Abstract
Objectives:
Virtual 3D specimen mapping of oncologic surgical specimens provides a visual record of the specimen and margin sampling sites which can be utilized in a variety of cancer care settings. Our objective was to perform a retrospective review of head and neck surgical oncology cases where the specimen was mapped post-operatively and to evaluate the utility of these 3D specimen maps amongst the multidisciplinary cancer care team.
Methods:
A retrospective review of our 3D specimen model biorepository was performed. Surgical specimens were 3D scanned and then graphically annotated (or “mapped”) during routine pathologic processing. The resulting 3D specimen maps were distributed to the multidisciplinary oncologic care team. Final margin status and any use of the 3D specimen maps were recorded.
Results:
A total of 28 cases were included. Virtual 3D specimen maps were utilized by the cancer care team in 8 cases (29%), including 2 positive margin cases, 2 close margin cases, and 4 indeterminate margin cases. 3D specimen maps were used to visualize positive margin sites for pathologist-surgeon communication as a visual reference during tumor board discussions and to inform radiation treatment planning.
Conclusion:
Post-operative virtual 3D specimen mapping of oncologic specimens creates a permanent visual record of the specimen and the margins sampled and may serve as a beneficial tool for communication amongst the multidisciplinary cancer care team.
Keywords: 3D scanning, surgical margins, head and neck cancer, pathology communication
Lay Summary:
Virtual 3D specimen maps provide a permanent visual record of the resected cancer specimen and its pathology processing. This retrospective review of a virtual 3D model biorepository, 3D specimen maps were often used for margin localization and surgeon-pathologist communication.
INTRODUCTION
After surgical resection, oncologic specimens are sent to a surgical pathology lab for gross examination, sectioning, and microscopic evaluation. Surgical pathologists are responsible for reporting a final margin status from the three-dimensional (3D) specimen. The resulting surgical margin status is used among other pathologic risk factors to guide adjuvant treatment. When a final positive margin is present, the anatomic location is used as a target for re-resection and adjuvant radiation therapy. Unfortunately, oral cavity squamous cell carcinoma (SCC) has the highest positive surgical margin rate among solid malignancies that occur in both men and women.1
The current standard-of-care pathology report relies on written descriptions of the specimen and sites of margin sampling. While two dimensional (2D) photos of the specimen are sometimes obtained beforehand, the specimen itself is typically destroyed during sectioning, leaving no visual representation of margin sampling location. Thus, the anatomic localization of margin sampling sites is limited to verbal and written descriptions which can be challenging and frustrating for surgeons and pathologists alike. Accurately communicating margin location is particularly difficult for head and neck surgical specimens given their anatomic 3D complexity and frequent mixture of margin tissue types.2
To address this unmet need, we have developed a protocol to create a 3D model of the resected cancer specimen using 3D scanning.3 In the current study, we expand upon our previous report4 to include virtual 3D specimen mapping of post-operative surgical specimens. Our goal was to provide a permanent 3D record of the surgical specimen and clearly demonstrate the sites of inking and margin sampling, serving as an adjunctive communication tool between surgeons and pathologists.
MATERIALS AND METHODS
Patient Population
This is a retrospective review of a 3D specimen model biorepository that was approved by the Vanderbilt University Medical Center Institutional Review Board (IRB #221597). Patients provided written consent for 3D scanning of their surgical specimen prior to surgery. Inclusion criteria were patients 18 and older with a suspected or biopsy-proven head and neck neoplasm undergoing surgical resection. 3D specimen maps were created based on surgeon and pathologist preference and staff availability. The authors recognize the improved oncologic outcomes associated with a specimen-based approach to margin analysis.4,5 However, the approach to margin analysis for this retrospective review was surgeon-dependent with a combination of defect (tumor bed)- and specimen-based approaches.
3D Scanning
As detailed in our previous work,3,6 surgical specimens were 3D scanned by a research team member prior to handing off the specimen to the pathology team. A commercially available, structured-light 3D scanner (EinScan SP, Shining 3D, Hangzhou, China) and the accompanying software (EXScan, Shining 3D) were used. To improve image quality, the specimens were rinsed and patted dry prior to scanning. The specimen was placed on a turntable and its top surface was scanned. Our previously published median 3D scanning time was 8 minutes which is time that is added to the frozen section analysis process in a specimen-driven approach to margins.6 The specimen was then flipped over, and the 3D scanning process was repeated. The two virtual halves of the specimen were aligned using three-point cross-registration, yielding a virtual 3D model of the surgical specimen. Once the 3D scan was completed, the surgical specimen was returned to the pathology team and processed per standard of care.
Post-Operative 3D Specimen Mapping
Surgical specimens were most commonly processed the day after surgery, following overnight formalin fixation, by a member of the pathology team (often a resident physician or pathologists’ assistant). A research team member was present during inking and sectioning of the specimen and directly observed alongside the prosector (Figure 1). In our experience the entire 3D specimen mapping process took 30 to 60 minutes, varying for each specimen and prosector, for a research team member alongside the prosector. The 3D specimen model was exported into 3MF file format for use in computer-aided design (CAD) software (Meshmixer, Autodesk Inc., San Rafael, CA).
Figure 1.
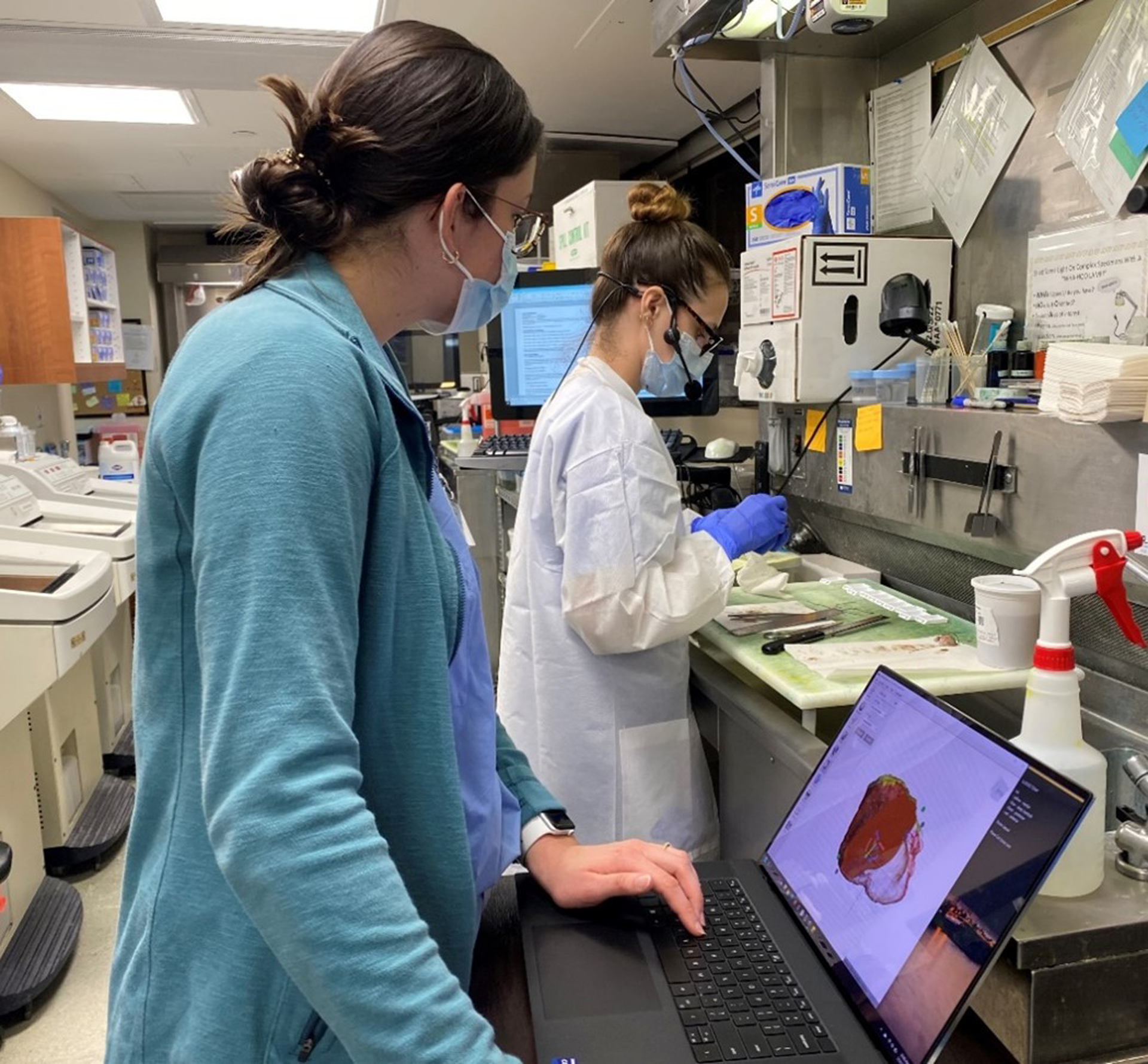
Research team member works alongside pathologists’ assistant during specimen processing to virtually map inking of specimen and sites of margin sampling.
Graphical annotation of the pathologic processing onto the surface of the 3D specimen model, i.e. virtual 3D specimen mapping, was performed (Figure 2). The “Paint Vertex” virtual paintbrush was used for most virtual 3D specimen mapping purposes, including inking the specimen, labeling margins with letters, and adding patterns to denote the type of section. The ideal size setting was 15 to 20 for marking and labeling margins, while a larger size was used for inking. The “Plane Cut” tool was used to mark cross-sectional cuts made during sectioning.
Figure 2.
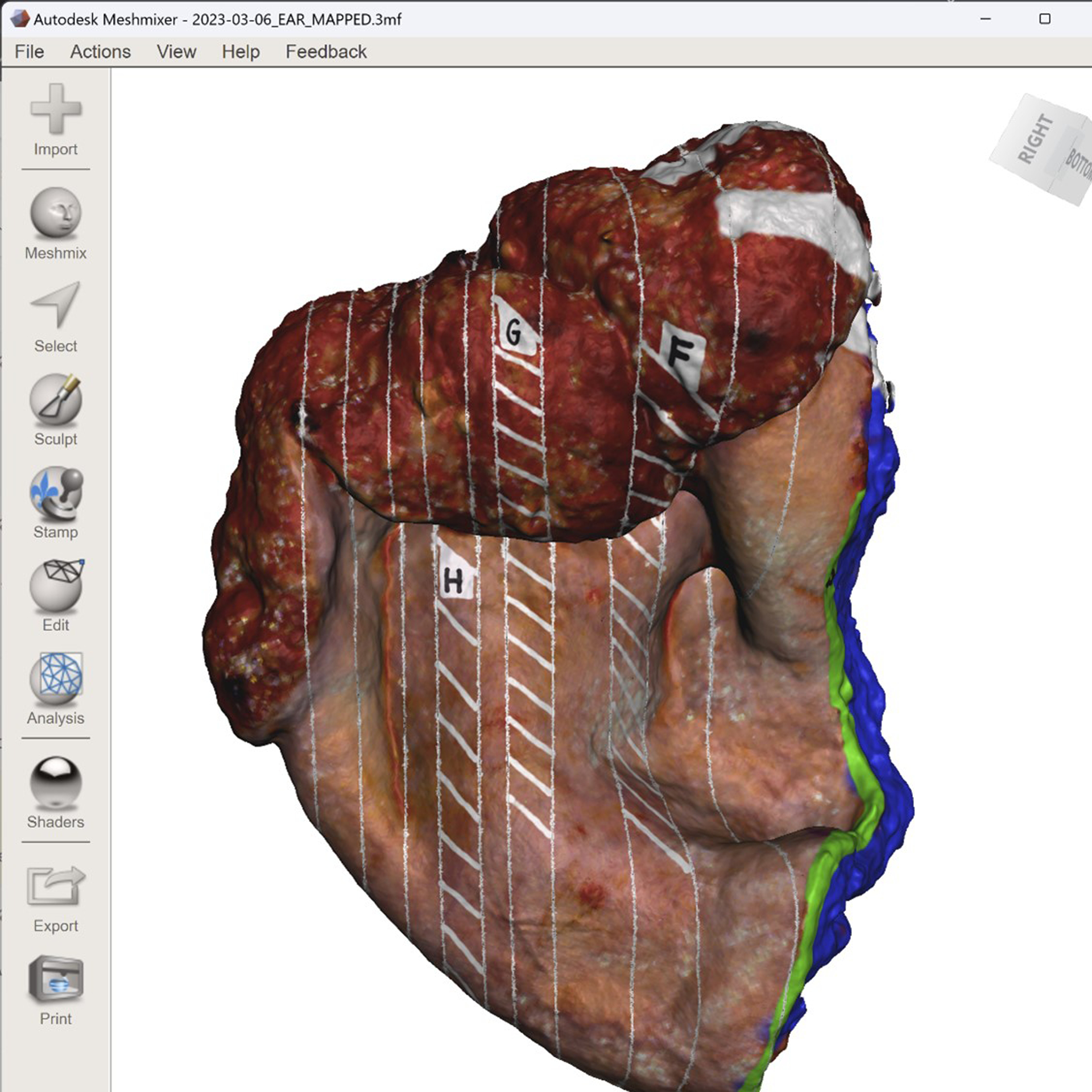
Completed three-dimensional (3D) specimen map in the computer-aided design software used to virtually annotate the specimen model.
Shave and perpendicular margins were marked by a line corresponding to margin length and location. Once plane cuts were made to indicate cross-sectional cuts, cross hatches (or similar patterns), seen in Figure 2, were applied to indicate the part of that section placed into the cassette for analysis. In addition, the 3D specimen was virtually inked in corresponding color and location to that of the actual inked specimen. The anatomic orientation denoted by each ink color was recorded (Figure 3).
Figure 3.
Example of a video distributed to members of the oncologic care team after specimen mapping is completed and prior to release of final pathology report. The video contains a key and the virtual three-dimensional (3D) specimen which is rotated in the video to show all aspects.
Final 3D specimen maps were distributed to attending pathologists and surgeons to serve as a visual tool for communication, including in-person or teleconference discussion of any close or positive margins when indicated (Figure 4). 3D specimen maps were sometimes shared by the pathologist at multidisciplinary head and neck tumor board when discussing adjuvant treatment recommendations. The 3D map can be rotated to show the specimen from any angle. Positive or close margins can be indicated by the pathologist on the specimen using the annotation tools.
Figure 4.
Virtual three-dimensional (3D) specimen map is used as a communication tool and visual reference in a teleconference between surgeons and pathologists establishing the final diagnosis for a given case.
Of note, 17 of the patients in the present study were previously published in our initial feasibility study and briefly discussed in a different context.6
RESULTS
From October 2021 to April 2023, 28 head and neck oncologic specimens were 3D scanned and virtually mapped. Most of the surgical specimens were squamous cell carcinoma (SCC) (86%, n=24), with the most common anatomic subsites being oral cavity (54%, n=15) and larynx (29%, n=8). All 28 specimen maps were distributed to attending head and neck surgeons and pathologists prior to review of the slides and release of the final pathology report. The 3D specimen maps were utilized to facilitate communication of a margin between surgeons and pathologists in 29% (n=8) of cases. Detailed information for all cases can be found in Table 1.
Table 1:
Description of 3D Specimen Mapping Cases
| Case # | Pathology | Anatomic Subsite | Margin Status |
|---|---|---|---|
| 1 | SCC | Oral cavity | Negative |
| 2 | Giant cell tumor | Larynx | Negative |
| 3 | SCC | Larynx | Negative |
| 4 | SCC | Oral cavity | Indeterminate |
| 5 | Chondrosarcoma | Larynx | Close |
| 6 | SCC | Oropharynx | Negative |
| 7 | SCC | Oral cavity | Negative |
| 8 | SCC | Larynx | Negative |
| 9 | SCC | Larynx | Negative |
| 10 | SCC | Oral cavity | Indeterminate |
| 11 | SCC | Oral cavity | Negative |
| 12 | SCC | Oral cavity | Indeterminate |
| 13 | SCC | Oral cavity | Negative |
| 14 | Chondrosarcoma | Larynx | Negative |
| 15 | SCC | Oral cavity | Negative |
| 16 | SCC | Oral cavity | Negative |
| 17 | SCC | Larynx | Negative |
| 18 | SCC | Oropharynx | Negative |
| 19 | SCC | Larynx | Negative |
| 20 | SCC | Oral cavity | Negative |
| 21 | SCC | Oral cavity | Negative |
| 22 | SCC | Cutaneous | Negative |
| 23 | SCC | Cutaneous | Negative |
| 24 | SCC | Oral cavity | Negative |
| 25 | SCC | Oral cavity | Indeterminate |
| 26 | BCC | Cutaneous | Positive |
| 27 | SCC | Oral cavity | Positive |
| 28 | SCC | Oral cavity | Close |
Cases tended to fall into four categories based on final margin status. 1) Positive: Positive margins on the main specimen or in the separate defect margin specimens (if tumor bed approach to margin analysis was utilized by surgeon) which were not re-resected and covered by additional specimens. 2) Close: Negative margins on the main specimen but with a clearance of less than 5 mm. 3) Indeterminate: Positive margins on the main specimen where separate defect margin specimens were sent, but it was not clear that they superseded the area of positivity due to lack of clear co-registration for the anatomic site. 4) Negative: Negative margins on the main specimen and negative separate defect margin specimens (if tumor bed approach to margin analysis was utilized by surgeon) with adequate clearance (typically > 5 mm) of the closest margin.
Positive Margins
Two (7%) of the cases that underwent 3D specimen mapping had final positive surgical margins. Case #26 was a scalp and calvarium resection for basal cell carcinoma (BCC) with final margins showing tumor involving full thickness calvarium with gross dural involvement over the sagittal sinus. The patient is currently receiving adjuvant chemotherapy and radiation therapy for this unresectable positive margin. In Case #27 a hemi-glossectomy was performed for a right oral tongue SCC with area of leukoplakia on the ventral tongue which was included in the en bloc specimen. Final margins of the right oral tongue SCC were negative for carcinoma, however there was a, previously unknown second primary 4 mm focus of invasive cancer at the deep margin of the ventral tongue leukoplakia. The surgeon noted difficulty localizing this focus of cancer within a large area of severe dysplasia resected in this area. The site of the positive margin, as demonstrated by the 3D specimen map, will be closely monitored for further treatment.
Close Margins
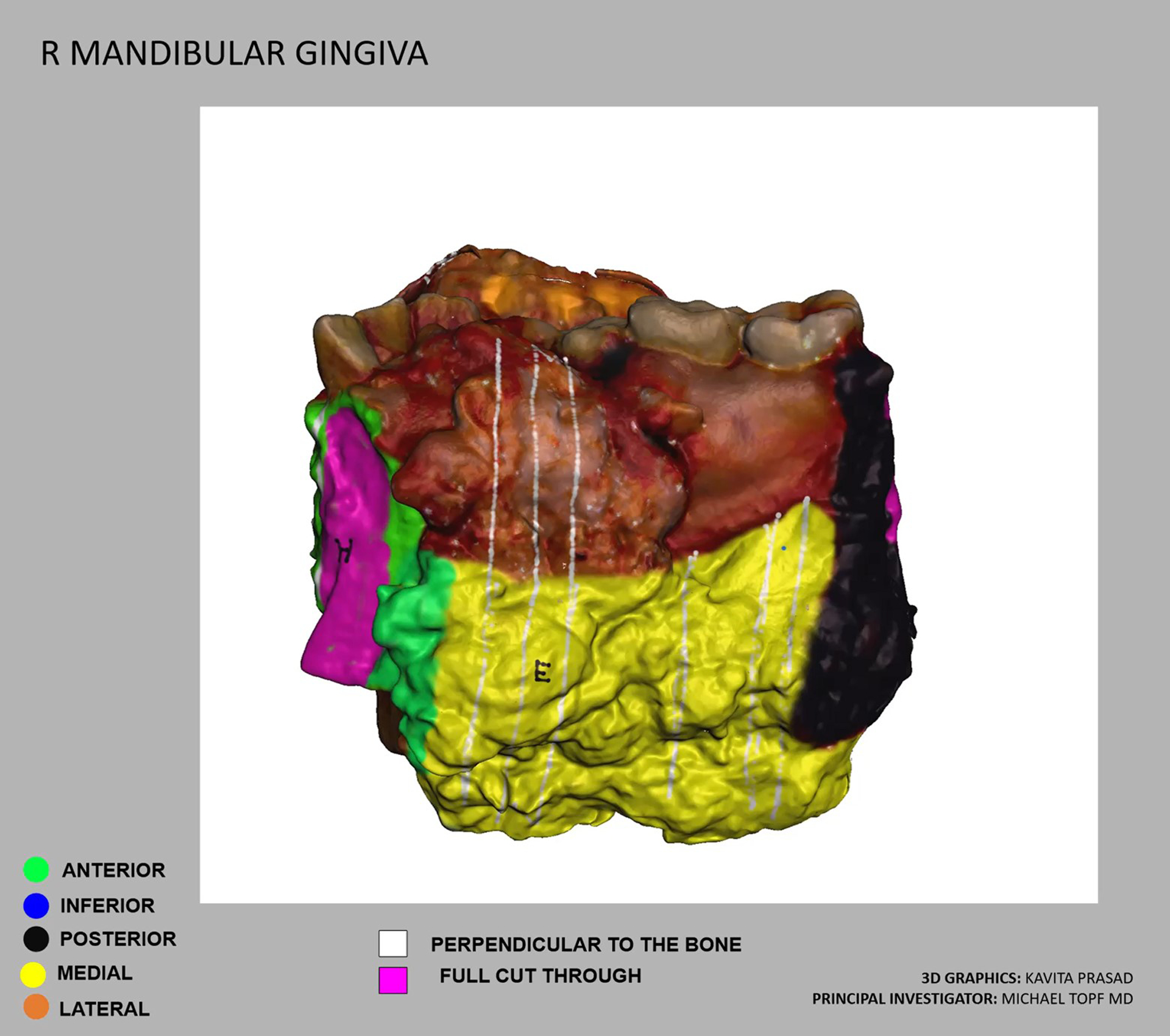
Similarly, two (7%) cases had close margins on final pathology. Case #5 was a total laryngectomy for chondrosarcoma with a close margin of <1 mm on final pathology. The specimen map was used to visually demonstrate the location of the close margin during postoperative discussion between surgeon and pathologist. Given that the surgery was performed for low grade chondrosarcoma, the patient was recommended for close observation of this close anterior soft tissue margin. Case #28 was a mandibular alveolar ridge oral cavity composite resection which included the ipsilateral level Ib lymph dissection en bloc. Final pathology revealed a close lateral soft tissue margin of 1 mm. The pathologist localized the area of concern on the virtual 3D specimen map (Figure 5) and shared this with the surgeon to communicate the close margin location. The surgeon confirmed that additional buccal fat was resected in this area and was sent as a separate specimen which was negative for carcinoma. The patient has been recommended for adjuvant radiation therapy based on the close margin and other pathologic risk factors.
Figure 5.
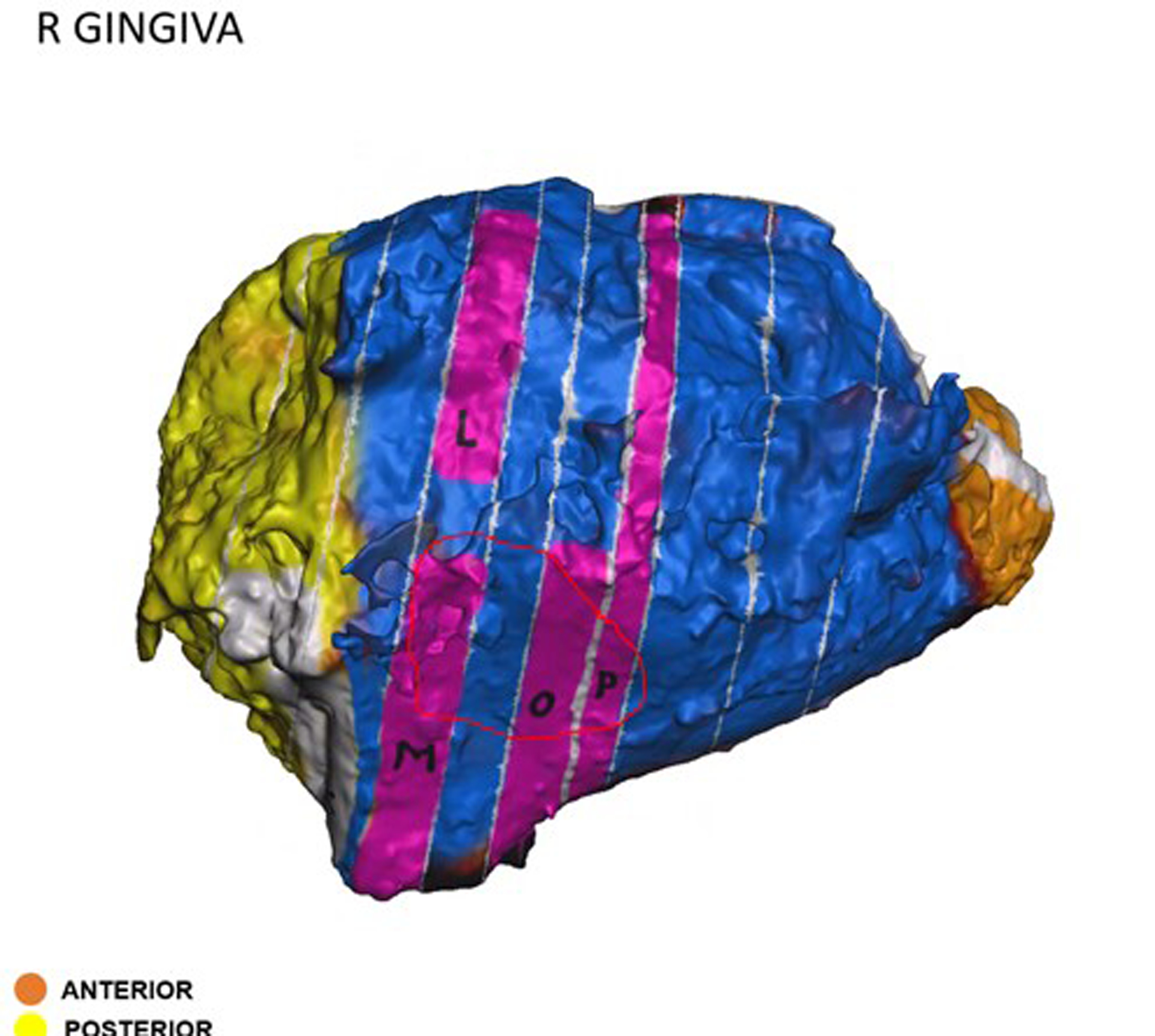
Virtual three-dimensional (3D) specimen map from a mandibular alveolar ridge oral cavity composite resection. A close margin, indicated by the red circle, was localized on the specimen map by the pathologist and communicated via email to the surgeon.
Indeterminate Margins
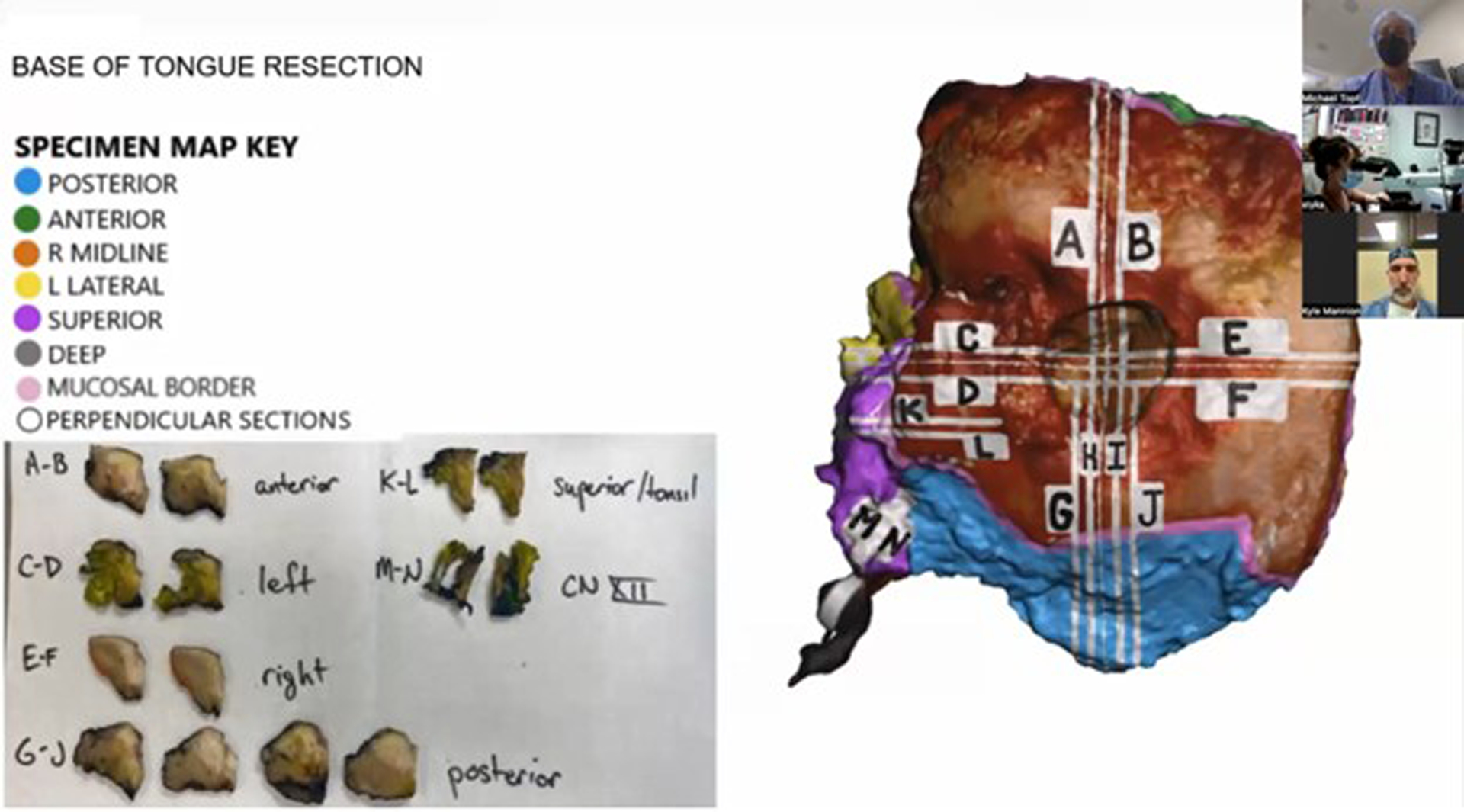
Four of the 28 cases (14%) had indeterminate margins, requiring additional communication between surgeon and pathologist to determine final margin status. Case #4 was an oral cavity composite resection with a positive inferior/anterior soft tissue margin on the main specimen. The location of the true margin, either from the main specimen or additional genioglossus resection, was uncertain. Using the 3D specimen map as a visual guide, the surgeon and pathologist determined the true margin to be on the additional specimen, superseding the main specimen and yielding a negative final margin. Case #10 was an oral cavity composite resection with a hemi-mandibulectomy. Carcinoma was present at the posterior margin, but the surgeon reported the true posterior margin was on an additional specimen. This margin was discussed by surgeon and pathologist, using the 3D specimen map, and was determined to be superseded by an additional defect-sampled tissue. Despite the final margin status, the patient required adjuvant treatment due to high-risk pathologic features including positive margin on the main specimen. The 3D specimen map was referenced during the multidisciplinary discussion to plan for adjuvant treatment. Case #12 was an oral cavity composite resection with mandibulectomy found to have invasive carcinoma present at the posterior-lateral mucosal and posterior soft tissue margins. It was uncertain if these were the true margins and required surgeon-pathologist discussion. The main specimen margin was determined to be superseded by additional frozen section margins, using the 3D specimen map to co-register margin location (Figure 6). Case #25 was also an oral cavity composite resection with mandibulectomy, reported to have a positive inferolateral soft tissue and anterior bone margin. The surgeon and pathologist discussed both margins, using the specimen map as a reference (Figure 7), and determined that the soft tissue margin was superseded by another specimen containing level I soft tissue and lymph nodes. The bone margin was deemed a final positive margin, requiring additional treatment. The 3D specimen map was shared at multidisciplinary tumor board, and the patient ultimately underwent additional mandibular resection.
Figure 6.
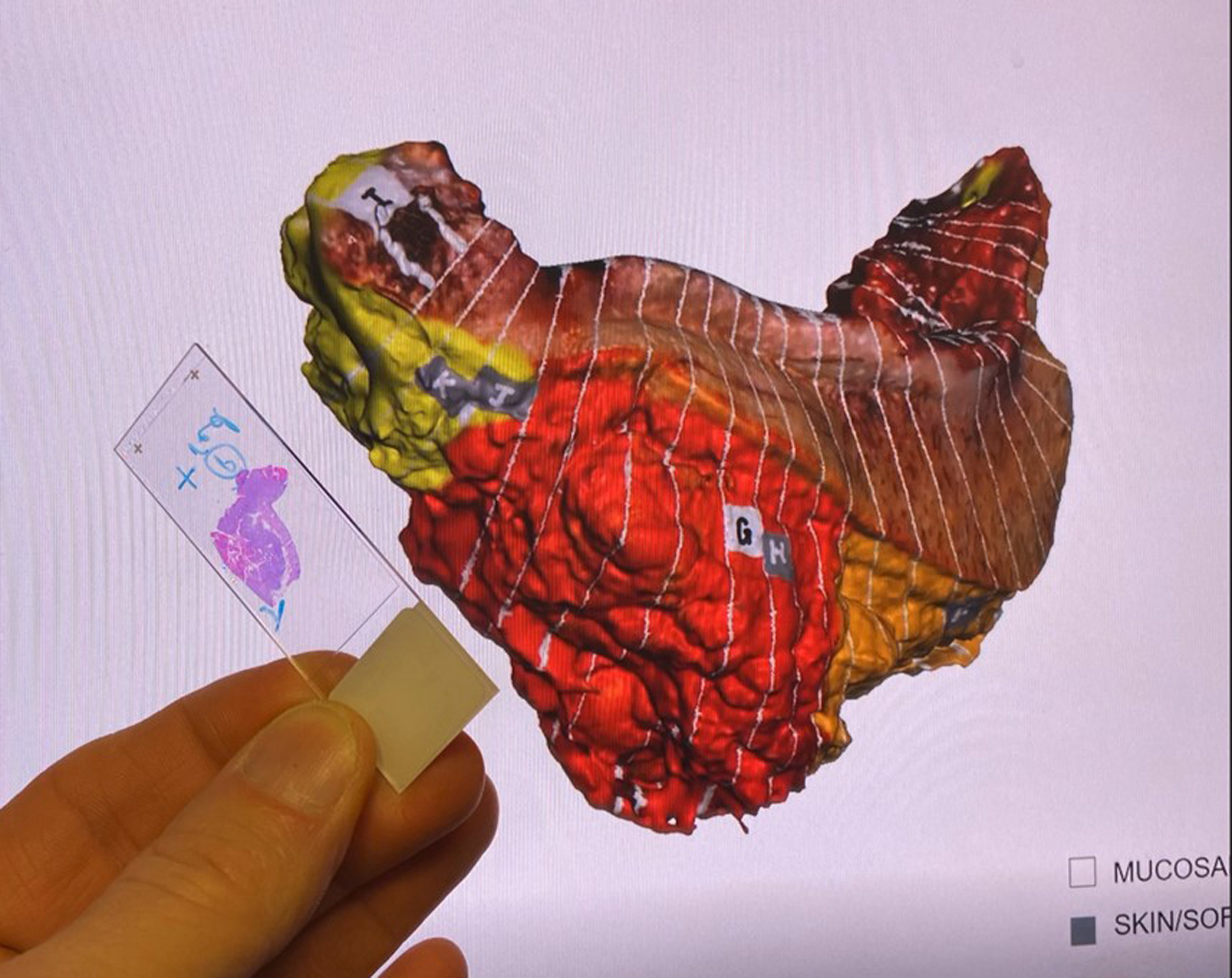
Pathologist co-registers and compares the positive margin on a histology slide and the margin location on the three-dimensional (3D) specimen map.
Figure 7.
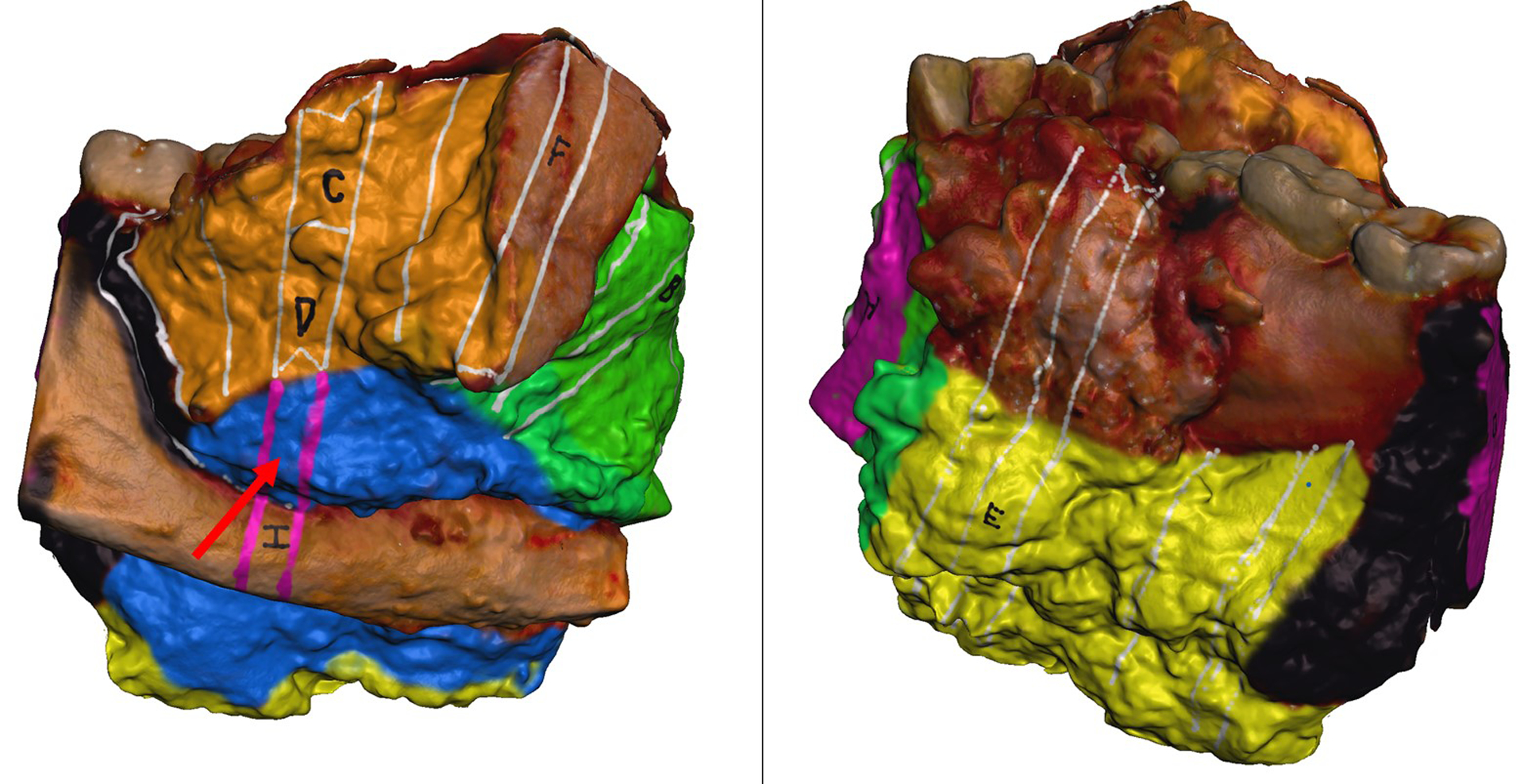
Virtual three-dimensional (3D) specimen map of an oral cavity composite resection with mandibulectomy. Site of an indeterminate margin indicated by red arrow. After postoperative discussion between surgeon and pathologist, this margin was determined to be superseded by another specimen.
Negative Margins
A majority of cases had negative margins on final pathology report (71%, n=20). In these cases, discussion of margins post-operatively is typically more straightforward and often does not require further discussion by surgeon or pathologist. While 3D specimen maps may prove less critical for surgeon-pathologist communication for these cases, they could demonstrate value for adjuvant radiation therapy treatment planning. We are currently investigating the impact of 3D specimen maps on adjuvant radiation therapy treatment fields (NCT #05743569). Case #19 was a total laryngectomy case with invasive poorly differentiated SCC on final pathology with negative margins. The patient was recommended for adjuvant radiation therapy based on high-risk pathologic features including perineural invasion. A 3D printed model and virtual model of the 3D specimen map were provided to the treating radiation oncologist to improve understanding of the surgical resection.
DISCUSSION
In this paper we detail the use of virtual 3D specimen maps for post-operative communication between surgeons and pathologists. We created post-operative 3D specimen maps for 28 head and neck cancer resections. The 3D specimen maps were utilized as an adjunct for communication of a margin between surgeons and pathologists in almost a third of cases in the setting of visualizing positive margin sites, tumor board discussions, and radiation treatment planning. For all cases, the 3D specimen maps serve as a permanent record of the specimen and its processing by pathology.
For cases in which there was a positive, close, or indeterminate margin, surgeons and pathologists were able to reference the margin sampling site on the main specimen, utilizing the 3D specimen map as a visual aid. Since the physical specimen is often destroyed during pathologic processing, the 3D specimen map allowed the surgeon to visualize the specimen and positive margin site before performing re-resection. Additionally, the precise location of the area of concern was referenced for planning of adjuvant therapies. In cases with an indeterminate margin, the specimen map was used to clarify whether the true margin was the positive margin from the main specimen or if this was superseded by additional resected tissue. Of note, given the existing data on positive specimen margins superseded by additional negative tissue,5 this is often considered an indication for adjuvant radiation therapy at our multidisciplinary head and neck tumor board.
Regardless of margin status or specimen type, the specimen maps were a useful resource for interdisciplinary communication. The specimen maps were distributed to all surgeons and pathologists involved, serving as a visual key for discussion. This was an improvement from the standard of care, which relies on microscopic slides and written margin names from the pathology gross description and/or final diagnosis lines, to orient and contextualize the exact location of the margins. We envision the pathology report of the future will include screenshots of 3D specimen maps or a video file that can be manipulated in real time by the provider.7 Additionally, the scans were used to facilitate discussion during multidisciplinary head and neck tumor board, as final margin status is a key factor in determining a patient’s adjuvant treatment course.8,9
The 3D specimen maps can be used to guide surgical re-resection. Accurately relocating the anatomic location of a positive margin for additional resection is challenging. A prospective study of 14 oropharyngeal SCC cases found that surgeons were unable to reliably locate margin sampling sites with error exceeding 1 cm in 32% of attempts.10 Coutu and colleagues found that only 20% of re-resections had further malignancy identified on pathology.11 Given these findings, it is not surprising that re-resection to negative margins does not reliably improve local recurrence rates.12–15 Furthermore, as time lapses between the initial surgery and re-resection, there is likely an increased need for a visual reference for the anatomic area of concern. The virtual 3D specimen maps may also be useful for coordinating cancer care for patients at multiple institutions as patients often receive adjuvant therapy outside of the medical center that performed their surgery. Brandwein and colleagues describe the difficult-to-decipher nature of operative and pathology reports, suggesting 3D images as a valuable adjunct to traditional methods, especially when coordinating cancer care at multiple hospitals.16
Positive surgical margins are an important prognostic factor for head and neck cancer and are associated with increased risk for cancer recurrence and mortality.17–19 Despite the challenges facing surgeons with margin relocation, Banoub et al. found that re-localization of margins by radiation oncologists was more variable than surgeons.20 Given the importance of radiation therapy in cancer care, we have a forthcoming study to investigate the utility of these virtual specimen maps in adjuvant radiation planning (NCT #05743569). We hypothesize that radiation oncologists will benefit from a visual reference for sites of margin sampling and could tailor locations and doses of radiation based on detailed margin distance measurements annotated onto 3D images.
The most notable limitations of this study are the retrospective nature and small sample size with limited variety of specimen types. These factors were primarily limited by cases available for research team and pathology coordination and will expand as we continue to map specimens. While all technology and software used for our 3D specimen mapping protocol is commercially available and relatively affordable, 3D specimen maps require several hours of work from a trained research team member. This does limit transferability of this technique to other institutions to some degree, though the authors report a reasonable learning curve. Additionally, the accuracy of the 3D specimen map is limited by the manual mapping by the research team member, requiring careful attention to the pathology processing and communication with the prosector to be as accurate as possible. Future work should focus on further evaluation of specimen map utility using a control group and quantifiable outcomes. It would also be worthwhile to quantify the impact of these specimen maps on radiation planning and surgical re-resection in the future. While this study has found the 3D specimen maps to be a helpful visual adjunct when discussing margins post-operatively, it remains to be investigated how frequently 3D specimen maps alter clinical care. Notably, the approach to margin analysis remains surgeon-dependent, and many of the cases presented in this retrospective review used a tumor-bed approach to margin analysis. The authors recognize the improved outcomes associated with a specimen-based approach.4,5 This limitation is particularly relevant for our indeterminate margins. While there are multiple cases with defect-sampled margins presented in this retrospective review, the authors are not recommending a defect-based approach to margin analysis.
CONCLUSION
Virtual 3D specimen maps are a useful visual adjunct to the standard of care in surgical pathology. The 3D specimen maps serve as a permanent record of the surgical specimen and its processing by pathology. These maps can be leveraged as a tool for communication amongst the multidisciplinary cancer care team, proving to be a particularly utilized tool when a positive, close, or indeterminate surgical margin is present on final pathology.
Funding:
This work was supported by a Vanderbilt Clinical Oncology Research Career Development Program – K12 NCI 2K12CA090625–22A1 and an ACS Institutional Research Grant (#IRG-19-139-60).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Meeting Information:
Oral Presentation
The Triological Society
Boston, Massachusetts, USA
May 3–5, 2023
Level of evidence: Level 4
REFERENCES
- 1.Orosco RK, Tapia VJ, Califano JA, et al. Positive Surgical Margins in the 10 Most Common Solid Cancers. Sci Rep. Apr 9 2018;8(1):5686. doi: 10.1038/s41598-018-23403-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black C, Marotti J, Zarovnaya E, Paydarfar J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. Dec 15 2006;107(12):2792–800. doi: 10.1002/cncr.22347 [DOI] [PubMed] [Google Scholar]
- 3.Sharif KF, Prasad K, Miller A, et al. Enhanced Intraoperative Communication of Tumor Margins Using 3D Scanning and Mapping: The Computer-Aided Design Margin. Laryngoscope. Dec 19 2022;doi: 10.1002/lary.30511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amit M, Na’ara S, Leider-Trejo L, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: A prospective randomized controlled study. Head Neck. Apr 2016;38 Suppl 1:E1803–9. doi: 10.1002/hed.24320 [DOI] [PubMed] [Google Scholar]
- 5.Maxwell JH, Thompson LD, Brandwein-Gensler MS, et al. Early Oral Tongue Squamous Cell Carcinoma: Sampling of Margins From Tumor Bed and Worse Local Control. JAMA Otolaryngol Head Neck Surg. Dec 2015;141(12):1104–10. doi: 10.1001/jamaoto.2015.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif KF, Lewis JS Jr, Ely KA, et al. The computer-aided design margin: Ex vivo 3D specimen mapping to improve communication between surgeons and pathologists. Head Neck. Jan 2023;45(1):22–31. doi: 10.1002/hed.27201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez AN, Sharif KF, Guelfi E, et al. Ex vivo 3D scanning and specimen mapping in anatomic pathology. J Pathol Inform. 2023;14:100186. doi: 10.1016/j.jpi.2022.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. [DOI] [PubMed] [Google Scholar]
- 10.Kerawala CJ, Ong TK. Relocating the site of frozen sections--is there room for improvement? Head Neck. Mar 2001;23(3):230–2. doi: [DOI] [PubMed] [Google Scholar]
- 11.Coutu B, Ryan E, Christensen D, et al. Positive margins matter regardless of subsequent resection findings. Oral Oncol. May 2022;128:105850. doi: 10.1016/j.oraloncology.2022.105850 [DOI] [PubMed] [Google Scholar]
- 12.Ettl T, El-Gindi A, Hautmann M, et al. Positive frozen section margins predict local recurrence in R0-resected squamous cell carcinoma of the head and neck. Oral Oncol. 2016;55:17–23. [DOI] [PubMed] [Google Scholar]
- 13.Patel RS, Goldstein DP, Guillemaud J, et al. Impact of positive frozen section microscopic tumor cut-through revised to negative on oral carcinoma control and survival rates. Head Neck. 2010;32:1444–51. [DOI] [PubMed] [Google Scholar]
- 14.Tasche KK, Buchakjian MR, Pagedar NA, Sperry SM. Definition of “Close Margin” in Oral Cancer Surgery and Association of Margin Distance With Local Recurrence Rate. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125:2298–307. [DOI] [PubMed] [Google Scholar]
- 16.Brandwein-Weber M, Urken ML, Topf MC, et al. Radical shift in the communication paradigm in head and neck frozen section analysis: Intraoperative three-dimensional specimen scanning. Head Neck. Jan 2023;45(1):7–9. doi: 10.1002/hed.27247 [DOI] [PubMed] [Google Scholar]
- 17.Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003;32(1):30–4. 10.1054/ijom.2002.0313. [DOI] [PubMed] [Google Scholar]
- 18.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg. 1990. Oct 1;160(4):410–4. [DOI] [PubMed] [Google Scholar]
- 19.Jesse RH, Sugarbaker EV. Squamous cell carcinoma of the oropharynx: Why we fail. Am J Surg. 1976;132(4):435–8. 10.1016/0002-9610(76)90314-7. [DOI] [PubMed] [Google Scholar]
- 20.Banoub RG, Crippen MM, Fiorella MA, et al. Variance in 3D anatomic localization of surgical margins based on conventional margin labeling in head and neck squamous cell carcinoma. Oral Oncol. Mar 14 2023;139:106360. doi: 10.1016/j.oraloncology.2023.106360 [DOI] [PMC free article] [PubMed] [Google Scholar]


