Abstract
Hydroxychloroquine (HCQ) is obtained by hydroxylation of chloroquine (CQ) and the first indication was malaria. Nowadays, HCQ is commonly used in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) with favorable results. Antiphospholipid syndrome (APS) is an autoimmune disease characterized by thrombosis and/or pregnancy morbidity and persistent positivity of antiphospholipid antibodies. Around 20–30% of pregnant women with APS develop adverse pregnancy outcomes despite conventional treatment with aspirin and heparin, called refractory obstetric APS. Interestingly, HCQ has shown positive effects on top of the standard of care in some refractory obstetric APS patients. HCQ mechanisms of action in APS comprise its ability to bind sialic acid present in cell membranes, its capacity to block the binding of antiphospholipid antibodies to the cell and the induced increase of pH in extracellular and intracellular compartments. However, the precise mechanisms of HCQ in the specific situation of refractory APS still need to be fully clarified. Therefore, this review summarizes the known modulating effects of HCQ and CQ, their side effects and use in APS and different pathologies to understand the benefit effects and the mechanism of action of HCQ in refractory obstetric APS.
Keywords: Hydroxychloroquine, Chloroquine, Antiphospholipid syndrome, Refractory, Pregnancy
Introduction
Hydroxychloroquine (HCQ) is a drug obtained by beta hydroxylation of chloroquine (CQ), an alkaloid, similar in structure to the active compounds present in the cinchona bark [1]. Drugs derived from cinchona bark have been used for treating several diseases, mainly malaria, a disease caused by parasites from the Plasmodium genus [2]. CQ was first synthesized in 1934 and HCQ in 1960 by chemical modification of CQ [3]. HCQ and CQ have similar pharmacokinetic properties; however, HCQ is more polar and less lipophilic, resulting in less diffusion across biological membranes and thus less toxicity [4].
In the twentieth century, widespread use of quinine by the US Army as a prophylaxis for malaria was accompanied by observations that this drug had positive effects on other diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [5, 6]. HCQ was implemented in the treatment of these autoimmune diseases afterwards, and it induced favorable effects on diabetes and dyslipidemia in SLE and RA patients [4, 7]. More recently, the use of HCQ and CQ was proposed as a treatment for COVID-19 with controversial results [8].
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by thrombosis (vascular APS) and/or pregnancy morbidity (obstetric APS) and persistent positivity of moderate to high titers of antiphospholipid antibodies (aPL) such as anti-cardiolipin (aCL), anti-ß2 glycoprotein 1 (aß2GP1) or the lupus anticoagulant (LA) in a 12-week interval. APS-related pregnancy morbidity is characterized by consecutive spontaneous abortions before 10th week of gestation, unexplained fetal deaths at or beyond the 10th week of gestation and premature births before the 34th week of gestation because of placental insufficiency [9]. Recently, vascular and obstetric APS have been described as separate entities due to differences in their pathophysiology [10]. Moreover, APS can be primary, in the absence of other autoimmune diseases, or secondary when it is related to another autoimmune diseases, mainly SLE and RA [11].
Around 20–30% of pregnant APS patients develop adverse pregnancy outcomes despite conventional treatment with heparin and low-dose aspirin which is called refractory obstetric APS [12, 13]. The European Alliance of Associations for Rheumatology (EULAR) guidelines recently considered HCQ addition in the first trimester for women with refractory obstetric APS [14]. Adding HCQ to conventional treatment in those patients has led to a 34% live birth achievement increase in the subsequent pregnancy [12]. Indeed, a recent systematic review confirms live birth rate improvement after adding HCQ [15]. However, HCQ effects have been discussed mainly for vascular APS manifestations and the mechanisms of these beneficial effects are still unclear in obstetric APS [16–18]. Therefore, this review describes the effects of HCQ and CQ in APS and different pathologies to propose possible mechanisms of action of HCQ and its promising modulatory effects in refractory obstetric APS.
Search strategy
A literature electronic search through Medline/PubMed, Scopus, and Directory of Open Access Journals (DOAJ) was carried out for this narrative review. The following keywords were used: ‘Antiphospholipid syndrome’, ‘Obstetric antiphospholipid syndrome’, ‘Refractory’, ‘Hydroxychloroquine’, ‘Chloroquine’, ‘Pregnancy’. We included original articles, narrative and systematic reviews relevant to our review aim. No limits on publication date were placed and publications in languages other than English or Spanish were excluded which was a limitation of our study. All relevant studies until August 2023 were included.
Pharmacokinetics of HCQ
HCQ is administered orally and is mostly absorbed in the upper intestinal tract with a subsequent bioavailability of 0.7–0.8 and a binding to blood proteins of 40%, of which albumin is the main one [4, 19]. This binding is reversible and creates a balance between the bound drug fraction and the free or pharmacologically active drug fraction. Moreover, HCQ is metabolized in the liver resulting in metabolites like desethylhydroxychloroquine, desethylchloroquine, and bidesethylhydroxychloroquine. These metabolites have a half-life between 30 to 60 days. HCQ is excreted in the urine in a concentration of 15–25% as metabolites and 60% in its unchanged form [19]. Pharmacokinetics of HCQ have been described during pregnancy; higher volume of distribution, higher clearance due to increased glomerular filtration, and increased metabolism by liver enzymes have been described [20].
Safety of HCQ use
Retinopathy risk is the most important side effect of HCQ. Risk factors for retinopathy are high daily doses (> 5 mg/kg/day), duration of use > 5 years, concomitant tamoxifen treatment, and a decrease in drug elimination due to renal impairment [21, 22]. Furthermore, HCQ is associated with benign corneal deposits, decreased visual acuity, and color vision deficiency. Because of these side effects, the American Academy of Ophthalmology (AAO) recommends a baseline fundus examination to rule out preexisting maculopathy and an extended screening with visual fields and spectral-domain optical coherence tomography in case of abnormalities. Depending on major risk factors, following ophthalmological evaluation should be performed sooner or after five years of use [21, 23].
HCQ overdose toxicity manifestations include cardiovascular shock, hypokalemia, psychosis and seizures due to sodium and potassium channel blockade [24]. Medical care for HCQ overdose is based on supportive treatment [25]. Furthermore, HCQ and CQ can be ineffective or toxic in patients with different genetic conditions [26].
HCQ treatment can be initiated or continued during pregnancy and breastfeeding, and its use has not been associated with adverse pregnancy outcomes [27]. Furthermore, the discontinuation of HCQ therapy before or during pregnancy in patients with SLE leads to increased disease activity which is associated with adverse pregnancy outcomes [28]. Several studies have shown no significant risk of congenital malformations, retinopathy or ototoxicity in pregnancy [29–31].
Effects of HCQ from malaria to autoimmune diseases
HCQ and CQ are weak bases with a positive charge and concentrate in acidic organelles such as endosomes, the Golgi apparatus, and lysosomes. Both molecules are unable to cross the cell membrane in their protonated form; however, the unprotonated portion enters the cell and is protonated inversely to pH; once protonated it is trapped in the acidic organelles [32].
Below, we present some modulating effects of HCQ that could explain its utility in different diseases.
HCQ increases endoplasmic reticulum pH: effects on dyslipidemias
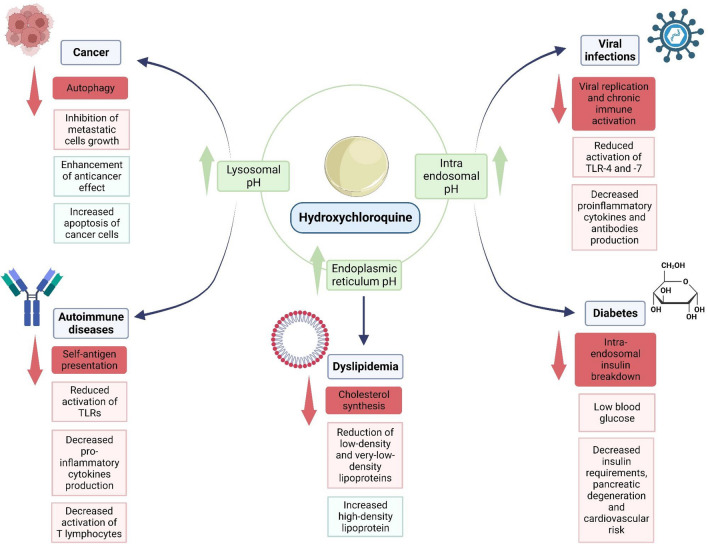
APS and SLE patients have higher cardiovascular morbidity and mortality than the general population, and hyperlipidemia control is essential to decrease it [33]. In a cohort of 264 SLE patients, treatment with 200–400 mg of HCQ daily resulted in a decrease in plasma cholesterol levels [34]. Furthermore, an increase in cholesterol after discontinuation of HCQ has been observed [35]. A systematic review that included 559 patients from eight studies found a reduction in low-density lipoprotein levels by using HCQ [36]. The exact effect of HCQ on the enzymes involved in cholesterol synthesis is unclear. However, CQ disables acetyl-coenzyme A, required in initial events of cholesterogenesis in the endoplasmic reticulum, suggesting that HCQ lowers cholesterol levels by increasing the pH in the endoplasmic reticulum [37]. Therefore, HCQ treatment could be co-adjuvant to conventional cardiovascular risk control tools in patients with APS and/or SLE.
HCQ increases endosomal pH: effects on diabetes and viral infections
Case reports have described hypoglycemia in patients with RA treated with HCQ, with or without diabetes [38, 39]. Interestingly, the magnitude of the effect of HCQ on glucose levels has been sufficient to suppress the need for insulin [40]. The mechanism of HCQ on glucose metabolism may be related to inhibition of intracellular insulin degradation in human adipose tissue, by changes in lysosomal enzyme activity and intra-endosomal pH [41, 42].
In viral infections, it has been established that CQ inhibits pH-dependent steps in the replication of flavivirus, retrovirus, and coronavirus [32, 43]. In treating patients with HIV infection, 400 mg of HCQ has been included as an adjuvant for six months, as this resulted in an increase in the CD4-positive T lymphocyte count [44]. Furthermore, use of HCQ combined with hydroxycarbamide and didanosine for 48 weeks decreased viral load and increased CD4-positive T lymphocyte count [45]. HCQ could inhibit replication of the virus by increasing endosomal pH, which inhibits post-translational modifications of the gp120 glycoprotein, essential for the fusion of viral and cellular membranes [46]. Moreover, HCQ suppressed HIV replication by 50% as demonstrated in T lymphocytes obtained from pleural fluid from an individual infected with HIV and monocyte cell line 63HIV [46]. Thus, through endosomal pH increase, HCQ seems to play an immunomodulatory role, which could lead to beneficial effects in patients with chronic inflammatory diseases such as obstetric APS without seeming to interfere with responses against pathogens [10].
Another important pathway modulated by HCQ is the pathway of Toll-like receptors (TLRs). TLRs in endosomes transmit signals that are regulated by pH. Among these TLRs are the TLR-4, -7, -8, and -9. In APS, TLRs are activated in the absence of pathogens and can lead to the production of pro-inflammatory cytokines and aPL [47]. Blocking in endosomal acidification by CQ inhibits TLR-3 and TLR-4 signal by attenuating the adapter-dependent signaling pathway that contains the Toll/IL-1 receptor domain (TIR), interferon-inducing and independently of myeloid differentiation primary response gene 88 (MyD88) [48–50]. Moreover, CQ decreases the activation of interferon response factors, nuclear factor kappa B (NF-kB) and activator protein 1 (AP-1) in monocyte-derived macrophages [48]. These events reduce the production of proinflammatory cytokines [51]. Finally, other pathways that CQ can inhibit involve the activation of p38MAPK by stress in the endoplasmic reticulum leading to increased proinflammatory cytokines [52, 53].
HCQ increases lysosomal pH: effects in cancer
The effects of HCQ in cancer have been mainly related to the inhibition of autophagy and proliferation of cancer cells and induction of apoptosis [54, 55]. Autophagy is related to cell protection as a survival mechanism for tumor cells [56]. Preclinical studies indicate that combining HCQ with chemotherapy or radiotherapy can contribute to higher response rates of treatments against different types of cancer, even in advanced disease stages [57, 58]. For instance, HCQ and CQ reduced the proliferation of cells from human bladder cancer in a dose and time-dependent manner [54]. Besides, in metastatic kidney cancer cells, HCQ inhibited cell growth, oxygen consumption and mitochondrial respiration [59].
HCQ effects in autoimmune diseases
In 1894, quinine was first used for the treatment of discoid lupus [6]. In this context, CQ was observed to interfere with phagocytosis due to increased lysosomal pH, causing an interruption in the presentation of self-antigens both in vitro and in vivo [60, 61]. The lysosomes contain acidic hydrolases that are active at a pH of 5 and are necessary for the degradation of proteins, nucleic acids, lipids, and carbohydrates [62]. For digestion of captured components after phagocytosis, a pH of 4.8 is required in these acidic organelles in contrast to the cytosol, which has a pH of 7.2 [62]. Interestingly, in an experimental autoimmune model of multiple sclerosis, inhibition of lysosomal proteases caused a reduction in the proinflammatory response and autoantigens presentation [63].
On the other hand, lysosomal pH is decreased in the immune response as it is required in antigenic processing, but the addition of compounds with tropism to lysosomes, such as CQ, inhibit catabolism and antigenic presentation to T lymphocytes by mouse macrophages [60]. HCQ modulates pH in lysosomes, which could alter the processing and presentation of self-antigens and, consequently, activation of macrophages and T lymphocytes in APS. Furthermore, HCQ modulates the activation of T lymphocytes by inhibiting calcium mobilization in the endoplasmic reticulum [64].
The efficacy of HCQ in the treatment of RA has been reported in randomized controlled clinical trials, in which it has been used in combination with methotrexate and sulfasalazine and is considered one of the safest and best-tolerated drugs licensed for the treatment of RA [65]. Furthermore, the efficacy of HCQ has especially been recognized in treating SLE patients both with and without secondary APS. [66]. Nowadays, HCQ is recommended for all SLE patients, especially during pregnancy and breastfeeding, where it is safe and effective in preventing disease flares and adverse pregnancy outcomes [67, 68]. HCQ use has been described in vascular and obstetric APS, especially in patients with SLE-related secondary APS. Nevertheless, its therapeutic potential has been linked to obstetric APS due to inflammation during placentation [14].
In summary, the modulating effects of HCQ in several conditions have been associated with increased pH in different cellular compartments such as endoplasmic reticulum, endosomes and lysosomes reducing TLR activation, increasing apoptosis of cancer cells and decreasing pro-inflammatory cytokines which leads to positive effects in diabetes, dyslipidemias, cancer, viral infections, and autoimmune diseases such as APS, SLE and RA. Underlying mechanisms of HCQ involved in these processes are described in Fig. 1. In the remaining part of this review, we will summarize published studies describing these and other modulating effects of HCQ in APS, especially in refractory obstetric APS.
Fig. 1.
HCQ effects in different diseases mediated by pH modifications. HCQ induces beneficial effects in cancer, dyslipidemia, viral infections, diabetes and autoimmune diseases through pH modifications. Abbreviations. TLRs, toll-like receptors. This figure was created with BioRender.com
HCQ possible modulating effects in obstetric APS
APS-related pregnancy morbidity is associated with the presence of aPL that induces complement activation, cytokine production and placental dysfunction [69, 70]. Therefore, obstetric APS is mainly characterized by a proinflammatory state during placentation related to reduced trophoblast invasion and impaired spiral artery remodeling at early pregnancy stages [10]. Moreover, aß2GP1 antibodies have been identified as key pathogenic factors due to their effects on the maternal–fetal interface [70]. In APS, HCQ can modulate pH by similar pathways as described above [71, 72]. Furthermore, other mechanisms are described in patients with APS, as HCQ inhibits complement activation, reduces production of tissue factor (TF), disintegrates binding of aß2GP1 to ß2GP1 and complexes of phospholipid bilayers, inhibits production of tumor necrosis factor α (TNFα) in monocytes, restores anticoagulant action of annexin A5, reduces trophoblast alteration, fetal death, placental insufficiency, thrombosis, endothelial dysfunction and decreased LA levels (Table 1). Furthermore, our Reproduction research group found that both aPL from primary and secondary refractory APS patients induced endothelial dysfunction in vitro, and HCQ restored endothelial function in cells treated with aPL from women with refractory primary obstetric APS [73].
Table 1.
In vitro and in vivo effects of HCQ in antiphospholipid syndrome
| APS classification | Clinical manifestation | Source and dose of aPL | Number of patients | Study model | HCQ dosage | Time of exposition | Mechanism of modulation | References |
|---|---|---|---|---|---|---|---|---|
| Secondary APS | PM and VT | LA, aCL and aß2GP1 (100 μg/ml) | 20 (14 women and 6 men) | In vitro (monocytes) | 1 μM | 6 h and 15 m | Inhibition of TNFα production (without modulating effects of some aß2GP1) | [87] |
| Not specified | PM and VT | Monoclonal antibody (0.1 mg/ml) and aPL-NS (0.5 mg/ml) | 3 (gender not specified) | In vitro (monocytes and phospholipid bilayer) | > 1 μg/ml | 0–4 h | Reduction of aPL/PL/ß2GP1 complex formation | [76] |
| Catastrophic APS | PM and VT | LA, aCL and aβGP1 (0.5 mg/ml) | 3 (2 women and 1 men) | In vitro (endothelium, trophoblast, and phospholipid bilayer) | 1 μg/ml and 1 mg/ml | 0–4 h | Restoration of the anticoagulant action of Annexin A5 | [88] |
| NA | NA | Monoclonal antibody aß2GP1 (20 μg/ml) | NA (antibodies produced in mice) | In vitro (trophoblast) | 1 μg/ml | 72 h | Reversal of IL-6 inhibition and partial modulation of impaired migration. It does not modulate the secretion of proinflammatory cytokines such as IL-8 and IL-1β | [89] |
| Not specified | PM and VT | LA, aCL and aß2GP1 (4.2 mg/mouse) | 5 (women) | In vitro (syncytiotrophoblast) | 0.1, 0.2, 0.5 and 1 μg/ml−1 | 48 h | Restoration of trophoblastic fusion by a decrease of TLR-4 | [90] |
| Primary APS | VT | Monoclonal antibody aß2GP1 (0.1 mg/ml) | 1 woman | In vitro (phospholipid vesicles) | 0,01- 0,5 mg/ml | 0–250 min | Reduction of aPL/PL/ß2GP1 complex formation | [91] |
| Refractory primary/secondary, and non-refractory APS (thrombotic or obstetric) | PM and VT | LA, aCL and aß2GP1 | 21 women | In vitro (endothelium) | 1 μg/ml | 1 h | Partial restoration of endothelial function altered by aPL from patients with refractory primary obstetric APS | [73] |
| Primary APS | PM and VT | aß2GP1 (100 μg/mouse) | 7 (5 women and 2 men) | In vivo (C57BL/6 mice) | In vivo (200 μg/mouse) | Insertion with a micro-osmotic pump on day 8 of gestation | Prevention of fetal death, placental insufficiency, cortical development abnormality, decreases C5a levels, and C´ activation. It does not inhibit the binding of aPL to brain tissue but impede their pathological effect | [69] |
| Ex vivo (mouse tissue) | Ex vivo (20–50 and 100 ng/ml) | |||||||
| Primary APS | Not specified | aCL and aß2GP1 | 6 (gender not specified) | In vivo (C57BL/6 mice) | In vivo 12 μg/g/day | 7 days | Reduction of thrombosis by decreased TF and modulation of endothelial dysfunction and p-eNOS expression | [92] |
| Ex vivo (mouse tissue mesenteric arteries) | Ex vivo or in vitro 1 and 10 mg/ml | |||||||
| In vitro (human aortic endothelial cells) | ||||||||
| Not specified | PM and VT | LA, aCL and aß2GP1 | 22 (20 women and 2 men) | Prospective observational study | 200 mg/day | 3 months | Reduction of TF levels. It did not affect Annexin A5 activity, on aß2GP1, thromboelastography, on CRP, C´, and VEGF levels | [93] |
| Primary APS | PM and VT | LA, aCL and aß2GP1 | 114 (100 women and 14 men) | Retrospective study | Not specified | 12 months | Reduction of aCL, aß2GP1, and thrombotic events | [94] |
APS antiphospholipid syndrome, NS not specified, NA not applicable, PM pregnancy morbidity, VT vascular thrombosis, LA lupus anticoagulant, aCL anti-cardiolipin antibodies, aß2GP1 anti-ß2 glycoprotein 1 antibodies, μM micromolar, μg micrograms, ml milliliters, mg milligrams, ng nanogram, g grams, h hours, m minutes, TNFα tumor necrosis factor alpha, PL phospholipids, ß2GP1 ß2 glycoprotein 1, IL interleukin, C´ complement, TLR-4 Toll-like receptor 4, TF tissue factor, p-eNOS phosphorylated endothelial nitric oxide synthase, CRP C-reactive protein, VEGF vascular endothelial growth factor
HCQ also plays a role in the disintegration of autoimmune complexes, since, in the antigen–antibody reaction, the effect of pH on the equilibrium constant is characterized by a curve between pH 6.5 and 8.4 [74]. At different values, the equilibrium constant is 100 times lower, since these extreme conditions generate conformational changes in the antibody that alter its complementarity with the antigen [74]. Depending on the type of antibody, it will have a specific pH at which it can bind to the antigen in different intracellular compartments [75]. Interestingly, HCQ could avoid binding of aPL to the plasma membrane or cause breakdown of aPL complexes, but the pH values at which these aPL bind to antigens are unknown [76, 77]. The disintegration of the complexes by HCQ prevents the different cascades triggered by the aPL such as the decrease of AnxA5, complement activation, decrease of endothelial nitric oxide synthase, TF production, thrombosis, and placental dysfunction. Furthermore, HCQ inhibits translocation of the catalytic subunit of NADPH oxidase 2 (NOX2) from the cell membrane to the endosome, consequently blocking the production of endosomal superoxide mediated by aPL [78]. Finally, HCQ effects on TLR could inhibit pro-inflammatory responses following aPL and ß2GP1 interaction in the trophoblast [79, 80].
Clinical effects of HCQ in obstetric APS
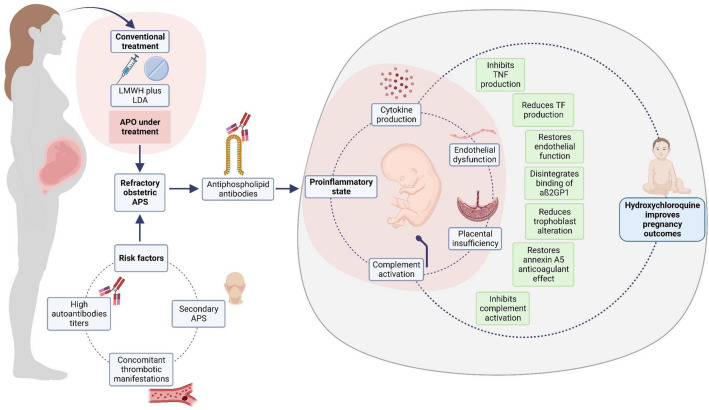
The conventional treatment of obstetric complications in APS patients is based on the daily use of low-dose aspirin (75–100 mg) and low molecular weight heparin (LMWH) [14]. Nevertheless, patients with refractory obstetric APS do not respond to this conventional therapy. These patients most often have higher aPL titers, concomitant clinical manifestations of thrombosis, and other autoimmune diseases [81].
Five retrospective studies showed an improvement in live birth rate in women with persistent aPL positivity when HCQ was added to conventional treatment regimens [82]. In 87 patients with refractory primary obstetric APS, women treated with 60 mg of enoxaparin, low-dose aspirin and 400 mg of HCQ had a live birth rate of 97.1% (67/69) compared with 62% (20/30) in those who did not receive HCQ. Moreover, a low prevalence of other pregnancy complications in APS patients treated with HCQ was reported [83]. Interestingly, in a cohort of aPL-positive women, pregnancy failures occurred in 30.9% under conventional treatment with low-dose aspirin and LMWH. A reduction of pregnancy failures to 22% was observed in the subsequent pregnancies of those refractory patients after adding HCQ [84]. Hooper et al. described an increase in the achievement of live births and better maternal and neonatal outcomes after adding HCQ in patients with refractory obstetric APS [15].
In different reports, HCQ modulated pathological effects generated by aPL in men and women with primary APS, secondary APS, catastrophic APS, and clinical manifestations of thrombosis and pregnancy morbidity. The beneficial effects of HCQ and their underlying mechanisms in refractory obstetric APS are summarized in Fig. 2. Some results are controversial between studies and, lack of stratification between different aPL and clinical manifestations has made it difficult to understand the exact mechanism of action of HCQ in obstetric APS. It is possible that, according to these characteristics, different doses, and periods of exposure to the drug are required. Furthermore, the need to start treatment with HCQ in the preconception period rather than the first trimester and pharmacokinetic changes due to pregnancy should be addressed in the treatment schemes for APS patients.
Fig. 2.
HCQ benefic effects in refractory obstetric APS. HCQ improves pregnancy outcomes in patients with refractory obstetric APS by restoring endothelial function, decreasing complement and cytokine production, and preventing placental damage. LMWH low molecular weight heparin, LDA low-dose aspirin, APO adverse pregnancy outcomes, APS antiphospholipid syndrome, TNF tumor necrosis factor, TF tissue factor, aß2GP1 anti-ß2 glycoprotein 1 antibodies. This figure was created with BioRender.com
These results suggested that HCQ might be used in patients with refractory obstetric APS, and we encourage its implementation in clinical practice. However, available data mainly come from small retrospective studies; hence, future prospective studies and randomized clinical trials with a larger population of patients, including primary and secondary APS, are required to demonstrate which patients would benefit most and set the best suitable treatment scheme. Currently, efforts assessing the HCQ use in patients with obstetric APS occur through the HYPATIA and HIBISCUS clinical trials [85, 86]. Finally, the effects of usually added treatments in obstetric refractory APS such as glucocorticoids and intravenous immunoglobulin should be addressed in future studies.
Conclusion
In this review, we described distinct positive effects of HCQ in different pathologies as well as in obstetric APS. These results suggest that HCQ should be a highly valuable addition to the treatment of refractory obstetric APS. Although most of the in vitro information is based on studies conducted with CQ, HCQ is the more favorable alternative due to lower risks of adverse effects, lower dosage requirements, and the greater identified effectiveness in some essays. Nevertheless, HCQ-related effects in different subgroups of APS patients remain unclear. Thus, we encourage a detailed classification of APS subgroups in further studies.
Acknowledgements
The authors acknowledge BioRender.com as the software used for figure-making.
Author contributions
Conceptualization: JJF, MV. Methodology: JJF, MV, AO. Writing: original draft: JJF, MV. Writing—review and editing: SH, KdL, AC. All authors have read and agreed to the final version of the manuscript. All authors take full responsibility for the integrity and accuracy of all aspects of the work.
Funding
This work was supported by Ministerio de Ciencia, Tecnología e Innovación (Minciencias, Colombia), Grant 111580762949. JJF is a recipient of a doctoral scholarship from MinCiencias (906-2021). MV was a recipient of a doctoral scholarship from MinCiencias (757-2016). The funders had no role in study design, data interpretation or writing the report.
Declarations
Conflict of interest
All authors declare that they have no competing interests.
Declarations
Not applicable.
Disclaimer
No part of the review, including its graphics, are copied or published elsewhere in whole or in part.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kalia S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther. 2007;20(4):160–174. doi: 10.1111/j.1529-8019.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim HS, Im JS, Cho JY, Bae KS, Klein TA, Yeom JS, Kim TS, Choi JS, Jang IJ, Park JW. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother. 2009;53(4):1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabourel JD. Effects of hydroxychloroquine on the growth of mammalian cells in vitro. J Pharmacol Exp Ther. 1963;141:122–130. [PubMed] [Google Scholar]
- 4.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 5.Haladyj E, Sikora M, Felis-Giemza A, Olesinska M. Antimalarials—are they effective and safe in rheumatic diseases? Reumatologia. 2018;56(3):164–173. doi: 10.5114/reum.2018.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CD, Cyr M. The history of lupus erythematosus. From hippocrates to osler. Rheum Dis Clin North Am. 1988;14(1):1–14. doi: 10.1016/S0889-857X(21)00942-X. [DOI] [PubMed] [Google Scholar]
- 7.Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16(3):411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 8.Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2(2):CD013587. doi: 10.1002/14651858.CD013587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 10.Meroni PL, Raschi E, Grossi C, Pregnolato F, Trespidi L, Acaia B, Borghi MO. Obstetric and vascular APS: same autoantibodies but different diseases? Lupus. 2012;21(7):708–710. doi: 10.1177/0961203312438116. [DOI] [PubMed] [Google Scholar]
- 11.Pons-Estel GJ, Andreoli L, Scanzi F, Cervera R, Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun. 2017;76:10–20. doi: 10.1016/j.jaut.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Gerde M, Ibarra E, Mac Kenzie R, Fernandez Suarez C, Heer C, Alvarez R, Iglesias M, Balparda J, Beruti E, Rubinstein F. The impact of hydroxychloroquine on obstetric outcomes in refractory obstetric antiphospholipid syndrome. Thromb Res. 2021;206:104–110. doi: 10.1016/j.thromres.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxford) 2010;49(2):281–288. doi: 10.1093/rheumatology/kep373. [DOI] [PubMed] [Google Scholar]
- 14.Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, Cuadrado MJ, Dorner T, Ferrer-Oliveras R, Hambly K, Khamashta MA, King J, Marchiori F, Meroni PL, Mosca M, Pengo V, Raio L, Ruiz-Irastorza G, Shoenfeld Y, Stojanovich L, Svenungsson E, Wahl D, Tincani A, Ward MM. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper A, Bacal V, Bedaiwy MA. Does adding hydroxychloroquine to empiric treatment improve the live birth rate in refractory obstetrical antiphospholipid syndrome? A systematic review. Am J Reprod Immunol. 2023;90(3):e13761. doi: 10.1111/aji.13761. [DOI] [PubMed] [Google Scholar]
- 16.Mekinian A, Costedoat-Chalumeau N, Masseau A, Tincani A, De Caroli S, Alijotas-Reig J, Ruffatti A, Ambrozic A, Botta A, Le Guern V, Fritsch-Stork R, Nicaise-Roland P, Carbonne B, Carbillon L, Fain O. Obstetrical APS: is there a place for hydroxychloroquine to improve the pregnancy outcome? Autoimmun Rev. 2015;14(1):23–29. doi: 10.1016/j.autrev.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Belizna C. Hydroxychloroquine as an anti-thrombotic in antiphospholipid syndrome. Autoimmun Rev. 2015;14(4):358–362. doi: 10.1016/j.autrev.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Ambati A, Knight JS, Zuo Y. Antiphospholipid syndrome management: a 2023 update and practical algorithm-based approach. Curr Opin Rheumatol. 2023;35(3):149–160. doi: 10.1097/BOR.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tett SE. Clinical pharmacokinetics of slow-acting antirheumatic drugs. Clin Pharmacokinet. 1993;25(5):392–407. doi: 10.2165/00003088-199325050-00005. [DOI] [PubMed] [Google Scholar]
- 20.Balevic SJ, Green TP, Clowse MEB, Eudy AM, Schanberg LE, Cohen-Wolkowiez M. Pharmacokinetics of hydroxychloroquine in pregnancies with rheumatic diseases. Clin Pharmacokinet. 2019;58(4):525–533. doi: 10.1007/s40262-018-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinger G, Morad Y, Westall CA, Laskin C, Spitzer KA, Koren G, Ito S, Buncic RJ. Ocular toxicity and antenatal exposure to chloroquine or hydroxychloroquine for rheumatic diseases. Lancet. 2001;358(9284):813–814. doi: 10.1016/S0140-6736(01)06004-4. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye (Lond) 2017;31(6):828–845. doi: 10.1038/eye.2016.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, Academy A, of O, Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision) Ophthalmology. 2016;123(6):1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 24.Doyno C, Sobieraj DM, Baker WL. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin Toxicol (Phila) 2021;59(1):12–23. doi: 10.1080/15563650.2020.1817479. [DOI] [PubMed] [Google Scholar]
- 25.Marquardt K, Albertson TE. Treatment of hydroxychloroquine overdose. Am J Emerg Med. 2001;19(5):420–424. doi: 10.1053/ajem.2001.25774. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Vinayagamoorthy N, Han K, Kwok SK, Ju JH, Park KS, Jung SH, Park SW, Chung YJ, Park SH. Association of polymorphisms of cytochrome P450 2D6 with blood hydroxychloroquine levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2016;68(1):184–190. doi: 10.1002/art.39402. [DOI] [PubMed] [Google Scholar]
- 27.Birru Talabi M, Clowse MEB. Antirheumatic medications in pregnancy and breastfeeding. Curr Opin Rheumatol. 2020;32(3):238–246. doi: 10.1097/BOR.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 28.Buchanan NM, Toubi E, Khamashta MA, Lima F, Kerslake S, Hughes GR. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55(7):486–488. doi: 10.1136/ard.55.7.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huybrechts KF, Bateman BT, Hernandez-Diaz S. Hydroxychloroquine early in pregnancy and risk of birth defects: absence of evidence is not the same as evidence of absence. Am J Obstet Gynecol. 2021;224(5):549–550. doi: 10.1016/j.ajog.2020.12.1219. [DOI] [PubMed] [Google Scholar]
- 30.Berard A, Sheehy O, Zhao JP, Vinet E, Quach C, Bernatsky S. Chloroquine and hydroxychloroquine use during pregnancy and the risk of adverse pregnancy outcomes using real-world evidence. Front Pharmacol. 2021;12:722511. doi: 10.3389/fphar.2021.722511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers CD, Johnson DL, Xu R, Luo Y, Felix R, Fine M, Lessard C, Adam MP, Braddock SR, Robinson LK, Burke L, Jones KL. Birth outcomes in women who have taken hydroxycholoroquine during pregnancy: a prospective cohort study. Arthritis Rheumatol. 2022;74(4):711–724. doi: 10.1002/art.42015. [DOI] [PubMed] [Google Scholar]
- 32.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/s1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drosos GC, Vedder D, Houben E, Boekel L, Atzeni F, Badreh S, Boumpas DT, Brodin N, Bruce IN, Gonzalez-Gay MA, Jacobsen S, Kerekes G, Marchiori F, Mukhtyar C, Ramos-Casals M, Sattar N, Schreiber K, Sciascia S, Svenungsson E, Szekanecz Z, Tausche AK, Tyndall A, van Halm V, Voskuyl A, Macfarlane GJ, Ward MM, Nurmohamed MT, Tektonidou MG. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. 2022;81(6):768–779. doi: 10.1136/annrheumdis-2021-221733. [DOI] [PubMed] [Google Scholar]
- 34.Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am J Med. 1994;96(3):254–259. doi: 10.1016/0002-9343(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 35.Rahman P, Gladman DD, Urowitz MB, Yuen K, Hallett D, Bruce IN. The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol. 1999;26(2):325–330. [PubMed] [Google Scholar]
- 36.Babary H, Liu X, Ayatollahi Y, Chen XP, Doo L, Uppaluru LK, Kwak MK, Kulaga C, Modjinou D, Olech E, Yoo JW. Favorable effects of hydroxychloroquine on serum low density lipid in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Int J Rheum Dis. 2018;21(1):84–92. doi: 10.1111/1756-185X.13159. [DOI] [PubMed] [Google Scholar]
- 37.Beynen AC, van der Molen AJ, Geelen MJ. Inhibition of hepatic cholesterol biosynthesis by chloroquine. Lipids. 1981;16(6):472–474. doi: 10.1007/BF02535017. [DOI] [PubMed] [Google Scholar]
- 38.Shojania K, Koehler BE, Elliott T. Hypoglycemia induced by hydroxychloroquine in a type II diabetic treated for polyarthritis. J Rheumatol. 1999;26(1):195–196. [PubMed] [Google Scholar]
- 39.Cansu DU, Korkmaz C. Hypoglycaemia induced by hydroxychloroquine in a non-diabetic patient treated for RA. Rheumatology (Oxford) 2008;47(3):378–379. doi: 10.1093/rheumatology/kem378. [DOI] [PubMed] [Google Scholar]
- 40.Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas–a randomized trial. Diabetes Res Clin Pract. 2002;55(3):209–219. doi: 10.1016/s0168-8227(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 41.Blazar BR, Whitley CB, Kitabchi AE, Tsai MY, Santiago J, White N, Stentz FB, Brown DM. In vivo chloroquine-induced inhibition of insulin degradation in a diabetic patient with severe insulin resistance. Diabetes. 1984;33(12):1133–1137. doi: 10.2337/diab.33.12.1133. [DOI] [PubMed] [Google Scholar]
- 42.Contreres JO, Faure R, Baquiran G, Bergeron JJ, Posner BI. ATP-dependent desensitization of insulin binding and tyrosine kinase activity of the insulin receptor kinase. The role of endosomal acidification. J Biol Chem. 1998;273(34):22007–22013. doi: 10.1074/jbc.273.34.22007. [DOI] [PubMed] [Google Scholar]
- 43.Naghipour S, Ghodousi M, Rahsepar S, Elyasi S. Repurposing of well-known medications as antivirals: hydroxychloroquine and chloroquine—from HIV-1 infection to COVID-19. Expert Rev Anti Infect Ther. 2020;18(11):1119–1133. doi: 10.1080/14787210.2020.1792291. [DOI] [PubMed] [Google Scholar]
- 44.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, Meraviglia P, Capetti A, Biasin M, Trabattoni D, Clerici M. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118(12):3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 45.Paton NI, Aboulhab J, Karim F. Hydroxychloroquine, hydroxycarbamide, and didanosine as economic treatment for HIV-1. Lancet. 2002;359(9318):1667–1668. doi: 10.1016/S0140-6736(02)08557-4. [DOI] [PubMed] [Google Scholar]
- 46.Chiang G, Sassaroli M, Louie M, Chen H, Stecher VJ, Sperber K. Inhibition of HIV-1 replication by hydroxychloroquine: mechanism of action and comparison with zidovudine. Clin Ther. 1996;18(6):1080–1092. doi: 10.1016/s0149-2918(96)80063-4. [DOI] [PubMed] [Google Scholar]
- 47.Cheng S, Wang H, Zhou H. The role of TLR4 on B cell activation and anti-beta2GPI antibody production in the antiphospholipid syndrome. J Immunol Res. 2016;2016:1719720. doi: 10.1155/2016/1719720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H, Kozicky L, Saferali A, Fung SY, Afacan N, Cai B, Falsafi R, Gill E, Liu M, Kollmann TR, Hancock RE, Sly LM, Turvey SE. Endosomal pH modulation by peptide-gold nanoparticle hybrids enables potent anti-inflammatory activity in phagocytic immune cells. Biomaterials. 2016;111:90–102. doi: 10.1016/j.biomaterials.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 49.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9(4):361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutz M, Metzger J, Gellert T, Luppa P, Lipford GB, Wagner H, Bauer S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur J Immunol. 2004;34(9):2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- 51.Cervera R. Estrategias terapéuticas en el síndrome antifosfolipídico. Reumatol Clin. 2010;6(1):37–42. doi: 10.1016/j.reuma.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res. 2008;77(2):150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simoncini S, Sapet C, Camoin-Jau L, Bardin N, Harle JR, Sampol J, Dignat-George F, Anfosso F. Role of reactive oxygen species and p38 MAPK in the induction of the pro-adhesive endothelial state mediated by IgG from patients with anti-phospholipid syndrome. Int Immunol. 2005;17(4):489–500. doi: 10.1093/intimm/dxh229. [DOI] [PubMed] [Google Scholar]
- 54.Lin YC, Lin JF, Wen SI, Yang SC, Tsai TF, Chen HE, Chou KY, Hwang TI. Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis. Kaohsiung J Med Sci. 2017;33(5):215–223. doi: 10.1016/j.kjms.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer. 2018;124(16):3307–3318. doi: 10.1002/cncr.31335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y, Eom JI, Jeung HK, Jang JE, Kim JS, Cheong JW, Kim YS, Min YH. Induction of cytosine arabinoside-resistant human myeloid leukemia cell death through autophagy regulation by hydroxychloroquine. Biomed Pharmacother. 2015;73:87–96. doi: 10.1016/j.biopha.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Patel S, Hurez V, Nawrocki ST, Goros M, Michalek J, Sarantopoulos J, Curiel T, Mahalingam D. Vorinostat and hydroxychloroquine improve immunity and inhibit autophagy in metastatic colorectal cancer. Oncotarget. 2016;7(37):59087–59097. doi: 10.18632/oncotarget.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HO, Mustafa A, Hudes GR, Kruger WD. Hydroxychloroquine destabilizes phospho-S6 in human renal carcinoma cells. PLoS ONE. 2015;10(7):e0131464. doi: 10.1371/journal.pone.0131464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu M, Wu X, Lin B, Han J, Yang L, Han S. Lysosomal pH decrease in inflammatory cells used to enable activatable imaging of inflammation with a sialic acid conjugated profluorophore. Anal Chem. 2015;87(13):6688–6695. doi: 10.1021/acs.analchem.5b00847. [DOI] [PubMed] [Google Scholar]
- 62.Takenouchi T, Sekiyama K, Tsukimoto M, Iwamaru Y, Fujita M, Sugama S, Kitani H, Hashimoto M. Role of autophagy in P2X7 receptor-mediated maturation and unconventional secretion of IL-1β in microglia. Autophagy: cancer, other pathologies, inflammation, immunity, infection, and aging. Amsterdam: Elsevier; 2015. pp. 211–222. [Google Scholar]
- 63.Fissolo N, Kraus M, Reich M, Ayturan M, Overkleeft H, Driessen C, Weissert R. Dual inhibition of proteasomal and lysosomal proteolysis ameliorates autoimmune central nervous system inflammation. Eur J Immunol. 2008;38(9):2401–2411. doi: 10.1002/eji.200838413. [DOI] [PubMed] [Google Scholar]
- 64.Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. 2000;95(11):3460–3466. doi: 10.1182/blood.V95.11.3460. [DOI] [PubMed] [Google Scholar]
- 65.O'Dell JR, Leff R, Paulsen G, Haire C, Mallek J, Eckhoff PJ, Fernandez A, Blakely K, Wees S, Stoner J, Hadley S, Felt J, Palmer W, Waytz P, Churchill M, Klassen L, Moore G. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(5):1164–1170. doi: 10.1002/art.10228. [DOI] [PubMed] [Google Scholar]
- 66.Pons-Estel GJ, Alarcon GS, McGwin G, Jr, Danila MI, Zhang J, Bastian HM, Reveille JD, Vila LM. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009;61(6):830–839. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, Houssiau F, Jayne D, Kouloumas M, Kuhn A, Larsen JL, Lerstrom K, Moroni G, Mosca M, Schneider M, Smolen JS, Svenungsson E, Tesar V, Tincani A, Troldborg A, van Vollenhoven R, Wenzel J, Bertsias G, Boumpas DT. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 68.Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, Doria A, Fischer-Betz R, Forger F, Moraes-Fontes MF, Khamashta M, King J, Lojacono A, Marchiori F, Meroni PL, Mosca M, Motta M, Ostensen M, Pamfil C, Raio L, Schneider M, Svenungsson E, Tektonidou M, Yavuz S, Boumpas D, Tincani A. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. 2017;76(3):476–485. doi: 10.1136/annrheumdis-2016-209770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertolaccini ML, Contento G, Lennen R, Sanna G, Blower PJ, Ma MT, Sunassee K, Girardi G. Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J Autoimmun. 2016 doi: 10.1016/j.jaut.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fierro JJ, Velasquez M, Cadavid AP, de Leeuw K. Effects of anti-beta 2-glycoprotein 1 antibodies and its association with pregnancy-related morbidity in antiphospholipid syndrome. Am J Reprod Immunol. 2022;87(1):e13509. doi: 10.1111/aji.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meroni PL. Prevention and treatment of obstetrical complications in APS: is hydroxychloroquine the Holy Grail we are looking for? J Autoimmun. 2016 doi: 10.1016/j.jaut.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 72.De Carolis S, Botta A, Salvi S, di Pasquo E, Del Sordo G, Garufi C, Lanzone A, De Carolis MP. Is there any role for the hydroxychloroquine (HCQ) in refractory obstetrical antiphospholipid syndrome (APS) treatment? Autoimmun Rev. 2015;14(9):760–762. doi: 10.1016/j.autrev.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Velásquez M, Peláez LF, Rojas M, Narváez-Sánchez R, Velásquez JA, Escudero C, San Martín S, Cadavid ÁP. Differences in endothelial activation and dysfunction induced by antiphospholipid antibodies among groups of patients with thrombotic, refractory, and non-refractory antiphospholipid syndrome. Front Physiol. 2021;12:764702–764702. doi: 10.3389/fphys.2021.764702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reverberi R, Reverberi L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007;5(4):227–240. doi: 10.2450/2007.0047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Igawa T, Mimoto F, Hattori K. pH-dependent antigen-binding antibodies as a novel therapeutic modality. Biochim Biophys Acta. 2014;1844(11):1943–1950. doi: 10.1016/j.bbapap.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Rand JH, Wu XX, Quinn AS, Chen PP, Hathcock JJ, Taatjes DJ. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood. 2008;112(5):1687–1695. doi: 10.1182/blood-2008-03-144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lackner KJ, Manukyan D, Muller-Calleja N. Endosomal redox signaling in the antiphospholipid syndrome. Curr Rheumatol Rep. 2017;19(4):20. doi: 10.1007/s11926-017-0647-7. [DOI] [PubMed] [Google Scholar]
- 78.Müller-Calleja N, Manukyan D, Canisius A, Strand D, Lackner KJ. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann Rheum Dis. 2017;76(5):891–897. doi: 10.1136/annrheumdis-2016-210012. [DOI] [PubMed] [Google Scholar]
- 79.Mulla MJ, Brosens JJ, Chamley LW, Giles I, Pericleous C, Rahman A, Joyce SK, Panda B, Paidas MJ, Abrahams VM. Antiphospholipid antibodies induce a pro-inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. Am J Reprod Immunol. 2009;62(2):96–111. doi: 10.1111/j.1600-0897.2009.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poulton K, Ripoll VM, Pericleous C, Meroni PL, Gerosa M, Ioannou Y, Rahman A, Giles IP. Purified IgG from patients with obstetric but not IgG from non-obstetric antiphospholipid syndrome inhibit trophoblast invasion. Am J Reprod Immunol. 2015;73(5):390–401. doi: 10.1111/aji.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sciascia S, Hunt BJ, Talavera-Garcia E, Lliso G, Khamashta MA, Cuadrado MJ. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol. 2016;214(2):273.e1–273.e8. doi: 10.1016/j.ajog.2015.09.078. [DOI] [PubMed] [Google Scholar]
- 82.Yuan T, Xu J, Chen D, Yang C, Peng B. The additional use of hydroxychloroquine can improve the live birth rate in pregnant women with persistent positive antiphospholipid antibodies: a systematic review and meta-analysis. J Gynecol Obstet Hum Reprod. 2021;50(8):102121. doi: 10.1016/j.jogoh.2021.102121. [DOI] [PubMed] [Google Scholar]
- 83.Gerde M, Ibarra E, Mac Kenzie R, Suarez CF, Heer C, Alvarez R, Iglesias M, Balparda J, Beruti E, Rubinstein F. The impact of hydroxychloroquine on obstetric outcomes in refractory obstetric antiphospholipid syndrome. Thromb Res. 2021;206:104–110. doi: 10.1016/j.thromres.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Khizroeva J, Bitsadze V, Tincani A, Makatsariya A, Arslanbekova M, Babaeva N, Tsibizova V, Shkoda A, Makatsariya N, Tretyakova M. Hydroxychloroquine in obstetric antiphospholipid syndrome: rationale and results of an observational study of refractory cases. J Matern Fetal Neonatal Med. 2021;35(25):6157–6164. doi: 10.1080/14767058.2021.1908992. [DOI] [PubMed] [Google Scholar]
- 85.Schreiber K, Breen K, Cohen H, Jacobsen S, Middeldorp S, Pavord S, Regan L, Roccatello D, Robinson SE, Sciascia S, Seed PT, Watkins L, Hunt BJ. HYdroxychloroquine to Improve Pregnancy Outcome in Women with AnTIphospholipid Antibodies (HYPATIA) Protocol: a multinational randomized controlled trial of hydroxychloroquine versus placebo in addition to standard treatment in pregnant women with antiphospholipid syndrome or antibodies. Semin Thromb Hemost. 2017;43(6):562–571. doi: 10.1055/s-0037-1603359. [DOI] [PubMed] [Google Scholar]
- 86.Belizna C, Pregnolato F, Abad S, Alijotas-Reig J, Amital H, Amoura Z, Andreoli L, Andres E, Aouba A, Apras Bilgen S, Arnaud L, Bienvenu B, Bitsadze V, Blanco P, Blank M, Borghi MO, Caligaro A, Candrea E, Canti V, Chiche L, Chretien JM, Cohen Tervaert JW, Damian L, Delross T, Dernis E, Devreese K, Djokovic A, Esteve-Valverde E, Favaro M, Fassot C, Ferrer-Oliveras R, Godon A, Hamidou M, Hasan M, Henrion D, Imbert B, Jeandel PY, Jeannin P, Jego P, Jourde-Chiche N, Khizroeva J, Lambotte O, Landron C, Latino JO, Lazaro E, de Leeuw K, Le Gallou T, Kilic L, Limper M, Loufrani L, Lubin R, Magy-Bertrand N, Mahe G, Makatsariya A, Martin T, Muchardt C, Nagy G, Omarjee L, Van Paasen P, Pernod G, Perrinet F, Pires Rosa G, Pistorius MA, Ruffatti A, Said F, Saulnier P, Sene D, Sentilhes L, Shovman O, Sibilia J, Sinescu C, Stanisavljevic N, Stojanovich L, Tam LS, Tincani A, Tollis F, Udry S, Ungeheuer MN, Versini M, Cervera R, Meroni PL. HIBISCUS: Hydroxychloroquine for the secondary prevention of thrombotic and obstetrical events in primary antiphospholipid syndrome. Autoimmun Rev. 2018;17(12):1153–1168. doi: 10.1016/j.autrev.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Müller-Calleja N, Hollerbach A, Häuser F, Canisius A, Orning C, Lackner KJ. Antiphospholipid antibody-induced cellular responses depend on epitope specificity: implications for treatment of antiphospholipid syndrome. J Thromb Haemost. 2017;15(12):2367–2376. doi: 10.1111/jth.13865. [DOI] [PubMed] [Google Scholar]
- 88.Rand JH, Wu XX, Quinn AS, Ashton AW, Chen PP, Hathcock JJ, Andree HA, Taatjes DJ. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood. 2010;115(11):2292–2299. doi: 10.1182/blood-2009-04-213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albert CR, Schlesinger WJ, Viall CA, Mulla MJ, Brosens JJ, Chamley LW, Abrahams VM. Effect of hydroxychloroquine on antiphospholipid antibody-induced changes in first trimester trophoblast function. Am J Reprod Immunol. 2014;71(2):154–164. doi: 10.1111/aji.12184. [DOI] [PubMed] [Google Scholar]
- 90.Marchetti T, Ruffatti A, Wuillemin C, de Moerloose P, Cohen M. Hydroxychloroquine restores trophoblast fusion affected by antiphospholipid antibodies. J Thromb Haemost. 2014;12(6):910–920. doi: 10.1111/jth.12570. [DOI] [PubMed] [Google Scholar]
- 91.Bezati E, Wu XX, Quinn AS, Taatjes DJ, Rand JH. A new trick for an ancient drug: quinine dissociates antiphospholipid immune complexes. Lupus. 2015;24(1):32–41. doi: 10.1177/0961203314547792. [DOI] [PubMed] [Google Scholar]
- 92.Miranda S, Billoir P, Damian L, Thiebaut PA, Schapman D, Le Besnerais M, Jouen F, Galas L, Levesque H, Le Cam-Duchez V, Joannides R, Richard V, Benhamou Y. Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome Role of reduced inflammation and endothelial dysfunction. PLoS ONE. 2019;14(3):e0212614. doi: 10.1371/journal.pone.0212614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schreiber K, Breen K, Parmar K, Rand JH, Wu XX, Hunt BJ. The effect of hydroxychloroquine on haemostasis, complement, inflammation and angiogenesis in patients with antiphospholipid antibodies. Rheumatology (Oxford) 2018;57(1):120–124. doi: 10.1093/rheumatology/kex378. [DOI] [PubMed] [Google Scholar]
- 94.Nuri E, Taraborelli M, Andreoli L, Tonello M, Gerosa M, Calligaro A, Argolini LM, Kumar R, Pengo V, Meroni PL, Ruffatti A, Tincani A. Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. Immunol Res. 2017;65(1):17–24. doi: 10.1007/s12026-016-8812-z. [DOI] [PubMed] [Google Scholar]