Abstract
Streptococcal pyrogenic exotoxin B (SpeB), a conserved cysteine protease expressed by virtually all Streptococcus pyogenes strains, has recently been shown to be an important virulence factor (S. Lukomski, S. Sreevatsan, C. Amberg, W. Reichardt, M. Woischnik, A. Podbielski, and J. M. Musser, J. Clin. Invest. 99:2574–2580, 1997). Genetic inactivation of SpeB significantly decreased the lethality of a serotype M49 strain for mice and abolished the lethality of a serotype M3 strain after intraperitoneal (i.p.) injection. In the present study, a wild-type M3 isolate and an M3 speB mutant derivative were used to investigate the mechanism responsible for altered virulence. Following i.p. injection, the mutant and wild-type strains induced virtually identical cellular inflammatory responses, characterized largely by an influx of polymorphonuclear leukocytes (PMNs). In addition, the mutant and wild-type strains rapidly entered the blood and were recovered from all organs examined. However, significantly fewer (P < 0.05) CFUs of the isogenic mutant derivative than of the wild-type parent strain were recovered from blood and organs. PMNs effectively cleared the M3 speB mutant from the peritoneum by 22 h, thereby sparing the host. In contrast, the wild-type M3 strain continued to replicate intraperitoneally and had the ability to kill phagocytes. This process allowed the wild-type strain to continuously disseminate, resulting in host death. Our results indicate that genetic inactivation of the cysteine protease decreased the resistance of the mutant to phagocytosis and impaired its subsequent dissemination to organs. These results provide insight into the detrimental effect of SpeB inactivation on virulence.
Group A Streptococcus (GAS) is a gram-positive bacterium that causes diverse human infections. The pathogen is the most common cause of childhood pharyngitis, an infection responsible for considerable morbidity and health care costs in the United States and other countries. GAS also causes infections that are characterized by invasion and destruction of host tissue. This category of diseases includes impetigo, cellulitis, erysipelas, and necrotizing fasciitis. Patients with severe forms of some of these diseases can have GAS bloodstream infections, a condition that may result in dissemination of the organism to virtually any organ. In addition, GAS is responsible for causing acute rheumatic fever and glomerulonephritis, diseases that occur in patients with antecedent streptococcal infection. These two diseases, referred to as postinfection sequelae, are major causes of heart and kidney disease in developing countries worldwide. Despite the immense human toll this organism extracts, we have only a rudimentary understanding of pathophysiologic processes underlying the host-pathogen interaction.
One GAS product that has been the subject of investigation by several laboratories is streptococcal pyrogenic exotoxin B (SpeB) (4, 17). This molecule is a highly conserved 28-kDa extracellular cysteine protease that is initially made as a 40-kDa zymogen. Several lines of evidence have suggested that this molecule participates in host-pathogen interactions in some GAS diseases (17). The speB gene is ubiquitously distributed in GAS, and virtually all organisms express SpeB (14). Patients with GAS infections such as pharyngitis, acute rheumatic fever, and invasive diseases seroconvert to SpeB (7, 27). Moreover, patients with fatal invasive streptococcal disease have lower acute-phase antibody levels against SpeB than do individuals with less serious infections, an observation indicating that anti-SpeB antibody may play a protective role in humans (10). This idea is supported by the results of mouse immunization studies (12). Several in vitro activities of SpeB also suggest that the enzyme is important in GAS pathogenesis (17). The cysteine protease releases biologically active fragments of the streptococcal cell surface molecules M protein and C5a peptidase (1), both considered to be virulence factors. SpeB cleaves human fibronectin and degrades vitronectin (14), molecules necessary for maintaining extracellular matrix integrity. The streptococcal cysteine protease also processes human interleukin-1β precursor to mature active interleukin-1β, a major inflammatory mediator (13). In addition, SpeB activates a human matrix metalloprotease (2) that may contribute to bacterial invasion and tissue destruction. Purified cysteine protease cleaves and releases urokinase plasminogen activator receptor from the surface of mononuclear phagocytes (33) and releases biologically active kinins from their precursors (8).
Relatively recently, genetic tools have been formulated to assist in the molecular dissection of GAS pathophysiologic processes. The use of isogenic strains has been a particularly important strategy to probe GAS host-pathogen interactions. For example, inactivation of M protein by transposon mutagenesis resulted in a loss of resistance to phagocytosis by human neutrophils (18). Subsequent reintroduction of a functional emm6 gene restored the wild-type level of resistance (20), thereby unambiguously proving the long-postulated anti-phagocytic role of this surface protein (15). Similarly, molecular genetic studies have shown that the hyaluronic acid capsule and extracellular C5a peptidase participate in virulence (11, 28, 29). A capsule-negative isogenic mutant strain generated by transposon mutagenesis was no longer resistant to phagocytosis in vitro and was also less virulent for mice (28, 29). Moreover, a C5a peptidase-deficient mutant obtained by insertional mutagenesis was effectively cleared by neutrophils whereas the wild-type isolate avoided phagocytosis by retarding the influx of inflammatory cells to the site of infection (11).
The putative involvement of SpeB in streptococcal virulence was recently proven by construction and testing of isogenic mutant strains deficient in streptococcal extracellular cysteine protease production (16). Comparison of the virulence of two pairs of GAS isogenic strains (serotypes M3 and M49) in an intraperitoneal (i.p.)-injection mouse model demonstrated that the mutant derivatives were significantly altered in their ability to kill the host animal. The M3 speB mutant strain lost virtually all ability to cause mouse death. Similarly, the virulence of the serotype M49 speB mutant was also substantially reduced. The cause of the alteration in virulence was not investigated.
To address this issue, we studied the differences in pathogenesis between the virulent parental M3 isolate and the avirulent cysteine protease-deficient mutant derivative. The M3 strain pair was selected for analysis because strains of this serotype are frequent causes of human severe invasive disease episodes, many characterized by septicemia, tissue destruction, and patient death. The M3 isogenic strain pair was also used because of the pronounced difference in mouse virulence created by inactivation of SpeB (16). We report here that soon after i.p. injection, the parent and mutant strains induced a similar or identical cellular inflammatory response. However, the mutant was phagocytosed and cleared more efficiently by polymorphonuclear leukocytes (PMNs) than was the parental organism. As a consequence, the level of bacteremia, dissemination to organs, and subsequent host death were substantially greater for the wild-type M3 parent strain. These data suggest that some of the decreased virulence of S. pyogenes deficient in cysteine protease function is due to altered resistance to phagocytosis and dissemination.
MATERIALS AND METHODS
Bacterial strains and growth.
Wild-type S. pyogenes AM3 and its isogenic mutant derivative, deficient in production of the active extracellular cysteine protease SpeB, were used. The parental wild-type strain, originally recovered from a patient with puerperal sepsis (26), was obtained from the National Collection of Type Cultures, Public Health Laboratory Services, London, United Kingdom. The speB knockout mutant strain was generated by insertional mutagenesis as previously described (16). The wild-type parental M3 organism also has the following genetic characteristics: (i) an emm sequence identical to the emm3 allele encoding serotype M3 protein (31), (ii) the speB3 allele typically occurring in all M3 strains (14), and (iii) the speC gene encoding streptococcal pyrogenic exotoxin type C. Strains were grown in brain heart infusion broth (Remel, Lenexa, Kans.) or on tryptose agar plates with 5% sheep blood (Becton Dickinson, Cockeysville, Md.) at 37°C in a 5% CO2–20% O2 atmosphere. Erythromycin (3 μg/ml) was used for selection of the M3 speB mutant strain.
Bacterial dissemination to organs.
The M3 wild-type and M3 speB mutant strains were grown overnight in brain heart infusion broth. The bacteria were harvested by centrifugation and washed once with ice-cold phosphate-buffered saline (PBS). Inocula of ∼106 CFU were prepared in 0.25 ml of PBS. Groups of 20 adult (18- to 20-g) male outbred CD-1 Swiss mice (Harlan, Houston, Tex.) were injected i.p. as described previously (16). The actual number of CFU injected was verified for each experiment by performing colony counts on blood agar plates. Culture purity was assessed by examination of colony morphology and the presence of beta-hemolysis. Control mice were injected with 0.25 ml of lipopolysaccharide-free PBS.
To assay bacterial dissemination to organs, groups of five animals were sacrificed at 4 h postinoculation and daily thereafter. The mice were anesthetized by inhalation of Metofane (Mallinckrodt Veterinary, Mundelein, Ill.) and then euthanized by cervical dislocation. Before sacrifice, blood samples were collected by retroorbital puncture. The blood was diluted 10-fold in PBS, and 100-μl aliquots were cultured on blood agar plates and incubated overnight at 37°C. The growth of one colony on plates prepared in this manner indicated the presence of 100 CFU per ml of blood and defined the minimum detectable level of bacteria present in the blood.
To determine the organ burden of GAS, the right kidney, the right lung, and portions of the spleen and liver of each mouse were collected. Care was taken to remove the same portions of the organs from each mouse. The tissue specimens were weighed and homogenized in 1 ml of PBS by being passed several times through a three-way stop-cock attached to two syringes. Homogenized tissue was then diluted in PBS, and 100-μl aliquots were cultured overnight at 37°C. As described above, the presence of one colony, obtained by plating undiluted homogenate, represented the minimum detectable level of bacteria present in an organ sample. However, due to the differences in average organ weight, one CFU per plate corresponded to 43 CFU/g of kidney, 73 CFU/g of lung, 170 CFU/g of spleen, and 35 CFU/g of liver.
Statistical analysis of wild-type and mutant infection levels was performed by Student’s t test, and P values less than 0.05 were considered to be significant. Samples in which bacteria were not detected (organs from mice which cleared infection with the M3 speB mutant strain) were considered “zero” for statistical analyses.
Analysis of peritoneal exudate cells (PECs).
Mice were injected i.p. with ∼106 CFU of wild-type M3 or M3 speB mutant or with PBS, as described above. At 1, 4, and 22 h postinfection, five mice injected with bacteria and two control mice injected with PBS were sacrificed. PECs were obtained from these animals by lavage. Briefly, 3 ml of RPMI medium (Gibco BRL, Gaithersburg, Md.) supplemented with 100 U of heparin (Sigma, St. Louis, Mo.) per ml was injected into the peritoneum. After gently massaging the abdomen, an incision was made and the lavage fluid was collected by aspiration. The number of viable host cells in each suspension was determined microscopically with a standard hemocytometer and trypan blue staining (22). Differential counts of the host cells in these suspensions were performed after staining with acridine orange as described by Golstein and Blomgren (6).
Bacterial clearance in vivo.
Bacterial killing was assayed by determining the number of GAS interacting with the inflammatory cells of the host present inside the peritoneum. First, the PEC suspensions recovered from the animals injected with GAS were centrifuged for 5 min at 110 × g. The resulting supernatant fluid was cultured on blood agar plates (PEC-free GAS) overnight at 37°C. The bacteria associated with PECs (representing the combination of ingested and adherent GAS) were pelleted by centrifugation. The cells were resuspended at their original volume in sterile PBS. The PECs in the suspension were lysed by the addition of Triton X-100 to a final concentration of 0.01%. There was no difference in the survival of the mutant and wild-type strains after Triton X-100 exposure. Serial 10-fold dilutions of the supernatant and lysed cell pellet were made in PBS, and aliquots of 0.1 ml were cultured on blood agar plates overnight at 37°C. To verify that infection was progressing as anticipated, spleens were harvested from each animal and processed as described above.
Microscopy.
To assess if the GAS associated with PECs were intracellular, PECs were stained with Leukostat (Fisher Scientific, Orangeburg, N.Y.) or acridine orange (0.01% suspension in PBS) and examined under a light or fluorescence microscope, respectively. The Leukostat stain is a modified Wright stain. The acridine orange stain differentiates between dead (orange) and live (green) bacteria (19).
RESULTS
Bacterial dissemination to organs.
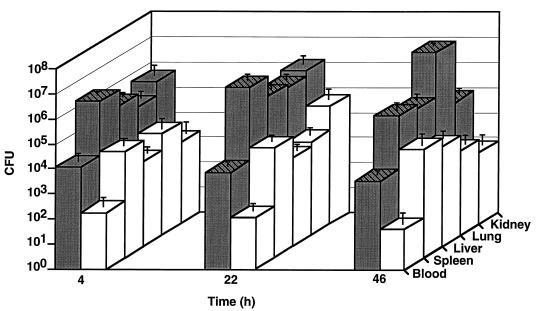
Previous studies found that 106 CFU of the wild-type GAS serotype M3 injected i.p. caused the death of at least 90% of mice within 5 days, although most animals died 16 to 48 h after inoculation (16). In contrast, the isogenic M3 speB mutant was virtually unable to kill any animals. The molecular mechanism responsible for this detrimental effect on virulence was not investigated. We hypothesized that decreased dissemination to organs may account for some of the altered virulence observed in the isogenic mutant strain. Therefore, the time course and magnitude of dissemination of wild-type M3 and M3 speB mutant strains from the peritoneum to blood and organs (kidney, lungs, spleen, and liver) were compared.
The magnitude of spread of the pathogen from the peritoneum to blood and organs was assessed 4 h after injection and then at daily intervals (Fig. 1). As rapidly as 4 h postinfection, beta-hemolytic streptococci were recovered from the blood, kidney, lung, spleen, and liver of all animals challenged with the wild-type M3 strain. All mice had the M3 speB mutant strain in all organs tested, and three of five animals had bacteria recovered from blood collected 4 h after injection. However, the M3 speB mutant was present in smaller numbers, on average, than was the wild-type strain. The difference in CFU between the wild-type and mutant strains was statistically significant for the spleen (P = 0.0016) and liver (P = 0.0038).
FIG. 1.
Bacterial dissemination to organs. Inocula of approximately 106 CFU of the wild-type M3 strain and the M3 speB mutant derivative were injected i.p. into groups of 20 mice. Blood and organ samples were collected at 4 h post-inoculation and then at daily intervals. Aliquots of blood and homogenized organ tissue were plated on blood agar and incubated overnight at 37°C. Shaded bars represent the wild-type strain, and open bars represent an M3 speB mutant strain; they show mean values of the CFU of GAS present in 1 ml of blood or 1 g of the organ tissue collected from five animals. Statistically significant results (Student’s t test, P < 0.05) are denoted by cross-hatches.
At 22 h postinjection, all the organs from mice infected with wild-type GAS had more CFU than at 4 h (kidney, 3-fold increase; lung, 6-fold increase; spleen, 3.5-fold increase; and liver, 2.5-fold increase), a result indicating progression of the infection process. The level of bacteremia at 22 h was not different from that at 4 h. In contrast, for the M3 speB mutant, the bacterial counts in blood and organs did not differ significantly from those at the 4-h time point. The differences in CFU between the parental and mutant strains were statistically significant for all samples (blood, P = 0.015; lung, P = 0.018; spleen, P = 0.043; liver, P = 0.001), except for the kidney (P = 0.1).
As anticipated, at 46 h, all the samples taken from mice infected with the M3 parental strain yielded positive bacterial cultures. Interestingly, very high levels of CFU (on average, ∼2 × 107 CFU/g) were present in lungs. The mutant derivative behaved very differently. For example, only one of five blood cultures collected at 46 h postinfection was positive and GAS were not grown from organs obtained from two mice. Moreover, the CFU counts of samples obtained from the other three animals were low. These observations were in striking contrast to the high CFU levels obtained from the blood and organs of mice challenged with the parental wild-type M3 isolate. On average, at the same time point, animals injected with the wild-type organism had 102 to 104 more CFU than did those injected with the speB mutant. The differences between the CFU present in tissues collected from mice injected with either the wild-type M3 or M3 speB mutant were highly significant for all samples tested (blood, P = 0.007; kidney, P = 0.006; lung, P = 0.000; spleen, P = 0.01; liver, P = 0.037).
Due to the mortality rate of the mice challenged with the wild-type strain, no animals were available for analysis 70 h after inoculation. In contrast, after 70 h GAS were not recovered from the blood or organs of mice injected with the M3 speB mutant strain.
No beta-hemolytic microorganisms were isolated from the blood or organs of the control mice injected with PBS at any time point.
Clearance of GAS in vivo.
The studies documented that GAS disseminate to the organs after i.p. inoculation and that mice given the wild-type M3 organism had higher bacterial burdens than did those injected with the mutant GAS strain. These results suggested that the mutant organism was being effectively cleared in the peritoneum whereas the wild type was not. To test this hypothesis, we first studied the influx of inflammatory cells induced by injection of the isogenic pair of GAS strains. Groups of mice were injected i.p. with the isogenic GAS strains as described in Materials and Methods. At 1, 5, and 22 h after inoculation, PECs were collected from the infected mice and the numbers of GAS present in the peritoneal lavage fluid (PEC free) or PEC pellets (PEC associated) were analyzed. To verify that infection was progressing appropriately, spleen samples and PECs were examined in parallel.
The association of the wild-type M3 and mutant M3 speB strains with PECs after i.p. inoculation is shown in Table 1. At 1 h after pathogen injection, compared to the mutant strain, approximately 40 times more wild-type M3 strain was free in the peritoneum (P = 0.0001). At this time point, approximately 7 times more CFU of wild-type M3 were PEC associated than PEC free. In striking contrast, 2,000 times more CFU of the M3 speB mutant were PEC associated than PEC free. These data suggested that the mutant strain was phagocytosed and subsequently killed more efficiently than was the wild-type isolate. The high standard deviation associated with some of the mean CFU values is due to the use of outbred mice and the relatively small sample size of animals.
TABLE 1.
Distribution of wild-type M3 and mutant M3 speB inside the peritoneum after injection
| Time (h) | Inoculum typea | No. of GAS in peritoneum (102 CFU/ml)b
|
No. of positive spleen cultures/total no. | |
|---|---|---|---|---|
| PEC free | PEC associated | |||
| 1 | M3 | 136 ± 38 | 900 ± 122 | 5/5 |
| M3 speB | 3.3 ± 2.6 | 6,975 ± 5,451 | 5/5 | |
| 4 | M3 | 124,000 ± 45,607 | 164,000 ± 21,909 | 5/5 |
| M3 speB | 13 ± 5 | 1,038 ± 942 | 5/5 | |
| 22 | M3 | 120,000 ± 35,000 | 160,000 ± 48,000 | 4/4 |
| M3 speB | 0.08 ± 0.2c | 31 ± 66d | 4/5 | |
Mice were injected i.p. with 0.25 ml of PBS, 1.4 × 106 CFU of the M3 strain, or 1.1 × 106 CFU of the M3 speB strain.
Values are means ± standard deviations (five animals per group).
Four of five mice had no detectable GAS.
Four of five mice had <6 × 102 CFU/ml, and one mouse had 150 × 102 CFU/ml, thereby causing the large standard deviation.
The number of PEC-free CFU of the wild-type strain was approximately 1,000-fold greater at 4 h after inoculation than at 1 h, a result indicating progression of the infection. A substantial increase (∼180-fold) in the PEC-associated CFU was also recorded. In contrast, very little increase in the PEC-free CFU was observed in mice injected with the speB mutant. Moreover, there was a considerable decrease in the PEC-associated CFU compared to the 1-h time point. These observations are also consistent with the hypothesis that the M3 speB mutant strain was being efficiently phagocytosed and cleared compared to the wild-type parental organism.
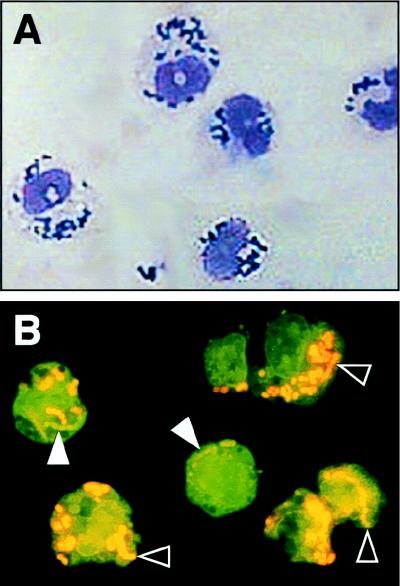
To determine if the bacteria were directly associated with PECs, and not merely present in the same cell fraction, microscopic analysis of the PEC pellets was conducted at the 4-h time point. The mutant GAS were apparently located within the cytoplasm of PMNs rather than being randomly associated with the cell surface (Fig. 2). Moreover, differential staining with acridine orange showed that most of the ingested mutant GAS were dead. Few GAS were located outside PMNs, a result consistent with the data presented in Table 1. Analysis of the PEC suspensions made from mice infected with the wild-type isolate revealed that many GAS were not associated with PECs, and, as assessed by acridine orange staining, these bacteria were viable. Wild-type GAS that were apparently located intracellularly were also stained orange, indicating that they were nonviable (data not shown).
FIG. 2.
Microscopic analysis of the interaction of PECs and speB mutant GAS. (A) The PEC suspension was stained with Leukostat, a modified Wright stain. The mutant bacteria are apparently located in the cytoplasm. Note that no bacteria overlie the host cell nuclei, the result that would be expected if the GAS were merely associated with the host cell surface. (B) Most mutant GAS have been killed by PMNs, as assessed by differential staining with acridine orange. Solid (white) arrowheads designate the live (green) organisms; black arrowheads identify the dead (orange) bacteria.
At 22 h, very high and approximately equal CFU counts (>107 CFU/ml) of the wild-type strain were identified in the PEC-free and PEC-associated specimens. In contrast, only one of five mice had PEC-free GAS (0.08 × 102 CFU/ml), and very low counts (approximately 102 CFU/ml) of PEC-associated bacteria were detected in all five mice.
All spleen specimens taken from mice infected with the M3 wild-type strain were culture positive for GAS through the 22-h time course of the experiment. Although all the spleen samples obtained from mice injected with the speB mutant strain M3 were also culture positive at 1 and 4 h, only four of five specimens grew GAS at 22 h. Moreover, two of the four positive spleen cultures had low colony counts compared to colony counts obtained from spleens of animals receiving the wild-type parent (data not shown).
Analysis of PECs from mice injected with isogenic strains of GAS.
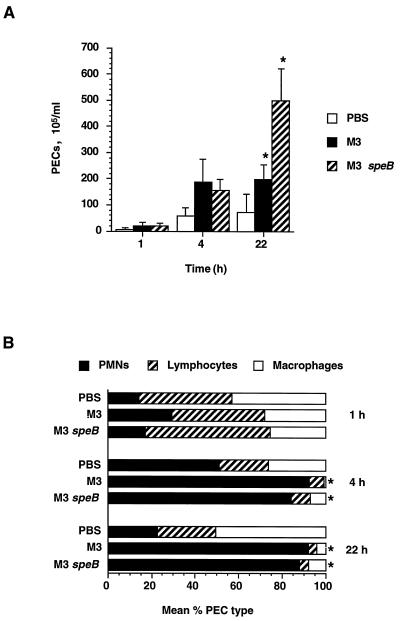
The observation that the course of i.p. infection by the parental M3 strain is different from that of infection by the mutant M3 speB strain prompted us to investigate the cellular inflammatory response of the host. As described above, PECs were isolated from the peritoneum at 1, 4, and 22 h after infection. The total number of host cells and the host cell viability were determined, and differential counts were performed. Because GAS is a classic pyogenic organism, we expected that infection would cause a substantial influx of PMNs into the peritoneum, thereby markedly changing the PEC composition.
The differential counts of the PECs are shown in Fig. 3. No significant changes in the number or composition of PECs were observed 1 h after infection. However, 4 h after injection, there was a significant absolute increase in the number of PECs recorded in the animals receiving either the wild-type or a speB mutant strain. Virtually all of this increase was caused by PMN influx. As assessed by trypan blue staining, all the PECs were viable. The population of PMNs in the peritoneal cavity corresponded to 84% (M3 speB) to 92% (M3 wild type) of the total PECs, compared to 51% in a control PBS sample (both differences were statistically significant: M3, P = 0.00002; M3 speB, P = 0.002).
FIG. 3.
PMN influx and differential counts of PECs. Mice were injected i.p. with 1.4 × 106 CFU of the wild-type M3 strain and 1.1 × 106 CFU of the M3 speB mutant derivative or PBS alone. PECs were isolated from five mice at 1, 4, and 22 h postinoculation. (A) PEC influx into the peritoneum after GAS inoculation. Statistically significant differences (Student’s t test, P < 0.05) in PEC numbers between samples from animals injected with the wild-type or mutant strain are marked with asterisks. (B) Differential counts of PMN, lymphocyte, and macrophage populations. The graph shows percent mean cell populations in total PECs. Statistically significant differences (Student’s t test, P < 0.05) in PMN numbers between samples from animals injected with bacteria or PBS are marked with asterisks.
The dominant presence of PMNs in the peritoneum remained at 22 h after bacterial injection (wild-type, 92% [P = 0.0003]; mutant, 88% [P = 0.0003]). Compared to the 4-h time point, the most striking change recorded was an increase in the total number of PECs in mice receiving the speB mutant strain. Animals receiving the M3 speB mutant had PEC counts of 498 × 105/ml, whereas the mice injected with the wild-type parental organism had PEC counts of 199 × 105/ml, a number essentially identical to that at the 4-h time point. Moreover, only 53% of the PMNs from animals injected with the wild-type strain were viable, compared with >99% PMN viability assessed in mice given the speB mutant strain. These results suggest that the significant difference (P = 0.001) between total PEC numbers in the peritoneum 22 h after inoculation with the M3 or M3 speB strain may be due to both a difference in the level of PMN influx into the peritoneum and PMN viability.
DISCUSSION
A recent study showed that genetic inactivation of the SpeB cysteine protease had a remarkably detrimental effect on mouse virulence after i.p. injection (16). Compared to the parental wild-type M3 strain, an isogenic mutant derivative was virtually unable to kill mice. In addition, the mouse virulence of an M49 organism with the cysteine protease inactivated was decreased significantly. These observations provided a critical unambiguous demonstration that the cysteine protease is an important GAS virulence factor and stimulated the investigations reported in this study. A key finding from the present analysis is that inactivation of SpeB results in altered interactions between bacterial cells and host PMNs. Our data demonstrate that inactivation of the S. pyogenes cysteine protease results in (i) decreased resistance to phagocytosis, (ii) loss of toxicity to PMNs, and (iii) decreased dissemination to organs, resulting in a loss of the ability to kill mice.
Research conducted over many decades has suggested that resistance to phagocytosis is mediated by the important surface components M protein and hyaluronic acid capsule (3, 5, 9, 15, 23, 24, 28, 29, 32). Generation of isogenic strains by molecular genetic strategies and analysis in model systems have confirmed this idea (3, 18, 20, 21, 28, 29), although the exact mechanism of the antiphagocytic activity of these molecules is not fully understood. Similarly, we do not yet know the mechanism by which SpeB contributes to GAS resistance to phagocytosis. Inasmuch as Elliott (4) and Berge and Bjorck (1) showed that SpeB cleaves M protein, it is tempting to speculate that our observations are due to failure of the mutant to degrade this molecule. However, this hypothesis seems counterintuitive because the effect of SpeB on M protein is release of amino-terminal fragments presumably required for the antiphagocytic effect. Hence, it is hard to envision a process whereby failure to cleave and release an antiphagocytic molecule results in enhanced phagocytosis. One possible mechanism might involve binding of protease-cleaved M protein fragments to the bacterial surface, a process that could result in a greater effective surface density of an antiphagocytic molecule. We note, however, that there has not yet been a formal demonstration that SpeB protease cleaves M protein made by serotype M3 strains. Similarly, at present there is no evidence that SpeB is involved in capsule production, but our observations suggest that this could be a fruitful area of investigation.
The streptococcal cysteine protease may influence professional phagocyte function by other mechanisms. The activity of SpeB may influence phagocytosis through a direct proteolytic effect on C5a peptidase. The streptococcal surface protein C5a peptidase cleaves human serum chemotaxin C5a (30), which decreases the influx of inflammatory cells to the site of infection (11). Berge and Bjorck (1) reported that in vitro, SpeB cleaved and released a 116-kDa internal fragment of C5a peptidase. The solubilized C5a peptidase fragment blocked directed leukocyte migration in an agarose matrix. The investigators hypothesized that release of the C5a peptidase fragment might inhibit phagocytes from reaching the infection site. Taken together, these data suggest that the most likely effect of inactivation of SpeB would be to decrease the release of the biologically active C5a peptidase fragment, a process that in principle could result in a reduced influx of phagocytes and decreased phagocytosis. It is therefore noteworthy that in vivo, at 1 h after injection, there are already 8 times as many PEC-associated M3 speB mutant cells as wild-type parental cells (P = 0.02). These data underscore the fact that far more cells of the protease-negative mutant are apparently phagocytosed than are cells of the wild-type strain.
Explanation of the exact molecular mechanism underlying our results must clearly await additional investigations. We believe that one particularly important observation is that at 22 h, the number of PECs recovered from mice infected with the speB mutant was more than twice the number recovered from animals given the wild-type M3 organism. Essentially all of this difference was due to PMNs. Moreover, only half of the PMNs from animals receiving the wild-type organism were viable whereas all the PMNs from mice injected with the speB mutant were viable. These observations suggest that the wild-type organism is killing PMNs, thereby reducing the total number of PECs. Although we cannot formally rule out the possibility that the speB mutant has an increased ability to cause PMN influx, it is a less likely mechanism since no difference in PMN influx was seen at 1 and 4 h after inoculation. Detailed analysis of the interactions of the isogenic pair of strains with PMNs and of the cysteine protease with PMNs should provide further insight into the mechanism responsible for our observations.
The discovery that the speB mutant is deficient in resistance to phagocytosis suggests that the production of cysteine protease in the context of human infection assists GAS survival and dissemination. Indeed, bacterial dissemination to organs differs between the wild-type M3 isolate and protease-deficient M3 speB mutant strain. When the data are pooled over time (4 to 46 h postinoculation), significantly more wild-type GAS are present in the kidney (P = 0.001), lung (P = 0.04), spleen (P = 0.002), and liver (P = 0.00). There are several lines of evidence supporting the idea that SpeB participates in disease pathogenesis in some patients (17). First, Holm et al. (10) demonstrated that patients with low SpeB antibody levels in serum were more likely to have a bad clinical outcome than patients with high antibody levels. This observation suggests that inactivation of SpeB by serum antibody provides protection against GAS in humans. Second, Simor et al. (25) reported that GAS strains recovered from patients with severe soft tissue infections produced higher levels of protease than did organisms isolated from less severe infections. Third, humans with invasive and other GAS infections seroconvert to SpeB, which means that the molecule is made in vivo (7, 27). Fourth, the ability of the enzyme to degrade extracellular matrix proteins such as fibronectin and vitronectin (14) and to activate matrix metalloprotease-2 (2) may enhance the persistence of the wild-type strain and its dissemination through tissue. Additional in vitro and in vivo studies are required to define the precise molecular mechanism responsible for the decreased virulence of the SpeB isogenic organism.
ACKNOWLEDGMENTS
The assistance of G. J. Adams with statistical analyses and G. Mardon with graphics is gratefully acknowledged.
These studies were supported by NIH grant AI-33119. J.M.M. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Berge A, Bjorck L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J Biol Chem. 1995;270:9862–9867. doi: 10.1074/jbc.270.17.9862. [DOI] [PubMed] [Google Scholar]
- 2.Burns E H, Jr, Marciel A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dale J B, Washburn R G, Marques M B, Wessels M R. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun. 1996;64:1495–1501. doi: 10.1128/iai.64.5.1495-1501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott S D. A proteolytic enzyme produced by group A streptococci with special reference to its effect on the type-specific M antigen. J Exp Med. 1945;81:573–592. doi: 10.1084/jem.81.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golstein P, Blomgren H. Further evidence for autonomy of T-cell mediating specific in vitro cytotoxicity: efficiency of very small amounts of highly purified T cells. Cell Immunol. 1973;9:127–141. doi: 10.1016/0008-8749(73)90174-3. [DOI] [PubMed] [Google Scholar]
- 7.Gubba S, Low D E, Musser J M. Expression and characterization of group A Streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human invasive disease episodes. Infect Immun. 1998;66:765–770. doi: 10.1128/iai.66.2.765-770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herwald H, Collin M, Muller-Ester W, Bjorck L. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J Exp Med. 1996;184:1–9. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch J G, Church A B. Studies of phagocytosis of group A streptococci by polymorphonuclear leukocytes in vitro. J Exp Med. 1960;111:309–322. doi: 10.1084/jem.111.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm S E, Norrby A, Bergholm A M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, McLandsborough L, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapur V, Maffei J T, Greer R S, Li L-L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin-1β convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 13.Kapur V, Majesky M W, Li L-L, Black R A, Musser J M. Cleavage of interleukin 1 β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapur V, Topouzis S, Majesky M W, Li L-L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 15.Lancefield R C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 16.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Invest. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musser J M. Streptococcal superantigen, mitogenic factor, and pyrogenic exotoxin B expressed by Streptococcus pyogenes. Structure and function. In: Leung D Y M, Huber B T, Schlievert P M, editors. Superantigens. Molecular biology, immunology, and relevance to human disease. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 281–310. [DOI] [PubMed] [Google Scholar]
- 18.Norgren M, Caparon M G, Scott J R. A method for allelic replacement that uses the conjugative transposon Tn916: deletion of the emm6.1 allele in Streptococcus pyogenes JRS4. Infect Immun. 1989;57:3846–3850. doi: 10.1128/iai.57.12.3846-3850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pantazis C G, Kniker W T. Assessment of blood leukocyte microbial killing by using a new fluorochrome microassay. J Reticuloendothel Soc. 1979;26:155–170. [PubMed] [Google Scholar]
- 20.Perez-Casal J, Caparon M G, Scott J R. Introduction of the emm6 gene into an emm-deleted strain of Streptococcus pyogenes restores its ability to resist phagocytosis. Res Microbiol. 1992;143:549–558. doi: 10.1016/0923-2508(92)90112-2. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Casal J, Price J A, Maguin E, Scott J R. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol. 1993;8:809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 22.Phillips H J, Tarryberry J E. Counting actively metabolizing tissue culture cells. Exp Cell Res. 1957;13:341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- 23.Podbielski A, Schnitzler N, Beyhs P, Boyle M D P. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol Microbiol. 1996;19:429–441. doi: 10.1046/j.1365-2958.1996.377910.x. [DOI] [PubMed] [Google Scholar]
- 24.Schrager H M, Rheinwald J G, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Invest. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simor A E, Louie L, Schwartz B, McGeer A, Scriver S, Low D E the Ontario GAS Study Project. Program and Abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Association of protease activity with group A streptococcal (GAS) necrotizing fasciitis (NF), abstr. 1164. [Google Scholar]
- 26.Stamp T C, Hendry E B. The immunizing activity of certain chemical fractions isolated from haemolytic streptococci. Lancet. 1937;i:257–259. [Google Scholar]
- 27.Todd E W. A study of the inhibition of streptococcal proteinase by sera of normal and immune animals and of patients infected with group A hemolytic streptococci. J Exp Med. 1947;85:591–606. doi: 10.1084/jem.85.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessels M R, Goldberg J B, Moses A E, DiCesare T J. Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect Immun. 1994;62:433–441. doi: 10.1128/iai.62.2.433-441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wexler D E, Chenoweth D E, Cleary P P. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci USA. 1985;82:8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whatmore A M, Kapur V, Sullivan D J, Musser J M, Kehoe M A. Non-congruent relationships between variation in emm gene sequences and the population genetic structure of group A streptococci. Mol Microbiol. 1994;14:619–631. doi: 10.1111/j.1365-2958.1994.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 32.Whitnack E, Bisno A L, Beachey E H. Hyaluronate capsule prevents attachment of group A streptococci to mouse peritoneal macrophages. Infect Immun. 1981;31:985–991. doi: 10.1128/iai.31.3.985-991.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf B B, Gibson C A, Kapur V, Hussaini I M, Musser J M, Gonias S L. Proteolytically active streptococcal pyrogenic exotoxin B cleaves monocytic cell urokinase receptor and releases an active fragment of the receptor from the cell surface. J Biol Chem. 1994;269:30682–30687. [PubMed] [Google Scholar]