Abstract
Axial spondyloarthritis (axSpA) is a chronic, inflammatory rheumatic disease that primarily affects the axial skeleton, often inflicting severe pain, diminished mobility, and a compromised quality of life. The advent of Assessment of SpondyloArthritis international Society (ASAS) classification criteria for spondyloarthritis (SpA) have enabled the classification of patients with axSpA in the non-radiographic stage but poorly perform if mistakenly used for diagnostic purposes. Despite notable progress in early diagnosis facilitated by referral strategies and extensive magnetic resonance imaging (MRI) utilization, diagnostic delays persist as a concerning issue. This underscores the urgency to narrow the diagnostic gap and highlights the critical role of early diagnosis in mitigating the long-term structural damage associated with this condition. Research into the impact of non-steroidal anti-inflammatory drugs (NSAIDs) and biologic disease-modifying antirheumatic drugs (bDMARDs) on inflammatory symptoms and radiographic progression has been extensive. A compelling body of evidence suggests that early intervention leads to superior disease outcomes. However, most of these studies have centered on patients with established diseases rather than those in the early stages. Consequently, findings from studies on early pharmacological intervention remain inconclusive, and the potential for modifying the disease trajectory is still debatable. Without precise data from clinical trials, insights from basic science regarding the pathogenic mechanisms might point toward potential targets that warrant early intervention in the disease process. This review underscores the urgency of early diagnosis and intervention in axSpA, highlighting ongoing research gaps and the need for further exploration to improve patient outcomes.
Keywords: Early axial spondyloarthritis, TNF-alpha, IL-17 pathway, Disease progression, Therapeutic intervention
Key Summary Point
| Early axial spondyloarthritis (axSpA) poses significant challenges and opportunities for intervention in disease progression. |
| Early interventions in axSpA through pathways like tumor necrosis factor-alpha and interleukin-17 inhibitors could hold promise for modifying disease progression. However, the available evidence is still evolving and requires further exploration. |
| The ASAS spondyloarthritis EARly definition (ASAS-SPEAR) definition of early axSpA, indicating less than 2 years from symptom onset, opens doors for tailored interventions and research. |
| The new early axSpA definition will pave the way for dedicated clinical trials focusing on this patient group, offering the potential for more substantial insights into a therapeutic window of opportunity. |
Introduction
Historically, diagnosing axial spondyloarthritis (axSpA) took up to 7 years from symptom onset [1]. Increased awareness, early referral strategies, and the definition of non-radiographic axSpA (nr-axSpA) aim to progressively narrow this diagnostic gap over time, however, with limited success so far [1, 2].
The current Assessment of SpondyloArthritis international Society-Spondyloarthritis (ASAS) classification for axSpA, including nr-axSpA in addition to radiographic axSpA (r-axSpA), permits the diagnosis and treatment of individuals who may never develop apparent radiographic spondyloarthritis (SpA) features, allowing early identification of patients yet to develop radiographic changes (~ 28%) [3].
Among the evolving paradigms in axSpA treatment, the prospect of early intervention has garnered substantial attention. However, evaluating early treatment’s impact on axSpA long-term course is challenging due to limited and inconsistent data. Older trials, excluding patients without radiographic sacroiliitis, hinder findings’ applicability. It’s noteworthy that in 2023, the ASAS (EARLY definition) steering committee convened an international working group to establish such a definition for early axSpA [4].
In light of these considerations, this review aims to examine the potential role of early treatment in axSpA and its potential impact on patient outcomes. We will delve into the challenges of diagnosis, the underlying pathophysiology of early disease stages, and the evidence supporting the role of early treatment in curtailing radiographic progression and structural damage.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Pathophysiological Aspects of Early axSpA
While the precise hierarchy of pathophysiological mechanisms driving SpA remains incompletely elucidated, multiple lines of evidence suggest that early stage axSpA is characterized by a complex interplay of different mechanisms.
Diverse cytokine axes come into play during the initial phases of axSpA, with notable attention on the tumor necrosis factor-alpha (TNF-α) signaling pathway and the interleukin-23/interleukin-17 (IL-23/IL-17) axis. The role of TNF-α in experimental SpA models is well established, and these models are mechanically dependent [5]. In addition to the well-described role as an inflammatory mediator, several evidence cases pointed out the contribution of the TNF signaling pathway to new bone formation: data have shown that TNF-α is a crucial inducer of DKK-1 in mouse inflammatory arthritis models [6]. Subsequent studies have firmly confirmed its pivotal role in axSpA pathogenesis, resulting in the approval of TNF-α inhibitors that have transformed the prognosis of patients with axSpA. Notably, several studies have demonstrated their impact on radiographic progression, and many clinical trials have been conducted in early disease stages with promising results.
The IL-23/IL-17 axis is widely acknowledged for its central role in orchestrating type 3 immune response and perpetuating immune-related conditions, including axSpA. IL-23 is a pro-inflammatory cytokine that urges the polarization of T cells into T helper 17 (Th17) cells, a significant cell subtype of T cells producing IL-17. However, despite the importance of IL-23 in disease pathogenesis, the IL-12/23 inhibition and the IL-23 specific inhibition did not show clinical efficacy in clinical trials in patients with AS [7, 8].
On the contrary, recent advancements have unveiled that IL-17 production extends beyond Th17 cells, with innate immune cells such as innate lymphoid cells (ILC) and innate-like T cells emerging as significant contributors to axSpA pathogenesis [9]. Mucosa-associated immune cells, specifically ILC3, mucosal-associated invariant T (MAIT) cells, and gamma delta (γδ) T cells, are also reported to be expanded in the inflamed gut in inflammatory bowel disease (IBD) and SpA, serving as sources of IL-17 [10, 11]. The significance of the IL-17 cytokine pathway gains further weight from a recent study, which demonstrated heightened IL-17-producing CD4+ T cells in patients with early active axSpA [12]. This underlines the critical role of IL-17 in the early stages of the disease and supports the encouraging data arising from the therapeutic blockade of IL-17A in clinical settings.
New insights are emerging about enthesis immunology since enthesitis is regarded to be a hallmark of the early stages of axSpA. The discovery of a vast pool of resident immune cells in the enthesis has opened new scenarios [13]. These populations of immune cells seem to activate in response to chronic biomechanical stress, and evidence of a bidirectional gut-enthesis axis has been described [14, 15]. In this context, a contribution to type 3 immunity is given from the gut and other barrier sites, confirming the existence of a gut–joint and gut–entheseal axis [10, 14, 16, 17].
In recent years, growing evidence has recognized stromal cells as essential contributors to structural damage in SpA [11]. These cells have been implicated in mediating the inflammatory response to mechanical stress [5]. Moreover, it has been demonstrated that stromal cells can directly stimulate Th17 production of IL-17 independently of IL-23 by releasing prostaglandin E2 (PGE2) [18]. PGE2 and its receptor prostaglandin E receptor 4 (EP4) have also been implicated in the pathogenesis of axiaSpA, with EP4 being upregulated in patients with axSpA compared to healthy controls, psoriatic arthritis (PsA), and patients with rheumatoid arthritis (RA) and playing a role in Th17 development [19, 20]. A recent study has additionally demonstrated that circulating levels of EP4 can predict radiographic progression and that EP4 activation in monocytes induces bone formation from mesenchymal stem cells [21]. These findings support the idea that the PGE2/EP4 axis is involved in both the inflammatory phase of early axSpA and the later ossification stage, further strengthening the rationale for non-steroidal anti-inflammatory drugs (NSAIDs) use in patients with axSpA.
Definition of Early axSpA
In recent years, the concept of “early axSpA” has gained prominence, reflecting the need to identify and treat the disease in its initial stages. Although evidence supporting specific symptom duration remains limited due to the historical predominance of longstanding disease cases in research studies, the need for a standardized framework for defining early axSpA is crucial.
However, until the ASAS-SPEAR (ASAS SPondyloarthritis EARly definition) project, a universally accepted definition for early axSpA had been lacking [22]. This project sought to address this gap by formulating a definition by expert consensus, informed by a systematic literature review (SLR) and a Delphi survey [4, 22]. The new definition was based largely on expert opinion agreeing that the definition should be based solely on the axial symptom duration [4]. Notably, there was consensus among ASAS members in selecting a symptom duration cut-off of ≤ 2 years for the definition [4]. This duration, though relatively longer compared to other rheumatic conditions, reflects an aspirational standpoint considering the current diagnostic delays associated with axSpA. It is important to note that the ASAS definition of early axSpA is designed to facilitate research endeavors by classifying patients in clinical trials based on homogeneous criteria [4]. As far the early diagnosis capability will increase worldwide, this definition is also meant to be revised.
Challenges in Diagnosis and Monitoring
The diagnosis of axSpA remains a complex issue, characterized by delayed identification of the disease [23]. Nearly two decades ago, the mean diagnostic delay for ankylosing spondylitis (AS), a subset of axSpA with definite structural damage visible on X-rays of sacroiliac joints, was approximately 10 years [24]. While strides have been made in improving early diagnosis through referral strategies [25], the conceptualization of the early disease stage [4], the definition of nr-axSpA [26], and the widespread utilization of MRI, a substantial diagnostic delay (5 to 10 years across most of the countries) still persists in axSpA [27] making it one of the longest diagnostic lags in rheumatology [27, 28]. While commendable, the push for timely detection may inadvertently lead to an increased risk of erroneously diagnosing non-inflammatory conditions as axSpA, particularly when misapplying existing classification criteria for primary diagnosis.
Clear differentiation between diagnostic and classification approaches is imperative [29]. The diagnostic approach involves considering a suspicion of axSpA, evaluating pre-test probabilities, analyzing positive and negative test outcomes, exploring alternative explanations for symptoms (differential diagnoses), and concluding with post-test probability assessment.
Test results possess varying weight; for example, the absence of radiographic sacroiliitis does not significantly diminish the likelihood of axSpA as the disease might be in an early stage. In contrast, the absence of active inflammation and structural changes on MRI strongly argues against axSpA diagnosis [30, 31]. The classification approach (e.g., by applying the ASAS classification criteria for axSpA [32] necessitates an existing definitive diagnosis before applying classification criteria. The classification approach predominantly considers positive test results, neglects differential diagnoses, and provides a Boolean outcome indicating fulfillment or non-fulfillment of criteria, characterized by specific sensitivity and specificity levels.
Clinical parameters for diagnosing axSpA encompass various signs and manifestations. These include inflammatory back pain (with specific characteristics), lasting three months or longer, often responsive to NSAIDs. Peripheral manifestations like arthritis, enthesitis, and dactylitis can occur, along with extra-musculoskeletal signs such as uveitis, psoriasis, and inflammatory bowel disease. HLA-B27, a genetic marker, is a specific but population-dependent diagnostic factor, while acute phase reactants like CRP and ESR provide insights into systemic inflammation with lower sensitivity. A recently developed polygenic risk score showed a good diagnostic performance in differentiating AS from patients with non-specific back pain [33] but has not entered a broad clinical practice yet.
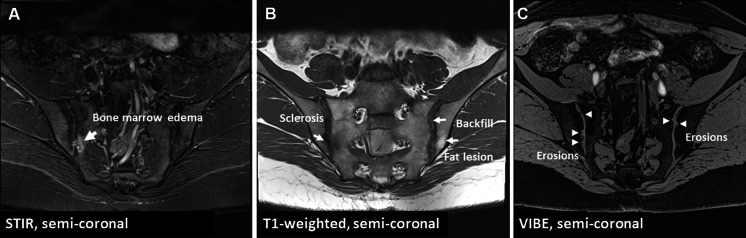
Imaging is pivotal in the diagnostic process of axSpA. Conventional radiography of sacroiliac joints lacks sensitivity and specificity for axSpA diagnosis [30]. MRI of sacroiliac joints, however, can capture active inflammation (osteitis) and post-inflammatory structural changes (erosions, fat lesions, backfill, sclerosis, and ankylosis)—Fig. 1. Importantly, the interpretation of MRI findings should always be done in the context of clinical presentation, as bone marrow edema is also observed in healthy individuals, athletes, and those with mechanical/degenerative issues in the axial skeleton, such as osteitis condensans ilii [34–37]. Notably, typical structural changes (especially the highly specific ones, such as erosions, backfill, and ankylosis) boost the specificity of bone marrow edema. Moreover, the site of inflammatory and structural changes aids in differential diagnosis; mechanically induced bone marrow edema often occurs in anterior and caudal joint segments, whereas axSpA primarily affects the middle joint portion [38]
Fig. 1.
MRI of sacroiliac joints of a 38-year-old male patient with inflammatory back pain for years, HLA-B27 positivity and elevated CRP. A STIR sequence shows subchondral bone marrow edema and a subchondral cyst or an erosion localized in the mid part of the joint (arrow). B T1-weighted sequence demonstrates erosions with fat metaplasia in the erosion cavity (backfill)—bold arrow, as well as a fat lesion (thin arrow) and sclerosis (dotted arrow). C The erosion-sensitive sequence (VIBE) confirms the presence of erosions (arrows). CRP C-reactive protein, MRI magnetic resonance imaging, STIR short tau inversion recovery, VIBE volumetric interpolated breath-hold examination. Poddubnyy D. Joint Bone Spine 2023 Jan;90(1):105,468.
Published under a Creative Commons license (CC BY 4.0)
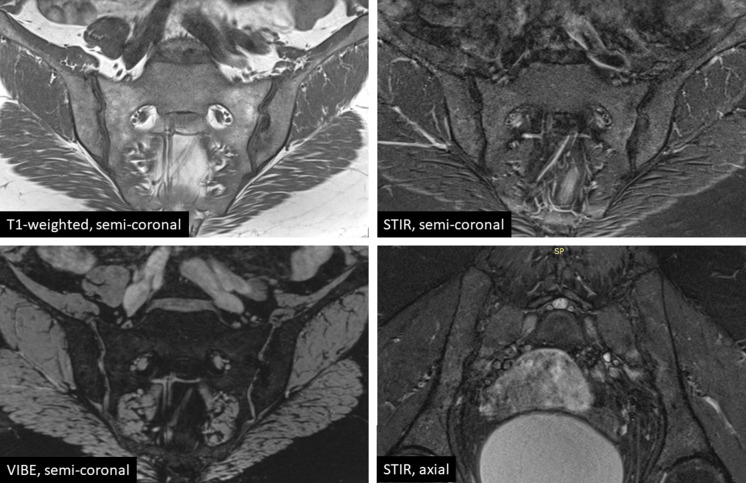
For reliable detection of inflammatory and structural sacroiliac joint changes, a recommended set by the radiologists of the European Society of Skeletal Radiology (ESSR) Arthritis Subcommittee [39] and most recently—by ASAS and the Spondyloarthritis Research and Therapy Network (SPARTAN) of four sequences has emerged: (1) semi-coronal T1-weighted for the assessment of structural changes, (2) semi-coronal T2-weighted with fat suppression (e.g., Short Tau Inversion Recovery—STIR) for the assessment of active inflammatory changes, (3) semi-coronal cartilage-sensitive sequence (T1 fat-suppressed gradient-echo sequence, such as Volumetric Interpolated Breath-hold Examination—VIBE) for erosion depiction, and (4) an additional T2-weighted semi-axial sequence with fat suppression for better identifying active inflammatory changes, particularly in the posterior joint region [40]. An example of the MRI performed in accordance with the above-mentioned protocol is presented in Fig. 2. No contrast medium (Gd) is usually needed. Computed tomography (CT) of sacroiliac joints is less sensitive but more specific, identifying structural post-inflammatory changes [30]. CT is recommended when MRI results are inconclusive about structural damage.
Fig. 2.
The currently recommended MRI sequences the diagnostic evaluation of sacroiliac joints in patients with suspected axial spondyloarthritis. MRI magnetic resonance imaging, STIR short tau inversion recovery, VIBE volumetric interpolated breath-hold examination
MRI of the spine can be considered in addition to MRI of sacroiliac joints depending on the pain type, localization, and suspected diagnosis: while in the vast majority of the primary axSpA cases, the disease starts in the sacroiliac joints, in patients with psoriasis/psoriatic arthritis, inflammatory affection of the spine may occur without involvement of sacroiliac joints [41]. In summary, for an accurate axSpA diagnosis, an objective confirmation of axial skeleton inflammation is crucial. The complexity stems from the interplay between pre-test probability, diverse test results, exclusion of differential diagnoses, and estimation of disease probability. While classification criteria aid research, a tailored diagnostic framework that accommodates variations in diagnostic performance is essential for timely and accurate diagnosis.
Role of Early Treatment in the Management of axSpA
Examining the impact of early treatment on radiographic progression is crucial in axSpA, but the broader question is whether early intervention can genuinely alter disease trajectories.
It is conceivable that early access to treatment will be associated with an earlier reduction of symptoms and manifestations rather than no intervention. Limited data are available informing on higher efficacy of the treatment in early disease compared to long-lasting, and this will be hereby discussed for each of the current first-line bDMARDs [42–44]. However, whether early treatment may change the disease trajectories is still a matter of discussion. There is a lack of data on the impact of early treatment in changing patients-reported outcome long term, and similarly regarding the occurrence of extra-articular manifestations (EAMs). Limited data exist regarding the influence of early treatment on the development of EAMs. However studies suggest that EAM can occur at any point in the disease course, with a higher prevalence in patients with longer disease duration [45, 46]. Conversely, one surrogate parameter possibly able to inform on the impact of early treatment on the disease trajectories is the articular damage accrual measured as radiographic progression.
This is vital as stopping radiographic progression is paramount, and currently, no treatment have proved to reverse accrued structural damage. The influence of early treatment on future radiographic changes is still debated.
Over time, data regarding inhibiting radiographic progression and accumulating damage in axSpA have yielded mixed findings, often originating from studies that focused on patients who already had established radiographic signs at recruitment. The effects of continuous therapy using non-steroidal NSAIDs on bone damage have shown variability in different studies, with conflicting results [47, 48]. Establishing the effectiveness of TNF blockade in slowing damage accrual has been challenging. Encouraging results emerged with early and prolonged anti-TNF therapy, often exceeding 4 years, primarily in ankylosing spondylitis (AS) with initial radiographic damage [49, 50]. Interestingly, more favorable responses were seen in early non-radiographic axSpA and those with shorter symptom durations [51].
Evidence on improved treatment responses in early axSpA is limited and conflicting. Unlike rheumatoid arthritis (RA), axSpA displays slower and variable structural changes, making radiographic progression heterogeneous [52]. In addition, clinical trials often focus on patients with pre-existing syndesmophytes, boosting statistical power but introducing bias by potentially excluding early axSpA (pre-radiographic axSpA) cases. This hinders evaluating treatment effectiveness during the transition from pre-radiographic to radiographic axSpA. In a prospective cohort with early axSpA (disease duration < 1 year), the percentage of nr-axSpA decreased progressively from nearly 67 to 29% [53] in line with other reports [54]. This distinction is pivotal; some patients initially diagnosed as nr-axSpA are, in fact, in the pre-radiographic stage—diagnosed with axSpA before radiographic changes manifest if left untreated. Conversely, some patients classified as nr-axSpA will remain in this category for extended durations or indefinitely [54]. The omission of true non-radiographic cases obstructs a genuine assessment of the therapeutic impact of early intervention in the disease (early axSpA).
In addition, early pharmacologic treatment seems to have a positive effect on quality of life. Some data confirm that treatment with the anti-TNF certolizumab pegol in patients with nr-axSpA with shorter symptom duration improves symptoms and quality of life [44]. However, a trend toward higher responses was evident in those with shorter disease duration with younger patients having better responses, likely due to their shorter disease duration [42].
In another study involving both patients with rheumatoid arthritis and r-axSpA, bDMARDs improve quality of life, especially in those with shorter disease duration and younger age [55].
Could Anti-TNFα be Used Earlier in axSpA?
Despite the excellent effect of NSAIDs in controlling disease signs and symptoms, nearly 50% of patients with axSpA still have active disease despite treatment with NSAIDs [56]. For such patients, TNF-α inhibitors (TNFi) have represented a breakthrough in managing active axSpA [57–59]. Determining whether early intervention with TNFi can alter the response poses a complex challenge. A recent systematic literature review dealt with this matter realizing that evidence on better response to treatment in early axSpA is very limited [51]. The same work calculated the relative risk ratio (RRR): the ratio between relative risk (RR) of active treatment in early vs. the RR in established disease [51]. In patients with nr-axSpA early symptom duration was associated with an up to five-time higher change to achieving ASAS40 (≥ 40% improvement in three of the four domains with an absolute improvement of at least 2 on a 0 to 10 scale, and no worsening in the remaining domain) following TNFi treatment [44, 60].
Considering disease duration for comparison offers more data, particularly for what concerns r-axSpA. In this case even using multiple cut-off point (≃ 2, ≃ 5, and ≃ 10 years) in randomized controlled trials (RCTs), the analysis suggested a numerically higher proportion of patients achieving remission in early axSpA but formal analysis did not show any advantage in term of reaching ASAS response in treating with TNFi earlier, with the paradox that in some trial the response was in favor of long disease duration [61, 62]. Conversely, cohort studies suggested a shorter disease duration as a factor possibly predicting better improvement in Bath Ankylosing Spondylitis Metrology Index (BASMI), Bath Ankylosing Spondylitis Functional Index (BASFI) or Short Form Health Survey 36 (SF-36) [55, 63].
The Infliximab (IFX) as First Line Therapy in Patients with Early Active Axial Spondyloarthritis Trial (INFAST) study demonstrated better outcomes on a variety of efficacy measures in patients with early axSpA who were treated with IFX + NPX (Naproxen) than in those treated with NPX alone [64]. In the latter study symptom duration in patients with early axSpA was less than 3 years and the authors concluded that better response to NSAIDs and TNFi was higher in patients with shorter disease duration [64]. Similarly, among patients with nr-axSpA, those enrolled in the ABILITY-3 study and receiving adalimumab were more likely to achieve remission if they were younger, which might serve as a proxy for a shorter symptom duration [65].
It is important to note that all these efficacy comparisons even if are not based on head-to-head trials and they suggest a difference in treatment response based on the duration of the disease. A 2-year study on three independent cohorts showed blockage of TNF does not stop radiographic progression [66–68]. Subsequent studies, however, have pointed out that the use of TNFi could impact radiographic progression [49, 50, 69–72]. Overall, the available data were meta-analyzed by Boer et al. that reached to the conclusion that during the initial 2 years, no significant distinction in spinal radiographic progression was observed between patients who were administered TNFi and those who were not [73]. Nevertheless, beyond the 2-year mark, there emerged a potential protective influence associated with TNFi treatment [50]. Limited studies have assessed the radiographic progression in relation to TNFi use during the early stages of the disease. One of the most significant is the one from Haroon, that already in 2014 reported that early initiation of TNFi was associated with a reduction in the rate of radiographic progression in AS when early was considered before 10 year of disease onset [50]. After adjusting for baseline Modified Stoke Ankylosing Spondylitis Spine Score (mSASSS), patients initiating therapy over a decade after symptom onset displayed an increased likelihood of progression, contrasting those who started treatment earlier. This underscores the significance of both TNFi usage and the timing of therapy initiation influencing the rate of mSASSS progression [50]. Worth noting that this and similar studies used 10 years from symptoms onset as cut-off for defining early axSpA [50, 71] this is well beyond our current definition of early axSpA. Conversely, patients with shorter disease duration were investigated by Dougados et al. that compared patients with axSpA receiving etanercept in the EMBARK trial with similar patients in the untreated DESIR (DEvenir des Spondylarthropathies Indifférenciées Récentes) cohort, mean symptoms duration 2.4 and 1.7 years, respectively. This study suggested a lower rate of progression in the sacroiliac joints with etanercept than without anti-tumor necrosis factor therapy [74].
A contribution to this topic is also offered by the data available from the trials conducted on nr-axSpA. In the Rheumatoid Arthritis Prevention of structural Damage (RAPID)-axSpA trial on patients with AS and nr-axSpA treated with certolizumab pegol (CZP), the median symptom duration in nr-axSpA was 5.8 years. Overall, in 4 years of treatment the progression was very limited and virtually absent in nr-axSpA [75]. Conversely, in other cohort of untreated nr-axSpA the progression to AS was between 10% and 12% in 2 years [76–78].
Considering these findings, it becomes plausible to speculate that early intervention might indeed play a substantial role in shaping the trajectory of disease progression in axSpA even if not formally fully supported by the available literature. The temporal dimension of treatment initiation coupled with the employment of TNFi appear to possibly have an impact on the rate of mSASSS progression. These observations suggest the potential benefits of timely therapeutic measures while acknowledging that the current evidence may not offer conclusive comparisons between early and late intervention with TNFi.
Early Intervention Targeting IL-17
The IL-17 axis plays a cardinal role in the pathogenesis of SpA [79–81]. While the scarcity of data for TNFi posed challenges in drawing conclusions, the situation becomes even more intricate when considering IL-17 inhibitors. In fact, very few trials have provided the opportunity to directly compare early intervention in early axial SpA (axSpA) with established axSpA.
Selective inhibitors of IL-17A, such as secukinumab (SEC) and ixekizumab (IXE), have emerged as significant treatment options in SpA [82–84] but their efficacy in early disease stages remains largely unexplored, as seen in a recent literature review [51]. In this SLR work led by Ramiro, earlier mentioned, only a few works on IL-17i were included [42, 43, 85]. Specifically, looking at the r-axSpA early (< 2 years) administration of SEC did not increase the chance to obtain ASAS40 compared to patients with over 2 years of disease durations [43].
In Deohar et al.’s study, data from MEASURE 1–4 trials pooled patients with active r-axSpA were split into two groups by disease duration, receiving either SEC or placebo. SEC improved all measures, with established disease showing a greater burden. Still, SEC was effective in both groups, with slightly better results in shorter disease duration [42].
Shifting the focus to nr-axSpA, an analysis conducted by Navarro-Compán et al. delved into the data gleaned from the COAST-X phase 3 RCT comparing placebo against IXE administered every 4 (Q4W) or every 2 (Q2W) weeks. Notably, at the currently approved dosage (Q4W), a statistically significant divergence in the ASAS40 response rate at week 16, compared to the placebo, was discerned solely among patients with a disease duration of less than 5 years [85]. This trend echoed similar observations for BASDAI50 (≥ 50% improvement of the initial BASDAI [Bath Ankylosing Spondylitis Disease Activity Index]). However, it is important to note that a direct formal comparison between the two patient groups (> 5 years vs. < 5 years) was not conducted [85]. The RRR calculated by Ramiro et al. stood at 3.62, though it remained statistically non-significant.
Also considering the other possible opportunities offered by early treatment, such as the inhibition of long-term structural damage, the data on IL17i are limited. Data at 104 weeks from a phase 3, placebo-controlled RCT comparing SEC with placebo up to week 16 and then crossed over SEC in r-axSpA initially showed promising results. The rate of mSASSS-measured spinal radiographic changes was very low, but no direct comparator was available [86]. Similar results were obtained in nr-axSpA at 2 years [87]. A historical comparison with NSAID-treated biologic-naïve patients (ENRADAS) revealed a higher non-progression rate in the SEC group, but it was not significant. Interpret with caution, as NSAIDs may affect radiographic progression [86, 88]. The open-label extension of the same RCT (MEASURE-1) confirmed minimal progression after 4 years of continuous SEC treatment; mean mSASSS change at week 208 was 1.2 ± 3.91 in SEC 150 mg patients [89].
As for IXE, in r-axSpA, data from the COAST-V and COAST-W phase 3 trials, long-term extension study, available at 2 years demonstrated, in the absence of a comparator, that IXE-treated patients show an overall low rate of radiographic progression, where still age is a negative predictor [90]. In the COAST-X RCT in which it was compared to placebo in nr-axSpA, a significant reduction in erosion in the sacroiliac joints and an increase of fat lesions and backfill compared to placebo at week 16 was observed [91]. In general, the clinical meaning of this observation may be limited, but it could be very relevant in the context of early treatment and progression of structural damage.
Following the promise offered by the trials on SEC, a trial comparing the effects of SEC and Adalimumab on radiographic Progression in Patients with AS was conducted [92]. Initial data from the SURPASS trial have now emerged: spinal radiographic progression over 2 years was low with no significant difference between SEC and adalimumab [92].
The current dearth of extensive studies focusing on patients with early SpA restricts our ability to definitively endorse the application of anti-IL17A agents in the initial phases of the disease.
Conclusions
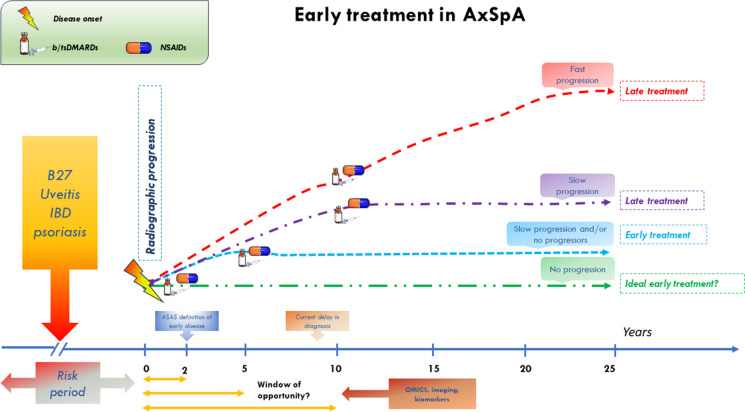
The prospects of early treatment in axSpA open the doors to the concept of the “window of opportunity” (Fig. 3). This concept, well established in rheumatoid arthritis, is less clear in axSpA, primarily due to challenges in defining early SpA. Assessing early treatment impact on axSpA long-term course faces hurdles, including limited data, inconsistent outcomes, and the absence of a universal early axSpA definition. Additionally, assessing treatment effects on structural damage varies across interventions, complicating evaluation. In this context, the sensitivity of mSASS might not fully capture current interventions impact given axSpA complex disease dynamics.
Fig. 3.
Pitfalls, opportunities, and challenges in the early treatment of AxSpA. ASAS Assessment of spondyloarthritis international society-spondyloarthritis, AxSpA axial Spondyloarthritis, bDMARD biologic disease-modifying antirheumatic drugs, IBD inflammatory bowel disease, NSAID non-steroidal anti-inflammatory drugs, tsDMARD targeted synthetic disease-modifying antirheumatic drug
In summary, clarifying the “window of opportunity” and demonstrating early treatment superiority in axSpA remain ongoing challenges. However, ongoing research efforts using refined definitions and comprehensive clinical data offer hope for better understanding and managing this condition. Although, while comprehensive clinical data on early axSpA management is awaited, we must acknowledge the valuable contributions of basic science in uncovering pathogenic mechanisms. Discoveries regarding IL-17, TNF-α, Janus Kinases, and PGE2 suggest potential avenues for reshaping disease trajectories by mitigating new bone formation and structural damage.
Author Contribution
Francesco Ciccia conceived the review’s framework and designed Fig. 3. Daniele Mauro, Giulio Forte, and Denis Poddubnyy were responsible for drafting the manuscript, with Denis Poddubnyy also preparing Figs. 1 and 2. Daniele Mauro, Giulio Forte, and Francesco Ciccia provided critical revisions. All authors contributed to the article’s development and approved the final version for submission.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Denis Poddubnyy: Research support from AbbVie, Eli Lilly, MSD, Novartis, Pfizer; Consulting fees from AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Janssen, Moonlake, Novartis, Pfizer, and UCB; Speaker fees from AbbVie, Canon, DKSH, Eli Lilly, Janssen, MSD, Medscape, Novartis, Peervoice, Pfizer, and UCB; Member of the executive committee of ASAS; Member of steering committee of GRAPPA. Francesco Ciccia: Research support from AbbVie, Eli Lilly, Novartis, Pfizer; Consulting and speaking fees from AbbVie, Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, and UCB; Full member of ASAS. Daniele Mauro and Guilio Forte have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Francesco Ciccia and Denis Poddubnyy shared co-senior authorship.
References
- 1.Hay CA, Packham J, Ryan S, Mallen CD, Chatzixenitidis A, Prior JA. Diagnostic delay in axial spondyloarthritis: a systematic review. Clin Rheumatol. 2022;41:1939–1950. doi: 10.1007/s10067-022-06100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol. 2012;8:262–268. doi: 10.1038/nrrheum.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Rudwaleit M, Khan MA, Sieper J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 2005;52:1000–1008. doi: 10.1002/art.20990. [DOI] [PubMed] [Google Scholar]
- 4.Navarro-Compán V, Benavent D, Capelusnik D, van der Heijde D, Landewé RB, Poddubnyy D, et al. ASAS consensus definition of early axial spondyloarthritis. Ann Rheum Dis. 2023 doi: 10.1136/ard-2023-224232. [DOI] [PubMed] [Google Scholar]
- 5.Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014;73:437–445. doi: 10.1136/annrheumdis-2013-203643. [DOI] [PubMed] [Google Scholar]
- 6.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 7.Baeten D, Østergaard M, Wei JCC, Sieper J, Järvinen P, Tam LS, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. 2018;77:1295–1302. doi: 10.1136/annrheumdis-2018-213328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deodhar A, Gensler LS, Sieper J, Clark M, Calderon C, Wang Y, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 2019;71:258–270. doi: 10.1002/art.40728. [DOI] [PubMed] [Google Scholar]
- 9.Rosine N, Miceli-Richard C. Innate cells: the alternative source of IL-17 in axial and peripheral spondyloarthritis? Front Immunol. 2021 doi: 10.3389/fimmu.2020.553742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauro D, Macaluso F, Fasano S, Alessandro R, Ciccia F. ILC3 in axial spondyloarthritis: the gut angle. Curr Rheumatol Rep. 2019 doi: 10.1007/s11926-019-0834-9. [DOI] [PubMed] [Google Scholar]
- 11.Mauro D, Simone D, Bucci L, Ciccia F. Novel immune cell phenotypes in spondyloarthritis pathogenesis. Semin Immunopathol. 2021;43(2):265–277. doi: 10.1007/s00281-021-00837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen DTSL, Hameetman M, van Bergen J, Huizinga TWJ, van der Heijde D, Toes REM, et al. IL-17-producing CD4+ T cells are increased in early, active axial spondyloarthritis including patients without imaging abnormalities. Rheumatology. 2015;54:728–735. doi: 10.1093/rheumatology/keu382. [DOI] [PubMed] [Google Scholar]
- 13.Sharif K, Bridgewood C, Dubash S, McGonagle D. Intestinal and enthesis innate immunity in early axial spondyloarthropathy. Rheumatology. 2020;59:iv67–78. doi: 10.1093/rheumatology/keaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauro D, Nakamura A, Haroon N, Ciccia F. The gut-enthesis axis and the pathogenesis of Spondyloarthritis. Semin Immunol. 2021;58:101607. doi: 10.1016/j.smim.2022.101607. [DOI] [PubMed] [Google Scholar]
- 15.Rosine N, Rowe H, Koturan S, Yahia-Cherbal H, Leloup C, Watad A, et al. Characterization of blood <scp>mucosal-associated</scp> invariant T cells in patients with axial spondyloarthritis and of resident <scp>mucosal-associated</scp> invariant T cells from the axial entheses of <scp>non-axial</scp> spondyloarthritis control patients. Arthritis Rheumatol. 2022;74:1786–1795. doi: 10.1002/art.42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costello M-E, Elewaut D, Kenna TJ, Brown MA. Ankylosing spondylitis microbes, the gut and ankylosing spondylitis review. Arthritis Res Ther. 2013 doi: 10.1186/ar4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauro D, Gandolfo S, Tirri E, Schett G, Maksymowych WP, Ciccia F. The bone marrow side of axial spondyloarthritis. Nat Rev Rheumatol. 2023 doi: 10.1038/s41584-023-00986-6. [DOI] [PubMed] [Google Scholar]
- 18.Paulissen SMJ, van Hamburg JP, Davelaar N, Asmawidjaja PS, Hazes JMW, Lubberts E. Synovial fibroblasts directly induce Th17 pathogenicity via the cyclooxygenase/prostaglandin E2 pathway, independent of IL-23. J Immunol. 2013;191:1364–1372. doi: 10.4049/jimmunol.1300274. [DOI] [PubMed] [Google Scholar]
- 19.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Aoki T, Thumkeo D, Siriwach R, Yao C, Narumiya S. T cell–intrinsic prostaglandin E 2 -EP2/EP4 signaling is critical in pathogenic T H 17 cell–driven inflammation. J Allergy Clin Immunol. 2019;143:631–643. doi: 10.1016/j.jaci.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauro D, Srinath A, Guggino G, Nicolaidou V, Raimondo S, Ellis JJ, et al. Prostaglandin E2/EP4 axis is upregulated in spondyloarthritis and contributes to radiographic progression. Clin Immunol. 2023;251:109332. doi: 10.1016/j.clim.2023.109332. [DOI] [PubMed] [Google Scholar]
- 22.Benavent D, Capelusnik D, van der Heijde D, Landewé R, Poddubnyy D, van Tubergen A, et al. How is early spondyloarthritis defined in the literature? Results from a systematic review. Semin Arthritis Rheum. 2022;55:152032. doi: 10.1016/j.semarthrit.2022.152032. [DOI] [PubMed] [Google Scholar]
- 23.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73–84. doi: 10.1016/S0140-6736(16)31591-4. [DOI] [PubMed] [Google Scholar]
- 24.Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–66. doi: 10.1007/s00296-002-0237-4. [DOI] [PubMed] [Google Scholar]
- 25.Poddubnyy D, van Tubergen A, Landewé R, Sieper J, van der Heijde D. Development of an ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483–1487. doi: 10.1136/annrheumdis-2014-207151. [DOI] [PubMed] [Google Scholar]
- 26.Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker-Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German spondyloarthritis inception cohort. Arthritis Rheum. 2009;60:717–727. doi: 10.1002/art.24483. [DOI] [PubMed] [Google Scholar]
- 27.Garrido-Cumbrera M, Navarro-Compán V, Bundy C, Mahapatra R, Makri S, Correa-Fernández J, et al. Identifying parameters associated with delayed diagnosis in axial spondyloarthritis: data from the European map of axial spondyloarthritis. Rheumatology (United Kingdom) 2022;61:705–712. doi: 10.1093/rheumatology/keab369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redeker I, Callhoff J, Hoffmann F, Haibel H, Sieper J, Zink A, et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatology (Oxford) 2019;58:1634–1638. doi: 10.1093/rheumatology/kez090. [DOI] [PubMed] [Google Scholar]
- 29.Poddubnyy D. Classification vs diagnostic criteria: the challenge of diagnosing axial spondyloarthritis. Rheumatology (Oxford) 2020;59:iv6–17. doi: 10.1093/rheumatology/keaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diekhoff T, Eshed I, Radny F, Ziegeler K, Proft F, Greese J, et al. Choose wisely: imaging for diagnosis of axial spondyloarthritis. Ann Rheum Dis. 2022;81:237–242. doi: 10.1136/annrheumdis-2021-220136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komsalova LY, Martínez Salinas MP, Jiménez JFG. Predictive values of inflammatory back pain, positive HLA B27 antigen and acute and chronic magnetic resonance changes in early diagnosis of spondyloarthritis. A study of 133 patients. PLoS ONE. 2020;15:e0244184. doi: 10.1371/journal.pone.0244184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Wu X, Leo PJ, De Guzman E, Akkoc N, Breban M, et al. Polygenic risk scores have high diagnostic capacity in ankylosing spondylitis. Ann Rheum Dis. 2021;80:1168–1174. doi: 10.1136/annrheumdis-2020-219446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poddubnyy D, Weineck H, Diekhoff T, Redeker I, Gobejishvili N, Llop M, et al. Clinical and imaging characteristics of osteitis condensans ilii as compared with axial spondyloarthritis. Rheumatology. 2020;59:3798–3806. doi: 10.1093/rheumatology/keaa175. [DOI] [PubMed] [Google Scholar]
- 35.Weber U, Jurik AG, Zejden A, Larsen E, Jørgensen SH, Rufibach K, et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring ‘Background noise’ toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol. 2018;70:736–745. doi: 10.1002/art.40429. [DOI] [PubMed] [Google Scholar]
- 36.de Winter J, de Hooge M, van de Sande M, de Jong H, van Hoeven L, de Koning A, et al. Magnetic resonance imaging of the sacroiliac joints indicating sacroiliitis according to the assessment of spondyloarthritis international society definition in healthy individuals, runners, and women with postpartum back pain. Arthritis Rheumatol. 2018;70:1042–1048. doi: 10.1002/art.40475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baraliakos X, Richter A, Feldmann D, Ott A, Buelow R, Schmidt CO, et al. Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. Ann Rheum Dis. 2020;79:186–192. doi: 10.1136/annrheumdis-2019-215553. [DOI] [PubMed] [Google Scholar]
- 38.Weber U, Jurik AG, Zejden A, Larsen E, Jørgensen SH, Rufibach K, et al. Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes. Arthritis Rheumatol. 2018;70:736–745. doi: 10.1002/art.40429. [DOI] [PubMed] [Google Scholar]
- 39.Sudoł-Szopińska I, Jurik A, Eshed I, Lennart J, Grainger A, Østergaard M, et al. Recommendations of the ESSR arthritis subcommittee for the use of magnetic resonance imaging in musculoskeletal rheumatic diseases. Semin Musculoskelet Radiol. 2015;19:396–411. doi: 10.1055/s-0035-1564696. [DOI] [PubMed] [Google Scholar]
- 40.Lambert R, Baraliakos X, Bernard S, Carrino J, Diekhoff T, Eshed I, Hermann K, Herregods N, Jaremko J, Jans L, Jurik A, O'Neill J, Reijnierse M, Tuite M, Maksymowych W. Development of international consensus on a standardized image acquisition protocol for diagnostic evaluation of the sacroiliac joints by MRI – an ASASSPARTAN Collaboration [abstract]. Arthritis Rheumatol. 2022;74(suppl 9). https://acrabstracts.org/abstract/development-of-international-consensus-on-a-standardized-image-acquisition-protocol-for-diagnostic-evaluation-of-the-sacroiliac-joints-by-mri-an-asas-spartan-collaboration/. Accessed 10 Nov 2023.
- 41.Poddubnyy D. Managing psoriatic arthritis patients presenting with axial symptoms. Drugs. 2023;83:497–505. doi: 10.1007/s40265-023-01857-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deodhar A, Boonen A, Ferraccioli G, van den Bosch F, Martinez D, Porter B, et al. FRI0388 secukinumab improves health-related quality of life in patients with ankylosing spondylitis, irrespective of time since first diagnosis: pooled results from the secukinumab phase 3 trial program. Ann Rheum Dis. 2019;78:878–879. [Google Scholar]
- 43.Deodhar A, Mease P, Machado P, Meng X, Strand V, Magrey M. Impact of age and disease duration on the response to IL-17A inhibitor (Secukinumab) treatment in ankylosing spondylitis: pooled results from the phase 3 measure studies [abstract]. Arthritis Rheumatol. 2019;71(suppl 10). https://acrabstracts.org/abstract/impact-of-age-and-disease-duration-on-the-response-to-il-17a-inhibitor-secukinumab-treatment-in-ankylosing-spondylitis-pooled-results-from-the-phase-3-measure-studies/. Accessed 10 Nov 2023.
- 44.Kay J, Gensler L, Deodhar A, Maksymowych W, Haroon N, Auteri S, de Peyrecave N, Kumke T, Hoepken B, Bauer L, Rudwaleit M. Earlier treatment of non-radiographic axial spondyloarthritis with certolizumab pegol results in improved clinical and patient-reported outcomes [abstract]. Arthritis Rheumatol. 2019;71(suppl 10). https://acrabstracts.org/abstract/earlier-treatment-of-non-radiographic-axial-spondyloarthritis-with-certolizumab-pegol-results-in-improved-clinical-and-patient-reported-outcomes/. Accessed 10 Nov 2023.
- 45.Bilgin E, Kalyoncu U, Gossec L. SAT0367 extra-articular manifestations in early axial spondyloarthritis: what is their frequency? A systematic literature review including 2854 patients. Ann Rheum Dis. 2020;79:1130–1131. doi: 10.1136/annrheumdis-2020-eular.3067. [DOI] [Google Scholar]
- 46.Bilgin E, Kalyoncu U, Gossec L. Prevalence of Extra-articular manifestations in early ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a systematic literature review and meta-analysis of 1504 patients [abstract]. Arthritis Rheumatol. 2020;72(suppl 10). https://acrabstracts.org/abstract/prevalence-of-extra-articular-manifestations-in-early-ankylosing-spondylitis-versus-non-radiographic-axial-spondyloarthritis-a-systematic-literature-review-and-meta-analysis-of-1504-patients/. Accessed 10 Nov 2023.
- 47.Sieper J, Listing J, Poddubnyy D, Song I-H, Hermann K-G, Callhoff J, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS) Ann Rheum Dis. 2016;75:1438–1443. doi: 10.1136/annrheumdis-2015-207897. [DOI] [PubMed] [Google Scholar]
- 48.Braun J, Baraliakos X. Do NSAIDs affect radiographic progression in axial SpA? Nat Rev Rheumatol. Nat Res. 2020;16:9–10. doi: 10.1038/s41584-019-0341-4. [DOI] [PubMed] [Google Scholar]
- 49.Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014;73:710–715. doi: 10.1136/annrheumdis-2012-202698. [DOI] [PubMed] [Google Scholar]
- 50.Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65:2645–2654. doi: 10.1002/art.38070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capelusnik D, Benavent D, van der Heijde D, Landewé R, Poddubnyy D, van Tubergen A, et al. Treating spondyloarthritis early: does it matter? Results from a systematic literature review. Rheumatology. 2023;62:1398–1409. doi: 10.1093/rheumatology/keac532. [DOI] [PubMed] [Google Scholar]
- 52.Ramiro S, Stolwijk C, van Tubergen A, van der Heijde D, Dougados M, van den Bosch F, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis. 2015;74:52–59. doi: 10.1136/annrheumdis-2013-204055. [DOI] [PubMed] [Google Scholar]
- 53.Poddubnyy D, Brandt H, Vahldiek J, Spiller I, Song I-H, Rudwaleit M, et al. The frequency of non-radiographic axial spondyloarthritis in relation to symptom duration in patients referred because of chronic back pain: results from the Berlin early spondyloarthritis clinic. Ann Rheum Dis. 2012;71:1998–2001. doi: 10.1136/annrheumdis-2012-201945. [DOI] [PubMed] [Google Scholar]
- 54.Protopopov M, Poddubnyy D. Radiographic progression in non-radiographic axial spondyloarthritis. Expert Rev Clin Immunol. 2018;14:525–533. doi: 10.1080/1744666X.2018.1477591. [DOI] [PubMed] [Google Scholar]
- 55.Chen M-H, Lee M-H, Liao H-T, Chen W-S, Lai C-C, Tsai C-Y. Health-related quality of life outcomes in patients with rheumatoid arthritis and ankylosing spondylitis after tapering biologic treatment. Clin Rheumatol. 2018;37:429–438. doi: 10.1007/s10067-017-3965-2. [DOI] [PubMed] [Google Scholar]
- 56.Baraliakos X, Kiltz U, Peters S, Appel H, Dybowski F, Igelmann M, et al. Efficiency of treatment with non-steroidal anti-inflammatory drugs according to current recommendations in patients with radiographic and non-radiographic axial spondyloarthritis. Rheumatology. 2017;56:95–102. doi: 10.1093/rheumatology/kew367. [DOI] [PubMed] [Google Scholar]
- 57.Braun J, Baraliakos X, Golder W, Brandt J, Rudwaleit M, Listing J, et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: Evaluation of a new scoring system. Arthritis Rheum. 2003;48:1126–1136. doi: 10.1002/art.10883. [DOI] [PubMed] [Google Scholar]
- 58.Rudwaleit M. Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept. Ann Rheum Dis. 2005;64:1305–1310. doi: 10.1136/ard.2004.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baraliakos X, Davis J, Tsuji W, Braun J. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor ? Receptor fusion protein etanercept. Arthritis Rheum. 2005;52:1216–1223. doi: 10.1002/art.20977. [DOI] [PubMed] [Google Scholar]
- 60.Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1) Ann Rheum Dis. 2013;72:815–822. doi: 10.1136/annrheumdis-2012-201766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baraliakos X, Koenig AS, Jones H, Szumski A, Collier D, Bananis E. Predictors of clinical remission under anti-tumor necrosis factor treatment in patients with ankylosing spondylitis: pooled analysis from large randomized clinical trials. J Rheumatol. 2015;42:1418–1426. doi: 10.3899/jrheum.141278. [DOI] [PubMed] [Google Scholar]
- 62.Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis. 2014;73:587–594. doi: 10.1136/annrheumdis-2012-202533. [DOI] [PubMed] [Google Scholar]
- 63.Lubrano E, Perrotta FM, Manara M, D’Angelo S, Ramonda R, Punzi L, et al. Improvement of function and its determinants in a group of axial spondyloarthritis patients treated with TNF inhibitors: a real-life study. Rheumatol Ther. 2020;7:301–310. doi: 10.1007/s40744-020-00197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sieper J, Lenaerts J, Wollenhaupt J, Rudwaleit M, Mazurov VI, Myasoutova L, et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, Part 1. Ann Rheum Dis. 2014;73:101–107. doi: 10.1136/annrheumdis-2012-203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sieper J, Landewé R, Magrey M, Anderson JK, Zhong S, Wang X, et al. Predictors of remission in patients with non-radiographic axial spondyloarthritis receiving open-label adalimumab in the ABILITY-3 study. RMD Open. 2019;5:e000917. doi: 10.1136/rmdopen-2019-000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Heijde D, Salonen D, Weissman BN, Landewé R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11:R127. doi: 10.1186/ar2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Heijde D, Landewé R, Baraliakos X, Houben H, van Tubergen A, Williamson P, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008;58:3063–3070. doi: 10.1002/art.23901. [DOI] [PubMed] [Google Scholar]
- 68.van der Heijde D, Landewé R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58:1324–1331. doi: 10.1002/art.23471. [DOI] [PubMed] [Google Scholar]
- 69.Maas F, Arends S, Brouwer E, Essers I, van der Veer E, Efde M, et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with tumor necrosis factor inhibitors. Arthritis Care Res (Hoboken) 2017;69:1011–1019. doi: 10.1002/acr.23097. [DOI] [PubMed] [Google Scholar]
- 70.Haroon N, Maksymowych WP, Rahman P, Tsui FWL, O’Shea FD, Inman RD. Radiographic severity of ankylosing spondylitis is associated with polymorphism of the large multifunctional peptidase 2 gene in the spondyloarthritis Research Consortium of Canada cohort. Arthritis Rheum. 2012;64:1119–1126. doi: 10.1002/art.33430. [DOI] [PubMed] [Google Scholar]
- 71.Park JW, Kim MJ, Lee JS, Ha Y-J, Park JK, Kang EH, et al. Impact of tumor necrosis factor inhibitor versus nonsteroidal antiinflammatory drug treatment on radiographic progression in early ankylosing spondylitis: its relationship to inflammation control during treatment. Arthritis Rheumatol. 2019;71:82–90. doi: 10.1002/art.40661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molnar C, Scherer A, Baraliakos X, de Hooge M, Micheroli R, Exer P, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis. 2018;77:63–69. doi: 10.1136/annrheumdis-2017-211544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boers N, Michielsens CAJ, van der Heijde D, den Broeder AA, Welsing PMJ. The effect of tumour necrosis factor inhibitors on radiographic progression in axial spondyloarthritis: a systematic literature review. Rheumatology. 2019;58:1907–1922. doi: 10.1093/rheumatology/kez363. [DOI] [PubMed] [Google Scholar]
- 74.Dougados M, Maksymowych WP, Landewé RBM, Moltó A, Claudepierre P, de Hooge M, et al. Evaluation of the change in structural radiographic sacroiliac joint damage after 2 years of etanercept therapy (EMBARK trial) in comparison to a contemporary control cohort (DESIR cohort) in recent onset axial spondyloarthritis. Ann Rheum Dis. 2018;77:221–227. doi: 10.1136/annrheumdis-2017-212008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sampaio-Barros PD, Bertolo MB, Kraemer MHS, Marques-Neto JF, Samara AM. Undifferentiated spondyloarthropathies: a 2-year follow-up study. Clin Rheumatol. 2001;20:201–206. doi: 10.1007/s100670170066. [DOI] [PubMed] [Google Scholar]
- 77.Poddubnyy D, Sieper J. Radiographic progression in ankylosing spondylitis/axial spondyloarthritis. Curr Opin Rheumatol. 2012;24:363–369. doi: 10.1097/BOR.0b013e328352b7bd. [DOI] [PubMed] [Google Scholar]
- 78.Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Marker-Hermann E, Zeidler H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:1369–1374. doi: 10.1136/ard.2010.145995. [DOI] [PubMed] [Google Scholar]
- 79.Taams LS, Steel KJA, Srenathan U, Burns LA, Kirkham BW. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol. 2018;14:453–466. doi: 10.1038/s41584-018-0044-2. [DOI] [PubMed] [Google Scholar]
- 80.Lubberts E. Erratum: The IL-23–IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11:562–562. doi: 10.1038/nrrheum.2015.128. [DOI] [PubMed] [Google Scholar]
- 81.Wendling D, Cedoz J-P, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–305. doi: 10.1016/j.jbspin.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 82.van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441–2451. doi: 10.1016/S0140-6736(18)31946-9. [DOI] [PubMed] [Google Scholar]
- 83.Braun J, Kiltz U, Bühring B, Baraliakos X. Secukinumab in axial spondyloarthritis: a narrative review of clinical evidence. Ther Adv Musculoskelet Dis. 2021;13:1759720X2110418. doi: 10.1177/1759720X211041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atzeni F, Carriero A, Boccassini L, D’Angelo S. Anti-IL-17 agents in the treatment of axial spondyloarthritis. Immunotargets Ther. 2021;10:141–153. doi: 10.2147/ITT.S259126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navarro-Compán V, Reveille JD, Rahman P, et al. OP0034 Ixekizumab improves signs, symptoms, and quality of life in patients with axial SpA irrespective of disease duration: results from the COAST-V, COAST-W and COAST-X trials. Ann Rheum Dis. 2022;81:24–25. doi: 10.1136/annrheumdis-2022-eular.164. [DOI] [Google Scholar]
- 86.Braun J, Baraliakos X, Deodhar A, Baeten D, Sieper J, Emery P, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070–1077. doi: 10.1136/annrheumdis-2016-209730. [DOI] [PubMed] [Google Scholar]
- 87.Braun J, Blanco R, Marzo-Ortega H, Gensler LS, Van den Bosch F, Hall S, et al. Two-year imaging outcomes from a phase 3 randomized trial of secukinumab in patients with non-radiographic axial spondyloarthritis. Arthritis Res Ther. 2023;25:80. doi: 10.1186/s13075-023-03051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braun J, Haibel H, de Hooge M, Landewé R, Rudwaleit M, Fox T, et al. Spinal radiographic progression over 2 years in ankylosing spondylitis patients treated with secukinumab: a historical cohort comparison. Arthritis Res Ther. 2019;21:142. doi: 10.1186/s13075-019-1911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braun J, Baraliakos X, Deodhar A, Poddubnyy D, Emery P, Delicha EM, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology. 2019;58:859–868. doi: 10.1093/rheumatology/key375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van der Heijde D, Østergaard M, Reveille JD, Baraliakos X, Kronbergs A, Sandoval DM, et al. Spinal radiographic progression and predictors of progression in patients with radiographic axial spondyloarthritis receiving ixekizumab over 2 years. J Rheumatol. 2022;49:265–273. doi: 10.3899/jrheum.210471. [DOI] [PubMed] [Google Scholar]
- 91.Maksymowych WP, Baraliakos X, Lambert RG, Landewé R, Sandoval D, Carlier H, et al. Effects of ixekizumab treatment on structural changes in the sacroiliac joint: MRI assessments at 16 weeks in patients with non-radiographic axial spondyloarthritis. Lancet Rheumatol. 2022;4:e626–e634. doi: 10.1016/S2665-9913(22)00185-0. [DOI] [PubMed] [Google Scholar]
- 92.Baraliakos X, Østergaard M, Gensler LS, Poddubnyy D, Lee EY, Kiltz U, et al. Comparison of the effects of secukinumab and adalimumab biosimilar on radiographic progression in patients with ankylosing spondylitis: design of a randomized, phase IIIb study (SURPASS) Clin Drug Investig. 2020;40:269–278. doi: 10.1007/s40261-020-00886-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.