Abstract
We previously reported important differences in resistance to Taenia crassiceps murine cysticercosis between BALB/c substrains. It was suggested that resistance might correlate with expression of the nonclassic class I major histocompatibility complex (MHC) Qa-2 antigen; BALB/cAnN is Qa-2 negative and highly susceptible to T. crassiceps, whereas BALB/cJ expresses Qa-2 and is highly resistant. In this study, we investigated the role of Qa-2 in mediating resistance to cysticercosis by linkage analysis and infection of Qa-2 transgenic mice. In BALB/cAnN × (C57BL/6J × BALB/cAnN)F1 and BALB/cAnN × (BALB/cJ × BALB/cAnN)F1 backcrosses, the expression of Qa-2 antigen correlated with resistance to cysticercosis. Significantly fewer parasites were recovered from infected Qa-2 transgenic male and female mice than from nontransgenic mice of similar genetic background. These results clearly demonstrate that the Qa-2 MHC antigen is involved in resistance to T. crassiceps cysticercosis.
Taenia solium cysticercosis is a parasitic disease that seriously affects human health (24) and causes important economic losses in pig farming of developing countries (1) where conditions that favor parasite transmission persist. The essential role of pigs as an obligatory intermediate host in the parasite life cycle offers the opportunity to interfere with transmission by inducing acquired immunity through vaccination (10, 13, 18), by decreasing susceptibility through genetic manipulation (14), or both. Systematic exploration of the role of genetic factors in cysticercosis and the identification of protective immunogens are hampered by the high costs and slow data retrieval involved in studies with pigs. However, another cestode, Taenia crassiceps, that naturally infects rodents (3) is highly suitable for experimentation. It shows extensive antigenic cross-reactivity and cross-protective immunity with T. solium (7, 21); the antigenic similarity is such that T. crassiceps antigens can be used for immunodiagnosis of human cysticercosis (9). Furthermore, T. crassiceps and T. solium both have a typical two-host taeniid life cycle and morphologically and structurally related larval stages. Since T. crassiceps can reproduce asexually, experimental infection is readily attained by injecting the cysticerci in the peritoneal cavity of the mouse (3). Thus, T. crassiceps murine cysticercosis has been shown to be a useful experimental model of metacestode infection in the study of genetic factors involved in host resistance (2, 20) and underlying immunological mechanisms (19, 23, 25).
Initial findings showed that genes linked to H-2 affect T. crassiceps growth in mice (20). Thus, significant differences in the extent of the parasitosis were found between mice carrying the H-2d (BALB/cAnN and DBA/2) haplotype, which were the most susceptible, and mice with H-2b (BALB/B, C57BL/6J, and C57BL/10J) or H-2k (BALB/K, C3H/HeJ, and C3H/FeJ) haplotype, which were comparatively resistant. Further studies (2) showed low susceptibility of congenic and recombinant B10 mice, regardless of H-2 haplotype, indicating that genes in C57BL background confer resistance to the parasitosis such that they override the effect of H-2. The effect of genes outside H-2 on the control of parasite growth was also revealed by the differential susceptibility of three H-2d BALB/c substrains, of which BALB/cAnN was highly susceptible, whereas BALB/cJ was highly resistant and BALB/cByJ displayed and intermediate degree of susceptibility (2). BALB/cAnN and BALB/cJ, which are genetically quite similar strains, differ in several phenotypes, including the expression of the Qa-2 antigen (11, 16). This antigen is a nonclassical class I major histocompatibility complex (MHC) molecule encoded by four genes (Q6 to Q9) located telomeric to the H-2D loci (11, 22). BALB/cJ (Qa-2low), a Qa-2 expressor substrain, has only active Q6 and Q7 genes because Q8 and Q9 have fused, resulting in an inactive Q8/Q9d gene (11, 15). In BALB/cAnN (Qa-2null), an additional deletion of genomic DNA has occurred between the Q6 and Q7 genes, leading to their inactivation and accounting for the Qa-2 null expression (11). We proposed previously that differences in susceptibility to T. crassiceps observed between BALB/cJ and BALB/cAnN might be related to Qa-2 antigen expression (2). Here we describe that results of genetic linkage studies are entirely consistent with this hypothesis. Furthermore, a role of Qa-2 in mediating resistance to T. crassiceps was directly established by the diminution of parasite loads in infected Qa-2 transgenic mice.
MATERIALS AND METHODS
Mice.
C57BL/6J, BALB/cAnN, and BALB/cJ strains were obtained from our animal facility. Original stocks were from the Jackson Laboratory (Bar Harbor, Maine), Michael Bevan (Seattle University), and the National Institute for Medical Research, Animal Centre, London, England, respectively. BALB/cAnN × (C57BL/6J × BALB/cAnN)F1 and BALB/cAnN × (BALB/cJ × BALB/cAnN)F1 backcross male and female mice were produced in our animal facility. The experiments reported herein were conducted according to the principles set forth in reference 15a.
Parasites and infections.
T. crassiceps ORF (3) has been maintained by serial intraperitoneal passage in BALB/cAnN female mice for 10 years in our institute. Parasites for infection were harvested from the peritoneal cavities of mice, 1 to 3 months after inoculation of 10 cysticerci per mouse as described elsewhere (19, 20). For the evaluation of susceptibility to cysticercosis, mice were injected intraperitoneally with 10 small (2-mm-diameter), nonbudding T. crassiceps larvae, suspended in phosphate-buffered saline. Thirty days after infection, mice were sacrificed and T. crassiceps cysts inside the peritoneal cavity were counted as previously described (19, 20).
Production of Q9 transgenic mice.
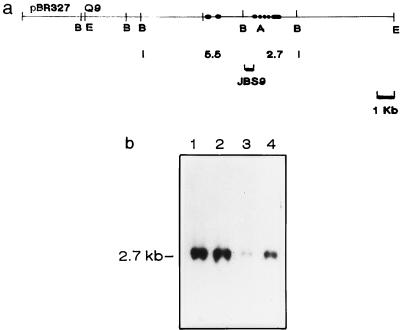
Q9 transgenic mice were derived by introducing the Q9b gene cloned from the C57BL/10 strain (27) into pronuclei of fertilized oocytes from (C57BL/6J × BALB/cAnN)F1 mice by using standard procedures (4). Transgenic mice were identified by Southern blot analysis, hybridizing a probe from the large intron of the Q9 gene (JBS9; see Fig. 2a) to BamHI-digested DNA extracted from tail biopsy samples. To evaluate the effect of Qa-2 on susceptibility to T. crassiceps, two male transgenic (C57BL/6J × BALB/cAnN)F1 founder mice (Tg1 and Tg2) were backcrossed to BALB/cAnN female mice, and their progeny were infected with T. crassiceps as described above. Expression of Qa-2 antigen was determined as described below. For controls, nontransgenic (C57BL/6J × BALB/cAnN)F1 male mice similarly backcrossed to female BALB/cAnN mice were used.
FIG. 2.
Southern blot analysis of transgenic mice produced by injection of the Q9 gene in (C57BL/6J × BALB/cAnN)F1 embryos. (A) Map of microinjected Q9 gene. Relevant restriction sites: B, BamHI; A, ApaI; E, EcoRI. The JBS9 probe is a 500-bp BamHI-SalI fragment corresponding to a segment of the large intron between exons 3 and 4 (16) which hybridizes with a 2.7-kb BamHI fragment. (B) Genomic DNA isolated from tail biopsies was digested with BamHI and hybridized with the JBS9 probe. Lane 1, DNA from a Q9 transgenic Tg1 male mouse; lane 2, DNA from a Q9 transgenic Tg2 male mouse; lane 3, DNA from a nontransgenic littermate mouse; lane 4, DNA from a C57BL6/J mouse.
Qa-2 antigen detection.
Expression of Qa-2 antigen was determined by flow cytometric analysis with a FACScan (Becton Dickinson, Palo Alto, Calif.). For genetic linkage studies, mice were classified as Qa-2 positive (Qa-2+) or null (Qa-2−). For this, peripheral blood lymphocytes from BALB/cAnN × (C57BL/6J × BALB/cAnN)F1 and BALB/cAnN × (BALB/cJ × BALB/cAnN)F1 were stained with fluorescein isothiocyanate-conjugated anti-mouse Qa-2a (Pharmingen, San Diego, Calif.). Ten thousand cells were analyzed with a lymphocyte gate. To determine the expression of Qa-2 protein in CD4+ and CD8+ T cells, two-color fluorescence-activated cell sorting (FACS) analysis of thymus, spleen, lymph nodes, and blood cells from transgenic and nontransgenic mice was performed. Cells were stained with monoclonal antibodies for Qa-2a, CD4, and CD8. Cell suspensions from 6-week-old Tg1-derived transgenic and nontransgenic mice were stained with the following monoclonal antibodies: phycoerythrin-conjugated anti-CD4, fluorescein isothiocyanate-conjugated anti-CD8, and biotin-conjugated anti-Qa-2a (all from Pharmingen) followed with streptavidin-Quantum red (Sigma Chemical Co., St. Louis, Mo.). Single-cell suspensions were prepared and stained with the antibodies, using standard procedures. Ten thousand cells were analyzed with a lymphocyte gate as defined by light scatter.
Statistical analysis.
Statistical comparisons between groups were carried out by the Mann-Whitney and Kruskal-Wallis nonparametric tests.
RESULTS AND DISCUSSION
Linkage analysis between Qa-2 expression and resistance to T. crassiceps.
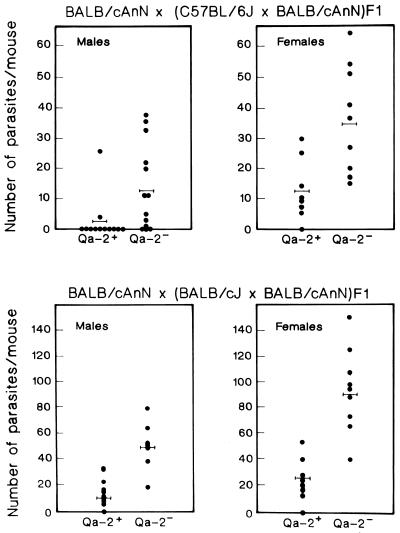
BALB/cAnN × (C57BL/6J × BALB/cAnN)F1 and BALB/cAnN × (BALB/cJ × BALB/cAnN)F1 male and female backcross mice were infected to assess the level of resistance to T. crassiceps and simultaneously classified according to their Qa-2 phenotype as Qa-2− or Qa-2+ by flow cytometric analysis. Figure 1 shows that in both backcrosses, Qa-2+ male mice were significantly more resistant to T. crassiceps cysticercosis than null male mice (P < 0.01). Similarly, Qa-2+ female backcross mice harbored fewer parasites than their Qa-2 null littermates (P < 0.01). These data strongly indicate that the presence of the Qa-2 protein correlates with resistance to cysticercosis. Males were less susceptible than females of the same Qa-2 phenotype with the exception of BALB/cAnN × (BALB/cJ × BALB/cAnN)F1 Qa-2+ mice, where females and males showed similar parasite burdens. As can also be seen in Fig. 1, BALB/cAnN × (C57BL/6J × BALB/cAnN)F1 mice had lower parasite burdens than their BALB/cAnN × (BALB/cJ × BALB/cAnN)F1 counterparts, probably due to the effect of additional C57BL/6J background genes conferring resistance to the parasitosis (2).
FIG. 1.
Association between Qa-2 expression and resistance to T. crassiceps cysticercosis in BALB/cAnN × (C57BL/6J × BALB/cAnN)F1 and BALB/cAnN × (BALB/cJ × BALB/cAnN)F1 backcross male and female mice. Mice were classified according to the expression of Qa-2 protein as determined by flow cytometric analysis. Each point corresponds to the individual number of parasites recovered from each mouse 30 days after intraperitoneal infection with 10 T. crassiceps cysticerci. The bars represent mean parasite numbers for each experimental group. The numbers of cysticerci found in Qa-2+ mice were statistically lower (P < 0.01) than those found in Qa-2 null mice.
Increased resistance of Qa-2 transgenic mice to T. crassiceps.
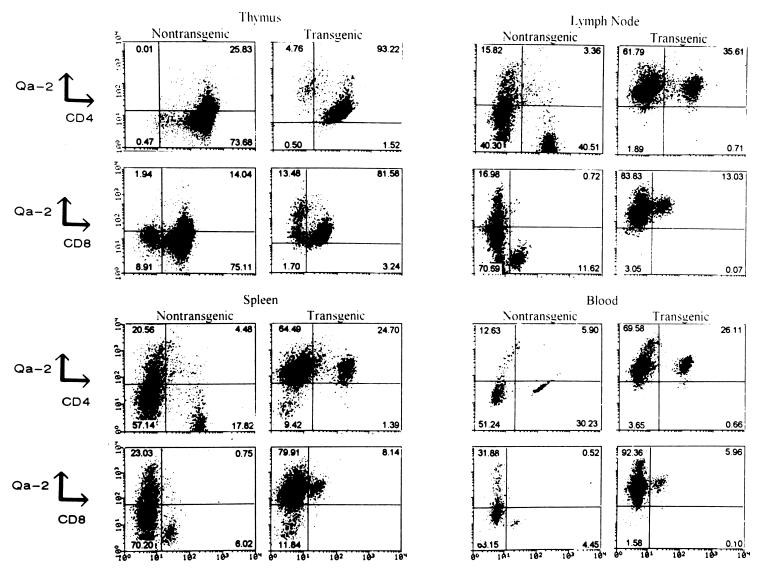
A genomic clone containing the Q9 gene (Fig. 2a) was used to generate (C57BL/6J × BALB/cAnN)F1 transgenic mice. Two male transgenic founders (Tg1 and Tg2) were genotyped by Southern analysis (Fig. 2b). A much higher intensity of the band corresponding to the Q9 gene was observed in the Tg1 and Tg2 transgenic mice compared with nontransgenic mice, indicating the presence of multiple copies of the transgene. Increased expression of Qa-2 antigen in T cells of various lymphoid tissues from Tg1- and Tg2-derived mice was found by FACS analysis; no significant changes in the relative size of CD4+ and CD8+ T-cell subpopulations could be detected (Fig. 3).
FIG. 3.
Two-color FACS analysis of thymus, spleen, lymph nodes, and blood cells from transgenic and nontransgenic mice. Cells were stained with monoclonal antibodies for Qa-2, CD4, and CD8 to identify the expression of Qa-2 protein in both T-cell types. The percentage of cells in each quadrant is indicated. Equal numbers of events are plotted for each staining.
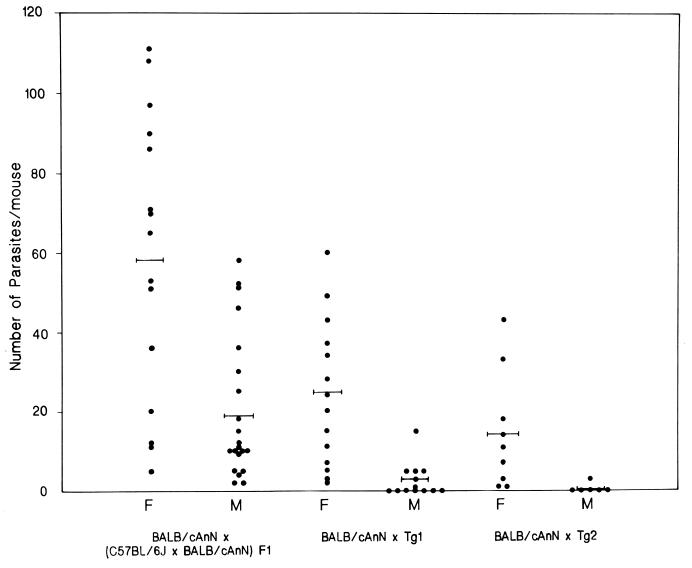
To investigate the role of Qa-2 in mediating resistance against T. crassiceps, both female and male backcross progeny of Tg1 and Tg2 founder mice were infected. As shown in Fig. 4, the number of parasites recovered in mice derived from the transgenic founders was significantly lower than that observed in similarly backcrossed nontransgenic mice (P < 0.01). The higher susceptibility of females noted before (2, 20) was maintained.
FIG. 4.
Individual numbers of parasites recovered from Qa-2 transgenic and nontransgenic mice. Cysticerci in the peritoneal cavity were counted 30 days after infection. Tg1 and Tg2 transgenic founder mice were (C57BL/6J × BALB/cAnN)F1 males. Both female (F) and male (M) progeny from transgenic and nontransgenic (C57BL/6J × BALB/cAnN)F1 male mice backcrossed to BALB/cAnN females were infected. The bars represent mean parasite numbers for each experimental group. The numbers of cysticerci found in Tg1- and Tg2-derived mice were statistically lower (P < 0.01) than those in nontransgenic mice.
The data presented here clearly demonstrate that Qa-2 is involved in resistance to T. crassiceps cysticercosis, although the mechanisms underlying the capacity of Qa-2 to control the extent of this parasitosis remain obscure. The role of Qa-2 might be immunologically mediated, since Qa-2 is a class I MHC protein, serves as a peptide receptor (5, 17), and can behave as a transplantation antigen (12). Thus, Qa-2 may be involved in presentation of cysticercal antigens to T cells, including perhaps those expressing γδ receptors or other unique subsets which may participate, probably through the production of cytokines, in the destruction of the larvae at early stages during their cycle, when they are presumably more sensitive to immune attack. It is also plausible that Qa-2 could influence resistance by mediating the selection of relevant effector T cells during intrathymic maturation or by affecting the shaping of the natural killer cell repertoire during development. Although the mechanism(s) has to be determined, our results constitute strong evidence that expression of Qa-2 in vivo is capable of modifying the course of a parasitic disease and suggest an important biological role for these nonclassical MHC molecules in immunity.
The observed gender-associated differences in susceptibility to T. crassiceps reveal that besides Qa-2, other biological factors such as hormonal environment also modulate the outcome of infection. Thus, it has been reported that 17-β-estradiol promotes whereas androgens restrict the growth of cysticerci (8). In addition, preliminary findings suggest that the differential susceptibility between females and males may be immunologically mediated (23) as has been proposed for other diseases (26).
Considering the extensive antigenic cross-reactivity and cross-immunity between T. solium and T. crassiceps cysticerci, it seems possible that T. solium cysticercosis resistance may also be associated to the expression in human and pigs of a protein similar to Qa-2. In this regard, it has been reported that a human nonclassical class I MHC gene product (HLA-G) could be a functional homolog of the mouse Qa-2 antigen (6). Our results suggest the importance of examining the expression levels of HLA-G in cells from cysticercotic and noncysticercotic individuals from an area of endemicity which could reveal genetic differences associated to human cysticercosis. The identification of this resistance gene also suggests possible practical applications in the production of transgenic pigs with increased resistance to T. solium cysticercosis by the transfer of the gene which could have additional potential benefits in pig rearing (28).
ACKNOWLEDGMENTS
We are indebted to Carlos Larralde and Michael Parkhouse for their help and many useful discussions. We thank Victor E. Gould, José Moreno, and Rose G. Mage for comments on the manuscript. We also thank Gerardo Arrellín and Georgina Díaz for help with animal care.
This work was supported in México by grant 3427N from Consejo Nacional de Ciencia y Tecnología and by grant 208395 from Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México.
REFERENCES
- 1.Aluja A. Frequency of porcine cysticercosis in Mexico. In: Flisser A, Willms K, Laclette J P, Larralde C, Ridaura C, Beltrán F, editors. Cysticercosis: present state of knowledge and perspectives. New York, N.Y: Academic Press; 1982. pp. 53–62. [Google Scholar]
- 2.Fragoso G, Lamoyi E, Mellor A, Lomelí C, Govezensky T, Sciutto E. Genetic control of susceptibility to Taenia crassiceps cysticercosis. Parasitology. 1996;112:119–124. doi: 10.1017/s003118200006515x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman R S. Studies of the biology of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda) Can J Zool. 1962;40:969–990. [Google Scholar]
- 4.Hogan B, Costantini F, Lacy E. Manipulating the mouse embryo. A laboratory manual. Cold Spring Harbor Laboratory, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 5.Joyce S, Tabaczewski P, Hogue Angeletti R, Nathenson S G, Stroynowski I. A nonpolymorphic major histocompatibility complex class Ib molecule binds a large array of diverse self-peptides. J Exp Med. 1994;179:579–588. doi: 10.1084/jem.179.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurisicova A, Casper R F, MacLusky N J, Mills G B, Librach C L. HLA-G expression during preimplantation human embryo development. Proc Natl Acad Sci USA. 1996;93:161–165. doi: 10.1073/pnas.93.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larralde C, Montoya R M, Sciutto E, Díaz M L, Govezensky T, Coltorti E. Deciphering western blots of tapeworm antigens (Taenia solium, Echinococcus granulosus, and Taenia crassiceps) reacting with sera from neurocysticercosis and hydatid disease patients. Am J Trop Med Hyg. 1989;40:282–290. doi: 10.4269/ajtmh.1989.40.282. [DOI] [PubMed] [Google Scholar]
- 8.Larralde C, Morales J, Terrazas I, Govezensky T, Romano M C. Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. J Steroid Biochem Mol Biol. 1995;52:575–580. doi: 10.1016/0960-0760(95)00062-5. [DOI] [PubMed] [Google Scholar]
- 9.Larralde C, Sotelo J, Montoya R M, Palencia G, Padilla A, Govezensky T, Diaz M L, Sciutto E. Immunodiagnosis of human cysticercosis in cerebrospinal fluid. Arch Pathol Lab Med. 1990;114:926–928. [PubMed] [Google Scholar]
- 10.Manoutcharian K, Rosas G, Hernández M, Fragoso G, Aluja A, Villalobos N, Rodarte L F, Sciutto E. Cysticercosis: identification and cloning of protective recombinant antigens. J Parasitol. 1996;82:250–254. [PubMed] [Google Scholar]
- 11.Mellor A L, Antoniou J, Robinson P J. Structure and expression of genes encoding murine Qa-2 class I antigens. Proc Natl Acad Sci USA. 1985;82:5920–5924. doi: 10.1073/pnas.82.17.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellor A L, Tomlinson P D, Antoniou J, Chandler P, Robinson P, Felstein M, Sloan J, Edwards A, O’Reilly L, Cook A, Simpson E. Expression and function of Qa-2 major histocompatibility complex class I molecules in transgenic mice. Int Immunol. 1991;3:493–502. doi: 10.1093/intimm/3.5.493. [DOI] [PubMed] [Google Scholar]
- 13.Molinari J L, Soto R, Tato P, Rodríguez D, Retana A, Sepúlveda J, Palet A. Immunization against porcine cysticercosis in an endemic area in Mexico: a field and laboratory study. Am J Trop Med Hyg. 1993;49:502–512. doi: 10.4269/ajtmh.1993.49.502. [DOI] [PubMed] [Google Scholar]
- 14.Muller M, Brem G. Disease resistance in farm animals. Experientia. 1991;47:923–934. doi: 10.1007/BF01929883. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama K, Tokito S, Pannetier C, Nakauchi H, Gachelin G. MHC gene Q8/9d of the BALB/cJ strain cannot encode a Qa-2,3 class I antigen. Immunogenetics. 1991;33:225–234. doi: 10.1007/BF00230499. [DOI] [PubMed] [Google Scholar]
- 15a.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Research Council; 1985. [Google Scholar]
- 16.Potter M. History of the BALB/c family. Curr Top Microbiol Immunol. 1985;122:1–5. doi: 10.1007/978-3-642-70740-7_1. [DOI] [PubMed] [Google Scholar]
- 17.Rotzschke O, Falk K, Stevanovic S, Grahovac B, Soloski J J, Jung G, Rammensee H G. Qa-2 molecules are peptide receptors of higher stringency than ordinary class I molecules. Nature. 1993;361:642–644. doi: 10.1038/361642a0. [DOI] [PubMed] [Google Scholar]
- 18.Sciutto E, Aluja A, Fragoso G, Rodarte L F, Hernández M, Villalobos M N, Padilla A, Keilbach N, Baca M, Govezensky T, Díaz S, Larralde C. Immunization of pigs against Taenia solium cysticercosis: factors related to effective protection. Vet Parasitol. 1995;60:53–67. doi: 10.1016/0304-4017(94)00781-7. [DOI] [PubMed] [Google Scholar]
- 19.Sciutto E, Fragoso G, Baca M, De la Cruz V, Lemus L, Lamoyi E. Depressed T-cell proliferation associated with susceptibility to experimental Taenia crassiceps infection. Infect Immun. 1995;63:2277–2281. doi: 10.1128/iai.63.6.2277-2281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sciutto E, Fragoso G, Díaz M L, Valdez F, Montoya R M, Govezensky T, Lomelí C, Larralde C. Murine Taenia crassiceps cysticercosis: H-2 complex and sex influence on susceptibility. Parasitol Res. 1991;77:243–246. doi: 10.1007/BF00930866. [DOI] [PubMed] [Google Scholar]
- 21.Sciutto E, Fragoso G, Trueba L, Lemus D, Montoya R M, Díaz M L, Govezensky T, Lomelí C, Tapia G, Larralde C. Cysticercosis vaccine: cross protecting immunity with T. solium antigens against experimental murine T. crassiceps cysticercosis. Parasite Immunol. 1990;12:687–696. doi: 10.1111/j.1365-3024.1990.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 22.Stroynowski I. Tissue-specific, peptide-binding transplantation antigens: lessons from the Qa-2 system. Immunol Rev. 1995;147:91–108. doi: 10.1111/j.1600-065x.1995.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 23.Terrazas, L. I., R. Bojalil, T. Govezensky, and C. Larralde. Shift from an early protective Th1-type immune response to a late permissive Th2-type response in murine cysticercosis (Taenia crassiceps). J. Parasitol., in press. [PubMed]
- 24.Vazquez V, Sotelo J. The course of seizures after treatment for cerebral cysticercosis. N Engl J Med. 1992;327:696–701. doi: 10.1056/NEJM199209033271005. [DOI] [PubMed] [Google Scholar]
- 25.Villa O F, Kuhn R E. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th-1 associated phenomena. Parasitology. 1996;112:561–570. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- 26.Walker W, Roberts C W, Ferguson D J, Jebbari H, Alexander J. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect Immun. 1997;65:1119–1121. doi: 10.1128/iai.65.3.1119-1121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss E H, Golden L, Fahner K, Mellor A L, Devlin J J, Bullman H, Tiddens H, Budand H, Flavell R A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984;310:650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Jin P, Mellor A L, Warner C M. Identification of the Ped gene at the molecular level: the Q9 MHC class I transgene converts the Ped slow to the Ped fast phenotype. Biol Reprod. 1994;51:695–699. doi: 10.1095/biolreprod51.4.695. [DOI] [PubMed] [Google Scholar]