling can help the patient to cope with or adjust to their current health situation (poor mental health, upsetting physical health condition, and difficult emotions). Studies have reported that nurse-led counseling is beneficial for the improvement of mental health status and QOL for patients with HF.42 Experts have unanimously suggested encouraging nurses to be involved in HF care, since they are an integral part of the patient journey in HF management; moreover, they are skilled at navigating both the emotional and physical needs of their patients.
Improving Quality of Life in Patients with Heart Failure Based on Outcomes from Evidence-Based assessment
Maintaining QOL is a robust and independent predictor of all-cause death and HF hospitalization across all regions of the world in mildly and severely symptomatic HF.43 In MEA, particularly in Africa, compared to their western counterparts, health-related QOL is markedly lower among patients with HF.43 A good QOL is as imperative, if not more, as survival for most patients living with HF.44 Guideline-recommended medical and behavioral interventions for HF, including self-care interventions, exercise training, and cardiac rehabilitation, can help to improve QOL.45
Self-Care Management (Maintenance and Management)
Growing evidence suggests that patients with HF who demonstrate self-care deficits in activities such as treatment compliance, maintaining fluid restrictions, and not identifying the warning symptoms of worsening HF early have frequent hospitalizations and decreased QOL.46 Most HF management programs emphasize that improved self-care is the key to success in order to improve adherence, QOL, and reduce mortality, morbidity, and ultimately health-care costs47,48 (Table 1). 37,49–56
Table 1.
Studies Reporting Improved Quality of Life in Patients with Heart Failure
| Author | Approach | Study Design, Population and Sample Size | Objective | Intervention | Findings |
|---|---|---|---|---|---|
| Abbasi et al, 2018 51 | Self-care | Non RCT, chronic HF (n = 111) | To compare the effects of the self-management education program using the multimethod approach and multimedia on QOL among patients with chronic HF. | Control group (n = 38): received routine education consisting of face-to-face education by staff nurses and an educational pamphlet that was offered at discharge. Multimedia group (n = 37): Received routine education using multimedia, including photos, PPTs, animations, video clips, and texts. The session lasted for 15-20 minutes, and the patients and 1 of their family members were taught how to use multimedia. All are requested to use what they were taught at home for 3 months after discharge from the hospital. Multimethod group (n = 36): Received routine education and were invited to take part in 3 education sessions using a combination of education methods (face-to-face, PPT, photos, video clips, problem solving, and tutorials were used to provide education regarding self-management interventions) scheduled on 3 consecutive days with the presence of one of their family members. Each session lasted for 45-60 minutes at the time of admission and before discharge from the hospital. |
The education program improved QOL in patients with chronic HF. There were statistically significant differences in the mean score of changes in the psychological (P = .035), self-efficacy and knowledge (P < .001), and life satisfaction (P = .047) domains between the groups. The multimethod approach was more effective compared to other methods. |
| Jovicic et al, 200649 | Self-care | Systematic review: 6 RCTs, HF (n = 857) |
To determine the effectiveness of self-management interventions on hospital readmission rates, mortality, and HR QOL in patients diagnosed with HF. |
Self-management interventions. | Self-management reduced all-cause hospital readmissions (OR = 0.59; 95% CI, 0.44-0.80, P = .001) and HF-related readmissions (OR = 0.44; 95% CI, 0.27-0.71, P = .001). There was no significant effect on mortality, functional capabilities, symptom status, or QOL. |
| Dewalt et al, 201255 | Self-care | RCT, HF (n = 605) | To determine the effectiveness of a single session versus a more intensive multisession education program. | Single-session group: a 40-minute in-person, literacy-sensitive training. Multisession group: 40-minutes of in-person, literacy-sensitive training plus ongoing telephone-based support. |
Intensive multisession intervention did not change all-cause hospitalization or death (0.75 vs. 0.73 incidence rate per year; unadjusted IRR: 1.01), HF-related hospitalization (0.30 vs. 0.27 incidence rate; unadjusted IRR: 0.92) or HF-related QOL (after 12 months) compared with a single session intervention (P = .082). |
| Otsu and Moriyama, 201156 | Self-care | RCT Chronic HF (n = 102) |
To provide an educational self-management program to Japanese outpatients with chronic HF in order to improve their clinical outcomes. | Control group (n = 52): medical treatment and standard care. Intervention group (n = 50): An educational program for 6 months (6 nurse-directed sessions). |
In either group, there was no fatality or hospitalization due to chronic HF during the program. After 12 months, the QOL (6.20 ± 0.60 vs. 5.65 ± 0.85; P = .002) and compliance behavior with regard to sodium restriction (2.47 ± 0.69; 1.73 ± 0.96; P = .000) and activities/exercises (2.36 ± 0.67 vs. 0.73 ± 0.86; P = .000) are significantly better in the intervention group compared to the control group. |
| Wang et al, 201152 | Self-care | Quasiexperimental design Congestive HF (n = 27) |
To determine if participants with HF who were managed under the HF self-care program had fewer distressing symptoms, better functional status, improved QOL, and reduced hospital and emergency readmission rates compared with control group participants. | Control group (n = 13): routine care during hospitalization. Intervention group (n = 14): intensive education. Before discharge, an informal meeting was conducted with patients and their families to remind them of their medication and the date of their next clinical follow-up. In addition, an education brochure was provided. Telephone call was initiated 3 or 4 days after discharge (out-of-hospital care). Home visits were arranged to assess the self-care performance of patients. The education content included the pathophysiology of disease, risk factors, signs, and symptoms; stressing medication and exercise regimens; importance of adherence to diet; implementing lifestyle changes; and daily weight measurement habit. |
A significant improvement in symptom distress (P < .01) and QOL (SF36 score: 118.75 ± 11.20 vs. 89.00 ± 28.47; P < .05) was observed among patients who received self-care education compared to the control group. However, there were no significant differences in emergency department visits (P = .26) and hospital readmissions (P = .06). |
| Donner Alves et al, 201253 | Self-care (Nutritional guidance) | RCT HF (n = 46) |
To evaluate if a global nutritional orientation could affect nutritional knowledge, adherence to food guidelines, anthropometrics, and QOL in HF patients. | Control group (n = 23): usual care with medical and nursing staff. Intervention group (n = 23): usual care plus additional nutritional guidance about diet and its relationship with disease, sources of nutrients, and reduction of dietary sodium and fats. |
After 6 months of follow-up, the nutritional knowledge of the intervention group increased. Also, caloric, fat, and sodium intake decreased in the intervention group compared to the control (P < .05). No significant difference in QOL was found between the control and the intervention group. |
| Kutzleb et al, 200650 | Self-care | Prospective quasiexperimental study HF (n = 23) |
To evaluate the impact of a nurse-directed approach to patient education focused on lifestyle modification, diet, daily weight management, and medication compliance to improve the QOL and functional capacity in people with HF. | Routine care group (n = 10): Protocol-driven medical management along with smoking cessation, medications, diet, and nutritional counseling provided with each 3-month visit. Nurse-directed care group (n = 13): Routine care plus comprehensive disease management education over 12 months, and weekly telephone follow-up between monthly follow-ups. The patient education included daily weight charting and an education booklet for patients and their family. Medication compliance counseling consisted of the development of an individualized medication grid sheet listing each medication, dosage strength, administration schedule, etc. Diet and nutrition counseling incorporated a food exchange list, food preparation tips, and a 4-step approach to managing a low-salt diet. Individualized counseling concentrated on exercise, smoking cessation, and the elimination of alcohol intake. |
There was statistically significant improvement in QOL in the nurse-directed patient education group compared to routine care group (F = 3.569, P = .000; social and economic: F = 14.109, P = .000; health and function: F = 3.995, P = .003; psychological and spiritual: F = 13.212, P = .000; and family: F = 2.384, P = .048). |
| Doughty et al, 200254 | Self-care education and integrated HF management |
RCT HF (n = 197) |
To determine the effect of an integrated HF management program involving patient and family, primary and secondary care, on QOL and death or hospital readmissions in patients with chronic HF. | Control group (n = 97): usual care. Intervention group (n = 100): individual and group education sessions (each lasting 1.5-2 hours, 2 within 6 weeks of hospital discharge, and another after 6 months). All these patients are provided with a personal diary to record medication and body weight, information booklets, and regular clinical follow-up alternating between the general practitioner and HF clinic. |
Integrated management program for patients with chronic HF improved QOL, reduced total hospital admissions, and reduced total bed days as well. However, for mortality or hospital readmission, there is no significant difference between the intervention and control groups (68 vs. 61, respectively, χ 2 = 0.95, P = .33). |
| Smeulders et al, 201037 | Self-care | RCT, Congestive HF (n = 317) | To evaluate the effects of the Chronic Disease Self-Management Program on psychosocial attributes, self-care behavior, and QOL among congestive HF patients who experienced a slight to marked limitation of physical activity. | Control group patients (n = 131): usual care, consisting of regular outpatient checkups. Intervention group patients (n = 186): usual care and participated in the 6-week self-management program. |
Self-management program resulted in statistically significant effects on cognitive symptom management (P < .001), self-care behavior (P = .008), and cardiac-specific QOL (P = .005). |
| Piotrowicz et al, 201564 | Exercise-based cardiac rehabilitation | RCT HF (n = 131) |
To assess QOL changes in HF patients after HTCR) vs. outpatient basedSCR. | Control group (n = 56): SCR. Intervention group (n = 75): HTCR. |
Both groups achieved a significant improvement in QOL [69.2 ± 26.4 (before rehabilitation) vs. 70.5 ± 25.4, P = .0074 (after rehabilitation completion)]. However, patients who underwent HTCR showed an improvement mainly in the mental categories [21.68 ± 12.46 (before rehabilitation) vs. 18.56 ± 9.18 (after rehabilitation completion)]. Patients in the SCR group showed improvement in their general physical well-being (23.20 ± 10.71 vs. 21.60 ± 9.65). |
| Long et al, 201961 | Exercise-based cardiac rehabilitation | Meta-analysis 44 trials; HF (n = 5,783) |
To determine the effects of exercise-based cardiac rehabilitation on mortality, hospital admission, and health-related QOL of people with HF. | Control group: usual medical care. Intervention group: exercise‐based interventions given alone or as a component of comprehensive cardiac rehabilitation. |
A clinically important improvement in short-term disease-specific health-related QOL is evident [SMD 0.60, 95% CI, 0.82-(−0.3)9; I² = 87%; χ
2 = 215.03]. Cardiac rehabilitation improved all-cause mortality in the long term (RR 0.88, 95% CI, 0.75-1.02), reduced overall hospital admissions (RR 0.70, 95% CI, 0.60-0.83), and HF-specific hospitalization in the short term (RR 0.59, 95% CI, 0.42-0.84). |
| Taylor et al, 201962 | Exercise-based cardiac rehabilitation | Meta-analysis 19 RCTs chronic HF (n = 3990) |
To obtain definitive estimates of the impact of exercise-based cardiac rehabilitation interventions compared with no exercise intervention (control) on mortality, hospitalization, exercise capacity, and health-related QOL in HF patients. | Control group: usual medical care. Intervention group: exercise-based cardiac rehabilitation for at least 3 weeks with 6 months’ follow-up. |
There was a statistically significant difference in favor of exercise-based cardiac rehabilitation for QOL [Minnesota Living with HF Questionnaire mean score −5.94, 95% CI, −1.0-(−10.9)]. However, no significant difference in pooled time-to-event estimates in favor of exercise-based cardiac rehabilitation was found for mortality or hospitalization [All-cause mortality (HR = 0.83, 95% CI, 0.67-1.04); HF-related mortality (HR = 0.84, 95% CI, 0.49-1.46); all-cause hospitalization (HR = 0.90, 95% CI, 0.76-1.06); and HF-related hospitalization (HR = 0.98, 95% CI, 0.72-1.35)]. |
| Davies et al, 201063 | Exercise-based cardiac rehabilitation | Meta-analysis 19 RCTs; Systolic HF (n = 3647) |
To determine the effect of exercise training on clinical events and health-related QOL of patients with systolic HF. | Control group: usual medical care. Intervention group: exercise-based cardiac rehabilitation with 6 months’ follow-up. |
Compared with usual care, in selected HF patients, exercise training reduces HF-related hospitalizations (RR = 0.72, 95% CI, 0.52-0.99) and results in clinically important improvements in health-related QOL [mean difference: −0.63, 95% CI, (−0.80)-(−0.37)]. With respect to all-cause mortality or overall hospital admissions there was no significant difference between the groups. |
HF: heart failure, HR: hazards ratio, HTCR: home-based telemonitored cardiac rehabilitation, IRR: incidence rate ratio, OR: odds ratio, PPT: PowerPoint presentation, QOL: quality of life, RCT: randomized controlled trial, RR: relative ratio, SCR: standard cardiac rehabilitation, SF36: 36-item Short Form Survey, SMD: standardized mean difference.
A longer duration of self-management interventions was found to be more effective in improving several outcomes.57 Promoting effective self-care practices by all clinicians in patients with HF could improve QOL and reduce the economic and personal burden of recurrent hospitalizations. Hence, the concept of self-care is supported by international guidelines.58 Interventions using face-to-face communication59 and a multidisciplinary team of interventionists60 were found to be more effective than interventions without these strategies. However, in MEA countries like Lebanon, self-care remains suboptimal and warrants the development of novel strategies to improve it.26
Exercise Training Cardiac Rehabilitation
Research has repeatedly reinforced the usefulness of exercise training cardiac rehabilitation interventions in patients with stabilized HF in decreasing symptoms, improving QOL, reducing hospital admissions, and consequently reducing financial burden (Table 1).61-64
It was found to be a clinically effective and economical intervention for patients with HF.62 Additionally, providing group exercise training would allow patients to meet individuals who are experiencing similar life challenges and thus offer an additional network of support.
In MEA countries, currently, there are no national strategies regarding cardiac rehabilitation; however, very few health-care institutions have implemented this program.
Remote Health Monitoring
Technological advances have allowed increasingly sophisticated attempts to remotely monitor and manage HF.65 A meta-analysis has concluded that remote patient monitoring (RPM) programs for HF patients can reduce hospital admissions and mortality and simultaneously improve health-related QOL.66,67 Prior studies have demonstrated that the RPM of homebound HF patients significantly reduced home visits by trained nurses and reduced hospital readmissions.68 A recently updated Cochrane review that investigated the use of structured telephone support or noninvasive telemonitoring has demonstrated a modest beneficial effect of remote monitoring on all-cause mortality and HF-related hospitalizations compared with standard HF care.69 In another meta-analysis, a structured telephone support program delivered through human-to-human contact or human-to-machine interface showed beneficial trends, particularly in reducing all-cause mortality for recently discharged patients with HF.70 Remote monitoring is often done in patients with HF who have an implantable device (resynchronization pacing and/or defibrillator), and feedback given to them after alerts are noted after transmissions.
Patient Activation in Heart Failure
Patient activation or active engagement in their health management is critical for those with HF to improve their condition, given the complex and potentially progressive nature of the disease, the high presence of comorbidities, and the financial and emotional stress the disease places on patients and their families. Growing evidence suggests that patient education and activation in understanding their disease and imparting the knowledge, skills, and confidence to engage in managing one's health favorably impact patient behaviors and health outcomes among patients with HF.46,49,71 Low activation has been associated with depression, anxiety, and worse clinical course,72 and high activation has been associated with better self-care behaviors and more favorable outcomes.73,74
Educational interventions may hold promise for improving patient activation.75 A randomized clinical trial (RCT) conducted on patients with HF to determine the efficacy of a patient activation intervention (a 6-month program delivered by advanced practice nurses) compared with usual care on activation, self-care management, hospitalizations, and emergency department visits demonstrated a significant increase in patient activation with the targeted interventions compared to usual care.76 A systematic review indicated strategies such as patient education to enhance self-care, follow-up monitoring by specially trained staff, and access to specialized HF clinics as the most efficacious approaches for the management of patients with HF to reduce HF hospitalization.60
The literature has revealed a variety of choices for self-management education interventions, including face-to-face education, multimedia-based education, telemonitoring, education accompanied by telephone support, nurse case management, and telemonitoring for activating or improving self-care among patients with HF (Supplementary Table 1).77- 85
Supplementary Table 1.
Studies reporting improved outcomes with patient activation
| Author | Approach | Study Design, Population and sample size | Objective | Intervention | Findings |
|---|---|---|---|---|---|
| Young et al, 201679 | Self-care education | RCT chronic HF (n = 105) | To evaluate the mechanism of the patient activation intervention, comparing the intervention and usual care (UC) groups on self management knowledge, self-efficacy for selfmanagement, patient activation at the end of intervention (3 months). |
UC group (n = 51): UC, the standard discharge teaching for HF that includes written and verbal information about HF self-care and scheduled follow-up doctor appointments. Intervention group (n = 54): Usual care and the 12-week PATCH intervention, ie, a oneon-one in-hospital self-management training session and postdischarge reinforcement sessions (twice a week for the first 2 weeks, once a week for weeks 3 to 6, and every other week for weeks 7 to 12) delivered by telephone. |
PATCH intervention showed significantly greater improvement in self efficacy in for self-management (taking prescribed medication, a lowsodium diet, and exercising daily, weighing themselves) (P = 0.034), selfmanagement strategies (P <0.0005), and patient activation scores (P = 0.0693). compared to usual care. However, the 30-day hospital readmission rate was significantly higher in the PATCH group (n = 10 vs. 3; χ2 with continuity correction = 2.914, P = 0.088) than in the UC group. |
| González et al, 200578 | Nurse-guided self-care education | Prospective study HF (n = 298) |
To evaluate nurse education programme in an outpatient HF population. | Face to face education with printed leaflets for patients and their families, and with posters in the waiting room reminding them of signs of worsening HF. At every visit, to reinforce self-care behavior, patients are reinstructed about the disease. Patients were instructed to do daily activities to improve the respiratory work, and they were involved in a 4-month exercise programme. |
Nurse-guided education has changed self-care behavior of patients with HF in several important aspects, as weight and blood controls, and has increased their knowledge and understanding of the disease and treatment. |
| Boyde et al, 201880 | Self-care | RCT, HF (n = 200) |
To determine the effectiveness of a multimedia educational intervention for patients with heart failure in reducing unplanned hospital readmissions. |
Control group (n = 100): Usual education Intervention group (n = 100): Multimedia educational intervention (viewing a DVD, and verbal discussion supported by a written manual with a teach-back evaluation strategy). |
The self-care educational intervention reduced the risk of readmission at 12 months by 30% (relative risk: 0.703; 95% CI, 0.548 to 0.903). |
| Sherwood et al, 201781 | Self-care and telephone monitoring | RCT, HF (n = 180) | To evaluate efficacy of a coping skill training intervention, delivered over the telephone to HF patients, on 3 outcomes: postintervention QOL, HF disease biomarkers, and longerterm clinical outcomes defined by hospitalisation or death. |
Control group (n = 90): HF education delivered by a Physician’s Assistant plus weekly individual phone calls of up to 30minutes (16 calls). Intervention group (N = 90): Coping skills training delivered by a clinical psychologist and was comprised of 16 weekly 30-minute individual phone calls. Initial 4 sessions were on health behaviors diet and salt restriction, daily weighing, physical activity, medication adherence. The remaining 12 sessions were on specific coping techniques. |
Both the groups showed improvements in HF self-management scores postintervention. Coping skills training resulted in greater improvements in QOL compared to routine HF education (P <0.01) also lowered risk of HF hospitalisations (HR = 0.65, 95% CI, 0.44 to 0.98, P = 0.040) and mortality (HR = 0.84, 95% CI, 0.59 to 1.21) in patients. |
| Baker et al, 201177 | Self-care and telephone monitoring | RCT HF (n = 605) |
To examine the effect of 2 different levels of self-care training on the adoption of key self-care behaviors and on QOL: A single educational session delivered by a health educator vs A single educational session and a series of follow-up phone calls. |
Control group (n = 302): Brief (1 hour) educational intervention. Intervention group (n = 303): More intensive educational intervention group plus reinforcing telephone education (5 to 8 follow-up phone calls from the educator with each call lasting about 10 minutes over 4 weeks). |
The intervention group had greater improvements in general and salt knowledge (P < .001) and greater increases in self-care behaviors (from mean of 4.8 to 7.6 for the intervention group vs. 5.2-6.7 for the control group; P < .001). QOL improved from 58.5 to 64.6 for the intervention group but did not change for the control group (64.7-63.9; P < .001 for the difference in change scores). Telephone reinforcement of learning goals and self-care behaviors improved knowledge, health behaviors, and HF-related QOL compared to a single education session. |
| Ong et al, 201682 | Combination of telephone support and telemonitoring | RCT Older adults hospitalised with HF (n = 1437) |
To evaluate the effectiveness of a care transition intervention using RPM in reducing 180-day all-cause readmissions. |
UC group: (n = 722). Intervention group (health coaching telephone calls and telemonitoring): (n = 715). |
No significant difference in 30-day readmission or 180-day mortality between the intervention and usual care group. But there was a significant difference in 180-day QOL. |
| Yu et al, 201583 | Combination of nurse home visits and telephone support | RCT Chronic HF (n = 178) |
To determine the effect of nurse-implemented transitional care on readmission and mortality rates in Chinese individuals with chronic HF in Hong Kong. |
UC group (Controls): (n = 88). TC group: (n = 90). |
No significant differences in eventfree survival, hospital readmission, or mortality between the TC and UC groups, although the TC group had a lower hospital readmission rate at 6 weeks (8.1% vs. 16.3%, P = 0.048) and lower mortality at 9 months (4.1% vs. 13.8%, P = 0.03). The TC group also had a shorter hospital stay (P = 0.006) and significantly better self-care and QOL. |
| Kalter-Leibovici et al, 201784 | Disease management programs | Open-label trial, Chronic HF (n = 1,360) |
This evaluated whether a countrywide disease management program is superior to usual care in reducing adverse health outcomes and improving well-being among community-dwelling adult patients with moderate to severe chronic HF who have universal access to advanced healthcare services and technologies. |
UC group (n = 678). Intervention group (n = 682): Disease management, delivered by multidisciplinary team. |
The disease management intervention was not superior to usual care with respect to the primary composite endpoint, but it improved QOL and depression. |
| Bekelman et al, 201585 | Disease management clinics | RCT HF (n = 392) |
To determine the effectiveness of a collaborative care patient-centered disease management (PCDM) intervention to improve the health status of patients with HF. |
PCDM intervention group: (n = 187). UC group: (n = 197). |
Multifaceted PCDM intervention demonstrated improved patient health status compared with usual care; however, the improvement observed was nonsignificant. |
RCT: Randomized controlled trial, HF: Heart failure, CI: Confidence interval, QOL: Quality of life, HR: Hazards ratio, PATCH: Patient AcTivated Care at Home, PCDM: Patient-centered disease management, RPM: remote patient monitoring, TC: Transitional care, UC: Usual care group.
In MEA, patient activation programs are conducted in some countries like Lebanon, Kuwait, Morocco, Egypt, Türkiye, and Saudi Arabia. In Lebanon and Türkiye, a program, named “Heart Failure Awareness Days” was conducted regularly before the coronavirus disease 2019 pandemic. As a part of the program activities such as lectures to patients, their relatives, and the general population about HF, workshops about “HF diets,” “open house” in HF clinics, panel debates with HF cardiologists, etc., were organized. In Kuwait, a monthlong program named “strengthen your heart” was conducted by visiting hospitals to educate patients and caregivers about different HF symptoms, self-care maintenance, etc. In Saudi Arabia, campaigns were conducted during special occasions like Ramadan, Women’s Day, etc. via social media through social media influencers (Supplementary Table 2).
Supplementary Table 2.
Patient activation programmes conducted in MEA to motivate patient with HF
|
|
|
|
|
|
|
|
|
|
|
|
HF, Heart failure, HFA, Heart Failure Association, HFWG, Heart failure working group, MEA, Middle East and Africa, PCP, Primary care physician, PHC, Primary health care, TSC, Turkish Society of Cardiology, TV, television.
Some of the real-world initiatives to leverage patient activation in HF recommended by experts are described in Box 1.
Box 1. Practical Initiatives to Leverage Patient Activation
Campaigns through lectures and discussions, visiting hospitals and primary care centers, and organizing programs for educating the patients and caregivers
Campaigns through a newspaper, social media influencers (Twitter and Instagram), radio, and television
Collaborate with the cardiology societies and pharmaceutical companies to create awareness among patients, caregivers, and PCPs.
Individual patient care and education via presentations, counseling, lectures, and individual sessions with the patient and caregivers with the support of health-care workers (2-3 HF nurses, physical therapists, nutritionist pharmacists, and psychologists).
Utilize specific occasions (Ramadan period) to leverage patient activation by inviting a group of experts (cardiologists, physicians, dietitians, nurses, educationists, and psychologists) to a discussion.
Conduct awareness campaign in local language by inviting groups of patients and caregivers on 1 platform by a group of PCPs, internists, and cardiologists to discuss about HF-related issues
Introduce centers of excellence in different regions for helping patients manage their disease condition, discuss HF-related treatment issues, and promote patient-physician communication.
Maintain a scorecard with the help of a pharmacist to track the performance of patient. The scorecard may comprise all the details, including the diagnosis, the medication details, the doses suggested, etc.
HF, heart failure, PCP, primary care physician.
Role of Caregivers and Educators in Patient Support
Role of Caregivers
Caregivers play an important part in improving patient outcomes since the majority of patients with HF depend on caregivers’ support for successful HF self-care, which is essential for optimal patient outcomes.86,87 Caregivers not only support lifestyle changes that go along with a diagnosis of HF and personal care but also help with symptom management, medical coordination, and treatment decision-making, in addition to emotional support.88 Clinical practice guidelines from the American Heart Association recommend the inclusion of caregivers along with the patient for individualized education and counseling on self-care.89 Studies reported significant improvement in patient self-care, reductions in hospitalization, readmission, and mortality rate in HF with the involvement of caregivers in patient care.90,91 The experts also recommended the involvement and empowerment of caregivers and collaboration with health-care workers (PCPs, cardiologists, nurses, and psychologists) through an open discussion that may help in improving patient outcomes.
Heart Failure Educators
Patient education is an essential component in HF management; hence, an HF educator who has knowledge and skills in the medical sciences, pedagogy, communication, and counseling plays a major role in empowering patients to manage their disease. A narrative review has identified patient educators as a potential option for improving patient health and well-being.92
In many Western countries, nurses experienced in HF management are playing an educator role to create awareness among patients, similar to certified diabetes educators. These education programs provided by nurses on the self-care behavior of HF patients were very effective in improving patient outcomes.48,93 Studies demonstrated a significant improvement in the mortality rate among patients who received HF education through a certified nurse practitioner.48 In MEA, there is a scarcity of trained HF educators. Most of the time, the clinicians are playing the educator role as well, due to the shortage of nurses who have experience in HF management. The need for training qualified HF educators is ever-increasing in MEA due to the upsurge in disease burden. Incorporating HF educators into practice settings adds significantly to HF care and will improve communication between patients and health-care providers. It will serve to improve clinical and QOL outcomes for people with HF.
In South Africa, nurses provide support to the patients in HF education and medication adherence, giving more insights to the insurance companies about medications because, in South Africa, HF medication costs are not always covered by the funder. The nurse practitioners in this country are trying to extend their support to the patients by providing insights to the insurance companies about the critical need for these medications.
Role of Patient Advocacy Groups and Heart Failure Societies in Patient Support
Role of Patient Advocacy Groups
Patient advocacy groups (PAGs) are nonprofit groups that represent patients with a health condition or their caregivers. Patient advocacy groups are playing a crucial role in providing peer support for patients and families, reducing stigma, raising awareness, educating, raising funds for nonaffording patients, influencing policymakers, and impacting national research agendas by bringing the public's concerns about the disease to policymakers and the medical community.94 In addition, they also help patients manage their finances by assisting them in understanding medical bills and insurance coverage.94 Studies have also demonstrated positive effects of peer support on self-efficacy, activity, reducing pain, and decreasing emergency room visits.95
In Western countries, several PAGs, such as European Patient Advocacy Groups, Heart Failure Society of America, The Mended Hearts and the Mended Little Hearts Advocacy, and Global Heart Hub, are providing peer support for patients and families of those affected by cardiovascular disease. However, in MEA, PAGs were established only in countries like Egypt, Lebanon, and Israel [the Egyptian Association for Care of Heart Failure Patients, the Israeli Heart Association, and Heart Failure in Lebanon, a nongovernmental organization (NGO)].
Despite a significant body of evidence that reinforced HF self-management is key for improving patient outcomes and decreasing hospitalizations, to date, there are major gaps in representing the voice of the HF patient in the MEA region. Though NGOs and medical societies from MEA are performing many activities in the health-care sector, the activities are still at a nascent stage in many countries. There is a need to have a separate group of people who will advocate the cause of patient support, and will work explicitly in these areas to increase transparency and credibility. The World Heart Federation also emphasized the need to involve PAGs, raise the HF profile on national agendas, raise awareness among patients, and influence decisions and policymakers.4
In Egypt, a few NGOs (the Egyptian Friends of National Heart Foundation of National Heart Institute and the Egyptian Society for Patients with HF) are supporting patients by promoting and facilitating health and educational activities (healthy living, medication adherence, lifestyle modifications, and using home monitoring devices) to improve adherence to therapy and clinical outcomes. Besides, they are also strengthening health systems by influencing policymakers. For example, in Egypt, with the support of PAGs, an NGO was able to procure HF medications free of cost for financially challenged patients by reaching out to the Egyptian Health Ministry and insurance providers.
Heart Failure Societies
Heart failure societies play a key role in the development and implementation of guidelines, increasing public and physicians’ awareness regarding prevention, investment in research, support in continuous medical education, organization of cardiology symposia and congresses, and achievement of national registries regarding main cardiac conditions to decrease the burden of cardiovascular diseases. Besides, the organizations can also enhance awareness among lawmakers and policymakers while advocating for changes to protect and improve the health of people. Globally, the European Society of Cardiology (ESC) is actively conducting several programs or campaigns in different countries to create awareness at the community, patient, and PCP levels.
In MEA countries like Lebanon, Jordan, Morocco, and Türkiye, the cardiology societies (members of ESC) are actively conducting programs or campaigns to create awareness at the patient level, community level and PCP level. The “Heart Failure Awareness Days” campaign organized by the HF Working Group of the Lebanese Society of Cardiology (LSC) in collaboration with the Lebanese Ministry of Health and Lebanese Nursing Order is an example of such a program designed to create awareness among patients and caregivers.96 This can has been initiated in 2011 by the HF Working Group of LSC and has been awarded by HFA-ESC 5 times up to date (best overall campaign, best poster, best social media activities, etc.). Further, case-based clinical presentations and lectures are organized by medical societies for physicians’ internists, pulmonologists, endocrinologists, and other specialists to increase awareness at the primary care level. Since 2012, the Turkish Society of Cardiology has actively participated in the initiative “HF Awareness Day” activities, organized press conferences, printed and distributed posters and booklets for patients, and arranged webinars for physicians. This significant event is now included in the regular activity program of the HF Working Group. Also, the Turkish HF Working Group has published national guidelines on specific HF topics in order to improve the implementation of optimal HF therapy. 97- 100
The experts recommend all the HF societies in MEA collaborate with international medical societies to organize activities at the local and national levels to improve patient care in HF management.
Heart Failure Programs and Clinics
In the MEA region, mostly the Gulf countries like Saudi Arabia, Qatar, and the UAE have established structured HF programs with specialized clinics run by cardiologists who work with professionals like nurses, pharmacists, physiotherapists, and others certified in treating HF.14 For example, Saudi Arabia has at least 10 HF clinics dispersed throughout the country, and they have had a significant impact on the way patients with HF are treated and the patient outcomes.10,14 HF clinics are endorsed by international recommendations; other nations in the region are yet to establish them.14
In most MEA countries, nongovernmental organizations like cardiac medical societies have conducted several campaigns to increase awareness among the general population, HF patients, and GPs. In some countries, like Jordan, the UAE, Türkiye, and Saudi Arabia, governments have launched some programs to increase awareness and control cardiovascular disease. For example, in Türkiye, in 2017, the Turkish Ministry of Health (MoH), in colloboration with Turkish Society of Cardiology, has established a national heart health policy to decrease the burden of cardiovascular disease and its risk factors. The MoH leads the main public awareness campaigns, projects, and educational activities.101 Similar programs were conducted in countries like the UAE, Lebanon,102 Egypt,103 and Jordan.104
Additionally, in many countries, workshops and seminars on heart health and HF prevention are conducted in collaboration with health-care professionals and medical organizations. Some countries have dedicated national or regional heart health days during which various activities and events are organized to focus on heart health and HF prevention. In addition to public awareness initiatives, some governments in Gulf countries are offering training and continuing education programs for health-care professionals to improve HF management.
Conclusion
Heart failure remains a major public health problem in the MEA. Despite the continued impressive advances in the therapeutic management of HF, there is a high unmet need for patient support for HF in the MEA. In this region, up to three-fourths of patients with HF were diagnosed with NYHA class III and IV disease. There is low awareness about HF at the patient as well as PCP levels; thus, the probability of an early diagnosis is limited. Effective strategies at the patient and PCP levels are essential to bridge the gaps in HF patient support. Patient education is a crucial component in HF care and should be provided through effective and well-evaluated strategies through the adoption of digital health technologies. Patient support programs have been considered a promising approach in reducing rehospitalizations and even major adverse cardiovascular events. Incorporating HF educators into routine practice settings can add significantly to HF care and can improve communication between patients and the health-care provider. Patient advocacy groups can play a vital role in easing the burden by providing peer support for patients and their families. Given the important role that PSPs play in creating value for patients in terms of patient support, comprehensive efforts should be made to expand and endorse PSPs in the MEA.
Although some MEA nations have taken measures to increase public awareness and offer HF care, these initiatives have not been evaluated for their effectiveness, and the lack of literature on the outcomes of public awareness remains a significant gap. Monitoring and evaluating the effectiveness of these programs are essential to gauge their impact on public health and to identifying areas for improvement. By prioritizing the assessment of public awareness programs, MEA countries can strengthen their efforts to combat HF.
The recommendations by the experts for improved patient support for patients with HF in the MEA region are presented in the box below:
Run national awareness campaigns.
Develop clear region- or country-specific referral protocols.
Facilitate interaction between primary-level health-care workers and specialists.
-
Establish HF clinics and specialist nurse follow-up.
Improve communication between the different health-care sectors and through education.
Establish formal pathways for patient education.
Promote patient-driven HF associations and family and community engagement.
Implement health-care worker outreach programs.
Develop industry-related initiatives to reduce the cost of devices and equipment.
Encourage private fundraising initiatives.
Collaborate with nongovernmental organizations and the government.
HF: Heart failure.
Funding Statement
This is funded by AstraZeneca, Middle East and Africa.
Footnotes
Availability of data material: All data generated or analyzed during this study are included in this published article and its supplementary data file.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Design – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Supervision – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Resources – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Materials – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Data Collection and/or Processing – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Analysis and/or Interpretation – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Literature Search – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Writing – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.; Critical Review – H.N.S., Y.Ç., A.B., E.K., E.N.O., F.B., H.B.A.S., H.R., K.A.A., M.A., M.B.Y., R.T.
Acknowledgments: The authors acknowledge Dr. Kamal Waheeb AlGhalayini for his contributions to this manuscript. The authors would also like to thank Dr. Sasikala Somara of Fortrea Scientific Pvt. Ltd. (formerly Labcorp Scientific Services & Solutions Pvt. Ltd.) for medical writing support, which is done in accordance with Good Publication Practice 2022 guidelines.
Declaration of Interests: H.N.S. received speaker honorarium from Novartis, AstraZeneca, Boehringer Ingelheim, Abbott, Bayer and Vifor. Y.Ç. received a speaker honorarium or consultation fee from AstraZeneca, Novartis, Servier, Boehringer Ingelheim and Roche Diagnostics. A.B. received institutional fee from Novartis, Bayer, Amgen, AstraZeneca, Boehringer Ingelheim, Servier and Merck. E.K. received honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim, Novartis, Novo-Nordisk, Pfizer, Roche, Sanofi and Servier. E.N.O. received research grants from AstraZeneca and Novartis; also received speaker fees from AstraZeneca, Servier, Novartis, Boehringer Ingelhaim, Pfizer and Merck. F.B. declares no conflict of interest. H.B.A.S. received honoraria from AstraZeneca and Merck for congress presentations and advisory boards. H.R. received honoraria from Novartis and AstraZeneca and Menarinin for congress presentations and advisory boards. K.A.A. as advisor received honorarium from Boehringer Ingelheim, AstraZeneca, Pfizer, Vifor, Novartis, Janssen, Merck, Bayer, Abbott (Heartmate3, CardioMEMS), Medtronic (Heartware) and Syncardia TAH. M.A. received speaker honoraria from Bayer, AstraZeneca, Novartis and Boehringer Ingelheim. M.B.Y. received institutional fee from Novartis, Bayer, Amgen, AstraZeneca, Boehringer Ingelheim, Servier, Roche Diagnostics and Albert Health. R.T. received honoraria as a speaker and advisory board member form AstraZeneca, BI, Pfizer, Merck, Novartis, Hikma, MSPhrma. Grant recepient from Pfizer.
References
- 1. Bragazzi NL, Zhong W, Shu J, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682 1690. ( 10.1093/eurjpc/zwaa147) [DOI] [PubMed] [Google Scholar]
- 2. Sulaiman K, Panduranga P, Al-Zakwani I, et al. Clinical characteristics, management, and outcomes of acute heart failure patients: observations from the Gulf acute heart failure registry (Gulf CARE). Eur J Heart Fail. 2015;17(4):374 384. ( 10.1002/ejhf.245) [DOI] [PubMed] [Google Scholar]
- 3. Dokainish H, Teo K, Zhu J, et al. Heart failure in Africa, Asia, the Middle East and South America: the INTER-CHF study. Int J Cardiol. 2016;204:133 141. ( 10.1016/j.ijcard.2015.11.183) [DOI] [PubMed] [Google Scholar]
- 4. Ferreira JP, Kraus S, Mitchell S, et al. World Heart Federation roadmap for heart failure. Glob Heart. 2019;14(3):197 214. ( 10.1016/j.gheart.2019.07.004) [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4 25. ( 10.1002/ehf2.12005) [DOI] [PubMed] [Google Scholar]
- 6. Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from global burden of disease study 2017. BMC Public Health. 2021;21(1):401. ( 10.1186/s12889-021-10429-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Heart Federation. Middle East & North Africa. World Heart Federation. https://world-heart-federation.org/where-we-work/middle-east-north-africa/ . ; Accessed March 7, 2022. [Google Scholar]
- 8. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123 1133. ( 10.1016/j.jacc.2013.11.053) [DOI] [PubMed] [Google Scholar]
- 9. Moertl D, Altenberger J, Bauer N, et al. Disease management programs in chronic heart failure: position statement of the heart failure working group and the working group of the Cardiological assistance and care personnel of the Austrian Society of Cardiology. Wien Klin Wochenschr. 2017;129(23-24):869 878. ( 10.1007/s00508-017-1265-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Shamiri MQ. Heart failure in the Middle East. Curr Cardiol Rev. 2013;9(2):174 178. ( 10.2174/1573403X11309020009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alhabeeb W, Elasfar A, AlBackr H, et al. Clinical characteristics, management and outcomes of patients with chronic heart failure: results from the heart function assessment registry trial in Saudi Arabia (HEARTS-chronic). Int J Cardiol. 2017;235:94 99. ( 10.1016/j.ijcard.2017.02.087) [DOI] [PubMed] [Google Scholar]
- 12. Assiri AS. Clinical and therapeutic profiles of heart failure patients admitted to a Tertiary hospital, Aseer region, Saudi Arabia. Sultan Qaboos Univ Med J. 2011;11(2):230 235 [PMC free article] [PubMed] [Google Scholar]
- 13. Karaye KM, Dokainish H, ElSayed A, et al. Clinical profiles and outcomes of heart failure in five African countries: results from INTER-CHF study. Glob Heart. 2021;16(1):50. ( 10.5334/gh.940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elasfar AA, Alhabeeb W, Elasfar S. Heart failure in the Middle East Arab countries: current and future perspectives. J Saudi Heart Assoc. 2020;32(2):236 241. ( 10.37616/2212-5043.1040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hassanein M, Abdelhamid M, Ibrahim B, et al. Clinical characteristics and management of hospitalized and ambulatory patients with heart failure-results from ESC heart failure long-term registry-Egyptian cohort. ESC Heart Fail. 2015;2(3):159 167. ( 10.1002/ehf2.12046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Çavuşoğlu Y, Altay H, Aras D, et al. Cost-of-disease of heart failure in Turkey: A Delphi panel-based analysis of direct and indirect costs. Balkan Med J. 2022;39(4):282 289. ( 10.4274/balkanmedj.galenos.2022.2022-3-97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association | Circulation. 2022. https://www.ahajournals.org/doi/10.1161/CIR.0000000000001052. Accessed September 2, 2022. [DOI] [PubMed] [Google Scholar]
- 18. Sinan ÜY, Ekmekçi A, Özbay B, et al. The real-life data of hospitalized patients with heart failure: on behalf of the journey HF-TR study investigators. Anatol J Cardiol. 2019;21(1):25 30. ( 10.14744/AnatolJCardiol.2018.50880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El Hadidi S, Samir Bazan N, Byrne S, Darweesh E, Bermingham M. Heart failure prescribing quality at discharge from a critical Care Unit in Egypt: the impact of multidisciplinary care. Pharmacy (Basel). 2020;8(3):159. ( 10.3390/pharmacy8030159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abid L, Charfeddine S, Kammoun I, et al. Epidemiology of heart failure and long-term follow-up outcomes in a north-African population: results from the NAtional TUnisian REgistry of Heart Failure (NATURE-HF). PLoS One. 2021;16(5):e0251658. ( 10.1371/journal.pone.0251658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Komajda M, Cowie MR, Tavazzi L, et al. Physicians’ guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail. 2017;19(11):1414 1423. ( 10.1002/ejhf.887) [DOI] [PubMed] [Google Scholar]
- 22. Fonseca C. Diagnosis of heart failure in primary care. Heart Fail Rev. 2006;11(2):95 107. ( 10.1007/s10741-006-9481-0) [DOI] [PubMed] [Google Scholar]
- 23. Ganguli A, Clewell J, Shillington AC. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review. Patient Prefer Adherence. 2016;10:711 725. ( 10.2147/PPA.S101175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbas H, Yehya L, Kurdi M, Karam R. Patients’ knowledge and awareness about patient support programs: a cross-sectional study on Lebanese adults with chronic diseases. Int J Technol Assess Health Care. 2021;37:e34. ( 10.1017/S0266462321000040) [DOI] [PubMed] [Google Scholar]
- 25. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256 262 [PubMed] [Google Scholar]
- 26. Massouh A, Abu Saad Huijer H, Meek P, Skouri H. Determinants of self-care in patients with heart failure: observations from a developing country in the Middle East. J Transcult Nurs. 2020;31(3):294 303. ( 10.1177/1043659619865587) [DOI] [PubMed] [Google Scholar]
- 27. Raffaa HSM, Alasmari BA, Abadi SA, et al. Adherence of heart failure patients to heart failure medications and its determinants in the Aseer region, Southern Saudi Arabia. J Fam Med Prim Care. 2020;9(9):5041 5045. ( 10.4103/jfmpc.jfmpc_904_20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olano-Lizarraga M, Wallström S, Martín-Martín J, Wolf A. Causes, experiences and consequences of the impact of chronic heart failure on the person´s social dimension: a scoping review. Health Soc Care Community. 2022;30(4):e842 e858. ( 10.1111/hsc.13680) [DOI] [PubMed] [Google Scholar]
- 29. Tsabedze N, Kinsey JH, Mpanya D, Mogashoa V, Klug E, Manga P. The prevalence of depression, stress and anxiety symptoms in patients with chronic heart failure. Int J Ment Health Syst. 2021;15(1):44. ( 10.1186/s13033-021-00467-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527 1537. ( 10.1016/j.jacc.2006.06.055) [DOI] [PubMed] [Google Scholar]
- 31. Easton K, Coventry P, Lovell K, Carter LA, Deaton C. Prevalence and measurement of anxiety in samples of patients with heart failure: meta-analysis. J Cardiovasc Nurs. 2016;31(4):367 379. ( 10.1097/JCN.0000000000000265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and anxiety in heart failure: a review. Harv Rev Psychiatry. 2018;26(4):175 184. ( 10.1097/HRP.0000000000000162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dickens C, Cherrington A, Adeyemi I, et al. Characteristics of psychological interventions that improve depression in people with coronary heart disease: a systematic review and meta-regression. Psychosom Med. 2013;75(2):211 221. ( 10.1097/PSY.0b013e31827ac009) [DOI] [PubMed] [Google Scholar]
- 34. Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9(6):360 370. ( 10.1038/nrcardio.2012.45) [DOI] [PubMed] [Google Scholar]
- 35. Tully PJ, Baumeister H. Collaborative care for comorbid depression and coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2015;5(12):e009128. ( 10.1136/bmjopen-2015-009128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang PS, Aguilar-Gaxiola S, Alonso J, et al. Worldwide use of mental health services for anxiety, mood, and substance disorders: results from 17 countries in the WHO world mental health (WMH) surveys. Lancet. 2007;370(9590):841 850. ( 10.1016/S0140-6736(07)61414-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smeulders ESTF, van Haastregt JCM, Ambergen T, et al. Nurse-led self-management group programme for patients with congestive heart failure: randomized controlled trial. J Adv Nurs. 2010;66(7):1487 1499. ( 10.1111/j.1365-2648.2010.05318.x) [DOI] [PubMed] [Google Scholar]
- 38. Johnston M, Foulkes J, Johnston DW, Pollard B, Gudmundsdottir H. Impact on patients and partners of inpatient and extended cardiac counseling and rehabilitation: a controlled trial. Psychosom Med. 1999;61(2):225 233. ( 10.1097/00006842-199903000-00015) [DOI] [PubMed] [Google Scholar]
- 39. Dracup K, Baker DW, Dunbar SB, et al. Management of heart failure. II. Counseling, education, and lifestyle modifications. JAMA. 1994;272(18):1442 1446. ( 10.1001/jama.1994.03520180066037) [DOI] [PubMed] [Google Scholar]
- 40. Powell LH, Calvin JE, Richardson D, et al. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304(12):1331 1338. ( 10.1001/jama.2010.1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Friedman M, Thoresen CE, Gill JJ, et al. Alteration of type A behavior and its effect on cardiac recurrences in post myocardial infarction patients: summary results of the recurrent coronary prevention project. Am Heart J. 1986;112(4):653 665. ( 10.1016/0002-8703(86)90458-8) [DOI] [PubMed] [Google Scholar]
- 42. Mo Y, Chu M, Hu W, Wang H. Association between the nurse-led program with mental health status, quality of life, and heart failure rehospitalization in chronic heart failure patients. Med (Baltim). 2021;100(10):e25052. ( 10.1097/MD.0000000000025052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johansson I, Joseph P, Balasubramanian K, et al. Health-related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation. 2021;143(22):2129 2142. ( 10.1161/CIRCULATIONAHA.120.050850) [DOI] [PubMed] [Google Scholar]
- 44. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20(9):1016 1024. ( 10.1016/s1053-2498(01)00298-4) [DOI] [PubMed] [Google Scholar]
- 45. Freedland KE, Rich MW, Carney RM. Improving quality of life in heart failure. Curr Cardiol Rep. 2021;23(11):159. ( 10.1007/s11886-021-01588-y) [DOI] [PubMed] [Google Scholar]
- 46. Chriss PM, Sheposh J, Carlson B, Riegel B. Predictors of successful heart failure self-care maintenance in the first three months after hospitalization. Heart Lung. 2004;33(6):345 353. ( 10.1016/j.hrtlng.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 47. Lee CS, Tkacs NC, Riegel B. The influence of heart failure self-care on health outcomes: hypothetical cardioprotective mechanisms. J Cardiovasc Nurs. 2009;24(3):179 87; quiz 188. ( 10.1097/JCN.0b013e31819b5419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strömberg A. The crucial role of patient education in heart failure. Eur J Heart Fail. 2005;7(3):363 369. ( 10.1016/j.ejheart.2005.01.002) [DOI] [PubMed] [Google Scholar]
- 49. Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. ( 10.1186/1471-2261-6-43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kutzleb J, Reiner D. The impact of nurse-directed patient education on quality of life and functional capacity in people with heart failure. J Am Acad Nurse Pract. 2006;18(3):116 123. ( 10.1111/j.1745-7599.2006.00107.x) [DOI] [PubMed] [Google Scholar]
- 51. Abbasi A, Najafi Ghezeljeh T, Ashghali Farahani M, Naderi N. Effects of the self-management education program using the multi-method approach and multimedia on the quality of life of patients with chronic heart failure: a non-randomized controlled clinical trial. Contemp Nurse. 2018;54(4-5):409 420. ( 10.1080/10376178.2018.1538705) [DOI] [PubMed] [Google Scholar]
- 52. Wang SP, Lin LC, Lee CM, Wu SC. Effectiveness of a self-care program in improving symptom distress and quality of life in congestive heart failure patients: a preliminary study. J Nurs Res. 2011;19(4):257 266. ( 10.1097/JNR.0b013e318237f08d) [DOI] [PubMed] [Google Scholar]
- 53. Donner Alves F, Correa Souza G, Brunetto S, Schweigert Perry ID, Biolo A. Nutritional orientation, knowledge and quality of diet in heart failure: randomized clinical trial. Nutr Hosp. 2012;27(2):441 448. ( 10.1590/S0212-16112012000200014) [DOI] [PubMed] [Google Scholar]
- 54. Doughty RN, Wright SP, Pearl A, et al. Randomized, controlled trial of integrated heart failure management. The Auckland heart failure management study. Eur Heart J. 2002;23(2):139 146. ( 10.1053/euhj.2001.2712) [DOI] [PubMed] [Google Scholar]
- 55. DeWalt DA, Schillinger D, Ruo B, et al. A multisite randomized trial of a single- versus multi-session literacy sensitive self-care intervention for patients with heart failure. Circulation. 2012;125(23):2854 2862. ( 10.1161/CIRCULATIONAHA.111.081745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Otsu H, Moriyama M. Effectiveness of an educational self-management program for outpatients with chronic heart failure. Jpn J Nurs Sci. 2011;8(2):140 152. ( 10.1111/j.1742-7924.2010.00166.x) [DOI] [PubMed] [Google Scholar]
- 57. Jonkman NH, Westland H, Groenwold RHH, et al. What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. J Card Fail. 2016;22(11):861 871. ( 10.1016/j.cardfail.2016.06.422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42(36):3599 3726. ( 10.1093/eurheartj/ehab368) [DOI] [PubMed] [Google Scholar]
- 59. Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health Aff (Proj Hope). 2009;28(1):179 189. ( 10.1377/hlthaff.28.1.179) [DOI] [PubMed] [Google Scholar]
- 60. McAlister FA, Stewart S, Ferrua S, McMurray JJJV. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810 819. ( 10.1016/j.jacc.2004.05.055) [DOI] [PubMed] [Google Scholar]
- 61. Long L, Mordi IR, Bridges C, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1(1):CD003331. ( 10.1002/14651858.CD003331.pub5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taylor RS, Walker S, Ciani O, et al. Exercise-based cardiac rehabilitation for chronic heart failure: the EXTRAMATCH II individual participant data meta-analysis. Health Technol Assess. 2019;23(25):1 98. ( 10.3310/hta23250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12(7):706 715. ( 10.1093/eurjhf/hfq056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Piotrowicz E, Stepnowska M, Leszczyńska-Iwanicka K, et al. Quality of life in heart failure patients undergoing home-based telerehabilitation versus outpatient rehabilitation--a randomized controlled study. Eur J Cardiovasc Nurs. 2015;14(3):256 263. ( 10.1177/1474515114537023) [DOI] [PubMed] [Google Scholar]
- 65. Brahmbhatt DH, Cowie MR. Remote management of heart failure: an overview of telemonitoring technologies. Card Fail Rev. 2019;5(2):86 92. ( 10.15420/cfr.2019.5.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roccaforte R, Demers C, Baldassarre F, Teo KK, Yusuf S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. Eur J Heart Fail. 2005;7(7):1133 1144. ( 10.1016/j.ejheart.2005.08.005) [DOI] [PubMed] [Google Scholar]
- 67. Clark RA, Inglis SC, McAlister FA, Cleland JGF, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334(7600):942. ( 10.1136/bmj.39156.536968.55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Myers S, Grant RW, Lugn NE, Holbert B, Kvedar JC. Impact of home-based monitoring on the care of patients with congestive heart failure. Home Health Care Manag Pract. 2006;18(6):444 451. ( 10.1177/1084822306289991) [DOI] [Google Scholar]
- 69. Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JGF. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;2015(10):CD007228. ( 10.1002/14651858.CD007228.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pandor A, Gomersall T, Stevens JW, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart (Br Card Soc). 2013;99(23):1717 1726. ( 10.1136/heartjnl-2013-303811) [DOI] [PubMed] [Google Scholar]
- 71. Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. patient ed. 2010;78(3):297 315. ( 10.1016/j.pec.2010.01.016) [DOI] [PubMed] [Google Scholar]
- 72. Smith SG, Curtis LM, Wardle J, Wagner von C, Wolf MS. Skill set or mind set? Associations between health literacy, patient activation and health. PLoS One. 2013;8(9):e74373. ( 10.1371/journal.pone.0074373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Masterson Creber R, Chen T, Wei C, Lee CS. Brief report: patient activation among urban hospitalized patients with heart failure. J Card Fail. 2017;23(11):817 820. ( 10.1016/j.cardfail.2017.08.452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27(5):520 526. ( 10.1007/s11606-011-1931-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Poon BY, Shortell SM, Rodriguez HP. Patient activation as a pathway to shared decision-making for adults with diabetes or cardiovascular disease. J Gen Intern Med. 2020;35(3):732 742. ( 10.1007/s11606-019-05351-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shively MJ, Gardetto NJ, Kodiath MF, et al. Effect of patient activation on self-management in patients with heart failure. J Cardiovasc Nurs. 2013;28(1):20 34. ( 10.1097/JCN.0b013e318239f9f9) [DOI] [PubMed] [Google Scholar]
- 77. Baker DW, DeWalt DA, Schillinger D, et al. The effect of progressive, reinforcing telephone education and counseling vs. brief educational intervention on knowledge, self-care behaviors and heart failure symptoms. J Card Fail. 2011;17(10):789 796. ( 10.1016/j.cardfail.2011.06.374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. González B, Lupón J, Herreros J, et al. Patient’s education by nurse: what we really do achieve? Eur J Cardiovasc Nurs. 2005;4(2):107 111. ( 10.1016/j.ejcnurse.2005.03.006) [DOI] [PubMed] [Google Scholar]
- 79. Young L, Hertzog M, Barnason S. Effects of a home-based activation intervention on self-management adherence and readmission in rural heart failure patients: the PATCH randomized controlled trial. BMC Cardiovasc Disord. 2016;16(1):176. ( 10.1186/s12872-016-0339-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Boyde M, Peters R, New N, Hwang R, Ha T, Korczyk D. Self-care educational intervention to reduce hospitalisations in heart failure: A randomised controlled trial. Eur J Cardiovasc Nurs. 2018;17(2):178 185. ( 10.1177/1474515117727740) [DOI] [PubMed] [Google Scholar]
- 81. Sherwood A, Blumenthal JA, Koch GG, et al. Effects of coping skills training on quality of life, disease biomarkers and clinical outcomes in patients with heart failure: a randomized clinical trial. Circ Heart Fail. 2017;10(1):e003410. ( 10.1161/CIRCHEARTFAILURE.116.003410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition–heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176(3):310 318. ( 10.1001/jamainternmed.2015.7712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yu DSF, Lee DTF, Stewart S, Thompson DR, Choi KC, Yu CM. Effect of nurse-implemented transitional care for Chinese individuals with chronic heart failure in Hong Kong: a randomized controlled trial. J Am Geriatr Soc. 2015;63(8):1583 1593. ( 10.1111/jgs.13533) [DOI] [PubMed] [Google Scholar]
- 84. Kalter-Leibovici O, Freimark D, Freedman LS, et al. Disease management in the treatment of patients with chronic heart failure who have universal access to health care: a randomized controlled trial. BMC Med. 2017;15(1):90. ( 10.1186/s12916-017-0855-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bekelman DB, Plomondon ME, Carey EP, et al. Primary results of the patient-centered disease management (PCDM) for heart failure study: a randomized clinical trial. JAMA Intern Med. 2015;175(5):725 732. ( 10.1001/jamainternmed.2015.0315) [DOI] [PubMed] [Google Scholar]
- 86. Dunbar SB, Clark PC, Quinn C, Gary RA, Kaslow NJ. Family influences on heart failure self-care and outcomes. J Cardiovasc Nurs. 2008;23(3):258 265. ( 10.1097/01.JCN.0000305093.20012.b8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hwang B, Fleischmann KE, Howie-Esquivel J, Stotts NA, Dracup K. Caregiving for patients with heart failure: impact on patients’ families. Am J Crit Care. 2011;20(6):431 441; quiz 442. ( 10.4037/ajcc2011472) [DOI] [PubMed] [Google Scholar]
- 88. Wingham J, Frost J, Britten N, et al. Needs of caregivers in heart failure management: a qualitative study. Chronic Illn. 2015;11(4):304 319. ( 10.1177/1742395315574765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Barnason S, White-Williams C, Rossi LP, et al. Evidence for therapeutic patient education interventions to promote cardiovascular patient self-management: a scientific statement for healthcare professionals from the American Heart association. Circ Cardiovasc Qual Outcomes. 2017;10(6):e000025. ( 10.1161/HCQ.0000000000000025) [DOI] [PubMed] [Google Scholar]
- 90. Alnomasy N. Impact of caregiver support on patient self-care outcomes with heart failure: a systematic review. J Nurs Pract. 2020;3(1):151 161 [Google Scholar]
- 91. Deek H, Chang S, Newton PJ, et al. An evaluation of involving family caregivers in the self-care of heart failure patients on hospital readmission: randomised controlled trial (the FAMILY study). Int J Nurs Stud. 2017;75:101 111. ( 10.1016/j.ijnurstu.2017.07.015) [DOI] [PubMed] [Google Scholar]
- 92. Jeon YH, Kraus SG, Jowsey T, Glasgow NJ. The experience of living with chronic heart failure: a narrative review of qualitative studies. BMC Health Serv Res. 2010;10:77. ( 10.1186/1472-6963-10-77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kollia ZA, Giakoumidakis K, Brokalaki H. The effectiveness of nursing education on clinical outcomes of patients with heart failure: a systematic review. Jundishapur J Chronic Dis Care. 2016;5(2):1 11. ( 10.17795/jjcdc-35881) [DOI] [Google Scholar]
- 94. Shah K, Garg S. Patient advocacy groups: need and opportunity in India. Perspect Clin Res. 2011;2(1):4 7. ( 10.4103/2229-3485.76283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Parry M, Watt-Watson J. Peer support intervention trials for individuals with heart disease: a systematic review. Eur J Cardiovasc Nurs. 2010;9(1):57 67. ( 10.1016/j.ejcnurse.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 96. Ministry Of Health. Lebanon. The national campaign to raise awareness of heart failure. http://www.moph.gov.lb. Accessed March 17, 2022. [Google Scholar]
- 97. Çavuşoğlu Y, Alper AT, Altay H, et al. Natriuretic peptides in clinical practice. Anatol J Cardiol. 2019;21(suppl 1):1 40. ( 10.14744/AnatolJCardiol.2019.55623) [DOI] [PubMed] [Google Scholar]
- 98. Çavuşoğlu Y, Altay H, Cahn A, et al. Sodium glucose co-transporter 2 inhibitors in heart failure therapy. Turk Kardiyol Dern Ars. 2020;48(3):330 354. ( 10.5543/tkda.2020.74332) [DOI] [PubMed] [Google Scholar]
- 99. Altay H, Çavuşoğlu Y, Çelik A, et al. Management of hyperkalemia in heart failure. Turk Kardiyol Dern Ars. 2021;49(suppl1):1 32. ( 10.5543/tkda.2021.S1) [DOI] [PubMed] [Google Scholar]
- 100. Çavuşoğlu Y, Özpelit E, Çelik A, et al. Cardiac amyloidosis: recent advances in the diagnosis and therapy. Turk Kardiyol Dern Ars. 2019;47(suppl 2):1 34. ( 10.5543/tkda.2019.28035) [DOI] [PubMed] [Google Scholar]
- 101. EAPC Country of the Month - Turkey. https://www.escardio.org/Sub-specialty-communities/European-Association-of-Preventive-Cardiology-(EAPC)/Advocacy/Prevention-in-your-country/Country-of-the-Month-Turkey. https://www.escardio.org/Sub-specialty-communities/European-Association-of-Preventive-Cardiology-(EAPC)/Advocacy/Prevention-in-your-country/Country-of-the-Month-Turkey. Accessed July 25, 2023. [Google Scholar]
- 102. Ministry Of Health. Lebanon. The National Campaign to Raise Awareness of Heart Failure. http://www.moph.gov.lb. Accessed March 17, 2022. [Google Scholar]
- 103. Heart disease awareness initiatives on the rise, prevention remains critically important in Egypt. EgyptToday. https://www.egypttoday.com/Article/1/84502/Heart-disease-awareness-initiatives-on-the-rise-prevention-remains-critically. Accessed July 20, 2023. [Google Scholar]
- 104. Jordan implements WHO HEARTS in primary health care to strengthen management of cardiovascular diseases and related risks. World Health Organization - Regional Office for the Eastern Mediterranean. Publisher e. http://www.emro.who.int/jor/jordan-infocus/jordan-implements-who-hearts-in-primary-health-care-to-strengthen-management-of-cardiovascular-diseases-and-related-risks.html. Accessed July 20, 2023. [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a