Abstract
Objective
Olaparib is a PARP (poly-ADP-ribose polymerase) inhibitor used for maintenance therapy in BRCA-mutated cancers. Metformin is a first-choice drug used in the treatment of type 2 diabetes. Both drugs are commonly co-administered to oncologic patients with add-on type 2 diabetes mellitus. Olaparib is metabolized by the CYP3A4 enzyme, which may be inhibited by metformin through the Pregnane X Receptor. In vitro studies have shown that olaparib inhibits the following metformin transporters: OCT1, MATE1, and MATE2K. The aim of the study was to assess the influence of ‘the perpetrator drug’ on the pharmacokinetic (PK) parameters of ‘the victim drug’ after a single dose. To evaluate the effect, the AUC0→∞ (area under the curve) ratio was determined (the ratio between AUC0→∞ in the presence of the perpetrator and AUC0→∞ without the presence of the perpetrator).
Methods
Male Wistar rats were assigned to three groups (eight animals in each group), which were orally administered: metformin and olaparib (IMET+OLA), vehiculum with metformin (IIMET), and vehiculum with olaparib (IIIOLA). Blood samples were collected after 24 h. HPLC was applied to measure the concentrations of olaparib and metformin. The PK parameters were calculated in a non-compartmental model.
Results
Metformin did not affect the olaparib PK parameters. The AUC0→∞ IMET+OLA/IIIOLA ratio was 0.99. Olaparib significantly increased the metformin Cmax (by 177.8%), AUC0→t (by 159.8%), and AUC0→∞ (by 74.1%). The AUC0→∞ IMET+OLA/IIMET ratio was 1.74.
Conclusions
A single dose of metformin did not affect the PK parameters of olaparib, nor did it inhibit the olaparib metabolism, but olaparib significantly changed the metformin pharmacokinetics, which may be of clinical importance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00280-023-04591-y.
Keywords: Olaparib, Metformin, Pharmacokinetics, Drug–drug interactions
Introduction
Olaparib is a medication used for maintenance therapy in BRCA-mutated cancers. It inhibits poly-ADP ribose polymerase (PARP) – an enzyme involved in DNA repair. This drug is mainly indicated for ovarian cancer, fallopian tube cancer, peritoneal cancer, pancreatic cancer, and prostate cancer with hereditary or somatic BRCA1 or BRCA2 mutation [1, 2]. It was first approved as a single agent by the European Medicines Agency (EMA) in the European Union and by the Food and Drug Administration (FDA) in the United States in 2014. It was first approved as a maintenance therapy for recurrent high-grade platinum-sensitive ovarian cancer in BRCA1/2-mutated patients. The maintenance therapy should be started 8 weeks after the last course of platinum-based chemotherapy if there was a partial or complete response to the treatment [3, 4]. Olaparib is an oral drug – 50-mg hard capsules which were mainly changed to film-coated tablets with a starting dose of 300 mg twice a day, in combination with bevacizumab are recommended in the treatment of high-risk ovarian cancer. The main side effects of olaparib are: anemia, leukopenia, nausea, vomiting, and fatigue [1, 4].
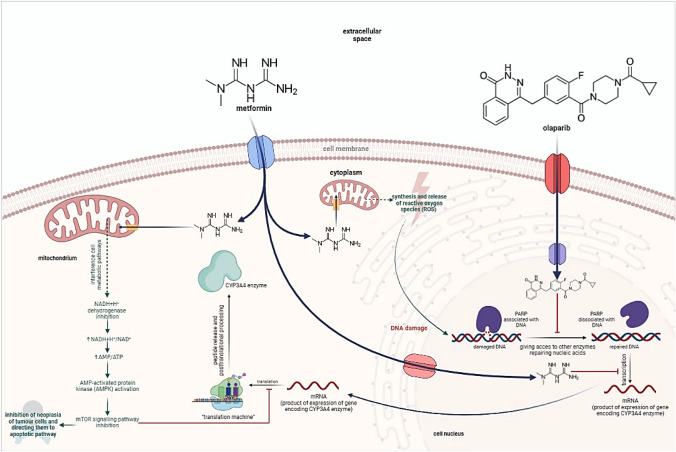
Metformin, which is a biguanide derivative, is a first-line drug applied to patients with type 2 diabetes or prediabetes. It is assumed that the antihyperglycemic effect of metformin is achieved through the inhibition of the mitochondrial complex I (NADH + H+ dehydrogenase), which changes not only the NAD+/NADH + H+ ratio, but also the AMP/ATP ratio. An increase in the AMP/ATP ratio is the driving force for AMP-activated protein kinase (AMPK – 5’-adenosinemonophosphate-activated protein kinase). AMPK is also significant in the activation of the p53 protein, which is colloquially known as ‘the guardian of the genome’. AMPK acts as a suppressor in cells exposed to mutations or damage. This leads to their apoptosis when the repair processes fail. AMPK is also associated with other cellular pathways, e.g., STAT, and it regulates the levels of their most important proteins. Research has shown that metformin significantly reduces the levels of p-STAT3 and C-MYC proteins. This decrease is even greater in the presence of a PARP inhibitor. As a result, the proliferative properties of neoplastic cells are suppressed [5]. Apart from that, metformin induces mitochondrial shock and thus causes additional DNA damage by redirecting metabolism in favor of the formation of reactive oxygen species [6]. Metformin is also considered to be involved in the inhibition of genes encoding the CYP3A4 enzyme. For this reason, the metabolism of this enzyme’s substrates, such as olaparib, can be expected to decrease significantly [7].
The affinity of PARP inhibitors for transporters involved in passive transport into and out of cells can be used as a desirable interaction in the pharmacokinetics of drugs (especially olaparib [1] and rucaparib [8]). Many drugs, including those exhibiting cytostatic activity, are substrates for these protein transporters, e.g., oxaliplatin and metformin for OCT1, cisplatin, metformin, and propranolol for OCT2, topotecan and metformin for MATE1, oxaliplatin, topotecan, and metformin for MATE2K [9]. The blockage of the transporter protein responsible for the transport from hepatocytes or into to the renal tubules may cause the accumulation of the drug in the body.
Olaparib is a drug which can inhibit such proteins. When the pathways responsible for the distribution of anticancer drugs in the tissues overlap, the antitumor activity of both drugs increases and there is a risk of more severe adverse reactions. The interaction of metformin with olaparib is an example of the interaction whose pharmacodynamic mechanism has been thoroughly investigated. This interaction is particularly important due to the fact that it is very likely that both drugs may be combined with each other, because type II diabetes, to which metformin is dedicated, poses a significant risk of ovarian cancer [7, 10, 11]. In addition, olaparib raises the blood glucose level by blocking GLUT2 transporters, which increases the fasting glucose level. Therefore, it is very likely that antihyperglycemic treatment will be implemented [12].
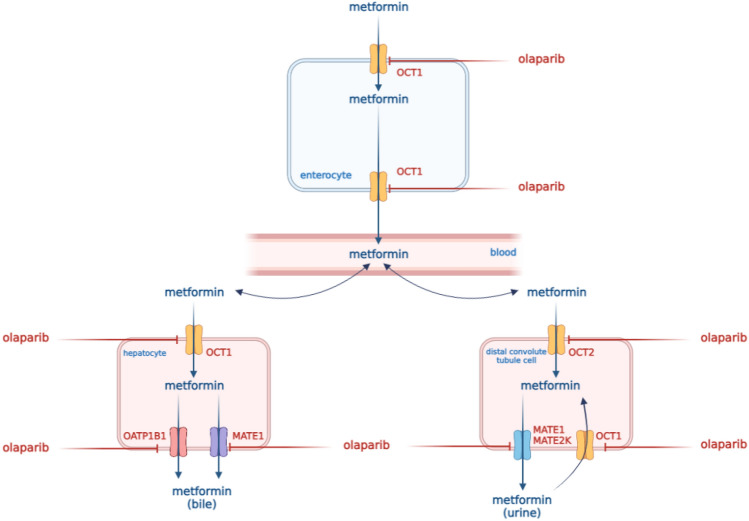
What is important to manage, metformin and olaparib synergistically inhibit tumor growth by blocking the cell cycle, especially in the S phase, when the synthesis of histone proteins and DNA replication play the most significant role – during the phase specific to PARP inhibitors [13]. The pharmacokinetics and pharmacodynamic interaction of olaparib with metformin are presented in Figs. 1 and 2.
Fig. 1.
Pharmacokinetic interaction of olaparib with metformin
Fig. 2.
Pharmacodynamic interaction of olaparib with metformin
In view of the aforementioned facts, there is a risk of interaction between olaparib and metformin. The aim of our study on animals was to investigate this risk.
Reagents
Metformin (CAS number 1115–70-4) and olaparib (CAS number 763113–22-0) were purchased from LGC Standards (Łomianki, Poland). Paracetamol (CAS number 103–90-2), olaparib-d4, methanol, acetonitrile, ammonium formate, ammonium acetate, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (Poznań, Poland). Water used in the mobile phase was deionized, distilled, and filtered through a Millipore system (Direct Q3, Millipore, USA) before use. Olaparib (Lynparza®, batch number RR214) was purchased from AstraZeneca Pharma Poland Sp. z o.o. (Warsaw, Poland). Metformin (Metformax, batch number 16518316) was purchased from Teva Pharmaceuticals Polska Sp. z o.o. (Warsaw, Poland).
Animal experiments
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The animals were given a standard diet and water ad libitum, and the experimental protocol for this study was approved by the Local Ethics Committee (No. 45/2022, of 27 May 2022), Poznań University of Life Sciences, Department of Animal Physiology and Biochemistry, Wołyńska 35, 60–637 Poznań, Poland. Adult male Wistar rats (weight 380–530 g) were used in the study. The animals were maintained under standard breeding conditions with a 12/12 h light–dark cycle (lights on at 06.00, lights off at 18.00) at constant room temperature (23 ± 2 °C), relative humidity of 55 ± 10% and given ad libitum access to food and water. The animals were allowed to acclimatize for a week before the beginning of the experiments. The rats were divided into three groups. Olaparib was formulated in 10% dimethyl sulfoxide (DMSO) – 90% saline at a single dose of 100 mg/kg [14]. The solution for oral metformin administration was prepared in saline at a single dose of 100 mg/kg [15]. Drugs were orally administered to rats by gavage between 7:00 a.m. and 8:00 a.m. Before administration (0 h) and at different time intervals after the administration, i.e., 0.085, 0.5, 1, 2, 4, 6, 8, 10, and 24 h [16], blood was collected from the tail vein of each rat into 1.5-ml heparinized Eppendorf (EP) tubes. The blood samples were centrifuged at 5000 rpm for 10 min, and the plasma was transferred to new centrifuge tubes and then stored at – 80 °C. However, after performing the tests, it turned out that sampling should be extended in order to achieve lower values of the residual field. Unfortunately, the authors did not indicate the existence of such a problem in their studies.
There were a few cases of high values of the residual area in both groups (> 20%). Therefore, the AUC- or kel-dependent results of analyses are additionally shown in Table 1B (Table 1A shows Cmax, tmax and AUC0-t).
Table 1.
Plasma pharmacokinetic parameters of metformin (MET) after the oral administration of a single dose of metformin (100 mg/kg b.w.) to the IIMET group and metformin + olaparib (100 mg/kg b.w. + 100 mg/kg b.w.) to the IMET+OLA group
| Pharmacokinetic parameters | IIMET (n = 8) | IMET+OLA (n = 8) | p value IMET+OLA vs. IIMET | Gmean ratio* (90% CI) IMET+OLA vs. IIMET |
|---|---|---|---|---|
| Table A | ||||
| Cmax (µg/ml) | 0.45 ± 0.29 (64.4) | 1.25 ± 0.80 (64.2) | 0.0117 | 2.73 (1.56; 4.79) |
| tmax (h) | 1.09 ± 0.57 (51.7) | 2.63 ± 1.85 (70.4) | 0.0580 | 2.26 (1.23; 4.17) |
| Table B | ||||
| AUC0-t (µg × h/ml) | 3.83 ± 1.23 (32.1) | 9.95 ± 5.09 (32.1) | 0.0016 | 2.45 (1.72; 3.50) |
| AUC0-∞ (µg × h/ml) | 7.90 ± 1.93 (24.4) | 13.75 ± 5.51 (40.1) | 0.0117 | 1.67 (1.26; 2.22) |
| ka (h−1) | 0.41 ± 0.25 (62.4) | 0.59 ± 0.17 (29.7) | 0.1170 | 1.60 (1.09; 2.37) |
| kel (h−1) | 0.032 ± 0.014 (42.7) | 0.054 ± 0.022 (40.6) | 0.0294 | 1.72 (1.17; 2.53) |
| t0.5 (h) | 26.54 ± 14.52 (54.7) | 14.95 ± 6.04 (40.4) | 0.0587 | 0.58 (0.39; 0.86) |
| Cl/F (l/h) | 6.31 ± 1.48 (23.5) | 3.57 ± 1.26 (35.34) | 0.0014 | 0.55 (0.41; 0.72) |
| Vd/F (l) | 227.66 ± 90.97 (40.0) | 79.61 ± 43.04 (54.1) | 0.0010 | 0.32 (0.20; 0.51) |
Arithmetic means and standard deviations (SD) are shown with coefficients of variation CV (%) in brackets
Cmax the maximum plasma concentration; AUC0-t area under the plasma concentration–time curve from zero to the time of the last measurable concentration; AUC0-∞ area under the plasma concentration–time curve from zero to infinity; tmax time to the first occurrence of Cmax; ka absorption rate constant; kel elimination rate constant; t0.5 half-life in the elimination phase; Cl/F apparent plasma drug clearance, Vd/F apparent volume of distribution
*Geometric mean (Gmean) ratio between the IMET+OLA and IIMET groups (%) with a 90% confidence interval (CI) in the brackets. Table 1A was shown Cmax, tmax and Table 1B was shown the AUC- or kel-dependent results of analyses
HPLC–UV assay of metformin
High-performance liquid chromatography (HPLC) with ultraviolet (UV) detection (HPLC Waters 2695 Separations Module with autosampler, Waters 2487 Dual Absorbance Detector) [17] after a liquid–liquid extraction with a mixture of 1-butanol:n-heptane (50:50, v/v) was applied to measure the concentrations of metformin in the rats’ plasma. An analytical Symmetry® C8 column (250 × 4.6 mm, 5.0 μm; Waters Corporation, Milford, MA, USA) and a mobile phase consisting of 0.1 M ammonium formate solution, pH 6.3 with an isocratic flow rate of 1.0 ml/min were used. The volume of each injection was 20 µl, and the retention times for metformin and internal standard (acetaminophen) were 3.5 and 11.2 min, respectively.
UPLC-MS/MS assay
Olaparib in the plasma samples was quantified with an ACQUITY 1 plus ultra-high-performance liquid chromatograph combined with a Xevo TQ-S micro triple quadrupole mass spectrometer (Waters Corporation, Milford, MA, USA) [18]. A Cortecs UPLC C18 column (2.1 × 50 mm, 1.6 µm, Waters Corporation, Milford, MA, USA) was used for chromatographic separation. The column temperature and injection volume were set at 40 °C and 1 µl, respectively. The mobile phase comprised acetonitrile (eluent A) and 2 mM ammonium acetate in water (eluent B) with 0.1% 98–100% formic acid. The flow rate was maintained at 0.3 ml/min. The gradient elution was as follows: 0–3 min, 5% A; 4–5 min, 95%, A; 3–5 min, linear from 5 to 95% A; 5–6 min, linear from 95 to 5% A. The mass spectrometer operated in the multiple reaction monitoring mode. Two transitions for olaparib and olaparib-d4 (IS) were monitored: m/z 435.1 → 367.1 and 435.1 → 281.0 (qualifier transition) for olaparib, and m/z 439.1 → 367.1 and 439.1 → 281.0 for IS.
Pharmacokinetic evaluation
The following pharmacokinetic parameters of olaparib and metformin were calculated with the Pkanalix 2023R1 software (Lixoft, France): the elimination rate constant (ke), the absorption rate constant (ka), the half-life in the elimination phase (t1/2), the area under the concentration–time curve from zero to the last measurable concentration (AUC0–t), the area under the plasma concentration–time curve from zero to infinity (AUC0–∞), the apparent plasma drug clearance (Cl/F), and the apparent volume of distribution (Vd/F). The maximum plasma concentration (Cmax) and the time to reach the Cmax (tmax) were obtained directly from the measured values. All of the above-mentioned parameters underwent statistical analysis.
Statistical analysis
The SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. The Shapiro–Wilk test was used to determine the normality. Two pairs of groups were analyzed: IOLA+MET vs. IIIOLA and IOLAR+MET vs. IIMET independently. The differences between the normally distributed variables were determined with the Student’s t test. The variables which were not normally distributed were analyzed with the Kruskal–Wallis test at a significance level of p < 0.05.
Results
Analytical method validation
The methods were validated according to the published European Medicines Agency guideline [19]. The calibration curves for metformin were linear (r > 0.997) within concentration ranges of 0.1–4.0 µg/ml. Within- and between-run precision (coefficient of variation, CV) and accuracy (%bias) were determined for the following metformin plasma concentrations: 0.1, 0.3, 2.0, and 3.2 µg/ml. The CV was less than 13% and 10%, whereas the accuracy was less than 8% and 5% for all within- and between-run concentrations, respectively. The calibration curves for olaparib were prepared within a range of 10–20,000 ng/ml with a correlation coefficient r > 0.99. The lower limit of quantification (LLOQ) was determined at 10 ng/ml with acceptable precision and accuracy and S/N > 10. The accuracy, determined as %bias, was ≤ 13% across three quality control (QC) levels and < 20% for the LLOQ. The intra- and inter-run precision of the assay (coefficient of variation) was within 15% for the QC samples and below 20% for the LLOQ. Pkanalix software was used for pharmacokinetic analyses and to analyze the plasma concentration-versus-time data. A non-compartmental model was used to calculate the values of the pharmacokinetic parameters. The results are listed in Tables 1 and 2. Figures 3 and 4 represent the mean plasma concentration–time curves after the oral administration of metformin and olaparib to the rats.
Table 2.
Plasma pharmacokinetic parameters of olaparib (OLA) after the oral administration of a single dose of olaparib (100 mg/kg b.w.) to the IIIOLA group and metformin + olaparib (100 mg/kg b.w. + 100 mg/kg b.w.) to the IMET+OLA group
| Pharmacokinetic parameters | IIIOLA (n = 8) | IMET+OLA (n = 8) | p value IMET+OLA vs. IIIOLA | Gmean ratio*** (90% CI) IMET+OLA vs. IIIOLA |
|---|---|---|---|---|
| Cmax (ng/ml) | 8716.73 ± 6798.10 (78.0) | 9416.94 ± 6330.79 (67.2) | 0.8342* | 1.22 (0.50; 2.99) |
| AUC0-t (ng × h/ml) | 28,276.56 ± 28,159.91 (99.6) | 28,253.68 ± 21,387.83 (75.7) | 0.7527** | 1.38 (0.49; 3.83) |
| AUC0-∞ (ng × h/ml) | 28,575.50 ± 28,313.46 (99.1) | 29,016.69 ± 21,601.76 (74.4) | 0.7527** | 1.41 (0.51; 3.90) |
| tmax (h) | 1.18 ± 0.91 (77.8) | 1.11 ± 0.75 (67.7) | 0.7087** | 0.94 (0.43; 2.07) |
| ka (h−1) | 0.92 ± 0.29 (32.1) | 1.23 ± 0.15 (41.6) | 0.1509* | 1.28 (0.88; 1.87) |
| kel (h−1) | 0.44 ± 0.34 (77.5) | 0.34 ± 0.22 (65.6) | 0.4784* | 0.97 (0.41; 2.31) |
| t0.5 (h) | 4.79 ± 6.06 (126.5) | 3.18 ± 2.28 (71.7) | 0.7527** | 0.97 (0.41; 2.31) |
| Cl/F (l/h) | 7.84 ± 15.21 (193.9) | 2.62 ± 2.07 (79.0) | 0.8336** | 0.73 (0.26; 2.03) |
| Vd/F (l) | 22.49 ± 24.81 (110.3) | 12.51 ± 11.61 (92.8) | 0.5995** | 0.76 (0.22; 2.62) |
Arithmetic means and standard deviations (SD) are shown with coefficients of variation CV (%) in brackets
Cmax the maximum plasma concentration; AUC0-t area under the plasma concentration–time curve from zero to the time of the last measurable concentration; AUC0-∞ area under the plasma concentration–time curve from zero to infinity; tmax – time to the first occurrence of Cmax; ka absorption rate constant; kel elimination rate constant; t0.5 half-life in the elimination phase; Cl/F apparent plasma drug clearance, Vd/F apparent volume of distribution
*t test for equal variance
**Kruskal–Wallis for no normality
***Geometric mean (Gmean) ratio between the IMET+OLA and IIIOLA groups (%) with a 90% confidence interval (CI) in the brackets
Fig. 3.
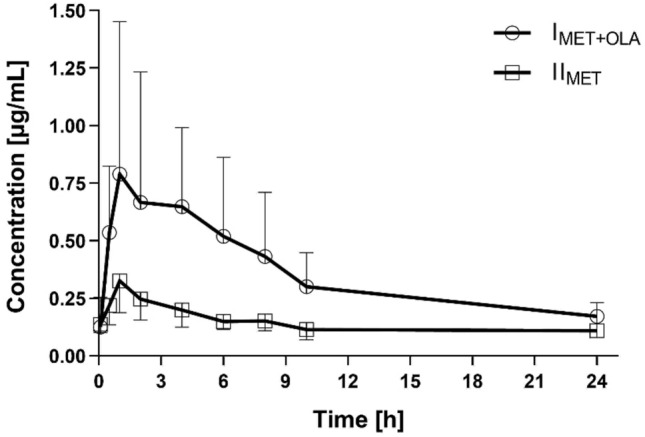
Metformin plasma concentration–time profiles (mean ± SD) in the rats which received metformin (IIMET) and metformin + olaparib (IMET+OLA)
Fig. 4.
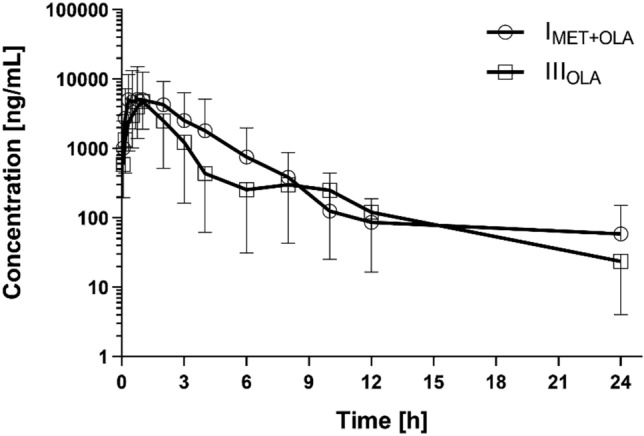
Olaparib plasma concentration–time profiles (mean ± SD) in the rats which received olaparib (IIIOLA) and metformin + olaparib (IMET+OLA)
The influence of olaparib on the pharmacokinetics of metformin
When metformin was co-administered with olaparib, the AUC0-t and AUC0-∞ of metformin increased by 159.8% and 74.1%, respectively, as compared with the administration of metformin alone. In the presence of olaparib, the Cmax of metformin increased by 177.8%, whereas the Vd/F (65.1%) and Cl/F (43.4%) of metformin decreased. However, there were not significant differences between the two groups in the values of the other pharmacokinetic parameters, including tmax (p = 0.0580), ka (p = 0.1170), and t0.5 (p = 0.0587). There was wide intersubject variability in the pharmacokinetic parameters, as evidenced by the coefficients of variation (CV%) (Table 1: Table 1A was shown Cmax, tmax, and Table 1B was shown the AUC- or kel-dependent results of analysis.). The values of the IMET+OLA/IIMET ratio for Cmax, AUC0-t, and AUC0→∞ were 2.78, 2.59, and 1.74, respectively.
The influence of metformin on the pharmacokinetics of olaparib
In comparison with the control group, the Vd/F (from 22.49 ± 24.81 to 12.51 ± 11.61 l) and Cl/F (from 7.84 ± 15.21 to 2.62 ± 2.07 l/h) of olaparib decreased when it was co-administered with metformin, but there was no statistical significance (p = 0.5995 and 0.8336, respectively). There were no significant changes in the other main pharmacokinetic parameters of olaparib. The values of the IMET+OLA/IIMET ratio for Cmax, AUC0-t, and AUC0→∞ were: 1.08, 0.99, and 1.02, respectively.
Discussion
As there is a high risk of drug interactions with olaparib, it is important to investigate interactions with other drugs, including metformin, because it is administered to diabetic patients with ovarian cancer [20, 21]. Research has proven that metformin lowers the risk of liver, pancreatic, and breast cancers [21, 22], inhibits the growth of existing cancer cells, and reduces mortality in the course of ovarian, endometrial, and colorectal cancers [23]. Moreover, cell line studies have shown that metformin and olaparib synergistically inhibit tumor growth by blocking the cell cycle [13]. Research on animals assessing the interaction between sorafenib and metformin showed reduced exposure to the anticancer drug, but no changes in the PK parameters of metformin [24]. Moreover, as metformin is believed to inhibit the activation of genes encoding the CYP3A4 enzyme, a significant decrease in the metabolism of substrates (e.g., olaparib [7]) of this enzyme can be expected [25]. It is assumed that interactions occurring at the level of transporters increase the metformin concentration (and thus increase the risk of adverse effects) and decrease exposure to olaparib. This problem is important because it may be necessary to investigate the use of olaparib during the treatment of ovarian cancer and due to the presence of diabetes and the administration of metformin in cancer patients. There have been reports on the increased risk of ovarian cancer among diabetic patients. Diabetes, endometriosis, polycystic ovarian syndrome, as well as several genetic polymorphisms significantly increase the risk of ovarian cancer [26]. Cohort and nested case–control studies conducted by Lee showed that patients with diabetes were at statistically significantly higher risk of ovarian cancer (RR, 1.16; 95% CI, 1.01–1.33), without significant heterogeneity (I = 27; P = 0.172) [27].
For all these reasons, the pharmacodynamic mechanism of the interaction of metformin with olaparib should be investigated. Additionally, as olaparib raises the blood glucose level by blocking GLUT2 transporters, it results in overestimated fasting glucose level. Therefore, it is very likely that antihyperglycemic treatment will be implemented. It all shows that both drugs can be combined with each other, because type 2 diabetes, to which metformin is dedicated, poses a significant risk of ovarian cancer [7, 10, 11].
The influence of olaparib on the pharmacokinetics of metformin
There is a high risk of drug interactions with olaparib. In vitro studies have shown that olaparib inhibits BCRP, OATP1B1, OCT1, OCT2, OAT3, MATE1, and MATE2K, which may result from increased exposure to the substrates of these transporters [1]. OCT1, MATE1, and MATE2K are important transporters for the pharmacokinetics of metformin. Metformin is a drug which does not have metabolites. It is excreted in an unchanged form with urine by glomerular filtration and tubular secretion. Metformin only minimally binds to blood proteins. However, it also binds to erythrocytes, which are its second distribution compartment. The role of transporters in the pharmacokinetics of metformin is very complex. OCT1 in enterocytes may influence the transport of metformin into the interstitial fluid. Additionally, the hepatic uptake is also mediated by OCT1. Therefore, the inhibition of OCT1 may decrease the effect of metformin. OCT2 is involved in the uptake of metformin from the blood into the kidney. MATE1 and MATE2K (efflux transporters) are responsible for the elimination of metformin from renal cells to the urine [28–30]. Additionally, the interaction with OCT2 in proximal tubule epithelial cells may increase the systemic disposition of metformin by reduced renal clearance. In our study, the co-administration of a single dose of olaparib with metformin significantly decreased the metformin clearance from 6.31 ± 1.48 l/h to 3.57 ± 1.26 l/h (p = 0.0014). The concomitant application of olaparib and metformin increased the metformin Cmax 2.8 times and its AUC 2.6 times. Such an increase in exposure to metformin may cause the risk of side effects, particularly in the gastrointestinal tract. According to some researchers, the weakening of the OCT1 function or the reduction of drug transport through OCT1 may result in gastrointestinal intolerance due to increased metformin concentration in the intestine [31, 32]. It is known that the gastrointestinal distress of metformin seems to be locally driven, hence it is hard to rationalize how do the higher systemic concentrations cause gastrointestinal side effects. Also, there is no clear evidence that bile excretion component of metformin increases in presence of olaparib. However, inhibition of the activity of the OCT1 transporter is an important issue.
Therefore, the effect of olaparib, which is an OCT1 and OCT2 inhibitor, on the increased risk of GI intolerance of patients taking metformin cannot excluded.
It is noteworthy that in the absence of hypersensitivity to metformin, it is used as a first-choice drug in the treatment of type 2 diabetes. Additionally, metformin has been proved to reduce the risk of liver, pancreatic, and breast cancers [21, 22], inhibit the growth of existing cancer cells, and reduce mortality in the course of ovarian, endometrial, and colorectal cancers [23]. Moreover, cell line studies have shown that metformin and olaparib synergistically inhibit tumor growth by blocking the cell cycle [13]. Therefore, metformin is a promising drug in the treatment of cancer.
The influence of metformin on the pharmacokinetics of olaparib
Metformin is not expected to be involved in many drug–drug interactions (DDIs) but there are studies showing that it has the potential to be the perpetrator in DDIs. Metformin reduced the Cmax and AUC24 of aliskiren but the changes were not significant, so clinical DDIs are not expected. There have been studies showing that metformin affects phenprocoumon and warfarin. It is known that the Cmax and AUC24 of trospium decreased when it was combined with metformin – probably metformin can inhibit the oral absorption of the drug. On the other hand, metformin was found to increase the exposure to topiramate [33]. Vuu et al. [34] observed that the co-administration of metformin and sotorasib did not affect the sotorasib exposure to a clinically significant extent. It also did not affect the hypoglycemic effect of metformin, although it was different from the one observed in vitro and its duration was shorter.
Chinese researchers hypothesized that the combination of sorafenib and metformin may have a synergistic effect in the treatment of colorectal cancer while reducing the severity of side effects [35]. However, when vandetanib is combined with metformin, the latter may require additional monitoring and periodic dose escalation [36]. The authors of the METAL (METformin in Advanced Lung Cancer) study [37], which was a phase I-II trial, hypothesized that the administration of metformin to non-diabetic patients may revert resistance to gefitinib, which is a selective epidermal growth factor receptor (EGFR) and tyrosine kinase inhibitor applied in non-small cell lung cancer. The researchers observed that a stable blood glucose level was maintained in the non-diabetic population. At the same time, during the 30-week observation period the neoplastic disease became stabilized in 50% of the patients. The combination of metformin with gefitinib inhibits cell proliferation and induces apoptosis, particularly in cell lines harboring the wild-type LKB1 gene. This dependence can also be observed in another tyrosine kinase inhibitor – erlotinib. The time-to-progression median was 20 weeks. This effect may have been caused by the fact that metformin may activate AMP-activated protein kinase and thus inhibit the mTOR and block the MAPK signaling. The relationships between metformin and tyrosine kinase inhibitor are constantly being investigated [37].
Clinical trials on metformin have not shown any influence of this drug on the efficacy of the following medications: alogliptin, dapagliflozin, dutogliptin, gemigliptin, linagliptin, lobeglitazone, rosiglitazone, rosuvastatin, saxagliptin, sitagliptin, and vildagliptin [33]. As metformin is believed to inhibit the activation of the genes encoding the CYP3A4 enzyme, a significant decrease in the metabolism of substrates (e.g., olaparib [7]) of this enzyme can be expected [25]. Gralewska et al. found that the treatment with olaparib and metformin increased oxidative stress and decreased the mitochondrial membrane potential. The co-administration of metformin and olaparib may result in almost two times greater early apoptosis than when the drugs are administered individually. After the co-administration of olaparib with metformin the percentage of late apoptotic cells was significantly higher than when the drugs were given separately (28.4% for co-administration vs. 5.1% for olaparib and 8.2% for metformin) [6]. Another study showed that biguanides in combination with PARP inhibitors synergistically reduced the epithelial-mesenchymal transition, proliferation, and survival of ovarian drug-resistant cancer cells [38].
Our research showed that a single dose of metformin did not have inhibitory effect on olaparib and did not affect its PK parameters. However, olaparib significantly changed the pharmacokinetics of metformin. The Cmax of metformin increased by 177.8%, whereas the Vd/F and Cl/F of metformin decreased. There were no significant differences between the two groups (metformin co-administered with olaparib and metformin administered alone) in the other pharmacokinetic parameters, including tmax (p = 0.0580), ka (p = 0.1170), and t0.5 (p = 0.0587. The values of the IMET+OLA/IIMET ratio for Cmax, AUC0-t, and AUC0→∞ were 2.78, 2.59, and 1.74, respectively. Investigations in human in vitro systems indicated phase I metabolism of olaparib was CYP mediated and that CYP3A4 and 3A5 were the dominant metabolic enzymes. As expression of CYPs 3A4 and 3A5 is highly variable in human and olaparib clearance in human was primarily metabolic, this may explain some of the variability observed in clinical pharmacokinetics. Perhaps, in the study, the high variability contributed to the lack of statistically significant differences in the PK parameters of olaparib.
There were some limitations to our study, such as the small size of the sample, which was limited by the Local Ethics Committee (No. 45/2022, of 27 May 2022). The lack of using a model is also a significant limitation of the study. Another limitation was the fact that both drugs (not only metformin but also olaparib) were administered only once and at the same dose. If the experiment had been continued to the steady state (as in patients), there might have been changes in the olaparib PK as well. If the experiment had been conducted on pre-diabetic or diabetic animals, the effect of the pathological condition on the PK of both drugs might also have been observed.
Conclusions
In conclusion, we showed that metformin had no effect on the pharmacokinetics and metabolism of olaparib, but olaparib significantly increased the body’s exposure to metformin, which may be of significant clinical relevance and may be associated with the risk of adverse effects. The presented results require confirmation in a clinical trial.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
The study concept and design were prepared by JS-R, AK, and DS-F. Data acquisition was prepared by JS-R, AK, DS-F, ES, TG, and AW. Validation of analytical method and measurements of drug concentrations were done by AK, and DS-F, FO. Data analysis and interpretation were prepared by JS-R, AK, DS-F, FO, TG, AW, ES. Writing of the manuscript were prepared by JS-R, AK, DS-ES. Final corrections were done by JS-R, AK, DS-F, FO, TG, AW, EG, and ES.
Funding
The study was financed with an academic grant of the Poznań University of Medical Sciences (grant No. NSB0000015). The funding source had no effect on any part of the study, preparation, or submission of the manuscript.
Data availability
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines concerning the care and use of animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lynparza, 100-mg film-coated tablets - summary of product characteristics. https://www.ema.europa.eu Accessed 13 Apr 2020
- 2.Baum J, Zickler D, Bolbrinker J, Richter R, Braicu EI, Grabowski J, Sehouli J. Olaparib in an ovarian cancer patient with end-stage renal disease and hemodialysis. Cancer Chemother Pharmacol. 2023;4:325–330. doi: 10.1007/s00280-023-04514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Gao Y, Luan X, Li K, Wang J, Dai Y, Kang M, Lu C, Zhang M, Lu CX, Kang Y, Xu C. An effective AKT inhibitor-PARP inhibitor combination therapy for recurrent ovarian cancer. Cancer Chemother Pharmacol. 2022;89(5):683–695. doi: 10.1007/s00280-022-04403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora S, Balasubramaniam S, Zhang H, Berman T, Narayan P, Suzman D, Bloomquist E, Tang S, Gong Y, Sridhara R, Turcu FR, Chatterjee D, Saritas-Yildirim B, Ghosh S, Philip R, Pathak A, Gao JJ, Amiri-Kordestani L, Pazdur R, Beaver JA. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist. 2021;26(1):e164–e172. doi: 10.1002/onco.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi BJ, Sun Y, Quan LL, Zhao JT, Wei B, Wang SQ. Metformin can enhance the inhibitory effect of olaparib in bladder cancer cells. Dis Markers. 2022;2022:5709259. doi: 10.1155/2022/5709259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gralewska P, Gajek A, Marczak A, Rogalska A. Metformin affects olaparib sensitivity through induction of apoptosis in epithelial ovarian cancer cell lines. Int J Mol Sci. 2021;22(19):10557. doi: 10.3390/ijms221910557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilla Reddy V, Bui K, Scarfe G, Zhou D, Learoyd M. Physiologically based pharmacokinetic modeling for olaparib dosing recommendations: bridging formulations, drug interactions, and patient populations. Clin Pharmacol Ther. 2019;105(1):229–241. doi: 10.1002/cpt.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubraca 250-mg film-coated tablets - summary of product characteristics.https://www.ema.europa.eu Accessed 29 Jun 2022
- 9.Tátrai P, Zolnerciks J K, Gáborik Z, Roelof de Wilde & Petró N (2021) The transporter book, 4th edition (SOLVO biotechnology): 168–171;246–252
- 10.McCormick A, Swaisland H. In vitro assessment of the roles of drug transporters in the disposition and drug–drug interaction potential of olaparib. Xenobiotica. 2017;47(10):903–915. doi: 10.1080/00498254.2016.1241449. [DOI] [PubMed] [Google Scholar]
- 11.Choi MK, Song IS. Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug Metab Pharmacokinet. 2008;23(4):243–253. doi: 10.2133/dmpk.23.243. [DOI] [PubMed] [Google Scholar]
- 12.Romero I, Rubio MJ, Medina M, Matias-Guiu X, Santacana M, Schoenenberger JA, Guerra EM, Cortés A, Minig L, Coronado P, Cueva JF, Gómez L, Malfettone A, Sampayo M, Llombart-Cussac A, Poveda A. An olaparib window-of-opportunity trial in patients with early-stage endometrial carcinoma: POLEN study. Gynecol Oncol. 2020;159(3):721–731. doi: 10.1016/j.ygyno.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Hijaz M, Chhina J, Mert I, Taylor M, Dar S, Al-Wahab Z, Ali-Fehmi R, Buekers T, Munkarah AR, Rattan R. Preclinical evaluation of olaparib and metformin combination in BRCA1 wildtype ovarian cancer. Gynecol Oncol. 2016;142(2):323–331. doi: 10.1016/j.ygyno.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Australian Public Assessment Report for Olaparib, February 2018. https://www.tga.gov.au Accessed 13 Apr 2020
- 15.Chen M, You G, Xie C, Yang R, Hu W, Zheng Z, Liu S, Ye L. Pharmacokinetics of metformin in collagen-induced arthritis rats. Biochem Pharmacol. 2021;185:114413. doi: 10.1016/j.bcp.2021.114413. [DOI] [PubMed] [Google Scholar]
- 16.Su G, Qin L, Su X, Tao C, Wei Y. Gender-dependent pharmacokinetics of olaparib in rats determined by ultra-high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Biomed Chromatogr. 2020;34(3):e4791. doi: 10.1002/bmc.4791. [DOI] [PubMed] [Google Scholar]
- 17.Gabr RQ, Padwal RS, Brocks DR. Determination of metformin in human plasma and urine by high-performance liquid chromatography using small sample volume and conventional octadecyl silane column. J Pharm Pharm Sci. 2010;13(4):486–494. doi: 10.18433/j32c71. [DOI] [PubMed] [Google Scholar]
- 18.Fu Q, Chen M, Hu S, McElroy CA, Mathijssen RH, Sparreboom A, Baker SD. Development and validation of an analytical method for regorafenib and its metabolites in mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1090:43–51. doi: 10.1016/j.jchromb.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICH guideline M10 on bioanalytical method validation Step 5 https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf Accessed 25 January 2023
- 20.Dębska S, Kubicka J, Czyżykowski R, Habib M, Potemski P. PARP inhibitors–theoretical basis and clinical application. Postepy Hig Med Dosw (Online) 2012;66:311–321. doi: 10.5604/17322693.999033. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(8):707–710. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 24.Karbownik A, Szkutnik-Fiedler D, Czyrski A, Kostewicz N, Kaczmarska P, Bekier M, Stanisławiak-Rudowicz J, Karaźniewicz-Łada M, Wolc A, Główka F, Grześkowiak E, Szałek E. Pharmacokinetic interaction between sorafenib and atorvastatin, and sorafenib and metformin in rats. Pharmaceutics. 2020;12(7):600. doi: 10.3390/pharmaceutics12070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020;34:101517. doi: 10.1016/j.redox.2020.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanha K, Mottaghi A, Nojomi M, Moradi M, Rajabzadeh R, Lotfi S, Janani L. Investigation on factors associated with ovarian cancer: an umbrella review of systematic review and meta-analyses. J Ovarian Res. 2021;14(1):153. doi: 10.1186/s13048-021-00911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Jeon I, Kim JW, Song YS, Yoon JM, Park SM. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2013;23(3):402–412. doi: 10.1097/IGC.0b013e31828189b2. [DOI] [PubMed] [Google Scholar]
- 28.Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74(2):359–371. doi: 10.1016/j.bcp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Minematsu T, Giacomini KM. Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol Cancer Ther. 2011;10(3):531–539. doi: 10.1158/1535-7163.MCT-10-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genom. 2012;22(11):820–827. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426–435. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dujic T, Zhou K, Donnelly LA, Tavendale R, Palmer CN, Pearson ER. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: a GoDARTS study. Diabetes. 2015;64(5):1786–1793. doi: 10.2337/db14-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stage TB, Brøsen K, Christensen MM. A comprehensive review of drug–drug interactions with metformin. Clin Pharmacokinet. 2015;54(8):811–824. doi: 10.1007/s40262-015-0270-6. [DOI] [PubMed] [Google Scholar]
- 34.Vuu I, Wahlstrom J, Houk BE. Impact of Sotorasib on the pharmacokinetics and pharmacodynamics of metformin, a MATE1/2K Substrate, in healthy subjects. Clin Pharmacokinet. 2023;62(2):267–275. doi: 10.1007/s40262-022-01192-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Cao L, Xu G, He H, Zhao H, Liu T. Co-delivery of sorafenib and metformin from amphiphilic polypeptide-based micelles for colon cancer treatment. Front Med (Lausanne) 2022;9:1009496. doi: 10.3389/fmed.2022.1009496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson S, Read J, Oliver S, Steinberg M, Li Y, Lisbon E, Mathews D, Leese PT, Martin P. Pharmacokinetic evaluations of the co-administrations of vandetanib and metformin, digoxin, midazolam, omeprazole or ranitidine. Clin Pharmacokinet. 2014;53(9):837–847. doi: 10.1007/s40262-014-0161-2. [DOI] [PubMed] [Google Scholar]
- 37.Morgillo F, Fasano M, Della Corte CM, Sasso FC, Papaccio F, Viscardi G, Esposito G, Di Liello R, Normanno N, Capuano A, Berrino L, Vicidomini G, Fiorelli A, Santini M, Ciardiello F. Results of the safety run-in part of the METAL (METformin in advanced lung cancer) study: a multicentre, open-label phase I-II study of metformin with erlotinib in second-line therapy of patients with stage IV non-small-cell lung cancer. ESMO Open. 2017;2(2):e000132. doi: 10.1136/esmoopen-2016-000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, López-Ozuna VM, Baloch T, Bithras J, Amin O, Kessous R, Kogan L, Laskov I, Yasmeen A. Biguanides in combination with olaparib limits tumorigenesis of drug-resistant ovarian cancer cells through inhibition of Snail. Cancer Med. 2020;9(4):1307–1320. doi: 10.1002/cam4.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.