Abstract
Objectives
To assess cigarette smoking’s effects on efficacy of the preferential Janus kinase (JAK) 1 inhibitor filgotinib and drug persistence in patients with rheumatoid arthritis (RA).
Methods
Efficacy in non-smokers, former smokers, and current smokers from phase 3 filgotinib trials was analyzed, including patients with inadequate response (IR) to methotrexate (MTX) or biologic disease-modifying antirheumatic drugs (bDMARDs) or who were MTX-naïve. Proportions achieving Disease Activity Score in 28 joints with C-reactive protein (DAS28[CRP]) ≤ 3.2 were compared using logistic regression. Retrospective claims-based switching data were reviewed.
Results
Week 12 (W12) DAS28(CRP) ≤ 3.2 was achieved by 50, 61, and 62% of MTX-IR non-smokers, former smokers, and current smokers taking filgotinib 200 mg (FIL200) + MTX vs. 23, 16, and 32% taking placebo + MTX (p < 0.001, < 0.001, and 0.001) and 50, 34, and 33% taking adalimumab + MTX (p = 0.97, 0.013, and 0.006 vs. FIL200 + MTX). W12 DAS28(CRP) ≤ 3.2 was achieved by 46, 48, and 32% of bDMARD-IR non-smokers, former smokers, and current smokers taking FIL200 + conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) vs. 16, 23, and 5% taking placebo + csDMARD (p < 0.001, 0.077, and 0.051); 57, 58, and 59% of respective MTX-naïve smoking groups achieved W12 DAS28(CRP) ≤ 3.2 with FIL200 + MTX vs. 28, 37, and 18% with MTX (p < 0.001, 0.026, and < 0.001). Claims data showed former/current smokers were likelier than non-smokers to switch from adalimumab to other biologics or JAK inhibitors.
Conclusions
Greater proportions of MTX-IR current/former smokers responded to FIL200 + MTX vs. adalimumab + MTX. In non-smoking MTX-IR, bDMARD-IR, and MTX-naïve patients with RA, FIL200 + MTX demonstrated increased response vs. controls. Current/former smokers were likelier to discontinue adalimumab vs. non-smokers in real-world clinical settings.
Trial Registration
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-023-00619-0.
Keywords: Arthritis, Rheumatoid, Smoking, Tumor necrosis factor inhibitors, JAK inhibitors
Key Summary Points
| Why carry out this study? |
| Cigarette smoking is associated with greater disease activity in patients with rheumatoid arthritis (RA) and with a reduced response to some RA therapies. |
| The impact of smoking on the efficacy of filgotinib, an oral Janus kinase 1 inhibitor, in patients with RA is unknown. |
| What was learned from this study? |
| Filgotinib provides greater efficacy vs. placebo or methotrexate (MTX) to patients with RA who were MTX-naïve or had an inadequate response to MTX regardless of smoking status, as well as superior disease control vs. adalimumab among current/former smokers with an inadequate MTX response. |
| While smokers with RA may expect a reduced effect of MTX or adalimumab vs. non-smokers, patients receiving filgotinib may experience similar efficacy regardless of smoking status. |
Introduction
Cigarette smoking is an important risk factor for the development of rheumatoid arthritis (RA) [1] and is associated with higher RA disease activity [2]. Furthermore, smokers with RA have been described as less likely to respond to some treatments and more likely to discontinue or switch treatments compared with non-smokers [3–6]. Filgotinib, an oral preferential Janus kinase (JAK) 1 inhibitor, was evaluated in three phase 3 clinical studies in adults with moderately to severely active RA [7–9]. In subgroup analysis in patients with poor prognostic factors for disease progression (e.g., seropositivity, high-sensitivity C-reactive protein [CRP] ≥ 4 mg/l, high disease activity, and erosion score > 0 at baseline), filgotinib 200 mg (FIL200) + methotrexate (MTX) demonstrated similar efficacy as seen in the overall population [10]. However, the impact of smoking on the efficacy of filgotinib in patients with RA was not studied. We conducted a post hoc subgroup analysis of filgotinib phase 3 trial data to identify any associations of treatment efficacy with smoking status. We also performed an insurance claims-based study evaluating odds of switching treatment among smokers and non-smokers in real-world clinical settings.
Methods
Randomized Trial Analysis
Designs of the FINCH 1 (inadequate response to MTX [MTX-IR]; NCT02889796) [7], FINCH 2 (inadequate response to biologic disease-modifying antirheumatic drugs [bDMARD-IR]; NCT02873936) [8], and FINCH 3 (MTX-naïve; NCT02886728) [9] trials have been described previously. The FINCH 1 study randomized patients with MTX-IR active RA in a 3:3:2:3 ratio to FIL200 + MTX, FIL100 + MTX, subcutaneous adalimumab 40 mg every 2 weeks + MTX, or placebo + MTX [7]. Eligible patients had ≥ 6 swollen joints and ≥ 6 tender joints both at screening and day 1 despite ongoing MTX treatment for ≥ 12 weeks with stable treatment at 7.5–25 mg/week for ≥ 4 weeks. In FINCH 2, patients with active RA who were bDMARD-IR were randomized 1:1:1 to once-daily FIL200 + stable conventional synthetic DMARDs (csDMARDs, including MTX), FIL100 + csDMARDs, or placebo + csDMARDs [8]. Eligible patients had ≥ 6 swollen joints and ≥ 6 tender joints at both screening and baseline and a serum CRP level of 4 mg/l or greater based on central laboratory assessment at screening. The FINCH 3 study randomized patients who were MTX-naïve with active RA in a 2:1:1:2 ratio to FIL200 + MTX, FIL100 + MTX, FIL200 monotherapy, or MTX [9]. Eligible patients had limited (< 3 doses ≤ 25 mg) or no prior MTX exposure, swollen joint count ≥ 6 of 66 joints, and ≥ 6 tender joints of 68 joints at screening and day 1.
The randomized trials were conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation guidelines. Protocols were approved by the institutional review board or ethics committee at each site. All participants provided written informed consent on enrollment. FINCH 1 was approved by the Advarra Central Institutional Review Board (Reference # 00000971). FINCH 2 was approved by the Administrative Panel on Human Subjects in Medical Research (Reference # 4593). FINCH 3 was approved by the Ethics Committee Research UZ/KU Leuven (Reference # S59627).
Multivariable-adjusted logistic regression models were fitted to assess the effects of self-reported current, former, or never smoking status on the likelihood of obtaining low disease activity, as measured by week 12 Disease Activity Score in 28 joints with CRP (DAS28[CRP]) ≤ 3.2. Additional efficacy endpoints included American College of Rheumatology 20, 50, or 70% improvement (ACR20, ACR50, ACR70), Clinical Disease Activity Index (CDAI) low disease activity (≤ 10), and DAS28(CRP) remission (< 2.6). Missing patient-response data were imputed using non-responder imputation (109/1755 [6%] of patients with MTX-IR, 47/448 [10%] of patients with bDMARD-IR, and 99/1249 [8%] of patients who were MTX-naïve were non-responders imputed). Baseline factors (selected based on the authors’ subject-matter expertise) were tested for association with DAS28(CRP) ≤ 3.2 response at week 12 after stratification for smoking status (self-reported as no history of smoking, former history of smoking, or current smoking); those with significant (p < 0.05) association were included as covariates in each model (Supplementary Materials Table 1). Displayed response rates and confidence intervals were determined from the logistic regression models. All p values are nominal, without adjustment for multiple testing.
Table 1.
Baseline demographic and clinical characteristics among subgroups by smoking status across the FINCH 1, FINCH 2, and FINCH 3 randomized trials
| Non-smoker (n = 2488) | Former smoker (n = 477) | Current smoker (n = 481) | Total (N = 3446) | p value | |
|---|---|---|---|---|---|
| Baseline DAS28(CRP) ≤ 5.1 | 603 (24.2) | 144 (30.2) | 121 (25.2) | 868 (25.2) | 0.023 |
| Age, years, mean (SD) | 52.1 (13.5) | 57.7 (12.1) | 53.7 (10.4) | 53.1 (13.0) | < 0.001 |
| Sex, female | 2145 (86.2) | 308 (64.6) | 298 (62.0) | 2751 (79.8) | < 0.001 |
| Geographic region | < 0.001 | ||||
| Group Aa | 727 (29.2) | 293 (61.4) | 212 (44.1) | 1232 (35.8) | |
| Group Bb | 1180 (47.4) | 99 (20.8) | 164 (34.1) | 1443 (41.9) | |
| Group Cc | 329 (13.2) | 24 (5.0) | 41 (8.5) | 394 (11.4) | |
| Group Dd | 98 (3.9) | 10 (2.1) | 11 (2.3) | 119 (3.5) | |
| Group Ee | 154 (6.2) | 51 (10.7) | 53 (11.0) | 258 (7.5) | |
| Number of prior bDMARDs, mean (SD) | 0.2 (0.7) | 0.4 (1.0) | 0.3 (0.9) | 0.3 (0.8) | 0.004 |
| Duration of RA, mean (SD) | 6.5 (7.8) | 6.4 (8.5) | 5.7 (7.4) | 6.3 (7.8) | 0.147 |
| Baseline HAQ-DI, mean (SD) | 1.6 (0.6) | 1.5 (0.6) | 1.5 (0.6) | 1.6 (0.6) | < 0.001 |
| Oral corticosteroid use | 1097 (44.1) | 186 (39.0) | 230 (47.8) | 1513 (43.9) | 0.021 |
| Baseline comorbidity | 1189 (47.8) | 316 (66.2) | 271 (56.3) | 1776 (51.5) | < 0.001 |
| Baseline NSAID use | 1325 (53.3) | 275 (57.7) | 306 (63.6) | 1906 (55.3) | < 0.001 |
| CCP antibody positive | 1826 (73.4) | 352 (73.8) | 382 (79.4) | 2560 (74.3) | 0.021 |
| Rheumatoid factor positive | 1738 (69.9) | 349 (73.2) | 382 (79.4) | 2469 (71.6) | < 0.001 |
Data are presented as n (%) unless otherwise noted. p values are based on Pearson’s chi-squared test (categorical variable) or linear model ANOVA (continuous variable)
aGroup A consisted of Australia, Belgium, Canada, Germany, Ireland, Israel, Italy, Netherlands, New Zealand, Republic of Korea, South Africa, Spain, the UK, and the US (FINCH 1); Australia, Belgium, France, Germany, Israel, Italy, Netherlands, South Korea, Spain, Switzerland, the UK, and the US (FINCH 2); and Australia, Belgium, Canada, France, Germany, Ireland, Israel, Italy, Netherlands, New Zealand, Republic of Korea, Singapore, South Africa, Spain, Sweden, Switzerland, the UK, and the US (FINCH 3);
bGroup B consisted of Bulgaria, Czech Republic, Hungary, India, Poland, Romania, Russian Federation, Serbia, Slovakia, and Ukraine (FINCH 1); Hungary, Czech Republic, and Poland (FINCH 2); and Bulgaria, Croatia, Czech Republic, Estonia, Georgia, Hungary, India, Latvia, Moldova, Poland, Romania, Russian Federation, Serbia, Slovakia, and Ukraine (FINCH 3);
cGroup C consisted of Argentina and Mexico (FINCH 1); Argentina, Puerto Rico, and Mexico (FINCH 2); and Argentina, Brazil, Chile, Colombia, Mexico, Peru, and Puerto Rico (FINCH 3);
dGroup D consisted of Hong Kong, Taiwan, and Thailand (FINCH 1); China (originally planned but no patients were screened or enrolled from China; FINCH 2); and China, Hong Kong, Malaysia, Philippines, Taiwan, Thailand, and Vietnam (FINCH 3);
eGroup E consisted of Japan
ANOVA analysis of variance; bDMARD biologic disease-modifying antirheumatic drug; CCP cyclic citrullinated peptide; DAS28(CRP) Disease activity score in 28 joints with C-reactive protein; HAQ-DI health assessment questionnaire–disability index; NSAID non-steroidal anti-inflammatory drug; RA rheumatoid arthritis; SD standard deviation
Insurance Claims-Based Study
A retrospective, US-based cohort study was conducted using PharMetrics Plus data between January 1, 2006 and October 31, 2021. Patients with RA and a history of exposure to certolizumab, golimumab, etanercept, abatacept, infliximab, adalimumab, or tocilizumab were included in the cohort (baricitinib, tofacitinib, and rituximab were not approved for first-line biologic or targeted-therapy treatment in the US, and exposure to these drugs was not considered in the inclusion criteria) [11–13]. Patients aged < 18 years or with < 6 months of follow-up were excluded. A new-user design was employed in which patients were followed from the date of prescription of first biologic until the earliest date of switch to a different biologic or JAK inhibitor, loss of insurance coverage, or end of the study period. Recognizing that patients may switch medications for any number of possible reasons, treatment switching was the outcome of interest as a proxy for lack of medication effectiveness, as efficacy outcomes were not available from the claims data. A Cox proportional hazards model was used to estimate time to switch to a new RA biologic or JAK inhibitor drug in different smoking status groups (defined by ICD-9 or ICD-10 codes: 3051, 6490, 98,984, F172, 09933, P042, P9681, T652, Z716, and Z720 for current smokers; V1582 and Z878 for former smokers; patients with none of these codes were assumed to be non-smokers) while adjusting for sex, age, previous and ongoing csDMARD use, time since first RA diagnosis code appearing in the data, and corticosteroid use.
Patient and Public Involvement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination of plans of this research.
Results
Randomized Trial Analysis
Data from 3446 patients with RA with available smoking status from the FINCH studies were analyzed. Table 1 and Supplementary Materials Table 2 show baseline demographics and clinical characteristics. The population included 14.0% current smokers, 13.8% former smokers, and 72.2% non-smokers, with similar proportions across trials. A lower proportion of current and former smokers were female vs. non-smokers (62.0, 64.6, and 86.2%, respectively); current smokers had a mean disease duration of 5.7 years vs. 6.4 and 6.5 years among former smokers and non-smokers. Proportions of patients with bDMARD-IR with cyclic citrullinated peptide (CCP) antibody varied by smoking status (p = 0.026); 83.8% of current smokers were CCP antibody positive vs. 67.9% of former smokers and 68.3% of non-smokers (Supplementary Materials Table 3). Among patients with MTX-IR, 82.8, 82.3, and 78.4% of current smokers, former smokers, and non-smokers, respectively, were CCP antibody positive; respective percentages were 73.1, 67.4, and 67.8% among patients who were MTX-naïve.
Table 2.
Difference in response rate at week 12 vs. placebo + MTX in patients with MTX-IR by smoking status
| Smoking status | Adalimumab + MTX (n = 323) | Filgotinib 100 mg + MTX (n = 480) | Filgotinib 200 mg + MTX (n = 475) | |
|---|---|---|---|---|
| ACR20 | Non-smoker | + 22%a | + 16%a | + 23%a |
| Former smoker | + 19% | + 36%a | + 46%a | |
| Current smoker | + 17% | + 27%b | + 27%b | |
| ACR50 | Non-smoker | + 17%a | + 14%a | + 24%a |
| Former smoker | + 13% | + 33%a | + 45%a | |
| Current smoker | + 4% | + 17%c | + 27%b | |
| ACR70 | Non-smoker | + 9%a | + 9%a | + 18%a |
| Former smoker | + 6% | + 30%b | + 29%b | |
| Current smoker | − 3% | + 9% | + 15%c | |
| CDAI LDA | Non-smoker | + 18%a | + 11%b | + 22%a |
| Former smoker | + 14% | + 24%c | + 34%a | |
| Current smoker | + 7% | + 17%c | + 25%b | |
| DAS28(CRP) ≤ 3.2 | Non-smoker | + 27%a | + 14%a | + 27%a |
| Former smoker | + 18% | + 30%b | + 45%a | |
| Current smoker | + 1% | + 19%c | + 30%b | |
| DAS28(CRP) remission | Non-smoker | + 19%a | + 14%a | + 25%a |
| Former smoker | + 9% | + 13% | + 41%a | |
| Current smoker | + 6% | + 20%b | + 24%b |
ap < 0.001. bp < 0.01. cp < 0.05. p values based on logistic regression. Maximum likelihood estimation was used for all variables. CDAI LDA was defined as ≤ 10. DAS28(CRP) remission was defined as < 2.6
ACR20/50/70 American College of Rheumatology 20, 50, or 70% improvement; CDAI clinical disease activity index; DAS28(CRP) disease activity score in 28 joints with C-reactive protein; LDA low disease activity; MTX methotrexate; MTX-IR inadequate response to MTX
Table 3.
Difference in response rate at week 12 vs. placebo + csDMARD in patients with bDMARD-IR by smoking status
| Smoking status | Filgotinib 100 mg + csDMARD (n = 152) | Filgotinib 200 mg + csDMARD (n = 147) | |
|---|---|---|---|
| ACR20 | Non-smoker | + 26%a | + 34%a |
| Former smoker | + 35%b | + 35%c | |
| Current smoker | + 21% | + 38%b | |
| ACR50 | Non-smoker | + 22%a | + 32%a |
| Former smoker | + 4% | + 25% | |
| Current smoker | + 9% | + 22% | |
| ACR70 | Non-smoker | + 12%b | + 22%a |
| Former smoker | − 10% | − 2% | |
| Current smoker | + 4% | + 18% | |
| CDAI LDA | Non-smoker | + 27%a | + 24%a |
| Former smoker | + 17% | + 12% | |
| Current smoker | + 23% | + 32%b | |
| DAS28(CRP) ≤ 3.2 | Non-smoker | + 26%a | + 30%a |
| Former smoker | + 8% | + 25% | |
| Current smoker | + 23% | + 27% | |
| DAS28(CRP) remission | Non-smoker | + 22%a | + 22%a |
| Former smoker | + 4% | − 2% | |
| Current smoker | + 17% | + 18% |
ap < 0.001. bp < 0.05. cp < 0.01. Logistic regression was done with Firth correction for subgroups where the placebo response rate was 0 (ACR70 in current smokers, DAS28[CRP] remission in current smokers). CDAI LDA was defined as ≤ 10. DAS28(CRP) remission was defined as < 2.6. ACR20/50/70 American College of Rheumatology 20, 50, or 70% improvement; bDMARD-IR inadequate response to biologic disease-modifying antirheumatic drug; CDAI clinical disease activity index; csDMARD conventional synthetic disease-modifying antirheumatic drug; DAS28(CRP) disease activity score in 28 joints with C-reactive protein; LDA low disease activity
As shown in Supplementary Materials Table 1, baseline characteristics associated with achievement of DAS28(CRP) ≤ 3.2 at week 12 after stratification for smoking status were DAS28(CRP) ≤ 5.1 and lower baseline Health Assessment Questionnaire–Disability Index score (all trials), baseline CCP antibody positivity (MTX-IR and MTX-naïve trials), and lack of any comorbidity (bDMARD-IR and MTX-naïve trials). Additionally, age and use of non-steroidal anti-inflammatory drugs at baseline were associated with achievement of week 12 DAS28(CRP) ≤ 3.2 in patients with MTX-IRs, as were lower number of prior bDMARDs in patients with bDMARD-IR and both shorter duration of RA and rheumatoid factor positivity in patients who were MTX-naïve.
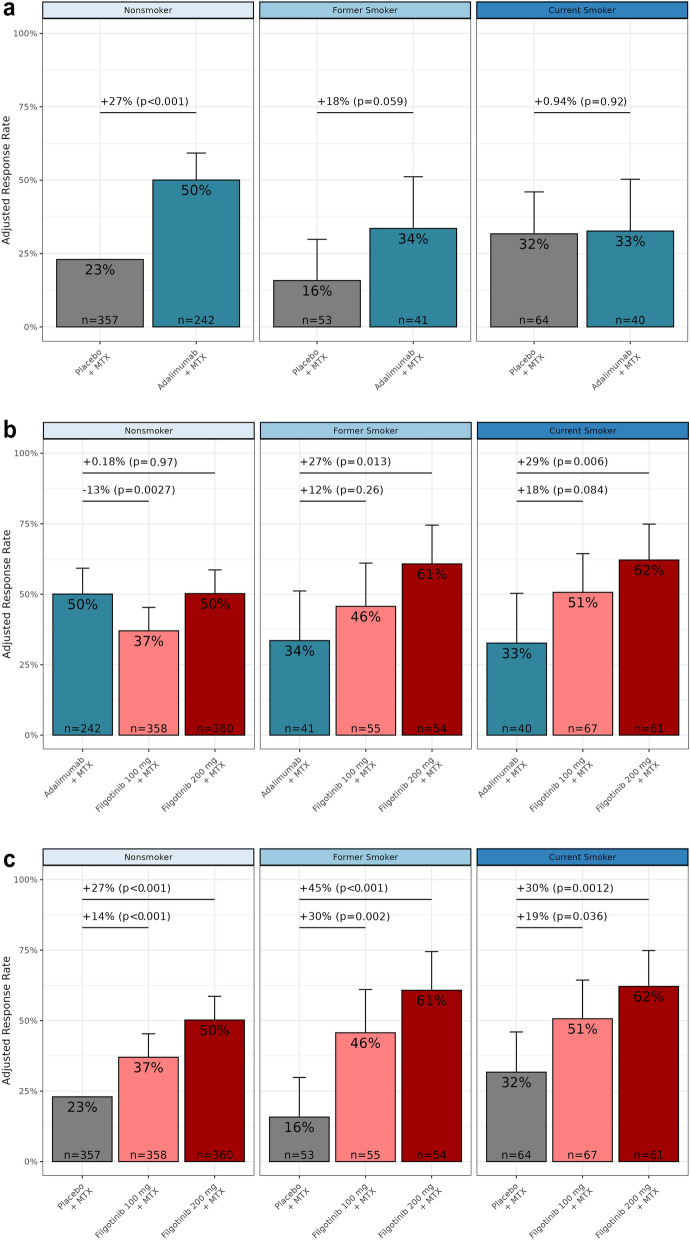
The placebo-adjusted DAS28(CRP) ≤ 3.2 response rate of adalimumab + MTX among MTX-IR current smokers was numerically lower compared with non-smokers (Fig. 1a). At week 12, 50% of non-smokers receiving adalimumab + MTX achieved DAS28(CRP) ≤ 3.2 (27% difference vs. placebo + MTX; p < 0.001), while rates among former and current smokers were 18 and 1% higher vs. placebo + MTX (p = 0.059 and p = 0.92). Compared with adalimumab + MTX, FIL200 + MTX exhibited higher response rates in MTX-IR former smokers (+ 27%; p = 0.013) and current smokers (+ 29%; p = 0.006), while non-smokers exhibited similar response rates between treatments (+ 0.18%; p = 0.97; Fig. 1b).
Fig. 1.
Week 12 DAS28(CRP) ≤ 3.2 response rate at week 12 by smoking status among patients with MTX-IR in a adalimumab and placebo groups, b filgotinib and adalimumab groups, and c filgotinib and placebo groups. p values refer to stratified comparison between treatment groups by smoking status (linear regression). p values are nominal, without adjustment for multiple testing. DAS28(CRP) disease activity score in 28 joints with C-reactive protein, MTX methotrexate, MTX-IR inadequate response to MTX
In contrast, patients who received filgotinib showed improved response rates vs. the control arms across trials and in different smoking subgroups. Among patients with MTX-IR, DAS28(CRP) ≤ 3.2 response rates at week 12 in the FIL200 + MTX treatment arm were significantly higher vs. placebo + MTX across smoking subgroups (Fig. 1c; 50 vs. 23% in non-smokers, 61 vs. 16% in former smokers, and 62 vs. 32% in current smokers; all p < 0.01). Response rates in the FIL100 + MTX arm were also significantly higher vs. placebo + MTX among the respective groups (14, 30, and 19% higher; p ≤ 0.036). Similarly, among patients with bDMARD-IR (Supplementary Materials Fig. 1a), FIL200 + csDMARD was associated with higher DAS28(CRP) ≤ 3.2 response rates vs. placebo + csDMARD among non-smokers and numerically higher rates of response among former and current smokers. Among patients who were MTX-naïve (Supplementary Materials Fig. 1b), FIL200 + MTX yielded greater proportions of patients achieving DAS28(CRP) ≤ 3.2 status at week 12 vs. MTX monotherapy in all smoking subgroups. Tables 2, 3, and 4 show additional endpoints at week 12: DAS28(CRP) < 2.6, ACR20, ACR50, ACR70, and CDAI ≤ 10 response rates. Among patients with MTX-IR, FIL200 + MTX was consistently associated with higher response rates across endpoints vs. placebo + MTX regardless of smoking status. Among patients with bDMARD-IR, non-smokers in the FIL200 + csDMARD treatment arm showed consistently higher rates of response vs. placebo + csDMARD in all endpoints, and in patients who were MTX-naïve, FIL200 + MTX was associated with higher response rates vs. MTX monotherapy in all endpoints regardless of smoking status.
Table 4.
Difference in response rate at week 12 vs. MTX monotherapy in patients who were MTX-naïve by smoking status
| Smoking status | Filgotinib 100 mg + MTX (n = 207) | Filgotinib 200 mg + MTX (n = 416) | Filgotinib 200 mg (n = 210) | |
|---|---|---|---|---|
| ACR20 | Non-smoker | + 11%a | + 19%b | + 15%c |
| Former smoker | + 22%a | + 14%a | + 24%a | |
| Current smoker | + 16% | + 21%a | + 1% | |
| ACR50 | Non-smoker | + 15%c | + 24%b | + 19%b |
| Former smoker | + 31%c | + 36%b | + 52%b | |
| Current smoker | + 16% | + 28%c | + 6% | |
| ACR70 | Non-smoker | + 11%c | + 18%b | + 15%b |
| Former smoker | + 23%a | + 27%c | + 46%b | |
| Current smoker | + 25%a | + 31%b | + 11% | |
| CDAI LDA | Non-smoker | + 19%b | + 23%b | + 17%b |
| Former smoker | + 25%a | + 25%c | + 50%b | |
| Current smoker | + 14% | + 32%c | + 4% | |
| DAS28(CRP) ≤ 3.2 | Non-smoker | + 24%b | + 29%b | + 22%b |
| Former smoker | + 17% | + 21%a | + 42%b | |
| Current smoker | + 28%a | + 40%b | + 20%a | |
| DAS28(CRP) remission | Non-smoker | + 14%c | + 22%b | + 14%c |
| Former smoker | + 16% | + 29%c | + 41%b | |
| Current smoker | + 24%a | + 27%c | + 5% |
ap < 0.05. bp < 0.001. cp < 0.01. p values based on logistic regression. Maximum likelihood estimation was used for all variables. CDAI LDA was defined as ≤ 10. DAS28(CRP) remission was defined as < 2.6
ACR20/50/70 American College of Rheumatology 20, 50, or 70% improvement, CDAI clinical disease activity index, DAS28(CRP) disease activity score in 28 joints with C-reactive protein, LDA low disease activity, MTX methotrexate
Claims-Based Study
A total of 148,275 patients with RA receiving their first biologic (baseline characteristics are shown in Supplementary Materials Table 4) were enrolled in the claims-based study. Of 66,466 patients prescribed adalimumab, 7% were identified as former smokers and 15% as current smokers. The likelihood of switching from an anti-tumor necrosis factor (TNF) bDMARD to a different bDMARD or a JAK inhibitor was higher among current or former smokers vs. non-smokers (Fig. 2), a finding consistent with previous reports [3–5] and with results seen with adalimumab in the randomized trial analysis. In contrast, no significant difference in the likelihood of switching between smokers and non-smokers was seen in patients who received abatacept or tocilizumab.
Fig. 2.
Risk of switching from first bDMARD in patients with RA by smoker status. Estimate of > 1 represents a greater likelihood of current/former smokers switching to a second biologic compared with non-smokers. Covariates included sex, age, disease duration, prior corticosteroid use, and previous/current csDMARD use. bDMARD biologic DMARD, csDMARD conventional synthetic DMARD, CTLA4 cytotoxic T-lymphocyte associated protein 4, DMARD disease-modifying antirheumatic drug, IL6R interleukin 6 receptor, RA rheumatoid arthritis, TNF tumor necrosis factor
Discussion
Cigarette smoking is associated with higher disease activity in patients with RA [1, 2] and with reduced efficacy of some DMARDs [3–6, 14]; in particular, smoking has been shown to be negatively associated with clinical response and persistence to adalimumab and other anti-TNFs in patients with RA [3–5]. Therefore, it is important to consider the effects of smoking when selecting DMARD options.
The relevance of smoking status to DMARD efficacy was reflected in this analysis of the FINCH 1 placebo-controlled, randomized clinical trial, which included an adalimumab active comparator arm in an MTX-IR RA population. Differences in response rates between patients taking adalimumab + MTX vs. placebo + MTX were greatest among non-smokers, with a smaller difference among former smokers and minimal difference among current smokers. In contrast, no clear relationship between smoking status and clinical response was observed among patients who received FIL200 + MTX. When comparing efficacy directly against adalimumab + MTX by smoking status, FIL200 + MTX response rates were significantly higher in current and former smokers but comparable among non-smokers. Our post hoc exploratory analyses additionally found that MTX-naïve and patients with MTX-IR taking FIL200 + MTX were more likely to achieve week 12 DAS28(CRP) ≤ 3.2 than those taking MTX monotherapy or placebo + MTX across all smoking subgroups. Although the bDMARD-IR trial was smaller than the others (447 vs. 1247 [MTX-naïve] or 1752 [MTX-IR]), numerically higher response rates were observed for FIL200 + csDMARD vs. placebo + csDMARD in all smoking subgroups [7–9].
The claims-based analysis reported here enrolled approximately 150,000 individuals to provide further evidence regarding the efficacy of biologics in patients with RA with a history of smoking. We demonstrate that both current and former smokers are more likely to switch from therapy with the anti-TNF DMARD therapies adalimumab, etanercept, certolizumab, golimumab, or infliximab than are non-smokers. While numeric trends toward a greater chance of switching among smokers vs. non-smokers receiving abatacept or tocilizumab were seen, no significant relationship between smoking status and likelihood of switching was observed for patients who received abatacept or tocilizumab, suggesting this relationship may be particularly associated with the TNF signaling blockade, as has been previously observed [15].
This exploratory post hoc analysis was limited by the small number of current and former smokers enrolled in the FINCH studies and the limitations associated with defined subgroups based on self-reported characteristics, such as history of smoking. Some unexpected results, including a 9 and 16% (adjusted) higher DAS28(CRP) response rate in MTX-IR smokers vs. non-smokers or former smokers (in contrast to previous studies [6, 14]), are likely due to chance based on the small sample size of the subgroup (32% responders among the 64 current smokers receiving placebo + MTX). As with other exploratory studies, results should be interpreted in light of their post hoc nature. Specifically, we note that smoking status was not used as a criterion for randomization. Although we applied multivariable adjustment to control for potentially important factors between smokers and non-smokers, the usual cautions related to the potential for unmeasured or residual confounding apply. Additionally, duration of smoking was not reported, and self-reported smoking status in administrative claims data is specific but not sensitive; some misclassification of smoking status reported in real-world data is likely. However, it seems implausible that such misclassification would be different by RA treatment, increasing confidence in our results. Finally, we note that this analysis was completed before the European Medicines Agency’s safety committee announced their recommendations concerning the use of JAK inhibitors by patients who smoke [16]. This recommendation followed the publication of clinical trial results showing risks of venous thromboembolism, major adverse cardiac events, and malignancies were higher with tofacitinib compared with TNF inhibitors in patients with cardiovascular risk factors such as smoking [17]. Further research is needed to determine the relative risk of venous thromboembolism, cardiovascular disease, or malignancy among patients at elevated risk taking each of the different JAK inhibitors [18].
Conclusions
Current and former smokers in a trial of patients with MTX-IR with RA who received adalimumab + MTX exhibited reduced efficacy vs. FIL200 + MTX or placebo + MTX compared with non-smokers, a finding consistent with both the switching patterns seen in the claims-based analysis and previous reports. In contrast, filgotinib efficacy was consistently higher vs. the control arms across smoking subgroups among all three RA populations. In direct comparison between FIL200 + MTX and adalimumab + MTX, similar response rates were observed among non-smokers, while significantly greater response rates were observed with FIL200 + MTX among former and current smokers. These findings provide further support for reduced efficacy of anti-TNF biologics in patients with RA with a history of smoking and suggest that those patients might see greater disease improvements via therapies with alternative mechanisms, such as JAK inhibition. The potential for adverse events associated with each individual JAK inhibitor should be determined and considered when identifying the best therapy for each patient.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and investigators who participated in the FINCH studies, who made this work possible.
Medical Writing, Editorial, and Other Assistance.
This study was designed by Gilead Sciences, Inc. Medical writing and editorial support was provided by Rob Coover, MPH, of AlphaScientia, a Red Nucleus company, and was funded by Gilead Sciences, Inc. The authors thank Jason Yuan for statistical programming.
Author Contributions
Jeffrey R. Curtis, Paul Emery, Bryan Downie, Rachael E. Hawtin, and Gerd Rüdiger Burmester were involved in the conception and design of the study/analyses. Bryan Downie, Yan Zhong, Jinfeng Liu, and Ling Han performed the data and statistical analyses. Jeffrey R. Curtis, Paul Emery, Bryan Downie, and Gerd Rüdiger Burmester contributed to the interpretation of the data. All authors critically revised the manuscript for important intellectual content and provided final approval for publication.
Funding
This study and its publication, including the journal’s Rapid Service Fee, was funded by Gilead Sciences, Inc., Foster City, CA, USA.
Data Availability
Gilead Sciences, Inc., shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non-conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Data requests should be sent to datarequest@gilead.com.
Declarations
Conflict of Interest
Jeffrey R. Curtis has served as a consultant for AbbVie, Amgen, Bendcare, Bristol Myers Squibb, Corrona, Eli Lilly, Janssen, Myriad, Novartis, Pfizer, Regeneron, Roche, and UCB; and has received grant/research support from AbbVie, Amgen, Bristol Myers Squibb, Corrona, Eli Lilly, Janssen, Myriad, Pfizer, Regeneron, Roche, and UCB. Paul Emery receives grant/research support from AbbVie, Bristol Myers Squibb, Eli Lilly, and Samsung; and is a consultant for AbbVie; Bristol Myers Squibb; Celltrion; Gilead Sciences, Inc.; Eli Lilly; Novartis; Roche; Samsung; and Sandoz. Bryan Downie, Yan Zhong, and Ling Han are shareholders and employees of Gilead Sciences, Inc. Rachael E. Hawtin is a former employee of Gilead Sciences, Inc., and a current shareholder, and is a current employee of Ultragenyx Pharmaceutical Inc. Jinfeng Liu is a shareholder and employee of Gilead Sciences, Inc., and shareholder of Roche. Gerd Rüdiger Burmester reports serving as a consultant and on a speakers bureau for AbbVie, Eli Lilly, Pfizer, and Gilead Sciences, Inc.
Ethical Approval
The randomized trials were conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation guidelines. Protocols were approved by the institutional review board or ethics committee at each site. All participants provided written informed consent on enrollment. FINCH 1 was approved by the Advarra Central Institutional Review Board (Reference # 00000971). FINCH 2 was approved by the Administrative Panel on Human Subjects in Medical Research (Reference # 4593). FINCH 3 was approved by the Ethics Committee Research UZ/KU Leuven (Reference # S59627).
Footnotes
Rachael E. Hawtin was an employee of Gilead Sciences, Inc. at time of research.
Prior Presentation: This work was presented as a poster at the EULAR European Congress of Rheumatology, Madrid, Spain, June 2–5, 2021. Emery P, Downie B, Liu J, et al. Ann Rheumatic Dis 2021;80 (suppl 1):502. Poster 0536.
References
- 1.Meer E, Thrastardottir T, Wang X, et al. Risk factors for diagnosis of psoriatic arthritis, psoriasis, rheumatoid arthritis, and ankylosing spondylitis: a set of parallel case-control studies. J Rheumatol. 2022;49(1):53–59. doi: 10.3899/jrheum.210006. [DOI] [PubMed] [Google Scholar]
- 2.Gianfrancesco MA, Trupin L, Shiboski S, et al. Smoking is associated with higher disease activity in rheumatoid arthritis: a longitudinal study controlling for time-varying covariates. J Rheumatol. 2019;46(4):370–375. doi: 10.3899/jrheum.180262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canhao H, Rodrigues AM, Mourao AF, et al. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford) 2012;51(11):2020–2026. doi: 10.1093/rheumatology/kes184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyrich KL, Watson KD, Silman AJ, Symmons DP, British Society for Rheumatology Biologics Register Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 5.McCulley CB, Barton JL, Cannon GW, et al. Body mass index and persistence of conventional DMARDs and TNF inhibitors in rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):422–428. [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui A, Totonchian A, Jabar Ali JB, et al. Risk factors associated with non-respondence to methotrexate in rheumatoid arthritis patients. Cureus. 2021;13(9):e18112. doi: 10.7759/cureus.18112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80(7):848–858. doi: 10.1136/annrheumdis-2020-219214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese MC, Kalunian K, Gottenberg JE, et al. Effect of filgotinib vs. placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA. 2019;322(4):315–325. doi: 10.1001/jama.2019.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westhovens R, Rigby WFC, van der Heijde D, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis. 2021;80(6):727–738. doi: 10.1136/annrheumdis-2020-219213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aletaha D, Westhovens R, Gaujoux-Viala C, et al. Efficacy and safety of filgotinib in methotrexate-naive patients with rheumatoid arthritis with poor prognostic factors: post hoc analysis of FINCH 3. RMD Open. 2021;7(2):e001621. doi: 10.1136/rmdopen-2021-001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tofacitinib [prescribing information]. New York, NY: Pfizer Inc.; 2020 [updated 1/2022; cited 6/5/2023]. Available from: https://labeling.pfizer.com/showlabeling.aspx?id=959.3/4/2022.
- 12.Baricitinib [prescribing information]. Indianapolis, IN: Eli Lilly & Co.; 2018 [updated 6/2022; cited 6/5/2023]. Available from: https://uspl.lilly.com/olumiant/olumiant.html#pi.3/4/2022.
- 13.Rituximab [prescribing information]. South San Francisco, CA: Genentech, Inc.; 1997 [updated 12/2021; cited 6/5/2023]. Available from: https://www.gene.com/download/pdf/rituxan_prescribing.pdf.3/4/2022.
- 14.Safy-Khan M, de Hair MJH, Welsing PMJ, van Laar JM, Jacobs JWG, Society for Rheumatology Research Utrecht Current smoking negatively affects the response to methotrexate in rheumatoid arthritis in a dose-responsive way, independently of concomitant prednisone use. J Rheumatol. 2021;48(10):1504–1507. doi: 10.3899/jrheum.200213. [DOI] [PubMed] [Google Scholar]
- 15.Roodenrijs NMT, Welsing PMJ, van Roon J, et al. Mechanisms underlying DMARD inefficacy in difficult-to-treat rheumatoid arthritis: a narrative review with systematic literature search. Rheumatology (Oxford) 2022;61(9):3552–3566. doi: 10.1093/rheumatology/keac114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Medicines Agency. EMA recommends measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders. 2022 [Available from: https://www.ema.europa.eu/en/news/ema-recommends-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic.]
- 17.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 18.Winthrop KL, Cohen SB. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol. 2022;18(5):301–304. doi: 10.1038/s41584-022-00767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gilead Sciences, Inc., shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non-conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Data requests should be sent to datarequest@gilead.com.