Abstract
It has long been appreciated that cues from the innate immune system orchestrate downstream adaptive immune responses. Although previous work has focused on the roles of Toll-like receptors in this regard, relatively little is known about how Nod-like receptors instruct adaptive immunity. Here we review the functions of different members of the Nod-like receptor family in orchestrating effector and anamnestic adaptive immune responses. In particular, we address the ways in which inflammasome and non-inflammasome members of this family affect adaptive immunity under various infectious and environmental contexts. Furthermore, we identify several key mechanistic questions that studies in this field have left unaddressed. Our aim is to provide a framework through which immunologists in the adaptive immune field may view their questions through an innate-immune lens and vice-versa.
Keywords: Innate immune system, Adaptive immune system, NOD-like receptors, Inflammasomes, Immune memory
Introduction
The innate immune system encompasses generalized defense mechanisms against pathogens and damaged cells and serves to direct adaptive immunity in vertebrates. Innate immune responses are coordinated by pattern-recognition receptors (PRRs), which are each activated in response to specific microbe- and danger-associated molecular patterns (MAMPs and DAMPs). The existence of PRRs was first proposed in 1989 by Charles Janeway [1]. Since then, dozens of PRRs have been discovered, beginning with Toll, which was described in Drosophila melanogaster as a critical mediator of the antifungal [2] and, later, Gram-positive bacterial defense responses [3]. The human homolog of Toll (now TLR4) was subsequently identified [4], and it was revealed that it initiates a pro-inflammatory immune response to lipopolysaccharide (LPS) [5]. Other sequence homology searches were likewise used to identify NOD-like receptors (NLRs), which are the focus of this review. Leveraging advances in genome sequencing and analyses, 22 human genes and 34 mouse genes encoding NLRs have since been identified [6]. NLRs are characterized by a carboxy-terminal leucine-rich repeat region and a central NACHT (NAIP, CIITA, HET-E, and TP1) domain, whereas amino-terminal domains vary between NLRs (Fig. 1) [6].
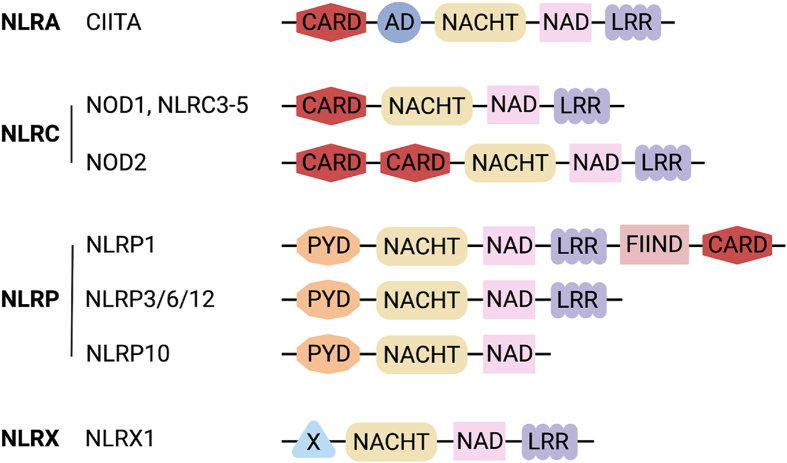
Fig. 1.
Structural schematics of select NLRs. Diagrams of the domain organization of all NLRs discussed in this review, organized into NLR families based on N-terminal domains.
Abbreviations: AD, acidic activation domain; CARD, caspase activating and recruitment domain; FIIND, function to find domain; LRR, leucine-rich repeat; PYD, pyrin domain; NACHT, domain present in NAIP, CIITA, HET-E, and TP-1; NAD, NACHT-associated domain. Created with BioRender.com.
Members of the NLR family of PRRs play diverse roles in innate immunity, from inflammasome formation for the production of IL-1β and IL-18 to NFκB activation (as reviewed in [7]). But beyond their immediate roles in the innate immune system, NLRs also fulfill vital functions in bridging innate-adaptive immunity to prime and shape antigen-specific T and B cell-coordinated responses with the potential to form immunological memory. NLRs have been implicated in the priming of adaptive immunity by orchestrating antigen presentation [8,9]. Furthermore, particular cytokine signals are also required to initiate adaptive immune responses and, crucially, to engage the appropriate effectors in each response; NLRs can indirectly provide some of these signals upon activation, as they potentiate responses from CD8+ T cells, various types of CD4+ T cells, and B cells under various infection and immunization conditions [10]. Herein, we will further discuss the roles of NLRs in orchestrating adaptive immune responses.
Non-inflammasome-forming NLRs
CIITA
The first NLR to be defined was class II transactivator (CIITA), or NLRA. Individuals with a form of severe combined immunodeficiency, now referred to as bare lymphocyte syndrome II, were shown to have a deficiency in MHC II expression that was caused by mutation in coding regions of CIITA [8]. Discovery of this association between CIITA and MHC II expression led to expansive research into the regulation and role of CIITA, including transcriptional control, protein domain features, and binding partners. Here, we will describe some of the known functions of CIITA in adaptive immune regulation [Fig. 2].
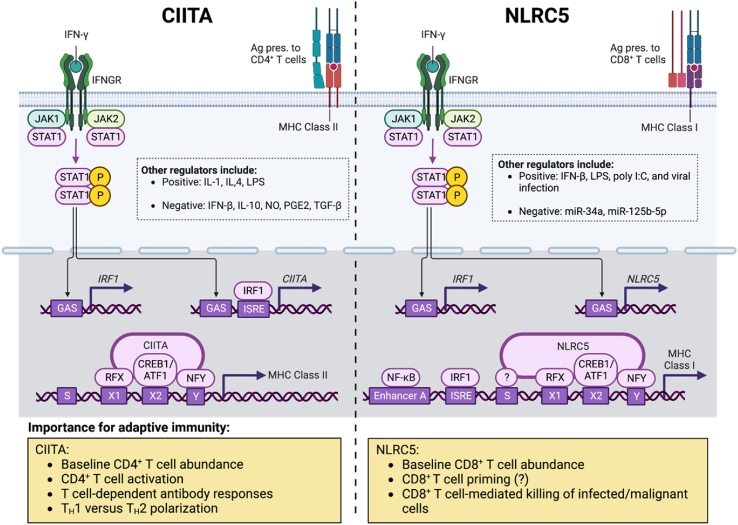
Fig. 2.
Control of MHC class I and II expression by NLRC5 and CIITA. Transcription of both CIITA (left) and NLRC5 (right) is induced by IFN-γ via JAK-STAT signalling and is also regulated by numerous other factors, including those listed in the dotted boxes [99]. CIITA and NLRC5 combine with transcription factors to assemble the CIITA and NLRC5 enhanceosomes, which induce the expression of MHC class II and I genes, respectively [99]. MHC class I and II are required for antigen presentation to CD8+ and CD4+ T cells, as diagrammed; therefore, CIITA and NLRC5 are important in T cell development and the coordination of adaptive immune responses (yellow boxes). Abbreviations: ATF1, activating transcription factor 1; CIITA, class II, major histocompatibility complex, transactivator; CREB1, CAMP responsive element binding protein 1; GAS, IFN-γ-activated site; IFNGR, IFN-γ receptor; IRF1, IFN-regulatory factor 1; ISRE, IFN-stimulated response element; JAK1, Janus kinase 1; JAK2, Janus kinase 2; MHC, major histocompatibility complex; NF-κB, nuclear factor-κB; NFY, nuclear transcription factor Y; NLRC5, NOD-, LRR- and CARD-containing 5; RFX, regulatory factor X; and STAT1, signal transducer and activator of transcription 1. Created with BioRender.com.
Unlike other NLRs, CIITA does not respond to particular MAMPs/DAMPs but rather interacts directly with transcriptional complexes to regulate gene expression [11]. This activity is constitutive in antigen presenting cells (APCs), including immature dendritic cells, macrophages, and B cells, or inducible by IFNγ signalling in hematopoietic and non-hematopoietic cell types [12]. The expression of MHC II on cortical thymic epithelial cells (cTECs) represents the first stage by which CIITA signalling regulates adaptive immunity. Specific deletion of promoter IV (pIV) on the mouse homolog of CIITA depletes expression in cTECs, while retaining expression in other thymic APCs [13,14]. These mice have substantially reduced populations of CD4+ T cells in the thymus and secondary lymphoid organs (SLOs) associated with a loss of positive selection and subsequent survival signals [13,14]. This deficiency also impairs T cell-dependent antibody responses following immunization with hapten-carrier complexes [13,14].
CD4+ T cell interaction with cognate antigen presented on dendritic cells (DCs) and B cells is required for mounting an effective adaptive immune response. Ciita-deficient mice show a loss of MHC II expression on DCs and B cells [15,16], and following immunization with a T cell-dependent antigen, lymph node T cells do not display an increased secondary response to in vitro antigen stimulation as apparent in wild type mice [16]. Deletion of pIII and pIV of Ciita depletes MHC II expression on B cells while maintaining expression in DCs and macrophages. These mice, similar to MHC II knockouts, have a nearly complete suppression of IgG production following hapten-carrier immunization [17]. T helper cell polarization and effector cytokine responses also appear to be influenced by CIITA transcriptional regulation. Naïve CD4+ T cells from CIITA knockout mice crossed with transgenic mice expressing MHC II under MHC I promoter control (reconstitutes the peripheral CD4+ T cell pool) express both IFNγ and IL-4 under Th1 polarizing conditions [18,19]. This response is maintained with subsequent stimulations, and upon re-expression of CIITA through vector transduction, type-2 cytokine levels are reduced [18]. Both Il4 and Gata3 expression are required for this response, demonstrated by a double knockout and RNA targeting, suggesting that these genes are regulated by CIITA [18].
A dysfunctional adaptive immune response can lead to increased susceptibility to pathogens and host disease. Ciita-deficient mice are markedly more susceptible to Mycobacterium tuberculosis and have a minimal IFNγ response during the infection [20]. Interestingly, M. tuberculosis also targets Ciita gene expression, and subsequently MHC II, through a specific cell surface lipoprotein to decrease antigen presentation and T cell activation [21]. Many other pathogens, including HIV [22], herpesviruses [23], and Toxoplasma gondii [24], contain virulence factors that interfere with CIITA signalling, highlighting its critical role in host immunity. Mutations in CIITA are also commonly found in B cell lymphomas, promoting immune evasion through the downregulation of MHC II and resulting changes to the tumor microenvironment (TME) [25,26].
NLRC5
NLRC5 is a master regulator of MHC class I gene transcription in both mice and humans [9,27,28] [Fig. 2]. NLRC5 expression is found primarily in immune cells, particularly those of the lymphoid lineage [[27], [28], [29], [30], [31]]. While NLRC5-deficiency only leads to modest decreases in splenic CD8+ T cells at homeostasis, strikingly, Nlrc5−/− lymphocytes are resistant to killing by cytotoxic T lymphocytes (CTLs), presumably by virtue of decreased surface MHC-I expression [27,28]. In terms of responses to infection, NLRC5-deficient mice are more susceptible to the deleterious effects of intravenous challenge with Listeria monocytogenes due to blunted CD8+ T cell expansion in the spleen and liver [32,33]. Intriguingly, a reduced intestinal antigen-specific CD8+ T cell response was found in NLRC5-deficient mice infected orally with rotavirus [34], indicating that the effects of NLRC5 on effector CTL responses in infection models apply broadly to mucosal tissues and are not restricted to systemic responses.
Despite its well-established role in regulating MHC-I expression and CTL responses, the effect of NLRC5-deficiency on initial T cell priming is somewhat unclear [Fig. 2]. For example, there are findings suggesting a null role for macrophage NLRC5 in CD8+ T cell priming [28]. Furthermore, DCs deficient in NLRC5 have a decreased ability to present endogenous antigen on MHC-I while not impacting CD8+ T cell priming, thereby uncoupling NLRC5 from effector CD8+ T cell activation [35]. However, NLRC5-deficient B cells display impaired antigen-specific stimulation of cognate CD8+ T cells [33], indicating that defects in antigen presentation may lie at the lymphoid rather than myeloid level.
An important consequence of MHC-I-CD8+ T cell interaction is the potential for target-specific lysis by the T cell. It is therefore conceivable that tumors with mutations in the MHC class I presentation machinery may evade CTL-mediated recognition and killing. Indeed, increased NLRC5 expression is positively associated with increased patient survival in melanoma, rectal, bladder, uterine, cervical, and head and neck cancers [36]. Direct evidence implicating NLRC5 in tumor cell killing has corroborated these findings. Indeed, murine melanoma cells stably transfected with NLRC5 display increased surface MHC class I, higher activation of anti-tumor CD8+ T cells, and lower tumor burden [37]. Based on these results, it is reasonable to expect that ectopic expression of NLRC5 in tumors may be an important component of future cancer vaccine formulations.
NOD1 and NOD2
NOD1 and NOD2 are both intracellular sensors of the γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP) bacterial peptidoglycan motifs, respectively [38]. Although both NOD1 and NOD2 have been implicated in the regulation of adaptive immunity, our discussion herein will primarily focus on the role of NOD2 in this regard due to its implication in inflammatory diseases such as Crohn's disease and Blau syndrome [39]. We will nonetheless note the shared roles of both NOD1 and NOD2 in orchestrating similar adaptive immune responses [ Fig. 3].
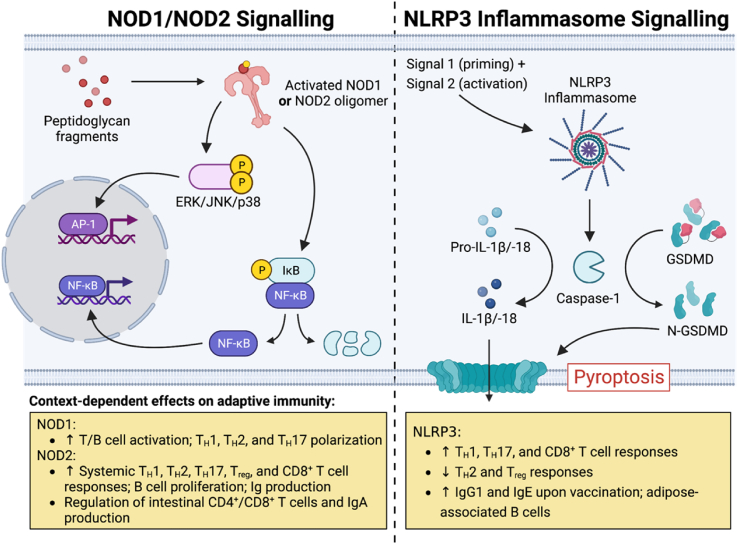
Fig. 3.
Overview of NOD1-, NOD2-, and NLRP3 inflammasome-mediated signalling. Upon activation in response to certain phosphorylated fragments of peptidoglycan, NOD1 and NOD2 trigger pro-inflammatory gene expression programs via NF-κB and mitogen-activated protein kinase pathways (left) [38]. The NLRP3 inflammasome (right) requires priming and then activation in response to signals 1 and 2, respectively [100]. Following inflammasome assembly, autoactivated caspase-1 cleaves (i) the precursors of IL-1β and IL-18, generating their mature forms, and (ii) Gasdermin D, liberating the N-terminal fragment that creates membrane pores and induces pyroptosis, an inflammatory mode of cell death [100]. Both NOD2 and NLRP3 activation have important consequences for adaptive immune responses (yellow boxes), as detailed in the main text. Abbreviations: AP-1, activator protein 1; ERK, extracellular signal-regulated kinase; GSDMD, Gasdermin D; IκB, inhibitor of NF-κB; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; N-GSDMD, N-terminal fragment of Gasdermin D; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; and NOD2, nucleotide-binding oligomerization domain-containing protein 2. Created with BioRender.com.
NOD1 and NOD2 as mediators of extraintestinal immune responses
Early studies of the impact of NOD1 and NOD2 agonists on adaptive immune responses revealed immunostimulatory effects on T cell function [[40], [41], [42]]. However, the exact mechanisms mediating these effects remain unclear. Murine studies administering model antigen intraperitoneally with pure FK156 (NOD1 agonist) and MDP as an adjuvant have implicated myeloid and stromal cell-derived NOD1 and NOD2-mediated signalling as important for Th2 polarization [[43], [44], [45], [46]]. However, the effects of NOD1/2 are unlikely to be restricted to polarization toward type 2 immune responses. In fact, NOD1/2 stimulation is likely to provide a broad adjuvant effect to induce type 1, 2, and 3 adaptive immunity. Indeed, induction of IFNγ+ CD4+, IL-4+ CD4+ and Il-17A secretion in the spleen in response to immunization with adjuvant and model antigen has been shown to be NOD1/2-dependent [43,[46], [47], [48]].
Further evidence pointing to the broad adjuvant effects of NOD1/2 on adaptive immunity are studies showing heightened CD8+ T cell and B cell responses following antigen encounter. For example, in vivo prime-boost immunization experiments with model antigen show NOD1/2-dependent effects on IFNγ and Ig production by memory CD8+ T cells and B cells, respectively [43,46]. Furthermore, NOD1/2 signalling has been shown to impact cross-presentation by DCs, with FK565 and MDP administration leading to greater in vivo CD8+ T cell-mediated target-specific killing [49]. Recently, it has been shown that NOD2 signalling in response to the gut microbiota is an important contributor to immune checkpoint blockade therapy in mice, increasing tumor infiltrating CD8+ T cells and decreasing tumour size [50]. This effect corresponded with broad changes in the myeloid intratumoral compartment [50]. Furthermore, murine infections with Influenza A virus (IAV) have uncovered a role for DC-derived NOD2 signals in mediating antiviral CD8+ T cell responses in the lung [51]. Finally, NOD1/2 is expressed within peripheral B cells and its stimulation along with BCR triggering marginally enhances B cell proliferation [52]. Taken together, it is well-appreciated that NOD1/2-mediated signalling can enhance a range of antigen-specific T cell and B cell responses, highlighting its role as a modulator of adaptive immunity.
NOD2-mediated T cell responses in the intestine
Mutations in NOD2 are the highest genetic risk factor for Crohn's disease development [53]. Therefore, the immunomodulatory effects of NOD2 have focused on its role in the small and large intestines. Although the functions of NOD2 in the intestine have implicated it in numerous pathways relating to intestinal homeostasis, as reviewed elsewhere [38], in this section we will only comment on its role in modulating T cell immunity at this site.
Aberrant CD4+ T cell dynamics are thought to underlie intestinal inflammation in IBD [54] and mutations in NOD2 may perturb intestinal T cell homeostasis prior to the onset of inflammation. In one of the first studies to examine altered intestinal T cell dynamics associated with NOD2 deficiency, WT and NOD2-knockout mice were immunized with OVA/CFA subcutaneously following adoptive transfer of OVA-specific T cells and subsequently challenged intrarectally with a recombinant Escherichia coli expressing OVA. NOD2-deficient mice lost significantly more weight and had overall greater intestinal pathology in an antigen-specific manner. Furthermore, this effect was mediated by increased polarization of colonic CD4+ T cells to an IFNγ-secreting phenotype [55], suggesting a lack of immunoregulation of inflammatory T cells in NOD2-deficient animals. In line with this, defective CD4+ T cell responses in NOD2-deficient mice correlate with increased intestinal permeability and IFNγ levels in their Peyer's patches [56]. These effects are likely dependent on microbial factors as Helicobacter hepaticus infection of NOD2-deficient mice induces Peyer's patch enlargement and an induction of Th1 responses in this compartment [57]. Together, these studies indicate that NOD2 deficiency is linked with increases in inflammatory Th1 cells in both the small intestine and colon. However, other studies examining the role of NOD2 in modulating intestinal T cell dynamics have revealed different, but not necessarily antagonistic, viewpoints. For example, in models of intestinal C. rodentium and S. typhimurium infections, NOD1/2 signalling was found to be important for protective T cell-dependent IL-17 responses [58].
Another potential mechanism by which NOD2 modulates intestinal T cell responses is through the maintenance of established resident T cell populations. Indeed, NOD2-deficient mice display lower levels of intraepithelial lymphocytes (IELs) in the small intestine and colon, due to a defect in intestinal macrophage production of IL-15. As a result, NOD2-deficient mice are more susceptible to 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis, an effect that can be rescued by reconstitution of these mice with additional CD8+ IELs [59]. The idea that NOD2 may mediate protection in the intestine via CD8+ T cells is particularly interesting and has been corroborated by a further study in the context of ɑCD3-induced small intestinal injury. Intraperitoneal administration of ɑCD3 induces ileal inflammation that is partially resolved due to increased trafficking of immunoregulatory IL-10+ CD8+ T cells into the lamina propria. Intriguingly, NOD2-deficient mice had fewer of these immunoregulatory CD8+ T cells traffic to the small intestine [60].
Altogether, these studies implicate NOD2 in orchestrating CD4+ and CD8+ T cell functions in the intestine both during homeostasis and during inflammatory perturbations. However, there are several inconsistencies across studies, perhaps pointing to differences in experimental design and mouse husbandry; specifically, studies often fail to use littermate control mice, a condition where the gut microbiota composition between deficient and sufficient animals is consistent. Indeed, given the broad effects of the microbiota on host health and disease, lack of littermate mice as controls continues to be a significant caveat in many studies [61,62].
Inflammasome-forming NLRs
Inflammasome-forming NLRs respond to a diverse set of infection- or damage-associated stimuli to orchestrate activation of pro-inflammatory caspases. The caspases induced by inflammasome activation include caspase-1, caspase-4/5 (in humans), caspase-8, and caspase-11 (in mice) [63]. Caspase-1 activation is perhaps the most well-studied consequence of inflammasome oligomerization [ Fig. 3]. Active caspase-1 leads to the cleavage of the pro forms of IL-1β, IL-18, and GSDMD into their active forms [63]. Cleavage of GSDMD causes the oligomerization of its N-terminus that then forms pores on the plasma membrane of the cell [63]. This ultimately results in the release of active IL-1β and IL-18 into the extracellular space, where both of these cytokines cause an array of downstream effects, not the least of which is pyroptotic cell death [63]. Active IL-1β and IL-18 also have profound effects on surrounding hematopoietic cells [63]. There is a paucity of literature detailing effects of NLRP1 and NLRP6 on adaptive immunity, therefore, in this section, we will review the ways in which NLRP3 and NLRC4 orchestrate downstream adaptive immune responses.
NLRP3
NLRP3 is the most well-studied inflammasome-forming NLR in the context of adaptive immunity. The post-priming oligomerization of NLRP3 depends on sensing of one of its many activators resulting from infection or cell damage: particulate matter (e.g., uric acid crystals), extracellular ATP, ion fluxes, and reactive oxygen species (ROS) [64]. It has been implicated in orchestrating responses to viral, bacterial, fungal, and protozoan pathogens, as well as in autoimmunity and cancer. Furthermore, NLRP3 may also play an important role in vaccine effectiveness due to its ability to be activated by vaccine components.
The role of NLRP3 in CD8+ T cell responses
The role of NLRP3 in CD8+ T cell responses was first elucidated by early studies of IAV infection in mice. Intranasal infection with IAV induces lung IL-1β release in an NLRP3-dependent manner via intracellular ROS induction [65]. However, the resulting CD4+ and CD8+ virus-specific responses were found to be independent of NLRP3 [66], but possibly requiring ASC and caspase-1, which are key components of the NLRP3 inflammasome [66]. Similarly, although alum-based vaccination activates NLRP3, the antigen-specific CD8+ T cell priming response remained similar between WT and NLRP3-deficient animals [67]. Furthermore, in cutaneous Leishmania braziliensis infection, NLRP3 has been shown to mediate skin pathology, albeit in a CD8+ T cell-independent manner [68]. However, these seemingly null effects of NLRP3 on CD8+ T cell function may be context-dependent. For example, a study of murine West Nile Virus (WNV) infection identified a role for IL-1β and NLRP3 in limiting encephalitis by augmenting antiviral CD8+ T cell immunity [69]. Similarly, a study using the myelin oligodendrocyte glycoprotein (MOG)-induced model of murine experimental autoimmune encephalomyelitis (EAE) showed that NLRP3-deficient mice have decreased neuroinflammation and lower infiltration of CD8+ T cells in their spinal cords [70]. Taken together, it remains a possibility that NLRP3 contributes to CD8+ T cell responses in particular tissues while having minimal effects in others.
In cancer, NLRP3 engagement has been found to mediate both pro- and anti-tumorigenic effects, depending on context. An early study determined that the tumor-specific CD8+ T cell priming response depends on NLRP3 in a mouse model of thymoma [71]. In contrast, NLRP3-deficiency was subsequently shown to increase tumor vaccine efficacy – a response mediated by increased intra-tumoral CD8+ T cell infiltration and decreased myeloid-derived suppressor cell migration in a mouse model of B16–F10 melanoma [72]. Similarly, murine NLRP3-deficiency augments anti-pancreatic ductal adenocarcinoma tumor responses via the reprogramming of intra-tumoral CD8+ T cells to a more immunogenic profile [73]. Increased CD8+ T cell infiltration and effector activity in the TME were further shown upon pharmacologic inhibition of NLRP3 alone [74] or in conjunction with PD-1 blockade [75]. In contrast, more recent studies examining the effects of immune checkpoint antagonists in tumor adaptive immunity found a role for CD39 [76] and TIM-3 [77] in negatively regulating NLRP3 activation, leading to decreased CD8+ T cell responses in the TME of MC38 colon carcinoma-bearing mice. However, further work in elucidating the effects of cancer cell-specific NLRP3 activation is required to bolster the mechanistic understanding of the ways in which NLRP3 inflammasome activation may impact tumor control.
NLRP3 and CD4+ T cell functions
Initial studies regarding the role of NLRP3 in CD4+ T cell function indicated myeloid-expressed NLRP3 is crucial for effective CD4+ cytokine [78] and proliferative [79] responses during T cell priming using model OVA antigen. However, subsequent studies examining the effect of NLRP3 in an IAV intranasal infection model revealed no discernible effect of NLRP3 in the resulting CD4+ T cell responses against IAV, despite a requirement for caspase-1 [66], ASC, and IL-1R signalling [66] in mouse survival. Together, this hints at a requirement for other caspase-1-dependent inflammasomes in the adaptive response against IAV. Given that in addition to TGFβ, IL-6, and IL23, inflammasome-dependent IL-1β drives Th17 polarization, most research on NLRP3 and CD4+ T cell responses focuses on Th17 cells in autoimmunity or non-viral infections. Indeed, in a murine model of Muckle-Wells syndrome where NLRP3 harbors missense mutations leading to its hyperactivity, mice present with spontaneous skin inflammation dependent on IL-17A [80]. In line with this observation, studies of murine EAE implicate NLRP3 in worsened disease progression due to heightened CD4+ T cell infiltration and IL-17 levels in the spinal cords of wild type mice, relative to NLRP3-deficient animals [70].
One of the first studies to examine the effects of NLRP3 in modulating the adaptive response to bacterial infection used a murine model of Bordetella pertussis infection [81]. The authors found that B. pertussis adenylate cyclase toxin induces IL-1β release by dendritic cells and subsequent CD4+ T cell-driven release of IL-17, dependent on NLRP3 and IL-1R, respectively, and this response was important for control of B. pertussis infection. Furthermore, murine immunization with CFA has been shown to induce Th17 polarization partially via NLRP3 [82]. Subsequent work has shown that NLRP3-NLRC4 dual deficient mice mount lower splenic effector Th1 responses following intravenous S. typhimurium infection implicating these two inflammasomes in anti-bacterial Th1 functions [83]. Together, these studies implicate NLRP3 in Th1 and Th17 responses against specific bacterial pathogens.
NLRP3 has also been shown to mediate both innate and adaptive responses to protozoa and helminth infections. During murine footpad Leishmania major infection, NLRP3 allows for greater protozoan burden by promoting Th2 instead of protective Th1 responses [84]. In Schistosoma mansoni helminth infection, liver granuloma size partially depends on NLRP3, which induces immunopathological Th1, Th2, and Th17 responses [85]. Similarly, during murine gastrointestinal infection with Trichuris muris, NLRP3 limits protective Th2 responses and promotes Th1 responses, leading to increased intestinal pathology [86]. Together, these results indicate that the NLRP3 inflammasome may mediate worse disease outcomes during infection with protozoa and helminths.
NAIP-NLRC4
NLR apoptosis inhibitory proteins (NAIPs) recognize intracellular bacterial needle proteins (mouse NAIP1), rod proteins (mouse NAIP2), and flagellin (mouse NAIP5/6). Humans have only one NAIP protein that may be a sensor for all of these ligands. Upon ligand binding, NAIPs activate NLRC4 to form the NAIP-NLRC4 inflammasome complex [87]. Although there has been some research regarding the effects of the NAIP-NLRC4 inflammasome in mediating adaptive immune responses, its role in this regard remains poorly understood. Early studies indicated that NLRC4 is both a potent inducer [88] and repressor [83,89] of T cell-mediated adaptive immunity. Indeed, in the context of different infection models, NLRC4 has been shown to dampen CD4+ and CD8+ T cell function [83]. One of the most convincing demonstrations of this was in a model of L. monocytogenes infection. This bacterium is normally a poor inducer of inflammasome activation, but upon ectopic expression of Legionella pneumophila flagellin, was found to repress expansion of protective antigen-specific CD8+ T cells in an NLRC4-dependent manner [89]. In contrast, the NAIP-NLRC4 inflammasome has been shown to induce robust protective adaptive immune responses when triggered by vaccine adjuvants. Murine vaccination with tumor cells expressing flagellin induces CD4+ and CD8+ T cell mediated protection from subsequent tumor challenge in a process requiring both TLR5 and NAIP-NLRC4 [90]. Furthermore, intranasal administration of a vaccine encoding recombinant vaccinia virus (VACV) and flagellin-OVA conjugate in mice induced robust anti-OVA CD8+ T cell responses in the lungs, spleen, and intestines in an NLRC4-dependent manner, as compared to vaccination with recombinant VACV-OVA alone [91]. In the context of viral infection, NLRC4 is protective in a murine model of IAV infection [92]. NLRC4-deficient mice were found to have increased mortality and an associated decrease in IAV-specific effector CD4+ T cells. Curiously, these effects were independent of NLRC4-inflammasome formation. Overall, although NLRC4 has been described as an activator and repressor of adaptive immune responses to bacterial, fungal, and viral infections, the mechanistic insight into the ways in which NLRC4 is activated in response to non-bacterial microbes remains unclear.
Contributions of other NLRs
Despite previous investigations into the effects of other NLRs, specifically, NLRX1, NLRC3, NLRP10, and NLRP12, on adaptive immune responses, there have been far too few studies to clearly define their functions. Regardless, previous work has identified NLRX1 [93] and NLRC3 [94] as negative regulators of CD4+ T cell functions. NLRP10 was initially thought to be a global regulator of adaptive immunity, with its deficiency leading to generalized defects in T cell and B cell effector functions [95]. However, subsequent studies confirmed that these effects were due to a deficiency in DOCK8 that arose during the generation of the NLRP10-deficient mice [96]. This case highlights, again, the critical need for littermate controls in all experiments involving transgenic mice. Finally, NLRP12 has also been implicated as a negative regulator of CD4+ T cell functions [97]. However, further investigation is needed to understand the precise role of NLRX1, NLRC3, NLRP10, and NLRP12 on adaptive immunity.
Conclusions and outstanding questions
Despite the large body of work on NLR-mediated effects on innate immune responses, there is a surprising paucity of research on their effects on adaptive immunity. Moreover, even among the literature examining the function of NLRs in this regard, there is a lack of research that bridges the depth of understanding brought about by the two “separate” fields of 1) NLRs and 2) adaptive immune responses. To exemplify this, among studies examining the effects of inflammasomes on effector and anamnestic immunity, few bring forward a mechanistic understanding of the requirement of caspases, ASC, and downstream IL-1β and IL-18 for these responses. On the other end of the spectrum, it is appreciated that the extent of IL-2 signalling to T cells may affect their propensity to become memory cells [98]. However, there has been no investigation of the ways in which lone or combinatorial NLR engagement in myeloid cells may, in turn, affect the levels of IL-2R/STAT5 signalling on T cells. Furthermore, perhaps due to technical limitations, the understanding of the ways in which NLR–NLR interactions shape adaptive immune responses is understudied. Examining NLRs using reductionist approaches may lead to incomplete conclusions relating to their physiological functions, as multiple NLRs are likely engaged during infectious contexts. Lastly, there is a need for further work examining the effects of NLRs on adaptive immunity at peripheral tissue sites. Investigating this is especially important given that infections are primarily mucosal or epidermal before spreading systemically. Overall, however, we are hopeful that the field will work to bridge the knowledge gained from the separate studies of NLRs and adaptive immunity. Such research will provide a framework for the design of NLR-targeting therapies in both infectious and auto-inflammatory contexts.
Funding
B.K.T is supported by the Canadian Association of Gastroenterology (CAG) and TRIANGLE Canada PhD student fellowships. C.C is supported by a Natural Sciences and Engineering Research Council of Canada Undergraduate Student Research Award. Work in the Philpott laboratory is supported by the Canadian Institutes for Health Research and the Natural Sciences and Engineering Research Council of Canada.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 3.Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414(6865):756–759. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Geddes K, Magalhães JG., Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8(6):465–479. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 7.Duxbury Z., hang Wu C., Ding P. A comparative Overview of the intracellular Guardians of plants and animals: NLRs in innate immunity and beyond. Annu Rev Plant Biol. 2021;72(1):155–184. doi: 10.1146/annurev-arplant-080620-104948. [DOI] [PubMed] [Google Scholar]
- 8.Steimle V, Otten LA., Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75(1):135–146. [PubMed] [Google Scholar]
- 9.Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, Bayir E, et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci U S A. 2010;107(31):13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227(1):221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 11.León Machado JA, Steimle V. The MHC Class II transactivator CIITA: Not (Quite) the Odd-One-Out Anymore among NLR Proteins. Int J Mol Sci. 2021;22(3):1074. doi: 10.3390/ijms22031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhlethaler-Mottet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16(10):2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldburger JM, Suter T, Fontana A, Acha-Orbea H, Reith W. Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene. J Exp Med. 2001;194(4):393–406. doi: 10.1084/jem.194.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldburger JM, Rossi S, Hollander GA, Rodewald HR, Reith W, Acha-Orbea H. Promoter IV of the class II transactivator gene is essential for positive selection of CD4+ T cells. Blood. 2003;101(9):3550–3559. doi: 10.1182/blood-2002-06-1855. [DOI] [PubMed] [Google Scholar]
- 15.Williams GS, Malin M, Vremec D, Chang CH, Boyd R, Benoist C, et al. Mice lacking the transcription factor CIITA--a second look. Int Immunol. 1998;10(12):1957–1967. doi: 10.1093/intimm/10.12.1957. [DOI] [PubMed] [Google Scholar]
- 16.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4(2):167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 17.LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5(9):899–908. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- 18.Gourley T, Roys S, Lukacs NW, Kunkel SL, Flavell RA, Chang CH. A novel role for the major histocompatibility complex class II transactivator CIITA in the repression of IL-4 production. Immunity. 1999;10(3):377–386. doi: 10.1016/s1074-7613(00)80037-0. [DOI] [PubMed] [Google Scholar]
- 19.Patel DR, Kaplan MH, Chang CH. Altered Th1 cell differentiation programming by CIITA deficiency. J Immunol. 2004;173(9):5501–5508. doi: 10.4049/jimmunol.173.9.5501. [DOI] [PubMed] [Google Scholar]
- 20.Repique CJ, Li A, Brickey WJ, Ting JPY, Collins FM, Morris SL. Susceptibility of mice deficient in the MHC class II transactivator to infection with Mycobacterium tuberculosis. Scand J Immunol. 2003;58(1):15–22. doi: 10.1046/j.1365-3083.2003.01266.x. [DOI] [PubMed] [Google Scholar]
- 21.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J Immunol. 2003;171(1):175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa S, Okamoto T, Peterlin BM. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity. 2000;12(1):61–70. doi: 10.1016/s1074-7613(00)80159-4. [DOI] [PubMed] [Google Scholar]
- 23.Le Roy E, Mühlethaler-Mottet A, Davrinche C, Mach B, Davignon JL. Escape of human cytomegalovirus from HLA-DR-restricted CD4(+) T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J Virol. 1999;73(8):6582–6589. doi: 10.1128/jvi.73.8.6582-6589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lüder CG, Lang C, Giraldo-Velasquez M, Algner M, Gerdes J, Gross U. Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J Neuroimmunol. 2003;134(1–2):12–24. doi: 10.1016/s0165-5728(02)00320-x. [DOI] [PubMed] [Google Scholar]
- 25.Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mottok A, Woolcock B, Chan FC, Tong KM, Chong L, Farinha P, et al. Genomic alterations in CIITA are frequent in primary mediastinal large B cell lymphoma and are associated with diminished MHC class II expression. Cell Rep. 2015;13(7):1418–1431. doi: 10.1016/j.celrep.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Neerincx A, Rodriguez GM, Steimle V, Kufer TA. NLRC5 controls basal MHC class I gene expression in an MHC enhanceosome-dependent manner. J Immunol. 2012;188(10):4940–4950. doi: 10.4049/jimmunol.1103136. [DOI] [PubMed] [Google Scholar]
- 28.Staehli F, Ludigs K, Heinz LX, Seguín-Estévez Q, Ferrero I, Braun M, et al. NLRC5 deficiency selectively impairs MHC class I- dependent lymphocyte killing by cytotoxic T cells. J Immunol. 2012;188(8):3820–3828. doi: 10.4049/jimmunol.1102671. [DOI] [PubMed] [Google Scholar]
- 29.Benkő S, Benko S, Magalhaes JG, Philpott DJ, Girardin SE. NLRC5 limits the activation of inflammatory pathways. J Immunol. 2010;185(3):1681–1691. doi: 10.4049/jimmunol.0903900. [DOI] [PubMed] [Google Scholar]
- 30.Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, et al. NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell. 2010;141(3):483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuenzel S, Till A, Winkler M, Häsler R, Lipinski S, Jung S, et al. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J Immunol. 2010;184(4):1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Wang Y, Chen F, Huang Y, Zhu S, Leng Q, et al. NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res. 2012;22(5):836–847. doi: 10.1038/cr.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas A, Meissner TB, Kawai T, Kobayashi KS. Cutting edge: impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator. J Immunol. 2012;189(2):516–520. doi: 10.4049/jimmunol.1200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun T, Ferrero RL, Girardin SE, Gommerman JL, Philpott DJ. NLRC5 deficiency has a moderate impact on immunodominant CD8+ T-cell responses during rotavirus infection of adult mice. Immunol Cell Biol. 2019;97(6):552–562. doi: 10.1111/imcb.12244. [DOI] [PubMed] [Google Scholar]
- 35.Rota G, Ludigs K, Siegert S, Tardivel A, Morgado L, Reith W, et al. T cell priming by activated Nlrc5-deficient dendritic cells is unaffected despite partially reduced MHC class I levels. J Immunol. 2016;196(7):2939–2946. doi: 10.4049/jimmunol.1502084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshihama S, Roszik J, Downs I, Meissner TB, Vijayan S, Chapuy B., et al. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc Natl Acad Sci USA. 2016;113(21):5999–6004. doi: 10.1073/pnas.1602069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez GM, Bobbala D, Serrano D, Mayhue M, Champagne A, Saucier C, et al. NLRC5 elicits antitumor immunity by enhancing processing and presentation of tumor antigens to CD8+ T lymphocytes. OncoImmunology. 2016;(6):5. doi: 10.1080/2162402X.2016.1151593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee T, Hovingh ES, Foerster EG, Abdel-Nour M, Philpott DJ, Girardin SE. NOD1 and NOD2 in inflammation, immunity and disease. Arch Biochem Biophys. 2019;670:69–81. doi: 10.1016/j.abb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Caso F, Galozzi P, Costa L, Sfriso P, Cantarini L, Punzi L. Autoinflammatory granulomatous diseases: from Blau syndrome and early-onset sarcoidosis to NOD2-mediated disease and Crohn’s disease. RMD Open. 2015;1(1) doi: 10.1136/rmdopen-2015-000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tada H, Aiba S, Shibata KI, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005;73(12):7967–7976. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto M, Germain RN, Chedid L, Benacerraf B. Enhancement of carrier-specific helper T cell function by the synthetic adjuvant, N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP) J Immunol. 1978;120(3):980–982. [PubMed] [Google Scholar]
- 42.Iribe H, Koga T, Onoue K. Production of T cell-activating monokine of Guinea pig macrophages induced by MDP and partial characterization of the monokine. J Immunol. 1982;129(3):1029–1032. [PubMed] [Google Scholar]
- 43.Fritz JH, Bourhis LL, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, et al. Nod1-Mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26(4):445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Magalhaes JG., Lee J, Geddes K, Rubino S, Philpott DJ, Girardin SE. Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands. Eur J Immunol. 2011;41(5):1445–1455. doi: 10.1002/eji.201040827. [DOI] [PubMed] [Google Scholar]
- 45.Magalhaes JG, Rubino SJ, Travassos LH, Bourhis L, Duan W, Sellge G, et al. Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc Natl Acad Sci USA. 2011;108(36):14896–14901. doi: 10.1073/pnas.1015063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magalhaes JG, Fritz JH, Bourhis L., Sellge G, Travassos LH, Selvanantham T, et al. Nod2-Dependent Th2 polarization of antigen-specific immunity. J Immunol. 2008;181(11):7925–7935. doi: 10.4049/jimmunol.181.11.7925. [DOI] [PubMed] [Google Scholar]
- 47.Kim D, Kim YG, Seo SU, Kim DJ, Kamada N, Prescott D, et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med. 2016;22(5):524–530. doi: 10.1038/nm.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D, Kim YM, Kim WU, Park JH, Núñez G, Seo SU. Recognition of the microbiota by Nod2 contributes to the oral adjuvant activity of cholera toxin through the induction of interleukin-1β. Immunology. 2019;158(3):219–229. doi: 10.1111/imm.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asano J, Tada H, Onai N, Sato T, Horie Y, Fujimoto Y, et al. Nucleotide oligomerization binding domain-like receptor signaling enhances dendritic cell-mediated cross-priming in vivo. J Immunol. 2010;184(2):736–745. doi: 10.4049/jimmunol.0900726. [DOI] [PubMed] [Google Scholar]
- 50.Griffin ME, Espinosa J, Becker JL, Luo JD, Carroll TS, Jha JK, et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science. 2021;373(6558):1040–1046. doi: 10.1126/science.abc9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lupfer C, Thomas PG, Kanneganti TD. Nucleotide oligomerization and binding domain 2-dependent dendritic cell activation is necessary for innate immunity and optimal CD8+ T cell responses to influenza A virus infection. J Virol. 2014;88(16):8946–8955. doi: 10.1128/JVI.01110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petterson T, Jendholm J, Månsson A, Bjartell A, Riesbeck K, Cardell LO. Effects of NOD-like receptors in human B lymphocytes and crosstalk between NOD1/NOD2 and Toll-like receptors. J Leukoc Biol. 2011;89(2):177–187. doi: 10.1189/jlb.0210061. [DOI] [PubMed] [Google Scholar]
- 53.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imam T, Park S, Kaplan MH, Olson MR. Effector T helper cell subsets in inflammatory bowel diseases. Front Immunol. 2018;9:1212. doi: 10.3389/fimmu.2018.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25(3):473–485. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Barreau F, Madre C, Meinzer U, Berrebi D, Dussaillant M, Merlin F, et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut. 2010;59(2):207–217. doi: 10.1136/gut.2008.171546. [DOI] [PubMed] [Google Scholar]
- 57.Biswas A, Liu YJ, Hao L, Mizoguchi A, Salzman NH, Bevins CL, et al. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci USA. 2010;107(33):14739–14744. doi: 10.1073/pnas.1003363107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17(7):837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 59.Jiang W, Wang X, Zeng B, Liu L, Tardivel A, Wei H, et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med. 2013;210(11):2465–2476. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Lahiri A, Haines GK, Flavell RA, Abraham C. NOD2 regulates CXCR3-dependent CD8+ T cell accumulation in intestinal tissues with acute injury. J Immunol. 2014;192(7):3409–3418. doi: 10.4049/jimmunol.1302436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robertson SJ, Lemire P, Maughan H, Goethel A, Turpin W, Bedrani L, et al. Comparison of Co-housing and littermate methods for microbiota standardization in mouse models. Cell Rep. 2019;27(6):1910–1919.e2. doi: 10.1016/j.celrep.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 62.Stappenbeck TS, Virgin HW. Accounting for reciprocal host–microbiome interactions in experimental science. Nature. 2016;534(7606):191–199. doi: 10.1038/nature18285. [DOI] [PubMed] [Google Scholar]
- 63.Deets KA, Vance RE. Inflammasomes and adaptive immune responses. Nat Immunol. 2021;22(4):412–422. doi: 10.1038/s41590-021-00869-6. [DOI] [PubMed] [Google Scholar]
- 64.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206(1):79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKee AS, Munks MW, MacLeod MK., Fleenor CJ, Van Rooijen N, Kappler JW, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183(7):4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Novais FO, Carvalho AM, Clark ML, Carvalho LP, Beiting DP, Brodsky IE, et al. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, et al. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8(11) doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185(2):974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 72.van Deventer HW, Burgents JE, Wu QP, Woodford RM, Brickey WJ, Allen IC, et al. The inflammasome component Nlrp3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 2010;70(24):10161–10169. doi: 10.1158/0008-5472.CAN-10-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daley D, Mani VR, Mohan N, Akkad N, Pandian GSDB, Savadkar S, et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214(6):1711–1724. doi: 10.1084/jem.20161707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu F, Zhao Y, Pang Y, Ji M, Sun Y, Wang H, et al. NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett. 2021;497:178–189. doi: 10.1016/j.canlet.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 75.Tengesdal IW, Menon DR, Osborne DG, Neff CP, Powers NE, Gamboni F, et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A. 2021;118(10) doi: 10.1073/pnas.2000915118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, et al. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov. 2019;9(12):1754–1773. doi: 10.1158/2159-8290.CD-19-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D, et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature. 2021;595(7865):101–106. doi: 10.1038/s41586-021-03626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181(6):3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 80.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30(6):860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, et al. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185(3):1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 82.Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol. 2013;190(11):5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tourlomousis P, Wright JA, Bittante AS, Hopkins LJ, Webster SJ, Bryant OJ, et al. Modifying bacterial flagellin to evade Nod-like Receptor CARD 4 recognition enhances protective immunity against Salmonella. Nat Microbiol. 2020;5(12):1588–1597. doi: 10.1038/s41564-020-00801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gurung P, Karki R, Vogel P, Watanabe M, Bix M, Lamkanfi M, et al. An NLRP3 inflammasome–triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest. 2015;125(3):1329–1338. doi: 10.1172/JCI79526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci USA. 2010;107(47):20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alhallaf R, Agha Z, Miller CM, Robertson AAB, Sotillo J, Croese J, et al. The NLRP3 inflammasome suppresses protective immunity to gastrointestinal helminth infection. Cell Rep. 2018;23(4):1085–1098. doi: 10.1016/j.celrep.2018.03.097. [DOI] [PubMed] [Google Scholar]
- 87.Paidimuddala B, Cao J, Nash G, Xie Q, Wu H, Zhang L. Mechanism of NAIP—NLRC4 inflammasome activation revealed by cryo-EM structure of unliganded NAIP5. Nat Struct Mol Biol. 2023;30(2):159–166. doi: 10.1038/s41594-022-00889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nyström S, Bråve A, Falkeborn T, Devito C, Rissiek B, Johansson DX, et al. DNA-encoded flagellin activates toll-like receptor 5 (TLR5), Nod-like receptor family CARD domain-containing protein 4 (NRLC4), and acts as an epidermal, systemic, and mucosal-adjuvant. Vaccines. 2013;1(4):415–443. doi: 10.3390/vaccines1040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B., Lauer P, et al. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc Natl Acad Sci USA. 2011;108(30):12419–12424. doi: 10.1073/pnas.1019041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of Toll- and Nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med. 2012;4(120):120ra16. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 91.Sanos SL, Kassub R, Testori M, Geiger M, Pätzold J, Giessel R, et al. NLRC4 inflammasome-driven immunogenicity of a recombinant MVA mucosal vaccine encoding flagellin. Front Immunol. 2018;8:1988. doi: 10.3389/fimmu.2017.01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hornick EE, Dagvadorj J, Zacharias ZR, Miller AM, Langlois RA, Chen P, et al. Dendritic cell NLRC4 regulates influenza A virus–specific CD4+ T cell responses through FasL expression. J Clin Invest. 2019;129(7):2888–2897. doi: 10.1172/JCI124937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J Clin Invest. 2020;130(4):1635–1652. doi: 10.1172/JCI129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uchimura T, Oyama Y, Deng M, Guo H, Wilson JE, Rampanelli E, et al. The innate immune sensor NLRC3 acts as a rheostat that fine-tunes T cell responses in infection and autoimmunity. Immunity. 2018;49(6):1049–1061.e6. doi: 10.1016/j.immuni.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eisenbarth SC, Williams A, Colegio OR, Meng H, Strowig T, Rongvaux A, et al. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature. 2012;484(7395):510–513. doi: 10.1038/nature11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krishnaswamy JK, Singh A, Gowthaman U, Wu R, Gorrepati P, Sales Nascimento M, et al. Coincidental loss of DOCK8 function in NLRP10-deficient and C3H/HeJ mice results in defective dendritic cell migration. Proc Natl Acad Sci USA. 2015;112(10):3056–3061. doi: 10.1073/pnas.1501554112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prado DS, Veras FP, Ferreira RG, Damasceno LEA, Melo PH, Zamboni DS, et al. NLRP12 controls arthritis severity by acting as a checkpoint inhibitor of Th17 cell differentiation. Faseb J. 2020;34(8):10907–10919. doi: 10.1096/fj.202000795R. [DOI] [PubMed] [Google Scholar]
- 98.Osum KC, Jenkins MK. Toward a general model of CD4+ T cell subset specification and memory cell formation. Immunity. 2023;56(3):475–484. doi: 10.1016/j.immuni.2023.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shukla A, Cloutier M, Appiya Santharam M, Ramanathan S, Ilangumaran S. The MHC class-I transactivator NLRC5: implications to cancer immunology and potential applications to cancer immunotherapy. Int J Mol Sci. 2021;22(4):1964. doi: 10.3390/ijms22041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]