Abstract
Fecal calprotectin (FC) is a promising biomarker for diagnosis and treatment of inflammatory bowel disease, ulcerative colitis (UC), and Crohn’s disease. An enzyme immunoassay (EIA) is widely used for FC detection, though the considerable lag time, up to several days, causes clinical management delay. This study was performed to examine the new rapid kit fCAL-turbo, which is based on a particle-enhanced turbidimetric immunoassay (15 min), by comparing FC values with other EIAs (EliA, PhiCal, Bühlmann) and endoscopic scores. Using 94 samples, fCAL-turbo showed strong significant positive correlations with the other kits (Spearman’s r = 0.9178–0.9886). Of 74 UC patients, 69 underwent an endoscopy and fCAL-turbo reflected endoscopic activity with a moderate correlation with Mayo endoscopic subscore (MES) (r = 0.6945, others r = 0.6682–0.7013). Receiver operating characteristic analyses based on MES 0 versus 1–3 showed a similar efficacy as compared to the other kits (cut-off and area under the curve: 89.70 µg/g and 0.8592, respectively, others 62.35–138.4 µg/g and 0.8280–0.8611, respectively). Furthermore, multiple regression analysis confirmed that fCAL-turbo results significantly contributed to prediction of MES 0 with a higher t-value as compared to the other biomarkers. fCAL-turbo showed strong correlations with the other kits and also demonstrated excellent performance for predicting endoscopic remission of UC.
Subject terms: Biomarkers, Gastroenterology
Introduction
Inflammatory bowel disease (IBD), represented by ulcerative colitis (UC) and Crohn's disease (CD), is a group of chronic immune-mediated intestinal inflammatory diseases1–3. The goal of IBD treatment is to achieve mucosal healing, which reduces hospitalization and need for bowel resection4–7. Thus, several endoscopic and histological scoring systems for evaluating mucosal healing have been established and shown to contribute to improvement of prognosis of IBD cases8,9. However, frequent use of endoscopy and mucosal biopsy procedures is relatively invasive, especially in children and elderly patients10. Therefore, recent research findings have led to development of less-invasive biomarkers for examinations of serum or fecal samples11.
Fecal calprotectin (FC) is one of the most promising biomarkers for diagnosis and treatment of IBD11. It is primarily derived from granulocytes and intestinal epithelial cells, and has a direct antibacterial effect to regulate inflammatory processes12,13. The level of FC reflects migration of granulocytes responding to inflammatory stimuli, thus this biomarker has been used to differentiate intestinal inflammatory disorders, including IBD, from functional intestinal disorders, such as irritable bowel disease, with high levels of sensitivity and specificity noted11,14,15. Use of this less-invasive biomarker can help to avoid overlooking of IBD, and also reduce frequent or unnecessary colonoscopy procedures10. In addition to screening, FC has been found to be useful for monitoring disease activity and based on strong evidence recommended for clinical practice focused on IBD cases4,9,16–27. We and others have also confirmed that FC is correlated with endoscopic remission in a manner superior to other blood biomarkers, such as C-reactive protein (CRP), hemoglobin, platelets, and white blood cell count (WBC)16–19,21,27. Moreover, it has been reported that its level shows elevation at two to three months before clinical relapse in IBD patients28. Thus, FC is regarded as one of the best biomarkers for prediction of endoscopic remission and clinical relapse in patients with IBD.
Several kits for detection of FC are available. Because of its accuracy and accumulated evidence, enzyme immunoassay (EIA) testing procedures, such as enzyme-linked immunosorbent assay (ELISA), and fluoroenzyme and chemiluminescence immunoassays, are considered to represent the gold standard11. EIA testing uses batch analysis and is normally performed by a specialist outside of the treating hospital29, thus results can be delayed for up to several days despite the 2.5-h EIA run-time. The present study was conducted to validate fCAL-turbo, a newly introduced rapid automated FC detection kit, in an IBD patient population. An fCAL-turbo assay is conducted as a particle-enhanced turbidimetric immunoassay and can be performed by a general chemical analyzer typically employed by a hospital, with the result available in approximately 15 min30. However, its clinical utility for IBD cases has not been fully elucidated. Therefore, we aimed to validate the performance of the fCAL-turbo assay by comparing results with other standard EIA assays in IBD patients. Furthermore, its efficacy for correlations with endoscopic activity31–33 and prediction of endoscopic remission in those patients was investigated.
Results
Patient demographics
A total of 94 IBD patients (74 UC, 20 CD) were included in this study. Patient profiles are shown in Table 1. Wide range of patients in age (range 20–83 years old), disease activity (78.2% and 75.0% of remission, and 21.8% and 25.0% of active in UC and CD, respectively) and medications (i.e. immunomodulators, steroid, biological drug) were included, allowing more universal validation of FC assays in variety of patients’ conditions. All fecal samples (n = 94) were included in the first comparison analysis (FC values comparison among four kits) (Fig. 1). For the second comparison (FC values vs. clinical and endoscopic activities), results of 22 cases were excluded, as one had multiple inflammatory polyps found by colonoscopy, which can elevate the fecal calprotectin level34, and one had collagen disease (polyarteritis nodosa), which can elevate the FC level, which left the remaining sample size of 20 CD patients too small for this comparison analysis. For comparison with endoscopic activities, results from three patients unable to undergo an endoscopy colonoscopy examination for personal reasons were excluded. Finally, a total of 69 UC patients were analyzed using Mayo endoscopic subscore (MES) (Fig. 1).
Table 1.
Clinical and demographic characteristics of IBD patients in this study.
| UC | CD | |
|---|---|---|
| Samples, n | 74 | 20 |
| Sex (female/male) | 25/49 | 7/13 |
| Age median [IQR] | 49.0 [39.8–58.0] | 41.5 [36.0–60.3] |
| Disease activitya (%) | ||
| Remission stage | 78.2 | 75.0 |
| Active stage | 21.8 | 25.0 |
| Duration of disease (median years) [IQR] | 6.7 [2.3–11.6] | 10.4 [4.4–18.9] |
| Disease extentb (%) | ||
| Proctitis [E1] | 16.2 | − |
| Left-sided-colitis [E2] | 31.1 | − |
| Pancolitis [E3] | 52.7 | − |
| Disease locationb (%) | ||
| Ileum [L1] | − | 30.0 |
| Colon [L2] | − | 0.0 |
| Ileum + colon [L3] | − | 70.0 |
| Upper GI [L4] | − | 0.0 |
| Behaviourb (%) | ||
| Non-constricting, non-penetrating [B1] | − | 20.0 |
| Stricturing [B2] | − | 60.0 |
| Penetrating [B3] | − | 10.0 |
| Stricturing and penetrating [B2 + 3] | − | 10.0 |
| Medication (%) | ||
| 5-aminosalicylate | 83.0 | 88.2 |
| Azathioprine/6-mercaptopurine | 39.7 | 11.8 |
| Anti-TNFα agents | 9.4 | 41.2 |
| Vedolizumab | 1.9 | 0.0 |
| Steroid | 11.3 | 5.9 |
| Tacrolimus | 6.8 | 0.0 |
| Endoscopic activity | ||
| MES = 0/1/2/3 | 23/19/20/7 | − |
| mSES-CD = 0–2/3–6/7– | − | 10/4/6 |
IBD inflammatory bowel disease, UC ulcerative colitis, CD Crohn’s disease, IQR interquartile range, GI gastrointestinal. aAssessment of clinical disease activity by using partial Mayo score for UC (remission: 0–2 points, active: 3–9 points) and Crohn's Disease Activity Index (CDAI, remission: 0–150 points, active: > 150 points). bAssessment of disease extent, disease location, and disease behaviour by using the Montreal classification. TNF tumor necrosis factor, MES Mayo endoscopic subscore, mSES-CD modified simple endoscopic score for CD.
Figure 1.
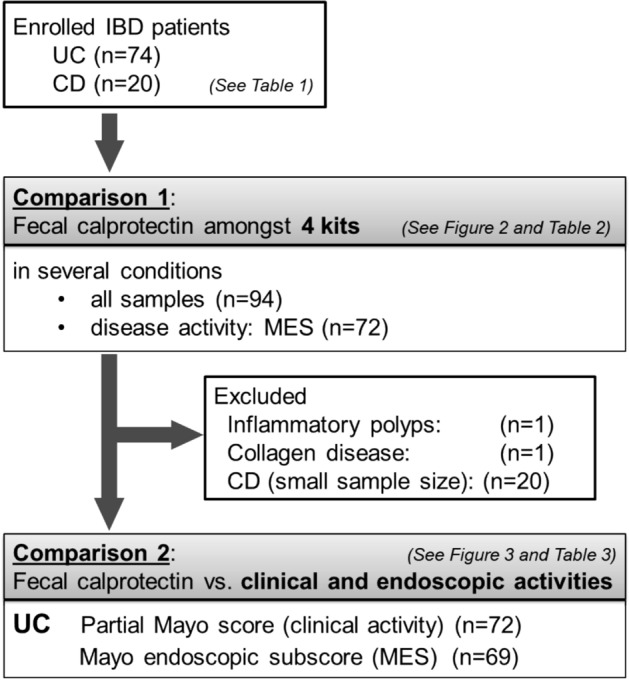
IBD patients included in this study. IBD: inflammatory bowel disease, UC: ulcerative colitis, CD: Crohn’s disease, MES: Mayo endoscopic subscore.
fCAL-turbo kit shows strong positive correlations with other kits
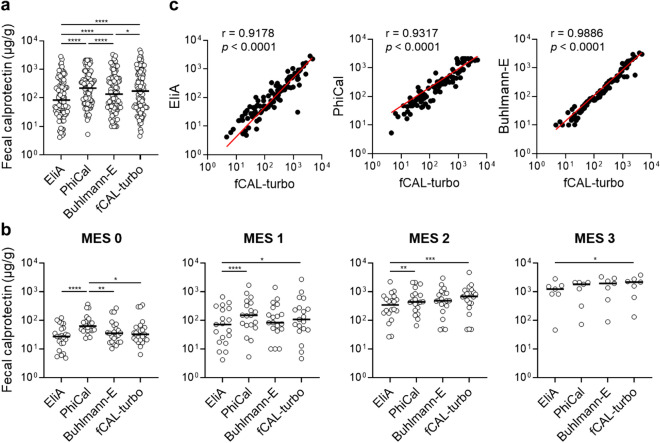
All four of the examined kits detected calprotectin in each of the fecal samples (n = 94). In the first comparison analysis using all samples, the median FC value varied significantly among the kits [median values: EliA 84.35 [95% confidence interval (CI) 49.80–165.5], PhiCal 220.1 (95% CI 140.6–322.4), BÜHLMANN fCAL® ELISA (hereinafter referred to “Buhlmann-E”) 135.5 (95% CI 77.80–231.2), fCAL-turbo 173.8 (95% CI 89.50–295.4) (Fig. 2a). As for average fold changes, fCAL-turbo showed a 2.0-, 1.2-, and 0.7-fold change of median FC value from EliA, Buhlmann-E, and PhiCal, respectively. Such variations among the kits were consistently observed for all disease conditions (MES 0–3) (Fig. 2b). Interestingly, the fCAL-turbo FC values tended to be lower with lower disease activities (MES 0–1) and higher with increased disease activities (MES 2–3), which suggests a characteristic advantage for use of fCAL-turbo to more specifically detect disease activities. Spearman’s rank correlation analysis demonstrated that fCAL-turbo had a significantly positive correlation with each of the other kits [compared to: EliA (r = 0.9178, 95% CI 0.8775–0.9453), PhiCal (r = 0.9317, 95% CI 0.8978–0.9546), Buhlmann-E (r = 0.9886, 95% CI 0.9827–0.9925)] (Fig. 2c, Table 2).
Figure 2.
Fecal calprotectin values obtained with examined kits. (a, b) Fecal calprotectin concentrations determined by use of the EliA, PhiCal, Buhlmann-E, and fCAL-turbo kits are shown for all of the samples (a. n = 94) and several disease conditions (b. n = 69; MES 0/1/2/3 = 23/19/20/7). Dunn’s multiple comparison test with Friedman’s test was used for the analyses. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (c) Spearman’s rank correlation analysis. Spearman’s correlation coefficient (r) values are shown. MES: Mayo endoscopic subscore.
Table 2.
Correlation analysis between FC kits in all samples.
| Assay X | Assay Y | n | Spearman's rank correlation | |
|---|---|---|---|---|
| r (95%CI) | P value | |||
| fCAL-turbo | EliA | 94 | 0.9178 (0.8775–0.9453) | < 0.0001 |
| fCAL-turbo | PhiCal | 94 | 0.9317 (0.8978–0.9546) | < 0.0001 |
| fCAL-turbo | Buhlmann-E | 94 | 0.9886 (0.9827–0.9925) | < 0.0001 |
| PhiCal | Buhlmann-E | 94 | 0.9265 (0.8901–0.9511) | < 0.0001 |
| PhiCal | EliA | 94 | 0.8970 (0.8472–0.9311) | < 0.0001 |
| Buhlmann-E | EliA | 94 | 0.9281 (0.8925–0.9522) | < 0.0001 |
FC fecal calprotectin, CI confidence interval.
fCAL-turbo kit results reflect clinical and endoscopic activities in UC patients
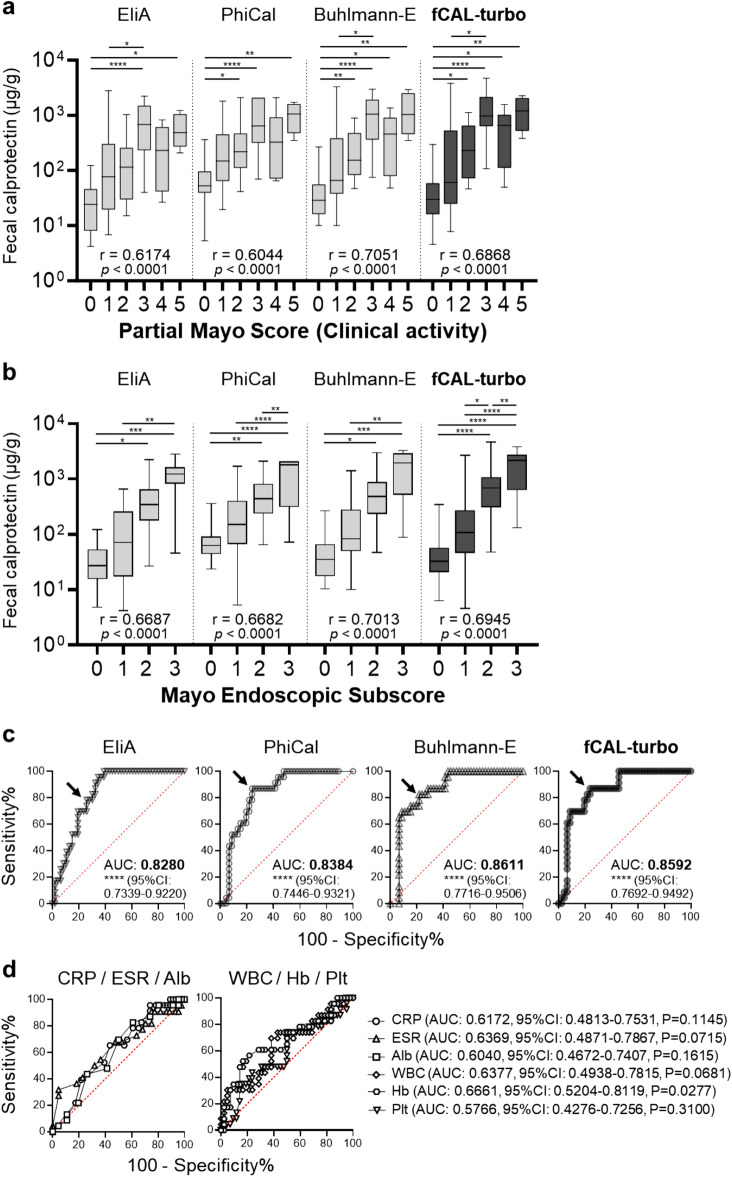
Results of the second analysis of correlation with UC clinical activity demonstrated that findings obtained with each kit reflected that activity, shown by partial Mayo score (pMayo), with a moderately significant correlation (Fig. 3a, Table 3). Spearman’s rank correlation analysis showed the following results: EliA, r = 0.6174 (95% CI 0.4445–0.7459); PhiCal, r = 0.6044 (0.4277–0.7366); Buhlmann-E, r = 0.7051 (0.5611–0.8077); and fCAL-turbo, r = 0.6868 (0.5363–0.7950). Since mucosal healing is the ideal goal of IBD therapy and endoscopic evaluation is more sensitive than clinical score, correlation analysis was also performed using endoscopic score (MES) (Fig. 3b, Table 4). Each kit reflected endoscopic activity, i.e., MES, with a moderately significant correlation. Spearman’s rank correlation analysis showed the following results: EliA, r = 0.6687 (95% CI: 0.4921–0.7925); PhiCal, r = 0.6682 (0.4913–0.7921); Buhlmann-E, r = 0.7013 (0.5372–0.8142); and fCAL-turbo, r = 0.6945 (0.5277–0.8097). As expected, FC values for endoscopic remission were significantly lower as compared to those for clinical remission (data not shown). Receiver operating characteristic (ROC) curve analysis for differentiation between endoscopic remission (MES = 0) and endoscopic activity (MES = 1–3) showed that the efficacy of the fCAL-turbo kit was similar to that of the other standard kits for the present UC cases (Fig. 3c, Table 5). The optimal cut-off values were 62.35, 138.4, 72.60, and 89.70 µg/g for the EliA (area under the curve, (AUC): 0.8280, 95% CI 0.7339–0.9220, p < 0.0001), PhiCal (AUC: 0.8384, 95% CI 0.7446–0.9321, p < 0.0001), Buhlmann-E (AUC: 0.8611, 95% CI 0.7716–0.9506, p < 0.0001) and fCAL-turbo (AUC: 0.8592, 95% CI 0.7692–0.9492, p < 0.0001) kits, respectively. Using the optimum cut-offs, FC values predicted endoscopic remission with a sensitivity of 78.26–86.96%, specificity of 73.91–78.26%, positive prediction value of 59.99–65.51%, negative prediction value of 87.17–92.10%, and accuracy of 75.36–79.71%. The AUC value obtained with the fCAL-turbo kit for predicting endoscopic remission was compared with values obtained with Elia, PhiCal, and Buhlmann-E, and found to be not significantly different (p = 0.251, p = 0.355, and p = 0.780, respectively). These results indicate that fCAL-turbo is a promising FC kit for predicting remission with an efficacy similar to the other FC kits tested. Finally, other clinical biomarkers for IBD, such as CRP, erythrocyte sedimentation rate (ESR), hemoglobin, and others, also useful for predicting remission, were examined, which showed that the AUC obtained with each of each of those was significantly smaller than the fCAL-turbo result (Fig. 3d). Moreover, multiple regression analysis confirmed that fCAL-turbo was the most effective, with a significantly higher t-value for the biomarkers CRP, ESR, hemoglobin, WBC, platelets, and albumin (Table 6).
Figure 3.
Comparison of fecal calprotectin with clinical and endoscopic activities in ulcerative colitis cases. (a) Fecal calprotectin and clinical activity score (partial Mayo score, pMayo). Spearman’s rank correlation analysis was used. Spearman correlation coefficient (r) values are shown (n = 72; pMayo 0/1/2/3/4/5 = 17/19/15/11/6/4). (b) Fecal calprotectin and Mayo endoscopic subscores. Spearman’s rank correlation analysis was used. Spearman correlation coefficient (r) values are shown. Kruskal–Wallis multiple comparison testing with ANOVA was performed (n = 69; MES 0/1/2/3 = 23/19/20/7). (c, d) Receiver operating characteristic curves. Arrows indicate data points with shortest distance from top left corner. Kruskal–Wallis multiple comparison testing with ANOVA was performed for data presented in panels (a) and (b). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, Alb: albumin, WBC: white blood cell, Hb: hemoglobin, Plt: platelet, AUC: area under the curve, CI: confidence interval.
Table 3.
Correlation analysis between FC values and pMayo score.
| vs. pMayo score | n | Spearman's rank correlation | |
|---|---|---|---|
| Assay | r (95% CI) | P value | |
| EliA | 72 | 0.6174 (0.4445–0.7459) | < 0.0001 |
| PhiCal | 72 | 0.6044 (0.4277–0.7366) | < 0.0001 |
| Buhlmann-E | 72 | 0.7051 (0.5611–0.8077) | < 0.0001 |
| fCAL-turbo | 72 | 0.6868 (0.5363–0.7950) | < 0.0001 |
FC fecal calprotectin, pMayo partial Mayo, CI confidence interval.
Table 4.
Correlation analysis between FC values and MES.
| vs. MES | n | Spearman's rank correlation | |
|---|---|---|---|
| Assay | r (95% CI) | P value | |
| EliA | 69 | 0.6687 (0.4921–0.7925) | < 0.0001 |
| PhiCal | 69 | 0.6682 (0.4913–0.7921) | < 0.0001 |
| Buhlmann-E | 69 | 0.7013 (0.5372–0.8142) | < 0.0001 |
| fCAL-turbo | 69 | 0.6945 (0.5277–0.8097) | < 0.0001 |
FC fecal calprotectin, MES Mayo endoscopic subscore, CI confidence interval.
Table 5.
Sensitivity, specificity, predictive value, and accuracy of FC kits for predicting endoscopic mucosal healing in UC patients.
| Assay | Cut-off (ug/g) | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | Accuracy (95%CI) |
|---|---|---|---|---|---|---|
| EliA | 62.35 | 78.26 (58.10–90.34) | 73.91 (59.74–84.40) | 59.99 (41.91–74.32) | 87.17 (74.03–94.58) | 75.36 (59.19–86.38) |
| PhiCal | 138.4 | 86.96 (67.87–95.46) | 76.09 (62.06–86.09) | 64.51 (47.21–77.43) | 92.10 (79.43–97.43) | 79.71 (63.99–89.21) |
| Buhlmann-E | 74.60 | 82.61 (62.86–93.02) | 78.26 (64.43–87.74) | 65.51 (46.91–79.13) | 90.00 (77.62–96.17) | 79.71 (63.90–89.50) |
| fCAL-turbo | 89.70 | 86.96 (67.87–95.46) | 76.09 (62.06–86.09) | 64.51 (47.21–77.43) | 92.10 (79.43–97.43) | 79.71 (63.99–89.21) |
FC fecal calprotectin, UC ulcerative colitis, CI confidence interval, PPV positive predictive value, NPV negative predictive value, CI confidence interval.
Table 6.
Multiple regression analysis of biomarkers for predicting endoscopic remission in UC patients.
| Factor | β-coefficient (95%CI) | t value | P value |
|---|---|---|---|
| WBC | − 0.00007 (− 0.00014 to 0.00000) | − 1.984 | 0.052 |
| Hb | 0.10134 (0.02030–0.18238) | 2.503 | 0.015 |
| Plt | 0.00804 (− 0.00513 to 0.02123) | 1.221 | 0.227 |
| Alb | − 0.01045 (− 0.38588 to 0.36496) | − 0.055 | 0.956 |
| CRP | − 0.13058 (− 0.60893 to 0.34775) | − 0.546 | 0.587 |
| ESR | 0.00571 (− 0.00871 to 0.02015) | 0.792 | 0.431 |
| fCAL-turbo | − 0.00018 (− 0.00030 to − 0.00006) | − 3.006 | 0.004 |
UC ulcerative colitis, CI confidence interval, CI confidence interval, WBC white blood cell, Hb hemoglobin, Plt platelet, Alb albumin, CRP C-reactive protein, ESR erythrocyte sedimentation rate.
Discussion
This relatively large comparative study was performed to examine the capabilities of fCAL-turbo, a new rapid FC detection kit. A strong correlation with other well-established EIA kits was noted and the findings indicate that fCAL-turbo accurately reflects endoscopic activity, thus providing useful results for detection of endoscopic remission. Accordingly, it is considered that the fCAL-turbo kit has a clinical efficacy similar to conventional FC kits when used for management of IBD patients. In the updated Selecting Therapeutic Targets in IBD initiative (STRIDE-II), which presents treat-to-target strategies associated with therapeutic goals, reduction of FC to an acceptable range has been proposed as a formal intermediate treatment target35. Because of its significant advantage of immediate turnaround time, as well as compatibility and cost performance, use of the fCAL-turbo can enhance the clinical efficacy of treat-to target strategies employed in IBD practice.
To date, three excellent studies have presented comparisons of values obtained with various kits including fCAL-turbo, though did not include endoscopic disease activity29,30,36. Oyaert et al.36 compared the diagnostic accuracy of six different assays using fecal samples from patients with CD (n = 15), UC (n = 12), gastrointestinal diseases used as a control (n = 52), and rheumatologic disease (n = 26). All six assays including fCAL-turbo demonstrated excellent diagnostic accuracy with similar AUCs. In the study by Noebauer et al.29, fecal samples from 95 symptomatic children suffering from chronic diarrhea, abdominal pain, and bloody stool were analyzed using fCAL-turbo, Buhlmann-E, and Quantum Blue, and a good correlation between fCAL-turbo and the other assays was found. Furthermore, Nilsen et al.30 compared FC values obtained with the fCAL-turbo and Buhlmann-E kits, and also analyzed variations between those two clinical chemistry analyzers, with a good correlation demonstrated. The present findings support those presented in these previous studies. In addition, we compared FC values and endoscopic disease activity to explore whether the fCAL-turbo assay can provide reliable detection at any disease stage. The findings showed detection of calprotectin at all stages of disease and clearly revealed endoscopic activity in IBD patients.
To the best of our knowledge, this is the first study to use endoscopic score for validation of performance of the fCAL-turbo kit. In UC patients, findings obtained with fCAL-turbo had a strong positive correlation with endoscopic activity (MES). The AUC value for the fCAL-turbo (0.85) for detecting endoscopic remission (MES = 0) was consistent with those of the other assays (0.77–0.86) examined in this study and presented in previous reports, such as PhiCal (cut-off: 180 µg/g; AUC 0.67)24, Buhlmann-E (cut-off: 201 µg/g, AUC 0.88) and Quantum Blue (cut-off: 150.5 µg/g; AUC 0.88)26, and EliA Calprotectin 2 (cut-off: 146.0 µg/g; AUC 0.777)25. Therefore, it is anticipated that the fCAL-turbo kit will be considered to be a reliable tool to monitor UC activity. While data for CD patients are not presented because of the small sample size (n = 20), promising results have shown that fCAL-turbo and other kits, except for EliA, reflect endoscopic activity well, as shown by modified simple endoscopic score for CD (mSES-CD), though the strength of the correlation was found to be moderate, weaker than that in UC patients, which is similar to results presented in previous reports37,38. Nevertheless, FC remains a promising biomarker for CD, as we and others have demonstrated its greater accuracy for predicting endoscopic remission in CD patients as compared to serum biomarkers17,35. Further comprehensive investigations are required35.
As noted in other reports39,40, the present findings showed kit-dependent variations, with a maximum 3.8-fold difference previously reported36,41. Several factors can influence the FC value, such as age42,43, obesity and diet43, physical inactivity44, and mucus or blood in the fecal sample45, though calprotectin itself remains stable for up to one week at room temperature22,46–48. Since the same fecal samples were examined with all of the assay kits in this study, those factors do not require consideration. Possible reasons for the variations noted include (1) different assay (method) principles, (2) different antibody for capture or detection, and/or (3) potentially different fecal supernatant extraction efficacy35,39. The Buhlmann-E and fCAL-turbo kits use the same antibody, and the strongest correlation was noted between them. Thus, even with different methods and extraction kits, the antibody may be an important factor for result consistency, as the various antibodies used in the different assays would be directed against different complexes of the FC protein.
A variety of cut-off levels for endoscopic remission detection have been reported35, which was also observed in the present study. FC cut-off values in UC patients range from 58 to 490 µg/g and in CD patients from 71 to 918 µg/g, though consensus has yet to be established35. The present findings suggest that the cut-off is correlated with the kit-dependent actual value for calprotectin, with the cut-off highest for PhiCal and followed in order by fCAL-turbo, Buhlmann-E, and EliA (Table 5), while the median level of actual calprotectin value was also highest for PhiCal, followed by fCAL-turbo, Buhlmann-E, and EliA (Fig. 2). To determine a more reliable cut-off level, investigators will need to consider kit-dependent variations as well. Clinicians are advised to use the same kit for monitoring of IBD activity in individual patients.
The present study has some limitations. First, other assays presently available were not included in the analyses, such as immuno-chromatographic49,50, colloidal gold aggregation51, and home-based52,53 assays, because of their lower frequency of use and level of accuracy. Furthermore, endoscopic score for IBD activity assessment was used, as that is the current gold standard for monitoring endoscopic remission in IBD patients31–33, though it would be better to add histological assessment. These limitations should be taken into account when interpreting the results regarding the clinical efficacy of FC for IBD endoscopic scores.
In conclusion, fCAL-turbo, a new rapid fully automated FC kit based on a particle-enhanced turbidimetric immunoassay method, showed strong and significant correlations with other established standard FC kits. Furthermore, moderate correlation with endoscopic activity in UC patients was noted, equivalent to that seen with the other kits. In particular, fCAL-turbo showed excellent performance for predicting endoscopic remission in UC patients, the same as the other standard assays. Based on its advantages including rapid results (15 vs. 150 min) and cost performance (random access testing vs. batch analysis) (Table S1)29, use of the fCAL-turbo kit can significantly augment the clinical efficacy of a treat-to-target strategy for IBD cases.
Materials and methods
Population
Patient enrollment was performed from October 2016 to November 2018 at Shimane University Hospital and Matsue Seikyo General Hospital in Japan as part of our previous prospective studies17,18. For the present investigation, fecal samples obtained in the previous studies were used. The maximum frozen storage period was three years and there were no more than two freeze–thaw cycles. Although calprotectin protein is known to remain stable for several days even at room temperature and after freeze–thaw cycles47,48, the quality of the fecal samples was verified, and there were no significant differences for representative FC values between those previous studies and the present. Information for the enrolled patients was analyzed in a retrospective manner. Enrolled patients, as in our previous studies17,18, were individuals with a previously established diagnosis of UC or CD, and scheduled to undergo a colonoscopy or balloon-assisted endoscopy (BAE). Excluded were those with colon cancer, acute or chronic gastrointestinal infection including Clostridioides difficile and cytomegalovirus, an artificial anus, regular intake of aspirin and/or other nonsteroidal anti-inflammatory drugs that can induce mucosal injury and might increase FC levels54, or unable to provide a fecal sample. As for patient demographics, gender, age, disease activity, duration, location, and behavior, and concomitant medications being taken at the time of fecal collection were noted. Clinical UC or CD disease activity was evaluated on the day of the endoscopy examination by use of a partial Mayo score (pMayo, endoscopic subscore removed, remission: 0–2 points, active stage: 3–9 points)55 or CD activity index (CDAI; remission: 0–150 points, active stage: > 150 points)56, respectively. Assessment of disease extent, location, and behavior was done using the Montreal classification57. The study protocol was approved by the institutional review board of both hospitals (Shimane University review board/Hospital review board) and performed in adherence to the Helsinki Declaration. Each patient provided written informed consent for participation.
Fecal samples
Fecal samples were collected at the first bowel movement in the morning22 within three days before a bowel preparation for a colonoscopy procedure, and brought to the hospital or sent by postal mail immediately after collection. Upon arrival, fecal samples were immediately frozen at − 20 °C without a preservative. The maximum time lag from fecal collection to frozen state was within 24 h, during which period calprotectin proteins in fecal samples have been proven to remain stable at room temperature22,46–48.
Measurement of fecal calprotectin level
FC levels were determined with the same fecal sample using each of the following four kits, according to the protocol of manufacturer. Those included three standard kits; EliA® Calprotectin 2 (Thermo Fisher Scientific, Sweden), PhiCal® Calprotectin ELISA (Immundiagnostik, Germany)49, and Buhlmann-E (BÜHLMANN fCAL® ELISA, Bühlmann, Switzerland), and also the new BÜHLMANN fCAL® turbo kit (Bühlmann, Switzerland)30. All samples were measured in duplicate under the same pre-analytical conditions, with the mathematical mean used to reflect any imprecision for the patient samples. The characteristics of each kit are shown in Table S1. PhiCal assays were performed by SRL Inc. (Tokyo, Japan) as part of our previous prospective study, while the others included in the present study were performed by Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan), after receiving the preserved fecal samples.
Endoscopic activity scoring
The UC and CD patients received a polyethylene glycol- or magnesium citrate-based electrolyte solution for bowel preparation prior to the endoscopy. In those with UC, a total colonoscopy was generally performed with a magnifying colonoscope (PCF 260AZI, Olympus, Tokyo, Japan). The findings were evaluated using MES for each of five portions of the colorectum (cecum to ascending colon, transverse, descending, sigmoid colon, rectum). Maximum MES in the colorectum was used as the final endoscopy score for the present study. In all patients with CD, ileocolonoscopy or retrograde BAE using a double-balloon enteroscope EN-450T5 (Fujifilm, Tokyo, Japan) examinations were performed. Endoscopic findings indicating CD were evaluated based on mSES-CD, in which the narrowing score is removed for CD cases. All of the endoscopic procedures were performed by experienced gastroenterologists, with the endoscopic scores independently re-assessed by two expert colonoscopists (K.K., A.O.) who were blinded to the FC results. If there were any discrepancies, the final accepted score was determined based on discussion with the two experts and their supervisor.
Statistical analysis
Nonparametric data are presented as median and interquartile range or 95% CI. For non-paired non-parametric comparisons, a Mann–Whitney test was performed (two-tailed). For non-paired non-parametric multiple comparisons, a Kruskal–Wallis test with ANOVA was performed (two-tailed), while correlation analyses were performed using Spearman’s rank correlation test (two-tailed). For paired non-parametric multiple comparisons, Dunn’s test with ANOVA was used to analyze differences among nonparametric data for paired samples. ROC curve analysis was utilized to determine AUC and optimal cut-off value for each kit for prediction of endoscopic remission. According to the optimal cut-off value, values for sensitivity, specificity, predictive value, and accuracy were also calculated, along with the 95% CI. Statistical analyses were performed using GraphPad Prism software (version 10.0.3), except for DeLong test and multiple regression analysis results, which were analyzed using the EZR software package (version 1.61, modified version of R commander version 2.8-0).
Supplementary Information
Acknowledgements
The authors express their appreciation to the staff members of Nissui Pharmaceutical Co., Ltd. and SRL Inc. for performing the FC detection assays, as well as Keiko Masuzaki for providing technical and administrative assistance.
Author contributions
K.K., A.O., and S.I. conceived the study and designed experiments. A.O., K.K., K.Ki., S.K., M.F., N.F., Y.M., N.O., N.I., and S.I. performed endoscopy and collected samples. A.O., K.K., and S.I. analyzed data. A.O. K.K, and M.A. performed statistical analysis. A.O., K.K., M.A., and S.I. wrote and edited the manuscript. S.I. supervised the study.
Funding
The cost of the fecal calprotectin assay, including EliA® Calprotectin 2, BÜHLMANN fCAL® ELISA, and BÜHLMANN fCAL® turbo, was paid by Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan).
Data availability
The data that support the findings of this study are available from the corresponding author, K.K., upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-51580-z.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Lee M, Chang EB. Inflammatory bowel diseases (IBD) and the microbiome—Searching the crime scene for clues. Gastroenterology. 2021;160:524–537. doi: 10.1053/j.gastro.2020.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng SC, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 4.Maaser C, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis. 2019;13:144–164K. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 5.Colombel JF, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 6.Shah SC, Colombel J-F, Sands BE, Narula N. Systematic review with meta-analysis: Mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 2016;43:317–333. doi: 10.1111/apt.13475. [DOI] [PubMed] [Google Scholar]
- 7.Naismith GD, et al. A prospective single-centre evaluation of the intra-individual variability of faecal calprotectin in quiescent Crohn’s disease. Aliment. Pharmacol. Ther. 2013;37:613–621. doi: 10.1111/apt.12221. [DOI] [PubMed] [Google Scholar]
- 8.De Cruz P, et al. Characterization of the gastrointestinal microbiota in health and inflammatory bowel disease. Inflamm. Bowel Dis. 2012;18:372–390. doi: 10.1002/ibd.21751. [DOI] [PubMed] [Google Scholar]
- 9.Turner D, et al. STRIDE-II: An update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Heida A, Holtman GA, Lisman-van Leeuwen Y, Berger MY, van Rheenen PF. Avoid endoscopy in children with suspected inflammatory bowel disease who have normal calprotectin levels. J. Pediatr. Gastroenterol. Nutr. 2016;62:47–49. doi: 10.1097/MPG.0000000000000939. [DOI] [PubMed] [Google Scholar]
- 11.Wagatsuma K, Yokoyama Y, Nakase H. Role of biomarkers in the diagnosis and treatment of inflammatory bowel disease. Life. 2021;11:1375. doi: 10.3390/life11121375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayling RM, Kok K. Fecal calprotectin. Adv. Clin. Chem. 2018;87:161–190. doi: 10.1016/bs.acc.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Lee JM, et al. Update of faecal markers of inflammation in children with cystic fibrosis. Mediat. Inflamm. 2012;2012:1–6. doi: 10.1155/2012/948367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rheenen PF, Van De Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ. 2010;341:188. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 2015;110:444–454. doi: 10.1038/ajg.2015.6. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima K, et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016;16:47. doi: 10.1186/s12876-016-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima K, et al. Fecal calprotectin more accurately predicts endoscopic remission of Crohnʼs disease than serological biomarkers evaluated using balloon-assisted enteroscopy. Inflamm. Bowel Dis. 2017;23:2027–2034. doi: 10.1097/MIB.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 18.Sonoyama H, et al. Capabilities of fecal calprotectin and blood biomarkers as surrogate endoscopic markers according to ulcerative colitis disease type. J. Clin. Biochem. Nutr. 2019;64:265–270. doi: 10.3164/jcbn.18-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawashima K, et al. Therapeutic efficacy of pH-dependent release formulation of mesalazine on active ulcerative colitis resistant to time-dependent release formulation: Analysis of fecal calprotectin concentration. Biomed Res. Int. 2014;2014:1–6. doi: 10.1155/2014/342751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colombel JF, et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 21.Schoepfer AM, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the lichtiger index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm. Bowel Dis. 2013;19:332–341. doi: 10.1097/MIB.0b013e3182810066. [DOI] [PubMed] [Google Scholar]
- 22.Lasson A, et al. The intra-individual variability of faecal calprotectin: A prospective study in patients with active ulcerative colitis. J. Crohn’s Colitis. 2015;9:26–32. doi: 10.1016/j.crohns.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Yasutomi E, et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep. 2021;11:11086. doi: 10.1038/s41598-021-90441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiraoka S, et al. Fecal immunochemical test and fecal calprotectin results show different profiles in disease monitoring for ulcerative colitis. Gut Liver. 2018;12:142–148. doi: 10.5009/gnl17013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naganuma M, et al. Significance of conducting 2 types of fecal tests in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020;18:1102–1111.e5. doi: 10.1016/j.cgh.2019.07.054. [DOI] [PubMed] [Google Scholar]
- 26.Lee YW, et al. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J. Intern. Med. 2019;34:72–80. doi: 10.3904/kjim.2016.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawashima K, et al. Low fecal calprotectin predicts histological healing in patients with ulcerative colitis with endoscopic remission and leads to prolonged clinical remission. Inflamm. Bowel Dis. 2022 doi: 10.1093/ibd/izac095. [DOI] [PubMed] [Google Scholar]
- 28.Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: A systematic review and practical guide. Inflamm. Bowel Dis. 2017;23:894–902. doi: 10.1097/MIB.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noebauer B, Ramic L, Konstantin A, Zachbauer C, Einwallner E. Analytical evaluation of a fully automated immunoassay for faecal calprotectin in a paediatric setting. Biochem. Med. 2017;27:030710. doi: 10.11613/BM.2017.030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsen T, Sunde K, Hansson L-O, Havelka AM, Larsson A. A novel turbidimetric immunoassay for fecal calprotectin optimized for routine chemistry analyzers. J. Clin. Lab. Anal. 2017;31:e22061. doi: 10.1002/jcla.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magro F, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohn’s Colitis. 2017;11:649–670. doi: 10.1093/ecco-jcc/jjx008. [DOI] [PubMed] [Google Scholar]
- 32.Peyrin-Biroulet L, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 33.Bouguen G, Levesque BG, Pola S, Evans E, Sandborn WJ. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2014;12:978–985. doi: 10.1016/j.cgh.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Hodgson-Parnell L, Spence O, Chapple K. Solitary juvenile polyp as a cause of elevated faecal calprotectin in an adult. BMJ Case Rep. 2018;2018:1–2. doi: 10.1136/bcr-2018-224770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.State M, et al. Surrogate markers of mucosal healing in inflammatory bowel disease: A systematic review. World J. Gastroenterol. 2021;27:1828–1840. doi: 10.3748/wjg.v27.i16.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyaert M, et al. Analytical performance and diagnostic accuracy of six different faecal calprotectin assays in inflammatory bowel disease. Clin. Chem. Lab. Med. 2017;55:1564–1573. doi: 10.1515/cclm-2016-1012. [DOI] [PubMed] [Google Scholar]
- 37.Lin J-F, et al. Meta-analysis: Fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm. Bowel Dis. 2014;20:1407–1415. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 38.D’Haens G, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 39.Mosli MH, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. Am. J. Gastroenterol. 2015;110:802–819. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 40.D’Amico F, Bonovas S, Danese S, Peyrin-Biroulet L. Review article: Faecal calprotectin and histologic remission in ulcerative colitis. Aliment. Pharmacol. Ther. 2020;51:689–698. doi: 10.1111/apt.15662. [DOI] [PubMed] [Google Scholar]
- 41.Whitehead SJ, French J, Brookes MJ, Ford C, Gama R. Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann. Clin. Biochem. Int. J. Lab. Med. 2013;50:53–61. doi: 10.1258/acb.2012.011272. [DOI] [PubMed] [Google Scholar]
- 42.Li F, et al. Fecal calprotectin concentrations in healthy children aged 1–18 months. PLoS ONE. 2015;10:e0119574. doi: 10.1371/journal.pone.0119574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caviglia GP, et al. Fecal calprotectin: Beyond intestinal organic diseases. Panminerva Med. 2018;60:29–34. doi: 10.23736/S0031-0808.18.03405-5. [DOI] [PubMed] [Google Scholar]
- 44.Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin. Cancer Epidemiol. Biomark. Prev. 2004;13:279–284. doi: 10.1158/1055-9965.EPI-03-0160. [DOI] [PubMed] [Google Scholar]
- 45.Calafat M, et al. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis. Inflamm. Bowel Dis. 2015;21:1072–1076. doi: 10.1097/MIB.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826.e2. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand. J. Gastroenterol. 1992;27:793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 48.Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176–180. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 49.Kittanakom S, et al. Comparison of fecal calprotectin methods for predicting relapse of pediatric inflammatory bowel disease. Can. J. Gastroenterol. Hepatol. 2017;2017:1–10. doi: 10.1155/2017/1450970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coorevits L, Baert FJ, Vanpoucke HJM. Faecal calprotectin: Comparative study of the Quantum Blue rapid test and an established ELISA method. Clin. Chem. Lab. Med. 2013;51:825–831. doi: 10.1515/cclm-2012-0386. [DOI] [PubMed] [Google Scholar]
- 51.Inoue K, et al. Usefulness of a novel and rapid assay system for fecal calprotectin in pediatric patients with inflammatory bowel diseases. J. Gastroenterol. Hepatol. 2014;29:1406–1412. doi: 10.1111/jgh.12578. [DOI] [PubMed] [Google Scholar]
- 52.Heida A, et al. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin. Gastroenterol. Hepatol. 2017;15:1742–1749.e2. doi: 10.1016/j.cgh.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Wei S-C, Tung C-C, Weng M-T, Wong J-M. Experience of patients with inflammatory bowel disease in using a home fecal calprotectin test as an objective reported outcome for self-monitoring. Intest. Res. 2018;16:546–553. doi: 10.5217/ir.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeuchi K, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2006;4:196–202. doi: 10.1016/S1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 55.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 56.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. doi: 10.1016/S0016-5085(76)80163-1. [DOI] [PubMed] [Google Scholar]
- 57.Satsangi J, Silverberg MS, Vermeire S, Colombel J-F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, K.K., upon reasonable request.