Abstract
Introduction
Current therapies for autoimmune rheumatic diseases (ARDs) have limited efficacy in certain patients, highlighting the need for the development of novel treatment approaches. This meta-analysis aims to assess the efficacy and safety of low-dose interleukin-2 (LD-IL-2) and evaluate the alterations in lymphocyte subsets in various rheumatic diseases following administration of different dosages of LD-IL-2.
Methods
A comprehensive search was conducted in PubMed, Web of Science, the Cochrane Library, Embase databases and CNKI to identify relevant studies. A total of 31 trials were included in this meta-analysis. The review protocols were registered on PROSPERO (CRD42022318916), and the study followed the PRISMA guidelines.
Results
Following LD-IL-2 treatment, patients with ARDs exhibited a significant increase in the number of Th17 cells and Tregs compared to their pre-treatment levels [standardized mean difference (SMD) = 0.50, 95% confidence interval (CI) (0.33, 0.67), P < 0.001; SMD = 1.13, 95% CI (0.97, 1.29), P < 0.001]. Moreover, the Th17/Tregs ratio showed a significant decrease [SMD = − 0.54, 95% CI (− 0.64, − 0.45), P < 0.001]. In patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), LD-IL-2 injection led to a significant increase in Treg numbers, and the Th17/Tregs ratio and disease activity scores, including Disease Activity Score-28 joints (DAS28), Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) and Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), were all significantly reduced. No serious adverse events were reported in any of the included studies. Additionally, 54.8% of patients with lupus nephritis achieved distinct clinical remission following LD-IL-2 treatment. Injection site reactions and fever were the most common side effects of LD-IL-2, occurring in 33.1% and 14.4% of patients, respectively.
Conclusion
LD-IL-2 treatment showed promise and was well tolerated in the management of ARDs, as it effectively promoted the proliferation and functional recovery of Tregs.
Trial Registration
Retrospectively registered (CRD42022318916, 21/04/2022).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-023-00620-7.
Keywords: Autoimmune rheumatic diseases, Tregs, Meta-analysis, Systematic review, Low-dose Interleukin-2 (LD-IL-2)
Key Summary Points
| Treatment with low-dose interleukin-2 (LD-IL-2) has been explored as a new clinical strategy for autoimmune rheumatic diseases (ARDs). However, there is currently no available information on evidence-based medicine regarding the changes in lymphocyte subsets in patients with autoimmune rheumatic diseases receiving LD-IL-2 therapy. |
| Disease activity scores, including Disease Activity Score-28 joints (DAS28), Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) and Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), were all significantly reduced following LD-IL-2 therapy. |
| LD-IL-2 was promising and well tolerated in treating ADRs, which could promote the proliferation and functional recovery of Tregs. |
| Continuous medication therapy with a dosage of 0.5 million IU (international unit) per day demonstrates a superior therapeutic effect compared to intermittent medication with a dosage of 1 million IU every other day. |
| Most adverse events associated with LD-IL-2 therapy were mild and resolved quickly after discontinuation. |
Introduction
Autoimmune rheumatic diseases (ARDs) are a group of autoimmune and/or inflammatory diseases characterized by immune dysfunction. These diseases can result in chronic systemic inflammation and damage to multiple organs. Previous studies have demonstrated high morbidity and disability rates associated with rheumatic diseases, imposing significant burdens on individuals, families and society [1]. Therefore, effective treatment of ARDs has always been a focus of clinical research.
T lymphocytes play a crucial role in adaptive immunity, and their dysregulation contributes to the pathogenesis of rheumatic diseases. T lymphocyte dysregulation includes cell dysfunction and quantitative imbalance. For example, in patients with SLE, quantitative imbalance manifests as expansion in some CD4+ cell subsets and reduction in regulatory T cells (Tregs) in the peripheral blood [2]. Cell dysfunction in patients with SLE manifests as the shifting of Th1 to Th2 immune responses and so on; these can be summarized as alterations within cytokine production, metabolism and epigenetic modifications in effector T cells, T helper cells (Th) and Tregs [3, 4]. Among these dysregulations, the hyperreactivity of Th cells and dysfunctional Tregs are particularly noteworthy. The balance between Th17 and Tregs is essential for maintaining immune homeostasis [5]. Thus, restoring the Th17/Tregs balance is crucial for the treatment of autoimmunity [6].
Interleukin-2 (IL-2), primarily produced by activated CD4+ T cells, is an indispensable cytokine for Tregs differentiation, survival and expansion [7]. Existing research has shown that IL-2 can dose-dependently promote the expansion and differentiation of different immune cell subsets. At low doses, IL-2 promotes Tregs, while at high doses, it promotes effector cells such as CD8+ T cells and natural killer (NK) cells [8]. Treatment with low-dose IL-2 (LD-IL-2) selectively expands Tregs, thereby restoring the Th17/Tregs balance. This approach has been explored as a new clinical strategy for ARDs [9]. However, there is currently no available information on evidence-based medicine regarding the changes in lymphocyte subsets in patients with autoimmune rheumatic diseases receiving LD-IL-2 therapy. The main purpose of this meta-analysis is to investigate the changes in lymphocyte subsets in patients with rheumatic diseases after LD-IL-2 therapy.
Methods
Data Sources and Search Strategy
This systematic meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines and registered on the International Prospective Register of Systematic Reviews (PROSPERO) trial registry (CRD42022318916). This article is based on a secondary analysis of previously conducted and published studies and does not contain any data on human participants or animals performed by any of the authors.
We searched PubMed, Web of Science, the Cochrane Library, Embase databases, WanFang database and CNKI for relevant articles published up to September 15, 2023. Our search strategy consisted of MeSH terms and entry terms with no restrictions on the language of the articles. We also scanned the reference lists of eligible articles for additional eligible articles that were not retrieved during the literature search. The relevant papers were identified using Medical Subject Headings (MeSH) terms: "Interleukin-2,″ "Rheumatic Diseases” and related free words.
Study Selection and Data Extraction
Inclusion criteria were original case reports, case series, observational studies (prospective and retrospective) and clinical trials that reported the changes in lymphocyte subsets in patients with ARDs receiving LD-IL-2 therapy. The criteria for the inclusion of studies in our meta-analysis were as follows: (1) the study population comprised participants aged 18 years old or older with ARDs; (2) the intervention was LD-IL-2 combined with conventional drug therapy. Treatment traditionally involves glucocorticoids and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs); (3) the levels of Treg cells, Th17 cells, Th1 cells, Th2 cells, CD4+ cells, CD8+ cells, NK cells or lymphocyte subset proportion in patients with autoimmune disease before and after treatment; (4) outcomes: Efficacy indicators, adverse event; (5) no limitation was applied for the disability level, sex and race of the study subjects. We excluded: (1) non-original studies or conference abstracts; (2) animal studies; (3) studies whose data were unreliable for extraction and analysis.
Two investigators independently assessed the studies based on the eligibility criteria; disagreements were resolved by a third investigator. A data collection sheet was used to record the first author's name, publication year, sample size, disease duration, treatment regimen, exposure duration, history of prior treatment, the levels of lymphocyte subsets proportion in patients before and after treatment, efficacy measures and treatment-related adverse events. Evidence was graded according to the Oxford Centre for Evidence-based Medicine 2011 Levels of Evidence. The quality of studies was assessed using the Newcastle-Ottawa quality assessment scale (NOS).
Statistical Analysis
We assessed the effect of LD-IL-2 therapy on the changes of lymphocyte subsets, such as the levels of Tregs, Th17 cells, Th1 cells, Th2 cells, CD4+ cells, CD8+ cells and NK cells. We also conducted subgroup analysis for drug dosage and different disease types of ARDs at the same time. Moreover, we evaluated the therapeutic efficacy and safety of LD-IL-2 therapy in ARDs. Stata 12. 0 software (Stata Corp, College Station, TX, USA) was used for the meta-analysis. Continuous data were summarized by using the standardized mean difference (SMD) with 95% CIs. Statistical significance was assumed for P < 0.05. We employed Stata 12.0 software for the meta-analysis. Subgroup analyses were used to further stratify the trials by treatment dose and combined administration. Heterogeneity was assessed by using the Cochran Q test and quantified with the I2statistic. The I-squared value (I2) was used to assess the statistical heterogeneity between studies. An I2 value of < 25% indicates low heterogeneity, 25%–75% moderate heterogeneity and > 75% considerable heterogeneity. In case of significant heterogeneity (I2 > 50%), the analysis was conducted under the random-effects model. Otherwise, a fixed-effect model was used. Sensitivity analysis was assessed by separately leaving each study out and rerunning the analysis with data. Funnel plots and Egger’s regression test were utilized to estimate the publication bias. Sensitivity analysis and Egger’s and Begg’s tests were conducted by STATA 12.0 software.
Result
Results of the Search Strategy
Through database searches, 18,766 records were found. We excluded 11547 titles and abstracts after initial screening, and 31 articles that met all eligibility criteria were finally selected for the meta-analysis. The specific screening process is shown in Fig. 1. The characteristics of the 31 included articles are shown in Tables 1, 2 and 3. Different kinds of ARDs were included in the selected studies: ten studies on systemic lupus erythematosus (SLE), seven studies on rheumatoid arthritis (RA), two studies on primary Sjogren's syndrome (pSS), three studies on psoriatic arthritis(PsA), five studies on myositis[including idiopathic inflammatory myopathies (IIM), polymyositis and dermatomyositis], one study on systemic sclerosis(SSc), one study on ANCA-associated vasculitis(AAV), one study on microscopic polyangiitis (MPA) and one study on Behcet’s disease (BD).
Fig. 1.
Study selection process. IL-2 Interleukin-2
Table 1.
Changes in the lymphocyte subsets and efficacy in patients with RA after LD-IL-2
| Study, year | No.of patients | Dosage | Th17 cells | Tregs | Th17/Tregs | DAS-28 |
|---|---|---|---|---|---|---|
|
Sheng-Xiao Zhang [10] 2021 |
233 | 0.5 million IU |
Before:6.27 ± 5.67 cells/μlA fter:10.98 ± 8.92 cells/μl |
Before:25.49 ± 27.94 cells/μlA fter:92.16 ± 79.41 cells/μl |
Before:0.30 ± 0.21A fter:0.17 ± 0.18 |
Before:5.54 ± 1.34 After:3.66 ± 1.09 |
|
X. Jia [11] 2018 |
75 | 0.5 million IU |
Before:0.88 ± 0.84 cells/μlA fter:1.98 ± 1.99 cells/μl |
|||
|
Sheng-Xiao Zhang [12] 2016 |
26 | 0.5 million IU |
Before:16.51 ± 19.06 cells/μlA fter:19 ± 11.38 cells/μl |
Before:18.67 ± 14.08 cells/μlA fter:78.55 ± 44.76 cells/μl |
Before:1.36 ± 1.49A fter:0.28 ± 0.20 |
Before:3.60 ± 0.96 After:2.85 ± 0.67 |
|
X. Jia [13] 2018 |
156 | 0.5 million IU |
Before:0.91 ± 0.79 cells/μlA fter:2.1 ± 1.81 cells/μl |
|||
|
Jia Wang [14] 2019 |
26 | 0.5 million IU |
Before:9 ± 8.81 cells/μlA fter:17.2 ± 12.81 cells/μl |
Before:22.6 ± 11.33 cells/μlA fter:65.2 ± 39.78 cells/μl |
Before:0.4 ± 0.39A fter:0.23 ± 0.24 |
Before:5.30 ± 0.89 After:2.70 ± 0.67 |
|
Jia Wang [15] 2022 |
26 | 0.5 million IU |
Before:9 ± 8.89 cells/μlA fter:17 ± 14.81 cells/μl |
Before:23 ± 11.85 cells/μlAf ter:65 ± 40 cells/μl |
Before:0.40 ± 0.39A fter:0.23 ± 0.24 |
|
|
Ming Yan [16] 2018 |
233 | 0.5 million IU |
Values are mean ± SD
RA rheumatoid arthritis, DAS-28 Disease Activity Score-28 joints, IL-2 interleukin-2, LD-IL-2 low-dose IL-2, 0.5 million IU 0.5 million international unit per day of IL-2 subcutaneous injection for 5 days. SD standard deviation
Table 2.
Changes in the lymphocyte subsets and efficacy in patients with SLE after LD-IL-2
| Study, year | No. of patients | Dosage | Th17 cells | Tregs | Th17/Tregs | SELENA-SLEDAI |
|---|---|---|---|---|---|---|
|
Jing He [17] 2019 |
30 | 1 million IU |
Before:12 ± 4.75 After:6 ± 4 |
|||
|
Jing He [18] 2016 |
23 | 1 million IU |
Before:12.77 ± 10.25 cells/μl After:14.19 ± 6.07 cells/μl |
Before:0.4 ± 0.35 cells/μl After:0.19 ± 0.51 cells/μl |
Before:11.14 ± 3.79 After:3.92 ± 2.23 |
|
|
Miao Shao [19] 2019 |
15 | 1 million IU |
Before:4.1 ± 1.28 cells/μl After:2.67 ± 2.07 cells/μl |
Before:10.64 ± 3.67 cells/μl After:14.11 ± 4.03 cells/μl |
||
|
Chunmiao [20] 2019 |
50 | 1 million IU |
Before:7.24 ± 5.21 cells/μl After:10.19 ± 5.69 cells/μl |
Before:0.83 ± 0.12 cells/μl After:0.34 ± 0.04cells/μl |
Before:5.92 ± 0.36 After:4.05 ± 0.31 |
|
|
Sheng-xiao Zhang [21] 2019 |
54 | 0.5 million IU |
Before:16.9 ± 1.69 cells/μl After:32.73 ± 8.27 cells/μl |
Before:0.22 ± 0.5 cells/μl After:0.19 ± 0.24 cells/μl |
||
|
Jing Wang [22] 2017 |
76 | 0.5 million IU |
Before:7.42 ± 0.62 cells/μl After:10.79 ± 10.67 cells/μl |
Before:34.02 ± 1.24 cells/μl After:57.55 ± 45.19 cells/μl |
Before:0.42 ± 0.53 cells/μl After:0.18 ± 0.23 cells/μl |
Before:10.87 ± 6.48 After:5.83 ± 4.18 |
|
Jia-Qian Zhang [23] 2022 |
41 | 0.5 million IU | Before:6.29 ± 6.01 cells/μl; After:10.02 ± 9.19 cells/μl |
Before:14.87 ± 11.4 cells/μl After:56.69 ± 40.76 cells/μl |
Before:0.3 ± 0.2 cells/μl After:0.19 ± 0.43 cells/μl |
|
|
Kai Fan [24] 2018 |
76 | 0.5 million IU |
Before:8.5 ± 6.6 cells/μl After:15.3 ± 21.4 cells/μl |
Before:28 ± 33 cells/μl After:80 ± 50 cells/μl |
||
|
J. Y. Humrich [25] 2022 |
24 | 1.5 million IU |
Before:11.30 ± 3.37 After:5.18 ± 1.75 |
|||
|
H. Zhou [26] 2023 |
11 | 1 million IU |
Before:9.72 ± 5.26 After:8.26 ± 3.36 |
Values are mean ± SD
SLE systemic lupus erythematosus, SELENA-SLEDAI Lupus Erythematosus National Assessment (SELENA) version of the SLE Disease Activity Index (SLEDAI), IL-2 interleukin-2, LD-IL-2 low-dose IL-2, 0.5 million IU 0.5 million international unit per day of IL-2 subcutaneous injection for 5 days, 1 million IU 1 million IU of IL-2 injection every other day for 2 weeks, followed by a 2-week break, 1.5 million IU 1.5 million IU of IL-2 injection per day for 5 days. SD standard deviation
Table 3.
Changes in the lymphocyte subsets and efficacy in patients with other autoimmune rheumatic diseases including pSS, PsA, DM, IIM, BD, MPA, AAV and SSc after LD-IL-2
| Study,.year | Illness | No. of patient | Dosage | Th17 cells | Treg cells | Th17/Treg cells | Efficacy |
|---|---|---|---|---|---|---|---|
|
Miao Miao [27] 2018 |
pSS | 82 | 0.5 million IU |
Before:8.44 ± 7.32 cells/μlA fter:11.47 ± 8.12 cells/μl |
Before:21.02 ± 15.29 cells/μlA fter:81.21 ± 58.28 cells/μl |
Before:0.68 ± 1.5A fter:0.22 ± 0.27 |
|
|
M. Miao [28] 2017 |
pSS | 88 | 0.5 million IU |
Before:6.49 ± 5.29 cells/μlA fter:9.74 ± 6.74 cells/μl |
Before:16.67 ± 12.35 cells/μlA fter:66.67 ± 52.47 cells/μl |
Before:0.33 ± 0.32A fter:0.22 ± 0.16 |
|
|
Jia Wang [29] 2020 |
PsA | 22 | 0.5 million IU |
Before:6.36 ± 6.39 cells/μlA fter:10 ± 9.76 cells/μl |
Before:26.26 ± 10.99 cells/μlA fter:70.48 ± 44.82 cells/μl |
Before:0.25 ± 0.23A fter:0.13 ± 0.19 |
|
|
Sheng-xiao Zhang [30] 2019 |
PsA | 22 | 0.5 million IU |
Before:6.71 ± 6.32 cells/μlA fter:10.37 ± 9.48 cells/μl |
Before:27.44 ± 11.3 cells/μlA fter:71.65 ± 44.04 cells/μl |
||
|
Z. Qiao [31] 2023 |
PsA | 45 | 0.5 million IU |
Before:0.62 ± 0.25 cells/μlA fter:0.26 ± 0.07 cells/μl |
|||
|
Min Feng [32] 2019 |
DM | 35 | 0.5 million IU |
Before:2.62 ± 2.82 cells/μlA fter:3.57 ± 6.70 cells/μl |
Before:8.1 ± 7.59 cells/μlA fter:20.71 ± 14.47 cells/μl |
||
|
X. Zheng [33] 2022 |
DM | 13 | 0.5 million IU |
Before:1.83 ± 5.03 cells/μlA fter:9.60 ± 4.58 cells/μl |
Before:10.52 ± 6.40 cells/μlA fter:29.73 ± 19.20 cells/μl |
Before:0.17 ± 0.79 cells/μlA fter:0.32 ± 0.24 cells/μl |
|
|
M. Miao [34] 2020 |
IIM | 15 | 1 million IU |
Before:8.18 ± 4.27 cells/μlA fter:15.2 ± 2.69 cells/μl |
CDASI Before:7 ± 5.19 After:2 ± 2.96 |
||
|
Miao Miao [34] 2021 |
IIM | 18 | 1 million IU |
Before:52.01 ± 8.21 cells/μlA fter:84.31 ± 20.25 cells/μl |
CDASI Before:7 ± 6.30 After:2 ± 5.19 |
||
|
Zhufeng, Y. Z [35] 2022 |
IIM | 13 | 1 million IU |
CDASI Before:12 ± 7.04 After:3 ± 4.07 |
|||
|
Liu, X [36] 2020 |
BD | 39 | 0.5 million IU |
Before:4.75 ± 5.86 cells/μlA fter:10.28 ± 11.13 cells/μl |
Before:13.45 ± 7.62 cells/μlA fter:56.17 ± 38.67 cells/μl |
Before:0.39 ± 0.19A fter:0.37 ± 0.29 |
|
|
Ruihe Wu [37] 2022 |
MPA | 15 | 0.5 million IU |
Before:4.58 ± 3.45 cells/μlA fter:6.76 ± 5.01 cells/μl |
Before:17.77 ± 10.5 cells/μlA fter:59.77 ± 49.83 cells/μl |
Before:0.31 ± 0.24A fter:0.14 ± 0.1 |
|
|
R. Wu [38] 2020 |
AAV | 13 | 0.5 million IU |
Before:2.71 ± 4.36 cells/μlA fter:3.21 ± 11.93 cells/μl |
Before:19.63 ± 4.79 cells/μlA fter:44.2 ± 50.94 cells/μl |
Before:0.17 ± 0.18A fter:0.09 ± 0.1 |
|
|
Zhen Yu [39] 2022 |
SSc | 23 | 0.5 million IU |
Before:3.78 ± 3.07 cells/μlA fter:7.68 ± 4.96 cells/μl |
Before:15.75 ± 15.02 cells/μlA fter:88.79 ± 78.74 cells/μl |
Before:0.22 ± 0.15A fter:0.16 ± 0.25 |
Values are mean ± SD
CDASI Cutaneous Dermatomyositis Disease Area and Severity Index, pSS primary Sjogren's syndrome, PsA psoriatic arthritis, DM dermatomyositis, IIM idiopathic inflammatory myopathies, BD Behcet’s disease, MPA microscopic polyangiitis, AAV ANCA-associated vasculitis, SSc systemic sclerosis, IL-2 interleukin-2, LD-IL-2 low-dose IL-2, 0.5 million IU 0.5 million international unit per day of IL-2 subcutaneous injection for 5 days, 1 million IU 1 million IU of IL-2 injection every other day for 2 weeks, followed by a 2-week break
Dynamic Changes of Lymphocyte Subsets in Patients with ARDs
Overall Change
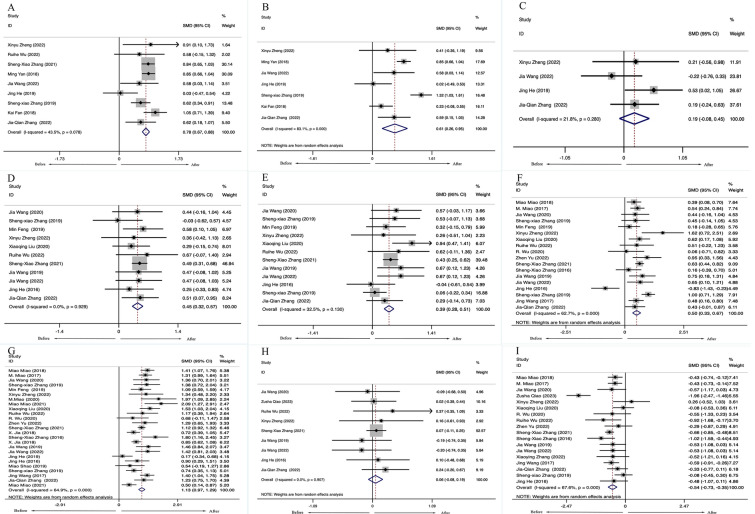
Thirty-one studies, including 1618 patients with ARDs, reported the changes in lymphocyte subset levels after LD-IL-2 therapy. The meta-analysis showed that patients with ARDs had a higher number of CD4+ T cells [SMD = 0.78, 95%CI (0.67, 0.88), P <0.001], CD8+ T cells [SMD = 0.61, 95% CI (0.26, 0.95), P < 0.001], Th1 cells [SMD = 0.45, 95% CI (0.32, 0.57), P < 0.001], Th2 cells [SMD = 0.39, 95% CI (0.28, 0.51), P < 0.001], Th17 cells [SMD = 0.50, 95% CI (0.33,0.67), P < 0.001] and Tregs [SMD = 1.13, 95% CI (0.97, 1.29), P < 0.001] after receiving LD-IL-2 compared with these patients before treatment. There were no statistical differences in the numbers of NK cells [SMD = 0.19, 95% CI (− 0.08, 0.45), P = 0.170] between before and after LD-IL-2 therapy. (Fig. 2).
Fig. 2.
Overall change in lymphocyte subsets in patients with ARDs. (A) CD4+. (B) CD8+. (C) NK. (D) Th1. (E) Th2. (F) Th17.(G) Treg. (H) Th1/Th2 (I) Th17/Treg. SMD standardized mean difference, CI confidence interval, ARDs autoimmune rheumatic diseases, NK natural killer, TH T helper cell, Treg regulatory T cells
Notably, a meta-analysis of 17 studies, including 817 patients with ARDs showed that the ratio between Th17 cells and Tregs (Th17/Tregs ratio) was decreased [SMD = − 0.54, 95% CI (− 0.64, − 0.45), P < 0.001] (Fig. 2I). There were no statistical differences in the ratio between Th1 cells and Th2 cells (Th1/Th2 ratio) [SMD = 0.06, 95% CI (− 0.08, 0.19, P = 0.907] (Fig. 2H). Significant heterogeneity across studies was observed in all the meta-analyses (range 21.8% to 95.6%). This may be due to different disease types of ARDs and drug dosages.
In the screening process, the vast majority of studies used LD-IL-2 combined with conventional drug therapy as a treatment approach. Treatment traditionally involves glucocorticoids and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs).
We found that there were only two studies that separately investigated LD-IL-2 in combination with other drugs [tumor necrosis factor-alpha inhibitor (TNFi) and rapamycin][40, 41]. These results also showed that, for patients with autoimmune rheumatic disease, the ratio between Th17 cells and Tregs was decreased after treatment.
Neither funnel plots (Supplementary Materials Figure S1) nor Egger tests showed evidence of publication bias in included studies of CD4+ cells (t = − 1.38, p = 0.209), CD8+ cells (t =− 1.19, p = 0.286), NK cells (t =− 0.12, 321 p = 0.915), Th1 cells (t = − 1.07, p = 0.314), Th2 cells (t = 0.69, p = 0.503), Th17 cells (t =− 0.94, p = 0.359), Treg cells (t = 1.31, p = 0.202), ratio of Th1/Th2 (t = − 0.21, p = 0.838) and the ratio of Th17/Treg (t = 0.14, p = 0.891). The sensitivity analyses did not affect the direction of results. (Supplementary Materials Figure S3).
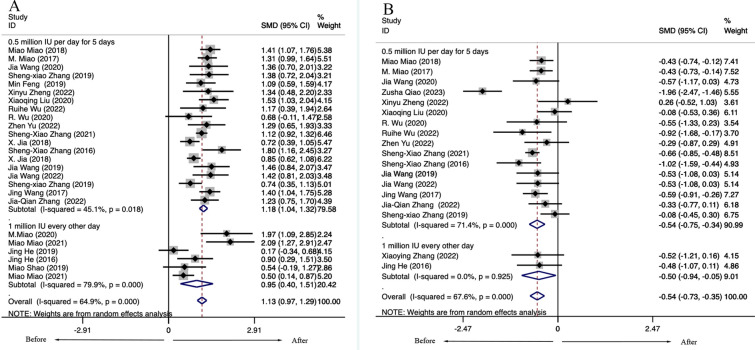
Variation Based on Different IL-2 Dosages
Considering different doses of LD-IL-2 on the treatment effect, we performed a subgroup analysis. Currently, the common drug regimens in clinics are 0.5 million IU (international unit) per day of IL-2 subcutaneous injection for 5 days or 1 million IU of IL-2 injection every other day for 2 weeks, followed by a 2-week break. The meta-analysis showed LD-IL-2 of patients with ARD results in both increased numbers of Tregs and decreased ratio of Th17/Tregs regardless of the method of LD-IL-2 administration. For patients receiving 0.5 million IU per day of IL-2 injection for 5 days, the ratio of Th17/Tregs was significantly decreased [Tregs: 0.5 million IU: SMD = 1.18, 95% CI (1.04, 1.32), P < 0.001; 1 million IU SMD = 0.95, 95%CI (0.40, 1.51), P < 0.001; Th17/Tregs: 0.5 million IU: SMD = − 0.54, 95% CI (− 0.75, − 0.34), P < 0.001; 1 million IU: SMD = − 0.50, 95% CI (− 0.94, − 0.05), P < 0.001] (Fig. 3).
Fig. 3.
Variation based on different IL-2 dosages in patients with autoimmune diseases. (A) Treg. (B) Th17/Treg. IL-2 interleukin-2, TH T helper cell, Treg regulatory T cells, SMD standardized mean difference, CI confidence interval, IU international unit
Variation Based on Different Rheumatic Diseases
Based on different rheumatic diseases, we performed a subgroup analysis. The subgroup analysis was mainly performed based on both RA and SLE.
Six studies, including 542 patients with RA, reported the absolute number of lymphocyte subsets in RA treated with LD-IL-2. The meta-analysis of the number of lymphocyte subsets showed that patients with RA had higher numbers of CD4+T cells [SMD = 0.83, 95% CI (0.70, 0.96), P = 0.668], Th1 cells [SMD = 0.49, 95% CI (0.32, 0.66), P = 0.995], Th2 cells [SMD = 0.48, 95% CI (0.31, 0.64), P = 0.552] and CD8+ T cells [SMD = 0.82, 95% CI (0.64, 1.00), P = 0.375] after receiving LD-IL-2 compared with these patients before treatment. There were no statistical differences in the ratio of Th1/Th2 [SMD = 0.02, 95% CI (− 0.14, 0.19), P = 0.473] and NK cells [SMD = − 0.22, 95% CI (− 0.76, 0.33), P = 0.868] before and after LD-IL-2 treatment. The numbers of Tregs [SMD = 1.12, 95% CI (0.86, 1.38), P < 0.001] and Th17 cells [SMD = 0.60, 95% CI (0.44, 0.76), P = 0.009] were significantly increased, while the ratio between Th17 cells and Tregs [SMD = − 0.66, 95% CI (− 0.82, − 0.50), P = 0.724] was decreased after LD-IL-2 injection (Fig. 4).
Fig. 4.
Variation based on different disease types. (A) CD4+. (B) CD8+. (C) NK. (D) Th1. (E) Th2. (F) Th17. (G) Treg. (H) Th1/Th2 (I) Th17/Treg. SMD standardized mean difference, CI confidence interval, RA rheumatoid arthritis, SLE systemic lupus erythematosus, DM dermatomyositis, PsA psoriatic arthritis, pSS primary Sjogren's syndrome, IM idiopathic inflammatory myopathies, AAV ANCA-associated vasculitis, NK natural killer
Seven studies, including 299 patients with SLE, reported the absolute number of lymphocyte subsets in SLE treated with LD-IL-2. Increased circulating Th1 [SMD = 0.41, 95% CI (0.06, 0.76), P = 0.484], CD4+T cells [SMD = 0.67, 95%CI (0.49, 0.85), P = 0.012] and NK cells [SMD = 0.33, 95% CI (0.00, 0.66), P = 0.324] were found in patients with SLE treated with LD-IL-2 therapy, while there were no significant changes in the CD8+ T cells [SMD = 0.56, 95% CI (− 0.06, 1.18), P < 0.001]. The number of and Th2 cells [SMD = 0.10, 95% CI (− 0.12, 0.32), P = 0.593] as well as the ratio of Th1/Th2 [SMD = 0.19, 95% CI (−0.16, 0.53), P = 0.712] did not show significant change before and after LD-IL-2 therapy of patients with SLE. In addition, the absolute number of Tregs in the peripheral blood of patients with SLE was significantly increased [SMD = 0.80, 95% CI (0.46, 1.14), P = 0.001], and Th17/Treg ratio was significantly decreased after treatment [SMD = -0.37, 95% CI (− 0.61, − 0.12), P = 0.239]. However, there were no significant changes in the number of Th17 cells [SMD = 0.31, 95% CI (− 0.30, 0.93), P < 0.001](Fig. 4).
Efficacy and Safety of LD-IL-2 Treatment in Patients with ARDs
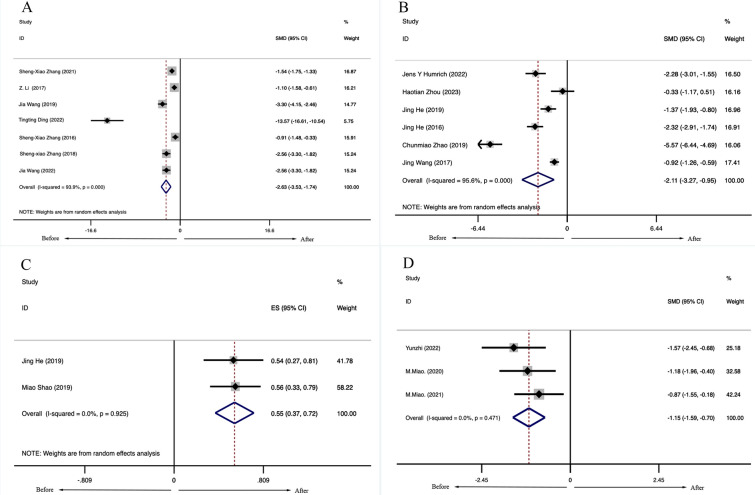
Among the articles included in the meta-analysis, some articles not only reported the changes in lymphocyte subsets after patients received LD-IL-2 treatment but also reported the changes in disease activity indicators and adverse events. Meta-analysis of 5 clinical studies, including 311 patients with RA, showed that the Disease Activity Score in 28 joints (DAS-28) of patients treated with LD-IL-2 was significantly decreased [SMD =− 2.63, 95% CI (− 3.53 − 1.74), P < 0.001] (Fig. 5A). There was statistically significant heterogeneity (I2 = 93.9%, P < 0.001). Meta-analysis of 6 observational studies, including 229 patients with SLE, showed that the Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index scores (SELENA-SLEDAI) were significantly decreased [SMD = − 2.11, 95% CI (− 3.72, − 0.95), P < 0.001] (Fig. 5B). Moreover, after the LD-IL-2 treatment, 54.8% of patients with lupus nephritis had distinct clinical remission. However, considerable heterogeneity (I2 = 81.9%, p < 0.001) may be due to the small number of studies (Fig. 5C). In addition, the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) scores of patients with IIM were significantly decreased after LD-IL-2 treatment [SMD = − 1.15, 95% CI (− 1.59, − 0.70), P = 0.471] (Fig. 5D).
Fig. 5.
Efficacy and safety of low-dose IL-2 (LD-IL-2). A Disease Activity Score-28 joints scores of RA treated with LD-IL-2. B The Lupus Erythematosus National Assessment (SELENA) version of the SLE Disease Activity Index scores of SLE treated with LD-IL-2. C The remission rate of patients with lupus nephritis after the LD-IL-2 treatment. D The Cutaneous Dermatomyositis Disease Area and Severity Index scores (CDASI) of patients with idiopathic inflammatory myopathies after LD-IL-2 treatment. RA rheumatoid arthritis, SLE systemic lupus erythematosus, IL-2 interleukin-2, SMD standardized mean difference, CI confidence interval
Three studies including 301 patients with RA reported adverse drug reactions (ADRs), and the overall rate was 0.083 (95% CI 0.051–0.114). No serious adverse events were reported in all these studies. Among the 48 patients with SLE identified from 2 studies reporting adverse events, the leading two common side effects were hematological injection site reaction and fever. Injection site reaction is a common dermatological change characterized by pain, redness and swelling at the site of injection. In patients with systemic lupus erythematosus (SLE), injection site reactions were observed in 33.1% of cases. Fever occurred in 14.4%, a common systemic effect of patients with SLE treated with LD-IL-2. Most of these adverse events were mild, and patients recovered quickly after discontinuation.
Neither funnel plots (Supplementary Materials Fig. S2) nor Egger tests showed evidence of publication bias in included studies of DAS-28 scores (t =− 2.47, p = 0.069) or SELENA-SLEDAI scores (t =− 1.49, p = 0.211). Egger tests showed publication bias in the studies of CDASI (t = − 54.64, p = 0.012), but this was probably due to the small number of patients enrolled in the study. The sensitivity analyses did not affect the direction of results. (Supplementary Materials Fig. S4).
Discussion
LD-IL-2 therapy has been shown to effectively counter-regulate inflammation in the treatment of ARDs while avoiding global immunosuppression, which is crucial for optimal treatment outcomes [42]. Our meta-analysis further supports the excellent therapeutic effect of LD-IL-2 in ARDs by upregulating Tregs to restore immune balance, with good tolerability.
The findings of our meta-analysis indicate that patients with ARDs experience an increase in the proportion of Th17 cells and Tregs after LD-IL-2 therapy. Additionally, the ratio of Th17/Tregs is significantly reduced, indicating a restoration of immune balance. Furthermore, other lymphocyte subsets also exhibit notable changes. Importantly, continuous medication therapy with a dosage of 0.5 million IU per day demonstrates a superior therapeutic effect compared to intermittent medication with a dosage of 1 million IU every other day.
We also investigated the effect of LD-IL-2 on lymphocyte subsets and the safety of patients with SLE and RA. Our results demonstrated that the ratio of Th17/Tregs cells decreased in patients with RA and SLE after LD-IL-2 treatment, indicating the superior ability of LD-IL-2 in expanding Tregs. However, we observed that only patients with RA showed a significant increase in Th1 and Th2 cells after treatment, with no statistical difference in the Th1/Th2 ratio. It has been reported that the pathogenesis of RA is also associated with an imbalance of Th1/Th2 cells, but LD-IL-2 treatment has a limited impact on Th1/Th2 regulation. Therefore, combining IL-2 with other drugs may lead to better therapeutic outcomes. In patients with RA, the DAS-28 scores were significantly reduced after medication, indicating that LD-IL-2 can effectively reduce disease activity. On the other hand, there was no statistical difference in Th17 cells after treatment in patients with SLE. For patients with SLE, the SELENA-SLEDAI scores were significantly decreased after medication. Previous research has shown that IL-2 can affect the disease activity of patients with SLE by reducing the level of Th17 cells, thereby decreasing SLEDAI [20]. However, our study found that IL-2 did not reduce Th17 cell levels in patients with SLE. It has been reported that patients with SLE treated with IL-2 may develop elevated levels of anti-IL-2 autoantibodies [43]. The induction of these autoantibodies may potentially affect IL-2-mediated changes in Th17 cells, which could explain our findings in patients with SLE.
Tregs play a crucial role in maintaining peripheral tolerance and controlling inflammation and autoimmunity in ARDs. However, disturbances in Treg biology, either numerically or functionally, are commonly observed in ARDs, leading to an imbalance between pathogenic and protective immune cells. Additionally, a relative deficiency of IL-2 in ARDs further disrupts Treg homeostasis, contributing to the breach of tolerance and chronic inflammation during disease pathogenesis [42].
Tregs express high-affinity IL-2 receptors, and LD-IL-2 promotes Treg differentiation through the STAT5 mechanism while inhibiting the differentiation of Th17 cells by preventing STAT3 activation. This promotes the balance between autoimmunity and immune tolerance in ARDs [44]. However, our study found that Tregs increased after LD-IL-2 treatment, but Th17 cells also increased. This finding is inconsistent with the proposed mechanism. Further investigation revealed that while Th17 cells increased or remained unchanged, the Th17/Tregs ratio decreased significantly. This indicates that the increase in Tregs was more significant than the increase in Th17 cells, resulting in a significant decrease in the Th17/Tregs ratio and thereby regulating the balance between effector T cells and Tregs.
In addition to supporting Tregs, IL-2 also plays a major role in promoting the proliferation of CD4+ T cells and the terminal differentiation of CD8+ T cells. This increased ability to fight infections should be taken into consideration [8]. Notably, IL-2 has a short half-life because of its low molecular weight. Therefore, to maintain efficient IL-2 bioavailability, administration must be repeated at close intervals [45].
LD-IL-2 therapy is generally well tolerated at lower dose ranges, with the most commonly reported adverse events being injection site reactions and flu-like syndromes. These adverse events were mostly mild and resolved quickly with symptomatic treatment or discontinuation. However, it should be noted that IL-2 dose escalation above 1 MIU/day may not be well tolerated in some patients and can lead to increased expansion of NK cells and higher frequency of side effects [46].
Our study had several limitations. First, we did not fully consider the effects of specific types of ARDs and disease duration, which may have contributed to the heterogeneity observed among the included studies. Second, the number of studies available for subgroup analysis was limited, which may have introduced bias to our results. Third, only published studies were included in the meta-analysis, and unpublished articles were excluded. Although no publication bias was detected through the funnel plot and Egger and Begg tests, the potential for publication and study selection bias still exists and may have influenced our findings. Therefore, further studies are needed to validate our results.
Conclusion
In conclusion, LD-IL-2 therapy has demonstrated favorable therapeutic effects on ARDs by modulating the proportion of lymphocyte subsets, and the incidence of adverse reactions among patients has been acceptable. LD-IL-2 treatment promotes the balance between effector T cells and regulatory T cells in patients with ARDs, resulting in reduced disease activity and restoration of immune balance. Our meta-analysis also provides evidence that daily injections of 0.5 million IU of IL-2 are more effective in inducing the balance of Th17/Treg and maintaining immune homeostasis compared to injections of 1 million IU every other day. Additionally, LD-IL-2 treatment can reduce disease activity in patients with RA and SLE by impacting the proportion of lymphocyte subsets. The majority of adverse events associated with LD-IL-2 therapy were mild and resolved quickly after discontinuation. In summary, LD-IL-2 therapy holds promise for revolutionizing the treatment of ARDs, but further intensive and long-term research is necessary to evaluate and enhance the effectiveness of this therapeutic approach.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Sheng-Xiao Zhang conceived, designed the study, and critically revised the manuscript. Qinyi Su collected the data, performed the statistical analysis, and drafted the manuscript. Xinmiao Wang, Yongzhi Li, Jiexiang Zhang, Cairui Bai, Xuechun Wang, Liu Yang, and Jingting Zhang contributed to the data collection and statistical analysis. All the authors read and approved the final manuscript.
Funding
This project and its publication, including the journal’s Rapid Service Fee, was supported by grants from the National Natural Science Foundation of China (no. 82001740) and the Natural Science Foundation of Shanxi Province (no. 202203021221269).
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files and text.
Declarations
Conflict of Interest
Qinyi Su, Xinmiao Wang, Yongzhi Li, Jiexiang Zhang, Cairui Bai, Xuechun Wang, Liu Yang, Jingting Zhang, Sheng-Xiao Zhang have nothing to disclose.
Ethical Approval
This article is based on a secondary analysis of previously conducted and published studies and does not contain any data on human participants or animals performed by any of the authors.
References
- 1.Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. 2019;78(11):1463–1471. doi: 10.1136/annrheumdis-2019-215920. [DOI] [PubMed] [Google Scholar]
- 2.Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol. 2020;21(6):605–614. doi: 10.1038/s41590-020-0677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenbrock K, Rauen T. T cell dysregulation in SLE. Clin Immunol. 2022;239:109031. doi: 10.1016/j.clim.2022.109031. [DOI] [PubMed] [Google Scholar]
- 4.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56(7):481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang YJ, Dai SM. Prevalence of rheumatic diseases and disability in China. Rheumatol Int. 2009;29(5):481–490. doi: 10.1007/s00296-008-0809-z. [DOI] [PubMed] [Google Scholar]
- 6.Shin JS, Kim I, Moon JS, et al. Intranuclear delivery of HIF-1α-TMD alleviates EAE via functional conversion of TH17 cells. Front Immunol. 2021;12:741938. doi: 10.3389/fimmu.2021.741938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasching P, Stradner M, Graninger W, et al. Therapeutic Potential of Targeting the Th17/Treg Axis in Autoimmune Disorders. Molecules 2017;22(1). [DOI] [PMC free article] [PubMed]
- 8.Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15(5):283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Kolios AGA, Liu Y, et al. Therapeutic potential of interleukin-2 in autoimmune diseases. Trends Mol Med. 2022;28(7):596–612. doi: 10.1016/j.molmed.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Zhang SX, Wang J, Wang CH, et al. Low-dose IL-2 therapy limits the reduction in absolute numbers of circulating regulatory T cells in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2021;13:1759720x211011370. [DOI] [PMC free article] [PubMed]
- 11.Jia X, An H, Fan K, Li F, Li X. Absolute number of peripheral CD4-CD25+FOXP3+T cells decreases and restores after low-dose interleukin-2 treatment in rheumatoid arthritis. Ann Rheum Dis. 2018;77:942–942. [Google Scholar]
- 12.Mang SX, Miao M, Liu XQ, Ma XW, Wu XY, Li XF. The Efficacy and Safety of Low Dose IL-2 Therapy in over-Treated Patients with Rheumatoid Arthritis: A Preliminary Study. Arthritis & Rheumatology 2016;68.
- 13.Jia X, An H, Fan K, Li F, Li X, Study on absolute number of peripheral CD8 + CD25 + Foxp3 + T cells in patients with rheumatoid arthritis and effect of low-dose interleukin-2 on it. International J Rheumatic Dis 2018. 21: 98–99.
- 14.Wang J, Sun HH, Qiao J, et al. Effect and clinical efficacy of low dose IL-2 on regulatory T cells in active rheumatoid arthritis. Chin Clin Res. 2019;32(10):1310–1314. [Google Scholar]
- 15.Wang J, Zhang SX, Chang JS, et al. Low-dose IL-2 improved clinical symptoms by restoring reduced regulatory T cells in patients with refractory rheumatoid arthritis: a randomized controlled trial. Front Immunol. 2022;13:947341. doi: 10.3389/fimmu.2022.947341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan, M. Low-Dose IL-2 Restored Reduced Regulatory T Cells in Patients with Rheumatoid Arthritis. Arthritis & Rheumatology, 2018. 70.
- 17.He J, Zhang R, Shao M, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79(1):141–149. doi: 10.1136/annrheumdis-2019-215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Zhang X, Wei Y, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 19.Shao M, He J, Zhang R, et al. Interleukin-2 deficiency associated with renal impairment in systemic lupus erythematosus. J Interferon Cytokine Res. 2019;39(2):117–124. doi: 10.1089/jir.2018.0016. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Chu Y, Liang Z, et al. Low dose of IL-2 combined with rapamycin restores and maintains the long-term balance of Th17/Treg cells in refractory SLE patients. BMC Immunol. 2019;20(1):32. doi: 10.1186/s12865-019-0305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SX, Wang J, Wang CH, et al. Low-dose il-2 therapy rescues decreased peripheral lymphocytes in SLE patients with different infectious status. Lupus Sci Med. 2019;6:A35–A36. [Google Scholar]
- 22.Jing Wang, Effect of IL-2 on Th17 and Treg cells in patients with systemic lupus erythematosus. 2017, Shanxi Medical University.
- 23.Zhang JQ, Zhang SX, Wang J, et al. Low-dose IL-2 therapy limits the reduction in absolute numbers of peripheral lymphocytes in systemic lupus erythematosus patients with infection. Curr Med Res Opin. 2022;38(6):1037–1044. doi: 10.1080/03007995.2022.2065145. [DOI] [PubMed] [Google Scholar]
- 24.Kai Fan, Expression of CD4~+Treg and CD8~+Treg cells in peripheral blood of patients with systemic lupus erythematosus and effect of low dose of IL-2 on their levels. 2018, Shanxi Medical University.
- 25.Humrich JY, et al. Low-dose interleukin-2 therapy in active systemic lupus erythematosus (LUPIL-2): a multicentre, double-blind, randomised and placebo-controlled phase II trial. Ann Rheum Dis. 2022;81(12):1685–1694. doi: 10.1136/ard-2022-222501. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, et al. Low-dose IL-2 mitigates glucocorticoid-induced Treg impairment and promotes improvement of SLE. Signal Transduct Target Ther. 2023;8(1):141. doi: 10.1038/s41392-023-01350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao M, Hao Z, Guo Y, et al. Short-term and low-dose IL-2 therapy restores the Th17/Treg balance in the peripheral blood of patients with primary Sjögren's syndrome. Ann Rheum Dis. 2018;77(12):1838–1840. doi: 10.1136/annrheumdis-2018-213036. [DOI] [PubMed] [Google Scholar]
- 28.Miao M, Hao Z, Guo Y, et al. The number of treg cells in peripheral blood in pss patients is decreased and low dose IL-2 can promote its proliferation. Ann Rheum Dis. 2017;76:869–869. [Google Scholar]
- 29.Wang J, Zhang SX, Hao YF, et al. The numbers of peripheral regulatory T cells are reduced in patients with psoriatic arthritis and are restored by low-dose interleukin-2. Ther Adv Chronic Dis. 2020;11:2040622320916014. doi: 10.1177/2040622320916014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SX, Hao YF, Wang J, Sun HH, Xiao-Feng LI. Imbalance of TH17 and regulatory T cells in patients with psoriatic arthritis and rebalance by low-dose IL-2. Ann Rheum Dis. 2019;78:1267–1268. [Google Scholar]
- 31.Qiao Z et al. Low-dose Interleukin-2 for psoriasis therapy based on the regulation of Th17/Treg cell balance in peripheral blood. Inflammation, 2023. [DOI] [PMC free article] [PubMed]
- 32.Feng M, Guo H, Zhang C, et al. Absolute reduction of regulatory T cells and regulatory effect of short-term and low-dose IL-2 in polymyositis or dermatomyositis. Int Immunopharmacol. 2019;77:105912. doi: 10.1016/j.intimp.2019.105912. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X, et al. Low-dose IL-2 therapy restores imbalance between Th17 and regulatory T cells in patients with the dermatomyositis combined with EBV/CMV viremia. Autoimmun Rev. 2022;21(11):103186. doi: 10.1016/j.autrev.2022.103186. [DOI] [PubMed] [Google Scholar]
- 34.Miao M, Li Y, Huang B, et al. Treatment of active idiopathic inflammatory myopathies by low-dose interleukin-2: a prospective cohort pilot study. Rheumatol Ther. 2021;8(2):835–847. doi: 10.1007/s40744-021-00301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhufeng Y, Xu J, Miao M, et al. Modification of intestinal microbiota dysbiosis by low-dose interleukin-2 in dermatomyositis: a post hoc analysis from a clinical trial study. Front Cell Infect Microbiol. 2022;12:757099. doi: 10.3389/fcimb.2022.757099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Li W, Liu X, Luo J, Gao C, Li X. Low-dose IL-2 effectively restored decreased regulatory T cells in patients with Behçet's disease. Clin Exp Rheumatol. 2021;39(4):746–752. doi: 10.55563/clinexprheumatol/lnn76t. [DOI] [PubMed] [Google Scholar]
- 37.Wu R, Mu Y, Zhao X, et al. Short-term and low-dose IL-2 therapy increases the reduced Treg cells in patients with microscopic polyangiitis. Autoimmun Rev. 2022;21(9):103156. doi: 10.1016/j.autrev.2022.103156. [DOI] [PubMed] [Google Scholar]
- 38.R Wu, R Su, T Ding, H Xue, C Wang. Low-Dose IL-2 restores treg-mediated immune tolerance in patients with anca-associated vasculitis. Ann Rheumatic Dis, 2020. 79: 1079–1080.
- 39.Yu Z, Cheng H, Ding T, et al. Absolute decrease in regulatory T cells and low-dose interleukin-2 therapy: restoring and expanding regulatory T cells to treat systemic sclerosis: a 24-week study. Clin Exp Dermatol. 2022;47(12):2188–2195. doi: 10.1111/ced.15345. [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Chu Y, Liang Z, Zhang B, Wang X, Jing X, et al. Low dose of IL-2 combined with rapamycin restores and maintains the long-term balance of Th17/Treg cells in refractory SLE patients. BMC Immunol. 2019;20(1):32. doi: 10.1186/s12865-019-0305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tingting D. Low dose IL-2 combined with TNF- α Clinical efficacy and immunomodulatory effects of inhibitors in the treatment of rheumatoid arthritis, Shanxi Medical University (2022)
- 42.Graßhoff H, Comdühr S, Monne LR, et al. Low-dose IL-2 therapy in autoimmune and rheumatic diseases. Front Immunol. 2021;12:648408. doi: 10.3389/fimmu.2021.648408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao M, Sun XL, Sun H, et al. Clinical relevance of autoantibodies against interleukin-2 in patients with systemic lupus erythematosus. Chin Med J (Engl) 2018;131(13):1520–1526. doi: 10.4103/0366-6999.235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu R, Li N, Zhao X, et al. Low-dose Interleukin-2: Biology and therapeutic prospects in rheumatoid arthritis. Autoimmun Rev. 2020;19(10):102645. doi: 10.1016/j.autrev.2020.102645. [DOI] [PubMed] [Google Scholar]
- 45.Pol JG, Caudana P, Paillet J, Piaggio E, Kroemer G. Effects of interleukin-2 in immunostimulation and immunosuppression. J Exp Med 2020;217(1). [DOI] [PMC free article] [PubMed]
- 46.Harris F, Berdugo YA, Tree T. IL-2-based approaches to Treg enhancement. Clin Exp [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files and text.