Abstract
There is evidence that physical activity (PA) has a long-term positive impact on disease. Whether PA is a risk factor for knee osteoarthritis (OA) is still controversial. The purpose of this study was to explore whether there is a causal relationship between PA and knee OA. We extracted PA and knee OA data from genome-wide association study (GWAS) databases. We used single-nucleotide polymorphisms (SNPs) as instrumental variables. We performed MR analysis by random-effects inverse-variance weighting (IVW), MR‒Egger, weighted median, simple mode, and weighted mode methods. We evaluated the stability and reliability of the results through sensitivity analysis. There was no significant association between PA and knee OA (p > 0.05). We did not detect any pleiotropy (MR‒Egger intercept test et al.: p > 0.05). The sensitivity analysis confirmed our results (p > 0.05). There is no causal relationship between PA and knee OA.
Subject terms: Diseases, Risk factors
Introduction
Osteoarthritis (OA), a common disease, not only causes pain in the joints but can also lead to a decrease in joint function, and further progression can cause disability1. Due to factors such as obesity and aging, the number of people suffering from OA is expected to rise in the future, and OA will afflict an increasing number of patients. OA not only causes physical pain to the patients themselves but also imposes a heavy financial burden on their families, with a noticeable impact in terms of socioeconomic costs and the health care system2,3. OA treatment methods include physical therapy, drug therapy, surgery and so on. However, these treatment methods only relieve pain symptoms and ultimately increase the economic burden of patients4. Therefore, Therefore, the pathogenesis and pathogenic factors of OA are receiving increasing attention to reduce its occurrence by controlling risk factors.
OA most commonly occurs in the knee. Previous knee injury, female sex, and obesity have been identified as potential risk factors for the development of knee OA5,6. Research has shown that physical activity (PA) has a long-term positive impact on diseases such as coronary heart disease7. PA also plays a role in the mental state of elderly people8. Despite the benefits associated with PA, whether it is a risk factor for OA (especially knee OA) is still highly controversial9,10. In addition, physiotherapists promote aerobic exercise in the treatment of OA11. Therefore, it is important to determine whether there is a causal relationship between PA and knee OA.
Currently, Mendelian randomization (MR) has been used extensively in genetic epidemiology research to explore risk factors associated with disease12. MR includes the use of genetic variants [instrumental variables (IVs)] to determine causality in the relationships between exposures and outcomes. The main advantage of this approach is that it avoids the creation of bias and the influence of potential confounding factors that are found in traditional research methods. Also, reverse causation does not typically occur in MR studies12,13.
Since it is still unclear whether PA is a risk factor for knee OA, there is a lingering concern that PA contributes to the development of knee OA. Therefore, we used two-sample MR analysis to explore whether there is a causal relationship between PA and knee OA.
Methods
Data availability
The data we used for this study were all obtained from published studies that were accessed from publicly available databases (most recent data publicly available in public databases). UK Biobank received ethical approval from the Research Ethics Committee, and all participants provided written, informed consent. The original studies also had ethical approval from the relevant institutions and consent from the participants themselves. This study was conducted in accordance with Burgess's guidelines and was reported in accordance with the STROBE-MR statement.
Exposure data
Single-nucleotide polymorphisms (SNPs) for this study were obtained from accessible and publicly available genome-wide association studies (GWAS) related to knee OA. Valid IVs should fulfill three assumptions: the IVs are strongly correlated with exposure; the IVs are not interfered with by any confounding factors; and the IVs affect the outcome only through exposure. We selected SNPs that were closely associated with exposure at the genome-wide significance level under stringent conditions (p < 5 × 10–8). For the independence of SNPs, we used the following conditions: clumping window, 10,000 kb; r2 0.001. The data for PA were derived from Wang et al.'s summary of relevant data from databases such as UK Biobank (https://www.ebi.ac.uk/gwas/publications/36071172)14. The primary study population for this GWAS comprised people of European ancestry. The number of PA patients was 606,820, and the number of non-PA patients was 526,725. The intensity of PA was self-reported as moderate-to-vigorous PA. In addition, to avoid the influence of SNPs related to aggravating existing/existing OA, we identified and excluded relevant SNPs by searching related articles published in PubMed.
Outcome data
The data for knee OA were derived from Zengini et al.'s summary of relevant data from databases such as UK Biobank (https://www.ebi.ac.uk/gwas/publications/29559693)15. Similarly, the main subjects of their research are people of European ancestry. There were 4672 knee OA patients and 172,791 individuals in the control group.
Although we used exposure and outcome data from papers published in UK Biobank, the original samples for both exposure and outcome data were all from different research institutions, and the original samples did not overlap. Therefore, there are no duplicate samples in the exposure and outcome data.
Statistical analysis
We analyzed the collected data with the “TwoSampleMR” package in R software (version 4.3.1). For exposed IVs, we selected SNPs with genome-wide significance (p < 5 × 10–8; r2 = 0.001, kb = 10,000)16. We then extracted IV-related data (without the use of proxy SNPs) from the knee OA outcome dataset. We harmonized exposure and outcome datasets. To determine causality in the association between PA and knee OA, we mainly used the random-effects inverse-variance weighting (IVW) model (for the IVW method, its accuracy and stability were based on the fact that all IVs are valid and there is no directional pleiotropy). MR‒Egger, weighted median, simple mode, and weighted mode were used to supplement our analysis. The primary method for detecting directional horizontal pleiotropy was the MR Egger intercept test (an intercept that was not equal to 0 was considered to be free of directional horizontal pleiotropy). In addition, the symmetry of the funnel plot was used to assess directional pleiotropy. For the assessment of pleiotropy, we also used the leave-one-out sensitivity test as well as the MR-PRESSO test. The heterogeneity of individual effects for each gene variant was assessed by using Cochran's Q statistic. A statistically significant difference was indicated by p < 0.05. The results were expressed as odds ratios (ORs) and corresponding 95% confidence intervals (Cis).
Results
MR analysis
Through rigorous screening, 17 SNPs with strong correlations were finally used as instrumental variables between PA and knee OA (F-statistic > 10). We did not find a statistically significant association between PA and knee OA by using the IVW model method (p = 0.918). Similarly, the MR‒Egger, weighted median, simple mode, and weighted mode methods used in this study revealed a statistically significant association between PA and knee OA (p > 0.05). Table 1 shows the results of the two-sample MR analysis between PA and knee OA. The included SNPs are shown in Supplementary Table 1.
Table 1.
The results of the two-sample mendelian randomization analysis.
| Exposures | Outcomes | IVW | Weighted median | MR Egger | Weighted mode | Simple mode |
|---|---|---|---|---|---|---|
| P | P | P | P | P | ||
| PA | Knee OA | 0.918 | 0.409 | 0.527 | 0.580 | 0.635 |
PA physical activity, knee OA knee osteoarthritis, IVW random-effect inverse variance weighted.
Sensitivity analysis
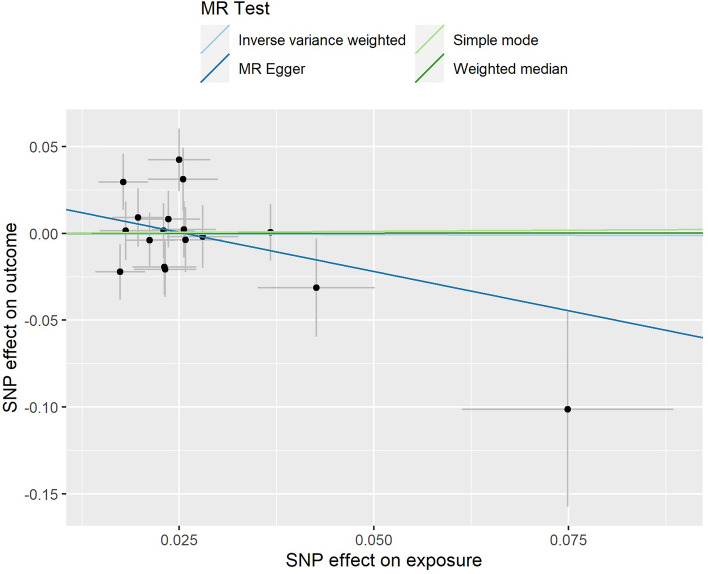
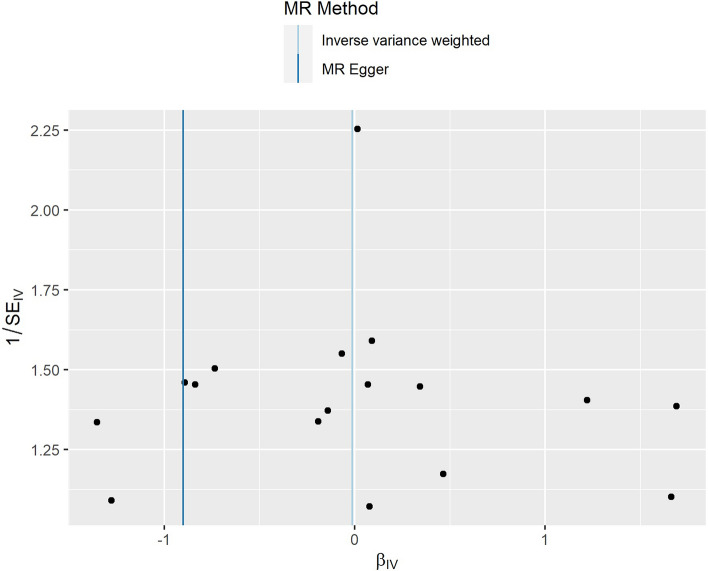
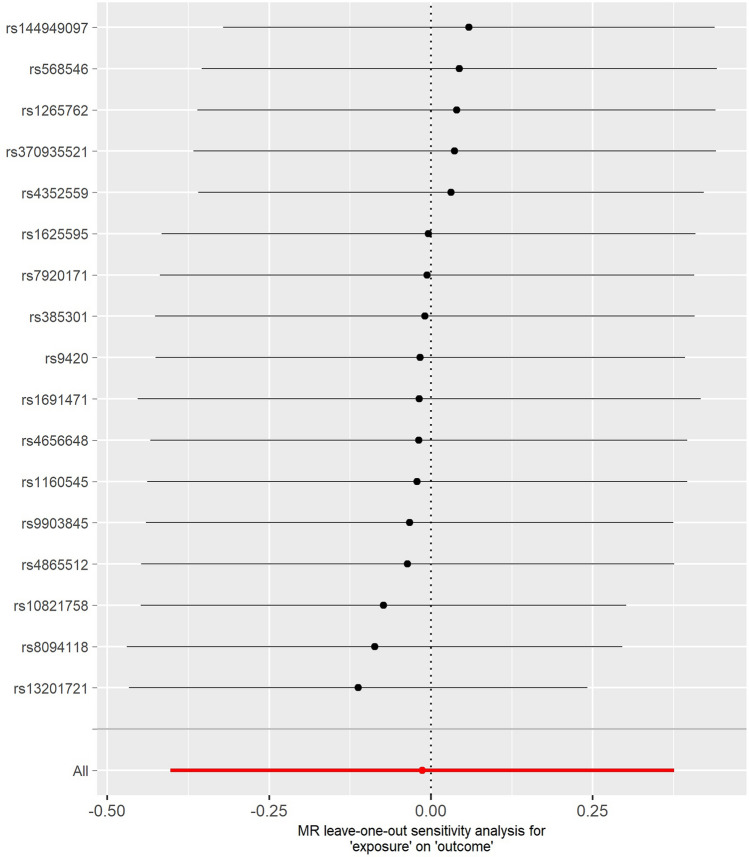
The results of the MR‒Egger intercept test suggested that no pleiotropy occurred (p > 0.05) (Fig. 1). Similarly, the results of the funnel plot suggested a very low risk of pleiotropy (Fig. 2). The results of the leave-one-out sensitivity tests and MR-PRESSO also revealed no pleiotropy (Fig. 3). There was also no statistical significance of the Cochran's Q statistic for heterogeneity.
Figure 1.
The results of MR‒Egger regression. MR Mendelian randomization.
Figure 2.
The results of funnel plot. MR Mendelian randomization.
Figure 3.
MR leave-one-out sensitivity analysis. MR Mendelian randomization.
Discussion
We used GWAS data from a large sample to explore the causal relationship between PA and knee OA. The results of this study suggest that there is no significant causal relationship between PA and knee OA. In other words, PA did not increase the risk of developing knee OA. In summary, PA was not a risk factor for the development of knee OA. Our results were also relatively stable in the sensitivity analysis.
There has been a well-known controversy over whether there is a causal relationship between PA and knee OA9,10. However, there were differences in the definition and degree of PA in different studies. In addition, there were differences in the methods used to study knee OA17. In addition, McAlindon et al.18 found an association between high-intensity PA and the risk of developing knee OA. While that association is possible, notably, the PA duration was a part of the equation that cannot be ignored. Some studies have shown that prolonged PA time will increase the probability of knee OA19,20. In addition, some of the current studies have included some self-reported data20. Self-reporting can also lead to differences in results due to the presence of subjective factors. For different populations, different occupations are also an important factor in the prevalence of knee OA. Manual workers have a higher risk of developing knee OA than nonmanual workers21.
The current research primarily suggests that PA will not increase the incidence rate of knee OA. The meta-analysis by Coburn et al. and Gates et al. yielded the same result: PA is not a risk factor for the development of knee OA22,23. Similarly, our two-sample MR analysis showed that PA does not lead to an increased prevalence of knee OA. In addition, according to our GWAS data source. In terms of PA intensity, our study population engaged in moderate-to-vigorous PA14. Therefore, our study can provide support that moderate-to-vigorous PA is not a risk factor for knee OA. We considered that PA did not lead to an increase in the prevalence of knee OA due to body mass index (BMI). It is now well established that obesity is an independent risk factor for the development of knee OA6. The effect of obesity on knee OA is not well understood, and the general mechanism is that obesity may affect the metabolic function of cells by altering the regulation of glucose metabolism. In addition, obesity alters mediators of oxidative stress and proinflammatory cytokines. Cytologic alterations lead to histologic cartilage damage and ultimately to the development of knee OA24. PA can effectively control BMI (especially moderate-to-vigorous PA)25, and since BMI is effectively controlled, obesity as an independent factor will no longer cause knee OA. Notably, although PA also has the potential to cause knee injury, knee injury is also a risk factor for knee OA5. Injury prevention programs can effectively reduce the possibility of knee injury26. For those concerned about knee OA caused by PA, effective injury prevention programs warrant attention.
Limitations
Our research has the following shortcomings: (1) The GWAS data we used were taken entirely from individuals of European ancestry, indicating that our results are only applicable to people with European ancestry; further research is needed to prove whether these results are also applicable to other populations. (2) There are various forms of PA, and because of the data sources, we can only analyze PA in general terms and cannot further refine the effects of the various types of PA on knee OA. (3) Because the PA GWAS data were pooled, the conditions for inclusion in the sample varied among institutions. As a result, we also did not have a way to conduct subgroup analyses of elderly people with high BMI or excessive PA. Subgroup analyses that cannot be refined can similarly lead to bias. (4) Our data only apply to moderate-to-vigorous PA, and further exploration is still needed for other intensities of PA.
Conclusion
In summary, we analyzed the GWAS data of PA and knee OA by using two-sample MR analysis. There was no obvious causal relationship between PA and knee OA. Therefore, PA is not a risk factor for the development of knee OA.
Supplementary Information
Acknowledgements
Data on PA associated single nucleotide polymorphisms and knee OA.
Abbreviations
- MR
Mendelian randomization
- IVs
Instrumental variables
- SNPs
Single-nucleotide polymorphisms
- GWAS
Genome-wide association studies
- PA
Physical activity
- OA
Osteoarthritis
- IVW
Inverse variance weighted
- ORs
Odds ratios
- Cis
Confidence intervals
- BMI
Body mass index
Author contributions
L.H. and Q.L. were responsible for the design. L.H. and Y.Z. was responsible of search. L.H. and Q.L. were involving in data screening and data extraction. L.H. was responsible in writing of the manuscript. All authors read and approve the final version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-52175-4.
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/s0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthr. Cartil. 2022;30(1):10–16. doi: 10.1016/j.joca.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020;79(6):819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 4.Abramoff B, Caldera FE. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Golightly YM, Shiue KY, Nocera M, Guermazi A, Cantrell J, Renner JB, et al. Association of traumatic knee injury with radiographic evidence of knee osteoarthritis in military officers. Arthritis Care Res. 2023;75(8):1744–1751. doi: 10.1002/acr.25072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salis Z, Gallego B, Nguyen TV, Sainsbury A. Association of decrease in body mass index with reduced incidence and progression of the structural defects of knee osteoarthritis: A prospective multi-cohort study. Arthritis Rheumatol. 2023;75(4):533–543. doi: 10.1002/art.42307. [DOI] [PubMed] [Google Scholar]
- 7.Bucciarelli V, Mattioli AV, Sciomer S, Moscucci F, Renda G, Gallina S. The impact of physical activity and inactivity on cardiovascular risk across women's lifespan: An updated review. J. Clin. Med. 2023;12(13):4347. doi: 10.3390/jcm12134347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laird E, Rasmussen CL, Kenny RA, Herring MP. Physical activity dose and depression in a cohort of older adults in the Irish longitudinal study on ageing. JAMA Netw. Open. 2023;6(7):e2322489. doi: 10.1001/jamanetworkopen.2023.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horga LM, Henckel J, Fotiadou A, Hirschmann A, Torlasco C, Di Laura A, et al. Can marathon running improve knee damage of middle-aged adults? A prospective cohort study. BMJ Open Sport Exerc. Med. 2019;5(1):e000586. doi: 10.1136/bmjsem-2019-000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2013;21(10):1558–1566. doi: 10.1016/j.joca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the US bone and joint initiative. Semin. Arthritis Rheum. 2014;43(6):701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 2016;27(11):3253–3265. doi: 10.1681/asn.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang D, Lin S, He J, Wang Q, Zhan Y. Association between COVID-19 and telomere length: A bidirectional Mendelian randomization study. J. Med. Virol. 2022;94(11):5345–5353. doi: 10.1002/jmv.28008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Emmerich A, Pillon NJ, Moore T, Hemerich D, Cornelis MC, et al. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat. Genet. 2022;54(9):1332–1344. doi: 10.1038/s41588-022-01165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP, Southam L, et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat. Genet. 2018;50(4):549–558. doi: 10.1038/s41588-018-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke L, Zheng-Bradley X, Smith R, Kulesha E, Xiao C, Toneva I, et al. The 1000 genomes project: Data management and community access. Nat. Methods. 2012;9(5):459–462. doi: 10.1038/nmeth.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, et al. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Joint Bone Spine. 2012;79(3):291–297. doi: 10.1016/j.jbspin.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 18.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: The Framingham study. Am. J. Med. 1999;106(2):151–157. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 19.Vincent KR, Vincent HK. Resistance exercise for knee osteoarthritis. PM & R J. Injury Funct. Rehabil. 2012;4(5 Suppl):S45–52. doi: 10.1016/j.pmrj.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Wang P, McGill B. The relationship between experience of knee pain and physical activity participation: A scoping review of quantitative studies. Int. J. Nurs. Sci. 2023;10(2):258–267. doi: 10.1016/j.ijnss.2023.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry TA, Wang X, Gates L, Parsons CM, Sanchez-Santos MT, Garriga C, et al. Occupation and risk of knee osteoarthritis and knee replacement: A longitudinal, multiple-cohort study. Semin. Arthritis Rheum. 2020;50(5):1006–1014. doi: 10.1016/j.semarthrit.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gates LS, Perry TA, Golightly YM, Nelson AE, Callahan LF, Felson D, et al. Recreational physical activity and risk of incident knee osteoarthritis: An international meta-analysis of individual participant-level data. Arthritis Rheumatol. 2022;74(4):612–622. doi: 10.1002/art.42001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coburn SL, Crossley KM, Kemp JL, Warden SJ, West TJ, Bruder AM, et al. Is running good or bad for your knees? A systematic review and meta-analysis of cartilage morphology and composition changes in the tibiofemoral and patellofemoral joints. Osteoarthr. Cartil. 2023;31(2):144–157. doi: 10.1016/j.joca.2022.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Batushansky A, Zhu S, Komaravolu RK, South S, Mehta-D'souza P, Griffin TM. Fundamentals of OA: An initiative of osteoarthritis and cartilage: Obesity and metabolic factors in OA. Osteoarthr. Cartil. 2022;30(4):501–515. doi: 10.1016/j.joca.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The effects of exercise and physical activity on weight loss and maintenance. Progress Cardiovasc. Dis. 2018;61(2):206–213. doi: 10.1016/j.pcad.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Al Attar WSA, Bakhsh JM, Khaledi EH, Ghulam H, Sanders RH. Injury prevention programs that include plyometric exercises reduce the incidence of anterior cruciate ligament injury: A systematic review of cluster randomised trials. J. Physiother. 2022;68(4):255–261. doi: 10.1016/j.jphys.2022.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data we used for this study were all obtained from published studies that were accessed from publicly available databases (most recent data publicly available in public databases). UK Biobank received ethical approval from the Research Ethics Committee, and all participants provided written, informed consent. The original studies also had ethical approval from the relevant institutions and consent from the participants themselves. This study was conducted in accordance with Burgess's guidelines and was reported in accordance with the STROBE-MR statement.
The data that support the findings of this study are available from the corresponding author upon reasonable request.