Abstract
The field of nuclear medicine is entering a new era of gamma-camera technology. Solid-state SPECT/CT systems will gradually replace the thallium-activated sodium-iodide NaI(Tl) systems. This digital technology allows drastic improvements in image quality, radiotracer dose reduction, and procedure efficiency. This pictorial review presents our initial experience on an NM/CT 870 CZT system (GE Healthcare), equipped with dual-head cadmium zinc telluride (CZT) detectors, for I-123 metaiodobenzylguanidine (MIBG) imaging in pediatric neuroblastoma. On planar imaging, CZT shows greater image quality than at conventional gamma-camera using the Infinia Hawkeye (GE Healthcare). Physiologic structures such as salivary glands and myocardium show sharper borders with a more notable signal-to-noise ratio at CZT than conventional gamma camera. On SPECT imaging, the CZT scanner, combined with resolution recovery, demonstrates either comparable or greater image quality at 80% of the conventional gamma camera’s acquisition time. Due to the 2.46-mm detector pixel with fully registered collimator holes matching each pixel and direct conversion of photons into electrical signals, the CZT gamma camera system provides significant advantages in photon localization and energy resolution.
Keywords: Neuroblastoma, I-123 MIBG, Gamma-camera, Cadmium zinc telluride detector
Introduction
Neuroblastoma, which constitutes approximately one-third of all infant malignancies, is the prevalent solid tumor found outside the brain in children [1, 2]. The majority of neuroblastomas are diagnosed during infancy and young childhood. The primary tumors can appear at different locations along the sympathetic chain in the trunk; nevertheless, they tend to arise in the abdomen, with the adrenal glands being the most common site of development (65–70%) [1, 3]. Thoracic primaries account for approximately 20% of neuroblastomas and are typically seen in infants [3]. Patients who have been diagnosed with low and intermediate-risk neuroblastoma have greater than 90% overall survival rate [4]. For high-risk patients, however, the long-term survival remains low at approximately 40% despite options for treatment intensification and immunotherapy [4].
I-123 metaiodobenzylguanidine (MIBG) scintigraphy is the preferred functional imaging modality for diagnosing, staging, and restaging neuroblastoma. Neuroblastomas show increased MIBG uptake in 90% of cases, allowing for detecting both the primary and metastatic disease with a sensitivity of 88–93% and specificity of 83–92% [5–8]. In addition, lesion detection and characterization can be improved beyond planar imaging when acquired with single photon emission computed tomography (SPECT), or SPECT/CT [6, 9, 10]. Recently, gamma-cameras based on cadmium-zinc-telluride (CZT) semiconductors have been introduced in clinical practice. They provide the advantages of shorter imaging time associated with greater system sensitivity and enhanced energy resolution as well as spatial resolution compared with thallium-activated sodium-iodide (NaI(Tl)) gamma-cameras [11–15]. However, although diagnostic capabilities of CZT gamma-cameras for Tc-99 m-based isotopes are well documented in the literature, clinical images on I-123 MIBG are limited, particularly in pediatric neuroblastoma [11, 16–21]. In this pictorial review, we showcase our initial clinical experience on an NM/CT 870 CZT scanner (GE Healthcare), a state-of-the-art SPECT/CT system with dual-head CZT detectors, for I-123 MIBG imaging in pediatric neuroblastoma.
Pictorial Review
For all clinical cases of this pictorial review, the dosage of administered I-123 MIBG is provided in MBq, and imaging was obtained 24 h after the radiotracer administration (Table 1 and Figs. 1, 2, 3, 4, and 5).
Table 1.
Scanner specifications, acquisition, and post-processing parameters. CZT scanning parameters were developed in collaboration with GE Healthcare in Haifa, Israel. WEHR, wide-energy high-resolution (2.26-mm hole diameter, 0.2-mm septal thickness, and 24.05-mm hole length); ELEGP, extended low-energy general-purpose (2.5-mm hole diameter, 0.4-mm septal thickness, and 40-mm hole length)
| GE 870 CZT | GE Infinia Hawkeye | ||
|---|---|---|---|
| System resolution, FWHM at 10 cm (mm) | 7.6 | 10.3 | |
| Energy resolution (keV) | 6.3 | 9.8 | |
| Radioactivity (MBq/kg weight) | 5.2 | 5.2 | |
| Collimator | WEHR | ELEGP | |
| Software package for processing | Q.VMI evolution for oncology | Evolution toolkit | |
| Planar | |||
| Energy (keV) + / − window (%) | 159 ± 10 | 159 ± 10 | |
| Whole-body, planar | Matrix | 256 × 1024 | 256 × 1024 |
| Mode | Continuous | Continuous | |
| Zoom | 1 | 1 | |
| Speed (cm/min) | 6 | 6 | |
| Static, planar | Matrix | 256 × 256 | 256 × 256 |
| Scan time (min) | 6 | 10 | |
| High-energy scatter correction | Yes | Not available | |
| 2D Clarity | No | Not available | |
| SPECT | |||
| Energy (keV) + / − window (%) | 159 − 10/ + 5 | 159 ± 10 | |
| Matrix | 128 × 128 | 128 × 128 | |
| Angular step (degrees) | 6 | 6 | |
| Mode | Step and shoot | Step and shoot | |
| Zoom | 1 | 1 | |
| Time/stop (sec/stop) | 40 | 50 | |
| Acquisition time (min) | 20 | 25 | |
| Post-processing | 4 iterations; 6 subsets; 1.5 post Gauss filter | 2 iterations; 10 subsets; 0.48 Butterworth filter | |
| Resolution recovery | Yes | No | |
| CT attenuation correction | Yes | Yes | |
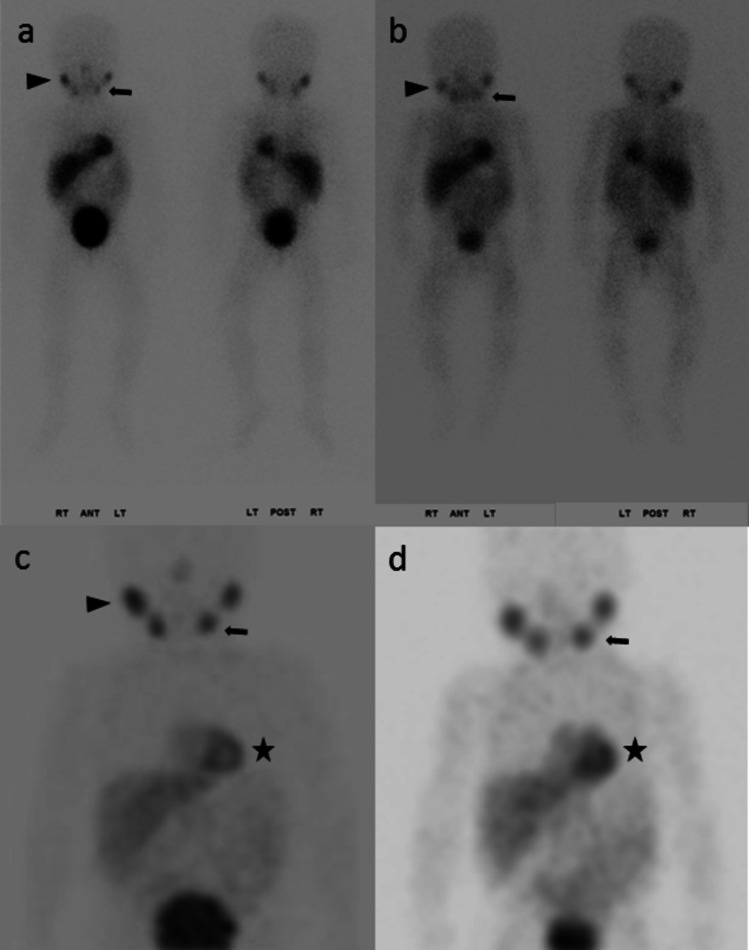
Fig. 1.
17-month-old girl, stage IV neuroblastoma, post-chemotherapy. MIBG scans at CZT (85 MBq) and conventional gamma camera 6 months earlier (81 MBq) were negative for tumors. However, physiologic structures, e.g., submandibular glands (arrow), parotid glands (arrowhead), and myocardium (star), were better delineated on CZT than on conventional camera. In the anterior planar image, the right parotid gland-to-background count ratio was 4.83 at CZT and 2.85 at conventional camera. Similarly, the count ratio for the left submandibular gland was 3.00 at CZT and 2.68 at conventional camera. Consistent region-of-interest sizes were employed for the parotid and submandibular glands, as well as for the background in the left frontal skull. The enhanced image quality is attributed to the intrinsically better spatial resolution associated with registered collimation, the greater energy resolution, and the high-energy scatter correction at the CZT scanner, which is unavailable on the conventional camera [12]. In addition, the narrower SPECT energy window provided by the CZT scanner contributes to the enhanced image quality of CZT relative to conventional camera, Table 1. Planar—a CZT; b conventional; maximum intensity projection (MIP)—c CZT; d conventional
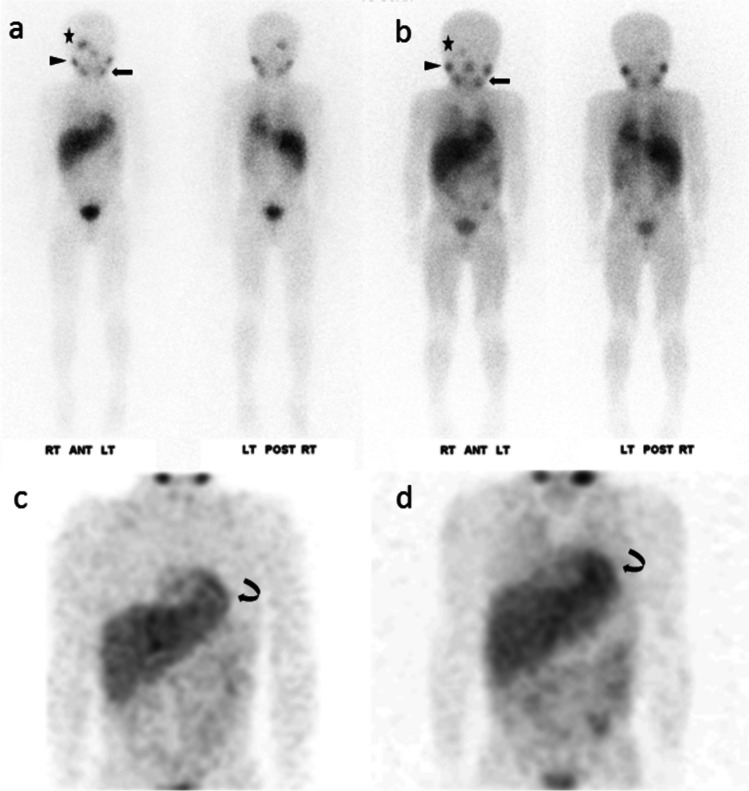
Fig. 2.
6-year-old boy, stage IV, n-Myc non-amplified neuroblastoma. CZT (130 MBq) demonstrated a focal lesion in the anterior right temporal lobe (star), positive for relapsed neuroblastoma at surgical resection. This lesion appeared smaller and less conspicuous five months earlier at conventional camera (141 MBq). The lesion-to-background count ratio was 3.71 at CZT and 1.55 at conventional camera based on the anterior planar image. The count ratio for the right parotid gland (arrowhead) was 3.31 at CZT and 2.67 at conventional camera. For the left mandibular gland (arrow), the count ratio was 3.37 at CZT and 2.79 at conventional camera. Consistent region-of-interest sizes were used for all measurements. On MIP, the outline of the myocardium (curved arrow) was more conspicuous and sharper at CZT than at conventional camera, and there was less background activity/noise. Planar—a CZT; b conventional; MIP—c CZT; d conventional
Fig. 3.
18-month-old girl with newly diagnosed low-risk neuroblastoma, with MIBG scan performed on the CZT (95 MBq). The 2-cm left paraspinal thoracic primary showed mild MIBG uptake, best seen on SPECT/CT (arrow). In addition, a 1.6-cm left supraclavicular lymph node showed moderate uptake (arrowhead). The scan was negative for bone marrow disease, confirmed by histopathology. a planar; b MIP; c and d axial SPECT and fused SPECT/CT
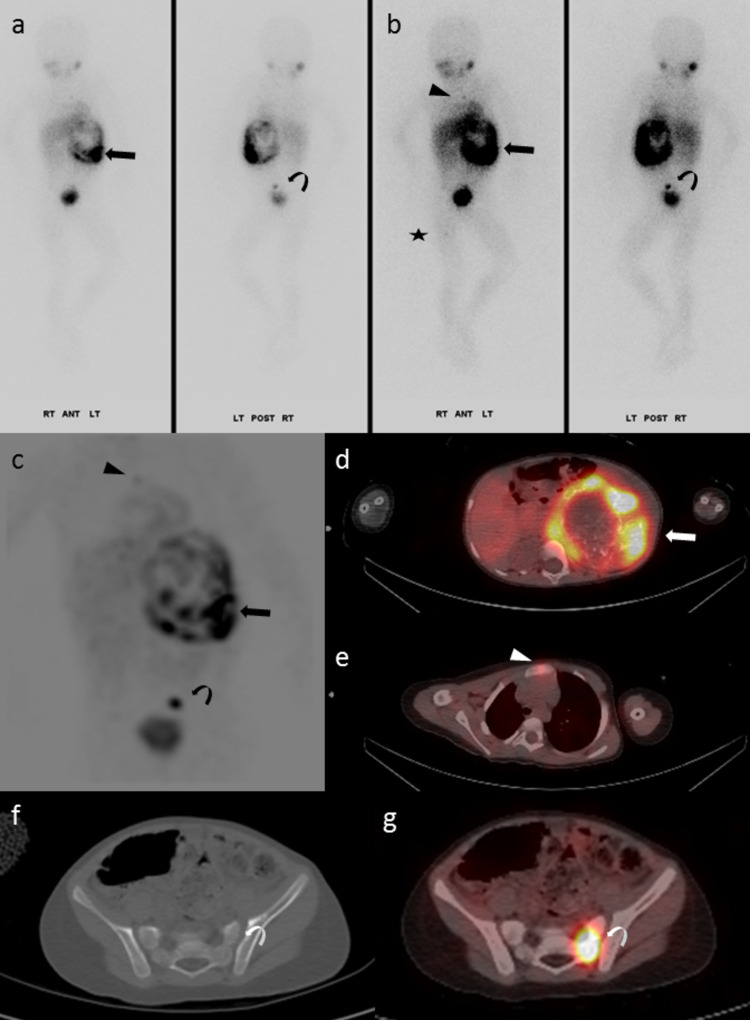
Fig. 4.
T3-year-old girl with newly diagnosed, n-Myc non-amplified neuroblastoma. The left retroperitoneal primary (arrow) showed heterogeneously increased MIBG uptake on CZT (104 MBq). In addition, there were multiple foci of bone marrow uptake, including the sternum (arrowhead), left sacrum (curved arrow), and proximal right femur (star). Planar—a low-intensity display; b high-intensity display; MIP—c; SPECT/CT—d, e, and g. The bone marrow lesions showed no structural changes on CT, including the left sacral lesion, f
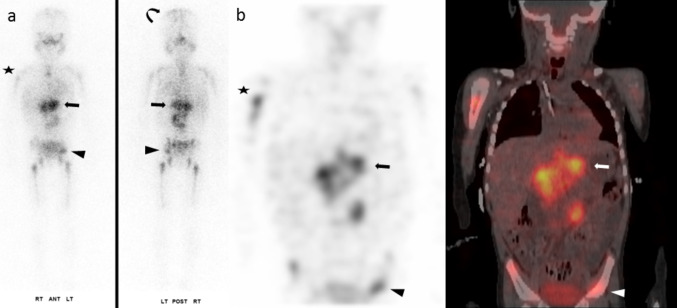
Fig. 5.
3-year-old girl with newly diagnosed poorly differentiated neuroblastoma. The 9 × 7-cm left retroperitoneal primary (arrow) displayed heterogeneous intense MIBG uptake (107 MBq). Widespread bone marrow disease included the bilateral humeri (star), pelvis (arrowhead), and calvarium (curved arrow), among others. Planar—a; SPECT and SPECT/CT, coronal—b
Discussion
Nuclear medicine technology has made significant advancements in the past decade, particularly in the area of digital imaging detectors for gamma-cameras and positron-emission tomography scanners. One of the most significant advancements in gamma-camera technology was the introduction of digital CZT detectors [22–24]. These detectors are lighter and more compact than analog photomultiplier-tube (PMT) detectors and have already gained popularity in cardiac imaging [25–27]. As CZT gamma-cameras do not require PMTs, they are more compact and have less dead space [11, 13]. Solid-state detectors are associated with reduced signal loss and noise, which are inherent to conventional PMT detectors due to the process of scintillation and light conversion to electrons within the PMT [11]. As a result, CZT detectors provide increased energy resolution, ranging from 5.4 to 6.3%, compared to 9% to 10% with NaI(Tl) detectors measured at 140 keV [11–13, 23, 24, 28].
One major advantage of CZT detectors is their ability to determine the location of the photons with high certainty by pixelating the detectors into small tiles instead of aligning large PMTs along the NaI(Tl) scintillation material. In 2016, GE Healthcare debuted the Discovery NM/CT 670 CZT system—the first commercial dual-head SPECT/CZT system for general-purpose imaging producing high-quality images for low-energy and medium-energy radiopharmaceuticals [19, 20, 29]. NM/CT 870 CZT (GE Healthcare) is a third-generation, state-of-the-art SPECT/CT system, with a dual-head gamma-camera and CZT detectors [12, 13]. This CZT gamma camera was designed for general-purpose imaging using low-energy and medium-energy isotopes with photopeak between 40 and 300 keV [12, 13]. This scanner is equipped with a propriety wide-energy, high-resolution (WEHR) collimator (2.26-mm hole diameter, 0.2-mm septal thickness, and 24.05-mm hole length) optimized for lower photopeaks such as Tc-99 m, Tl-201, I-123, Xe-133, and Lu-177 isotopes. Additionally, a medium-energy, high-resolution sensitivity (MEHRS) collimator (2.8-mm hole diameter, 0.9-mm septal thickness, and 40.25-mm hole length) is also available, which is more suitable for medium energy such as In-111 isotope [12, 30].
Each CZT detector head has a FOV of 39.4 × 51.2 cm and is equipped with 130 CZT detector modules measuring 39.36 × 39.36 × 7.25 mm. Each module consists of 16 × 16 detector pixels [13]. The 2.46-mm pixel is designed to match with a single collimator opening, resulting in an intrinsic spatial resolution is 2.46 mm, the same as the pixel size of individual detector pixels. The system sensitivity has been measured at 78–85 counts per second per megabecquerel (cps/MBq) at a distance of 100-mm per detector head for Tc-99 m. This value is similar to or slightly lower than some of the latest NaI(Tl) systems [12, 13]. Due to the registered collimator design, the CZT gamma camera shows a system spatial resolution of 6.3–7.9 mm for planar imaging of Tc-99 m isotope, compared with 7.2–7.9 mm for the conventional NaI(Tl) gamma-camera such as the E.cam (Siemens Healthcare). For I-123 isotope, the CZT system demonstrates similar or better sensitivity (117 cps/MBq vs. 116 cps/MBq) and spatial resolution (11.5–11.6 mm vs. 13.1–13.2 mm) than the analog system [12, 13]. Most importantly, the CZT system has a 5.6–6.3% energy resolution at 140 keV, improved from 9–10% on analog NaI(Tl) systems. This represents an approximately 1.7-fold improvement in energy resolution [11–13]. At 159 keV (photopeak of I-123), there is also a slight improvement in energy resolution at 9.1% (using WEHR collimator) compared to 10.4%. Diagnostic I-123 MIBG imaging can be challenging in young children with neuroblastoma, mainly due to low count statistics and low-dose administration (typically 5.2 MBq/kg body weight) [31]. The technical advantages of CZT detectors could prove beneficial in addressing these challenges.
The improved imaging properties of CZT detectors can reduce the acquisition time for a given administered radiotracer dose or improve count statistics and image quality. Alternatively, they can allow for a reduction in radiotracer dose while maintaining image quality for a given acquisition time. For instance, in one study, reducing the dosage from 4 MBq to 2.5 MBq per kilogram of body weight decreased the total effective radiation dose from 9.3 mSv to 5.8 mSv while preserving image quality [32]. The enhanced energy resolution with CZT detectors is also advantageous for dual-isotope imaging, such as simultaneous imaging of Tc-99 m (140 keV) and I-123 (159 keV) isotopes [11, 20, 33]. CZT detectors’ superior energy resolution allows for the use of narrower energy windows, resulting in better discrimination of different radiotracers’ individual energy peaks and reduced photon down-scatter from higher to lower energy peaks. In contrast to PMT detectors, CZT detectors can separate photopeak differences by a few keV (< 20 keV) instead of 20–80 keV [34]. As a result, the CZT gamma camera provides a better separation of the small peak difference (19 keV) between Tc-99 m and I-123 isotopes, leading to improved image quality [12]. CZT detectors are well suited for simultaneous imaging with Tc-99 m MDP and I-123 MIBG, especially when about 10% of neuroblastoma lesions are not I-123 MIBG avid [5, 35]. This approach could potentially enhance the detection and characterization of skeletal lesions, contributing to better diagnosis and patient management in both initial and subsequent treatment strategies.
Conclusion
The presented CZT gamma camera exhibits favorable imaging characteristics for low-energy and medium-energy isotopes, thereby improving the diagnostic confidence and accuracy of I-123 MIBG imaging for neuroblastomas compared with an analog PMT system. In addition, the system provides the opportunity for simultaneous imaging with Tc-99 m MDP bone scintigraphy with I-123 MIBG, potentially enhancing the detection and characterization of skeletal lesions. However, further clinical research is needed to evaluate this diagnostic potential. Undoubtedly, digital CZT systems will gradually replace the antiquated and bulky PMT gamma-cameras that have been the industry standard for more than 50 years.
Acknowledgements
We thank Bella Yuzefovich and Shira Klang at GE Healthcare in Haifa, Israel, for their technical assistance and collaboration in developing acquisition and postprocessing parameters.
Author Contribution
Conceptualization—NCN and AM; writing—original draft preparation, CS; resources—NS, JNS, MS, and ES; writing—review and editing—NCN, NS, ES, and AM; supervision—NCN. All authors read and approved the final manuscript.
Data Availability
Not applicable.
Compliance with Ethical Standards
Conflict of Interest
Natalie Shmuel is a clinical applications engineer, and Eli Stern is a research manager, both at GE Healthcare, Haifa, Israel. Cassidy Sweet, Jennifer N. Shoaf, Marcy Stoecklein, Ashok Muthukrishnan, and Nghi C. Nguyen declare that they have no conflict of interest.
Ethics Approval
Not applicable, as this is a pictorial review that is deemed exempt by the University of Pittsburgh Institutional Review Board.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Spix C, Pastore G, Sankila R, Stiller CA, Steliarova-Foucher E. Neuroblastoma incidence and survival in European children (1978–1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2081–2091. doi: 10.1016/j.ejca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: Radiologic-pathologic correlation. Radiographics. 2002;22:911–934. doi: 10.1148/radiographics.22.4.g02jl15911. [DOI] [PubMed] [Google Scholar]
- 4.Park JR, Eggert A, Caron H. Neuroblastoma: Biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24:65–86. doi: 10.1016/j.hoc.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Brisse HJ, McCarville MB, Granata C, Krug KB, Wootton-Gorges SL, Kanegawa K, et al. Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the International Neuroblastoma Risk Group Project. Radiology. 2011;261:243–257. doi: 10.1148/radiol.11101352. [DOI] [PubMed] [Google Scholar]
- 6.Vik TA, Pfluger T, Kadota R, Castel V, Tulchinsky M, Farto JC, et al. (123)I-mIBG scintigraphy in patients with known or suspected neuroblastoma: Results from a prospective multicenter trial. Pediatr Blood Cancer. 2009;52:784–790. doi: 10.1002/pbc.21932. [DOI] [PubMed] [Google Scholar]
- 7.Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer. 2010;102:1319–1326. doi: 10.1038/sj.bjc.6605621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decarolis B, Schneider C, Hero B, Simon T, Volland R, Roels F, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: Results of the Cologne interscore comparison study. J Clin Oncol. 2013;31:944–951. doi: 10.1200/JCO.2012.45.8794. [DOI] [PubMed] [Google Scholar]
- 9.Sharp SE, Trout AT, Weiss BD, Gelfand MJ. MIBG in neuroblastoma diagnostic imaging and therapy. Radiographics. 2016;36:258–278. doi: 10.1148/rg.2016150099. [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka M, Taki J, Mochizuki T, Kinuya S. Comparison of diagnostic value of I-123 MIBG and high-dose I-131 MIBG scintigraphy including incremental value of SPECT/CT over planar image in patients with malignant pheochromocytoma/paraganglioma and neuroblastoma. Clin Nucl Med. 2011;36:1–7. doi: 10.1097/RLU.0b013e3181feeb5e. [DOI] [PubMed] [Google Scholar]
- 11.Desmonts C, Bouthiba MA, Enilorac B, Nganoa C, Agostini D, Aide N. Evaluation of a new multipurpose whole-body CzT-based camera: Comparison with a dual-head Anger camera and first clinical images. EJNMMI Phys. 2020;7:18. doi: 10.1186/s40658-020-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Matsusaka Y, Onoguchi M, Ichikawa H, Okuda K, Shibutani T, et al. Experimental evaluation of the GE NM/CT 870 CZT clinical SPECT system equipped with WEHR and MEHRS collimator. J Appl Clin Med Phys. 2021;22:165–177. doi: 10.1002/acm2.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NM/CT 870 CZT. A Digital SPECT/CT. Product data sheet. DOC2109131 Rev. 4. GE Healthcare; 2023.
- 14.Ben-Haim S, Kennedy J, Keidar Z. Novel cadmium zinc telluride devices for myocardial perfusion imaging-Technological aspects and clinical applications. Semin Nucl Med. 2016;46:273–285. doi: 10.1053/j.semnuclmed.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Garcia EV, Faber TL, Esteves FP. Cardiac dedicated ultrafast SPECT cameras: New designs and clinical implications. J Nucl Med. 2011;52:210–217. doi: 10.2967/jnumed.110.081323. [DOI] [PubMed] [Google Scholar]
- 16.Bellevre D, Manrique A, Legallois D, Bross S, Baavour R, Roth N, et al. First determination of the heart-to-mediastinum ratio using cardiac dual isotope ((1)(2)(3)I-MIBG/(9)(9)mTc-tetrofosmin) CZT imaging in patients with heart failure: The ADRECARD study. Eur J Nucl Med Mol Imaging. 2015;42:1912–1919. doi: 10.1007/s00259-015-3141-3. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki Y, Kato Y, Imoto A, Fukuchi K. Imaging of the thyroid and parathyroid using a cardiac cadmium zinc telluride camera: Phantom studies. J Nucl Med Technol. 2018;46:39–44. [DOI] [PubMed]
- 18.Liu CJ, Cheng JS, Chen YC, Huang YH, Yen RF. A performance comparison of novel cadmium-zinc-telluride camera and conventional SPECT/CT using anthropomorphic torso phantom and water bags to simulate soft tissue and breast attenuation. Ann Nucl Med. 2015;29:342–350. doi: 10.1007/s12149-015-0952-z. [DOI] [PubMed] [Google Scholar]
- 19.Okano N, Osawa I, Tsuchihashi S, Takahashi M, Niitsu M, Matsunari I. High-speed scanning of planar images showing (123)I-MIBG uptake using a whole-body CZT camera: A phantom and clinical study. EJNMMI Res. 2019;9:22. doi: 10.1186/s13550-019-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada Y, Nakano S, Gatate Y, Okano N, Muramatsu T, Nishimura S, et al. Feasibility of simultaneous (99m)Tc-tetrofosmin and (123)I-BMIPP dual-tracer imaging with cadmium-zinc-telluride detectors in patients undergoing primary coronary intervention for acute myocardial infarction. J Nucl Cardiol. 2021;28:187–195. doi: 10.1007/s12350-018-01585-9. [DOI] [PubMed] [Google Scholar]
- 21.Rogasch JMM BS, Grosser OS, et al. Feasibility of iodine-123-mIBG SPECT/CT quantification in neuroblastoma using CZT and NaI detectors. Research Square. 2020. 10.21203/rs.3.rs-31865/v1
- 22.Sordo SD, Abbene L, Caroli E, Mancini AM, Zappettini A, Ubertini P. Progress in the development of CdTe and CdZnTe semiconductor radiation detectors for astrophysical and medical applications. Sensors (Basel) 2009;9:3491–3526. doi: 10.3390/s90503491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko T, Utanohara Y, Suzuki Y, Kurihara M, Iguchi N, Umemura J, et al. A preliminary feasibility study of simultaneous dual-isotope imaging with a solid-state dedicated cardiac camera for evaluating myocardial perfusion and fatty acid metabolism. Heart Vessels. 2016;31:38–45. doi: 10.1007/s00380-014-0578-4. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Matsunari I, Nishi K, Mizutani A, Miyazaki Y, Ogai K, et al. Simultaneous acquisition of (99m)Tc- and (123)I-labeled radiotracers using a preclinical SPECT scanner with CZT detectors. Ann Nucl Med. 2016;30:263–271. doi: 10.1007/s12149-015-1055-6. [DOI] [PubMed] [Google Scholar]
- 25.Tshori S, Livschitz S, Volodarsky I, Goland S, Shimoni S, Fabrikant J et al. Transthyretin cardiac amyloidosis scintigraphy using planar D-SPECT on dedicated cardiac CZT camera. J Nucl Cardiol. 2022;29:1995–2000. [DOI] [PubMed]
- 26.Agostini D, Marie PY, Ben-Haim S, Rouzet F, Songy B, Giordano A, et al. Performance of cardiac cadmium-zinc-telluride gamma camera imaging in coronary artery disease: A review from the cardiovascular committee of the European Association of Nuclear Medicine (EANM) Eur J Nucl Med Mol Imaging. 2016;43:2423–2432. doi: 10.1007/s00259-016-3467-5. [DOI] [PubMed] [Google Scholar]
- 27.Lima R, Peclat T, Soares T, Ferreira C, Souza AC, Camargo G. Comparison of the prognostic value of myocardial perfusion imaging using a CZT-SPECT camera with a conventional anger camera. J Nucl Cardiol. 2017;24:245–251. doi: 10.1007/s12350-016-0618-9. [DOI] [PubMed] [Google Scholar]
- 28.Bocher M, Blevis IM, Tsukerman L, Shrem Y, Kovalski G, Volokh L. A fast cardiac gamma camera with dynamic SPECT capabilities: Design, system validation and future potential. Eur J Nucl Med Mol Imaging. 2010;37:1887–1902. doi: 10.1007/s00259-010-1488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimelli A, Liga R, Bertasi M, Kusch A, Marzullo P. Head-to-head comparison of a CZT-based all-purpose SPECT camera and a dedicated CZT cardiac device for myocardial perfusion and functional analysis. J Nucl Cardiol. 2021;28:1323–1330. doi: 10.1007/s12350-019-01835-4. [DOI] [PubMed] [Google Scholar]
- 30.Deshayes E, Fersing C, Hebert K, Bardies M, Kotzki PO, Santoro L. Whole body planar and 3D quantitative imaging after (177)Lu-DOTATATE on CZT SPECT/CT device with MEHRS collimator. First report Hell J Nucl Med. 2021;24:165–166. doi: 10.1967/s002449912361. [DOI] [PubMed] [Google Scholar]
- 31.Gelfand MJ, Parisi MT, Treves ST, Reduction PNMD, W. Pediatric radiopharmaceutical administered doses, North American consensus guidelines. J Nucl Med. 2010;2011(52):318–322. doi: 10.2967/jnumed.110.084327. [DOI] [PubMed] [Google Scholar]
- 32.Oddstig J, Hedeer F, Jogi J, Carlsson M, Hindorf C, Engblom H. Reduced administered activity, reduced acquisition time, and preserved image quality for the new CZT camera. J Nucl Cardiol. 2013;20:38–44. doi: 10.1007/s12350-012-9634-6. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Miyagawa M, Nishiyama Y, Kawaguchi N, Ishimura H, Mochizuki T. Dual radioisotopes simultaneous SPECT of (99m)Tc-tetrofosmin and (123)I-BMIPP using a semiconductor detector. Asia Ocean J Nucl Med Biol. 2015;3:43–49. [PMC free article] [PubMed] [Google Scholar]
- 34.Cherry SR, Sorenson JA, Phelps, ME. Physics in nuclear medicine. 4th ed. Philadelphia: Elsevier Saunders. 2012.
- 35.Sharp SE, Gelfand MJ, Shulkin BL. Pediatrics: Diagnosis of neuroblastoma. Semin Nucl Med. 2011;41:345–353. doi: 10.1053/j.semnuclmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.