Abstract
Recent literature suggests that service dogs may be a valuable complementary intervention option for posttraumatic stress disorder (PTSD) among military veterans due to the potential influence on stress response dysregulation. The aim of this short-term longitudinal study was to quantify the impact of service dogs in US military veterans with PTSD with particular attention to the cortisol awakening response. A sub aim of the study was to empirically evaluate the physiological effects of PTSD service dogs on veteran partners. We conducted a clinical trial (ID: NCT03245814) that assessed the cortisol awakening response for 245 participants at baseline and 3 months follow-up across an intervention group (service dog: veterans n = 88, partners n = 46) and control group (usual care: n = 73, partners n = 38). A total of N = 161 veterans and N = 84 partners collected whole saliva samples via a passive drool collection immediately upon waking, 30 min after waking, and 45 min after waking on three consecutive weekdays at baseline and again at follow-up. Mixed model repeated measures (MMRM) with a fixed effect of the intervention group (service dog or control) were utilized. Covariates considered for the model included time of awakening, sleep duration, sleep efficiency, prior day experiences (measured via ecological momentary assessment), traumatic brain injury, age, gender, race, ethnicity, socioeconomic status, smoking status, alcohol use, physical health, and body mass index. A total of 3951 salivary samples were collected (veterans: 2613, partners: 1338). MMRM results demonstrated that veterans with a service dog had a statistically significant higher cortisol awakening response, including the area under the curve with respect to both increase (AUCi, β = 1.46, p = 0.046) and absolute increase (AINC, β = 0.05, p = 0.035). Results were not statistically significant for partners. Findings suggest that veterans with service dogs have a higher, less blunted CAR in comparison to veterans receiving usual care alone. In veterans with a blunted morning cortisol response, service dog placement could help boost their morning cortisol response.
Subject terms: Human behaviour, Endocrinology
Introduction
Service dogs have been investigated as a valuable complementary intervention option for posttraumatic stress disorder (PTSD) among some military veterans1. Though the literature on the benefits and challenges of service dogs for veterans with PTSD is growing, few studies explore the effects of the service dog on veterans from a physiological perspective. The current study empirically identifies the impact that service dogs may have on the stress physiology of veterans and veteran partners through analysis of the cortisol awakening response (CAR).
Service dogs for veterans with PTSD
Multiple studies using standardized clinical measures suggest that PTSD service dogs may be associated with lower PTSD severity, lower depression, and higher quality of life1,2. In addition, qualitative studies report improvements in personal wellbeing and relationship health3,4. Alongside positive findings, drawbacks such as relationship challenges and difficulties with access and acceptance in public spaces have also been found3,5–7.
Only three studies to date have incorporated physiological data to explore the effects of service dogs for veterans with PTSD. One longitudinal study looked at changes in physical activity and sleep after a veteran was paired with a service dog using actigraphy8. This study found that pairing a veteran with a PTSD service dog improved both physical activity and sleep quality. Two other cross-sectional studies examined the stress hormone cortisol9,10. The first study focused only on the CAR (0 and 30 min after waking) and found that veterans with service dogs had a higher CAR than veterans on the waitlist for a service dog9. The second study incorporated both CAR samples (0, 15, 30, and 60 min after waking) and an evening sample (right before going to sleep) and found no difference between groups10. More studies incorporating physiological metrics are needed to further understand the biological processes and potential mechanisms that may play a role in the service dog intervention. These mechanisms are important to uncover as historically, human-animal interaction literature has focused predominantly on psychological processes rather than biological processes11. The current analysis adds to the literature by exploring the CAR as a potential biological process affected by the interaction of veterans and service dogs in the context of a 3 month clinical trial.
PTSD service dogs and veteran families
The potential influence of a service dog on the family beyond the veteran (with whom the dog is matched), is an emerging area of research. Benefits of the service dog for the family include acting as a “relational bridge,” building resilience, and helping the veteran be more involved in family activities3,6,12. Alongside these family-focused benefits, challenges have emerged, including increased caregiver burden, decreased caregiver satisfaction, and jealousy7,13,14. Thus far, findings suggest that the benefits outweigh the challenges in most cases. The current literature on PTSD service dogs and veteran families has yet to use a physiological measure to explore potential family influences. This manuscript seeks to fill this gap in the current literature by measuring the CAR in both veterans and veterans’ partners.
Cortisol awakening response
The hypothalamic-pituitary-adrenocortical (HPA) axis is a cascade of endocrine pathways that help the body to maintain homeostasis when challenged by stressors15,16. Dysregulation of the HPA axis has been implicated in the relationship of chronic stress and negative health effects17,18. The hormone cortisol, commonly explored as a biomarker of stress, is a product of the HPA axis19. The HPA axis follows a diurnal pattern of secretion with a peak level of cortisol occurring in the early morning15. This early morning peak is an indicator of the dynamic changes that occur from the cortisol awakening response (CAR).
There is an increasing amount of evidence to suggest that PTSD is associated with a dysregulation of the HPA axis. Specifically, it is proposed that prolonged hyperarousal-induced cortisol production can have adverse effects on the body’s ability to regain homeostasis and result in persistent alterations in HPA axis functioning20,21. Therefore, while individuals with exposure to chronic stress or trauma initially exhibit higher cortisol levels, repeated disruptions to the HPA axis feedback mechanism can result in blunted cortisol production and lower CARs22–24. Indeed, a number of studies and meta-analyses suggest that individuals with PTSD have a significantly reduced CAR and daily cortisol output in comparison to trauma-exposed controls without PTSD25,26. However, regulation of the HPA axis and its response to traumatic events is complex and variable among individuals27. Some studies suggest that CAR curves are less sensitive and flatter in individuals with PTSD24,25,28,29, while others suggest no connection between PTSD status and CAR30–33. These mixed findings reflect complexity regarding the role of genetics, pre-traumatic risk factors, and aspects of PTSD pathology and measurement34,35. Despite mixed findings, the CAR is one of the most non-invasive, available metrics to study the stress response system in the context of trauma.
Theoretical basis
A prominent theory in the field of human-animal interaction is attachment theory, which suggests that humans are biologically motivated to form bonds with caregiving figures, creating a secure base throughout their lives to satisfy basic needs of safety36. The attachment system, as a mechanism for survival, turns on when an individual experiences stress37. Throughout life, an individual develops working models of attachment in terms of responsiveness to others’ bids for interactions and the ability to achieve sufficient attachment for oneself38. These models are critical in how individuals experience stress throughout their lives. The human-animal bond can provide the features of an attachment relationship in a similar way to a human–human bond39. Service dogs may influence these working models of attachment, potentially affecting stress levels40. This analysis adds another lens to understanding this potential theoretical mechanism underlying the interaction between veterans and service dogs by measuring the CAR as a physiological biomarker for stress.
The aim of this study was to empirically evaluate the physiological effects of service dogs on military veterans with PTSD through analysis of the CAR. The sub aim of the study was to investigate the physiological effects of PTSD service dogs on veteran partners. To address these aims, we conducted a clinical trial assessing the CAR at baseline and at 3 months follow-up across an intervention group (service dog) and control group (waitlisted with unrestricted access to usual care).
Methods
Participants
Participants were recruited from a United States, non-profit service dog provider, K9s For Warriors. A total of 245 individuals collected whole saliva samples, including 161 veterans and 84 partners. This sample is completely independent of the previous Rodriguez et al., 2018 study9. A power analysis (d = 0.40, power = 0.80, alpha = 0.05) was conducted on the primary outcome of the clinical trial, veteran PTSD severity, and suggested that the required number of participants was 50 per group (N = 100 veterans). Inclusion criteria for veterans consisted of a) a PTSD diagnosis verified by an independent clinician through the Clinician-Administered PTSD Scale, and b) an approved application for a service dog from K9s For Warriors. K9s for Warriors required the following criteria to be met for a veteran to receive a service dog: (a) military service on or after 9/11/2001, (b) honorable discharge or current honorable service, (c) a diagnosis of PTSD, traumatic brain injury or military sexual trauma from a medical professional, and (d) no conviction of any crimes against animals. Partners were invited to participate if they were cohabitating with a participating veteran.
Procedure
This analysis was part of a preregistered clinical trial (clinicaltrials.gov ID NCT03245814, 10/08/2017) which was approved by the Purdue University Human Research Protection Program Institutional Review Board (IRB Protocol 1702018766) and the Purdue University Institutional Animal Care and Use Committee (IACUC Protocol 1702001541). The trial began in July 2017 and was completed in June 2020. The study was monitored by an independent Data and Safety Monitoring Board (DSMB) and all methods were carried out in accordance with relevant guidelines. Informed consent was obtained from all study participants. Due to the long existing waitlists for service dogs, complete randomization was not possible. To account for time on the waitlist and order of applications, veterans were block randomized (block size = 4, time difference between waitlist placement or service dog allocation = 1–3 months). Participants were then placed into the service dog group if their randomized schedule included receiving a dog directly after baseline while participants were placed in the control group if their randomized schedule dictated that they would not receive a service dog during the 3 month study period.
Service dogs from K9s For Warriors were predominantly sourced from shelters after a temperament screening and ensuring they were of appropriate size (24 inches at the shoulder). Service dogs received a minimum of 120 h of training by K9s For Warriors trainers before placement, ensuring that the dogs knew both basic tasks and tasks specific to mitigate PTSD symptoms. The five key PTSD specific tasks trained by the provider included: alert to anxiety, comfort from anxiety, cover (standing behind the veteran to notify of people approaching from behind), make a friend (social initiation) and block (making space between a veteran and another person)41. After this initial training, veterans and service dogs were paired during a two-week intensive training camp where K9s For Warriors staff trained the veterans as to how to interact and work with their dogs. There were no significant changes made to service dog training or placement strategies by the organization for the duration of the study.
The clinical trial included a blinded clinician assessment, standardized self-report clinical assessments, ecological momentary assessment, actigraphy, and saliva collection at baseline and 3 months after baseline (follow up). The focus of the current manuscript was to analyze the salivary cortisol data of veterans. A sub focus of the manuscript was to analyze the salivary cortisol data of the veterans’ partners. Other data streams are published elsewhere6,13,14,42,43.
Salivary cortisol sampling protocol
Veterans and partners collected whole saliva via passive drool samples on three consecutive typical weekdays at baseline and again at follow up. Samples were requested to be collected immediately upon waking, 30 min after waking, and 45 min after waking following recommendations within the literature30. Participants were advised to refrain from eating, brushing their teeth, smoking, or drinking anything aside from water until the collection ended for the day44. Participants were told that they could take any necessary medication with water during the collection period.
Participants were sent reminder text messages (Zipwhip, 2017) to aid in collection compliance. Research assistants programmed the software to send reminders on collection days at times that were based upon the wake-up times that participants had previously shared with the research team. Upon receiving the reminders, participants were asked to obtain the samples and then reply to the message as a compliance marker and timestamp for each sample.
After collecting all three samples on a collection day, participants were instructed to keep the samples in their freezer until shipping all nine samples back to the research team after the final collection day. Samples were shipped overnight via pre-paid shipping envelopes. Upon arrival at the lab, the samples were kept frozen at − 80 °C until shipped for assay where they were kept at − 20 °C. Samples were assayed using a high sensitivity enzyme immunoassay at the Salimetrics’ Saliva Lab (Carlsbad, CA) using the Salimetrics Salivary Cortisol Assay Kit (Cat. No. 1-3002). The assay had an average intra-assay coefficient of variation of 4.60% and an average inter-assay coefficient of variation 6.00%.
Measures
Cortisol awakening response (CAR)
A CAR curve was created for each day using the three time points (upon waking (s1), 30 min after waking (s2), and 45 min after waking (s3)), totaling 3 CAR curves per participant over 3 days of collection. CAR was calculated as the “absolute increase of cortisol (AINC = (max value of s2, s3) − s1)” as used in previous literature focused on measuring CAR in a military population45,46. In addition to CAR, the area under the curve with respect to increase (AUCi) was calculated to measure cortisol awakening response over time30. AUCi (area under curve with respect to increase) was calculated with Pruessner’s formulas47.
Actigraphy
Sleep and activity were measured with the use of an Actiwatch Spectrum Plus (Philips Respironics). Actigraphy data was scored consistent with gold standard procedures, considering ecological momentary assessment morning sleep diary responses. All actigraphy scorers (n = 8) achieved 80% reliability and difficult cases were discussed as a research team. Actigraphy variables included rise time, sleep duration (the length of time the participant was asleep during their relative nighttime as defined by their own self-reported typical sleep–wake cycle) and sleep efficiency (percentage of time asleep between sleep onset and offset). Minute-by-minute (epoch-by-epoch) actigraphy was used to calculate the total number of minutes the participant was active in the 60 min prior to their time of awakening as an indication of participant compliance.
Ecological momentary assessment (EMA)
Participants filled out one EMA questionnaire in the morning, two at random times during the day, and one questionnaire before they went to bed. The morning survey was sent at the participant’s wakeup time. The random surveys were constrained to a window starting 2 h after wakeup until 2 h before bedtime separated by a minimum of 4 h. Expected wakeup and bedtimes were shared by participants prior to the study period start. This study uses data from the morning survey (if veteran had any nightmares the night before or panic attacks the day before) and the daily surveys (positive and negative affect). Nightmares were included as a binary yes/no variable, while panic attacks were captured both as a binary yes/no variable and as the number of panic attacks the participant experienced since the evening check-in the day prior. Affect was included in the model as total positive affect score minus total negative affect score, where higher scores indicate more positive affect (range: − 30 to 30)48. Affect was measured using a modified version of the Discrete Emotions Questionnaire and the Positive and Negative Affect Scale49,50.
Traumatic brain injury (TBI)
Screening for a TBI was conducted via the self-report Brief Traumatic Brain Injury Scale51, a three-question preliminary questionnaire for determining if a military member may be at risk for a deployment-related TBI.
Alcohol use
The Patient-Reported Outcomes Measurement Information System (PROMIS) Alcohol Use- Short Form 7a was used in the study to determine self-reported alcohol use of participants52. The survey is eight questions with the first question determining if the individual uses alcohol and the following seven questions determining the type and extent of use, if applicable.
Physical health
The Veterans’ Rand Survey (VR-12) Physical Health component score was used to measure self-reported physical health of participants53. This subscale focuses on physical abilities, physical limitations, and associated pain with physical exertion.
Demographics
Demographics included age, gender, race/ethnicity (aggregated into a binary score of Black, Indigenous, and Person of Color or White), socioeconomic status (comfortable, just enough to make ends meet, not enough to make ends meet), smoking status (binary yes/no), body mass index. Body mass index was calculated with patient reported weight and height (weight in kg/(height in meters)2).
Data analysis
Given that the peak CAR occurs between 30 and 45 min after awakening54, accurate sampling is critical because inaccurate sampling would occur outside of the response pattern of interest. Non-adherence was defined as the participant not complying with the time stamps (0, 30, 45 min) or not following the sampling protocol (e.g., eating or smoking). When these instances occurred, the 3 days sampling protocol was rescheduled, and new supplies were sent to the participant. Following data collection, suspected non-adherence was further addressed using protocols from prior literature by which values where s2 < s1 were removed55,56. Multiple imputation by chained equations (MICE) was used to impute dropped and/or missing values as well as missing covariate data using SAS 9.4. Data was winsorized (n = 9 veterans, n = 2 partners) based on +/− 3 standard deviations from the mean9,57. Participants were excluded if they were pregnant (n = 1 veteran, n = 2 partners) or if they were on glucocorticoid medications (n = 7 veterans, n = 1 partner). We assumed that the missingness is missing at random such that the missingness can be fully explained by our other covariates. Due to the large variability in our measurements (and our imputation model), a total of 100 datasets were imputed.
Analyses were implemented in SAS 9.4 using the PROC MIXED procedure to fit a mixed model repeated measure (MMRM). The veteran model had a fixed effect of the intervention group (service dog or usual care). Covariates considered for the model included (as fixed effects): actigraphy variables (morning rise time, sleep duration, sleep efficiency, pre-awakening activity (number of minutes active in the last 60 min of sleep)); prior day experiences as measured via EMA (nightmares, panic attacks, positive and negative affect); and self-reported demographic variables (traumatic brain injury, age, gender, race/ethnicity, socioeconomic status, smoking status, alcohol use, physical health, and body mass index)44. Covariates were included in the final model based on model fit. The included variables in all models were the baseline score (e.g., mean baseline AUCi for the 3 days for the AUCi model), positive and negative affect score, physical health, number of panic attacks, and pre-awakening activity. Variable selection was conducted using backward selection with a stopping threshold of p < 0.3. Pre-awakening activity was considered to be an important covariate and therefore not used in the backward selection procedure. There were no random effects, and the error matrix was modeled over time using a banded main diagonal structure which contains no correlation between timepoints. This covariance structure was selected based on AIC and BIC criterion that was compared against the unstructured covariance matrix, which estimated weak correlations between the timepoints. Diagnostic graphics such as the Q–Q plot of residuals were used to confirm the normality assumption for residuals. For each imputed dataset, the MMRM was fit. Rubin’s rule was used to account for the variability between datasets and conduct inference on parameter estimates. Analysis of the partner data followed a similar procedure, though the percent missingness was lower and therefore imputation was not conducted.
Results
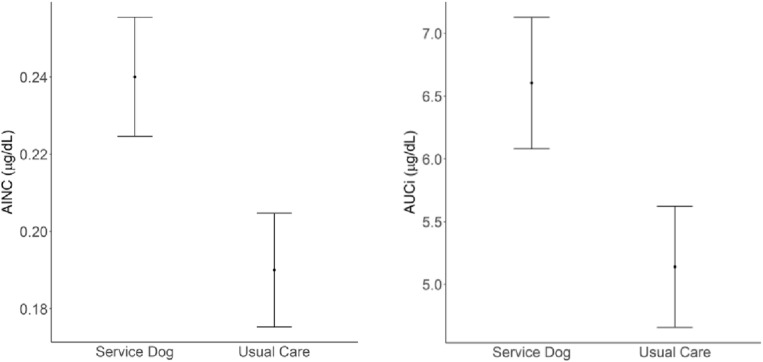
A total of 245 individuals participated in saliva sampling, including 161 veterans (n = 88 with a service dog, n = 73 receiving usual care) and 84 partners (n = 46 with a service dog, n = 38 receiving usual care; Table 1). Across both veterans and partners at baseline and 3 months follow up, a total of 3951 total samples were collected, representing 755 days of collection. Veterans collected 2613 salivary samples out of a possible 2898 samples planned (90%, three samples per day, over 3 days, at both timepoints). Samples represent collection from approximately 452 days across baseline and 3 months follow up. Partners collected 1338 salivary samples out of a possible 1512 samples planned (88%) and samples represent collection from 303 days across baseline and 3 months follow up. A total of 28.2% of veteran samples and 20.4% of partner samples were removed following the protocol for non-adherence. Results from the MMRM indicate that veterans with a service dog had a significantly higher CAR in comparison to the usual care group (Table 2). Both the area under the curve with respect to increase (AUCi, β = 1.46, SE = 0.73, p = 0.046) and the absolute increase (AINC, β = 0.05, SE = 0.02, p = 0.035) were significantly higher at follow up when controlling for baseline values (Fig. 1). PTSD Checklist (PCL-5) severity scores were not significantly correlated (< 0.1) with the AUCi or AINC. Results from the MMRM indicate that although the direction was positive (service dog group was higher), there was no significant difference in the CAR between partners of veterans with service dogs and partners of veterans without service dogs in terms of area under the curve with respect to increase (AUCi, β = 1.36, SE = 1.18, p = 0.255) or absolute increase (AINC, β = 0.04, SE = 0.04, p = 0.314).
Table 1.
Demographics of participants across groups at Baseline.
| Veterans | Partners | |||||||
|---|---|---|---|---|---|---|---|---|
| Waitlist (n = 73) | Service dog (n = 88) | t or χ2 | p | Waitlist (n = 38) | Service dog (n = 46) | t or χ2 | p | |
| Age, M (SD) | 38.2 (8.6) | 37.7 (8.5) | − 0.35 | 0.73 | 37.1 (8.8) | 36.2 (7.6) | − 0.52 | 0.6 |
| Gender, n (%) | 0.64 | 0.42 | - | 1.0 | ||||
| Female | 22 (30%) | 20 (23%) | 33 (87%) | 41 (89%) | ||||
| Male | 51 (70%) | 66 (77%) | 5 (13%) | 5 (11%) | ||||
| BIPOC, n (%) | 29 (40%) | 29 (34%) | 0.4 | 0.53 | 14 (37%) | 13 (29%) | 0.39 | 0.53 |
| Socioeconomic status, n (%) | – | 0.08 | – | 0.07 | ||||
| Comfortable | 35 (49%) | 26 (31%) | 21 (55%) | 13 (28%) | ||||
| Just enough to make ends meet | 32 (44%) | 51 (60%) | 14 (37%) | 27 (59%) | ||||
| Not enough to make ends meet | 5 (6.9%) | 8 (9.4%) | 3 (7.9%) | 6 (13%) | ||||
| Employed, n (%) | 25 (35%) | 29 (34%) | 0.02 | 0.89 | 27 (71%) | 28 (61%) | 0.47 | 0.49 |
| Education, n (%) | – | 0.44 | – | 0.6 | ||||
| Some high school | 0 | 0 | 0 (0%) | 1 (2.2%) | ||||
| High school/GED | 4 (5.6%) | 7 (8.2%) | 9 (24%) | 6 (13%) | ||||
| Some college | 22 (31%) | 32 (38%) | 11 (29%) | 16 (35%) | ||||
| 2 years degree | 11 (15%) | 17 (20%) | 5 (13%) | 6 (13%) | ||||
| 4 years degree | 19 (26%) | 18 (21%) | 8 (21%) | 13 (28%) | ||||
| Post-graduate degree | 16 (22%) | 11 (13%) | 5 (13%) | 4 (8.7%) | ||||
| Relationship status, n (%) | – | 0.09 | – | 0.44 | ||||
| Single (never married) | 6 (8.2%) | 14 (16%) | – | – | ||||
| Living with partner | 3 (4.1%) | 4 (4.7%) | 2 (5.3%) | 5 (11%) | ||||
| Married | 53 (73%) | 46 (53%) | 36 (95%) | 40 (87%) | ||||
| Divorced | 9 (12%) | 13 (15%) | 0 (0%) | 1 (2.2%) | ||||
| Separated | 2 (2.7%) | 9 (10%) | – | – | ||||
| Has pet(s), n (%) | 47 (65%) | 50 (59%) | 0.44 | 0.51 | 28 (74%) | 34 (74%) | 0.01 | 0.92 |
| Military branch, n (%) | – | 0.09 | – | – | ||||
| Air force | 5 (8.8%) | 5 (6.5%) | – | – | ||||
| Army | 31 (54%) | 45 (58%) | – | – | ||||
| Navy | 12 (21%) | 5 (6.5%) | – | – | ||||
| Marine corps | 7 (12%) | 16 (21%) | – | – | ||||
| Coast guard | 1 (1.8%) | 1 (1.3%) | – | – | ||||
| National guard | 1 (1.8%) | 5 (6.5%) | – | – | ||||
| Current PTSD treatment, n (%) | 62 (87%) | 75 (90%) | 0.12 | 0.73 | 1 (33%) | 2 (22%) | – | 1.00 |
| Number of PTSD treatments in the past 3 months, M (SD) | 9.1 (9.6) | 10.3 (11.3) | 0.69 | 0.49 | – | – | – | – |
| PTSD severity score, M (SD) | 56.3 (14.2) | 57.0 (11.0) | 0.36 | 0.72 | 16.7 (15.3) | 18.8 (16.3) | 0.61 | 0.55 |
| Veteran’s rand physical composite score, M (SD) | 38.8 (11.7) | 40.2 (9.4) | 0.83 | 0.41 | – | – | – | – |
| TBI, n (%) | 34 (47%) | 37 (44%) | 0.09 | 0.76 | – | – | – | – |
| BMI, M (SD) | 31.2 (6.8) | 31.6 (6.3) | 0.34 | 0.73 | 28.2 (4.7) | 30.2 (7.4) | 1.5 | 0.14 |
| Smokes, n (%) | 18 (25%) | 30 (36%) | 1.62 | 0.2 | 12 (32%) | 6 (13%) | 2.86 | 0.09 |
| PROMIS alcohol, M (SD) | 43.8 (7.3) | 44.8 (8.6) | 0.78 | 0.44 | 42.6 (5.9) | 42.4 (5.6) | − 0.16 | 0.87 |
| Steroid medication, n (%) | 6 (8.6%) | 1 (1.2%) | – | 0.05 | 0 (0%) | 0 (0%) | – | – |
| Pregnant, n (%) | 0 (0%) | 1 (5.0%) | – | 0.48 | 1 (3.1%) | 1 (5.9%) | – | 1.00 |
| Average sleep efficiency over baseline M (SD) | 81.0 (10.1) | 79.7 (10.1) | − 0.77 | 0.44 | 86.1 (4.9) | 84.8 (5.8) | − 1.02 | 0.31 |
| Average affect score over baseline M (SD) | − 2.1 (7.7) | − 5.9 (7.6) | − 3.11 | 0 | 7.3 (6.7) | 6.9 (8.7) | − 0.26 | 0.80 |
| Average s1 value over baseline, Mean (SD) | 0.3 (0.1) | 0.3 (0.1) | 0.95 | 0.34 | 0.34 (0.5) | 0.33 (0.2) | − 0.18 | 0.86 |
Statistical Tests performed: t-test (continuous), chi-squared test (binary categorical), Fisher’s exact test (non-binary categorical).
BIPOC black, indigenous, person of color, TBI deployment-related traumatic brain injury, BMI body mass index, PROMIS patient-reported outcomes measurement information system, S1 cortisol sample taken upon awakening.
– indicates that no data was available.
Table 2.
Veteran mixed model repeated measures analysis summary.
| AUCi | CAR (AINC) | |||||
|---|---|---|---|---|---|---|
| Variable | Estimate | SE | p value | Estimate | SE | p value |
| Intercept | 2.593 | 1.816 | 0.154 | 0.117 | 0.053 | 0.026 |
| Service dog | 1.459 | 0.731 | 0.046* | 0.045 | 0.021 | 0.035* |
| Baseline value | 0.174 | 0.087 | 0.047* | 0.141 | 0.079 | 0.077 |
| Affect | − 0.104 | 0.037 | 0.005** | − 0.003 | 0.001 | 0.006** |
| Physical health | 0.036 | 0.036 | 0.307 | 0.001 | 0.001 | 0.281 |
| Binary panic attack | 1.647 | 1.565 | 0.293 | 0.053 | 0.047 | 0.263 |
| # of panic attacks | − 0.951 | 0.680 | 0.162 | − 0.025 | 0.021 | 0.236 |
| Pre-awakening activity | − 0.003 | 0.036 | 0.932 | − 0.000 | 0.001 | 0.881 |
The usual care group is the reference group.
AUCi area under the curve with respect to increase, CAR (AINC) absolute increase of cortisol.
*p < 0.05, **p < 0.001.
Figure 1.
Mean Predicted CAR (AINC) and AUCi by group for veterans. Note Covariate adjusted means of CAR and AUCi controlling for baseline values, PANAS total score, VR-12 Physical Score as a measure of physical health, panic attack indicator, number of panic attacks, and pre-awakening activity. Bars represent standard error. *CAR (AINC) cortisol awakening response in (μg/dL), *AUCi area under the curve with respect to increase.
Discussion
The purpose of the current study was to empirically identify the impact that service dogs may have on the stress physiology of veterans and veteran partners through analysis of the cortisol awakening response (CAR). Results suggest that veterans with service dogs may have a significantly higher CAR in comparison to veterans receiving usual care alone. Given that individuals with PTSD may have dysregulation of the HPA axis, and subsequently dysregulation of the cortisol awakening response, results suggest that a service dog, as a complementary intervention, may offer a unique mechanism for regulation. These results are aligned with previous findings9 suggesting that veterans with a service dog have higher CAR than veterans on a waitlist for a service dog. Findings also suggest that affect may be minimally related to CAR. Though this finding could be considered counterintuitive to expectations, the size of the effect was negligible (− 0.104 and − 0.003 respectively), suggesting more targeted research is required to draw substantive conclusions, especially given the broader mixed findings in this domain58,59.Results from the veteran partners were not significant, indicating that the service dog may not influence partners through the same mechanism as veterans. This is not surprising as the role of the service dog is to offer individualized support to the veterans, not the partners.
Findings add information to previous studies that suggest a potential physiological mechanism for service dogs for veterans with PTSD. Previous studies have hypothesized that Attachment Theory and the role of a service dog as a secure base may help to lower stress levels by mitigating arousal, a common symptom associated with PTSD. It is possible that the severity of PTSD, and in particular the symptom of hyperarousal, plays an important role in this mechanism, potentially explaining differences in findings across studies9,10. Most of the current literature regarding the impact of service dogs on veterans focuses on psychological and social outcomes only. The addition of the biological findings in this study allow use of the biopsychosocial model of human health and wellbeing11. Understanding the interaction and outcomes of the three components is critical in identifying ways to individualize the intervention by highlighting specific mechanisms by which certain outcomes may occur.
Findings suggest that this potential mechanism is not found in military partners. The capacity by which partners interact with the service dogs is different than the interaction of the dog with the veterans. Service dogs are matched with veterans as an individualized complementary intervention for their PTSD. The service dogs are trained to do tasks that mitigate PTSD of the veterans, not to mitigate any specific need for the partners. Previous literature emerging from this same population of veteran partners suggests that partners may be influenced by the service dogs both positively in terms of higher positive emotions (e.g., calmness and confidence) and negatively in terms of lower caregiver satisfaction and higher caregiver burden13,14. Previous findings also suggest that there was no influence of service dogs on the anxiety and depression levels of partners14. Though previous literature suggests a potential indirect influence of the service dog on the partners, findings from this study suggest that the mechanism by which this influence is occurring may be different than what we are seeing with the veterans and not related to arousal dysregulation and the HPA axis.
Limitations and future research
Though this study strengthens our understanding of the influence of a service dog on the CAR, there are multiple limitations to consider. First, capturing additional covariates in the model may provide more specific insights. Though the model accounted for the majority of the recommended covariates in the expert guidelines44, future studies should capture a broader understanding of medication use (beyond the use of steroids). The severity and location of any traumatic brain injuries (TBI) should also be included, beyond a binary variable that only focuses on deployment related TBI. Additionally, future studies should ask questions regarding female participants’ menstrual cycle and breastfeeding status. Second, given the sensitivity of the CAR, adherence can be difficult. Though non-adherence was accounted for in multiple ways within the study design (e.g., text-message time stamps, actigraphy, morning sleep diaries), there was still unaccounted variance potentially because of the in-home study environment. Future studies should consider developing additional strategies regarding improving compliance, potentially collecting samples in a more controlled laboratory environment. Third, given the complexity of hyperarousal, additional physiological measures (e.g., heartrate) should be considered in tandem with CAR to more fully capture the concept of arousal in this context. Lastly, these findings capture the experience of individuals from one service dog provider. It is unknown whether these findings are similar across providers. Future studies could be designed and powered to look at the individual differences of CAR more specifically, perhaps focusing on differences between treatment non-responders and responders (as evidenced by PTSD changes and/or CAR status) or employing a latent state trait analysis for further understanding of the role of these individual differences60.
Conclusion
Results from this longitudinal clinical trial suggest that veterans with service dogs have a higher CAR than veterans in the usual care control group. When controlling for baseline values, both the area under the curve with respect to increase and the absolute increase were significantly higher at follow up for veterans with a service dog. These findings replicate a previous cross-sectional study with a similar population and suggest that service dogs may influence hyperarousal of veterans through modulation of the HPA axis9. Results for partners were null, suggesting that this potential mechanism is not found in military partners. Taken together, these findings advance the understanding of biological processes and potential mechanisms underlying the impact of service dogs for military veterans with PTSD.
Acknowledgements
We would like to thank all the veterans, partners and service dogs that made this study possible. Additionally, we would like to express gratitude to K9s For Warriors for their ongoing partnership. We thank E. Albrecht, K. Bauer, A. Brown, K. Burgess, E. Carranza, A. Evans, A. Guffey, A. Hungate, M. Huster, N. Kollars, S. Iyengar, P. Mbachu, E. Miller, K. Mummert, G. Ossello, A. Ramesh, S. Sheckell, P. Smith, A. Swain, S. Verdon, and J. Whitfield for their assistance in data collection and processing.
Author contributions
M.O. obtained funding and oversaw all aspects of the study. K.R. helped with conceptualizing the study and acquisition of data. L.N., R.Z., A.S. and E.M. analyzed the data. D.A.G. and A.J.S. assisted in interpretation of data. L.N. wrote the main manuscript text. All authors reviewed the manuscript.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health (NIH) under award number R21HD091896, Merrick PetCare and the Dogtopia Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
The data that support the findings of this study are available on request from the corresponding author, MO. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Competing interests
In the interest of full disclosure, DAG is founder and chief scientific and strategy advisor at Salimetrics LLC and Salivabio LLC. These relationships are managed by the policies on conflict of interest at Johns Hopkins University School of Medicine and the University of California at Irvine. AS is an equity-holding employee of Unlearn.AI, Inc., a company that creates software for clinical research. All other authors have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leighton SC, Nieforth LO, O’Haire ME. Assistance dogs for military veterans with PTSD: A systematic review, meta-analysis, and meta-synthesis. PLoS ONE. 2022;17:e0274960. doi: 10.1371/journal.pone.0274960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Haire ME, Rodriguez KE. Preliminary efficacy of service dogs as a complementary treatment for posttraumatic stress disorder in military members and veterans. J. Consult. Clin. Psychol. 2018;86:179–188. doi: 10.1037/ccp0000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowe TK, Sánchez V, Howard A, Western B, Barger S. Veterans transitioning from isolation to integration: A look at veteran/service dog partnerships. Disabil. Rehabil. 2018;40:2953–2961. doi: 10.1080/09638288.2017.1363301. [DOI] [PubMed] [Google Scholar]
- 4.Krause-Parello CA, Morales KA. Military veterans and service dogs: A qualitative inquiry using interpretive phenomenological analysis. Anthrozoös. 2018;31:61–75. doi: 10.1080/08927936.2018.1406201. [DOI] [Google Scholar]
- 5.Lessard G, et al. Psychiatric service dogs as a tertiary prevention modality for veterans living with post-traumatic stress disorder. Ment. Health Prev. 2018;10:42–49. doi: 10.1016/j.mhp.2018.01.002. [DOI] [Google Scholar]
- 6.Nieforth LO, Craig EA, Behmer VA, MacDermid Wadsworth S, O’Haire ME. PTSD service dogs foster resilience among veterans and military families. Curr. Psychol. 2021 doi: 10.1007/s12144-021-01990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarborough BJH, Stumbo SP, Yarborough MT, Owen-Smith A, Green CA. Benefits and challenges of using service dogs for veterans with posttraumatic stress disorder. Psychiatr. Rehabil. J. 2018;41:118–124. doi: 10.1037/prj0000294. [DOI] [PubMed] [Google Scholar]
- 8.Lessard G, Gagnon DH, Vincent C. Changes in physical activity and sleep among veterans using a service dog as a rehabilitation modality for post-traumatic stress disorder: An open-label single-arm exploratory trial Using actigraphy-based measures. J. Psychosoc. Rehabil. Ment. Health. 2020 doi: 10.1007/s40737-020-00187-4. [DOI] [Google Scholar]
- 9.Rodriguez KE, Bryce CI, Granger DA, O’haire ME. The effect of a service dog on salivary cortisol awakening response in a military population with posttraumatic stress disorder (PTSD) Psychoneuroendocrinology. 2018;98:202–210. doi: 10.1016/j.psyneuen.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Houtert EAE, Rodenburg TB, Vermetten E, Endenburg N. The impact of service dogs on military veterans and (Ex) first aid responders with post-traumatic stress disorder. Front. Psychiatry. 2022;13:834291. doi: 10.3389/fpsyt.2022.834291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gee NR, Rodriguez KE, Fine AH, Trammell JP. Dogs supporting human health and well-being: A biopsychosocial approach. Front. Vet. Sci. 2021;8:1–11. doi: 10.3389/fvets.2021.630465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitworth J, O’Brien C, Wharton T, Scotland-Coogan D. Understanding partner perceptions of a service dog training program for veterans with PTSD: Building a bridge to trauma resiliency. Soc. Work Ment. Health. 2020 doi: 10.1080/15332985.2020.1806181. [DOI] [Google Scholar]
- 13.Nieforth LO, et al. Quantifying the emotional experiences of partners of veterans with PTSD service dogs using ecological momentary assessment. Complement. Ther. Clin. Pract. 2022;48:101590. doi: 10.1016/j.ctcp.2022.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieforth LO, Miller EA, MacDermid Wadsworth S, O’Haire ME. Posttraumatic stress disorder service dogs and the wellbeing of veteran families. Eur. J. Psychotraumatol. 2022;13:2062997. doi: 10.1080/20008198.2022.2062997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. Int. J. Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Sheng JA, et al. The hypothalamic-pituitary-adrenal axis: Development, programming actions of hormones, and maternal-fetal interactions. Front. Behav. Neurosci. 2021;14:256. doi: 10.3389/fnbeh.2020.601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 18.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Stratakis C, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Stress. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- 20.Yehuda R, LeDoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol. Metab. Clin. N. Am. 2013;42:503. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr. Clin. N. Am. 2002;25:341–368. doi: 10.1016/S0193-953X(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 24.Rauch SAM, et al. Cortisol awakening response in PTSD treatment: Predictor or mechanism of change. Psychoneuroendocrinology. 2020 doi: 10.1016/j.psyneuen.2020.104714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan X, et al. The 24-hour urinary cortisol in post-traumatic stress disorder: A meta-analysis. PLoS ONE. 2020;15:e0227560. doi: 10.1371/journal.pone.0227560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro-Vale I, Carvalho D. The pathways between cortisol-related regulation genes and PTSD psychotherapy. Healthcare. 2020;8:376. doi: 10.3390/healthcare8040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauc G, Zvonar K, Vuks̆ić-Mihaljević Z, Flögel M. Post-awakening changes in salivary cortisol in veterans with and without PTSD. Stress Health. 2004;20:99–102. doi: 10.1002/smi.1001. [DOI] [Google Scholar]
- 29.Pan X, Wang Z, Wu X, Wen SW, Liu A. Salivary cortisol in post-traumatic stress disorder: A systematic review and meta-analysis. BMC Psychiatry. 2018;18:324. doi: 10.1186/s12888-018-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol. Psychol. 2008;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: A meta-analysis. Psychoneuroendocrinology. 2012;37:317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Laudenslager ML, et al. Salivary cortisol among American Indians with and without posttraumatic stress disorder (PTSD): Gender and alcohol influences. Brain Behav. Immun. 2009;23:658–662. doi: 10.1016/j.bbi.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacella ML, Feeny N, Zoellner L, Delahanty DL. The impact of PTSD treatment on the cortisol awakening response. Depress. Anxiety. 2014;31:862–869. doi: 10.1002/da.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speer KE, Semple S, Naumovski N, D’Cunha NM, McKune AJ. HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiol. Stress. 2019;11:100180. doi: 10.1016/j.ynstr.2019.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher S, et al. HPA axis regulation in posttraumatic stress disorder: A meta-analysis focusing on potential moderators. Neurosci. Biobehav. Rev. 2019;100:35–57. doi: 10.1016/j.neubiorev.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Bowlby J. Attachment and loss: Retrospect and prospect. Am. J. Orthopsychiatry. 1982;52:664–678. doi: 10.1111/j.1939-0025.1982.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 37.Simpson JA, StevenRholes W. Adult Attachment, Stress, and Romantic Relationships. Curr Opin Psychol. 2017;13:19–24. doi: 10.1016/j.copsyc.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowlby J. Attachment and Loss. Basic books; 1980. [Google Scholar]
- 39.Meehan M, Massavelli B, Pachana N. Using attachment theory and social support theory to examine and measure pets as sources of social support and attachment figures. Anthrozoös. 2017;30:273–289. doi: 10.1080/08927936.2017.1311050. [DOI] [Google Scholar]
- 40.Scotland-Coogan D. Relationships, socialization and combat veterans: The impact of receiving and training a service dog. Qual. Rep. 2019;24:1897–1914. [Google Scholar]
- 41.Rodriguez KE, LaFollette MR, Hediger K, Ogata N, O’Haire ME. Defining the PTSD service dog intervention: Perceived importance, usage, and symptom specificity of psychiatric service dogs for military veterans. Front. Psychol. 2020 doi: 10.3389/fpsyg.2020.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen CL, et al. Characterizing veteran and PTSD service dog teams: Exploring potential mechanisms of symptom change and canine predictors of efficacy. PLOS ONE. 2022;17:e0269186. doi: 10.1371/journal.pone.0269186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nieforth LO, Leighton SC, Schwichtenberg AJ, MacDermid Wadsworth S, O’Haire ME. A preliminary analysis of psychiatric service dog placements and sleep patterns of partners of veterans with PTSD. Anthrozoös. 2023 doi: 10.1080/08927936.2023.2268979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stalder T, et al. Evaluation and update of the expert consensus guidelines for the assessment of the cortisol awakening response (CAR) Psychoneuroendocrinology. 2022 doi: 10.1016/j.psyneuen.2022.105946. [DOI] [PubMed] [Google Scholar]
- 45.Clow A, et al. Post-awakening cortisol secretion during basic military training. Int. J. Psychophysiol. 2006;60:88–94. doi: 10.1016/j.ijpsycho.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Hellhammer J, et al. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 48.Leighton SC, et al. Psychiatric service dog placements are associated with better daily psychosocial functioning for military veterans with posttraumatic stress disorder. Psychol Trauma. 2023 doi: 10.1037/tra0001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harmon-Jones C, Bastian B, Harmon-Jones E. The discrete emotions questionnaire: A new tool for measuring state self-reported emotions. PLOS ONE. 2016;11:e0159915. doi: 10.1371/journal.pone.0159915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 51.Schwab KA, et al. The brief traumatic brain injury screen (BTBIS): Investigating the validity of a self-report instrument for detecting traumatic brain injury (TBI) in troops returning from deployment in Afghanistan and Iraq. Neurology. 2006;66:A235. [Google Scholar]
- 52.Cella D, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iqbal, S. U. et al. The veterans RAND 12 item health survey (VR-12): What it is and how it is used. CAPP Boston Univ. Sch. Public Health 1–12 (2007).
- 54.Stalder T, et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Kupper N, et al. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Tabachnick BG, Fidell LS. Using multivariate statistics. Allyn and Bacon; 2012. [Google Scholar]
- 58.Miller KG, et al. Trait positive and negative emotionality differentially associate with diurnal cortisol activity. Psychoneuroendocrinology. 2016;68:177–185. doi: 10.1016/j.psyneuen.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fink G. Neuroendocrine feedback control systems: An introduction. In: Fink G, Pfaff DW, Levine JE, editors. Handbook of Neuroendocrinology. Academic Press; 2012. pp. 55–72. [Google Scholar]
- 60.Doane LD, Chen FR, Sladek MR, Van Lenten SA, Granger DA. Latent trait cortisol (LTC) levels: Reliability, validity, and stability. Psychoneuroendocrinology. 2015;55:21–35. doi: 10.1016/j.psyneuen.2015.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, MO. The data are not publicly available due to their containing information that could compromise the privacy of research participants.