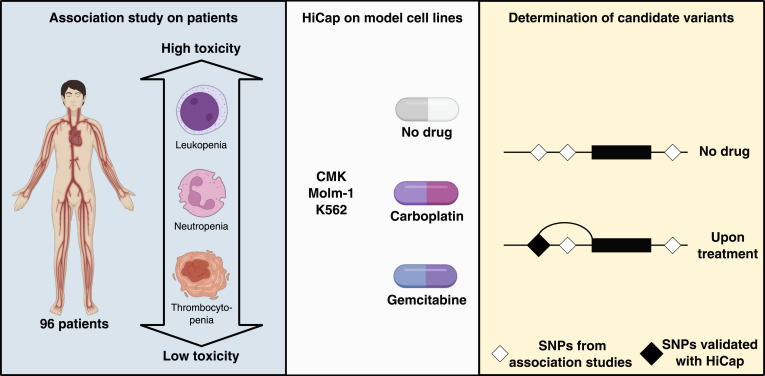
Using transcriptome and interactome maps with patients’ WGS, this research uncovers the role of noncoding mutations in the myelosuppression predisposition, advancing personalized cancer treatment.
Abstract
Non-small cell lung cancer is often diagnosed at advanced stages, and many patients are still treated with classical chemotherapy. The unselective nature of chemotherapy often results in severe myelosuppression. Previous studies showed that protein-coding mutations could not fully explain the predisposition to myelosuppression. Here, we investigate the possible role of enhancer mutations in myelosuppression susceptibility. We produced transcriptome and promoter-interaction maps (using HiCap) of three blood stem-like cell lines treated with carboplatin or gemcitabine. Taking advantage of publicly available enhancer datasets, we validated HiCap results in silico and in living cells using epigenetic CRISPR technology. We also developed a network approach for interactome analysis and detection of differentially interacting genes. Differential interaction analysis provided additional information on relevant genes and pathways for myelosuppression compared with differential gene expression analysis at the bulk level. Moreover, we showed that enhancers of differentially interacting genes are highly enriched for variants associated with differing levels of myelosuppression. Altogether, our work represents a prominent example of integrative transcriptome and gene regulatory datasets analysis for the functional annotation of noncoding mutations.
Graphical Abstract
Introduction
Cancer, a leading cause of death, is responsible for nearly one in six deaths worldwide (Ferlay et al, 2021). Lung cancer is the second most frequently diagnosed cancer, causing 1.8 million yearly deaths (Thai et al, 2021). Of those, 84% are diagnosed with non-small cell lung cancer (NSCLC) (Ganti et al, 2021), often at an advanced stage because of a lack of clinical symptoms and effective screening approaches (Gridelli et al, 2015). For many patients with advanced-stage NSCLC, where targeted therapies and immunomodulators are not indicated, the main treatment route remains traditional chemotherapy using the third generation chemotherapy (such as paclitaxel, docetaxel, gemcitabine, vinorelbine or irinotecan) in combination with platinum derivatives (carboplatin, cisplatin) (Baggstrom et al, 2007). The classical drug cocktail for NSCLC, gemcitabine in combination with carboplatin (Sederholm et al, 2005), is also widespread for treating other solid tumors such as bladder, ovarian, and breast cancers (Pittman et al, 2006). Despite their widespread application, these drugs can lead to severe adverse drug reactions (ADRs), often resulting in treatment cessation, treatment failure or even death (Testart-Paillet et al, 2007; Amjad et al, 2023).
Blood cell progenitors, located in the bone marrow, are fast-dividing cells that maintain the turnover of blood cells, such as lymphocytes, erythrocytes, platelets, and neutrophils (Carey, 2003). Their untargeted death leads to myelosuppression—dose-limiting toxicity in carboplatin/gemcitabine treatment (Chatelut et al, 2003). Notably, among platinum derivatives, it is carboplatin which is mainly associated with a higher risk of neurotoxicity and myelosuppression, whereas ADRs of cisplatin include a higher rate of nausea, vomiting, nephrotoxicity, and ototoxicity (Santana-Davila et al, 2014). So far, only neutropenia can be partially modulated in some cases by G-CSF (granulocyte colony-stimulating factor) (Pastor et al, 2013). As a result, around 55% of the patients receiving chemotherapy stopped it because of severe or life-threatening myelosuppression levels, even if the drug is effective for treating the tumor (Zatloukal et al, 2003; Sederholm et al, 2005; Rudd et al, 2005; Grønberg et al, 2009; Imamura et al, 2011). Therefore, it is imperative to devise tools to stratify patients with respect to their predisposition to experiencing ADRs, especially chemotherapy-induced myelosuppression. Genetic factors heavily modulate drug response phenotype, so patient genotype information could be used for therapy management and optimization (Trendowski et al, 2019; Mulford et al, 2021). A recent study by Swen et al (2023) validated this hypothesis and reported the use of a pharmacogenetics panel containing 12 genes. The use of the panel significantly reduced the number of patients with ADRs and was feasible across European healthcare system organizations (Swen et al, 2023).
The potential of using germline genetic markers for toxicity response prediction in the context of chemotherapy-induced myelosuppression was already reported in a set of our previous studies. After performing exome-sequencing of 215 NSCLC patients under carboplatin/gemcitabine treatment (Björn et al, 2020b; Svedberg et al, 2020) and whole-genome sequencing of 96 patients displaying the most extreme toxicity levels (neutropenia, leukopenia, and thrombocytopenia) (Björn et al, 2020a), very few coding variants were found to be associated with toxicity response. Almost all toxicity-associated variants were located in noncoding regions, suggesting a possible role for distal cis-regulatory elements in toxicity response regulation.
Enhancers are best-studied distal cis-regulatory elements containing clusters of binding sites for transcription factors (Banerji et al, 1981; Kleinjan & van Heyningen, 2005). Their disruption was shown to be a disease-driving or contributing mechanism via modulation of the expression of target genes, establishing a new disease group called enhanceropathies (Claringbould & Zaugg, 2021). The chromatin immunoprecipitation (ChIP) method can effectively locate active enhancers using antibodies against the relevant epigenomic marks such as H3K27Ac and H3K4me1 (Liang et al, 2004; Heintzman et al, 2009; Rada-Iglesias et al, 2011). However, their location provides limited insight into the functionality of enhancers (Blackwood & Kadonaga, 1998). Historically, enhancers were linked to their nearest gene. Still, various novel experimental assays showed that only one-third of the enhancers regulate their closest gene (Dostie et al, 2006; Åkerborg et al, 2019), necessitating methods to locate their target genes and their location within the same experiment.
Chromosome conformation capture for high-throughput sequencing (Hi-C) is the chief method to map the spatial conformation of genomes that mediates the promoter–enhancer contacts through looping (Lieberman-Aiden et al, 2009). However, its resolution does not allow for mapping interactions between individual promoter and enhancer elements because of the genome’s vast number of structural interactions and stochastic proximities (Forcato et al, 2017). The HiCap technology solves this problem by introducing a sequence capture step on the Hi-C material, enriching for interactions that involve specifically targeted regions (Sahlén et al, 2015). HiCap provides close to single-enhancer resolution (circa 860 bp), outperforming similar methods (Ma et al, 2015; Mifsud et al, 2015; Schoenfelder et al, 2015). This allows fine-mapping of sequence variants and target gene discovery in the same experiment (Åkerborg et al, 2019; Pradhananga et al, 2020; Sahlén et al, 2021; Zhigulev et al, 2022a). Because most variants associated with drug-induced myelosuppression were found within regulatory elements (Björn et al, 2020a), we reasoned that HiCap targeting all the promoters and selected mutations could help prioritize variants for clinical use and provide insights into the mechanisms by which they contribute to toxicity.
Results
HiCap depicts interactome dynamics of blood stem-like cells upon drug exposure
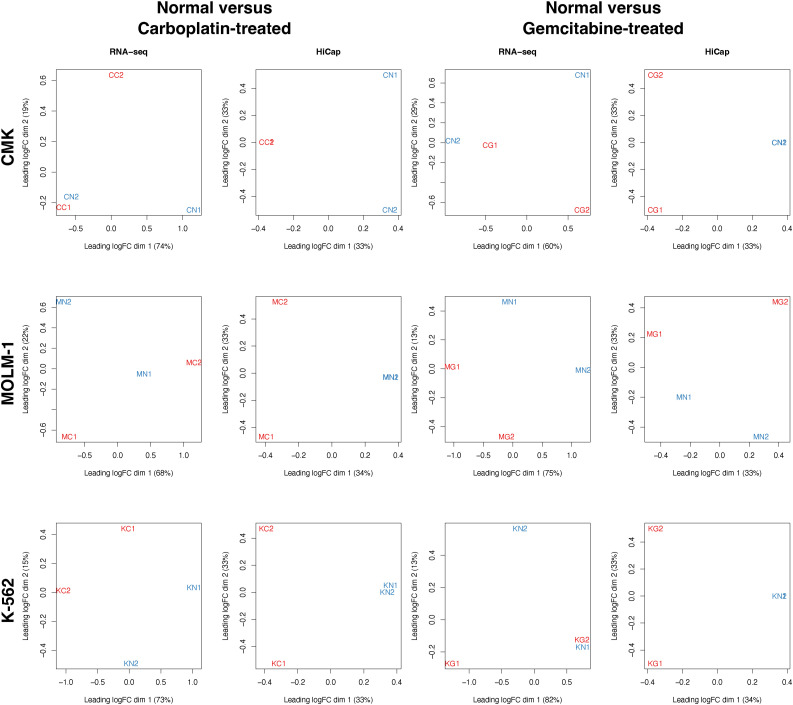
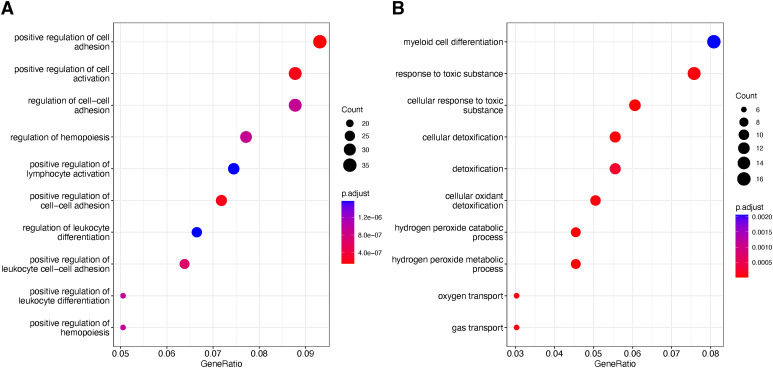
We processed 0.8 and 1.1 billion reads mapped on the transcriptome and interactome of 18 samples corresponding to normal and drug-treated counterparts of the three cell lines: CMK, MOLM-1, and K-562, in two replicates. These cell lines are a good and convenient surrogate model readily available in many research laboratories (Skopek et al, 2023). We first investigated gene expression changes upon drug exposure. The technical replicates of the experiments correlated well in all cases (coefficient of correlation >0.93 across all conditions) (Fig S1A–F). However, RNA profiles of samples were not always able to separate treated cells from their normal counterparts (Fig S2). Nevertheless, we detected differentially expressed (DE) genes (FDR < 0.1), absolute log-fold change (abs[logFC] > 1.2) belonging to relevant processes for drug exposure such as regulation of hemopoiesis (GO:1903706, FDR = 1 × 10−8) and response to toxic substances (GO:0009636, FDR = 5 × 10−4) (Fig S3A and B, Table S1), confirming the cells’ response to the treatment.
Figure S1. Correlation scatterplots of RNA-seq technical replicates.
(A, B, C, D, E, F) CMK, (C, D) MOLM-1, (E, F) K-562 cells. N, C, and G stand for non-treated, carboplatin- and gemcitabine-treated datasets.
Figure S2. The multidimensionality scaling (MDS) plots of the transcriptome (RNA-seq) and interactome (HiCap) profiles.
Profiles correspond to CMK, MOLM-1, and K-562 (C, M, K) cells upon carboplatin, gemcitabine or no drug (C, G, N) treatments in two replicates (1 and 2). Although normal and treated counterparts are separated in most of the cases, separation by the interactome profile is more effective.
Figure S3. GO term enrichment profiles of differentially expressed genes.
(A, B) GO term enrichments of DE genes upon (A) carboplatin and (B) gemcitabine treatments across all cells.
Table S1 RNA-seq dataset. (19.3MB, xlsx)
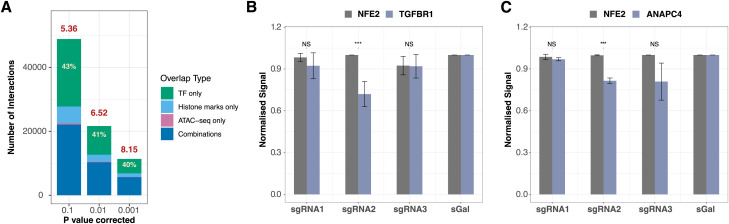
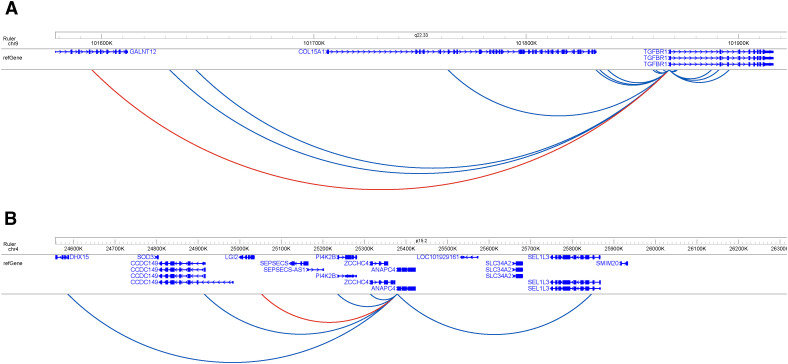
Next, we analyzed HiCap datasets to find genomic interactions of targeted promoters and selected variants (see the Materials and Methods section). We detected 114,365 distal regions (i.e., untargeted regions that are located distal to the promoters) interacting with 7,254 promoters (supporting pairs >4 and Bonferroni-adjusted P-value < 0.01) across all cell lines and treatments (Table S2). Promoter-interacting regions (PIRs) covered around 2.8% (84.4 Mb) of the genome. We also detected 18,024 interactions between promoters and 19,204 interactions between targeted variants and distal regions. Because K-562 is a tier 1 ENCODE cell line, a vast array of public ATAC-seq and ChIP-seq datasets is available (Table S3). We used these datasets to assess the enhancer potential of the PIRs. In K-562 cells, 51.2% (22,719/44,366) of the PIRs overlapped with at least one enhancer mark (6.52-fold enrichment) (Fig 1A). We found that 41% (18,190/44,366) of the PIRs overlapped only a TF-binding site, highlighting the importance of TF datasets during the evaluation of promoter–enhancer interactions. All super-enhancers (SEs) annotated in K-562 cells (Jiang et al, 2019) also overlapped with at least one PIR.
Figure 1. Validation of K-562 PIRs detected by HiCap.
(A) The enhancer element enrichment profile of PIRs at varying Bonferroni-corrected P-value thresholds. The numbers in red denote the fold enrichment for all enhancer elements using the BEDTools fisher tool. The percentages in white show the ratio of PIRs that only overlaps with TF binding sites. (B, C) enCRISPRi-LK validation of (B) chr9:101,595,333–101,595,751 region interacting with the TGFBR1 promoter and (C) chr4:25,051,758–25,052,804 region interacting with the ANAPC4 promoter. In both cases, different sgRNA2s show a significant effect. sGal represents non-targeting sgRNA. Data are presented as mean ± stdev. ***P ≤ 0.05 (t test).
Table S2 HiCap dataset. (79.6MB, xlsx)
Table S3 Enhancer datasets. (16.3KB, xlsx)
We further tested the potential of detected PIRs to modulate the activity of targeted genes using an inducible dual-effector epigenetic interference system enCRISPRi-LK (Li et al, 2020). We successfully reduced the gene expression in two out of three cases by repressing their enhancers (Fig 1B and C). The first gene, TGFBR1 (Transforming Growth Factor β Receptor 1), is a part of the transforming growth factor β signaling pathway known for supporting the maintenance of the self-renewal capacity of hematopoietic stem cells (Blank & Karlsson, 2015). It is regulated by an enhancer located 272 kb away in the chr9:101,595,333–101,595,751 region (Fig S4A). The second gene, ANAPC4 (Anaphase Promoting Complex Subunit 4), is part of a highly conserved protein complex that controls the amounts of the cyclins and other cell cycle regulators to ensure proper cell cycle transitions (Wäsch et al, 2010). Enhancer of ANAPC4 is located 326 kb away from it in the chr4:25,051,758–25,052,804 region (Fig S4B). None of these regions interact with NFE2 (Nuclear Factor Erythroid 2) promoter used as a negative control.
Figure S4. Interactome profiles of TGFBR1 and ANAPC4.
(A, B) Interactions present in the untreated K-562 cell line of (A) TGFBR1 and (B) ANAPC4. Interactions in red were validated with enCRISPRa/i.
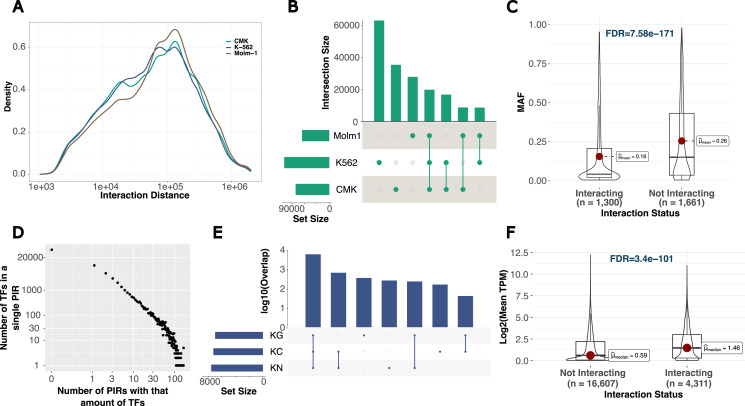
We then investigated different aspects of interaction data by looking at interaction statistics, functional enrichment of interacting regions, and expression profiles of interacting promoters. The average interaction distance across all the samples is 156 kb (Fig 2A). Our interactome maps showed close to single-enhancer resolution (average PIR length of 860 bp) and therefore facilitated the individual discovery of shared, and cell type-specific interactions (Fig 2B). Interactome profiles were more effective in separating untreated cells from their treated counterparts compared with that of the transcriptome data (Fig S2). Interestingly, 1,302 (43.9%) of targeted variants showed at least one interaction in one cell type, and interacting variants had significantly lower minor allele frequencies compared with those targeted variants without an interaction (Fig 2C, minor allele frequencies = 0.16 versus 0.26, FDR = 7.58 × 10−171).
Figure 2. Interactome organization across different cell lines and treatments.
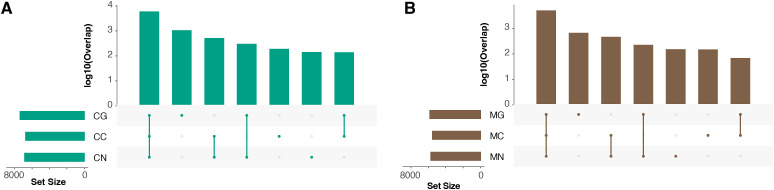
(A) Absolute interaction distance distribution of all interactions. (B) The overlap of interactions across cell types. (C) Dependence of targeted SNVs with different MAFs on their interaction status. (D) The distribution of the number of TFs found in each PIR. (E) The overlap statistics of interacting genes in K-562 cells (K) between treatments: carboplatin (C), gemcitabine (G), or no drug (N). (F) Dependence of gene expression on the interaction status of genes.
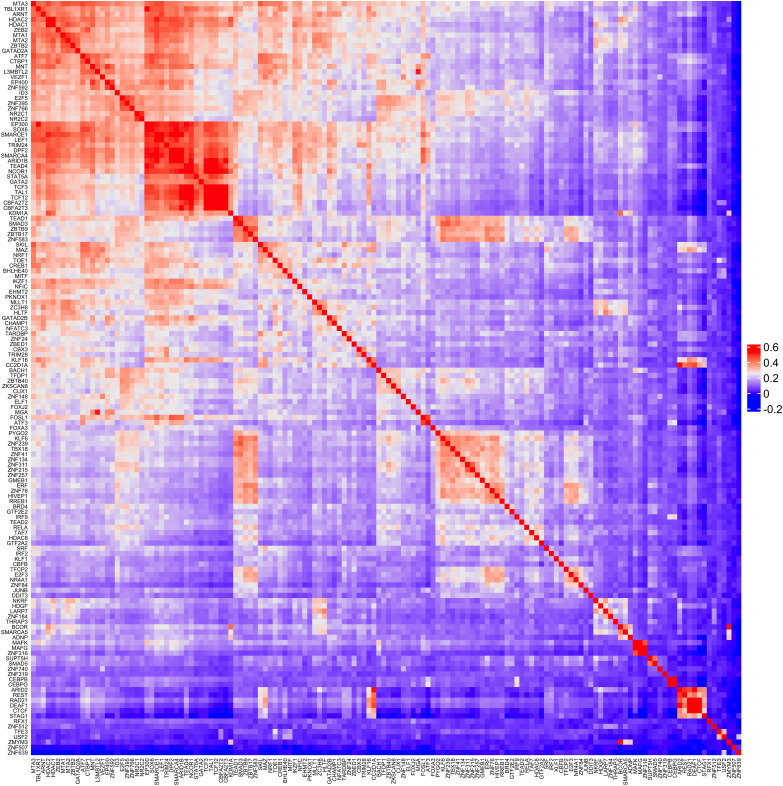
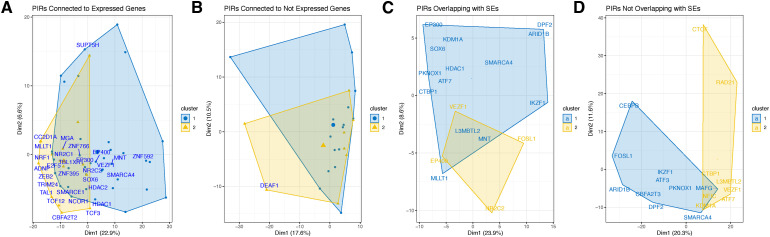
The ChIP-seq profiles of 218 TFs from K-562 cells (Table S3) were used to investigate the TF distribution across PIRs. The number of TF-binding sites per PIR followed an inverse power–law distribution; that is, most PIRs contained few TFs, and few PIRs contained many TFs (Fig 2D). Using the k-means clustering algorithm, we observed four main TF clusters that co-occur, whereas generally the binding patterns of most TFs were not correlated (Fig S5) suggesting a diversity of enhancers based on their TF-binding patterns. One of the clusters contained 49 TFs that are highly enriched for SWI/SNF superfamily (FDR = 6.3 × 10−16), NuRD complex (FDR = 3.8 × 10−13), and leukemogenesis (FDR = 5.8 × 10−11). That is in line with previous studies showing a high mutation rate of chromatin remodeling complexes in cancer (Bracken et al, 2019). We then separated PIRs connected to either expressed (top 75% quantile, 16,092 interactions) or not-expressed (bottom 25% quantile, 16,241 interactions) genes to see if there are any differential TF binding between the two groups. There were 52 and 24 TFs binding to PIRs connecting to high or low-expressed genes, respectively, and 23 TF were shared (Table S4). Transcriptional repressor DEAF1 (Michelson et al, 1999) was only found in PIRs connected to low-expressed genes. Moreover, 29 TFs were only found in PIRs connected to expressed genes (Fig S6A and B). Among them, members of the histone deacetylase family (HDAC1, HDAC2, EP400, NCOR1, and TBL1XR1) were present, confirming the role of histone deacetylation in gene activation (Jepsen et al, 2000; Bhaskara et al, 2008; LaBonte et al, 2009; Wang et al, 2009; Seto & Yoshida, 2014). Lastly, we looked at the TF-binding profiles of PIRs as a function of their overlap with SEs. Members of activator complexes such as EP300, EP400, TBL1XR1, NR2C2, and SOX6 were only found in PIRs overlapping with SEs (Fig S6C). However, CTCF and RAD21 (subunit of the cohesin complex [Hauf et al, 2001]) were found only in PIRs not overlapping with SEs (Fig S6D), which supports their role in regulating loop stability (Hansen et al, 2017).
Figure S5. Heatmap of TF co-occurrence in K-562 PIRs detected by HiCap.
Figure S6. The hierarchical clustering of the TFs in PIRs.
(A, B) Clustering of the TFs connected to (A) expressed genes or (B) not expressed genes. (C, D) Clustering of the TFs (C) overlapping or (D) not overlapping with SEs. Only genes that are expressed 90% quantile are taken for the sake of clarity.
Table S4 TF clusters. (20.5KB, xlsx)
Finally, we focused on the targeted genes across all cell lines and treatments. There were 8,251, 6,582, and 8,142 interacting genes in CMK, MOLM-1, and K-562 cells, respectively. We discovered cell type-specific and treatment-specific interacting genes (Figs 2E and S7A and B). In all cells, they were more likely to be expressed (Fig 2F). However, they were not more likely to be differentially expressed, supporting the different dynamics of the transcriptional regulation by enhancers and promoters (Larsson et al, 2019).
Figure S7. The overlap statistics of interacting genes.
(A, B) Gene sets for (A) CMK and (B) MOLM-1 cells.
Network analysis of interactome facilitates the discovery of differentially interacting genes
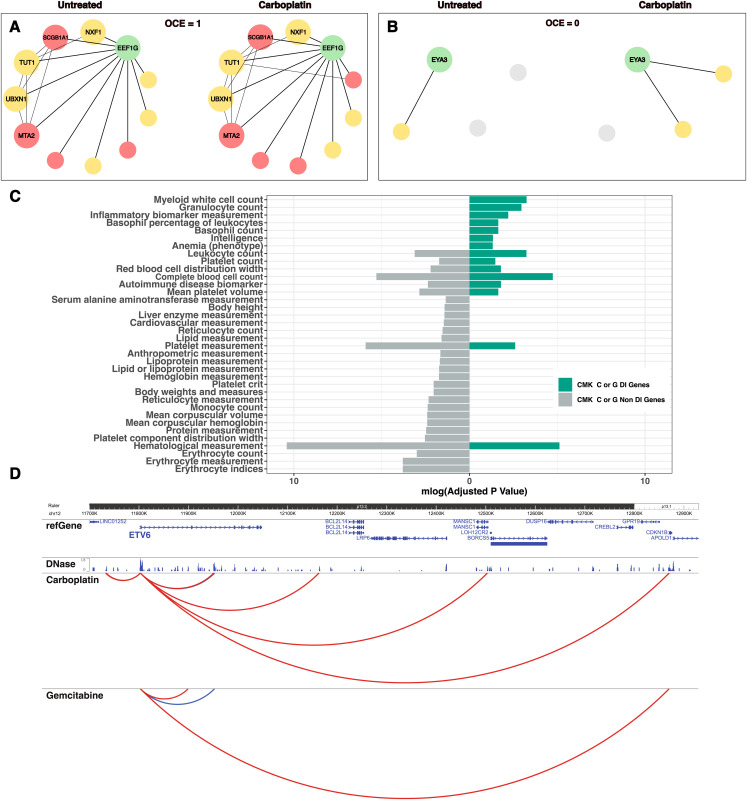
To track the dynamics of promoter–enhancer interactions, we constructed networks using promoters and PIRs as network nodes and connected them with an edge in case of interaction (see the Materials and Methods section). We generated a separate network for each replicate of cell type and state. We then calculated two parameters, namely, Overlap Coefficient (OCE) and Jaccard Similarity Index (JI), to quantify the connectivity differences for each node across different states for each cell type. If the OCE/JI is equal to one, it means that the gene did not change its interactions. In contrast, if the OCE/JI equals zero, then it means that the gene either changed all interactions, gained all new interaction(s) or lost all its interaction(s) upon drug exposure. As an example, EEFG1 (Eukaryotic Translation Elongation Factor 1 Gamma) is a housekeeping gene involved in the translation mechanisms (Kumabe et al, 1992), and its interaction profile did not change significantly in CMK cells upon carboplatin induction (Fig 3A). Meanwhile, EYA3 (EYA Transcriptional Coactivator And Phosphatase 3) plays a particular function as a distinguishing mark between apoptotic and repair responses to genotoxic stress (Cook et al, 2009; Krishnan et al, 2009). It entirely changed the interaction profile under the same conditions (Fig 3B). We named the promoter nodes that changed their connectivity as differentially interacting (DI) genes (see the Materials and Methods section). This approach revealed hundreds of genes with interaction changes upon treatment despite their relatively even steady-state expression levels (Table S5).
Figure 3. Differentially interacting genes in CMK cells.
(A, B) Overlap coefficient measures the similarity between connections of (A) EEF1G and (B) EYA3 in normal versus carboplatin-treated states. Node size reflects its type: gene or enhancer. The color represents the overlap status with DNase hypersensitivity sites: positive (red) or negative (yellow). (C) The enriched human phenotype terms in CMK cells treated with either carboplatin or gemcitabine using DI or non-DI genes. (D) The interaction profile of ETV6 upon treatment; static interactions are shown in blue, whereas gained interactions upon treatment are shown in red.
Table S5 Network statistics. (10.7MB, xlsx)
In CMK cells, there were 156/45 DE genes and 728/696 DI genes upon carboplatin and gemcitabine treatments, respectively. Generally, in CMK cells, only DI genes showed enrichments for hematological cell count traits. Therefore, we assessed the biological relevance of DI genes by comparing their enrichment for human phenotype terms with that of non-DI genes, that is, genes that did not change their interactions upon treatment. Indeed, DI genes in CMK cells were more enriched for relevant phenotypes (Fig 3C). For example, ETV6 (ETS Variant Transcription Factor 6) is a transcriptional repressor implicated in dominantly inherited thrombocytopenia (Hock & Shimamura, 2017). Despite no significant changes in steady-state expression levels, it underwent considerable interactome changes upon both carboplatin and gemcitabine exposure (Fig 3D).
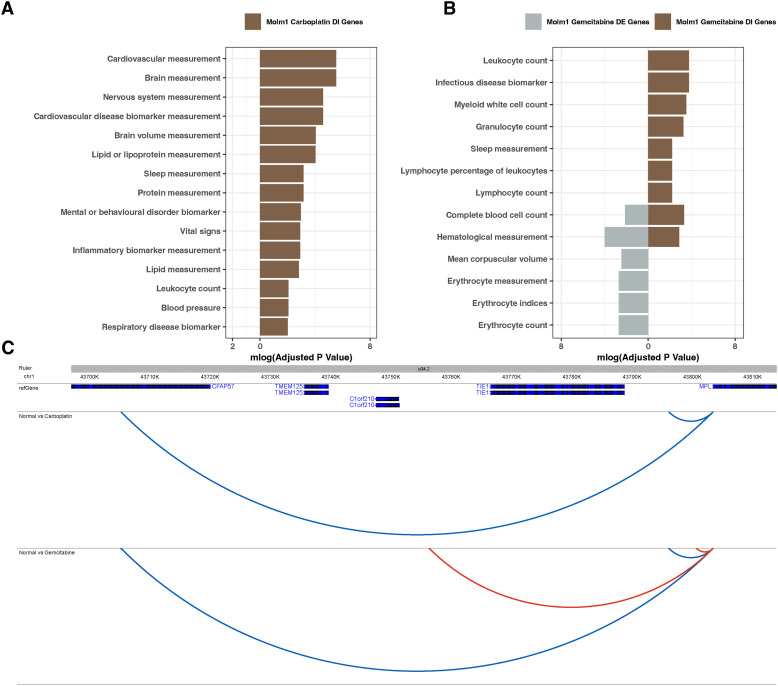
In MOLM-1 cells, we detected 11/252 DE genes and 464/625 DI genes upon exposure to carboplatin/gemcitabine, respectively. They were enriched for relevant traits except for DE genes upon exposure to carboplatin (Fig S8A and B). In particular, MPL (Proto-Oncogene, Thrombopoietin Receptor) is essential for the proliferation of megakaryocytes and platelet differentiation (Ng et al, 2014). It was not differentially expressed, but it changed its interaction landscape significantly upon exposure to both drugs (Fig S8C).
Figure S8. Comparison of DE and DI genes in MOLM-1 cells.
(A, B) Gene enrichments of DE (grey) and DI (brown) genes of MOLM-1 cells upon (A) carboplatin and (B) gemcitabine treatment. (C) The interaction profile of MPL upon carboplatin and gemcitabine treatment; static interactions are shown in blue, whereas gained interactions upon treatment are shown in red.
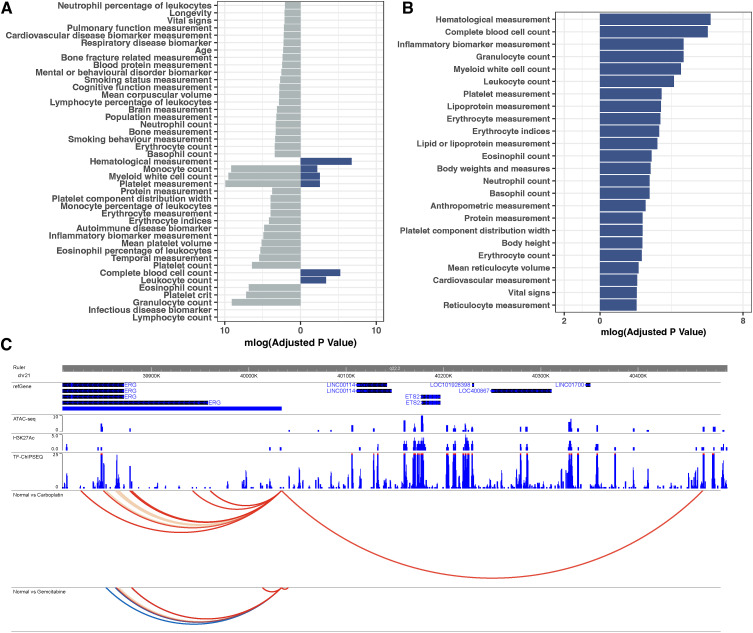
In K-562 cells, there were 555/44 DE genes and 921/932 DI genes upon treatment with carboplatin/gemcitabine, respectively. DE genes, upon carboplatin treatment, were enriched for multiple processes related to cell cycle regulation and hematological processes, whereas DI genes showed limited enrichments (Fig S9A). Conversely, very few genes were differentially expressed upon gemcitabine treatment, but DI genes were enriched for multiple processes related to lymphocyte and myeloid cell processes (Fig S9B). ERG (ETS Transcription Factor ERG), an essential gene for hematopoiesis (Knudsen et al, 2015), changed both its steady-state expression and interaction status in both treatments (Fig S9C). Concordantly, its haploinsufficiency impairs the self-renewal of hematopoietic stem cells under myelotoxic stress (Ng et al, 2011). The summary of DE and DI genes across cell types and treatments is presented in Table 1.
Figure S9. Comparison of DE and DI genes in K-562 cells.
(A, B) Gene enrichments of DE (grey) and DI (blue) genes of K-562 cells upon (A) carboplatin and (B) gemcitabine treatment. (C) The interaction profile of ERG upon carboplatin and gemcitabine treatment; static interactions are shown in blue, whereas gained interactions upon treatment are shown in red, whereas lost interactions are shown in yellow.
Table 1.
Comparison of DE and DI genes across cell lines and treatments.
| DE genes | DI genes | |||
|---|---|---|---|---|
| carboplatin | gemcitabine | carboplatin | gemcitabine | |
| CMK | 156 | 45 | 728 | 696 |
| MOLM-1 | 11 | 252 | 464 | 625 |
| K-562 | 555 | 44 | 921 | 932 |
Color reflects enrichment for relevant GO-terms (green–high, orange–medium, red–low).
Genes connected to candidate enhancer variants tend to differentially interact upon drug exposure
In our previous study, we characterized 8,072,672 single-nucleotide variants (SNVs) from 96 NSCLC patients with differing chemotherapy-induced myelosuppression levels. Only a few protein-coding mutations were associated with the toxicity response of the patients (Björn et al, 2020a). We hypothesized that some SNVs might modulate the activity of enhancers involved in myelosuppression or entirely disrupt them. We overlapped SNVs with 95,567 PIRs derived from HiCap experiments on model cell lines used here to test our hypothesis. Half of the PIRs (52% or 49,802 PIRs) contained at least one variant (50,119 SNVs), and 94.2% of those contained only a single variant.
To filter SNVs relevant to higher or lower risk of drug toxicity, we grouped variants based on their allele count (AC) and allele frequency (AF) differences between the two patient cohorts: high toxicity (HT) and low toxicity (LT) (Fig S10). Accordingly, there were 6,583 variants whose AC and AF differed at least by 25% (see the Materials and Methods section). We also required these variants to have a strong effect size (ES) on TF binding affinity. Using the motifbreakR package, we calculated the ES of the selected variants across a collection of PWMs. Moreover, we filtered only cases where both the interacting gene and TF were expressed (mean [transcripts per million (TPM) across the cell line under all treatments] > 0.2). That prioritized the top 2,720 variants further considered as candidate variants.
Figure S10. Clustering of patients based on toxicity response.
54 and 42 individuals are classified based on their blood cell counts as those with high- and low-toxicity response (HT versus LT, respectively).
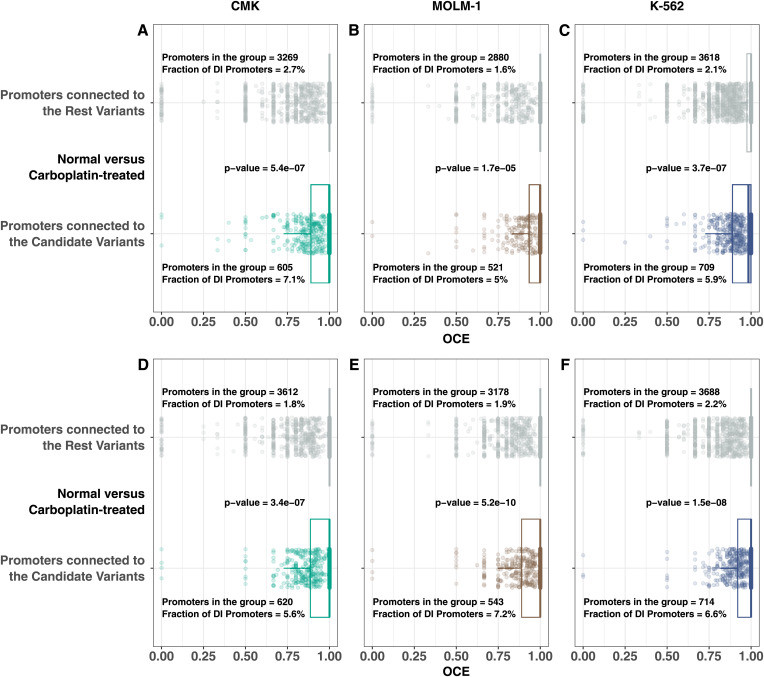
Afterward, we asked if promoters connected to PIRs carrying these candidate variants are likelier to change their interaction profile upon drug treatment than those connected to PIRs carrying other (rest) variants. Indeed, promoters interacting with PIRs carrying candidate variants were more likely to change their interactions upon exposure to carboplatin (Fig 4A–C) and gemcitabine (Fig 4D–F). The mean fraction of DI genes carrying variants in PIRs varied from 5.0–7.2% for candidate variants to 1.6–2.7% for the rest cases. The highest P-value between the two groups was 1.7 × 10−5 in MOLM-1 cells in normal versus carboplatin-treated cases (Fig 4B), proving that interactions bearing candidate variants are significantly enriched for DI genes. As a result, we report 196 candidate DI genes connected to the candidate variants, which may be involved in the genetic mechanisms of chemotherapy-induced myelosuppression (Table S6).
Figure 4. Overlap coefficient differences between genes connected to the candidate and rest enhancer mutations.
(A, B, C, D, E, F) Promoters connected to PIRs containing candidate variants are more likely to change their interaction profile upon carboplatin (A, B, C) and gemcitabine (D, E, F) induction than promoters connected to rest variants. Each dot represents a promoter connected to the PIR carrying the patient SNV. Candidate variants were filtered based on several steps described in the main text. The rest corresponds to filtered-out cases. Fisher’s exact test was used to calculate P-values.
Source data are available for this figure.
Table S6 Candidate genes. (18MB, xlsx)
We further investigated the relevance of the candidate gene set for the myelosuppression. We isolated genes associated (through mutations overlapping genes only) with the counts of lymphocytes, erythrocytes, platelets, and neutrophils from all associations NHGRI-EBI GWAS Catalogue v.1.0.2 (Sollis et al, 2023). Candidate genes showed a significant (P-value = 1.4 × 10−3) enrichment for blood cells count traits.
Finally, we investigated which TFs are disrupted by candidate variants in the enhancers of candidate genes. They showed significant enrichment for abnormal blood cell morphology/development (Bonferroni-adjusted P-value = 6.881 × 10−29) and other related mouse phenotype terms (Table S6). Moreover, they broadly overlapped with TFs previously associated with NSCLC and different stages of chemotherapy-induced response (Vishnoi et al, 2020; Wang et al, 2021; Otálora-Otálora et al, 2023).
Discussion
The establishment of Capture-C enabled the functional annotation of noncoding GWAS variants (Zhang & Lupski, 2015). With the development of different variations of capture Hi-C technology, most studies are still concentrated on the limited parts of the genome associated with the phenotypes of interest (Orozco et al, 2022). In this article, we performed a non-hypothesis-driven study linking SNVs associated with differing levels of chemotherapy-induced myelosuppression with their target genes at the whole-genome level. We take advantage of the HiCap technology (Sahlén et al, 2015) to prioritize candidate SNVs. Based on a 4-cutter DpnII enzyme, HiCap results in a close to single-enhancer resolution (860 bp). This precision is crucial for in vivo validations based on CRISPR genomic and epigenetic editing technologies, where correct gRNA targeting plays an important role. During our validations of detected enhancers in cells, only gRNAs targeting their centers affected the expression of targeted genes. Moreover, methods such as HiCap can identify causal variants, including those in linkage disequilibrium (Åkerborg et al, 2019); therefore, it can identify variants contributing to cellular phenotypes (Zaugg et al, 2022).
The main novelty of our approach is considering the interactome dynamics of normal versus, in this case, drug-treated cells. Whereas bulk RNA-seq profiles could not separate samples because of the stochastic RNA response upon treatments (Aissa et al, 2021), HiCap overcame this phenomenon and robustly separated samples. Our experimental design also facilitates a more comprehensive functional annotation of SNPs related to myelosuppression predisposition, whereas allowing the general description of DI genes. Importantly, in this study, we overlap SNVs of patients with relevant enhancers, however, derived from model blood stem-like cells, limiting the discovery of private enhancers for a patient.
Taking advantage of the experimental setup, we propose a new network algorithm to analyze promoter capture Hi-C data, prioritizing DI genes based on the interactome changes between two states. We show that DI genes consistently provide more information about myelosuppression predisposition genetics than DE genes. Numerous relevant genes, such as ETV6 and MPL, appeared to be DI, but not DE at bulk RNA-seq level. Nevertheless, in some cases, DI genes supported DE genes, for example, in the described case of ERG. Our results prove that genomic loop-based assays could rescue enhancer-mediated effects on gene expression, which can be lost during transcript abundance calculations in bulk RNA-sequencing experiments because of averaging out the expression levels over cells.
Finally, we highlight the importance of DI genes by showing their enrichment for myelosuppression level-associated candidate SNPs, suggesting enhancer mutations may modulate or entirely disrupt promoter–enhancer interactions. We present a catalog of candidate genes, connected to them candidate SNVs, and TFs disrupted by these mutations, emphasizing the robustness of our approach to study promoter–enhancer dysregulation at different levels. We expect these results to make the next step toward establishing personalized medicine.
Materials and Methods
Carboplatin/gemcitabine treatments and cell lines
Three human cell line models were used, including two with megakaryocyte-like properties: CMK (ACC-392) (Komatsu et al, 1989; Sato et al, 1989) and MOLM-1 (ACC-720) (Matsuo et al, 1991; Ogawa et al, 1996; Drexler et al, 1999) from the Leibniz-Institute DSMZ–German Collection of Microorganisms and Cell Cultures, and one with general myelogenous properties: K-562 (CCL-243) (Lozzio & Lozzio, 1975, 1979; Lozzio et al, 1981), from the American Type Culture Collection. Cells were cultured and exposed to carboplatin or gemcitabine, as previously explained (Björn et al, 2020a). In short, duplicates of 10 million cells in 15 ml RPMI 1640 supplemented with 10% FBS were treated for 24 h with the 72-h IC50 concentration of carboplatin, gemcitabine (both from Toronto Research Chemicals) or no drug (as a non-treated control). The drug concentrations used for K-562, CMK, and MOLM-1 were 14, 25, and 35 ng/ml for gemcitabine, and 30, 1.6, and 14 μg/ml for carboplatin, respectively. Only a minor reduction in cell viability was seen using the MTT assay at 24 h of incubation (Björn et al, 2020a). Subsequent laboratory procedures for RNAseq and HiCap (using the same cells) were immediately initiated to be as good of a snapshot of the cellular processes induced by the treatments as possible.
RNA-sequencing, alignment, and read summarization
RNA-seq data from our previous study (Björn et al, 2020a), generated from 1 ml of cell suspension from each of the treatments, were complemented with another round of sequencing of the previously prepared libraries using the same sequencing approach: HiSeq 2500 (Illumina) with HiSeq Rapid SBS Kit v2 chemistry and a 1 × 80 setup at Science for Life Laboratory (SciLifeLab), to increase the number of reads. Data were then merged. Briefly, TrimGalore! version 0.6.1 (Krueger, 2021) utilizing cutadapt version 2.3 (Martin, 2011) was used for quality and adapter trimming, STAR version 2.7.2b (Dobin et al, 2013) was used for alignment to GRCh37, RSEM package (Li & Dewey, 2011) version 2 was used to summarize the number of unique mapping reads per gene region (Table S1). The data quality was monitored using FastQC version 0.11.9 (Andrews, 2010), QualiMap version 2.2.1 (García-Alcalde et al, 2012), and MultiQC version 1.9 (Ewels et al, 2016).
Gene expression and enrichment analysis
The summarized read counts were further analyzed in R version 4.0.3 (R Core Team, 2021). Reads per kilobase million, counts per million, and TPM were calculated. Analysis of differentially expressed (DE) genes was conducted using edgeR version 3.18.1 (Robinson et al, 2010; McCarthy et al, 2012) and the TMM normalization method (Robinson et al, 2010). DE genes were extracted when comparing non-treated and carboplatin or gemcitabine-treated cells for the three cell lines. KEGG pathway and Gene Ontology (GO) enrichment analyses of various sets of genes were performed using the ToppGene suite (Chen et al, 2009) and the R package clusterProfiler version 3.12.0 (Yu et al, 2012), and StringDB (Szklarczyk et al, 2021), was used for analysis.
SNP selection
In our previous study, we analyzed 215 exomes of NSCLC patients (Björn et al, 2020b). All of them received at least one cycle of carboplatin/gemcitabine treatment, which was the standard of care for NSCLC patients at the time and place of the study. Of these, 96 patients were selected based on extreme toxicity, either low or high neutropenia, leukopenia, and thrombocytopenia. We classified patients into two groups (low and high toxicities, LT and HT, respectively) via unsupervised k-means clustering using blood cell count values of the three above phenotypes as input (Fig S10). The whole genomes of these 96 individuals were sequenced and analyzed (Björn et al, 2020a).
In this study, we overlapped SNVs detected in the 96 individuals (a total of 8,072,672 SNVs) with enhancer marks for blood cell lines collected from ENCODE (The ENCODE Project Consortium, 2012) and Fantom5 (Lizio et al, 2015) datasets available in 2017. In total, 3.27 million SNVs overlapped with at least one enhancer mark (Table S7).
Table S7 SNPs overlap with enhancer marks. (9.6KB, xlsx)
HiCap probe design
HiCapTools (Anil et al, 2018) was used to design probes against 22,171 promoters and 2,965 selected SNVs using default settings (Table S8). We selected 2,965 SNVs (the maximum number of variants we could target given the probe set size), with the largest frequency difference between patient groups and overlap with the multitude of enhancer marks to be included in the sequence probe capture set used for HiCap experiments. The probe set included 2,900 regions with no known promoter/enhancer activity. These regions were then used to generate the background interaction distribution and assign statistical significance for each observed proximity.
Table S8 Probes. (4.5MB, xlsx)
HiCap library preparation
The remaining 14 ml of cell suspension from each cell sample was used for HiCap, as previously explained (Åkerborg et al, 2019; Zhigulev et al, 2022b). This method is developed by us (Sahlén et al, 2015) and others (Mifsud et al, 2015) and provides high-resolution interaction data over genomic regions by hybridizing Hi-C material to probes targeting certain regions of interest, enabling the study of individual promoter–enhancer interactions. Briefly, the method starts with cross-linking DNA–protein–DNA complexes with formaldehyde, followed by roughly cutting DNA across the genome into ∼700-bp pieces using restriction endonucleases. Spatially close fragments are then ligated before capturing promoter–enhancer sequences using probes located in known genes’ promoters or probes containing selected SNPs associated with ADRs. Captured libraries were sequenced using HiSeq 2500 (Illumina) with HiSeq Rapid SBS Kit v2 chemistry and a 1 × 80 setup at Science for Life Laboratory (SciLifeLab). Table S9 shows the sequencing statistics for HiCap libraries. Lastly, sequencing data are analyzed for significant interactions.
Table S9 HiCap statistics. (23KB, xlsx)
Chromatin interaction calling
HiCapTools (Anil et al, 2018) was used to call interactions in all samples (Table S2). We required at least four pairs supporting each interaction. We then used three Bonferroni-adjusted P-value cutoffs (0.1, 0.01, 0.001) to filter interactions. We evaluated adjusted P-value cutoffs using the fold enrichment of distal regions for the publicly available enhancer datasets (Table S3), calculated by the BEDTools package version 2.30.0 (Quinlan & Hall, 2010). We used the adjusted P-value cutoff of 0.01 for all analyses throughout the article, except the network generation, where the threshold was 0.001. HiCap results were mapped to GRCh37.
CRISPR validation
Several PIRs were validated using the enCRISPRi-LK system (Li et al, 2020). Briefly, gRNAs (Table S10) targeting different parts of PIRs were cloned into the Lenti_sgRNA(MS2)_MCP-KRAB-IRES-zsGreen1 (#138460) backbone plasmid with BsmBI-v2 (NEB) Golden Gate assembly, using oligos ordered from Integrated DNA Technologies. Lentiviral particles containing either tet-inducible dCas9-LSD1 (#92362), inducer TetON3G-BFP (assembled from #128061 and #120577 plasmids using BamHI-HF and MluI-HF [both from NEB]), or targeting gRNA-KRAB plasmid were separately obtained by transfection of 293T cells using Lipofectamine 3000 Transfection Reagent (Thermo Fisher Scientific) and second generation lentiviral plasmids psPAX2 (#12260) and pMD2.G (#12259) according to the manufacturer’s instructions. A stable K-562-dCas9-LSD1-TetON3G-BFP cell line was obtained by transducing K-562 cells with the lentiviral particles for 6 h before changing the medium. Finally, gRNA-KRAB constructs were transduced to the previously obtained stable cell line. dCas9 expression was induced with 1 μg/ml of doxycycline 72 h before FACS at the Biomedicum Flow cytometry Core facility (Karolinska Institutet). After sorting for BFP, zsGreen, and mCherry expressing cells, RNA was extracted with the RNeasy Plus Mini kit. RNA integrity and yield were assessed by Bioanalyzer 2100 (RNA 6000 Pico Kit; Agilent). Gene expression changes were evaluated by triplicate RT-qPCR using DreamTaq Hot Start DNA Polymerase (Thermo Fisher Scientific) and an EvaGreen dye (Biotium).
Table S10 Primers. (10.1KB, xlsx)
Interactome network generation and calculation of network parameters
To generate interactome networks, we considered each interacting promoter or enhancer as a node in the network. We then connected two nodes if they had a significant interaction in at least one replicate. We used the igraph package version 1.4.2 (Csardi & Nepusz, 2006) distributed under R to generate the networks. We then created a summary of interactions for each node or gene by summing its interactions. We generated nine networks in total (Table S5).
To calculate the Jaccard index (JI) and overlap coefficient (OCE) of a node between the two networks (A, B), we used the following formulas:
where A and B denote the number of neighbors of node x in networks A and B, respectively.
The network is generated using non-replicated interactions (i.e., those that are called interactions in one of the replicates); therefore, JI and OCE parameters reflect the amount of replicated novel (gained) or lost interactions. Note that a node with a JI value less than one can have an OCE value equal to one. This is because OCE requires that the number of common nodes is more than the number of nodes in the smaller network. For example, a node with two connections in the untreated condition could lose one of its connections upon treatment and not gain any additional connection. The JI would be 0.5, but OCE would be 1 because the size of the network with minimum number of nodes is equal to the number of common nodes. Therefore, OCE is a stricter measure of connectivity change than JI.
We then defined differentially interacting (DI) genes as those OCE < 1 and JI <= 0.5. We excluded cases with JI = 0 to eliminate genes gaining or losing only one interaction. We only took genes with TPM > 0.2.
Allele count difference calculations
We calculated the allele count (AC) and frequencies (AF) of each alternative allele for both HT and LT groups of patients. We then took the absolute difference between LT and HT groups for AC and AF:
For candidate variants, we required them to be present in at least two individuals at a 25% AC and AF difference between the two groups. In short, that is represented by the following formula:
where allele counts difference threshold, ACEthr = 0.25.
The variants not fulfilling the following criteria are deemed as “rest”.
TF affinity predictions
To assess the potential effect of SNVs on the TF-binding affinity, we used the motifbreakR package (Coetzee et al, 2015). It directly weights the score by the importance of the position within a particular motif in a tested collection. We used a motifbreakR_motif object derived from MotifDb package version 1.40.0 (Shannon & Richards, 2023)—the collection of 2,817 position frequency matrices from four sources: ENCODE-motif, FactorBook, HOCOMOCO, and HOMER.
Data Availability
Whole-genome sequencing datasets analyzed during the current study are not publicly available because of reasons of sensitivity but are available from the corresponding author upon reasonable request. Raw sequence reads of RNA-seq and HiCap are submitted to NCBI Sequence Read Archive (PRJNA1012445).
Ethics statement
The 96 whole-genome sequences of NSCLC patients were diagnosed between 2006 and 2008 at Karolinska University Hospital, Stockholm, Sweden, and included after providing written informed consent in accordance with the Helsinki Declaration. The study received ethical approval from the Regional Ethics Committee in Stockholm (DNR-03-413 with amendment 2016/258-32/1). These patients are part of the material included in previously published studies (Björn et al, 2020a, 2020b; Svedberg et al, 2020).
Supplementary Material
Acknowledgements
We are grateful to Joakim Lundeberg (Royal Institute of Technology—KTH, Stockholm, Sweden) for fruitful discussions. We are also thankful to Bernhard Schmierer (SciLifeLab CRISPR Functional Genomics Unit, Solna, Sweden) for providing the 293T cell line and Ivan Kulakovskiy (Institute of Protein Research, Russian Academy of Sciences, Pushchino, Russia) for his suggestions on analyzing TF-binding affinity. The authors acknowledge support from the National Genomics Infrastructure in Stockholm funded by Science for Life Laboratory, the Knut and Alice Wallenberg Foundation, and the Swedish Research Council, and SNIC/Uppsala Multidisciplinary Center for Advanced Computational Science for assistance with massively parallel sequencing and access to the UPPMAX computational infrastructure. They also acknowledge the Biomedicum Flow Cytometry core facility (Karolinska Institutet), supported by KI/SLL, for providing cell sorting services and the KIGene core facility (Karolinska Institutet) for providing sequencing services. This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie Grant Agreement No 860002. The study was also funded by the Swedish Cancer Society, The Swedish Childhood Cancer Fond (barncancerfonden), and ALF Grants, Region Östergötland, and by the Swedish Research Council (Grant Agreement No. 78081). The information contained in this presentation reflects only the authors’ views. REA and EC are not responsible for any use that may be made of this information. This article is dedicated to the memory of EM, who passed away while the aricle was in preparation.
Author Contributions
A Zhigulev: data curation, software, formal analysis, visualization, project administration, and writing—original draft, review, and editing.
Z Norberg: validation and writing—review and editing.
J Cordier: validation and writing—review and editing.
R Spalinskas: investigation and writing—review and editing.
H Bassereh: software, formal analysis, and writing—review and editing.
N Björn: formal analysis, investigation, and writing—review and editing.
S Pradhananga: data curation and writing—review and editing.
H Gréen: conceptualization, resources, supervision, funding acquisition, methodology, and writing—review and editing.
P Sahlén: conceptualization, resources, data curation, supervision, funding acquisition, visualization, methodology, project administration, and writing—original draft, review, and editing.
Conflict of Interest Statement
P Sahlén holds a HiCap patent (EP2984182A1).
References
- Aissa AF, Islam ABMMK, Ariss MM, Go CC, Rader AE, Conrardy RD, Gajda AM, Rubio-Perez C, Valyi-Nagy K, Pasquinelli M, et al. (2021) Single-cell transcriptional changes associated with drug tolerance and response to combination therapies in cancer. Nat Commun 12: 1628. 10.1038/s41467-021-21884-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerborg Ö, Spalinskas R, Pradhananga S, Anil A, Höjer P, Poujade FA, Folkersen L, Eriksson P, Sahlén P (2019) High-resolution regulatory maps connect vascular risk variants to disease-related pathways. Circ Genom Precis Med 12: e002353. 10.1161/CIRCGEN.118.002353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjad MT, Chidharla A, Kasi A (2023) Cancer Chemotherapy. In StatPearls. St. Petersburg, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Andrews S (2010) FastQC - a quality control tool for high throughput sequence data. Babraham Bioinformatics http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Google Scholar]
- Anil A, Spalinskas R, Åkerborg Ö, Sahlén P (2018) HiCapTools: A software suite for probe design and proximity detection for targeted chromosome conformation capture applications. Bioinformatics 34: 675–677. 10.1093/bioinformatics/btx625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggstrom MQ, Stinchcombe TE, Fried DB, Poole C, Hensing TA, Socinski MA (2007) Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: A meta-analysis. J Thorac Oncol 2: 845–853. 10.1097/JTO.0b013e31814617a2 [DOI] [PubMed] [Google Scholar]
- Banerji J, Rusconi S, Schaffner W (1981) Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell 27: 299–308. 10.1016/0092-8674(81)90413-X [DOI] [PubMed] [Google Scholar]
- Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun Z-W, Hiebert SW (2008) Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell 30: 61–72. 10.1016/j.molcel.2008.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björn N, Badam TVS, Spalinskas R, Brandén E, Koyi H, Lewensohn R, De Petris L, Lubovac-Pilav Z, Sahlén P, Lundeberg J, et al. (2020. a) Whole-genome sequencing and gene network modules predict gemcitabine/carboplatin-induced myelosuppression in non-small cell lung cancer patients. NPJ Syst Biol Appl 6: 25. 10.1038/s41540-020-00146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björn N, Sigurgeirsson B, Svedberg A, Pradhananga S, Brandén E, Koyi H, Lewensohn R, de Petris L, Apellániz-Ruiz M, Rodríguez-Antona C, et al. (2020. b) Genes and variants in hematopoiesis-related pathways are associated with gemcitabine/carboplatin-induced thrombocytopenia. Pharmacogenomics J 20: 179–191. 10.1038/s41397-019-0099-8 [DOI] [PubMed] [Google Scholar]
- Blackwood EM, Kadonaga JT (1998) Going the distance: A current view of enhancer action. Science 281: 60–63. 10.1126/science.281.5373.60 [DOI] [PubMed] [Google Scholar]
- Blank U, Karlsson S (2015) TGF-β signaling in the control of hematopoietic stem cells. Blood 125: 3542–3550. 10.1182/blood-2014-12-618090 [DOI] [PubMed] [Google Scholar]
- Bracken AP, Brien GL, Verrijzer CP (2019) Dangerous liaisons: Interplay between SWI/SNF, NuRD, and polycomb in chromatin regulation and cancer. Genes Dev 33: 936–959. 10.1101/gad.326066.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey PJ (2003) Drug-induced myelosuppression: Diagnosis and management. Drug Saf 26: 691–706. 10.2165/00002018-200326100-00003 [DOI] [PubMed] [Google Scholar]
- Chatelut E, Delord J-P, Canal P (2003) Toxicity patterns of cytotoxic drugs. Invest New Drugs 21: 141–148. 10.1023/a:1023565227808 [DOI] [PubMed] [Google Scholar]
- Chen J, Bardes EE, Aronow BJ, Jegga AG (2009) ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37: W305–W311. 10.1093/nar/gkp427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claringbould A, Zaugg JB (2021) Enhancers in disease: Molecular basis and emerging treatment strategies. Trends Mol Med 27: 1060–1073. 10.1016/j.molmed.2021.07.012 [DOI] [PubMed] [Google Scholar]
- Coetzee SG, Coetzee GA, Hazelett DJ (2015) motifbreakR: an R/Bioconductor package for predicting variant effects at transcription factor binding sites. Bioinformatics 31: 3847–3849. 10.1093/bioinformatics/btv470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG (2009) Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458: 591–596. 10.1038/nature07849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csardi G, Nepusz T (2006) The igraph software package for complex network research. InterJournal Complex Systems 1695: 1–9. [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostie J, Richmond TA, Arnaout RA, Selzer RR, Lee WL, Honan TA, Rubio ED, Krumm A, Lamb J, Nusbaum C, et al. (2006) Chromosome conformation capture carbon copy (5C): A massively parallel solution for mapping interactions between genomic elements. Genome Res 16: 1299–1309. 10.1101/gr.5571506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler HG, MacLeod RAF, Uphoff CC (1999) Leukemia cell lines: In vitro models for the study of Philadelphia chromosome-positive leukemia. Leuk Res 23: 207–215. 10.1016/S0145-2126(98)00171-4 [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74. 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels P, Magnusson M, Lundin S, Käller M (2016) MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32: 3047–3048. 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F (2021) Cancer statistics for the year 2020: An overview. Int J Cancer 149: 778–789. 10.1002/ijc.33588 [DOI] [PubMed] [Google Scholar]
- Forcato M, Nicoletti C, Pal K, Livi CM, Ferrari F, Bicciato S (2017) Comparison of computational methods for Hi-C data analysis. Nat Methods 14: 679–685. 10.1038/nmeth.4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E (2021) Update of incidence, prevalence, survival, and initial treatment in patients with non–small cell lung cancer in the US. JAMA Oncol 7: 1824–1832. 10.1001/jamaoncol.2021.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alcalde F, Okonechnikov K, Carbonell J, Cruz LM, Götz S, Tarazona S, Dopazo J, Meyer TF, Conesa A (2012) Qualimap: Evaluating next-generation sequencing alignment data. Bioinformatics 28: 2678–2679. 10.1093/bioinformatics/bts503 [DOI] [PubMed] [Google Scholar]
- Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, Petrella F, Spaggiari L, Rosell R (2015) Non-small-cell lung cancer. Nat Rev Dis Primers 1: 15009. 10.1038/nrdp.2015.9 [DOI] [PubMed] [Google Scholar]
- Grønberg BH, Bremnes RM, Fløtten O, Amundsen T, Brunsvig PF, Hjelde HH, Kaasa S, von Plessen C, Stornes F, Tollåli T, et al. (2009) Phase III study by the Norwegian lung cancer study group: Pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 27: 3217–3224. 10.1200/JCO.2008.20.9114 [DOI] [PubMed] [Google Scholar]
- Hansen AS, Pustova I, Cattoglio C, Tjian R, Darzacq X (2017) CTCF and cohesin regulate chromatin loop stability with distinct dynamics. Elife 6: e25776. 10.7554/eLife.25776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Waizenegger IC, Peters JM (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293: 1320–1323. 10.1126/science.1061376 [DOI] [PubMed] [Google Scholar]
- Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, et al. (2009) Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459: 108–112. 10.1038/nature07829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H, Shimamura A (2017) ETV6 in hematopoiesis and leukemia predisposition. Semin Hematol 54: 98–104. 10.1053/j.seminhematol.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F, Nishio M, Noro R, Tsuboi M, Ikeda N, Inoue A, Ohsaki Y, Kimura Y, Nishino K, Uchida J, et al. (2011) Randomized phase II study of two schedules of carboplatin and gemcitabine for stage IIIB and IV advanced non-small cell lung cancer (JACCRO LC-01 study). Chemotherapy 57: 357–362. 10.1159/000330481 [DOI] [PubMed] [Google Scholar]
- Jepsen K, Hermanson O, Onami TM, Gleiberman AS, Lunyak V, McEvilly RJ, Kurokawa R, Kumar V, Liu F, Seto E, et al. (2000) Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102: 753–763. 10.1016/s0092-8674(00)00064-7 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Qian F, Bai X, Liu Y, Wang Q, Ai B, Han X, Shi S, Zhang J, Li X, et al. (2019) SEdb: A comprehensive human super-enhancer database. Nucleic Acids Res 47: D235–D243. 10.1093/nar/gky1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan DA, van Heyningen V (2005) Long-range control of gene expression: Emerging mechanisms and disruption in disease. Am J Hum Genet 76: 8–32. 10.1086/426833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KJ, Rehn M, Hasemann MS, Rapin N, Bagger FO, Ohlsson E, Willer A, Frank A-K, Søndergaard E, Jendholm J, et al. (2015) ERG promotes the maintenance of hematopoietic stem cells by restricting their differentiation. Genes Dev 29: 1915–1929. 10.1101/gad.268409.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Suda T, Moroi M, Tokuyama N, Sakata Y, Okada M, Nishida T, Hirai Y, Sato T, Fuse A (1989) Growth and differentiation of a human megakaryoblastic cell line, CMK. Blood 74: 42–48. 10.1182/blood.v74.1.42.bloodjournal74142 [DOI] [PubMed] [Google Scholar]
- Krishnan N, Jeong DG, Jung S-K, Ryu SE, Xiao A, Allis CD, Kim SJ, Tonks NK (2009) Dephosphorylation of the C-terminal tyrosyl residue of the DNA damage-related histone H2A.X is mediated by the protein Phosphatase eyes absent. J Biol Chem 284: 16066–16070. 10.1074/jbc.C900032200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F (2021) TrimGalore - A Wrapper Tool Around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files, with Some Extra Functionality for MspI-Digested RRBS-type (Reduced Representation Bisufite-Seq) Libraries. https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ [Google Scholar]
- Kumabe T, Sohma Y, Yamamoto T (1992) Human cDNAs encoding elongation factor 1 gamma and the ribosomal protein L19. Nucleic Acids Res 20: 2598. 10.1093/nar/20.10.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonte MJ, Wilson PM, Fazzone W, Groshen S, Lenz H-J, Ladner RD (2009) DNA microarray profiling of genes differentially regulated by the histone deacetylase inhibitors vorinostat and LBH589 in colon cancer cell lines. BMC Med Genomics 2: 67. 10.1186/1755-8794-2-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson AJM, Johnsson P, Hagemann-Jensen M, Hartmanis L, Faridani OR, Reinius B, Segerstolpe Å, Rivera CM, Ren B, Sandberg R (2019) Genomic encoding of transcriptional burst kinetics. Nature 565: 251–254. 10.1038/s41586-018-0836-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12: 323. 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Liu Y, Cao H, Zhang Y, Gu Z, Liu X, Yu A, Kaphle P, Dickerson KE, Ni M, et al. (2020) Interrogation of enhancer function by enhancer-targeting CRISPR epigenetic editing. Nat Commun 11: 485. 10.1038/s41467-020-14362-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Lin JCY, Wei V, Yoo C, Cheng JC, Nguyen CT, Weisenberger DJ, Egger G, Takai D, Gonzales FA, et al. (2004) Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci U S A 101: 7357–7362. 10.1073/pnas.0401866101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, et al. (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326: 289–293. 10.1126/science.1181369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizio M, Harshbarger J, Shimoji H, Severin J, Kasukawa T, Sahin S, Abugessaisa I, Fukuda S, Hori F, Ishikawa-Kato S, et al. (2015) Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol 16: 22. 10.1186/s13059-014-0560-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio CB, Lozzio BB (1975) Human chronic myelogenous leukemia cell line with positive Philadelphia chromosome. Blood 45: 321–334. 10.1182/blood.v45.3.321.321 [DOI] [PubMed] [Google Scholar]
- Lozzio BB, Lozzio CB (1979) Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leuk Res 3: 363–370. 10.1016/0145-2126(79)90033-X [DOI] [PubMed] [Google Scholar]
- Lozzio BB, Lozzio CB, Bamberger EG, Feliu AS (1981) A multipotential leukemia cell line (K-562) of human origin. Proc Soc Exp Biol Med 166: 546–550. 10.3181/00379727-166-41106 [DOI] [PubMed] [Google Scholar]
- Ma W, Ay F, Lee C, Gulsoy G, Deng X, Cook S, Hesson J, Cavanaugh C, Ware CB, Krumm A, et al. (2015) Fine-scale chromatin interaction maps reveal the cis-regulatory landscape of human lincRNA genes. Nat Methods 12: 71–78. 10.1038/nmeth.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17: 10. 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- Matsuo Y, Adachi T, Tsubota T, Imanishi J, Minowada J (1991) Establishment and characterization of a novel megakaryoblastic cell line, MOLM-1, from a patient with chronic myelogenous leukemia. Hum Cell 4: 261–264. [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297. 10.1093/nar/gks042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson RJ, Collard MW, Ziemba AJ, Persinger J, Bartholomew B, Huggenvik JI (1999) Nuclear DEAF-1-related (NUDR) protein contains a novel DNA binding domain and represses transcription of the heterogeneous nuclear ribonucleoprotein A2/B1 promoter. J Biol Chem 274: 30510–30519. 10.1074/jbc.274.43.30510 [DOI] [PubMed] [Google Scholar]
- Mifsud B, Tavares-Cadete F, Young AN, Sugar R, Schoenfelder S, Ferreira L, Wingett SW, Andrews S, Grey W, Ewels PA, et al. (2015) Mapping long-range promoter contacts in human cells with high-resolution capture Hi-C. Nat Genet 47: 598–606. 10.1038/ng.3286 [DOI] [PubMed] [Google Scholar]
- Mulford AJ, Wing C, Dolan ME, Wheeler HE (2021) Genetically regulated expression underlies cellular sensitivity to chemotherapy in diverse populations. Hum Mol Genet 30: 305–317. 10.1093/hmg/ddab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AP, Loughran SJ, Metcalf D, Hyland CD, de Graaf CA, Hu Y, Smyth GK, Hilton DJ, Kile BT, Alexander WS (2011) Erg is required for self-renewal of hematopoietic stem cells during stress hematopoiesis in mice. Blood 118: 2454–2461. 10.1182/blood-2011-03-344739 [DOI] [PubMed] [Google Scholar]
- Ng AP, Kauppi M, Metcalf D, Hyland CD, Josefsson EC, Lebois M, Zhang J-G, Baldwin TM, di Rago L, Hilton DJ, et al. (2014) Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc Natl Acad Sci U S A 111: 5884–5889. 10.1073/pnas.1404354111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Kurokawa M, Tanaka T, Mitani K, Inazawa J, Hangaishi A, Tanaka K, Matsuo Y, Minowada J, Tsubota T, et al. (1996) Structurally altered Evi-1 protein generated in the 3q21q26 syndrome. Oncogene 13: 183–191. [PubMed] [Google Scholar]
- Orozco G, Schoenfelder S, Walker N, Eyre S, Fraser P (2022) 3D genome organization links non-coding disease-associated variants to genes. Front Cell Dev Biol 10: 995388. 10.3389/fcell.2022.995388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otálora-Otálora BA, López-Kleine L, Rojas A (2023) Lung cancer gene regulatory network of transcription factors related to the hallmarks of cancer. Curr Issues Mol Biol 45: 434–464. 10.3390/cimb45010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor ML, Laffont CM, Gladieff L, Schmitt A, Chatelut E, Concordet D (2013) Model-based approach to describe G-CSF effects in carboplatin-treated cancer patients. Pharm Res 30: 2795–2807. 10.1007/s11095-013-1099-z [DOI] [PubMed] [Google Scholar]
- Pittman KB, Olver IN, Koczwara B, Kotasek D, Patterson WK, Keefe DM, Karapetis CS, Parnis FX, Moldovan S, Yeend SJ, et al. (2006) Gemcitabine and carboplatin in carcinoma of unknown primary site: A phase 2 adelaide cancer trials and education collaborative study. Br J Cancer 95: 1309–1313. 10.1038/sj.bjc.6603440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhananga S, Spalinskas R, Poujade F-A, Eriksson P, Sahlén P (2020) Promoter anchored interaction landscape of THP-1 macrophages captures early immune response processes. Cell Immunol 355: 104148. 10.1016/j.cellimm.2020.104148 [DOI] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM (2010) BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2021) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org/.
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J (2011) A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470: 279–283. 10.1038/nature09692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Oshlack A (2010) A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11: R25. 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RM, Gower NH, Spiro SG, Eisen TG, Harper PG, Littler JAH, Hatton M, Johnson PWM, Martin WMC, Rankin EM, et al. (2005) Gemcitabine plus carboplatin versus mitomycin, ifosfamide, and cisplatin in patients with stage IIIB or IV non-small-cell lung cancer: A phase III randomized study of the London lung cancer group. J Clin Oncol 23: 142–153. 10.1200/JCO.2005.03.037 [DOI] [PubMed] [Google Scholar]
- Sahlén P, Abdullayev I, Ramsköld D, Matskova L, Rilakovic N, Lötstedt B, Albert TJ, Lundeberg J, Sandberg R (2015) Genome-wide mapping of promoter-anchored interactions with close to single-enhancer resolution. Genome Biol 16: 156. 10.1186/s13059-015-0727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlén P, Spalinskas R, Asad S, Mahapatra KD, Höjer P, Anil A, Eisfeldt J, Srivastava A, Nikamo P, Mukherjee A, et al. (2021) Chromatin interactions in differentiating keratinocytes reveal novel atopic dermatitis- and psoriasis-associated genes. J Allergy Clin Immunol 147: 1742–1752. 10.1016/j.jaci.2020.09.035 [DOI] [PubMed] [Google Scholar]
- Santana-Davila R, Szabo A, Arce-Lara C, Williams CD, Kelley MJ, Whittle J (2014) Cisplatin versus carboplatin-based regimens for the treatment of patients with metastatic lung cancer. An analysis of veterans health administration data. J Thorac Oncol 9: 702–709. 10.1097/JTO.0000000000000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Fuse A, Eguchi M, Hayashi Y, Ryo R, Adachi M, Kishimoto Y, Teramura M, Mizoguchi H, Shima Y, et al. (1989) Establishment of a human leukaemic cell line (CMK) with megakaryocytic characteristics from a Down[R8S2Q1M7]s syndrome patient with acute megakaryoblastic leukaemia. Br J Haematol 72: 184–190. 10.1111/j.1365-2141.1989.tb07681.x [DOI] [PubMed] [Google Scholar]
- Schoenfelder S, Furlan-Magaril M, Mifsud B, Tavares-Cadete F, Sugar R, Javierre B-M, Nagano T, Katsman Y, Sakthidevi M, Wingett SW, et al. (2015) The pluripotent regulatory circuitry connecting promoters to their long-range interacting elements. Genome Res 25: 582–597. 10.1101/gr.185272.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederholm C, Hillerdal G, Lamberg K, Kölbeck K, Dufmats M, Westberg R, Gawande SR (2005) Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non–small-cell lung cancer: The Swedish lung cancer study group. J Clin Oncol 23: 8380–8388. 10.1200/JCO.2005.01.2781 [DOI] [PubMed] [Google Scholar]
- Seto E, Yoshida M (2014) Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6: a018713. 10.1101/cshperspect.a018713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Richards M (2023) MotifDb: An Annotated Collection of Protein-DNA Binding Sequence Motifs, R package version 1.44.0. 10.18129/B9.bioc.MotifDb. Available at: https://bioconductor.org/packages/MotifDb. [DOI] [Google Scholar]
- Skopek R, Palusińska M, Kaczor-Keller K, Pingwara R, Papierniak-Wyglądała A, Schenk T, Lewicki S, Zelent A, Szymański Ł (2023) Choosing the right cell line for acute myeloid leukemia (AML) research. Int J Mol Sci 24: 5377. 10.3390/ijms24065377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, Groza T, Güneş O, Hall P, Hayhurst J, et al. (2023) The NHGRI-EBI GWAS catalog: Knowledgebase and deposition resource. Nucleic Acids Res 51: D977–D985. 10.1093/nar/gkac1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedberg A, Björn N, Sigurgeirsson B, Pradhananga S, Brandén E, Koyi H, Lewensohn R, De Petris L, Apellániz-Ruiz M, Rodríguez-Antona C, et al. (2020) Genetic association of gemcitabine/carboplatin-induced leukopenia and neutropenia in non-small cell lung cancer patients using whole-exome sequencing. Lung Cancer 147: 106–114. 10.1016/j.lungcan.2020.07.005 [DOI] [PubMed] [Google Scholar]
- Swen JJ, van der Wouden CH, Manson LE, Abdullah-Koolmees H, Blagec K, Blagus T, Böhringer S, Cambon-Thomsen A, Cecchin E, Cheung K-C, et al. (2023) A 12-gene pharmacogenetic panel to prevent adverse drug reactions: An open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet 401: 347–356. 10.1016/S0140-6736(22)01841-4 [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, et al. (2021) The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49: D605–D612. 10.1093/nar/gkaa1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testart-Paillet D, Girard P, You B, Freyer G, Pobel C, Tranchand B (2007) Contribution of modelling chemotherapy-induced hematological toxicity for clinical practice. Crit Rev Oncol Hematol 63: 1–11. 10.1016/j.critrevonc.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS (2021) Lung cancer. Lancet 398: 535–554. 10.1016/S0140-6736(21)00312-3 [DOI] [PubMed] [Google Scholar]
- Trendowski MR, el Charif O, Dinh PC, Travis LB, Dolan ME (2019) Genetic and modifiable risk factors contributing to cisplatin-induced toxicities. Clin Cancer Res 25: 1147–1155. 10.1158/1078-0432.CCR-18-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnoi K, Viswakarma N, Rana A, Rana B (2020) Transcription factors in cancer development and therapy. Cancers (Basel) 12: 2296. 10.3390/cancers12082296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K (2009) Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138: 1019–1031. 10.1016/j.cell.2009.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Peng Q, Yin N, Xie Y, Xu J, Chen A, Yi J, Tang J, Xiang J (2021) Chromatin accessibility regulates chemotherapy-induced dormancy and reactivation. Mol Ther Nucleic Acids 26: 269–279. 10.1016/j.omtn.2021.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäsch R, Robbins JA, Cross FR (2010) The emerging role of APC/CCdh1 in controlling differentiation, genomic stability and tumor suppression. Oncogene 29: 1–10. 10.1038/onc.2009.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang L-G, Han Y, He Q-Y (2012) clusterProfiler: an R Package for comparing biological themes among gene clusters. OMICS 16: 284–287. 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukal P, Petruzelka L, Zemanová M, Kolek V, Skricková J, Pesek M, Fojtů H, Grygárková I, Sixtová D, Roubec J, et al. (2003) Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non-small cell lung cancer: A phase III randomized trial. Lung Cancer 41: 321–331. 10.1016/s0169-5002(03)00233-2 [DOI] [PubMed] [Google Scholar]
- Zaugg JB, Sahlén P, Andersson R, Alberich-Jorda M, de Laat W, Deplancke B, Ferrer J, Mandrup S, Natoli G, Plewczynski D, et al. (2022) Current challenges in understanding the role of enhancers in disease. Nat Struct Mol Biol 29: 1148–1158. 10.1038/s41594-022-00896-3 [DOI] [PubMed] [Google Scholar]
- Zhang F, Lupski JR (2015) Non-coding genetic variants in human disease. Hum Mol Genet 24: R102–R110. 10.1093/hmg/ddv259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhigulev A, Lång K, Pradhananga S, Spalinskas R, Franco-Cereceda A, Björck H, Eriksson P, Sahlén P (2022. a) The role of rare enhancer variants in bicuspid aortic valve pathology. Atherosclerosis 355: 37. 10.1016/j.atherosclerosis.2022.06.194 [DOI] [Google Scholar]
- Zhigulev A, Sahlén P, Clifton NJ (2022. b) Targeted chromosome conformation capture (HiCap). Methods Mol Biol 2532: 75–94. 10.1007/978-1-0716-2497-5_5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 RNA-seq dataset. (19.3MB, xlsx)
Table S2 HiCap dataset. (79.6MB, xlsx)
Table S3 Enhancer datasets. (16.3KB, xlsx)
Table S4 TF clusters. (20.5KB, xlsx)
Table S5 Network statistics. (10.7MB, xlsx)
Source Data for Figure 4LSA-2023-02244_SdataF4.xlsx (2.7MB, xlsx)
Table S6 Candidate genes. (18MB, xlsx)
Table S7 SNPs overlap with enhancer marks. (9.6KB, xlsx)
Table S8 Probes. (4.5MB, xlsx)
Table S9 HiCap statistics. (23KB, xlsx)
Table S10 Primers. (10.1KB, xlsx)
Data Availability Statement
Whole-genome sequencing datasets analyzed during the current study are not publicly available because of reasons of sensitivity but are available from the corresponding author upon reasonable request. Raw sequence reads of RNA-seq and HiCap are submitted to NCBI Sequence Read Archive (PRJNA1012445).
Ethics statement
The 96 whole-genome sequences of NSCLC patients were diagnosed between 2006 and 2008 at Karolinska University Hospital, Stockholm, Sweden, and included after providing written informed consent in accordance with the Helsinki Declaration. The study received ethical approval from the Regional Ethics Committee in Stockholm (DNR-03-413 with amendment 2016/258-32/1). These patients are part of the material included in previously published studies (Björn et al, 2020a, 2020b; Svedberg et al, 2020).