Abstract
Introduction
Vosoritide is the first precision medical therapy approved to increase growth velocity in children with achondroplasia. Sharing early prescribing experiences across different regions could provide a framework for developing practical guidance for the real-world use of vosoritide.
Methods
Two meetings were held to gather insight and early experience from experts in Europe, the Middle East, and the USA. The group comprised geneticists, pediatric endocrinologists, pediatricians, and orthopedic surgeons. Current practices and considerations for vosoritide were discussed, including administration practicalities, assessments, and how to manage expectations.
Results
A crucial step in the management of achondroplasia is to determine if adequate multidisciplinary support is in place. Training for families is essential, including practical information on administration of vosoritide, and how to recognize and manage injection-site reactions. Advocated techniques include establishing a routine, empowering patients by allowing them to choose injection sites, and managing pain. Patients may discontinue vosoritide if they cannot tolerate daily injections or are invited to participate in a clinical trial. Clinicians in Europe and the Middle East emphasized the importance of assessing adherence to daily injections, as non-adherence may impact response and reimbursement. Protocols for monitoring patients receiving vosoritide may be influenced by regional differences in reimbursement and healthcare systems. Core assessments may include pubertal staging, anthropometry, radiography to confirm open physes, the review of adverse events, and discussion of concomitant or new medications—but timing of these assessments may also differ regionally and vary across institutions. Patients and families should be informed that response to vosoritide can vary in both magnitude and timing. Keeping families informed regarding vosoritide clinical trial data is encouraged.
Conclusion
The early real-world experience with vosoritide is generally positive. Sharing these insights is important to increase understanding of the practicalities of treatment with vosoritide in the clinical setting.
Keywords: Achondroplasia, Assessments, Clinical practice, Early experience, Guidance, Management, Monitoring, Practicalities, Vosoritide
Key Summary Points
| Achondroplasia is the most common form of short stature skeletal dysplasia, caused by a gain-of-function mutation in the fibroblast growth factor receptor 3 (FGFR3) gene; it is associated with multisystem complications which may occur throughout the life course. Multidisciplinary care over the entire lifespan is needed to provide optimal care for individuals with achondroplasia; until recently there have been no medical therapies available that address the underlying pathophysiology of the condition. |
| Vosoritide is a modified recombinant human C-type natriuretic peptide (CNP) analogue that leverages the CNP pathway to counteract overactive FGFR3 signaling and allow endochondral bone growth; it was approved by the European Medicines Agency and the US Food and Drug Administration in 2021 for use in children with achondroplasia who have open epiphyses and are aged ≥ 5 years in the US, and ≥ 2 years in the European Union. |
| Practical guidance on the use of vosoritide is lacking; the aim of this publication was to share early experiences of vosoritide in clinical practice to provide a framework for the approach to management. |
| Multidisciplinary team support, infrastructure, appropriate training for patients and families, as well as expectation management are all key considerations for initiating vosoritide in clinical practice; core assessments to monitor efficacy and safety may include pubertal staging, anthropometry, and X-rays to confirm open physes, the review of adverse events, and discussion of concomitant or new medications. Timing and assessments may vary by region or healthcare system. |
| Sharing of early experience by providers treating children with achondroplasia is useful in developing standards on vosoritide treatment for patients and families; international consensus guidance is needed to support the use of vosoritide in clinical practice. |
Introduction
Achondroplasia is the most common form of short stature skeletal dysplasia, with an estimated prevalence of 3.72–4.6 per 100,000 births [1, 2]. It is an autosomal dominant condition, with approximately 80% of cases occurring de novo [1]. Achondroplasia is caused by a gain-of-function mutation in the fibroblast growth factor receptor 3 (FGFR3) gene [3, 4]. FGFR3 is a regulator of linear bone growth; however, the mutations observed in the FGFR3 gene in achondroplasia exaggerate its function as an inhibitor, leading to impaired endochondral ossification [5]. This is characterized by disproportionate short stature and significant medical complications [6–9]. Both annualized growth velocity (AGV) and height z-score decrease with age in childhood [10]. As a result, final adult height for people with achondroplasia in Caucasian populations is estimated to be 118–145 cm for men and 112–136 cm for women [11], although there is slightly less variance in a more recent study, showing 123–143 cm for men and 115–134 cm for women [12, 13]. Data from The Achondroplasia Natural History Study (CLARITY), an ongoing natural history study conducted in the USA, indicated that variability in adult height could be influenced by environmental factors, genetic differences in growth potential, and a lack of data in individuals aged > 18 years, among other factors [14]. In this study, data collected across 227 people from different ethnic backgrounds indicate an average final adult height of 129.9 cm for men and 122.4 cm for women [14].
Complications of achondroplasia can occur across the lifespan and may include foramen magnum stenosis, genu varum, kyphosis, lordosis and/or scoliosis, otitis media, sleep apnea, spinal stenosis, and pain [6–9, 15]. Short stature and limb disproportion may affect or require adaptations to perform activities of daily living [15, 16], potentially affecting quality of life [17, 18]. Therefore, improving physical functionality is a key treatment goal for people with achondroplasia.
Historically, therapeutic options for increasing height in achondroplasia have been limited to surgical lengthening or growth hormone (where licensed) [19]. Surgical limb lengthening can be used to increase bone length and address disproportion of the limbs [20]; however, it is an invasive procedure and patients may experience complications [21]. Growth hormone is approved in Japan for the treatment of achondroplasia in children aged 3 years and over but its impact on height is limited [22, 23]. Moreover, neither treatment modality addresses the underlying pathophysiology of achondroplasia.
Vosoritide is a modified recombinant human C-type natriuretic peptide (CNP) analogue that leverages the CNP pathway to counteract overactive FGFR3 signaling and allow more normalized endochondral bone growth [24]. It is the first disease-specific, precision medical therapy to increase growth velocity in children with achondroplasia. In a phase 2 dose-finding trial, an increase in AGV was observed for vosoritide at doses of 15 µg/kg and 30 µg/kg up to 42 months [25]. These results were reinforced by findings from a phase 3 randomized, double-blind, placebo-controlled trial, which demonstrated a statistically significant improvement in AGV of 1.57 cm/year for children aged 5 years and older treated with 15 µg/kg vosoritide versus placebo [26]. An open-label phase 3 extension study showed that these effects are sustained over 156 weeks, with mean cumulative AGV increasing from 4.26 cm/year at baseline to 5.57 cm/year in children randomized to vosoritide [27]. In 34 children with data available at 182 weeks, AGV was maintained at 5.45 cm/year. Mean increases in AGV were also noted in children who crossed over from the placebo group, increasing from 3.77 cm/year at baseline to 5.45 cm/year at week 104; this was maintained at 5.43 cm/year at week 130 [27].
On the basis of positive results and a generally mild safety profile demonstrated in the clinical trials [25–27], the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) approved vosoritide in 2021 for use in children with achondroplasia who have open epiphyses and are aged ≥ 5 years in the USA, and ≥ 2 years in the European Union (EU). Vosoritide is also approved for use in children aged ≥ 2 years in Brazil and Australia, and from birth in Japan.
Since the approval of vosoritide, little real-world data have been published. Early data from the French Temporary Authorization for Use cohort (ATU) showed a good safety profile in 29 treated patients, consistent with that observed in the clinical trials [28]. No patients discontinued treatment and there were no reports of any missed doses [28]. Subsequent follow-up of this cohort indicates that the safety profile remains consistent with that seen in clinical trials up to 12 months, with 21 adverse events reported in 57 treated patients, all of which were mild [29]. No patients discontinued treatment [29]. Data from the same study support the efficacy observed in the vosoritide trial program, with 22 patients treated for 12 months obtaining an AGV of 6.0 cm/year, and an absolute height increase of 6.2 cm [29]. In addition, CrescNet (www.crescnet.org), a web-based competence network for continuous and long-term monitoring of growth and weight development in children, has collected epidemiological data from Portugal, Austria, and Germany [30]. These data demonstrate that 164 patients who started vosoritide remained on treatment after 6 months with no significant safety events [20]. Data from CrescNet show that an increase of 0.45 H-SDS was observed in 85 patients treated with vosoritide after 1.23 years (SD 0.75) [31]. While these early data demonstrate safety, efficacy, and adherence in real-life clinical practice, practical guidance is lacking. As a result of the rarity of achondroplasia, many centers may manage only a small number of patients with the condition. It is therefore essential to explore aspects of using vosoritide in clinical practice and disseminate the knowledge gained through early experience.
Methods
Two meetings were held to gather insight and share knowledge among clinicians who had experience of using vosoritide in clinical practice. One meeting was held in Dublin, Ireland, in July 2022 and was attended by representatives from Europe and the Middle East. The second was a virtual meeting for participants in the USA, with attendance split over 2 days in September and October 2022. The group comprised nine geneticists (MBB, RC, VCD, DD, MF, JHF, SS, AT, WRW), six pediatric endocrinologists (GH, KM, TRR, OS, PS, AV), two pediatricians (JL, FR), two orthopedic surgeons (AJH, KW), and one geneticist and pediatrician (EL). There were representatives from Austria, France, Germany, Portugal, Saudi Arabia, and the USA. The aims of the meetings were (1) to share knowledge gained through the early clinical experience of prescribing vosoritide in real-world patients, and (2) to discuss practical considerations for those interested in prescribing vosoritide in their own centers.
At the time of the meetings the group was collectively managing over 220 patients receiving vosoritide, with an age range of 2–16 years. Several of the authors had also been involved in the vosoritide clinical trial program. At the time of the meeting, vosoritide was licensed by the EMA for use in children with achondroplasia with open epiphyses aged ≥ 2 years [32] and by the US FDA in children aged ≥ 5 years [33], so the experience of treating children with vosoritide aged 2–5 years differed in that aspect.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Informed by their shared experience, the group identified practical aspects of management that should be considered by centers planning to initiate vosoritide treatment. The aim of this article is to share these early experiences collected across different institutions and regions and begin to provide a framework for the approach to management when treating patients with vosoritide.
Results
Site Preparation
Multidisciplinary Care Team
Published protocols are available that provide recommendations on how to follow up people with achondroplasia over time [15, 34, 35]. Recent guidelines recommend that achondroplasia should be managed by an experienced multidisciplinary team (MDT) throughout the lifespan [34, 35]. The initiation of vosoritide does not replace the need for coordinated MDT management and follow-up, nor constitute an extra barrier to its adequate implementation. It is essential that adequate MDT support be established prior to treatment initiation. The lead clinician and specialties involved in the MDT may vary between centers and regions [35]; however, at a minimum the core MDT should ideally include clinical genetics and/or pediatric endocrinology and/or a pediatrician experienced in skeletal dysplasia, alongside specialists in pediatric pulmonology, neurosurgery, rehabilitation, and orthopedics. Possible additional members could include an ear, nose, and throat (ENT) specialist, and a specialist who can provide psychosocial support, such as a social worker.
Infrastructure Considerations
Prior to initiation of vosoritide there is a need to consider the infrastructure in place, including available clinic capacity and expertise for supporting patients and families, means to perform specific assessments, and ability to implement ongoing monitoring protocols. For example, the license granted by the EMA states that “the diagnosis of achondroplasia should be confirmed by appropriate genetic testing” [32]; this means that any center in Europe wishing to prescribe vosoritide must have access to genetic testing. If testing is not available within the center infrastructure, the prescribing clinician will need to outsource to a third party. This is also the case in the USA where some insurance companies require molecular diagnosis of achondroplasia to enable reimbursement of vosoritide. Pretreatment screening with baseline and follow-up monitoring may require the availability of specific equipment or assessments and expertise in their interpretation, such as calibrated stadiometers/scales, and access to a range of radiographic and laboratory studies needed to assess bone age and blood chemistry.
Sufficient clinic capacity and expertise required to carry out and interpret clinical assessments is also an important factor. Examples include experience in Tanner staging, the availability of genetic counselling, adequate nursing support for patients and families, and administrative capacity to manage insurance or reimbursement processes (where needed). Staffing capacity for monitoring patients receiving vosoritide should be considered, as frequent follow-up is needed, particularly in the first year (see “Clinical Assessments”). As a result of the multidisciplinary approach to management needed for people with achondroplasia, care is often provided in reference centers, of which there may be only one or two in a country or region. Accessibility to follow-up is vital. Distance from the center and travel time [36] may impact on the commitment of patients and caregivers to adhere to a medication, and to follow-up protocols.
Vosoritide is supplied as a powder and separate diluent. It should be stored at 2–8 °C (36–46 °F) [32, 33], and is administered via a daily subcutaneous injection. The packaging for vosoritide can be large and difficult to store in a family home, especially if prescribed in 30- to 90-day quantities. Local pharmacies with storage capacity may need to be identified and consideration given as to the quantity supplied, especially for initial doses while the child is acclimatizing to daily injections.
One prerequisite for initiation of vosoritide is a clear understanding of requirements for reimbursement or funding. Strict stipulations may be in place for reimbursement, such as how often prescriptions are to be renewed, and assessments to confirm efficacy. In some cases, insurance companies may require that the patient receive the first injection in the presence of a healthcare provider, and for this to be funded by the center. These factors need to be taken into consideration to enable effective, informed preparation for vosoritide treatment.
Patient Selection Criteria
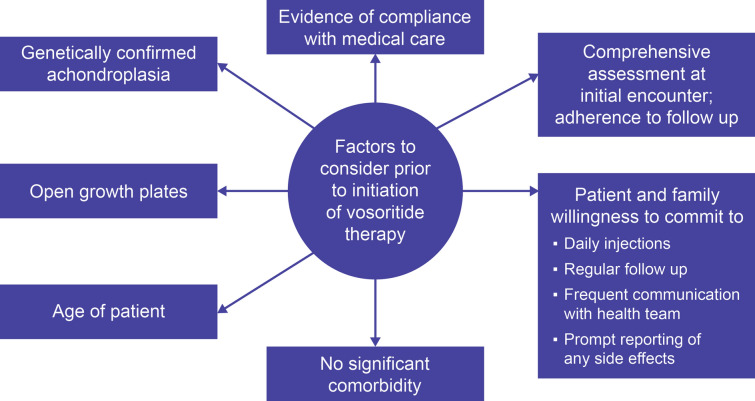
Although the option of vosoritide should be discussed with all who are eligible (children with achondroplasia with open epiphyses, age per country license), not all patients will wish to pursue therapy. It is important to identify barriers to treatment, and this should be assessed on an individual basis. Administration of vosoritide could potentially be burdensome if either the patient or caregiver is not committed to daily injections or the follow-up protocol. Identifying committed and potentially adherent patients and caregivers—as well as setting realistic expectations of treatment—will help ensure daily injections and monitoring are manageable. Figure 1 outlines some of the factors that may impact the decision to consider vosoritide for an individual patient.
Fig. 1.
Considerations to identify patients for treatment with vosoritide
Preparing for Treatment Initiation
An ongoing dialogue with patients and families is important prior to the start of vosoritide. Time should be allowed for patients and caregivers to process the information provided and to ask questions [37]. Preliminary conversations should include discussion of the level of interest in treatment, criteria for patient eligibility, potential benefits and risks of vosoritide, available data, potential timeframe for observing a response, practicalities of daily injections, and follow-up requirements. Vosoritide is an elective treatment and discontinuation of the medication will not cause harm to the patient—this should be stressed to patients and caregivers.
It is essential to understand the patient’s and/or caregiver’s motivation for starting treatment with vosoritide. In a recent qualitative study, the caregivers’ decision to initiate treatment was based on three variables: reduction in complications associated with achondroplasia, gain in height to increase functional independence, and perceived side effects of vosoritide [38].
Psychological counselling could be considered as part of pretreatment screening. This is particularly important for patients who lack routine follow-up but seek medical care for vosoritide once commercially available. It is vital to understand how each patient and caregiver views the effect a potential increase in height will have on their daily life and general well-being. Separating the psychology of a child from that of the caregiver can be difficult, particularly in children under the age of 5 years. Therefore, it may be helpful to have a separate conversation with the patient, away from the caregiver, to establish the child’s views and understanding.
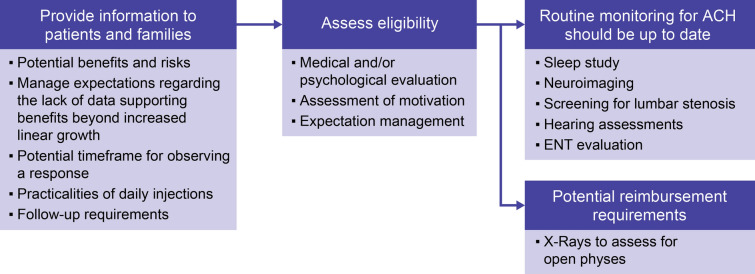
There is currently no published guidance available on the assessments that should be undertaken prior to initiation of vosoritide. Figure 2 is an example of a pretreatment protocol. Protocols will differ between centers and regions and may be dependent on factors such as the healthcare system, insurance or reimbursement authorities, available expertise, and accessibility of equipment and testing capability.
Fig. 2.
Example of a pretreatment protocol prior to initiation of vosoritide (ACH, achondroplasia; ENT, ear, nose, and throat)
Addressing Patient/Caregiver Concerns
The achondroplasia community is aware of vosoritide, with information gained from experience in clinical trials, social media, patient advocacy groups (PAGs), and the healthcare team. Some caregivers were aware of vosoritide from very early on, while the treatment was still in clinical trials [38]. However, conflicting information can lead to confusion or concerns, which must be addressed by the healthcare team. Patient concerns may include a potential future change in their physical appearance and the qualities they identify with, and unknown long-term safety and efficacy. Qualitative research to gain insight into caregivers’ experiences of vosoritide identified that their concerns may include the decision, on the child’s behalf, whether to start medication, when the child is too young to fully understand [38].
Supporting Patients and Families
Psychological support for both the patient and the caregiver(s) is an important part of pretreatment preparation and can be helpful for addressing concerns. Available data on efficacy and safety should be provided to patients and caregivers in a fit-for-purpose way to enable informed decision-making [37].
Patients and their caregivers need practical guidance on how to administer the medication. Vosoritide is supplied as a powder and diluent for reconstitution and is administered as a daily subcutaneous injection. Training can take approximately 1 h, and ideally this should take place in person. This can be difficult to achieve in a busy outpatient setting; thus, virtual training may be an option. Injection training for patients and caregivers can be provided by the manufacturer (BioMarin Pharmaceutical Inc) in some localities. Preparing and mixing vosoritide can be challenging for non-healthcare professionals, and there is a risk the process can take a long time and/or result in wasted syringes. There may be concerns over the injection equipment, including the retraction mechanism of the needle not working effectively in young children, the narrow gauge of the needle leading to breakage, or the potential for accidental intracutaneous injections. Injecting their own child can also be psychologically challenging for a parent [38].
The manufacturer (BioMarin Pharmaceutical Inc) recommends that the injection be administered in the front middle of the thigh, lower abdomen (at least 5 cm away from the navel), top of the buttocks, or on the back of the upper arms [32, 33]. It is also recommended to rotate the injection site and not use the same location on consecutive days [32, 33]. Daily injections can be burdensome for the patient and their caregivers. Therefore, empowering the patient can support their compliance. This could be done by allowing the child to select the injection site, having a pre-identified injection time each day, and establishing a routine for the process. Recent qualitative research on the experience of caregivers administering vosoritide indicates that involving children in preparing the treatment and decision-making about the injection site can be helpful in giving them a sense of ownership over the process [38]. Parents and caregivers also identified that strategies such as incentives, rewards, and distraction were beneficial in the early days, and that establishing vosoritide as a regular part of daily life can enable these strategies to be minimized over time [38].
During the phase 3 trial of vosoritide, Savarirayan et al. observed an injection-site reaction in 73% of patients receiving vosoritide, although all were non-serious and transient [26]. Real-world data from France supports this finding, with 21 adverse events observed in 57 patients, all of which were mild and 14 of which were injection-site reactions [29]. Patients and caregivers should be provided with information on common side effects of vosoritide and what to do in such cases. The burden of pain with each injection should not be underestimated, especially in younger patients. Pain from local injection reactions can be managed by using cold compresses, vibration tools, local anesthetic gels, and/or by distracting the child. Timing the injection to avoid early morning and ensuring the child is fed and well hydrated may preclude any dizziness following injection.
Clinical Assessments
There are currently no recommendations on specific assessments or timing of follow-up for patients receiving vosoritide. Monitoring protocols may vary by center and be influenced by regional differences in reimbursement and healthcare systems. Table 1 shows core assessments that may be included in vosoritide follow-up protocols, with day 0 relating to treatment initiation (not day 0 of life). Other assessments may be included, based on individual center protocols, such as X-rays of the spine and lower extremities, to establish baseline. In addition, routine monitoring for complications related to achondroplasia should still be performed.
Table 1.
Core assessments that may be included in vosoritide follow-up protocols, in addition to routine monitoring of achondroplasia
| Assessment | Considerations | Timing | ||||
|---|---|---|---|---|---|---|
| Day 0 | Month 1 | Month 3 | Every 6 months | Annually | ||
| Treatment-informing assessments | ||||||
| Molecular confirmation of achondroplasia | √ | |||||
| Parental height | √ | |||||
| Tanner staging | May be a requirement of reimbursement authorities | √ | √ | |||
| Bone age |
May be a requirement of reimbursement authorities Clinicians may choose to do less often (e.g., at baseline, then at signs of puberty) |
√ | √ | |||
| X-ray |
For assessment of growth plate (open or closed) To establish baseline (e.g., of spine and lower extremities) |
√ | √ | |||
| Vitamin D level | May be a requirement of reimbursement authorities | √ | √ | |||
| Status of achondroplasia complications | √ | √ | ||||
| Implementation of MDT management | √ | √ | ||||
| Efficacy assessments | ||||||
| Anthropometrics |
May include • Standing height • Sitting height • Arm span • Elbow range of motion • Upper and lower arm segment • May be a requirement of reimbursement authorities |
√ | √ | |||
| Annualized growth velocity | May be a requirement of reimbursement authorities | √ | √ | |||
| Symptoms (e.g., headaches, vision changes, worsening joint pain, lightheaded episodes, dizziness, nausea) | √ | √ | √ | |||
| Adherence (periods of pause/discontinuation) | √ | |||||
| Safety assessments | ||||||
| Review adverse events | √ | √ | √ | |||
| Discussion of concomitant/new medications | √ | √ | √ | √ | ||
| Discussion of other interventions | √ | |||||
Day 0 refers to treatment initiation, not day 0 of life
MDT multidisciplinary team
Table 2 illustrates an example of a local protocol, from the French Early Access Program [29, 39]. There is no right or wrong way to monitor patients receiving vosoritide, provided that established protocols are in place, safety and response to treatment are followed, and the patient has access to the MDT. There may not be capacity within the MDT to routinely follow up as frequently as the example given in Table 2. Some centers may choose to see patients every 3 months for the first year, then on a 6-monthly basis thereafter, while others may not schedule a first visit until 6 months after the initial dose. Centers may choose to vary the follow-up schedule depending on the age of the patient, with infants (license permitting) and younger children followed up on a more frequent basis than older children or adolescents. Whether consultations are held face-to-face or via telemedicine may also vary by center.
Table 2.
Vosoritide follow-up protocol from the French Early Access Program [39]
| Data elements of interest | Day 0 visit (Treatment start) |
Teleconsultation (Month 1) |
Month 3 | Month 6 and every 6 months | End of follow-up |
|---|---|---|---|---|---|
| Documentation of achondroplasia | √ | ||||
| Demographics | √ | ||||
| Medical and surgical history | √ | ||||
| Clinical exam | √ | √ | √ | ||
| Vital signs including blood pressure | √ | √ | √ | ||
|
Detailed anthropometric and morphological measurements |
√ | √ (annual) | |||
| X-ray of left hand and one knee | √ (annual) | ||||
| Tanner stages | √ | √ (annual) | |||
|
Medical and surgical events (incl. ACH-related interventions or investigations including blood test results) |
√ | √ | √ | ||
| Administration, prescribing and adherence information for vosoritide | √ | √ | √ | √ | √ |
| Visits healthcare professional | √ | √ | √ | ||
| Adverse event collection | √ | √ | √ | √ | √ |
| Concomitant and new medications | √ | √ | √ | √ | √ |
ACH achondroplasia
Treatment Response
Response to vosoritide treatment can vary in magnitude and timing. AGV was the primary endpoint in the phase 3 clinical trial [26] and is generally an accepted measure of meaningful response to vosoritide. Experience from clinical practice indicates that patients and families do not expect to see a response within 3–6 months of treatment initiation, but many will expect measurable results at 1 year. However, evidence from the vosoritide trial program combined with early clinical experience demonstrates a measurable response can be later than this—typically at 1–2 years [26, 27, 29]. Therefore, it is important to align with patients and families as to the definition of “response to treatment” prior to initiation of therapy, in an effort to manage expectations. Response to vosoritide will likely be affected by lack of adherence to daily injections and this may be a consideration if an expected increase in AGV is not achieved.
Managing Expectations
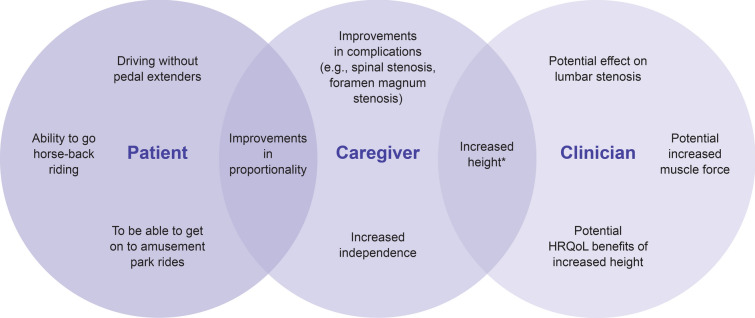
Open communication and an understanding of motivation are vital. Establishing the expectations of treatment outcomes with vosoritide is important to ascertain whether patients and their caregivers are aligned with one another and with the available evidence. There is currently no available data to support that vosoritide improves any aspect of achondroplasia other than linear growth. This should be clearly explained to patients and families. The goals of treatment may differ between the patient, caregiver, and clinician (Fig. 3).
Fig. 3.
Examples of different goals of vosoritide treatment (HRQoL, health-related quality of life) *Height is the only outcome with published data so clinicians considered this the only realistic goal
It is important to emphasize that treatment response to vosoritide can vary, and that ethnicity and parental height may impact achievable height gains. It may be useful to share individual spaghetti plots from the vosoritide clinical trial program [27] to demonstrate individual responses to treatment. The potential extent of linear growth should be clearly communicated, including demonstration of what a difference in height may mean to everyday life in the long-term. An apparent response to vosoritide treatment may not be immediately evident. Plotting the child’s growth on average-stature charts and sharing the curve in comparison to achondroplasia-specific curves may be useful to demonstrate response and provide motivation for continued treatment. Understanding the possibility of a varied, gradual response may prevent early discontinuation due to perceived lack of efficacy.
Although reimbursement authorities and insurance companies may require an appreciable treatment response by 1 year, there is general agreement that a longer timeframe of 2 years may be required to better assess efficacy. Assessment after 2 years of treatment can demonstrate whether the patient is responding to vosoritide, and this is a good timepoint at which to discuss with the patient and caregiver if they wish to continue with treatment.
Treatment Adherence, Pause or Discontinuation
Vosoritide is an elective medication that increases linear growth, with proven efficacy in patients who adhere to a schedule of daily subcutaneous injections [26, 27]. Non-adherence to daily injections may decrease the patient’s response to treatment. There is debate among those with experience of vosoritide in clinical practice as to whether adherence needs to be monitored given that it is an elective therapy. Anecdotal evidence suggests that adherence is not a requested parameter for insurance companies in the USA. However, clinicians in Europe and the Middle East may place greater emphasis on adherence because it can impact reimbursement. Real-world evidence identified that of 57 patients, 14 missed a total of 43 doses of vosoritide, with 23 missed doses occurring by month 12; one patient was responsible for 16 of these [29].
There may be instances in which a patient or caregiver chooses to pause vosoritide. Experience from clinical practice indicates that treatment pauses may occur in response to the burden of daily injections, other life circumstances, periods of time when a trained caregiver will not be present, or during surgical procedures. Planned bone surgery was an exclusion criterion for the phase 3 vosoritide clinical trial [26, 40] and to date there are no published data on the concomitant use of vosoritide during bone surgery, although orthopedic surgery is common in individuals with achondroplasia [19]. Until there is evidence to support or contradict a combined approach, continuation or pause of vosoritide during surgical procedures should be the decision of the treating physician, the patient, and the caregiver—and be supported by the best evidence available at the time.
Patients or caregivers may choose to discontinue treatment because of an inability to tolerate the injections or perceived lack of meaningful response. There may be instances in which the clinician suggests cessation of treatment for reasons including non-adherence, serious side effects or reactions, no change in growth velocity observed after 1–2 years of treatment, or closure of growth plates and achievement of near final adult height.
Pause or discontinuation of vosoritide may affect near final adult height, and there is a possibility of enhanced antibody production with a start-stop approach to treatment. However, patients and families should be assured that there will be no detrimental effect on the patient’s health if vosoritide treatment is paused or discontinued. Providing ongoing support and training for patients and caregivers may minimize stress associated with the medication administration.
When nearing completion of growth and cessation of therapy, assessment of the growth plates through imaging will be necessary. Ceasing treatment when AGV is less than 1.5 cm per year [32] is another potential option, when the patient has reached 99% of their adult height, or according to bone age, as assessed by the Greulich and Pyle method [41]. Insurance companies or reimbursement bodies may dictate the method by which to assess skeletal maturity prior to cessation of vosoritide treatment.
Concomitant Use with Other Treatments and Procedures
The multisystem complications associated with achondroplasia may necessitate other interventions, such as ENT procedures, neurosurgery, or orthopedic surgeries. In the scenario that interventions are required whilst a patient is receiving vosoritide, the prescribing clinician—as part of the wider MDT—should consider the best available evidence to support the decision-making process. However, there are currently no published data to either support or contradict the concomitant use of vosoritide with other medical interventions. In the absence of more evidence to support concomitant use, it is necessary to discuss the pros and cons of vosoritide treatment during other interventions with the patient and caregivers.
Discussion
Experience gained from clinical trials differs from the knowledge and experience gained when prescribing treatment in a real-world setting. Protocols that were followed in a clinical trial program may not be feasible in practice, and the size, location, and healthcare system may influence how a center introduces a new therapy. Vosoritide is the first approved, disease-specific, precision medicine to treat children with achondroplasia. Sharing early experiences from clinical practice can help support others wishing to prescribe vosoritide, by highlighting practical and logistical considerations.
Experience shows that establishing and implementing the correct infrastructure, equipment, staffing, expertise, and MDT support can help clinicians to prepare for prescribing vosoritide. Pretreatment screening and follow-up protocols ensure that patients are appropriately monitored for safety signals and response to treatment, and that treatment expectations are managed as part of an ongoing process. Capturing real-world treatment data in a future registry will improve our understanding as to how vosoritide could affect the natural history of achondroplasia, as well as determining safety and efficacy over time.
Conclusion
As more clinicians begin prescribing vosoritide in clinical practice, the need for international consensus guidelines will become more pressing. Therefore, sharing of early experiences by providers treating children with achondroplasia will prove invaluable when developing standards and guidelines for vosoritide therapy.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance
Medical writing and editorial assistance in the preparation of this article was provided by ELM Medical Ltd. Support for medical writing and editorial assistance was funded by BioMarin Pharmaceutical Inc.
Author Contributions
Oliver Semler, Valerie Cormier-Daire, Ekkhart Lausch, Michael B Bober, Ricki Carroll, Sergio B. Sousa, David Deyle, Maha Faden, Gabriele Hartmann, Aaron J. Huser, Janet M. Legare, Klaus Mohnike, Tilman R. Rohrer, Frank Rutsch, Pamela Smith, Andre M. Travessa, Angela Verardo, Klane, K. White, William R. Wilcox and Julie Hoover-Fong were present at the expert group meetings and made substantial contributions to the concept. All authors were involved in the creation and evolution of the subsequent manuscript. All authors reviewed and approved the outline, and all drafts. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. BioMarin Pharmaceutical Inc. funded and organized the expert group meetings, funded medical writing/editorial support, and the Rapid Service and Open Access fees.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
In addition to the individual conflict of interest outlined below, all authors participated in the expert group meetings, sponsored and organized by BioMarin Pharmaceutical Inc, on which this manuscript is based. Oliver Semler: has received grants or contracts from Kyowa Kirin, payment or honoraria for lectures/presentations from BioMarin and Kyowa Kirin, and support for attending meetings and/or travel from Pfizer. Valérie Cormier-Daire: has received payment or honoraria for lectures/presentations from BioMarin and Ipsen, and participated in Advisory Board meetings for BioMarin, QED-Propel and Mereo. Ekkehart Lausch: has received grants or contracts from DFG (SFB1453), H2020, consulting fees, support for attending meetings and/or travel, and has participated in Advisory Board meetings for BioMarin. Michael B. Bober: has received grants or contracts from BioMarin, Ascendis, QED and Pfizer (all Institutional), consulting fees from BioMarin and Tyra (Institutional), Ascendis and Bridge Bio, payment or honoraria for lectures/presentations from NovoNordisk and Tyra, has participated in Advisory Board meetings for BioMarin, and has a leadership role for the Skeletal Dysplasia Management Consortium. Ricki Carroll: has received grants or contracts from BioMarin, QED, Ascendis and Pfizer (all Institutional), and consulting fees from Guidepoint. Sérgio B. Sousa: has received payment or honoraria for lectures/presentations, support for attending meetings and/or travel, and has participated in Advisory Board meetings for BioMarin, and has a leadership role for the European Achondroplasia Forum and ERN-BOND. David Deyle: has received grants or contracts from Castle Creek. Maha Faden: has received support for attending meetings and/or travel, and has participated in Advisory Board meetings for BioMarin. Gabriele Hartmann: has received consulting fees, support for attending meetings and/or travel, and has participated in Advisory Board meetings for BioMarin. Aaron J. Huser: has received payment or honoraria for speaker bureau, has participated in Advisory Board meetings for BioMarin, and holds a leadership role for the MHE Research Foundation. Janet M. Legare: has received grants or contracts for clinical trials from Ascendis Pharma and Bridge Bio, and for a natural history study from BioMarin, and payment or honoraria for lectures/presentations from BioMarin. Klaus Mohnike: has received grants or contracts for clinical trials, payment or honoraria for lectures/presentations, support for attending meetings and/or travel and has participated in Advisory Board meetings for BioMarin. Tilman R. Rohrer: has received grants or contracts, consulting fees, payment or honoraria for lectures/presentations and support for attending meetings and/or travel from BioMarin and NovoNordisk. Frank Rutsch: has received grants or contracts (Institutional), consulting fees, payment or honoraria for lectures/presentations, support for attending meetings and/or travel, and has participated in Advisory Board meetings for BioMarin. Pamela Smith: has received payment or honoraria for lectures/presentations, and support for attending meetings and/or travel from BioMarin. Andre M. Travessa: has received consulting fees, support for attending meetings and/or travel, and participated in Advisory Board meetings. Angela Verardo: Has received payment or honoraria for speaker bureau from BioMarin. Klane K. White: Has received grants or contracts from BioMarin, Ascendis, Ultragenyx and Pfizer, royalties or licenses for UptoDate.com, payment or honoraria for lectures/presentations from BioMarin and has a leadership role for Little People of America, National MPS Society, Pediatric Orthopedic Society of North America, Scoliosis Research Society, Camp Korey, and the Skeletal Dysplasia Management Consortium. William R. Wilcox: has received grants or contracts (Institutional), and has participated in Advisory Board meetings for BioMarin. Julie Hoover-Fong: has received grants or contracts for clinical trials from BioMarin, QED and Pfizer, consulting fees from BioMarin, QED, NovoNordisk and Innoskel, payment or honoraria for lectures/presentations from Medscape and BioMarin, has participated in Advisory Board meetings for MCDS—Therapy Clinical Trial (unpaid), BioMarin and QED, and is an unpaid member of the Skeletal Dysplasia Management Consortium and of the Medical Advisory Board of the Little People of America.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Coi A, Santoro M, Garne E, et al. Epidemiology of achondroplasia: a population-based study in Europe. Am J Med Genet A. 2019;179:1791–1798. doi: 10.1002/ajmg.a.61289. [DOI] [PubMed] [Google Scholar]
- 2.Foreman PK, van Kessel F, van Hoorn R, van den Bosch J, Shediac R, Landis S. Birth prevalence of achondroplasia: a systematic literature review and meta-analysis. Am J Med Genet A. 2020;182A:2297–2316. doi: 10.1002/ajmg.a.61787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rousseau F, Bonaventure J, Legai-Mallet L, et al. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 4.Shiang R, Thompson LM, Zhu YZ, et al. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 5.Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162–172. doi: 10.1016/S0140-6736(07)61090-3. [DOI] [PubMed] [Google Scholar]
- 6.Hoover-Fong J, Cheung MS, Fano V, et al. Lifetime impact of achondroplasia: current evidence and perspectives on the natural history. Bone. 2021;146:115872. doi: 10.1016/j.bone.2021.115872. [DOI] [PubMed] [Google Scholar]
- 7.Wright MJ, Irving M. Clinical management of achondroplasia. Arch Dis Child. 2012;97:129–134. doi: 10.1136/adc.2010.189092. [DOI] [PubMed] [Google Scholar]
- 8.Pauli RM. Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis. 2019;14:1. doi: 10.1186/s13023-018-0972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maghnie M, Semler O, Guillen-Navarro E, et al. Lifetime impact of achondroplasia study in Europe (LIAISE): findings from a multinational observational study. Orphanet J Rare Dis. 2023;18:56. doi: 10.1186/s13023-023-02652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savarirayan R, Irving M, Harmatz P, et al. Growth parameters in children with achondroplasia: a 7-year, prospective, multinational, observational study. Genet Med. 2022;24:2444–52. doi: 10.1016/j.gim.2022.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Horton WA, Rotter JI, Rimoin DL, Scott CI, Hall JG. Standard growth curves for achondroplasia. J Pediatr. 1978;93:435–438. doi: 10.1016/S0022-3476(78)81152-4. [DOI] [PubMed] [Google Scholar]
- 12.Merker A, Neumeyer L, Hertel NT, et al. Growth in achondroplasia: development of height, weight, head circumference, and body mass index in a European cohort. Am J Med Genet A. 2018;176:1723–1734. doi: 10.1002/ajmg.a.38853. [DOI] [PubMed] [Google Scholar]
- 13.Merker A, Neumeyer L, Hertal NT, et al. Development of body proportions in achondroplasia: sitting height, leg length, arm span, and foot length. Am J Med Genet A. 2018;176:1818–1829. doi: 10.1002/ajmg.a.40356. [DOI] [PubMed] [Google Scholar]
- 14.Hoover-Fong JE, Schulze KJ, Alade AY, et al. Growth in achondroplasia including stature, weight, weight-for-height and head circumference from CLARITY: achondroplasia natural history study—a multi-center retrospective cohort study of achondroplasia in the US. Orphanet J Rare Dis. 2021;16:522. doi: 10.1186/s13023-021-02141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoover-Fong J, Scott CI, Jones MC, AAP Committee on Genetics Health supervision for people with achondroplasia. Pediatrics. 2020;145:e20201010. doi: 10.1542/peds.2020-1010. [DOI] [PubMed] [Google Scholar]
- 16.Alade Y, Tunkel D, Schulze K, et al. Cross-sectional assessment of pain and physical function in skeletal dysplasia patients. Clin Genet. 2013;84:237–43. doi: 10.1111/cge.12045. [DOI] [PubMed] [Google Scholar]
- 17.Constantinides C, Landis SH, Jarrett J, Quinn J, Ireland PJ. Quality of life, physical functioning, and psychosocial function among patients with achondroplasia: a targeted literature review. Disabil Rehabil. 2022;44:6166–6178. doi: 10.1080/09638288.2021.1963853. [DOI] [PubMed] [Google Scholar]
- 18.Maghnie M, Semler O, Guillen-Navarro E, et al. Health-related quality of life (HRQoL) in achondroplasia: findings from a multinational, observational study. Mol Genet Metabol. 2021;132:S127–S128. doi: 10.1016/S1096-7192(21)00280-8. [DOI] [Google Scholar]
- 19.Nahm NJ, Mackenzie WS, Mackenzie WG, et al. Achondroplasia natural history study (CLARITY): 60-year experience in orthopedic surgery from four skeletal dysplasia centers. Orphanet J Rare Dis. 2023;18(1):139. doi: 10.1186/s13023-023-02738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiva-Gea A, Delgado-Rufino FB, Queipo-de-Llano A, Mariscal-Lara J, Lombardo-Torre M, Luna-González F. Staged upper and lower limb lengthening performing bilateral simultaneous surgery of the femur and tibia in achondroplastic patients. Arch Orthop Trauma Surg. 2020;140:1665–1676. doi: 10.1007/s00402-020-03360-3. [DOI] [PubMed] [Google Scholar]
- 21.Hosny GA. Limb lengthening history, evolution, complications and current concepts. J Orthop Traumatol. 2020;21:3. doi: 10.1186/s10195-019-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yorifuji T, Higuchi S, Kawakita R. Growth hormone treatment for achondroplasia. Pediatr Endocrinol Rev. 2018;16(Suppl 1):123–128. doi: 10.17458/per.vol16.2018.yhk.ghachondroplasia. [DOI] [PubMed] [Google Scholar]
- 23.Harada D, Namba N, Hanioka Y, et al. Final adult height in long-term growth hormone-treated achondroplasia patients. Eur J Pediatr. 2017;176:873–879. doi: 10.1007/s00431-017-2923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorget F, Kaci N, Peng J, et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet. 2012;91:1108–1114. doi: 10.1016/j.ajhg.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savarirayan R, Irving M, Bacino CA, et al. C-type natriuretic peptide analogue therapy in children with achondroplasia. N Eng J Med. 2019;381:25–35. doi: 10.1056/NEJMoa1813446. [DOI] [PubMed] [Google Scholar]
- 26.Savarirayan R, Tofts L, Irving M, et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet. 2020;396:684–692. doi: 10.1016/S0140-6736(20)31541-5. [DOI] [PubMed] [Google Scholar]
- 27.Hoover-Fong J, Savarirayan R, Tofts L, et al. Persistent growth-promoting effects of vosoritide in children with achondroplasia for up to 35 years: update from phase 3 extension study. Genet Med. 2023;1(1):100222. doi: 10.1038/s41436-021-01287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cormier-Daire V, Cohen S, Edouard T, et al. Real-world experience with vosoritide for achondroplasia: interim findings from an early access programme in France. Horm Res Paediatr. 2022;95(Suppl 2):308. [Google Scholar]
- 29.Cormier-Daire V, Edouard T, Isidor B, et al. Real-world safety and effectiveness of vosoritide: Results from an early-access program in France. Presented at the 2023 ESPE European Society for Paediatric Endocrinology, 21–23 September 2023, The Hague, Netherlands.
- 30.Palm K, Bechthold-Dalla Pozza S, Gausche R, et al. Real-world data in children with achondroplasia after licensing of vosoritide. Horm Res Peadiatr. 2022;95(Suppl 2):151. [Google Scholar]
- 31.Kunkel P, Al Halak M, Bechthold-Dalla Pozza S, et al. Multidisciplinary approach in achondroplasia – real world experience after drug approval of vosoritide. Presented at the 2023 ESPE European Society for Paediatric Endocrinology, 21–23 September 2023, The Hague, Netherlands.
- 32.Voxzogo Summary of Product Characteristics 2021. https://www.ema.europa.eu/en/documents/product-information/voxzogo-epar-product-information_en.pdf. Accessed 05 May 2023
- 33.Voxzogo US Prescribing Information (2021, November). https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214938s000lbl.pdf Accessed 05 May 2023.
- 34.Savarirayan R, Ireland P, Irving M, et al. International consensus statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nat Rev Endocrinol. 2022;18:173–189. doi: 10.1038/s41574-021-00595-x. [DOI] [PubMed] [Google Scholar]
- 35.Cormier-Daire V, AlSayed M, Ben-Omran T, et al. The first European consensus on principles of management for achondroplasia. Orphanet J Rare Dis. 2021;16:333. doi: 10.1186/s13023-021-01971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Health Care Utilization and Adults with Disabilities. Health-Care Utilization as a Proxy in Disability Determination. Washington (DC): National Academies Press (US); 2018. [PubMed]
- 37.Katz AL, Webb SA, AAP Committee on Bioethics Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi: 10.1542/peds.2016-1485. [DOI] [PubMed] [Google Scholar]
- 38.NiMhurchadha S, Butler K, Argent R, et al. Parents’ experience of administering vosoritide: a daily injectable for children with achondroplasia. Adv Ther. 2023 doi: 10.1007/s12325-023-02496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Protocole d’utilisation thérapeutique et de recueil de données (PUT-RD). Accès précoce—VOXZOGO (vosoritide). https://has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=p_3304523 Accessed 17 May 2023.
- 40.Voxzogo exclusion criteria: an extension study to evaluate the efficacy and safety of BMN 111 in children with achondroplasia, 2018. https://www.clinicaltrials.gov/ct2/show/NCT03424018?term=302&cond=BMN111&draw=2&rank=1 Accessed 05 May 2023.
- 41.Greulich W, Pyle I. Radiographic atlas of skeletal development of the hand and wrist. London: Stanford University Press; 1959. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.