Abstract
Background: Transitioning patients between cholinesterase inhibitors was thought to require a washout period to avoid cholinergic toxicity; however, evidence suggests that abrupt discontinuation of donepezil may lead to cognitive decline. We evaluated the safety and tolerability of an immediate switch from donepezil to rivastigmine.
Method: This is an analysis of the safety and tolerability data from the first 28 days of an open-label, multicenter, prospective trial, conducted from August 2002 to August 2003, in which patients satisfying NINCDS-ADRDA criteria for probable Alzheimer's disease were administered rivastigmine 1.5 mg b.i.d. within 24 to 36 hours of donepezil discontinuation. Results are compared with adverse event rates from a retrospective analysis of a pivotal, placebo-controlled trial examining patients not previously treated with a cholinesterase inhibitor.
Results: Fifty-eight of 61 patients completed the first 28 days, with no suspected drug-related discontinuations during this period. Incidence of overall gastrointestinal adverse events at day 7 was 8.2%, and at day 28 was 11.5%. The corresponding rate for rivastigmine-treated patients in the retrospective analysis of the pivotal trial for day 7 was 3.3%.
Conclusion: These study results suggest that transitioning patients from donepezil to rivastigmine without a washout period is safe and well tolerated.
Alzheimer's disease is the most common form of dementia, estimated to affect over 4 million individuals in the United States.1–3 With the prevalence expected to increase 3 to 4 times over the next 50 years3,4 and no known cure to stop its aggressive assault on the brain,3 utilization of treatments that both control symptoms and target the pathologic disease process, and thus potentially slow disease progression, will be vital.
Several neurotransmitter deficits are evident in the Alzheimer's disease brain, but decreased concentration of acetylcholine (ACh) is most prominent, particularly in the neocortex and hippocampus.5 Studies on brain tissue from Alzheimer's disease patients have also revealed significant increase in butyrylcholinesterase (BuChE) activity and decrease in acetylcholinesterase (AChE) activity as severity of the disease progresses.5–8 The cholinesterase (ChE) inhibitors were developed to prevent the hydrolysis of ACh by ChE enzymes.1
Rivastigmine is a centrally selective ChE inhibitor with favorable pharmacologic characteristics for treating Alzheimer's disease.9–11 Classified as a ChE inhibitor, as opposed to an AChE inhibitor, rivastigmine inhibits both AChE and BuChE.5 It is brain-region selective, producing an effect on areas of the brain most impacted by Alzheimer's disease. This may be due to its preferential inhibition of the G1 form of AChE, which is found in high concentrations in the hippocampus and cortex.11 Furthermore, while the distribution of BuChE is widespread in the brain, there is abundant distribution in the temporal lobe and hippocampal formation.12,13 There is growing evidence supporting the role of BuChE in the pathology of Alzheimer's disease.12,14
Rivastigmine is metabolized primarily by its target enzymes, and a resulting inactive metabolite is renally excreted. It also binds weakly to plasma proteins. As a result, the probability that a drug-drug interaction will occur with rivastigmine is low.15 In addition, the plasma half-life is approximately 1.5 hours,16 but its duration of action extends to approximately 10 hours via its pseudoirreversible binding to cholinesterases.17–19 In contrast, donepezil, another frequently prescribed AChE inhibitor, is metabolized through the hepatic cytochrome P450 isoenzyme system, remains highly protein bound, and has a half-life of approximately 70 hours.20,21 The differences in half-lives between these 2 ChE inhibitors underlie the differences in dosing frequencies. Donepezil should be administered once a day, while rivastigmine should be given twice daily. Although it is a common belief that a once-daily dosing regimen can help with compliance of treatments in the general medical practice, no evidence in the literature supports that compliance of once-daily versus twice-daily dosing is affected in this particular studied population.
The pharmacologically diverse and clinically proven cholinergic therapies now available in the United States17,20,22,23—donepezil, rivastigmine, galantamine, and tacrine—allow clinicians the opportunity to offer long-term treatment to their patients with Alzheimer's disease. Over time, a once-beneficial medication may lose its efficacy due to drug- or disease-specific limitations, including the different pharmacologic properties of each agent or the possibility of the up-regulation of AChE. For example, data have suggested that the efficacy of donepezil decreases with time, due to its AChE up-regulation.24,25 Switching to another ChE inhibitor once the original drug is ineffective is a reasonable option that may prolong symptomatic control.21
BACKGROUND
In a large open-label study, a substantial proportion of patients previously unresponsive to or unable to tolerate donepezil experienced significant cognitive improvements following transition to rivastigmine.21 In their commentary, Auriacombe et al.21 suggested that dual inhibition of BuChE and AChE with rivastigmine may be responsible for the positive response. Postmortem examinations of the brains of patients with Alzheimer's disease have detected increased BuChE activity in those with more advanced disease. Although the clinical relevance of this increased activity in Alzheimer's disease has not been established, Perry et al.26 recently demonstrated a correlation between increased BuChE activity and increased rates of disease progression in Lewy body dementia. Furthermore, while the distribution of BuChE is widespread in the brain, there is abundant distribution in the temporal lobe and hippocampal formation.12,13 While other factors may come into play, it may be theorized that the effect of AChE-specific inhibitors may become diminished as Alzheimer's disease progresses and BuChE activity is enhanced relative to AChE activity.21
Molecular forms of AChE in the cerebrospinal fluid (CSF) and brain are understood to be similar; measurements of AChE in the CSF may be used as a biochemical marker for cholinergic function in Alzheimer's disease. Interestingly, studies on CSF demonstrate up-regulation of AChE during long-term therapy with donepezil, galantamine, and tacrine.25,27,28 In contrast, both AChE and BuChE activity levels in the CSF of patients with Alzheimer's disease following 12 months of rivastigmine treatment were lower than baseline by 36% and 45%, respectively.28 A feedback loop involving muscarinic or nicotinic AChE receptors29 may explain the AChE up-regulation observed in the CSF of patients treated with donepezil, galantamine, or tacrine. Additional well-controlled studies directly assessing CSF AChE during rivastigmine treatment may be helpful in further defining the mechanism behind sustained AChE inhibition.
Successful treatment with an alternative ChE inhibitor may be impacted by the way the initial switch is accomplished. The treatment objective is to avoid both rapid symptomatic worsening resulting from cessation of the first medication and adverse event emergence or reemergence secondary to initiation of the subsequent product. Several completed studies have been instrumental in providing information on effective switching strategies. Clinical trials with donepezil included a 3-or 6-week washout period and provided important data regarding loss of treatment effects associated with discontinuation of treatment. Although patients treated with donepezil demonstrated significant improvement on cognitive testing during active treatment, scores fell dramatically following the washout period.20,30,31 Rainer and associates32 also completed a small study (N = 47) that expanded the washout analysis to include a variety of drugs used to treat Alzheimer's disease. Again, the purpose was to evaluate effects on cognition following sudden medication discontinuation. As with the donepezil trials, the investigators observed considerable cognitive decline following termination of ChE inhibitors.32 Interestingly, a retrieved dropout analysis of the rivastigmine pivotal trials revealed that patients who discontinued rivastigmine secondary to withdrawal from the study did not experience a precipitous decline in cognition. Although still speculation, these findings suggest that rivastigmine not only alleviates the symptoms of Alzheimer's disease, but may also delay disease progression in some patients.33
The optimum approach for switching remains a topic of debate. Initially, investigators thought a washout period was necessary to avoid potential cholinergic adverse events; however, current data question the benefit of such an approach. Instead, an immediate transition from one product to the next has been advocated to preserve cognitive function, which may potentially decline secondary to the effect of cholinergic withdrawal during a washout period.34 To further evaluate the best switching approach, this comparative analysis was executed to determine if an immediate switch from donepezil to rivastigmine without a washout period could be accomplished safely. Results are compared with adverse event rates from a retrospective analysis of a placebo-controlled trial17 examining patients previously untreated with a ChE inhibitor prior to study entry.
METHOD
Patient Population
Men and women, 50 to 85 years of age, with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of mild to moderate dementia of the Alzheimer's type35 and an investigator-determined poor response to a minimum 6-month course of donepezil, were eligible to participate in the study. Poor response to donepezil was defined as a decline of at least 2 points on the Mini-Mental State Examination (MMSE)36 within the 12 previous months and investigator-determined clinical decline in one of the following areas: activities of daily living, behavior, or global functioning.
Patients, if mentally competent, provided written informed consent prior to participation in the study. Patients' caregivers and an appropriately responsible party on the patient's behalf also provided written informed consent. If the patient was not able to provide written informed consent, written informed consent was obtained from the caregiver and the authorized representative on the patient's behalf, and verbal assent was obtained from the patient if possible and permitted by state, local, and Institutional Review Board (IRB) regulations. Before implementing the study, the protocol and proposed informed consent form were reviewed and approved by a properly constituted IRB/Independent Ethics Committee/Research Ethics Board.
Patients included in the trial satisfied the criteria for the clinical diagnosis of probable/possible Alzheimer's disease as set forth by a work group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA)37 and presented with an MMSE score of 10 to 26 (inclusive), confirming mild-to-moderate Alzheimer's disease. Patients were excluded from the trial for advanced, severe, or unstable diseases of any type that may interfere with evaluations. Specific diagnoses included, but were not limited to, severe chronic obstructive pulmonary disease or asthma, unstable cardiovascular disease, stroke within 6 months of baseline, and uncontrolled peptic ulceration within 3 months of study participation. Patients could continue most medications, with the exception of nootropics, lithium, anticholinergics, and medication for Parkinson's disease. Previous exposure to rivastigmine, sensitivity to donepezil, and ingestion of an investigational drug 30 days prior to the screening visit precluded participation in the study; however, on a case-by-case basis, the medical monitor may have approved the inclusion of patients outside these ranges, provided there was a clear rationale for inclusion based on the clinical judgment of the principal investigator.
Study Design
This is an analysis of a prospective, open-label, multi-center study conducted in the United States from August 2002 to August 2003. Patients were screened approximately 14 days prior to baseline, during which time they continued their daily dose of donepezil. The evening before or the morning of their baseline visit (day 0), patients received their last dose of donepezil. They were then instructed to take their first dose of rivastigmine 1.5 mg on study day 1, between 24 and 36 hours after the last donepezil dose. Patients remained at the 1.5-mg b.i.d. dose for 28 days. Patients unable to tolerate rivastigmine 1.5 mg b.i.d. could decrease the dose to 1.5 mg once a day for up to 3 days, at which time the twice-daily dosing schedule was reinitiated. If the clinician desired, the patient could be titrated more rapidly with a 2-week titration, as allowed per the package insert.
Patients included in the cohort were contacted by telephone at week 1 (day 7) and returned for an office visit at week 4 (day 28). Safety and tolerability were assessed through collection of adverse event information and patient disposition.
Results from this open-label study are compared with findings from a retrospective analysis of data from one of the pivotal studies in the United States, a placebo-controlled clinical trial of previously untreated patients with Alzheimer's disease who were randomly assigned to receive rivastigmine treatment or placebo.17 The pivotal trial utilized a 1-week titration schedule, rather than the 4-week titration schedule employed in the open-label study. Only those patients receiving the starting dose of rivastigmine 1.5 mg b.i.d. or placebo in the pivotal trial are included in this comparative investigation.30,38
Statistical Methods
The safety population consisted of all patients who took at least 1 dose of study medication. Assessment of safety was based on the frequency of adverse events and patient disposition. Vital signs data were listed with notable values flagged and summary statistics reported for changes from baseline values. The study cohort size was planned to have a 95% upper confidence limit < 25% for the average of incidence rates of nausea and vomiting, assuming an average incidence rate of 15% estimated based on historical data and using a 2-sided confidence interval. All statistical tests were conducted against a 2-sided alternative hypothesis, employing a significance level of .05. Statistical analysis comparing rivastigmine treatment with placebo for safety in the retrospective analysis used analyses of variance for changes from baseline and Fisher exact test for the occurrence of abnormalities.17
RESULTS
At baseline, 61 patients with mild to moderately severe Alzheimer's disease who were responding poorly to donepezil were enrolled in the study. Fifty-eight patients (95%) completed 4 weeks of rivastigmine treatment. The premature discontinuations were secondary to adverse events, including irritability, confusion, vomiting, and, in 1 patient, worsening social withdrawal with verbal perseveration.
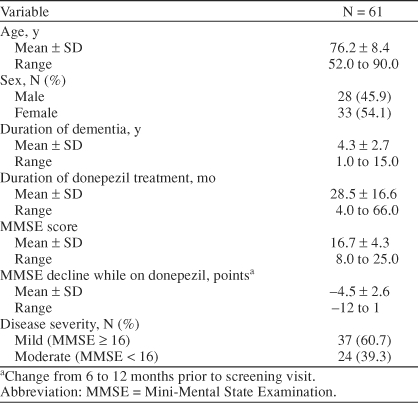
Demographic characteristics are presented in Table 1. Mean duration of treatment with donepezil was 28.5 months, with a range of 4 to 66 months. Prior to initiation of rivastigmine treatment, the mean rate of decline on the MMSE during 6 to 12 months of donepezil therapy was −4.5 points. Some exceptions were granted for patients with a decline of > 2 points within 6 months or a decline of > 4 points in up to 18 months.
Table 1.
Baseline Characteristics of Patients With Alzheimer's Disease: Open-Label Study Population
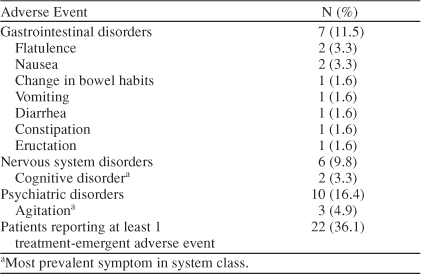
During the first 7 days of rivastigmine therapy following the switch from donepezil, 11 patients (18.0%) reported at least 1 treatment-emergent adverse event. Overall incidence of gastrointestinal (GI) disorders through day 7 was 8.2% (N = 5), with 2 reports of flatulence and 1 report each of constipation, diarrhea, eructation, nausea, and vomiting. The majority of GI adverse events were rated as mild. During the total 28 days of rivastigmine therapy, 22 patients (36.1%) reported at least 1 treatment-emergent adverse event, of which 10 cases were mild, 7 moderate, and 5 severe. Incidences of specific adverse events for days 7 and 28 of the open-label study are summarized in Tables 2 and 3.
Table 2.
Day 7 Incidence of the Most Common Adverse Events Following Administration of Rivastigmine in the Open-Label Study (N = 61)
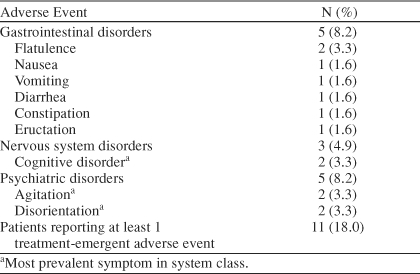
Table 3.
Day 28 Incidence of the Most Common Adverse Events Following Administration of Rivastigmine in the Open-Label Study (N = 61)
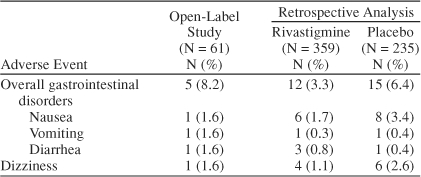
Tolerability and safety results from the immediate-switch protocol are compared with findings from a retrospective analysis of previously untreated patients initiated on rivastigmine from the pivotal trial17 and are presented in Table 4. The results from the immediate-switch protocol were comparable to the results from both the active and the placebo treatment arms in the pivotal study. Vital signs remained stable throughout the trial for all patient treatment groups.
Table 4.
Comparative Analysis: Adverse Event Rates in the Open-Label Study and Retrospective Analysis17
DISCUSSION
The results from the interim analysis of this clinical trial suggest that patients with a poor response to donepezil tolerate an immediate switch to rivastigmine. A comparison of tolerability findings from this open-label study with results for patients who were previously untreated17 suggests that the transition from donepezil to rivastigmine was safe and well tolerated in both studies. These results are in contrast to the commonly held belief that cholinergic adverse events may occur if a washout period is omitted prior to initiation of an alternative agent.34
The rationale for considering a switch from one drug to another relates to potentially important differences between drugs in this class. Despite the conception that drugs of this class are indistinguishable with regard to mechanism of action, the ChE inhibitors available maintain highly distinct pharmacokinetic, pharmacodynamic, and pharmacologic profiles. Such differences may explain the potential for a different ChE inhibitor to provide symptomatic improvement once the initially administered ChE inhibitor becomes ineffective.21
The interim data reported in the current clinical trial are in agreement with other data previously reported in poster presentations,39,40 which suggest that immediate-switch (with 24–36 hours between the last dose of donepezil and the first dose of rivastigmine) strategies from donepezil to rivastigmine were well tolerated; however, this is the first immediate-switch study to be published. Evidence from the present study suggests that immediate transition from donepezil to rivastigmine without a wash-out period is well tolerated and does not cause serious adverse events. Furthermore, the incidence of adverse events is comparable with the rates observed in previously untreated patients (data on file, Novartis Pharmaceuticals Corporation, East Hanover, N.J.). Theoretically, new adverse events do not occur because patients currently treated with a ChE inhibitor have already acclimated to their previous ACh levels. Thus, to avoid the potential of washout-induced cognitive decline, evidence supports an immediate switch from donepezil to rivastigmine, which can be accomplished without cholinergic-induced side effects.
This study is limited by its open-label design. Because a placebo group was not included in the open-label study, it is not possible to absolutely determine if the adverse event rates are as accurate as in a controlled trial. The retrospective analysis looked at data from a double-blind, placebo-controlled trial, but comparative analyses between the current, ongoing study and the retrospective collection of data are limited by potential differences in study design, patient population, and data collection techniques. In addition, although the results are compelling, this is both an interim analysis and a relatively small patient population designed to assess safety and tolerability of an immediate switch from donepezil to rivastigmine. The results of this study cannot be interpreted as supportive of a coadministration strategy of ChE inhibitors,16 since this is not recommended in the products' labeling information.41,42 Efficacy measures were not part of the interim analysis, because the study was designed to evaluate safety and tolerability rather than efficacy. Based on current findings, transition from donepezil to rivastigmine without a washout period is a well-tolerated approach.
CONCLUSION
Results of this study suggest that switching patients with Alzheimer's disease from donepezil to rivastigmine is well tolerated. Results are similar to initiation of rivastigmine in previously untreated patients. Immediate transition from one agent to another is preferred, and the rapid transition from donepezil to rivastigmine may be accomplished safely and with good tolerability. Moreover, switching to another ChE inhibitor may positively impact symptoms of Alzheimer's disease.21
Drug names: donepezil (Aricept), galantamine (Reminyl), lithium (Lithobid, Eskalith, and others), rivastigmine (Exelon), tacrine (Cognex).
Footnotes
Dr. Sadowsky has served as a consultant for or a speakers/advisory board member of Novartis, Pfizer, and Forest and has received honoraria from Novartis and Forest. Dr. Farlow has served as a consultant for Novartis, Forest, Pfizer, and Memory; has received grant/research support from Novartis, Forest, Pfizer, and Lilly Europe; and has received honoraria and served on the speaker/advisory boards of Novartis, Forest, and Pfizer. Drs. Atkinson, Chen, and Mirski and Mss. Steadman and Koumaras are all employees of Novartis.
REFERENCES
- Lamb HM, Goa KL.. Rivastigmine: a pharmacoeconomic review of its use in Alzheimer's disease. Pharmacoeconomics. 2001;19:303–318. doi: 10.2165/00019053-200119030-00008. [DOI] [PubMed] [Google Scholar]
- Evans DA.. Estimated prevalence of Alzheimer's disease in the United States. Milbank Q. 1990;68:267–289. [PubMed] [Google Scholar]
- Rice DP, Fillit HM, and Max W. et al. Prevalence, costs, and treatment of Alzheimer's disease and related dementia: a managed care perspective. Am J Manag Care. 2001 7:809–818. [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C.. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard CG.. Advances in the treatment of Alzheimer's disease: benefits of dual cholinesterase inhibition. Eur Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, and Blessed G. et al. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1978 4:273–277. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bruckner MK, and Lange M. et al. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer's disease resemble embryonic development: a study of molecular forms. Neurochem Int. 1992 21:381–396. [DOI] [PubMed] [Google Scholar]
- Davies P.. Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res. 1979;171:319–327. doi: 10.1016/0006-8993(79)90336-6. [DOI] [PubMed] [Google Scholar]
- Enz A, Amstutz R, and Boddeke H. et al. Brain selective inhibition of acetylcholinesterase: a novel approach to therapy for Alzheimer's disease. Prog Brain Res. 1993 98:431–438. [DOI] [PubMed] [Google Scholar]
- Cutler NR, Polinsky RJ, and Sramek JJ. et al. Dose-dependent CSF acetyl-cholinesterase inhibition by SDZ ENA 713 in Alzheimer's disease. Acta Neurol Scand. 1998 97:244–250. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Razin M, and Chorev M. et al. Pharmacological evaluation of phenyl-carbamates as CNS-selective acetylcholinesterase inhibitors. J Neural Transm Suppl. 1994 43:219–225. [PubMed] [Google Scholar]
- Darvesh S, Hopkins DA, Geula C.. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- Poirier J.. Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl. 2002;127:6–19. [PubMed] [Google Scholar]
- Greig NH, Lahiri DK, and Sambamurti K. Butyrylcholinesterase: an important new target in Alzheimer's disease therapy. Int Psychogeriatr. 2002 14suppl 1. 77–91. [DOI] [PubMed] [Google Scholar]
- Grossberg GT, Stahelin HB, and Messina JC. et al. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty-two classes of medications. Int J Geriatr Psychiatry. 2000 15:242–247. [DOI] [PubMed] [Google Scholar]
- Exelon [package, insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. 2001. [Google Scholar]
- Corey-Bloom J, Anand R, Veach J.. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetyl-cholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol. 1998;1:55–65. [Google Scholar]
- Anand R, Gharabawi G, Enz A.. Efficacy and safety results of the early phase studies with Exelon (ENA-713) in Alzheimer's disease: an overview. J Drug Dev Clin Pract. 1996;8:109–116. [Google Scholar]
- Anand R, Gharabawi G.. Clinical development of Exelon (ENA-713): the ADENA programme. J Drug Dev Clin Pract. 1996;8:117–122. [Google Scholar]
- Rogers SL, Farlow MR, and Doody RS. et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998 50:136–145. [DOI] [PubMed] [Google Scholar]
- Auriacombe S, Pere JJ, and Loria-Kanza Y. et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease who failed to benefit from treatment with donepezil. Curr Med Res Opin. 2002 18:129–138. [DOI] [PubMed] [Google Scholar]
- Knapp MJ, Knopman DS, and Solomon PR. et al. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. The Tacrine Study Group. JAMA. 1994 271:985–991. [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, and Wessel T. et al. Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000 54:2261–2268. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Amici S, and Lanari A. et al. Cerebrospinal fluid levels of biomarkers and activity of acetylcholinesterase (AChE) and butyryl-cholinesterase in AD patients before and after treatment with different AChE inhibitors. Neurol Sci. 2002 23:S95–S96. [DOI] [PubMed] [Google Scholar]
- Davidsson P, Blennow K, and Andreasen N. et al. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcho-linesterase inhibitors in patients with Alzheimer's disease. Neurosci Lett. 2001 300:157–160. [DOI] [PubMed] [Google Scholar]
- Perry E, McKeith I, Ballard C.. Butyrylcholinesterase and progression of cognitive deficits in dementia with Lewy bodies. Neurology. 2003;60:1852–1853. doi: 10.1212/01.wnl.0000068336.84399.9e. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Hellstrom-Lindahl E, and Almkvist O. et al. Activity of acetyl-cholinesterase in CSF increases in Alzheimer's patients after treatment with tacrine. Alzheimers Rep. 1999 2:347–352. [Google Scholar]
- Darreh-Shori T, Almkvist O, and Guan ZZ. et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology. 2002 59:563–572. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Rossner S, and Albrecht C. et al. Muscarinic acetylcholine receptors activate the acetylcholinesterase gene promoter. J Physiol Paris. 1998 92:257–264. [DOI] [PubMed] [Google Scholar]
- Aricept [package, insert]. New York, NY: Pfizer Inc. 2003. [Google Scholar]
- Greenberg SM, Tennis MK, and Brown LB. et al. Donepezil therapy in clinical practice: a randomized crossover study. Arch Neurol. 2000 57:94–99. [DOI] [PubMed] [Google Scholar]
- Rainer M, Mucke HA, and Kruger-Rainer C. et al. Cognitive relapse after discontinuation of drug therapy in Alzheimer's disease: cholinesterase inhibitors versus nootropics. J Neural Transm. 2001 108:1327–1333. [DOI] [PubMed] [Google Scholar]
- Farlow M, Potkin S, and Koumaras B. et al. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26-week, Alzheimer disease trial. Arch Neurol. 2003 60:843–848. [DOI] [PubMed] [Google Scholar]
- Edwards KR, Goodman W, and Khoury KN. et al. Rapid changeover from donepezil to rivastigmine well tolerated [poster]. Presented at the annual meeting of the American Geriatrics Society; May 9–13, 2001; Chicago, Ill. P251:S91–S92. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR.. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, and Folstein M. et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 34:939–944. [DOI] [PubMed] [Google Scholar]
- Galantamine [package, insert]. New York, NY: Pfizer Inc. 2003. [Google Scholar]
- Anand R, Shua-Haim J, and Sohn H. et al. Counterintuitive effects of a treatment washout in Alzheimer's disease patients switched from donepezil to rivastigmine [poster]. Presented at the 39th annual meeting of the American College of Neuropsychopharmacology; December 11–15, 2000; San Juan, Puerto Rico. Poster session 2. [Google Scholar]
- Shua-Haim J, Smith JM, and Amin S. Crossover results from donepezil (Aricept) to rivastigmine (Exelon) in Alzheimer's disease patients: an overall analysis of 3 prospective studies [poster]. Presented at the annual meeting of the American Geriatrics Society; May 9–13, 2001; Chicago, Ill. P330:S115. [Google Scholar]
- Aricept [package, insert]. New York, NY: Pfizer Inc. 2004. [Google Scholar]
- Exelon [package, insert]. East Hanover, NJ: Novartis. 2004. [Google Scholar]


