Abstract
We compared the abilities of different Salmonella enterica var. Typhimurium (S. typhimurium) strains harboring mutations in the genes aroA, aroAD, purA, ompR, htrA, and cya crp to present the heterologous antigen, C fragment of tetanus toxin, to the mouse immune system. Plasmid pTETtac4, encoding C fragment, was transferred into the various S. typhimurium mutants, and the levels of antigen expression were found to be equivalent. After primary oral immunization of BALB/c mice, all attenuated strains were capable of penetrating the gut epithelium and colonizing the Peyer’s patches and spleens of mice. Of all strains compared, the ΔpurA mutant colonized and persisted in the Peyer’s patches at the lowest level, whereas the ΔhtrA mutant colonized and persisted in the spleen at the lowest level. The level of specific antibody elicited by the different strains against either S. typhimurium lipopolysaccharide or tetanus toxoid was strain dependent and did not directly correlate to the mutants’ ability to colonize the spleen. The level of immunoglobulin G1 (IgG1) and IgG2a antibody specific for tetanus toxoid was determined in mice immunized with four S. typhimurium mutants. The level of antigen-specific IgG1 and IgG2a was significantly lower in animals immunized with S. typhimurium ΔpurA. Antigen-specific T-cell proliferation assays indicated a degree of variability in the capacity of some strains to elicit T cells to the heterologous antigen. Cytokine profiles (gamma interferon and interleukin-5) revealed that the four S. typhimurium mutants tested induced a Th1-type immune response. Mice were challenged with a lethal dose of tetanus toxin 96 days after oral immunization. With the exception of the S. typhimurium ΔpurA mutant, all strains elicited a protective immune response. These data indicate that the level of total Ig specific for the carried antigen, C fragment, does not correlate with the relative invasiveness of the vector, but it is determined by the carrier mutation and the background of the S. typhimurium strain.
Salmonella typhi is the predominant cause of enteric fever (17) and is transmissible via ingestion of contaminated food or water. S. typhi, once ingested, invades from the small bowel into the reticuloendothelial system, wherein the bacteria replicate in a variety of host cells (11). Live attenuated S. typhimurium strains have been studied extensively in the murine model to obtain insight into the optimal construction of live rationally attenuated S. typhi vaccines (30). Live attenuated Salmonella vaccines have been shown to confer better protection against virulent Salmonella infections than traditional whole-cell killed vaccines (5, 9). The best-characterized of the live rationally attenuated salmonellae are those harboring mutations in the prechorismate pathway (15). Prechorismate or aro mutants do not produce chorismate, an essential intermediate in the de novo synthesis of aromatic compounds including aromatic amino acids. These S. typhimurium aro mutant vaccines, as well as inducing protective immunity against virulent Salmonella infection, have been shown to elicit immune responses to a large number of heterologous antigens from a range of pathogens (30).
A number of nonaromatic rationally attenuated S. typhimurium mutants have been generated and studied in the murine model, albeit to various degrees. These mutants include deletions and insertions in genes encoding regulators (cya/crp and ompR) (6, 8), purine metabolism enzymes (purA and purE) (23, 26), and a heat shock protease (htrA) (4). Several of these nonaromatic mutants have been studied for their capacity to act as vaccine vectors. An S. typhimurium strain that harbors mutations in the genes cya and crp has been used extensively as a vaccine vector and for this reason has been included in our study (7, 16, 28, 31). The genes cya and crp encode adenylate cyclase and cyclic AMP receptor protein, respectively, which regulate expression of a number of Salmonella genes. Similarly, the htrA mutant has been investigated for its ability to carry heterologous antigens, although to a lesser extent. S. typhimurium ΔhtrA strains harbor a deletion in a serine protease gene, and their avirulence may be due to their relative incapacity to mount a complete stress response. Chabalgoity et al. (3) successfully used the htrA mutant as a vaccine vector and demonstrated protection against herpes simplex virus following immunization of mice with S. typhimurium ΔhtrA strains expressing a fusion protein comprising the C fragment of tetanus toxin and the glycoprotein D of herpes simplex virus.
The antigen selected for our study has been extensively investigated in aro-attenuated salmonellae. The C fragment of tetanus toxin (TT) is the immunogenic, nontoxic, binding portion of TT (14). Fairweather et al. (10) reported that two oral doses of an ΔaroA S. typhimurium mutant expressing C fragment from the expression plasmid pTETtac4 successfully immunized mice against lethal challenge with TT. The aim of this study was to assess the capacity of a number of isogenic attenuated Salmonella strains to act as vaccine vectors by correlating their ability to elicit immune responses with virulence, as measured by in vivo invasion and bacterial persistence.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used in this study are described in Table 1. Five of the six mutant strains studied are isogenic mutants of S. typhimurium SL1344, whereas one, harboring the cya crp mutation (χ4064), is in the S. typhimurium SR-11 background (Table 1.). S. typhimurium BRD175, BRD509, BRD578, BRD726, and LB5010 and pTETtac4 (10) were the generous gift of G. Dougan, Imperial College, London, England, and S. Chatfield and M. Roberts, Medeva Vaccine Research Unit, Imperial College. S. typhimurium SL3261 was kindly supplied by B. A. D. Stocker (Stanford University, Palo Alto, Calif.), and χ4064 was supplied by R. Curtiss III (Washington University, St. Louis, Mo.).
TABLE 1.
Attenuated S. typhimurium strains
| S. typhimurium vaccine strain | Wild-type parent strain | Mutated gene(s) | Source |
|---|---|---|---|
| BRD175 | SL1344 | purA | G. Dougan |
| BRD509 | SL1344 | aroA aroD | G. Dougan |
| BRD578 | SL1344 | ompR | G. Dougan |
| BRD726 | SL1344 | htrA | G. Dougan |
| SL3261 | SL1344 | aroA | B. A. D. Stocker |
| χ4064 | SR-11 | cya crp | R. Curtiss III |
Expression of C fragment by attenuated S. typhimurium strains.
Plasmid pTETtac4 was electroporated into the r− m+ strain S. typhimurium LB5010 (2) and then transduced by using bacteriophage P22 (Int−) as previously described (37) into BRD175, BRD509, BRD578, BRD726, and SL3261. S. typhimurium χ4064 was directly electrotransformed with pTETtac4 (1).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli (21) was performed with a 12.5% gel. Western immunoblot analyses were carried out by the method of Towbin et al. (35). Rabbit anti-TT antiserum (Calbiochem, San Diego, Calif.) was diluted at 1/1,000 in phosphate-buffered saline (PBS). Bound antibody was detected with sheep anti-rabbit–horseradish peroxidase (HRP) conjugate diluted 1/1,000 (Silenus Laboratories, Hawthorn, Victoria, Australia), and C fragment was visualized by the chromogen 4-chloro-1-naphthol (Bio-Rad, Hercules, Calif.), with H2O2 as the substrate.
Immunization of BALB/c mice with attenuated Salmonella strains.
Female 6- to 8-week-old BALB/c mice were obtained from The University of Melbourne, Department of Microbiology animal facility. For oral immunizations, bacteria were grown for 24 h without shaking before being resuspended in aliquots of PBS (200 μl/mouse). Mice were orally immunized via a gastric lavage needle. The dose given to each mouse was determined by retrospective viable count. Thirty minutes prior to oral inoculation, mice were administered 100 μl of 10% sodium bicarbonate to neutralize stomach acidity. For antibody and challenge studies, mice were immunized on day 0 and were boosted again on day 28. In colonization studies and in T-cell proliferation and cytokine assays, mice were immunized at day 0 only.
Isolation of salmonellae from spleens and Peyer’s patches of mice.
On days 7, 14, and 21 after oral administration of attenuated Salmonella strains (1 × 1010 to 3 × 1010 bacteria per mouse), mice were killed and their spleens and Peyer’s patches were removed. Spleens and Peyer’s patches were homogenized in 5 ml of sterile PBS, using a Stomacher 80 (Seward Medical, London, England) homogenizer. The number of salmonellae present in the organs was determined by viable counting of serial dilutions on Luria-Bertani (LB) agar plates containing antibiotic selection for the attenuated Salmonella strain with and without ampicillin (75 μg/ml).
Measurement of antibody responses by ELISA.
Following oral immunization on day 0 (7 × 109 to 2 × 1010 bacteria per mouse), mice were bled weekly from the retro-orbital sinus from days 14 to 56. The titers of antibody present in mouse sera were determined by using a standard enzyme-linked immunosorbent assay (ELISA) or a kinetic ELISA (20, 36). Ninety-six-well Maxisorp immunoplates (Nunc A/S, Kamstrup, Denmark) were coated overnight at 4°C with either S. typhimurium lipopolysaccharide (LPS; Sigma, St. Louis, Mo.) at a concentration of 10 μg/ml in PBS or tetanus toxoid (Commonwealth Serum Laboratories Ltd. [CSL], Melbourne, Victoria, Australia) at a concentration of 2 flocculating units/ml in carbonate coating buffer) (pH 9.6). In kinetic ELISAs, bound antibody was detected with either a sheep anti-mouse immunoglobulin (Ig)–HRP conjugate (Silenus Laboratories) or an affinity-purified rabbit anti-mouse IgA–HRP conjugate (ICN Biomedicals, Inc., Costa Mesa, Calif.). To determine IgG1 and IgG2a subclass titers by endpoint ELISA, rabbit anti-mouse IgG1 and IgG2a antibodies (Dako, Carpinteria, Calif.) were used, and bound antibody was detected with an anti-rabbit Ig–HRP conjugate (Silenus Laboratories). Serum subclass antibody titer was designated the reciprocal of the dilution of serum that gave an optical density at 492 nm (OD492) value threefold above the value obtained for preimmune serum. ELISAs were developed by using Immunopure OPD (Pierce, Rockford, Ill.), with H2O2 as the substrate.
Production of recombinant C fragment.
Recombinant C fragment was produced in Escherichia coli JM101 and purified by using a polyhistidine affinity tag located at the carboxy terminus of the protein. Briefly, a DNA sequence encoding a His6 tag was genetically fused to the 3′ end of the DNA encoding C fragment in the expression construct pTETtac115 (22) by PCR amplification. Mid-log 500-ml cultures of JM101 harboring the expression construct were grown in LB at 37°C with agitation, and C fragment expression was induced by the addition of isopropylthiogalactopyranoside. After a 4-h induction, cells were harvested and resuspended in 20 ml of lysis buffer (2 M urea, 0.1 M sodium phosphate, 0.01 M Tris-HCl [pH 8.0]). Cell lysis was completed by two passages through a French press at 1,000 lb/in2. Insoluble proteins were removed by centrifugation, and the supernatant containing soluble proteins was collected. A suspension of 50% Ni-nitrilotriacetic acid resin (2 ml; Qiagen, Chatsworth, Calif.) was added to the soluble protein fraction. Recombinant C fragment was then bound, washed under denaturing conditions, and eluted by using a pH gradient essentially as described by the manufacturer (Qiagen). The purified denatured protein was refolded by gradual dialysis against decreasing concentrations of urea and then freeze-dried prior to use. Purity was greater than 95% as determined by Coomassie blue staining of SDS-polyacrylamide gels.
Cytokine detection and antigen-induced T-cell proliferation. (i) Splenocytes.
Twenty-one days after oral immunization (1 × 1010 to 3 × 1010 bacteria per mouse), groups of three mice were killed and the spleens were removed. Single-cell suspensions of splenocytes were prepared by sieving spleens through wire mesh and washing cells three times in Hanks buffered saline containing 10% fetal calf serum. Cells were seeded in 48-well trays at a concentration of 5 × 106/ml in a volume of 0.5 ml of tissue culture medium (RPMI 1640; CSL), 10% fetal calf serum (Gibco BRL, Gaithersburg, Md.), l-glutamine (2 mM), pyruvate (1 mM), 2-mercaptoethanol (5 × 10−5 M) penicilin (100 U/ml), and streptomycin (100 μg/ml). C fragment was added to wells at concentrations of 10 and 1 μg/ml; media and concanavalin A (ConA; 5 μg/ml) were added to control wells. After incubation of cells for 42 h (37°C in 5% CO2 in air), supernatants were collected and used in cytokine ELISAs. Gamma interferon (IFN-γ) and interleukin-5 (IL-5) enzyme immunoassays were supplied by CSL and were carried out according to the manufacturer’s specifications. The detection limit of the IFN-γ ELISA was >0.014 ng/ml, whereas the detection limit of the IL-5 ELISA was >0.005 ng/ml.
(ii) Enriched T cells.
Twenty-eight days after oral immunization (2.5 × 1010 to 3.5 × 1010 bacteria per mouse), groups of five mice were killed and their spleens were removed and pooled. Single-cell suspensions of splenocytes were prepared by sieving spleens through wire mesh. Enriched T-cell suspensions were collected after passage of splenocytes through nylon wool columns (18). Enriched T cells were seeded into flat-bottom 96-well tissue culture trays (Nunc A/S) at a concentration of 3 × 105 in a volume of 100 μl in tissue culture medium. Gamma-irradiated (300 rads) syngeneic normal spleen cells were added at a concentration of 3 × 105 (in 100 μl of tissue culture medium) as a source of antigen-presenting cells. Recombinant C fragment (0.5 μg) was added to the first well and was twofold serially diluted across the plate. Control wells contained either 5 μg of ConA (Sigma) per ml or medium alone. Cells were incubated with antigen for 72 h at 37°C in 5% CO2 in air. Then 1 μCi of tritiated [3H]thymidine was added to all wells, and cells were incubated for a further 18 h before being harvested onto glass fiber filters (Packard, Meriden, Conn.). Incorporation of radioactive label was determined by direct beta counting in a Packard Matrix 9600.
Challenge of immunized mice with TT.
Mice were challenged subcutaneously with TT (batch SS620; CSL) which had been semipurified by ammonium sulfate precipitation and reconstituted in PBS at a concentration of approximately 500 50% lethal doses (LD50)/ml. Mice were given 200 μl of TT which contained 100 times the murine LD50. Mice were monitored regularly and at the first sign of paralysis were killed and designated not protected. Mice which showed no sign of paralysis for over 4 weeks were designated protected against lethal TT challenge.
Statistical analysis.
Unrelated groups of data were compared by using the unpaired Student t test. When the standard deviations of data groups were significantly different, the unrelated groups were compared by using the nonparametric Mann-Whitney test. A probability (P) value of less than 0.05 indicated that the groups were significantly different. To perform statistics on data groups which contained values below the point of detection for the particular assay, arbitrary values below the detection point were used. For example, when statistics were performed on the IgG2a subclass titers in Fig. 6, the points below the level of detection (100) were arbitrarily assigned values of 50.
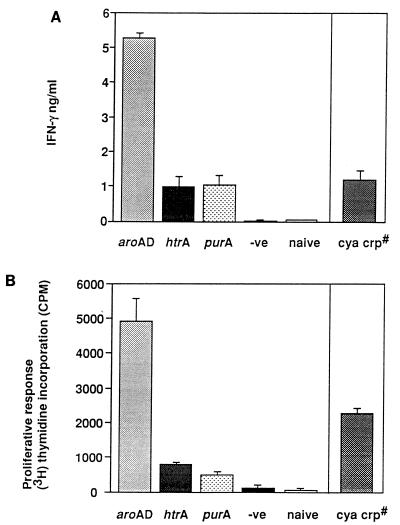
FIG. 6.
IFN-γ production and T-cell proliferation to recombinant C fragment. C-fragment-specific IFN-γ production by splenocytes isolated from mice 21 days after immunization (A) and the proliferative response to C fragment of T cells isolated from the spleens of mice 28 days after immunization (B) with attenuated S. typhimurium strains expressing C fragment: aroAD [BRD509(pTETtac4)], purA [BRD175(pTETtac4)], htrA [BRD726(pTETtac4)], and cya crp [χ4064(pTETtac4)] strains. For IFN-γ, the bar represents the mean and standard deviation of IFN-γ production in three mice. For T-cell proliferation, data are expressed as the mean counts/minute incorporated from triplicate wells (± standard deviation) minus the mean counts/minute incorporated from wells containing medium alone incubated with recombinant C fragment. The negative control (−ve) mice for each assay were immunized with BRD509 alone. #, nonisogenic mutant.
RESULTS
Expression of C fragment in S. typhimurium mutants.
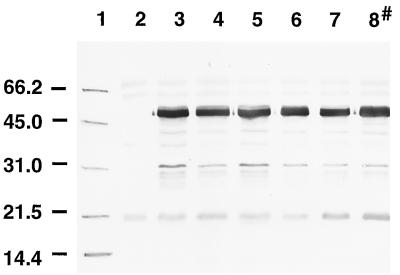
Plasmid pTETtac4 (10) was electrotransformed into S. typhimurium LB5010 (2) and transduced from this mutant into five different S. typhimurium attenuated strains by using Int− P22 bacteriophage-mediated transduction. One attenuated strain (χ4064) was electrotransformed with pTETtac4. C-fragment expression in the six S. typhimurium mutant strains, BRD509, SL3261, BRD726, BRD578, BRD175, and χ4064 harboring pTETtac4, was determined by immunoblotting. Whole-cell protein preparations of BRD509(pTETtac4), SL3261(pTETtac4), BRD726(pTETtac4), BRD578(pTETtac4), BRD175(pTETtac4), and χ4064(pTETtac4) were electrophoresed in an SDS–12.5% polyacrylamide gel, and the proteins were transferred to nitrocellulose. A protein of approximately 50 kDa of equivalent intensity was detected in all mutant strains containing pTETtac4 (Fig. 1), using rabbit polyclonal anti-TT antiserum, and was absent from the mutant strains alone (data not shown). The size of C fragment expressed by S. typhimurium corresponded to the size of purified commercial C fragment from Clostridium tetani on a Western immunoblot (data not shown).
FIG. 1.
Western blot showing the expression of C fragment by various S. typhimurium mutants. Cell lysates of S. typhimurium mutants containing pTETtac4 were subjected to SDS-PAGE, and the proteins were transferred to nitrocellulose which was subsequently probed with polyclonal anti-TT antiserum. Lanes: 1, molecular mass markers (positions are indicated in kilodaltons); 2, BRD509; 3, SL3261(pTETtac4); 4, BRD509(pTETtac4); 5, BRD726(pTETtac4); 6, BRD578(pTETtac4); 7, BRD175(pTETtac4); 8, χ4064(pTETtac4) (a nonisogenic mutant).
Persistence of the Salmonella mutants in the spleen and Peyer’s patches.
BALB/c mice were orally immunized with attenuated S. typhimurium expressing C fragment, and the kinetics of colonization and persistence of the bacteria in vivo was investigated. On days 7, 14, and 21, three to four mice from each group were killed and the numbers of bacteria present in the spleen (Fig. 2A) and Peyer’s patches (Fig. 2B) were determined by viable count. pTETtac4 appeared to be stably maintained by salmonellae in vivo since the numbers of bacteria isolated from the spleen and Peyer’s patches were equivalent on media with and without antibiotic selection for the plasmid.
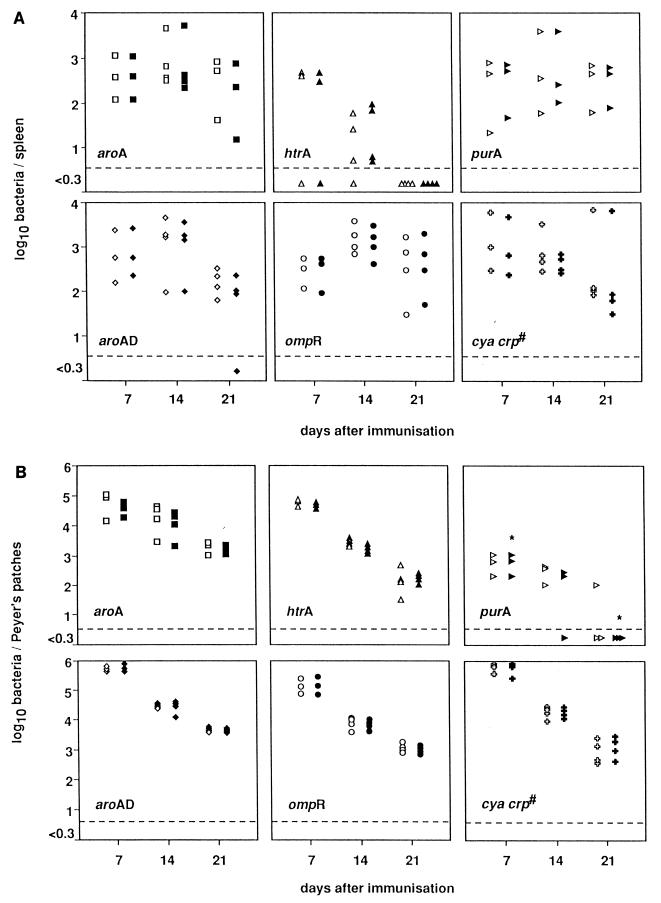
FIG. 2.
Time course of colonization and persistence of spleen and Peyer’s patches. Groups of mice were immunized orally with various S. typhimurium mutants expressing C fragment: aroA [SL3261(pTETtac4)], aroAD [BRD509(pTETtac4)], ompR [BRD578(pTETtac4)], purA [BRD175(pTETtac4)], htrA [BRD726(pTETtac4)], and cya crp [χ4064(pTETtac4)] strains. Seven, 14, and 21 days later, the mice were killed and the numbers of bacteria present in the spleen (A) and Peyer’s patches (B) of each animal were determined by isolating bacteria on LB agar (open shapes) or LB agar containing ampicillin (closed shapes). Each point denotes the number of bacteria isolated from either spleen or Peyer’s patches for one mouse. ∗, the number of bacteria isolated from the organ is significantly lower than the number of bacteria isolated from all other immunized mice. The dashed line in each graph represents the level of detection, which is <5 organisms. #, nonisogenic mutant.
Some variation was observed in the abilities of the different mutants to colonize and persist in the spleen (Fig. 2A). BRD726(pTETtac4), the htrA mutant, was the only strain which was unable to colonize the spleen of all inoculated mice. On day 14, the htrA mutant colonized the spleen at a significantly lower level than the aroA, aroAD, ompR, and cya crp mutants (P < 0.05), and by day 21 all BRD726(pTETtac4) cells had been cleared from the spleen. Even though BRD726(pTETtac4) was not detectable in the spleen on day 21, it was still possible to isolate the htrA mutant from the Peyer’s patches at this time point (Fig. 2B). All mutants were capable of colonizing the Peyer’s patches; however, the purA mutant [BRD175(pTETtac4)] was unable to persist in the mice for 21 days (Fig. 2B). BRD175(pTETtac4) colonized the Peyer’s patches on day 7 at a significantly lower level (10- to 100-fold) than all other Salmonella mutants tested (P < 0.05). By day 21, the level of bacteria detected in the Peyer’s patches of purA-immunized mice was comparatively low (P < 0.05), with all mice having cleared plasmid-bearing bacteria.
Anti-Salmonella and anti-tetanus toxoid antibody responses generated in BALB/c mice immunized with S. typhimurium mutants expressing C fragment.
Groups of five BALB/c mice were immunized orally with attenuated S. typhimurium expressing C fragment and were similarly boosted on day 28. All mice were bled weekly from days 14 to 56, and the serum antibody response was examined by using a kinetic ELISA against Salmonella LPS and TT (Fig. 3).
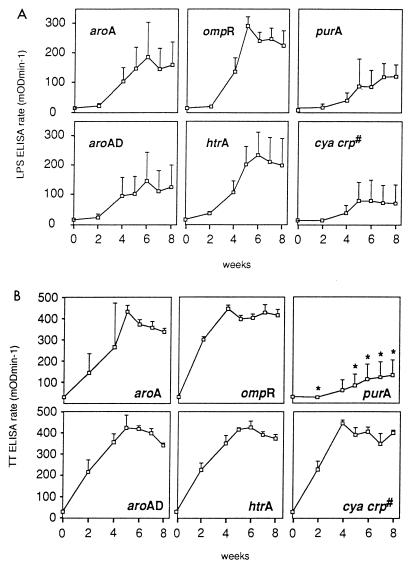
FIG. 3.
LPS-specific and tetanus toxoid-specific serum antibody responses. Groups of mice were immunized orally with various S. typhimurium mutants expressing C fragment (for identities, see the legend to Fig. 2). Serum samples were collected at two weekly intervals prior to secondary immunization on day 28; after the boost, sera were collected weekly until day 56. The amounts of S. typhimurium LPS-specific (A) and tetanus toxoid-specific (B) total Ig present in serum were determined by using a kinetic ELISA with sera diluted 1/1,000. Each point represents the mean and standard deviation of sera from five mice. #, nonisogenic mutant; ∗, the anti-tetanus toxoid antibody titer detected in the sera of purA [BRD175(pTETtac4)]-immunized mice is significantly different (P < 0.05) than the antibody titers detected in all other mice.
LPS-specific serum antibody (total Ig) was detected in all mice immunized with the six different attenuated S. typhimurium strains expressing C fragment (Fig. 3A). In all mice, the anti-LPS response took 14 days after immunization to increase, and then the antibody titer peaked either on day 35 or on day 42. On day 35, the ompR mutant induced the highest (P < 0.05) anti-LPS antibody titer compared with all other S. typhimurium mutants. The purA mutant induced significantly lower antibody titers than the ompR mutant on days 28 to 56 (P < 0.05). The htrA mutant which colonized and persisted poorly in the spleen was still capable of inducing an anti-LPS response equivalent to that induced by the four other isogenic mutants, ΔaroA, ΔaroAD, ΔompR, and ΔpurA, all of which demonstrated greater splenic persistence. These results suggest that the level of splenic colonization and persistence does not correlate with the homologous antibody response induced by the Salmonella carrier.
The levels of antibody induced against the heterologous antigen C fragment expressed in the six different mutants are shown in Fig. 3B. Four isogenic mutants, harboring mutations in aroA, aroAD, ompR, and htrA [SL3261(pTETtac4), BRD509(pTETtac4), BRD578(pTETtac4), and BRD726(pTETtac4), respectively], induced a higher level of anti-tetanus toxoid total Ig than the purA mutant [BRD175(pTETtac4)] (P < 0.05). This result suggests that the type of attenuating mutation does affect the strains’ ability to present a heterologous antigen to the host immune system. The htrA mutant, BRD726(pTETtac4), which colonized and persisted poorly in the spleen, induced a higher level of antibody against tetanus toxoid than the purA mutant [BRD175(pTETtac4)] (P < 0.05), which remained detectable in the spleen on day 21. This observation suggests that splenic persistence is not essential for the induction of a high antibody titer against the carried antigen. In addition to inducing the lowest level of antibody against tetanus toxoid (P < 0.05), the purA mutant [BRD175(pTETtac4)] colonized and persisted poorly in the Peyer’s patches compared with the other isogenic mutants. This result indicates that Peyer’s patch colonization and persistence of the Salmonella carrier may be necessary to induce a high antibody response against the carried antigen.
Similar levels of LPS-specific IgA antibody were detected in the sera of mice immunized with all of the isogenic S. typhimurium mutants expressing C fragment [SL3261(pTETtac4), BRD509(pTETtac4), BRD578(pTETtac4), BRD726(pTETtac4), and BRD175(pTETtac4)], whereas the level of tetanus toxoid-specific serum IgA antibody detected in mice immunized with the purA mutant expressing C fragment was considerably less than that detected in the serum of all other immunized mice (data not shown).
χ4064(pTETtac4) is a nonisogenic mutant harboring deletions in the genes cya and crp of S. typhimurium SR-11. This strain was capable of inducing a low-level antibody response to S. typhimurium LPS which was equivalent to the level induced by the purA mutant (P > 0.05); in contrast, χ4064(pTETtac4) induced a significantly higher anti-tetanus toxoid antibody titer than the purA mutant expressing C fragment (P < 0.05).
Challenge of mice immunized with S. typhimurium mutants expressing C fragment with 100 LD50 of tetanus toxin.
On day 96, mice that had previously been immunized with S. typhimurium mutants expressing C fragment on days 0 and 28 were challenged with 100 LD50 of TT. Five mice from each group were challenged subcutaneously with TT and then observed for signs of paralysis. Four of the five mice immunized with BRD175(pTETtac4), harboring a mutation in the purA gene, showed signs of paralysis. Two mice showed paralysis on day 1 after challenge, one showed paralysis on day 2, and one showed paralysis on day 5. The mice that displayed paralysis upon challenge corresponded to those animals with the lowest levels of anti-tetanus toxoid antibody detected in serum at day 56 (Fig. 4).
FIG. 4.
Challenge of immunized mice with TT. The amount of tetanus toxoid-specific total Ig in sera from individual mice on day 56 after immunization with S. typhimurium aroA [SL3261(pTETtac4)] (□), aroAD [BRD509 (pTETtac4)] (○), ompR [BRD578(pTETtac4)] (▵), htrA [BRD726(pTETtac4)] ( ), purA [BRD175(pTETtac4)] (▹), and cya crp [χ4064(pTETtac4)] (◂) strains expressing C fragment, as determined by kinetic ELISA when serum is diluted 1/1,000. The symbols that lie beneath the dashed line denote individual mice that showed signs of paralysis when challenged on day 96 with 100 LD50 of tetanus toxin; the symbols that lie above the dashed line denote mice that were protected against lethal TT challenge. #, nonisogenic mutant.
Anti-tetanus toxoid IgG subclass responses induced in the serum of BALB/c mice immunized with S. typhimurium mutants expressing C fragment.
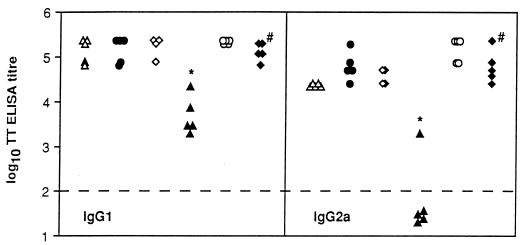
To further define humoral responses induced after S. typhimurium immunization, the titers of serum IgG subclass antibodies, IgG1 and IgG2a specific for tetanus toxoid, were determined in mice 42 days after immunization with the six different S. typhimurium mutants harboring pTETtac4 (Fig. 5). IgG1 antibody specific for tetanus toxoid was detected in the sera of all mice; however, the titer of IgG1 detected in mice immunized with BRD175(pTETtac4) was significantly lower than in all other immunized mice tested (P < 0.05). Mice immunized with SL3261(pTETtac4), BRD509(pTETtac4), BRD578(pTETtac4), BRD726(pTETtac4), and χ4064(pTETtac4) had detectable amounts of tetanus toxoid-specific IgG2a in serum on day 42. IgG2a specific for tetanus toxoid was detected in only one of five mice immunized with BRD175(pTETtac4). The subclass analysis revealed that the purA mutant induced a lower IgG1 and IgG2a antibody response (P < 0.05) than the five other mutants expressing C fragment, with a predominance of IgG1 specific for tetanus toxoid.
FIG. 5.
Anti-tetanus toxoid-specific IgG1 and IgG2a antibody. The amount of tetanus toxoid-specific IgG1 and IgG2a present in sera from individual mice on day 42 after immunization with aroA [SL3261(pTETtac4)] (▵), aroAD [BRD509(pTETtac4)] (•), htrA [BRD726(pTETtac4)] (◊), purA [BRD175(pTETtac4)] (▴), ompR [BRD578(pTETtac4)] (○), and cya crp [χ4064(pTETtac4)] (⧫) strains expressing C fragment was determined by ELISA. Each point represents a serum titer from an individual mouse. Serum subclass antibody titers were designated the reciprocal of the dilution of specific antibody that gave an OD492 value fivefold above the value obtained for preimmune sera. ∗, the anti-tetanus toxoid IgG1 and IgG2a titers detected in the sera of purA [BRD175(pTETtac4)]-immunized mice are significantly different (P < 0.05) than the IgG1 and IgG2a titers detected in all other mice. The dashed line represents the ELISA limit of detection. #, nonisogenic mutant.
IFN-γ production and T-cell proliferative responses to C fragment detected in mice immunized with S. typhimurium mutants expressing C fragment.
The inability of the purA mutant to act efficiently as a vaccine vector and provide protection against TT challenge led us to investigate whether this strain was deficient in its ability to induce T cells. The type of T helper (Th) response was initially assessed by detecting IFN-γ (a Th1 cytokine) or IL-5 (a Th2 cytokine) in supernatants of stimulated splenocyte cultures. After stimulation of splenocytes in vitro with recombinant C fragment, BRD509(pTETtac4)-immunized mice produced up to 5 ng of IFN-γ per ml, whereas BRD175(pTETtac4), BRD726(pTETtac4), and χ4064(pTETtac4) produced on average 1 ng/ml (Fig. 6A). Splenocytes from naive mice and mice immunized with BRD509 alone did not produce detectable levels of IFN-γ upon in vitro stimulation with C fragment (Fig. 6A). IL-5 was not detected in the supernatants of C fragment-stimulated splenocyte cultures derived from any Salmonella(pTETtac4)-immunized mice, naive control mice, or mice immunized with BRD509 alone (<4.58 pg/ml) (data not shown). However, IL-5 was detected in the supernatants of ConA (5 μg/ml)-stimulated splenocytes from all mice (from 40 to 240 pg/ml) (data not shown). Antigen-specific production of IFN-γ in the absence of IL-5 suggests all the Salmonella mutants expressing C fragment induced a Th1-type immune response against the carried antigen. These results suggest that it is not the type of Th response that limits the immunogenicity of C fragment when delivered by the purA mutant, BRD175(pTETtac4).
Analysis of cytokine production by unfractionated splenocytes was extended by examination of the ability of enriched T cells isolated from immunized mice to proliferate in the presence of recombinant C fragment. Mice were immunized with S. typhimurium BRD509(pTETtac4), BRD726(pTETtac4), BRD175(pTETtac4), and χ4064(pTETtac4). The results in Fig. 6B demonstrate that the four Salmonella mutants differ in the capacity to elicit C-fragment-specific T cells. Enriched T cells from the spleens of mice immunized with BRD509(pTETtac4) displayed the greatest proliferative response to C fragment, which was at least threefold higher than the response induced in χ4064(pTETtac4)-immunized mice. T cells from BRD726(pTETtac4)- and BRD175(pTETtac4)-immunized mice proliferated to a lesser extent but nevertheless proliferated marginally greater than T cells from BRD509-immunized or naive mice. Enriched T cells from the spleen of mice immunized with BRD509(pTETtac4) also displayed the greatest proliferative response to S. typhimurium soluble protein, which was approximately threefold higher than the response induced in χ4064(pTETtac4)-, BRD726(pTETtac4)-, and BRD175(pTETtac4)-immunized mice (data not shown). The finding that BRD509(pTETtac4) displayed the strongest proliferative response specific to C fragment correlates with the ability of this strain to induce the largest amount of IFN-γ from splenocytes isolated at day 21 postimmunization.
DISCUSSION
Insight into the potential of live, rationally attenuated S. typhi vaccines has been obtained through studies of attenuated S. typhimurium in the mouse model. While the ability of various mutants of S. typhimurium to act efficaciously as live vaccines has been intensively investigated in mice, only a limited number of these mutants have been evaluated as vaccine vectors (10, 28; reviewed in reference 30). This study describes the comparative ability of different attenuated S. typhimurium strains to elicit humoral and cellular immune responses against a heterologous antigen and to S. typhimurium LPS. Our study shows that S. typhimurium ΔaroA, ΔaroAD, ΔhtrA, ΔompR, and Δcya Δcrp mutants can all act efficiently as vaccine vectors for the delivery of C fragment and induce protective immune responses against TT challenge. In contrast, the immune response to C fragment when delivered by the S. typhimurium ΔpurA mutant was not protective. Our findings suggest that an effective Salmonella vaccine vector is one which efficiently colonizes the Peyer’s patches. In contrast, splenic colonization does not appear to be an essential characteristic. To our knowledge, this is the first study which compares isogenic Salmonella strains as vaccine vectors.
Wild-type S. typhimurium strains are highly virulent in the mouse model (27), and this virulence can differ between different wild-type S. typhimurium isolates (6, 8). The virulence of the wild-type background strain and the type of attenuating lesion can jointly influence the level of attenuation and immunogenicity of the resultant S. typhimurium mutant strain. The S. typhimurium mutants assessed in this study had been attenuated by either insertions or deletions in one of two classes of genes, metabolic (15, 26) and regulatory (6, 8). Five of the six mutants investigated are isogenic mutants, i.e., constructed in the same wild-type S. typhimurium strain, SL1344. The cya crp mutation was constructed in S. typhimurium SR-11. We examined the Δcya Δcrp mutant since it has been widely studied as a vaccine vector by a number of different workers (7, 16, 28, 31).
Karem et al. (19) have previously reported on the comparative abilities of two S. typhimurium mutants to act as vaccine vectors. The two nonisogenic ΔaroA ΔaroD and Δcya Δcrp mutants were constructed in the background strains, SL1344 and SR-11, respectively. It is difficult to attribute vaccine vector immunogenicity to differences in attenuating mutation, as the virulence of the wild-type background strain harboring the mutation influences the virulence and therefore immunogenicity of the strain. The heterologous antigen that was delivered in these two mutant strains, C fragment, was also expressed from two different promoters, the nirB promoter and the trc promoter. From this study, it was possible to determine which vaccine as a whole was more efficacious; however, the difference in background strain and expression level of the heterologous antigen made it not possible to attribute the differences in immunogenicity to the mutation used to attenuate the carrier bacterium.
In this study, we compared the levels of organ colonization and S. typhimurium-specific antibody responses only in Salmonella mutants that harbored the C-fragment expression plasmid and did not investigate the Salmonella mutants alone. Previously, we compared S. typhimurium without a C-fragment expression plasmid and S. typhimurium with a C-fragment expression plasmid and found no significant difference in the numbers of bacteria isolated from organs and no significant difference in the S. typhimurium-specific antibody responses (unpublished data). For this reason, the Salmonella mutants alone (without the C-fragment expression plasmid) were not further examined here.
This study investigated whether isogenic S. typhimurium mutants, harboring a C-fragment expression plasmid, differed in the ability to colonize mouse organs. All strains were capable of invading the Peyer’s patches and the spleen. The Salmonella mutants were able to translocate from the gut lumen to the intestinal mucosa and reside in Peyer’s patches, albeit the purA mutant did so to a lesser extent. The different mutants were also able to traffic to and/or survive within the spleen, with the htrA mutant displaying a reduced capacity in this regard. The inability of the ΔhtrA mutant to persist in the spleen after oral inoculation is consistent with previous studies demonstrating that the ΔhtrA mutant persisted in the spleens and livers of mice at a lower level than the ΔaroA mutant after intravenous immunization (4). Tacket et al. (33) also reported that S. typhi mutants harboring deletions in aroC, aroD, and htrA were well tolerated in human volunteers, were not detected in blood cultures, and were only transiently found in stool samples. The addition of the ΔhtrA mutation to the Δaro S. typhi strain CVD908 lowered the level of reactogenicity while retaining a potentially protective level of immunogenicity (33, 34).
We have shown, using isogenic mutants, that the type of mutation used to attenuate the Salmonella strains affects the vaccine’s immunogenicity, with regard to both the anti-Salmonella antibody response and the antibody response directed toward a heterologous antigen. The Salmonella ΔaroA, ΔaroAD, ΔompR, and ΔhtrA mutants expressing C fragment induced high levels of anti-tetanus toxoid antibody, whereas the ΔpurA mutant induced a significantly lower level. Of the mutants tested, only the purA mutant could not induce an antibody response sufficient to afford protection against 100 LD50 of TT. Previous studies have shown that the purA mutant, when injected intravenously, is unable to induce an immune response which protects mice against challenge with virulent salmonellae (26). Interestingly, the purA mutant initially colonized the Peyer’s patches at a level 10- to 100-fold lower than those of the other S. typhimurium mutants which were capable of inducing a protective response, and it was cleared from the Peyer’s patches by day 21. In contrast, the ΔpurA mutant colonized the spleen as well as, or at a higher level than, the other four isogenic mutant strains studied. In comparison to the other mutants, the ΔhtrA mutant colonized and persisted in the Peyer’s patches at a relatively high level but disseminated to or survived within the spleen at a comparatively low level. Given that the ΔhtrA mutant induced a higher level of antibody to tetanus toxoid than that elicited by the ΔpurA mutant, these observations suggest that splenic colonization is not necessary for efficient heterologous antigen delivery. Indeed, these results imply that a higher level of Peyer’s patch colonization and persistence may be an important characteristic of successful vaccination with attenuated S. typhimurium. The importance of Peyer’s patch colonization in induction of specific immune responses following oral immunization with attenuated S. typhimurium was originally indicated in studies by Galán and Curtiss (12). S. typhimurium harboring an attenuating mutation in phoP (of the phoP-phoQ two-component regulatory system) (24) were able to colonize the Peyer’s patches of orally immunized mice but were relatively deficient in splenic colonization. In the absence of efficient splenic colonization, this mutant was nevertheless capable of inducing an immune response which protected mice from wild-type S. typhimurium challenge (12).
The humoral immune response induced by the S. typhimurium mutants was further investigated through determining the IgG subclass of anti-tetanus toxoid antibody. Karem et al. (19) found that the anti-tetanus toxoid response in serum of mice immunized with an ΔaroA mutant of salmonellae expressing C fragment was predominantly IgG2a, suggestive of a Th1 type of immune response (32). Analysis of the subclass of the IgG antibody specific for tetanus toxoid induced by immunization with BRD175(pTETtac4) revealed significantly lower IgG1 and IgG2a titers compared to other isogenic mutants. Proportionally more tetanus toxoid-specific IgG1 was induced by the ΔpurA mutant than IgG2a. The overall amount of anti-tetanus toxoid antibody induced by the ΔpurA mutant was significantly lower than for other isogenic mutants. To determine whether this resulted from poor T-cell stimulation by the purA mutant expressing C fragment, the antigen-specific production of IFN-γ and IL-5 was investigated. All isogenic strains induced immune splenocytes which, when restimulated in vitro with recombinant C fragment, produced IFN-γ but no detectable IL-5. Our findings are indicative of a Th1 response (25) and are consistent with those of VanCott et al. (38), who detected high levels of IFN-γ but no IL-5 in the spleen and Peyer’s patch of mice immunized with an aromatic mutant of S. typhimurium expressing C fragment. The cytokine assays suggest that all strains including purA strains induce a Th1 type of immune response. The inability of the purA mutant expressing C fragment to protect mice from TT challenge was thus probably attributable to the quantitative (i.e., titer) rather than qualitative (i.e., IgG subclass or Th type) differences in the response induced and not directly attributable to reduced T-cell induction since htrA and purA mutants induced similar levels of IFN-γ from splenocytes and proliferative T-cell responses.
In our study, there was no direct correlation between the level of anti-Salmonella antibody induced by a particular mutant strain and the level of C-fragment antibody elicited. The S. typhimurium Δcya/Δcrp mutant strain induced high levels of anti-tetanus toxoid antibodies but a relatively low level of anti-LPS antibodies. The reasons for this are not obvious but suggest either that the two antigens studied (LPS and C fragment) are treated independently by the immune system or that different amounts of LPS are made by the strains in vivo. Adenylate cyclase (cya) and cyclic AMP receptor (crp) are required for the regulation of a large number of genes and operons which in turn control transport processes and the expression of flagella, fimbriae, and various outer membrane proteins. The cya crp mutation is not known to effect LPS biosynthesis, but given the large number of genes necessary for complete LPS biogenesis (29) and the global regulatory effects of crp, it is possible that Δcya crp affects LPS expression in vivo. Recent studies have shown a role for PhoP and PhoQ in determining the type of LPS produced by salmonellae and suggest that LPS biogenesis may not be a constitutive phenomenon (13).
This study demonstrates that the level of total Ig elicited against a carried antigen does not necessarily correlate with the ability of the Salmonella strain to colonize and persist in the spleen but does correlate with the ability of the strain to colonize and persist in the Peyer’s patches following oral immunization. The purA mutant expressing C fragment was unable to colonize and persist in the Peyer’s patches at a high level. Indeed, four of five mice immunized with this strain were not protected against lethal challenge with TT. These observations suggest that the Peyer’s patches are a primary inductive site for the protective antibody response elicited to C fragment when delivered orally by attenuated salmonellae. Further studies into why purA mutants poorly deliver heterologous antigens may provide insight into what properties are needed to obtain a more efficient vaccine vector. The ability of a bacterium to act efficiently as a vaccine vector is determined by the carrier mutation in conjunction with the background S. typhimurium strain, as both of these factors determine the ultimate level of residual virulence and consequently the strains’ immunogenicity. Finally, Salmonella carriers which invade and persist in the spleen at a low level, such as the ΔhtrA mutant, can deliver heterologous antigens to elicit serum Ig to antibody levels equivalent to that of carriers that colonize the spleen at a higher level. This may be advantageous in the construction of S. typhi vaccines where splenic invasion by the bacterium has been found to be an unwanted side effect of vaccination with live vaccines.
ACKNOWLEDGMENTS
BRD175, BRD509, BRD578, BRD726, LB5010, and pTETtac4 were generously donated by G. Dougan (Imperial College, London, England). S. typhimurium SL3261 was kindly supplied by B. A. D. Stocker (Stanford University, Palo Alto, Calif.), and χ4064 was supplied by R. Curtiss III (Washington University, St. Louis, Mo.).
S.J.D. is a recipient of an Australian Postgraduate Award. R.A.S. and C.P.S. are members of the Cooperative Research Centre for Vaccine Technology. This study was supported in part by the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Binotto J, MacLachan P, Sanderson K E. Electrotransformation in Salmonella typhimuriumLT2 strains. Can J Microbiol. 1991;37:474–477. doi: 10.1139/m91-078. [DOI] [PubMed] [Google Scholar]
- 2.Bullas L R, Ryu J I. Salmonella typhimurium LT2 strains which are r− m+for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983;156:471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabalgoity J A, Khan C M A, Nash A A, Hormaeche C E. A Salmonella typhimurium htrA live vaccine expressing multiple copies of a peptide comprising amino acids 8-23 of herpes simplex virus glycoprotein D as a genetic fusion to tetanus toxin fragment C protects mice from herpes simplex infection. Mol Microbiol. 1996;19:791–801. doi: 10.1046/j.1365-2958.1996.426965.x. [DOI] [PubMed] [Google Scholar]
- 4.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroAin the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 5.Collins F M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974;38:371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtiss R, III, Kelly S M. Salmonella typhimuriumdeletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtiss R, III, Goldschmidt R M, Fletchall N B, Kelly S M. Avirulent Salmonella typhimurium cya crporal vaccine strains expressing a streptococcal colonisation and virulence antigen. Vaccine. 1988;6:155–160. doi: 10.1016/s0264-410x(88)80020-3. [DOI] [PubMed] [Google Scholar]
- 8.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompRmutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenstein T K, Sultzer B M. Immunity to Salmonella infections. Adv Exp Med Biol. 1983;162:261–296. doi: 10.1007/978-1-4684-4481-0_26. [DOI] [PubMed] [Google Scholar]
- 10.Fairweather N F, Chatfield S, Makoff A J, Strugnell R A, Bester J, Maskell D J, Dougan G. Oral vaccination of mice against tetanus by use of a live attenuated Salmonellacarrier. Infect Immun. 1990;58:1323–1326. doi: 10.1128/iai.58.5.1323-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay B B. Molecular and cellular mechanisms of Salmonella pathogenesis. Curr Top Microbiol Immunol. 1994;192:163–185. doi: 10.1007/978-3-642-78624-2_8. [DOI] [PubMed] [Google Scholar]
- 12.Galán J E, Curtiss R., III Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989;6:433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 13.Guo L, Lim K B, Gunn J S, Bainbridge B, Darveau R P, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 14.Helting T B, Nau H H. Analysis of the immune response to papain digestion products of tetanus toxin. Acta Pathol Microbiol Immunol Scand Sect C. 1984;92:59–63. doi: 10.1111/j.1699-0463.1984.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimuriumare non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 16.Jagusztyn-Krynicka E, Clarke-Curtiss J E, Curtiss R I. Escherichia coli heat-labile toxin subunit B fusions with Streptococcus sobrinus antigens expressed by Salmonella typhimuriumoral vaccine strains: importance of the linker for antigenicity and biological activities of the hybrid proteins. Infect Immun. 1993;61:1004–1015. doi: 10.1128/iai.61.3.1004-1015.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones B D, Falkow S. Salmonellosis: Host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 18.Julius M H, Simpson E, Herzenberg L A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 19.Karem K L, Chatfield S, Kuklin N, Rousse B T. Differential induction of carrier antigen-specific immunity by Salmonella typhimuriumlive-vaccine strains after single mucosal or intravenous immunization of BALB/c mice. Infect Immun. 1995;63:4557–4563. doi: 10.1128/iai.63.12.4557-4563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kung V T, Humphries G M K. Kinetic ELISA in microtitre plates. Clin Chem. 1987;33:1573–1574. [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Makoff A J, Oxer M D, Romanos M A, Fairweather N F, Ballantine S. Expression of tetanus toxin fragment C in E. coli: high level expression by removing rare codons. Nucleic Acids Res. 1989;17:10191–10202. doi: 10.1093/nar/17.24.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McFarland W C, Stocker B A D. Effect of different purine auxotrophic mutations on mouse-virulence of a Vi-positive strain of Salmonella dublin and of two strains of Salmonella typhimurium. Microb Pathog. 1987;3:129–141. doi: 10.1016/0882-4010(87)90071-4. [DOI] [PubMed] [Google Scholar]
- 24.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimuriumvirulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.O’Callaghan D, Maskell D, Liew F Y, Easmon C S, Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attenuation, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988;56:419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plant J, Glynn A A. Genetics of resistance to infection with Salmonella typhimuriumin mice. J Infect Dis. 1976;133:72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- 28.Redman T K, Harmon C C, Lallone R L, Michalek S M. Oral immunization with recombinant Salmonella typhimurium expressing surface protein antigen A of Streptococcus sobrinus: dose response and induction of protective humoral response in rats. Infect Immun. 1995;63:2004–2011. doi: 10.1128/iai.63.5.2004-2011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves P R. Biosynthesis and assembly of lipopolysaccharide. New Compr Biochem. 1994;27:281–314. [Google Scholar]
- 30.Roberts M, Chatfield S N, Dougan G. Salmonella as carriers of heterologous antigens. In: O’Hagan D T, editor. Novel delivery systems for oral vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 27–58. [Google Scholar]
- 31.Stabel T J, Mayfield J E, Tabatabai L B, Wannemuehler M J. Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortis. Infect Immun. 1990;58:2048–2055. doi: 10.1128/iai.58.7.2048-2055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens T L, Bossie A, Sanders W M, Fernandez-Botran R, Coffman R L, Mosmann T R, Vitetta E S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 33.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroDand immune responses in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tacket C O, Hone D M, Losonsky G A, Guers L, Edelman R, Levine M M. Clinical acceptability and immunogenicity of CVD908 Salmonella typhivaccine strain. Vaccine. 1992;10:443–446. doi: 10.1016/0264-410x(92)90392-w. [DOI] [PubMed] [Google Scholar]
- 35.Towbin H T, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang V C, Wilson R C, Peralta J M. Quantitative single tube kinetic dependent K-ELISA assay. Methods Enzymol. 1983;92:391–403. doi: 10.1016/0076-6879(83)92033-5. [DOI] [PubMed] [Google Scholar]
- 37.Turner S J, Carbone F R, Strugnell R A. Salmonella typhimurium ΔaroA ΔaroDmutants expressing a foreign recombinant protein induce specific major histocompatibility complex class I-restricted cytotoxic T lymphocytes in mice. Infect Immun. 1993;61:5374–5380. doi: 10.1128/iai.61.12.5374-5380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages and derived cytokines following oral immunisations with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]