Abstract
Introduction
Bowel urgency (BU) is among the most disruptive of inflammatory bowel disease (IBD) symptoms. However, data on its prevalence and association with disease activity are limited. This real-world study of Japanese patients with IBD evaluated BU prevalence and compared clinical outcomes and health-related quality of life (HRQoL) between patients with and without BU.
Methods
Data were drawn from the Adelphi IBD Disease Specific Programme™, a cross-sectional survey of physicians and their patients with ulcerative colitis (UC) and Crohn’s disease (CD). Physicians reported demographic and clinical data, including disease activity measures (Mayo score and CD Activity Index [CDAI]), for consulting patients, who voluntarily completed a patient-reported questionnaire, including HRQoL measures (Short IBD Questionnaire [SIBDQ] and EQ-5D-5L). Outcomes were compared between patients with and without BU using t-, Fisher exact and Mann-Whitney U tests as appropriate.
Results
Of 120 UC patients, 27.5% (n = 33) self-reported BU; physicians were unaware of BU in 54.5% (n = 18) of these patients. Patients with BU had higher mean Mayo scores (p < 0.01) and lower mean SIBDQ scores (47.9 vs 56.6, p < 0.01) than patients without BU, with mean EQ-5D-5L scores 0.83 and 0.87, respectively (p = 0.06). Physicians were satisfied with treatment but believed better control could be achieved for 39.4% of patients with BU and 35.6% without. Of 114 CD patients, 17.5% (n = 20) self-reported BU; physicians were unaware of BU in 75.0% (n = 15) of these patients. Patients with BU had higher mean CDAI scores (p < 0.01) and lower mean SIBDQ (48.7 vs 56.2, p < 0.01) and EQ-5D-5L scores (0.81 vs 0.88, p < 0.01) than patients without BU. Physicians were satisfied but believed better control could be achieved for 40.0% of patients with BU vs 19.1% without.
Conclusions
Patients with BU have worse clinical outcomes and HRQoL than patients without, underlining the need for improved physician-patient communication regarding BU and new IBD therapeutic options.
Keywords: Ulcerative colitis, Crohn’s disease, IBD, Bowel urgency, Disease burden, Real-world, Survey, Japan
Key Summary Points
| Why carry out this study? |
| Bowel urgency (BU) is among the most disruptive symptoms of inflammatory bowel disease (IBD). |
| Although BU is associated with high patient burden, there are limited data on its prevalence, association with disease activity and patient-reported health-related quality of life (HRQoL) among patients with IBD in Japan. |
| What was learned from the study? |
| IBD patients with BU tended to have greater disease severity and disease activity and worse HRQoL than patients without BU. |
| IBD remained uncontrolled in some patients, with BU and other symptoms persisting regardless of treatment, resulting in both physicians and their patients believing that better disease control can be achieved. |
| Clinical and HRQoL burden associated with BU in IBD patients seen in routine clinical practice underlines the need for improved communication between physicians and their patients regarding BU as a symptom and new IBD therapeutic options. |
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory bowel diseases (IBDs) with no known cause; as such they are designated intractable by the Ministry of Health, Labour and Welfare of Japan [1]. Affecting 0.2% of the Japanese population [1], IBD has a substantial negative impact on patients’ health-related quality of life (HRQoL) [2–6].
UC is characterized by inflammation of the colonic mucosa and submucosa, which starts distally in the rectum and extends proximally [7–10]. Patients present with proctitis (22% of patients), left-sided colitis (37%) or pan-ulcerative colitis (extensive) disease (38%) [11]. CD is characterized by transmural inflammation in any area of the gastrointestinal tract [7, 9, 12], with disease classified as ileal (30% of patients), ileocolonic (40%) or colonic (30%) [12]. A nation-wide study of Japanese patients with CD reported prevalence rates of 23% for ileal, 60% ileocolonic and 16% colonic CD [13].
Clinical presentation varies depending on severity and extent of disease and commonly includes increased frequency of bowel movements, bowel urgency (BU) and incontinence [14–17]. BU, the sudden and immediate need for a bowel movement, is associated with symptoms of active IBD, such as increased average bowel movements per day, increased stool frequency relative to normal, rectal bleeding, moderate-severe abdominal pain and calprotectin ≥ 250 μg/g [18]. Proctitis may lead to BU in UC, while abnormal bowel motility due to extensive disease may lead to urgency in CD [14, 19]; BU may also occur regardless of perianal disease [20].
BU is associated with considerable morbidity and negatively impacts patient HRQoL, affecting emotional, psychological and social functioning [18, 20–22]. BU is considered the most disruptive IBD symptom [23], as well as one of the most frequent, severe and distressing [24]. It is significantly correlated with bowel incontinence [25] and reported to be one of the main worries of patients due to its unpredictable nature [24], with patients anxious about not reaching a toilet [25]. Rates of BU are also positively associated with bowel movement frequency and rectal bleeding [25].
Although commonly associated with symptoms of active IBD, Japanese guidelines [26] do not discuss BU or bowel incontinence. The guidelines do, however, report bowel frequency to be a determinant of disease severity. Moreover, regardless of high patient burden, there are limited data on the prevalence of BU, its association with IBD disease activity and patient-reported outcomes (PROs) including HRQoL.
The objectives of this study were to evaluate BU prevalence in a real-world, cross-sectional survey of patients with UC and CD and compare clinical outcomes including patient-reported HRQoL between patients with and without BU in a real-world setting in Japan.
Methods
Survey Design
Data were drawn from the Adelphi IBD Disease Specific Programme™ (DSP), a real-world, cross-sectional survey with retrospective data collection, completed by physicians and their consulting patients with IBD in Japan, November 2020—May 2021. The DSP comprised a physician survey, a physician-completed retrospective patient record form and a patient self-completion questionnaire. The DSP methodology has been previously published and validated [27–31].
Participant Selection and Data Collection
Physicians (gastroenterologists) were eligible to participate in the survey if they had a clinical workload of seven or more patients with UC and eight or more patients with CD in a typical month. Patients were eligible for inclusion if they were ≥ 18 years of age, had a physician-confirmed diagnosis of UC or CD and were not involved in a clinical trial. Patients with UC who currently had mild disease must have previously had moderate or severe disease at some point in their disease history.
Physicians completed patient record forms for the next seven and eight consecutive consulting patients with UC and CD, respectively, who visited for routine care. Physicians reported demographics, clinical characteristics, disease management, treatment satisfaction and healthcare resource utilization for each patient using existing patient clinical records, as well as their judgement and diagnostic skills consistent with decisions made in routine clinical practice.
Physicians then invited those patients for whom they completed a patient record form to complete a voluntary patient-reported form. Patients provided data on current symptoms, treatment satisfaction and HRQoL. The form included the short version of the Inflammatory Bowel Disease Questionnaire (SIBDQ) [32, 33], EQ-5D-5L and EQ-VAS [34] and the IBD-specific version of the Work Productivity and Activity Impairment (WPAI) questionnaire [35, 36]. Only currently employed patients completed the WPAI work-based questions.
All participating physicians and their patients were assigned a study number to assist anonymous data collection and enable data linkage during collection and analysis. This allowed patients’ responses to be matched with those of their corresponding physician and evaluation of how perceptions of disease severity and symptom burden aligned. Participating patients and physicians provided informed consent for their data to be collected and analyzed by academic researchers and analysts within pharmaceutical companies and used for publication. Patients who did not wish to participate did not return a completed patient self-completed form.
Study Measures
To assess BU, patients were asked to select the symptoms relevant to the question “Which symptoms do you currently suffer from?” BU was evaluated by patients checking the boxes for “Bowel movement urgency (suddenly/urgently need to poo)” and/or “Night-time urgency”. Physicians rated their patient’s current symptom severity as a result of their IBD, as ‘none’, ‘very mild’, ‘mild’, ‘moderate’, ‘severe’ or ‘extremely severe’. Disease activity was evaluated in UC using the Mayo score, calculated based on stool frequency, rectal bleeding, endoscopic findings and physician global assessment [37–39]. In CD, the Crohn’s disease activity index (CDAI) was used, with disease activity determined from eight items including number of stools, abdominal pain and antidiarrhoeal agents used in the previous 7 days [40, 41]. The Mayo score ranges 0–12, where a score of 0–2 indicates remission, 3–5 indicates mild disease activity, 6–10 indicates moderate disease activity and 10–12 indicates severe disease activity [37]. CDAI score ranges from 0 to 600, with a score of < 150 corresponding to relatively inactive disease (remission), 150–219 mildly active disease, 220 to 450 moderately active disease and > 450 severe disease activity [40].
HRQoL was measured by the SIBDQ total score and the EQ-5D-5L. SIBDQ assesses HRQoL in terms of social, emotional and physical well-being on a scale of 10 (poor) to 70 (good) [32, 33]. The EQ-5D-5L index evaluates health status/HRQoL across five levels: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Scores begin at 1 (the value of full health) and range below 0 (where 0 is the value of health state equivalent to dead, negative values representing worse than dead) [34]. The Japanese EQ-5D-5L value set was used to convert the responses into a health utility score, with a score range from − 0.091 to 1.000 [42]. The EQ-VAS rates the respondent’s current health state on a scale of 0 (worst imaginable health) to 100 (best imaginable health) [34, 43]. The WPAI (disease-specific version for IBD) questionnaire measures IBD-related time missed from work (absenteeism), impairment at work (presenteeism), work productivity (overall work impairment) and impairment of regular activities. WPAI scores are reported as percentage impairment [35, 36, 44, 45].
Permission was granted by the EuroQol Research Foundation for use of the EQ-5D-5L, undertaking number 165411. No permission was required for use of the WPAI. Use of the SIBDQ, authored by Dr. Jan Irvine et. al., was made under license from McMaster University, Hamilton, Canada.
Statistical Analysis
As the primary objective of the survey was descriptive (i.e., no a priori hypotheses specified), the sample size was fixed by the duration of the survey period. Therefore, formal sample size calculations were not applicable and were not performed. Data were summarized using descriptive analyses. Means and standard deviations (SD) were calculated for continuous variables (number of observations), and frequency and percentages were calculated for categorical variables (number of patients). Missing data were not imputed; therefore, the base number of patients included for analysis could vary from variable to variable and is reported separately for each analysis.
Demographics, clinical characteristics and PROs were compared between patients with and without BU, as reported by the patient using parametric tests and non-parametric tests as appropriate. t-tests were used to determine statistical differences between group means for continuous outcomes. Fisher’s exact test was carried out for categorical variables and Mann-Whitney U tests for ordinal variables where the assumptions for t-tests were violated. All analyses were conducted for UC and CD groups separately. Two-sided p values < 0.05 were considered statistically significant. No adjustment for multiplicity was made due to sample size limitations and the exploratory nature of the study.
Ethical Considerations
Data collection by DSP fieldwork teams was conducted in accordance with national market research and privacy regulations, including European Pharmaceutical Market Research Association (EphMRA) and the US Department of Health and Human Services National Institutes of Health, Health Insurance Portability and Accountability Act (HIPAA).
This research also obtained ethics approval from the Western Institutional Review Board, study protocol number 1-1238963-1 and was performed in accordance with the principles stated in the Declaration of Helsinki.
All responses captured on the data collection forms were anonymized to preserve respondent confidentiality. Responses were anonymized before aggregated reporting, the identity of the physicians was blinded, and no patient identifiers were collected. Physicians were compensated in line with fair market rates.
Results
Patient Characteristics and Self-Reported Prevalence of Bowel Urgency
Patients with Ulcerative Colitis
Data for 120 patients with UC included in this analysis were reported by 27 physicians across multiple centers. Of those 120 patients, 27.5% (n = 33) had self-reported BU (Table 1). In 54.5% of those cases (n = 18), physicians were unaware that their patients had BU.
Table 1.
Physician- and patient-reported bowel urgency in patients with ulcerative colitis and Crohn’s disease without and with bowel urgency
| Patient-reported BU | ||||||
|---|---|---|---|---|---|---|
| UC | CD | |||||
| Total (n = 120) | With BU (n = 33) | Without BU (n = 87) | Total (n = 114) | With BU (n = 20) | Without BU (n = 94) | |
| Physician-reported BU, n (%) | ||||||
| Without BU | 95 (79.2) | 18 (54.5) | 77 (88.5) | 98 (86.0) | 15 (75.0) | 83 (88.3) |
| With BU | 25 (20.8) | 15 (45.5) | 10 (11.5) | 16 (14.0) | 5 (25.0) | 11 (11.7) |
BU bowel urgency, UC ulcerative colitis, CD Crohn’s disease
Mean (SD) age of patients with self-reported BU and without BU were 45.0 (14.4) years and 38.9 years (12.6); 60.6% (n = 20) and 62.1% (n = 54) were male, respectively. While patients with BU were older, other demographic variables were similar between UC groups with and without BU (all p > 0.05; Table 2). Overall, most patients with and without BU had ulcerative proctitis (36.4% [n = 12] and 44.8% [n = 39], respectively) or left-sided colitis (36.4% [n = 12] and 25.3% [n = 22]). The proportion of patients with proctosigmoiditis was significantly greater in patients with BU compared to those without (30.3% [n = 10] vs 12.6% [n = 11], p = 0.03; Table 3). Excluding BU, the most prevalent symptoms reported by patients were abdominal pain (51.5% [n = 17]) and diarrhoea without blood (45.5% [n = 15]); the proportions of patients with abdominal pain (p = 0.02) and diarrhoea without blood (p < 0.01) were significantly greater among patients with BU than without BU. A higher proportion of patients with BU also reported experiencing passing wind (flatulence) (21.2% [n = 7] vs 6.9% [n = 6], p = 0.04) and passing of mucus (12.1% [n = 4] vs 2.3% [n = 2], p = 0.05) compared to patients without BU. No statistically significant differences were seen in the proportion of patients with and without BU reporting abdominal cramps, diarrhoea with blood, fatigue/tiredness, loss of appetite, rectal bleeding, stomach bloating (abdominal distension) or tenesmus (p > 0.05). No significant difference was seen between patients with and without BU in the prevalence of the concomitant condition IBS (Table 2).
Table 2.
Demographic characteristics of patients with ulcerative colitis or Crohn’s disease with and without bowel urgency
| UC | CD | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 120) | With BU (n = 33) | Without BU (n = 87) | p value (test) | Total (n = 114) | With BU (n = 20) | Without BU (n = 94) | p value (test) | |
| Age (years), mean (SD) | 40.6 (13.3) | 45.0 (14.4) | 38.9 (12.6) | 0.03 (TT) | 37.5 (12.2) | 38.9 (15.3) | 37.2 (11.6) | 0.58 (TT) |
| Sex, n (%) | 1 (FE) | 0.80 (FE) | ||||||
| Male | 74 (61.7) | 20 (60.6) | 54 (62.1) | 72 (63.2) | 12 (60.0) | 60 (63.8) | ||
| Female | 46 (38.3) | 13 (39.4) | 33 (37.9) | 42 (36.8) | 8 (40.0) | 34 (36.2) | ||
| Body mass index, mean (SD) | 21.1 (2.9) | 21.0 (4.0) | 21.1 (2.4) | 0.87 (TT) | 20.6 (2.6) | 20.8 (3.0) | 20.6 (2.5) | 0.68 (TT) |
| Smoking status, n (%) | 0.83 (FE) | 0.96 (FE) | ||||||
| n | 81 | 22 | 59 | 83 | 13 | 70 | ||
| Current smoker | 12 (14.8) | 3 (13.6) | 9 (15.3) | 8 (9.6) | 1 (7.7) | 7 (10.0) | ||
| Ex-smoker | 23 (28.4) | 5 (22.7) | 18 (30.5) | 18 (21.7) | 3 (23.1) | 15 (21.4) | ||
| Never smoked | 46 (56.8) | 14 (63.6) | 32 (54.2) | 57 (68.7) | 9 (69.2) | 48 (68.6) | ||
| Employment status, n (%) | 0.99 (FE) | 0.71 (FE) | ||||||
| n | 115 | 33 | 82 | 105 | 19 | 86 | ||
| Working full time | 70 (60.9) | 20 (60.6) | 50 (61.0) | 66 (62.9) | 11 (57.9) | 55 (64.0) | ||
| Working part time | 14 (12.2) | 4 (12.1) | 10 (12.2) | 9 (8.6) | 2 (10.5) | 7 (8.1) | ||
| On long-term sick leave | 1 (0.9) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Homemaker | 17 (14.8) | 6 (18.2) | 11 (13.4) | 17 (16.2) | 5 (26.3) | 12 (14.0) | ||
| Student | 7 (6.1) | 2 (6.1) | 5 (6.1) | 8 (7.6) | 1 (5.3) | 7 (8.1) | ||
| Retired | 5 (4.3) | 1 (3.0) | 4 (4.9) | 1 (1.0) | 0 (0.0) | 1 (1.2) | ||
| Unemployed | 1 (0.9) | 0 (0.0) | 1 (1.2) | 4 (3.8) | 0 (0.0) | 4 (4.7) | ||
| Prevalence of concomitant conditions, n (%) | ||||||||
| Irritable bowel syndrome | 1 (0.8) | 1 (1.1) | 0 (0.0) | 1.0 (FE) | 1 (0.9) | 1 (1.1) | 0 (0.0) | 1.0 (FE) |
UC ulcerative colitis, CD Crohn’s disease, BU bowel urgency, FE Fisher’s exact test, SD standard deviation, TT t-test
All data in the table are physician reported with the exception of patient-reported bowel urgency status
Table 3.
Clinical characteristics of patients with ulcerative colitis or Crohn’s disease with and without bowel urgency
| UC | CD | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 120) | With BU (n = 33) | Without BU (n = 87) | p-value (test) | Total (n = 114) | With BU (n = 20) | Without BU (n = 94) | p value (test) | |
| Patient-reported current symptomsa, n (%) | (FE) | (FE) | ||||||
| Abdominal cramps | 7 (5.8) | 4 (12.1) | 3 (3.4) | 0.09 | 9 (7.9) | 2 (10.0) | 7 (7.4) | 0.66 |
| Abdominal pain | 40 (33.3) | 17 (51.5) | 23 (26.4) | 0.02 | 43 (37.7) | 9 (45.0) | 34 (36.2) | 0.46 |
| Diarrhoea without blood | 30 (25.0) | 15 (45.5) | 15 (17.2) | < 0.01 | 43 (37.7) | 8 (40.0) | 35 (37.2) | 0.81 |
| Diarrhoea with blood | 24 (20.0) | 10 (30.3) | 14 (16.1) | 0.12 | 14 (12.3) | 6 (30.0) | 8 (8.5) | 0.02 |
| Fatigue/tiredness | 9 (7.5) | 5 (15.2) | 4 (4.6) | 0.11 | 17 (14.9) | 4 (20.0) | 13 (13.8) | 0.50 |
| Loss of appetite | 6 (5.0) | 0 (0.0) | 6 (6.9) | 0.19 | 8 (7.0) | 3 (15.0) | 5 (5.3) | 0.14 |
| Night-time urgency | 13 (10.8) | 13 (39.4) | 0 (0.0) | < 0.01 | 5 (4.4) | 5 (25.0) | 0 (0.0) | < 0.01 |
| Passing of mucus | 6 (5.0) | 4 (12.1) | 2 (2.3) | 0.05 | 8 (7.0) | 3 (15.0) | 5 (5.3) | 0.14 |
| Passing wind (flatulence) | 13 (10.8) | 7 (21.2) | 6 (6.9) | 0.04 | 12 (10.5) | 4 (20.0) | 8 (8.5) | 0.22 |
| Rectal bleeding | 12 (10.0) | 6 (18.2) | 6 (6.9) | 0.09 | 6 (5.3) | 3 (15.0) | 3 (3.2) | 0.07 |
| Stomach bloating (abdominal distension) | 23 (19.2) | 8 (24.2) | 15 (17.2) | 0.44 | 28 (24.6) | 6 (30.0) | 22 (23.4) | 0.57 |
| Tenesmus | 9 (7.5) | 4 (12.1) | 5 (5.7) | 0.26 | 7 (6.1) | 3 (15.0) | 4 (4.3) | 0.10 |
| Disease severity, n (%) | 0.01 (MW) | 0.09 (MW) | ||||||
| Mild | 79 (65.8) | 17 (51.5) | 62 (71.3) | 85 (74.6) | 12 (60.0) | 73 (77.7) | ||
| Moderate | 35 (29.2) | 16 (48.5) | 19 (21.8) | 23 (20.2) | 6 (30.0) | 17 (18.1) | ||
| Severe | 6 (5.0) | 0 (0.0) | 6 (6.9) | 6 (5.3) | 2 (10.0) | 4 (4.3) | ||
| UC disease activity (Mayo) score | ||||||||
| Mean (SD) | 2.4 (2.2) | 3.7 (2.1) | 1.9 (2.1) | < 0.01 (TT) | N/A | N/A | N/A | |
| Remission (0–2), n (%) | 69 (57.5) | 11 (33.3) | 58 (66.7) | < 0.01 (MW) | N/A | N/A | N/A | |
| Mild disease (3–5), n (%) | 38 (31.7) | 15 (45.5) | 23 (26.4) | N/A | N/A | N/A | ||
| Moderate/ severe disease (≥ 6), n (%) | 13 (10.8) | 7 (21.2) | 6 (6.9) | N/A | N/A | N/A | ||
| Crohn’s disease activity index, n | N/A | N/A | N/A | 105 | 16 | 89 | ||
| Mean (SD) | N/A | N/A | N/A | 69.7 (67.1) | 118.3 (85.7) | 61.0 (59.7) | < 0.01 (TT) | |
| Remission (< 150), n (%) | N/A | N/A | N/A | 90 (85.7) | 10 (62.5) | 80 (89.9) | < 0.01 (MW) | |
| Mild activity (150–219), n (%) | N/A | N/A | N/A | 8 (7.6) | 2 (12.5) | 6 (6.7) | ||
| Moderate/severe/very severe activity (≥ 220), n (%) | N/A | N/A | N/A | 7 (6.7) | 4 (25.0) | 3 (3.4) | ||
| Current UC disease group, n (%) | (FE) | |||||||
| Ulcerative proctitis | 51 (42.5) | 12 (36.4) | 39 (44.8) | 0.54 | N/A | N/A | N/A | |
| Proctosigmoiditis | 21 (17.5) | 10 (30.3) | 11 (12.6) | 0.03 | N/A | N/A | N/A | |
| Left-sided colitis | 34 (28.3) | 12 (36.4) | 22 (25.3) | 0.26 | N/A | N/A | N/A | |
| Pan-ulcerative colitis | 27 (22.5) | 5 (15.2) | 22 (25.3) | 0.33 | N/A | N/A | N/A | |
| Don’t know | 3 (2.5) | 2 (6.1) | 1 (1.1) | 0.18 | N/A | N/A | N/A | |
| Current CD disease group, n (%) | (FE) | |||||||
| Crohn’s ileitis | N/A | N/A | N/A | 29 (25.4) | 6 (30.0) | 23 (24.5) | 0.58 | |
| Crohn’s colitis | N/A | N/A | N/A | 49 (43.0) | 9 (45.0) | 40 (42.6) | 1 | |
| Crohn’s ileocolitis | N/A | N/A | N/A | 35 (30.7) | 5 (25.0) | 30 (31.9) | 0.61 | |
| Jejunoileitis | N/A | N/A | N/A | 1 (0.9) | 1 (5.0) | 0 (0.0) | 0.18 | |
| Gastroduodenal CD | N/A | N/A | N/A | 1 (0.9) | 0 (0.0) | 1 (1.1) | 1 | |
| Don’t know | N/A | N/A | N/A | 1 (0.9) | 0 (0.0) | 1 (1.1) | 1 | |
| Physician-reported assessment of patient current symptom status, n (%) | ||||||||
| Overall symptoms | < 0.01 (MW) | < 0.01 (MW) | ||||||
| None/very mild | 47 (39.2) | 7 (21.2) | 40 (46.0) | 52 (45.6) | 5 (25.0) | 47 (50.0) | ||
| Mild | 35 (29.2) | 10 (30.3) | 25 (28.7) | 39 (34.2) | 6 (30.0) | 33 (35.1) | ||
| Moderate/severe/extremely severe | 38 (31.7) | 16 (48.5) | 22 (25.3) | 23 (20.2) | 9 (45.0) | 14 (14.9) | ||
| Abdominal pain | < 0.01 (MW) | 0.05(MW) | ||||||
| None/very mild | 57 (47.5) | 8 (24.2) | 49 (56.3) | 58 (50.9) | 7 (35.0) | 51 (54.3) | ||
| Mild | 29 (24.2) | 9 (27.3) | 20 (23.0) | 37 (32.5) | 6 (30.0) | 31 (33.0) | ||
| Moderate/severe/extremely severe | 34 (28.3) | 16 (48.5) | 18 (20.7) | 19 (16.7) | 7 (35.0) | 12 (12.8) | ||
| Sleep disturbancea | < 0.01 (MW) | < 0.01(MW) | ||||||
| None/very mild | 74 (62.2) | 13 (40.6) | 61 (70.1) | 84 (73.7) | 11 (55.0) | 73 (77.7) | ||
| Mild | 28 (23.5) | 8 (25.0) | 20 (23.0) | 18 (15.8) | 3 (15.0) | 15 (16.0) | ||
| Moderate/severe/extremely severe | 17 (14.3) | 11 (34.4) | 6 (6.9) | 12 (10.5) | 6 (30.0) | 6 (6.4) | ||
| Fatigue/tiredness | < 0.01 (MW) | < 0.01 (MW) | ||||||
| None/very mild | 70 (58.3) | 12 (36.4) | 58 (66.7) | 66 (57.9) | 5 (25.0) | 61 (64.9) | ||
| Mild | 32 (26.7) | 11 (33.3) | 21 (24.1) | 30 (26.3) | 7 (35.0) | 23 (24.5) | ||
| Moderate/severe/extremely severe | 18 (15.0) | 10 (30.3) | 8 (9.2) | 18 (15.8) | 8 (40.0) | 10 (10.6) | ||
| Current treatment class, n (%) | (FE) | (FE) | ||||||
| 5-Aminosalicyclic acids | 99 (82.5) | 28 (84.8) | 71 (81.6) | 0.79 | 97 (85.1) | 18 (90.0) | 79 (84.0) | 0.73 |
| Corticosteroids | 25 (20.8) | 10 (30.3) | 15 (17.2) | 0.13 | 15 (13.2) | 5 (25.0) | 10 (10.6) | 0.14 |
| Immunomodulators | 28 (23.3) | 11 (33.3) | 17 (19.5) | 0.15 | 19 (16.7) | 4 (20.0) | 15 (16.0) | 0.74 |
| Biologics | ||||||||
| Tumour necrosis factor-alpha inhibitors | 41 (34.2) | 11 (33.3) | 30 (34.5) | 1 | 36 (31.6) | 10 (50.0) | 26 (27.7) | 0.07 |
| Anti-integrin | 13 (10.8) | 7 (21.2) | 6 (6.9) | 0.04 | 7 (6.1) | 1 (5.0) | 6 (6.4) | 1.00 |
| Anti-interleukin 12/23 | 1 (0.8) | 0 (0.0) | 1 (1.1) | 1 | 13 (11.4) | 3 (15.0) | 10 (10.6) | 0.70 |
| JAK inhibitors | 8 (6.7) | 3 (9.1) | 5 (5.7) | 0.68 | N/A | N/A | N/A | |
UC ulcerative colitis, CD Crohn’s disease, BU bowel urgency, FE Fisher’s exact test, MW Mann-Whitney U test, N/A not applicable, SD standard deviation, TT t-test
All data in the table are physician reported with the exception of patient-reported bowel urgency status, current symptoms and elements of the Crohn’s disease activity index
*Ten most frequently patient-reported symptoms by patients with UC or with CD, in alphabetical order
aFor UC only: total: n = 119; with BU: n = 32; without BU: n = 87
Patients with Crohn’s Disease
Data for 114 patients with CD included in this analysis were reported by 25 physicians. Of those 114 patients, 17.5% (n = 20) had self-reported BU (Table 1). In 75.0% (n = 15) of those cases, physicians were unaware that their patients had BU.
Mean age (SD) of patients with self-reported BU was 38.9 (15.3) years and 60.0% (n = 12) were male. For patients without BU this was 37.2 (11.6) years and 63.8% (n = 60) male. Demographic variables were similar between CD patients both with and without BU (Table 2). Overall, most CD patients with and without BU had colitis (45.0% [n = 9] and 42.6% [n = 40], respectively) or ileocolitis (25.0% [n = 5] and 31.9% [n = 30]; Table 3). Diarrhoea with blood was present in a significantly greater proportion of CD patients with BU compared to those without (30.0% [n = 6] vs 8.5% [n = 8]; p = 0.02). No statistically significant differences were seen in the proportion of patients with and without BU reporting abdominal cramps, abdominal pain, diarrhoea without blood, fatigue/tiredness, loss of appetite, passing of mucus, passing wind (flatulence), rectal bleeding, stomach bloating (abdominal distension) or tenesmus (p > 0.05). Similarly, no significant difference was seen between patients with and without BU in the prevalence of the concomitant condition IBS (Table 2).
Prevalence of Disease Severity and Disease Activity
Patients with Ulcerative Colitis
Of patients with BU, 51.5% (n = 17) had mild disease compared with 71.3% (n = 62) of patients without BU. Those with BU had significantly greater disease activity than patients without, as evidenced by higher Mayo scores (mean [SD] 3.7 [2.1] vs 1.9 [2.1]; p < 0.01; Table 3) and a lower proportion of patients in remission (33.3% [n = 11] vs 66.7% [n = 58]). Patients without BU had milder overall symptoms compared to patients with BU (p < 0.01 for all symptoms), with a higher proportion of patients without BU having none or very mild overall symptoms (46.0% [n = 40] vs 21.2% [n = 7]), abdominal pain (56.3% [n = 49] vs 24.2% [n = 8]), sleep disturbance (70.1% [n = 61] vs 40.6% [n = 13]) and fatigue/tiredness (66.7% [n = 58] vs 36.4% [n = 12]).
Patients with Crohn’s Disease
Of patients with CD and BU, 40.0% (n = 8) had moderate or severe disease compared with 22.3% (n = 21) of patients without BU. Those with BU had significantly greater disease activity than CD patients without, as evidenced by higher mean (SD) CDAI scores (118.3 [85.7] vs 61.0 [59.7]; p < 0.01; Table 3) and a lower proportion of patients in remission (62.5% [n = 10] vs 89.9% [n = 80]). Patients without BU had milder overall symptoms compared to patients with BU (p < 0.05 for all symptoms), with a higher proportion of patients without BU having none or very mild overall symptoms (50.0% [n = 47] vs 25.0% [n = 5]), abdominal pain (54.3% [n = 51] vs 35.0% [n = 7]), sleep disturbance (77.7% [n = 73] vs 55.0% [n = 11]) and fatigue/tiredness (64.9% [n = 61] vs 25.0% [n = 5]).
Prevalence of Bowel Urgency and Satisfaction/Dissatisfaction of Current Disease Control
Patients with Ulcerative Colitis
Overall, most patients with and without BU were receiving 5-aminosalicylic acids at time of data collection, (84.8% [n = 28] of patients with and 81.6% [n = 71] patients without BU), while approximately one-third of patients in each group received tumour-necrosis factor (TNF) inhibitors (33.3% [n = 11] of patients with and 34.5% [n = 30] patients without BU). Steroids were used in almost twice as many patients with BU (30.3% [n = 10]) versus patients without BU (17.2% [n = 15]). Treatment of patients with and without BU was similar, except for the use of anti-integrins, which were received by a significantly greater proportion of patients with BU (21.2% [n = 7] vs 6.9% [n = 6]; p = 0.04) (Table 3).
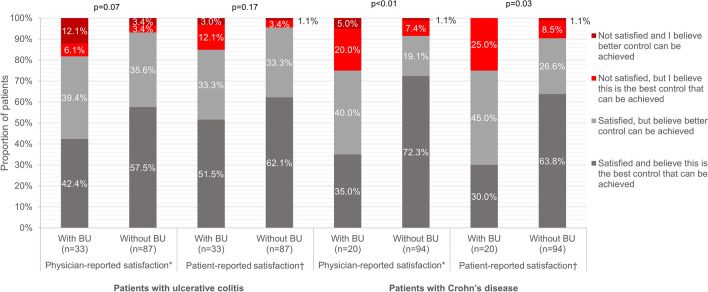
Physician satisfaction with current disease control was similar for patients with and without BU (p = 0.07). Physicians reported that they were satisfied but believed better control could be achieved for 39.4% (n = 13) of patients with BU and 35.6% (n = 31) of patients without. Likewise, physicians reported that they were satisfied and believed this was the best control that could be achieved for 42.4% of patients with BU and 57.5% of patients without (Fig. 1). Also, patients with BU reported similar satisfaction/dissatisfaction with the current disease control as patients without BU (p = 0.17); in both groups, 33.3% (patients with [n = 11] and without BU [n = 29]) of patients reported that they were satisfied but believed better control could be achieved, while 51.5% and 62.1% of patients with UC with and without BU reported that they were satisfied and believed this was the best control that could be achieved.
Fig. 1.
Satisfaction/dissatisfaction with current disease control in patients with ulcerative colitis and Crohn’s disease with and without bowel urgency. UC, ulcerative colitis; CD, Crohn’s disease; BU, bowel urgency; MW, Mann-Whitney U test. *Physicians were asked: Which of the following best describes your satisfaction with the current control? †Patients were asked: How satisfied are you with how well your medicine controls your IBD?
Patients with Crohn’s Disease
Overall, most patients received 5-aminosalicylic acids; 50.0% (n = 10) of patients with BU and 27.7% (n = 26) without used TNF inhibitors (p = 0.07). There was a greater than twofold increase in steroid use in patients with BU (25.0% [n = 5]) versus patients without BU (10.6% [n = 10]; p = 0.14). The difference in the proportion of patients with and without BU receiving each treatment class was similar (all p > 0.05; Table 3).
Physicians’ satisfaction/dissatisfaction with current disease control was significantly different for CD patients with and without BU (p < 0.01) (Fig. 1). Physicians reported they were satisfied but believed better control could be achieved for 40.0% (n = 8) of patients with BU and 19.1% (n = 18) of patients without. Patients’ satisfaction/dissatisfaction with current disease control achieved by their medication also significantly differed between those with and without BU (p < 0.01). Overall, 45.0% (n = 9) of patients with BU and 26.6% (n = 25) of patients without were satisfied but believed better control could be achieved.
Prevalence of Bowel Urgency and Health-Related Quality of Life Burden
Patients with Ulcerative Colitis
Patients with BU had a lower mean (SD) SIBDQ score compared with those without BU (47.9 [11.0] vs 56.6 [9.9], p < 0.01). The mean (SD) EQ-5D-5L score was lower for patients with BU compared to patients without (0.83 [0.10] vs 0.87 [0.11], p = 0.06), although the difference did not achieve statistical significance; however, the mean [SD] EQ-VAS score was significantly lower for patients with BU (65.6 [22.3] vs 82.3 [12.4], p < 0.01). Patients with BU also had a greater mean (SD) overall work impairment (24.8% [19.4] vs 14.0% [18.1], p = 0.04) and activity impairment (35.2% [27.2] vs 18.2% [20.9], p < 0.01) compared to those without BU (Table 4).
Table 4.
Patient-reported outcomes in patients with ulcerative colitis and Crohn’s disease without and with bowel urgency
| UC | CD | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | With BU | Without BU | p value (TT) | Total | With BU | Without BU | p value (TT) | |
| SIBDQ total score | < 0.01 | < 0.01 | ||||||
| n | 120 | 33 | 87 | 113 | 19 | 94 | ||
| Mean (SD) | 54.2 (10.9) | 47.9 (11.0) | 56.6 (9.9) | 54.9 (9.8) | 48.7 (11.8) | 56.2 (8.9) | ||
| EQ-5D-5L index score | 0.06 | < 0.01 | ||||||
| n | 117 | 32 | 85 | 109 | 20 | 89 | ||
| Mean (SD) | 0.86 (0.11) | 0.83 (0.10) | 0.87 (0.11) | 0.87 (0.10) | 0.81 (0.13) | 0.88 (0.09) | ||
| EQ-VAS score (how good/bad is your health today?) | < 0.01 | 0.29 | ||||||
| n | 120 | 33 | 87 | 114 | 20 | 94 | ||
| Mean (SD) | 77.7 (17.3) | 65.6 (22.3) | 82.3 (12.4) | 78.7 (15.1) | 75.5 (17.8) | 79.4 (14.4) | ||
| WPAI, percent work time missed because of disease | 0.94 | 0.44 | ||||||
| n | 72 | 18 | 54 | 70 | 10 | 60 | ||
| Mean (SD) | 3.0 (12.6) | 2.8 (4.8) | 3.0 (14.4) | 0.9 (2.6) | 1.5 (2.9) | 0.8 (2.5) | ||
| WPAI, percent impairment among working patients while working due to disease | 0.05 | 0.08 | ||||||
| n | 71 | 18 | 53 | 71 | 10 | 61 | ||
| Mean (SD) | 15.6 (17.9) | 22.8 (18.7) | 13.2 (17.1) | 17.7 (18.1) | 27.0 (25.4) | 16.2 (16.4) | ||
| WPAI, percent overall work impairment among working patients due to disease | 0.04 | 0.06 | ||||||
| n | 71 | 18 | 53 | 70 | 10 | 60 | ||
| Mean (SD) | 16.8 (18.9) | 24.8 (19.4) | 14.0 (18.1) | 17.7 (18.2) | 27.5 (26.1) | 16.0 (16.2) | ||
| WPAI, Percent activity impairment due to disease | < 0.01 | 0.01 | ||||||
| n | 120 | 33 | 87 | 114 | 20 | 94 | ||
| Mean (SD) | 22.8 (23.9) | 35.2 (27.2) | 18.2 (20.9) | 20.3 (20.5) | 30.5 (25.8) | 18.1 (18.6) | ||
UC ulcerative colitis, CD Crohn’s disease, BU bowel urgency, SIBDQ short version of the Inflammatory Bowel Disease, TT t test, VAS visual analogue scale, WPAI Work Productivity and Activity Impairment
EQ VAS scores range 0–100, with higher scores indicating better HRQoL
EQ-5D-5L index scores range 0–1, with higher scores indicating better HRQoL
SIBDQ total score ranges 10–70, with higher scores indicating better health
WPAI scores range 0–100%, with higher scores indicating greater impairment
Patients with Crohn’s Disease
The mean (SD) SIBDQ was lower in patients with BU than in patients without BU (48.7 [11.8] vs 56.2 [8.9], p < 0.01). The mean (SD) EQ-5D-5L index score was lower for CD patients with BU compared with patients without (0.81 [0.13] vs 0.88 [0.09], p < 0.01); however, the mean (SD) EQ-VAS scores were not significantly different (75.5 [17.8] vs 79.4 [14.4], p = 0.29). There was no significant difference in the mean (SD) WPAI percentage overall impairment between patients with and without BU (27.5% [26.1] vs 16.0% [16.2], p = 0.06), although work impairment in patients with BU tended to be greater. Mean (SD) WPAI activity impairment however was greater among patients with BU (30.5% [25.8] vs 18.1% [18.6], p = 0.01; Table 4).
Discussion
This real-world survey of physicians and their patients with UC and CD in Japan aimed to address the lack of data regarding BU prevalence and its association with clinical outcomes and HRQoL. This analysis compared IBD patients with and without BU and found that patients with BU were more likely to have greater disease severity and disease activity and worse HRQoL than patients without BU.
In this analysis, the prevalence of BU among patients with UC and CD was 27.5% (n = 33) and 17.5% (n = 20), respectively. These proportions are considerably lower than in previous reports from a recent Japanese survey in which 56.1% of 509 patients with UC who had visited medical providers regularly during the past year reported BU [25]. Similarly, previous cross-sectional, observational studies in Europe and the Americas have reported BU in 60–84% of patients with UC and 68%–74% of patients with CD [18, 20, 21, 46]. These differences may be attributed to differences in inclusion criteria; patients in the internet survey were only eligible if they had a recent hospital visit (within the last 3 months) for UC and were therefore likely to have more severe disease than the general consulting population used in our DSP analysis [25]. Similarly, inclusion criteria of the observational studies in Europe and the Americas also included patient populations with more severe cases than in our analysis [18, 20, 21, 46].
Moreover, we found there was a discordance between physician- and patient-reported BU. Discordance between physicians and their patients has previously been observed in UC symptom reporting, with patients reporting BU as the second-most commonly experienced symptom, while physicians reported BU as the fourth-most commonly reported symptom by patients [47]. Discordance in BU reporting in our study may have been due to patients’ embarrassment and hesitancy to mention such issues. Between 30–43% of patients are not comfortable reporting BU to their physician [47]. This may be exacerbated in Japanese patients as their character and culture make it difficult for them to discuss their symptoms [48].
Additionally, participating physicians may not have asked their patients specifically about BU as a symptom. A qualitative study focusing on communication between physicians and their patients with UC found that physicians typically used closed-ended questions when asking patients about their symptoms and spoke the most during the discussions [49]. Another study found that physicians often underestimate the impact of IBD symptoms on patients’ lives because they do not ask their patients about them directly [50]. Compounding the issue, Japanese IBD guidelines [26] currently include the Mayo score and CDAI scores—commonly used as markers of disease severity, treatment response and remission—which do not include BU as a symptom in their evaluation. These findings indicate approaches to physician-patient communication should be reviewed, with the aim of including active questioning and discussion about the presence and burden of BU when assessing IBD patients. The Urgency Numeric Rating Scale, which evaluates the severity of BU in adults with UC, was developed recently to address the lack of validated tools assessing this symptom. This tool allows physicians to assess and discuss BU in routine clinical practice and paves the way for the development of additional indices to assess BU [51].
Our analysis showed that patients with and without BU differed in some characteristics and symptoms. Patients with UC and BU were over twice as likely to have proctosigmoiditis (inflammation of the rectum and/or lower colon) than those without BU. In CD, colitis was the most frequent disease state among all patients. Abdominal pain was the most common symptom reported by both UC (51.5%) and CD (45.0%) patients with BU. Diarrhoea was also particularly problematic for patients with BU, with 30.3% and 45.5% of UC in addition to 30.0% and 40.0% of CD patients reporting diarrhoea with and without blood, respectively. Diarrhoea is caused by rectal mucosal inflammation in UC and is dependent on disease location in CD [20]; BU is due to the loss of rectal distensibility [21]. A study of clinical manifestations at IBD onset found that patients with left-sided colitis presented with a higher frequency of BU and of bloody diarrhoea compared to patients with proctitis. In the study, CD patients with isolated colonic involvement presented with a higher frequency of BU, diarrhoea and bloody diarrhoea, and faecal incontinence versus those with ileal or ileocolonic disease [21].
In the UC and CD cohorts, remission was reported for 33.3% and 62.5% of patients with BU, indicating that symptoms can persist despite disease inactivity. This is supported by studies in which patients have self-reported symptoms of IBD when they are in remission [16, 24]. Patients tend to evaluate their current health status relative to past experiences, with few patients equating remission with a complete return to a normal symptom-free state [16].
Our study indicated that the presence of BU was significantly associated with greater disease severity and reduced HRQoL in UC and CD. Well-known factors that reduce HRQoL, including pain, sleep disturbance and fatigue, were found to be more severe in patients with BU than in patients without. BU has previously been ranked the second most burdensome symptom of IBD, with effects on wellbeing that increase with severity [18, 24]. In Japan, the daily HRQoL impact of UC was highest amongst patients with BU with studies showing BU at least once a week led to half of patients missing appointments indicating disruption to day-to-day functioning [3, 25].
Despite reporting overall satisfaction, approximately 40% of physicians were satisfied (with response) but believed better control was achievable in IBD patients with BU. Physicians believed better disease control was possible for a higher proportion of CD patients with BU than without BU. However, for UC patients, physicians were satisfied and believed better control was possible for similarly high proportions of patients with and without BU. Since treatment is similar between patients regardless of BU, this finding may suggest that treatment is not optimal for patients with BU or that BU is particularly difficult to treat. However, BU may persist in patients with IBD regardless of optimal treatment [20], highlighting the need for more effective IBD treatments.
While patients with and without BU differed in clinical characteristics and HRQoL burden, this study found that there was no association between sex and presence/absence of BU. Although previous research has demonstrated sex-based differences in the presentation of IBD [52], sample sizes in our study were not sufficient to explore differences in patient characteristics and outcomes with and without BU disaggregated by sex. Further research is needed to assess differences in the presentation and BU among patients of different sex.
While BU is known to be a common and highly burdensome symptom in patients with irritable bowel syndrome with diarrhoea [53], < 1% of patients in our sample had diagnosed concomitant irritable bowel syndrome. Although this is likely underdiagnosed, this demonstrates that the BU within this study is likely to be a characteristic of patients’ CD or UC diagnosis.
As with all observational studies, this study has limitations. The patient inclusion criteria may result in the patient sample not representing the full IBD patient population. Generalizability may also be limited as the sample may consist of patients who most frequently visit their physicians and are more severely affected by their IBD than those who do not consult as often. Patient inclusion was based on the judgement of the physician and not a formalized diagnostic checklist; this is representative of physician’s real-world classification of the patient in clinical practice. The assessment of the presence/absence of BU was reliant on patients' understanding the questions and correctly completing the patient-reported form. Severity of BU was not captured within this secondary data source. Detailed studies that describe patients’ BU by severity are needed in the future to elucidate the unmet needs in different patient groups and understand its association with other clinical characteristics. A further limitation is that the cross-sectional design does not enable patients to be followed and assessed over time and prevents any conclusions about causal relationships; however, identification of significant associations is possible. Finally, due to sampling methodology, sample size is low for certain groupings, which should be accounted for when interpreting results. Some significant differences may have been missed because of the low number of patients; however, it does mean any significant differences we did identify are likely robust. Despite such limitations, real-world studies complement clinical trials since they lack strict eligibility criteria and patients are less likely to be adherent to medication.
Conclusions
This analysis showed that patients with BU differed in some of their clinical characteristics and had greater disease severity and disease activity and worse HRQoL than patients without BU. IBD remains uncontrolled, with BU and other symptoms persisting regardless of treatment, resulting in both physicians and their patients believing that better disease control can be achieved. Moreover, commonly used indicess often report patients in remission despite the presence of BU. The discordance between physician- and patient-reported BU indicates the need for improvement in communications regarding BU between physicians and their patients. Furthermore, the clinical and HRQoL burden associated with BU in IBD patients seen in routine clinical practice underlines the need for new therapeutic options for patients who experience sub-optimal response to currently available treatment approaches.
Acknowledgements
We thank the participants of the study.
Medical writing/editorial assistance
Medical writing support under the guidance of the authors was provided by Sue Libretto, PhD, of Sue Libretto Publications Consultant Ltd. (Hertfordshire, UK) and funded by Adelphi Real World in accordance with Good Publication Practice (GPP) guidelines [54].
Author Contributions
Chaochen Wang, Tomoko Ishizuka, Masaru Tanaka and Koji Matsuo of Eli Lilly Japan K.K., Kobe and Theresa Hunter Gibble of Eli Lilly and Company, Indianapolis, IN, USA, were involved in the analysis, design and editing of the manuscript. Survey design, data collection, data analysis, statistical analyses, data interpretation and editing were performed by Hannah Knight, Niamh Harvey and Liane Gillespie-Akar of Adelphi Real World. Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi IBD DSP. Eli Lilly and Company did not influence the original survey through either contribution to the design of questionnaires or data collection. All authors had access to the aggregated data, provided critical feedback and approved the final manuscript.
Funding
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Real World IBD Disease Specific Programme™ (DSP). Eli Lilly and Company did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here used data from the Adelphi Real World IBD DSP. The DSP is a wholly owned Adelphi Real World product. Eli Lilly and Company is one of multiple subscribers to the DSP. Publication of survey results was not contingent on the subscriber’s approval or censorship of the publication. Publication and open access were funded by Eli Lilly and Company.
Data Availability
All data, i.e., methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Hannah Knight at hannah.knight@adelphigroup.com. Hannah Knight is an employee of Adelphi Real World.
Declarations
Conflict of Interest
Chaochen Wang, Masaru Tanaka and Koji Matsuo are full-time employees of Eli Lilly Japan K.K., Kobe and minor shareholders of Eli Lilly and Company. Tomoko Ishizuka was a full-time employee of Eli Lilly Japan K.K., Kobe during the conduct of this study and is a current employee of Novo Nordisk Pharma Ltd. Theresa Hunter Gibble is a full-time employee and minor shareholder of Eli Lilly and Company. Hannah Knight, Niamh Harvey and Lianne Gillespie-Akar are employees of Adelphi Real World.
Ethical Approval
Data collection by DSP fieldwork teams was conducted in accordance with national market research and privacy regulations, including European Pharmaceutical Market Research Association (EphMRA) and the US Department of Health and Human Services National Institutes of Health, Health Insurance Portability and Accountability Act (HIPAA). This research also obtained ethics approval from the Western Institutional Review Board, study protocol number 1-1238963-1 and performed in accordance with the principles stated in the Declaration of Helsinki. All responses captured on the data collection forms were anonymized to preserve respondent confidentiality. Responses were anonymized before aggregated reporting, the identity of the physicians was blinded, and no patient identifiers were collected. Physicians were compensated in line with fair market rates.
References
- 1.Chiba M, Morita N, Nakamura A, Tsuji K, Harashima E. Increased incidence of inflammatory bowel disease in association with dietary transition (Westernization) in Japan. JMA J. 2021;4(4):347–357. doi: 10.31662/jmaj.2021-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamabe K, Liebert R, Flores N, Pashos CL. Health-related quality of life outcomes and economic burden of inflammatory bowel disease in Japan. Clinicoecon Outcomes Res. 2019;11:221–232. doi: 10.2147/CEOR.S179892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueno F, Nakayama Y, Hagiwara E, Kurimoto S, Hibi T. Impact of inflammatory bowel disease on Japanese patients' quality of life: results of a patient questionnaire survey. J Gastroenterol. 2017;52(5):555–567. doi: 10.1007/s00535-016-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto T, Yanai S, Toya Y, Ueno M, Nakamura S. Internet-orientated assessment of QOL and actual treatment status in Japanese patients with inflammatory bowel disease: the 3I survey. J Crohns Colitis. 2015;9:477–482. doi: 10.1093/ecco-jcc/jjv052. [DOI] [PubMed] [Google Scholar]
- 5.Le Berre C, Ananthakrishnan AN, Danese S, Singh S, Peyrin-Biroulet L. Ulcerative colitis and crohn's disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol. 2020;18(1):14–23. doi: 10.1016/j.cgh.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Dig Liver Dis. 2021;53(7):803–808. doi: 10.1016/j.dld.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Veauthier B, Hornecker JR. Crohn's disease: diagnosis and management. Am Fam Physician. 2018;98(11):661–669. [PubMed] [Google Scholar]
- 8.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 9.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl. 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter RJ, Kalla R, Ho GT. Ulcerative colitis: recent advances in the understanding of disease pathogenesis. F1000Res. 2020;9:294. doi: 10.12688/f1000research.20805.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato S, Kobayashi T, Kani K, Yamamoto R, Nagoshi S, Yakabi Y. Ulcerative colitis- review treatment with 5-aminosalicylic acid (including topical therapy) IBD Research. 2013;7(1):84–89. [Google Scholar]
- 12.Dulai PS, Singh S, Vande Casteele N, et al. Should we divide crohn's disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol. 2019;17(13):2634–2643. doi: 10.1016/j.cgh.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuoka K, Fujii T, Okamoto R, et al. Characteristics of adult patients newly diagnosed with Crohn's disease: interim analysis of the nation-wide inception cohort registry study of patients with Crohn's disease in Japan (iCREST-CD) J Gastroenterol. 2022 doi: 10.1007/s00535-022-01907-2. [DOI] [PubMed] [Google Scholar]
- 14.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 16.Norton C, Dibley LB, Bassett P. Faecal incontinence in inflammatory bowel disease: associations and effect on quality of life. J Crohn’s Colitis. 2013;7(8):e302–e311. doi: 10.1016/j.crohns.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Kamal N, Motwani K, Wellington J, Wong U, Cross RK. Fecal incontinence in inflammatory bowel disease. Crohns Colitis 360. 2021;3(2):otab013. doi: 10.1093/crocol/otab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawwas GK, Jajeh H, Shan M, Naegeli AN, Hunter T, Lewis JD. Prevalence and factors associated with fecal urgency among patients with ulcerative colitis and Crohn’s disease in the Study of a Prospective Adult Research Cohort With Inflammatory Bowel Disease. Crohns Colitis 360. 2021 doi: 10.1093/crocol/otab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchmann P, Kolb E, Alexander-Williams J. Pathogenesis of urgency in defaecation in Crohn’s disease. Digestion. 1981;22:310–316. doi: 10.1159/000198676. [DOI] [PubMed] [Google Scholar]
- 20.Petryszyn PW, Paradowski L. Stool patterns and symptoms of disordered anorectal function in patients with inflammatory bowel diseases. Adv Clin Exp Med. 2018;27(6):813–818. doi: 10.17219/acem/68986. [DOI] [PubMed] [Google Scholar]
- 21.Nóbrega VG, Silva INN, Brito BS, Silva J, Silva MCMD, Santana GO. The onset of clinical manifestations in inflammatory bowel disease patients. Arq Gastroenterol. 2018;55(3):290–295. doi: 10.1590/S0004-2803.201800000-73. [DOI] [PubMed] [Google Scholar]
- 22.Rangan V, Mitsuhashi S, Singh P, et al. Risk factors for fecal urgency among individuals with and without diarrhea, based on data from the national health and nutrition examination survey. Clin Gastroenterol Hepatol. 2018;16(9):1450–1458.e2. doi: 10.1016/j.cgh.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpio D, López-Sanromán A, Calvet X, et al. Perception of disease burden and treatment satisfaction in patients with ulcerative colitis from outpatient clinics in Spain: UC-LIFE survey. Eur J Gastroenterol Hepatol. 2016;28(9):1056–1064. doi: 10.1097/MEG.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 24.Farrell D, McCarthy G, Savage E. Self-reported symptom burden in individuals with inflammatory bowel disease. J Crohns Colitis. 2016;10(3):315–322. doi: 10.1093/ecco-jcc/jjv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hibi T, Ishibashi T, Ikenoue Y, Yoshihara R, Nihei A, Kobayashi T. Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a japanese internet survey. Inflamm Intest Dis. 2020;5(1):27–35. doi: 10.1159/000505092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489–526. doi: 10.1007/s00535-021-01784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 28.Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the disease specific programme. BMJ Open. 2016;6(8):e010352. doi: 10.1136/bmjopen-2015-010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi: 10.2147/DMSO.S120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dotson J, Hunter T, Lukanova R, et al. Symptomology and most bothersome symptoms among pediatric Crohn’s disease and ulcerative colitis patients: results from a physician and patient survey. J Crohns Colitis. 2021;15(Suppl. 1):S202–S203. doi: 10.1093/ecco-jcc/jjab076.237. [DOI] [Google Scholar]
- 31.Tillett W, Merola JF, Thaçi D, et al. Disease characteristics and the burden of joint and skin involvement amongst people with psoriatic arthritis: a population survey. Rheumatol Ther. 2020;7(3):617–637. doi: 10.1007/s40744-020-00221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96(3):804–810. doi: 10.1016/0016-5085(89)90905-0. [DOI] [PubMed] [Google Scholar]
- 33.Irvine EJ, Zhou Q, Thompson AK, CCRPT Investigators The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. Can Crohn's Relapse Prev Trial Am J Gastroenterol. 1996;91:1571–8. [PubMed] [Google Scholar]
- 34.The EuroQol Group EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 35.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 36.Reilly MC, Gerlier L, Brabant Y, Brown M. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn's disease. Clin Ther. 2008;30(2):393–404. doi: 10.1016/j.clinthera.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Italian Group for Inflammatory Bowel Disease. IG-IBD Scores. Mayo Full. https://www.igibdscores.it/en/info-mayo-full.html. Accessed June 2022.
- 38.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 39.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 40.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. Natl Coop Crohn's Dis Study Gastroenterol. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 41.Best WR. Predicting the Crohn's disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006;12(4):304–310. doi: 10.1097/01.MIB.0000215091.77492.2a. [DOI] [PubMed] [Google Scholar]
- 42.Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. 2016;25(3):707–719. doi: 10.1007/s11136-015-1108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512–530. doi: 10.1053/gast.2002.31072. [DOI] [PubMed] [Google Scholar]
- 45.Reilly MC, Brown MC, Brahant Y, et al. Defining the minimally important difference for WPAI: CD scores: what is a relevant impact on work productivity in active Crohn’s disease? Gut. 2007;56(Suppl. 3):A159. [Google Scholar]
- 46.Rubin DT, Sninsky C, Siegmund B, et al. International perspectives on management of inflammatory bowel disease: opinion differences and similarities between patients and physicians from the IBD GAPPS survey. Inflamm Bowel Dis. 2021;27(12):1942–1953. doi: 10.1093/ibd/izab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Travis S, Bleakman AP, Rubin D, et al. A216 bowel urgency communication gap between health care professionals and patients with ulcerative colitis in the US and Europe: communicating needs and features of ibd experiences (CONFIDE) survey. J Can Assoc Gastroenterol. 2023;6(Suppl. 1):52–54. doi: 10.1093/jcag/gwac036.216. [DOI] [Google Scholar]
- 48.Asai A, Okita T, Bito S. Discussions on present japanese psychocultural-social tendencies as obstacles to clinical shared decision-making in Japan. Asian Bioeth Rev. 2022;14(2):133–150. doi: 10.1007/s41649-021-00201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin DT, Dubinsky MC, Martino S, Hewett KA, Panés J. Communication between physicians and patients with ulcerative colitis: reflections and insights from a qualitative study of in-office patient-physician visits. Inflamm Bowel Dis. 2017;23(4):494–501. doi: 10.1097/MIB.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1(1):10–20. doi: 10.1016/j.crohns.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Dubinsky MC, Irving PM, Panaccione R, et al. Incorporating patient experience into drug development for ulcerative colitis: development of the Urgency Numeric Rating Scale, a patient-reported outcome measure to assess bowel urgency in adults. J Patient Rep Outcomes. 2022;6(1):31. doi: 10.1186/s41687-022-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rustgi SD, Kayal M, Shah SC. Sex-based differences in inflammatory bowel diseases: a review. Therap Adv Gastroenterol. 2020;13:1756284820915043. doi: 10.1177/1756284820915043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basilisco G, De Marco E, Tomba C, Cesana BM. Bowel urgency in patients with irritable bowel syndrome. Gastroenterology. 2007;132(1):38–44. doi: 10.1053/j.gastro.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 54.DeTora LM, Toroser D, Sykes A, et al. Good Publication Practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med. 2022;175(9):1298–1304. doi: 10.7326/M22-1460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, i.e., methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Hannah Knight at hannah.knight@adelphigroup.com. Hannah Knight is an employee of Adelphi Real World.