Abstract
Bacteria exert a variety of influences on the morphology and physiology of animal cells whether they are pathogens or cooperative partners. The association between the luminous bacterium Vibrio fischeri and the sepiolid squid Euprymna scolopes provides an experimental model for the study of the influence of extracellular bacteria on the development of host epithelia. In this study, we analyzed bacterium-induced changes in the brush borders of the light organ crypt epithelia during the initial hours following colonization of this tissue. Transmission electron microscopy of the brush border morphology in colonized and uncolonized hosts revealed that the bacteria effect a fourfold increase in microvillar density over the first 4 days of the association. Estimates of the proportions of bacterial cells in contact with host microvilli showed that the intimacy of the bacterial cells with animal cell surfaces increases significantly during this time. Antibiotic curing of the organ following colonization showed that sustained interaction with bacteria is essential for the retention of the induced morphological changes. Bacteria that are defective in either light production or colonization efficiency produced changes similar to those by the parent strain. Conventional fluorescence and confocal scanning laser microscopy revealed that the brush border is supported by abundant filamentous actin. However, in situ hybridization with β-actin probes did not show marked bacterium-induced increases in β-actin gene expression. These experiments demonstrate that the E. scolopes-V. fischeri system is a viable model for the experimental study of bacterium-induced changes in host brush border morphology.
Studies of the interactions between bacteria and animals have shown that bacteria strongly influence the morphology, biochemistry, and molecular biology of the tissues with which they associate, both during normal development and during the progression of a pathogenic infection (21, 22, 40, 46). For example, both pathogenic and benign bacteria alter the morphology of the villi and the underlying tissue layers of the intestine (21, 28, 42, 47), and bacteria appear to be requisite to the late development of the vertebrate immune system (10, 49, 54). At a microscopic level, bacterial cells mediate developmental changes in the host cell ultrastructure through changes in the patterns of gene expression and protein synthesis of these cells and/or posttranslational modifications in existing proteins (6, 12, 25, 33, 38).
Because the association of animal cells with bacteria most often involves a single host animal with a consortium of bacteria, such as that residing in the healthy mammalian intestine, it has been difficult to resolve which of these developmental changes result from interaction with the cooperative, essential microbiota and which occur in response to potential pathogens that are entering the habitat and threatening the community structure of the host and its critical prokaryotic partners. Several approaches to this problem have provided significant insight, most notably studies of germfree and gnotobiotic animal models (6, 8, 22, 24). Research with these model systems has confirmed that bacteria are essential both to the normal development and to the sustained health of the host (28, 33). The analyses of the behavior of individual microbe species introduced into the germfree environment have shown that specific bacteria have the potential to cause significant biochemical and molecular changes in host cells (6, 27, 39). However, using these models, it has been difficult to determine whether such changes actually occur in response to those specific microbes under normal conditions in which the entire consortium is present. Thus, complementary to research on germfree and gnotobiotic models are studies of simplified systems in which bacteria naturally occur in monospecific culture with a host. The association between the prokaryotic nitrogen-fixing bacteria and leguminous plant hosts has provided such a model of prokaryotic-eukaryotic interaction, and research of this partnership over several decades has resulted in a wealth of knowledge about how the symbiotic partners respond during the development of a stable relationship (19, 50).
The association between the marine luminous bacterium Vibrio fischeri and its host, the Hawaiian sepiolid squid Euprymna scolopes, provides an experimental model for the study of the persistent colonization of animal epithelia by extracellular bacteria (23, 32, 44). The relationship involves one host and one microbial species, both of which can be maintained independently under laboratory conditions. Further, molecular genetics has been successfully developed in V. fischeri (44), which will allow for the determination of symbiosis-specific phenotypic traits. The type of animal-bacterium relationship exemplified by the E. scolopes-V. fischeri association is similar in a number of aspects to the more common, perhaps ubiquitous, association of animals with resident intestinal bacteria. Specifically, similar to intestinal animal-bacterium alliances, the E. scolopes-V. fischeri relationship begins anew each generation with exposure to symbiotic bacterial cells from the external environment (32, 52) and the bacteria colonize highly polarized epithelial cells that are embryonically derived from gut primordia (34, 35).
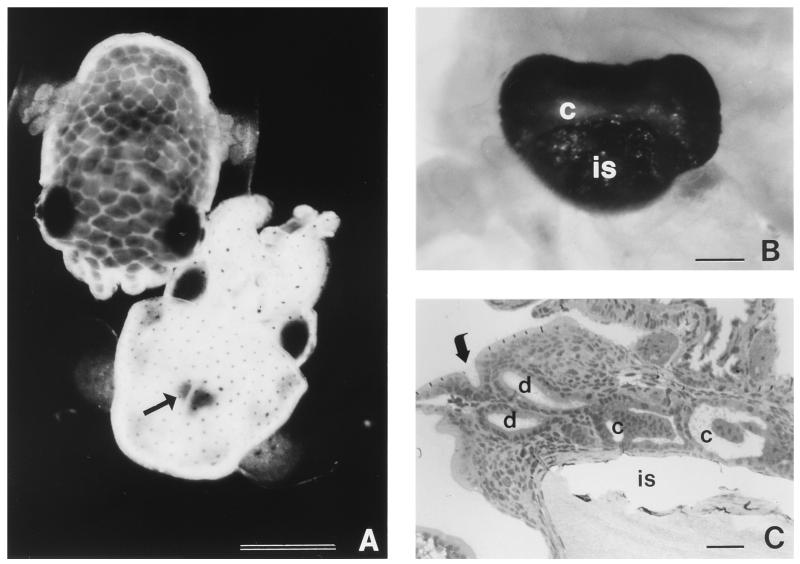
In the adult squid host, the colonized organ appears as a conspicuous bilobed structure in the center of the body cavity. It is comprised of a bacterium-containing core region surrounded by accessory tissues (31), which serve to modify the bacterial luminescence for use by the animal in its nocturnal behavior (37). The core of the adult organ houses on average a billion bacterial cells in labyrinthine crypts lined with highly polarized epithelial cells. During embryogenesis of the squid host (34), tissues of two types that play principal roles in the inoculation process are formed: (i) a transparent, superficial ciliated, microvillous epithelium that is not itself colonized but appears to potentiate the inoculation of the organ, and (ii) three simple, sacculate crypts, which are lined with polarized epithelia and are present on either side of the organ. These crypts are the sites of colonization by the bacterial partner (see Fig. 1 and 7).
FIG. 1.
Light organ of juvenile E. scolopes. (A) Photomicrographs of live, newly hatched squid. The upper left is a dorsal view of a healthy, resting individual. The lower right is a ventral view of an anesthetized animal; the light organ can be seen through the translucent mantle (arrow). Bar, 1 mm. (B) Photomicrograph of a juvenile light organ, revealed by ventral dissection of the mantle cavity. In the juvenile, the light organ consists of bacterium-containing ventral crypts (c) surrounded by the ink sac (is). Bar, 0.1 mm. (C) Light micrograph of a cross section of a symbiotic light organ at 1 day postinfection. The bacteria enter the light organ through pores (arrow) on the surface and travel along ciliated ducts (d) into the crypts (c) where colonization occurs. Each side of the organ bears three pores leading to three independent crypts. is, ink sac. Bar, 50 μm.
FIG. 7.
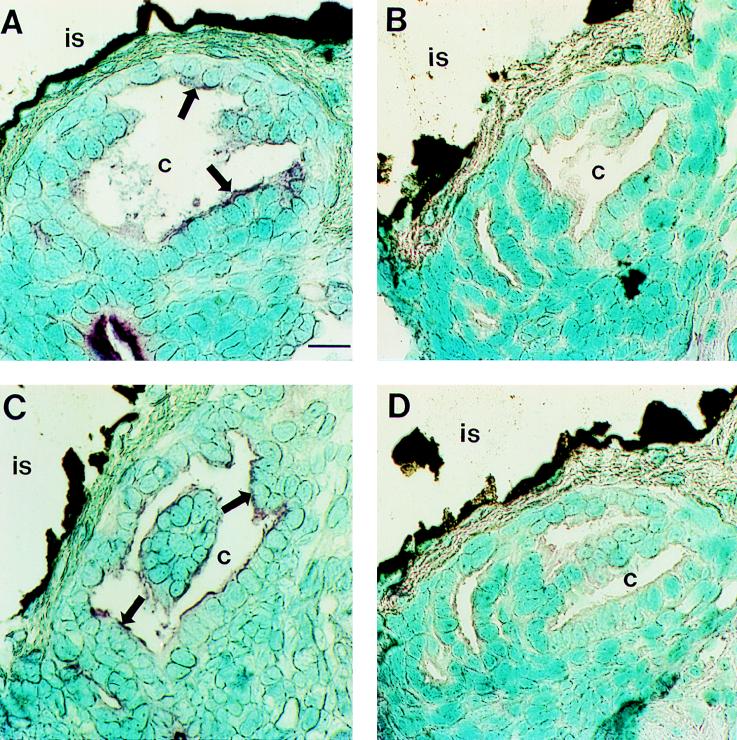
In situ hybridization of β-actin mRNA in the light organ crypts (c). (A) Light micrographs of the crypts of a symbiotic animal at 12 h postinfection reveal the presence of β-actin mRNA (purple staining [arrows]) along the apical surfaces of the crypt cells. (B) The negative controls in which tissue sections were incubated with the sense probe did not show any labeling. (C) The β-actin mRNA labeling in the crypts of an aposymbiotic animal at 12 h postinfection showed a pattern and intensity similar to those of the symbiotic animal at 12 h postinfection. (D) Negative control of a contiguous section. Bar, 20 μm.
The morphology and anatomy of the adult light organ are conspicuously different from those of the hatchling juvenile (31, 35). Superficially, the organ shape transforms from heart-like to bilobed, the accessory tissues associated with the modulation of bacterial luminescence are elaborated, and the superficial ciliated, microvillous surface is lost. In the light organ core, the bacterium-containing crypts ramify, the crypt cells swell, and the microvilli of the brush border change from being simple and sparse along hatchling crypt cells to highly dense and branched on the crypt cells of adult light organs. The goal of ongoing experimental studies is to reveal which of these developmental processes are bacterially induced and which are developmentally “hard-wired,” i.e., independent of interaction with bacterial symbionts. Comparison of newly hatched juveniles that either have or have not been exposed to V. fischeri have revealed that the regression of the superficial ciliated, microvillous field results from bacterium-induced cell death of these epithelial cells, a process that occurs over a period of 4 days (9, 35). Experiments in which the host light organ is cured of V. fischeri at various times following inoculation revealed that, although the cell death program requires 4 days for completion, the bacterial symbionts need only be present for an initial period of 12 h to induce the entire program (9). Thus, in this example, the bacteria appear to be sending an irreversible signal to the animal cells.
Here we report the results of experiments on the early development of the host cell brush border. Specifically, we determined whether (i) the elaboration of the highly complex brush border that is characteristic of the adult can be detected in the first few days of colonization; (ii) the bacteria are essential for these modifications in the brush border, and, if so, whether that involvement requires persistent interaction between host and symbiont cells; (iii) the normal developmental process of the host occurs when juveniles are colonized with specific cells of V. fischeri that are defective for certain symbiosis-associated phenotypic traits; and (iv) changes in β-actin production in host cells accompany alterations in the brush border. The results of our studies show that the bacteria have a profound effect on the development of the brush border during this early period and that sustained association with the bacterial cells is essential for the retention of these developmental modifications. Although the microvilli stain positively with fluorochromes that recognize actin, dramatic changes in expression of the β-actin gene, as revealed by in situ hybridization, were not observed during these first few days following colonization of the light organ. Experiments with V. fischeri mutants indicated that bacterium-induced alterations in the brush border do not require full colonization of the light organ and are independent of the presence of bacterial luminescence, the principal product of the bacteria used by the animal host.
MATERIALS AND METHODS
General procedures.
E. scolopes (Cephalopoda: Sepiolidae) squid were captured and maintained as previously described (31, 34). To ensure that newly hatched juvenile squid did not become prematurely infected with any residual bacteria that might be associated with the egg clutch, squid upon hatching were transferred immediately through three rinses of offshore seawater, which does not contain infective levels of V. fischeri (45). To render cohorts of hatchling squid symbiotic, V. fischeri cells were grown to log phase in seawater-based complex medium (45) and diluted to 1,000 cells per ml of seawater containing newly hatched squid. In experiments using aposymbiotic squid (uninfected because they had not been exposed to symbiotic strains of V. fischeri), the animals were exposed to offshore seawater alone. To prevent crosscontamination between individuals, symbiotic and aposymbiotic squid were kept individually in 5 ml of seawater in glass scintillation vials, and the water was changed daily. Colonization of the light organ by V. fischeri was monitored each day, following the water change, by measuring bacterial light emission with a photometer (model 3600; Biospherical Instruments, Inc., San Diego, Calif.); in previous studies, luminescence levels have been shown to be roughly proportional to the extent of symbiotic colonization (45).
All chemicals used were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise indicated.
Colonization treatments. (i) Normal development.
To characterize the effect of symbiotic bacteria on the development of the epithelial cell brush border of the host light organ crypts, we compared the ultrastructures of these cells in symbiotic and aposymbiotic animals during the establishment of the association. Hatchling squid were inoculated with V. fischeri and fixed at 12, 24, 48, 72, and 96 h. Control aposymbiotic animals were fixed at the same times. Both symbiotic and aposymbiotic juveniles were processed for analysis by transmission electron microscopy (TEM).
(ii) Transient versus continuous exposure to V. fischeri.
To determine whether a persistent interaction between the squid host and symbiotic bacteria is necessary to induce changes in the crypts of the light organ, we used the bacteriostatic antibiotic chloramphenicol (CM) to clear the light organ of symbionts. Previous experiments have shown that the light organ becomes free of viable symbionts 3 to 6 h after addition of CM to the seawater containing the juveniles (9). Fully infected animals were cured of bacteria with 10 μg of CM per ml of seawater at 12, 24, or 48 h after infection. A CM-resistant strain of V. fischeri was used as a control for any pharmacological effects that CM treatment may have on the crypt epithelium. The water was changed each day, and fresh CM was added at that time to those treatments that included the antibiotic. Animals were fixed at different times following antibiotic treatment and analyzed by TEM.
(iii) Inoculation with a luxA dark mutant and a leucine auxotroph of V. fischeri.
To determine the effect of light and colonization level on developmental changes in the host cell brush border, we used two mutant strains of the bacterial symbionts. The luxA dark mutant (51) produces no luminescence and has been classified as a “persistence mutant” (44); i.e., the level of colonization is the same as that of the wild type for the first 24 h but drops to 20% of the wild-type level by 48 h. The leucine auxotroph used in this study is fully luminous but has been classified as an “accommodation mutant” (44); i.e., it never reaches colonization levels typical of wild-type strains. This leucine auxotroph colonizes to only approximately 20% of the wild-type level. Hatchling squid were inoculated with these strains as described above. At 12, 24, and 48 h, samples were processed for analysis by TEM.
TEM.
To prepare samples of the light organ for TEM, the juvenile squid were anesthetized in iced seawater. Squid were fixed for 12 h at 4°C in 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4) with 0.45 M sodium chloride (marine PBS) and postfixed for 1 h at 25°C in 1% osmium tetroxide in marine PBS. The samples were dehydrated at 25°C through a graded ethanol series and embedded in Spurr resin (48). Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a JEOL CX-100 transmission electron microscope.
To study changes in the host brush border in response to interaction with bacterial symbionts, we chose to analyze only the largest pair of crypts of the juveniles. Each side of the juvenile light organ (Fig. 1) houses the symbionts in three crypts with different volumes (34). The largest crypt is the earliest to develop during embryogenesis, and at hatching it is the most differentiated. The density of microvilli per 25 μm2 of surface was derived by counting over a 5-μm length of membrane and assuming equal distribution in two dimensions. The microvillar density was measured in three different animals per time point, and for each animal, six distinct microscope fields were analyzed. Because the data for each time fell in a normal distribution, results were expressed as means ± standard deviations. In addition, the length and width of the microvilli were measured, compared among the various experimental treatments, and also expressed as means ± standard deviations.
To estimate the degree of intimacy of the bacterial cells with the animal cells, we first determined the number of bacterial cells in the crypts that were in direct contact with any microvilli of the brush border at a given time postinoculation. As a second indicator of this intimacy, we estimated the percentages of the bacterial surfaces in direct contact with the microvillar epithelia and assigned each measurement to a class of <30%, 30 to 50%, or >50%. These data were analyzed by chi-square approximations of a Kruskal-Wallis test (30) performed with the arc-sine-transformed percentages.
Fluorescent staining of F-actin.
To visualize the distribution and quantity of cytoskeletal actin filaments of the epithelial brush border, juvenile squid were stained with rhodamine-phalloidin (Molecular Probes, Inc., Eugene, Oreg.). Two-day-old symbiotic and aposymbiotic animals were anesthetized as previously described and fixed in 4% paraformaldehyde in marine PBS for 1 h at 25°C. They were rinsed in marine PBS and then permeabilized with marine PBS containing 1% Triton X-100 for 10 min. Finally, the samples were stained for 20 min in 2 ml of fluorescent solution containing 66 pg of rhodamine-phalloidin per ml of marine PBS to which 0.5% Triton X-100 had been added. The fluorescent labeling of filamentous actin (F-actin) was observed with an Olympus model BX60 epifluorescence microscope and an Olympus Fluoview model FVX-BX60 confocal laser scanning microscope.
In situ hybridization.
To determine whether any increase in microvillar density was the result of bacterium-induced changes in β-actin gene expression, we performed in situ hybridization using a β-actin riboprobe. Standard procedures that ensure RNase-free conditions were applied (1, 41, 53). Animals were anesthetized, and their light organs were removed and fixed for 12 h at 4°C in 4% paraformaldehyde in diethyl pyrocarbonate (DEPC)-treated and sterilized marine PBS. The tissue was rinsed three times for 10 min each in the same buffer, dehydrated through a graded ethanol series, and infiltrated with toluene. The tissue was then incubated in a 1:1 mixture of toluene and paraffin for 4 h at 58°C, followed by an overnight impregnation at 58°C in 100% paraffin. Light organs were then embedded in fresh paraffin. Sections of 4 μm were cut and mounted on aminopropyltriethoxysilane-coated microscope slides.
A 560-bp DNA fragment of the E. scolopes-specific β-actin gene was cloned in a pBluescript SK+ plasmid (Boehringer Mannheim, Inc., Indianapolis, Ind.) downstream of T3 and T7 promoters, in which T7 promoted the antisense riboprobe transcription. After linearization at a suitable site with appropriate restriction enzymes, the template DNA was purified by phenol-chloroform extraction and ethanol precipitation. In vitro transcription was performed with T3 and T7 RNA polymerases. Digoxigenin-labeled UTP was used as a substrate and incorporated into the transcript. The labeled sense and antisense RNA probes were purified by ethanol precipitation, aliquoted in DEPC water, and stored at −80°C. The final concentration of the probes was approximately 0.1 μg/μl.
Paraffin sections were dewaxed in toluene, rehydrated through decreasing percentages of ethanol from 100 to 0%, and rinsed in DEPC water. The sections were allowed to dry for 10 min at 42°C and incubated with 25 μg of proteinase K per ml of 10 mM sodium phosphate buffer with 0.15 M NaCl (PBS) and 10 mM EDTA (pH 7.4) (PBS-EDTA) for 10 min at 42°C. Sections were then rinsed once for 2 min in PBS-EDTA and three times for 2 min each in DEPC water, dehydrated throughout a graded ethanol series, and allowed to dry for 15 min at 42°C. Each section was incubated with 20 μl of a 1:10 dilution of the sense or anti-sense β-actin RNA probe in a hybridization medium comprised of 50% formamide, 20% dextran sulfate, 2.5 mg of salmon sperm DNA (5 Prime→3 Prime, Inc., Boulder, Colo.) per ml, and 0.03% Triton X-100 in 2× SSC (0.3 M NaCl and 0.03 M sodium citrate [pH 7.4]). The hybridization mixture was protected with a 22-mm2 coverslip and placed overnight in an oven at 50°C in a humidified chamber. Specimens were washed once for 5 min in PBS-EDTA containing 0.1% Triton X-100, once for 15 min at 55°C in 2× SSC containing 50% formamide, twice for 5 min each in 2× SSC, once for 5 min in 1× SSC, and once for 5 min in 0.5× SSC. After being rinsed in 100 mM Tris with 150 mM NaCl (pH 7.5) (TS), sections were then incubated for 1 h at 25°C with an antidigoxigenin antibody conjugated to alkaline phosphatase (Boehringer Mannheim, Inc.) diluted at 1:100 in TS containing 1% bovine serum albumin. Nitroblue tetrazolium and bromochloroindolyl phosphate substrates of alkaline phosphatase were used to develop the colorimetric reaction. The tissue was counterstained with Alcian blue and mounted in aqueous glycerol gelatin.
RESULTS
Effects of symbiosis on crypt epithelial brush border.
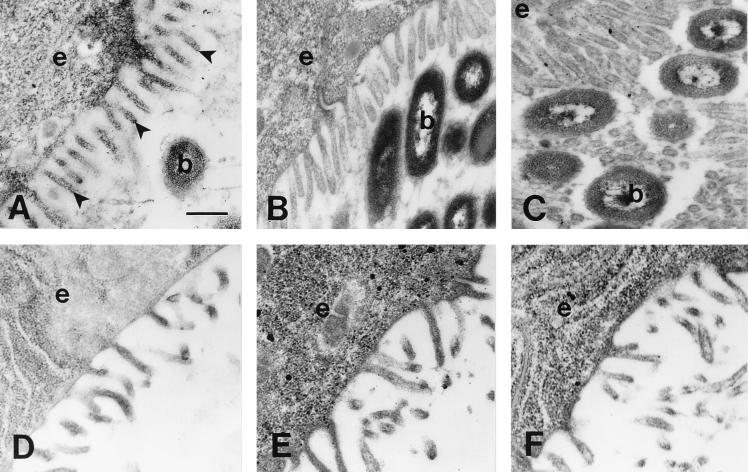
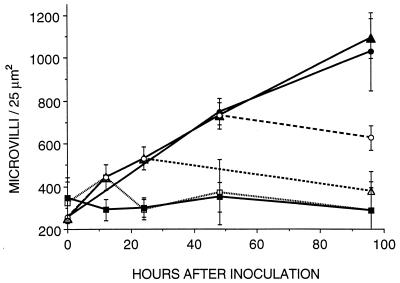
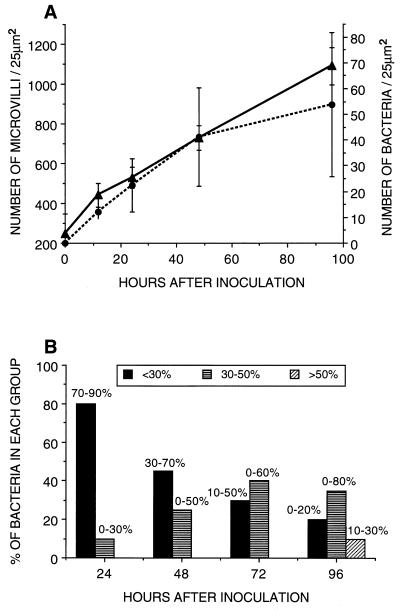
The density of microvilli in the brush border increased fourfold during the first 96 h of colonization with V. fischeri (Fig. 2 and 3). Newly hatched juveniles contained approximately 250 microvilli per 25 μm2 (x̄ = 250 ± 96) (Fig. 3). The increase in the density of microvilli averaged 1.7 times after a 12-h exposure to V. fischeri (x̄ = 442 ± 59) (Fig. 2A and 3), about 3 times after 48 h (x̄ = 730 ± 53) (Fig. 2B and 3), and more than fourfold after 96 h (x̄ = 1,090 ± 93) (Fig. 2C and 3). However, the increase of microvillar density was not observed in aposymbiotic animals of the same age. Newly hatched, aposymbiotic squid after 12 h (x̄ = 347 ± 96), 48 h (x̄ = 290 ± 50), and 96 h (x̄ = 289 ± 131) of exposure showed no significant differences in the density of microvilli of the crypt epithelial cells (Fig. 2D, E, and F and 3). Thus, the interaction of these cells with symbiotic V. fischeri appears to be responsible for the increase in microvillar density of the crypt brush border.
FIG. 2.
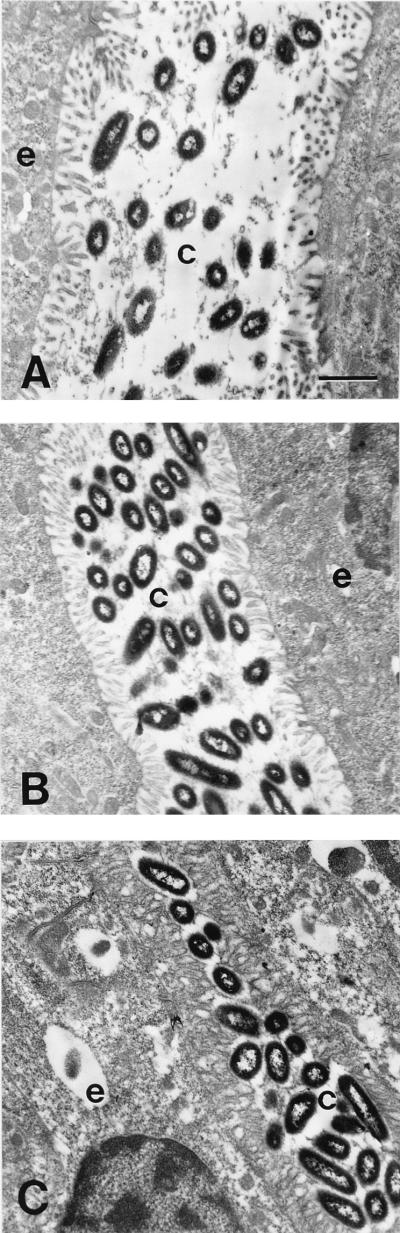
Bacterium-induced changes in microvillar density of the crypt brush border. Transmission electron micrographs of the crypt epithelia of animals at 12 h (A and D), 2 days (B and E), and 4 days (C and F) postinfection. In symbiotic animals (A to C), the number of microvilli (arrowheads) and the intimacy of the microvilli with the bacteria (b) increased for the first 4 days following inoculation. In the absence of symbiotic bacteria (D to F), the brush border remained unchanged. Bar, 0.5 μm.
FIG. 3.
Quantification of changes in microvillar density during normal development and after antibiotic treatment of the host epithelium. Crypts colonized by wild-type strains of V. fischeri (▴) showed a fourfold increase in microvillar density for the first 4 days of colonization. The microvillar density of the brush border of aposymbiotic squids (▪) did not change during the same time. When the light organ was cured of its bacteria by treatment with CM at 12 h (□), 1 day (▵), or 2 days (○) after inoculation, the microvillar density of the epithelial brush border started to decrease and slowly returned toward those of the newly hatched or aposymbiotic animals. Animals inoculated with CM-resistant strains of V. fischeri and treated for the 4-day period with CM (•) showed the same monotonic increase in microvillar density of the crypt brush border as animals inoculated with wild-type strains. Results are expressed as means; error bars indicate standard deviations.
This change in microvillar density over the first 4 days of infection was accompanied by an increase in the intimacy between the symbionts and the epithelial brush border. This modification in the relationship of host and bacterial cells was due to both a narrowing of the crypt spaces and an increase in the association of the bacterial surface with the brush border (Fig. 4). Twelve hours after bacterial inoculation (Fig. 4A and 5A), the number of bacteria in direct contact with a 25-μm2 area of host cell brush border averaged 12 (x̄ = 12 ± 6). After 24 h of infection, the average number doubled (x̄ = 22 ± 10) (Fig. 4B and 5A) and increased about four- to fivefold after 96 h (x̄ = 54 ± 27) (Fig. 4C and 5A). Of those bacteria that were in direct contact with the brush border, there was a significant increase in cell-cell contact (Fig. 5B). The proportion of the bacteria having less than 30% of their membrane surfaces in association with the brush border decreased approximately fourfold by 96 h postinoculation, from a median of 80% at 12 h to 20% at 96 h. The number of bacteria having between 30 and 50% of their membrane surfaces in contact with the epithelium increased three- to fourfold between 24 and 96 h after initiation of the symbiotic association. Bacteria with >50% of their surfaces associated with the brush border were found only in symbiotic animals at 96 h postinfection. Chi-square approximations of the Kruskal-Wallis test (30) demonstrated that all changes were statistically significant at the 95% level, except those in the 30 to 50% range, which were statistically significant at the 90% level.
FIG. 4.
Increasing intimacy between V. fischeri and the host brush border. Transmission electron micrographs of the crypts of symbiotic juveniles are shown at 12 h (A), 2 days (B), and 4 days (C) postinfection. During the first 4 days of the symbiotic association, while the number of bacteria increased, the crypts became narrower, enhancing the intimacy between the epithelial brush border and the symbionts. Bar, 1.5 μm.
FIG. 5.
Quantification of the changing intimacy between V. fischeri and the host brush border. (A) Parallel increase for the first 4 days of infection in microvillar density (▴) and in bacterial number in contact with the brush border (•). Results are expressed as means; error bars indicate standard deviations. A t test revealed that the increases in the number of microvilli measured between 12 and 48 h and between 24 and 96 h after inoculation were significant (P ≤ 0.025). (B) Changes in the proportion of bacterial membrane surface associated with the epithelial brush border during the first 4 days of infection. The percentage of bacteria having a small proportion (less than 30%) of their membrane surfaces associated with the epithelial brush border decreased, whereas the percentage of those having a larger proportion (more than 30%) of their membrane surfaces in contact with the microvilli increased significantly as determined by a chi-square approximation of the Kruskal-Wallis test. Bars represent median levels, with ranges noted above each bar.
Measurements of length and width of individual microvilli showed no statistically significant differences in any of the experimental treatments over these first few days of light organ colonization (data not shown).
Transient versus continuous exposure of crypt cells to V. fischeri.
Electron micrographs of CM-treated animals were also analyzed for the changes in the brush border. When CM was used to cure juveniles of their bacterial symbionts after either 12, 24, or 48 h of infection, their crypt microvillar density stopped increasing within a few hours after treatment and started to decrease slowly to numbers similar to those of aposymbiotic animals. The brush border of squid at 96 h postinfection cured with CM after 12 h of infection showed a number of microvilli per 25 μm2 (x̄ = 289 ± 104) similar to that of newly hatched juveniles (x̄ = 324 ± 96). The CM treatment itself had no evident pharmacological effect on the epithelial brush border of the crypt; the pattern of microvillar density of squid infected with CM-resistant strains of V. fischeri and treated with CM for the duration of the experiment was not different from that of symbiotic juveniles infected by the wild-type strain of V. fischeri and not treated with CM (at 48 h, x̄ = 748 ± 62; at 96 h, x̄ = 1,028 ± 181). These data suggest that the presence of V. fischeri in the light organ crypts is required to maintain the observed, induced, morphological changes.
Experiments with mutants of V. fischeri.
The microvillar density per 25 μm2 found in the crypts of juveniles infected for 48 h either by the luxA dark mutants (x̄ = 784 ± 85) or by leucine synthesis-deficient mutants (x̄ = 841 ± 63) of V. fischeri was similar to that of squid infected with the parent strain of symbiont (x̄ = 730 ± 53) (data not shown). These results show that neither the light emitted by the bioluminescence reaction of the normal strain of V. fischeri nor the presence of active luciferase is required for the symbiont-induced changes in the brush border. Moreover, the result obtained with leucine synthesis-deficient mutants suggests that an 80% reduction in the number of bacteria does not significantly affect the rate of change in the microvillar density of the host crypt epithelial cells for the first few days following colonization of the light organ.
Localization of F-actin and detection of β-actin mRNA by in situ hybridization.
Observations of host tissue by confocal microscopy showed that the brush borders of the light organ crypts were strongly labeled with rhodamine-phalloidin in both symbiotic and aposymbiotic animals, indicating that F-actin is abundant in the microvilli (Fig. 6). Similar amounts of β-actin mRNA were detected in symbiotic and aposymbiotic juveniles at the apical brush border of the crypt epithelium (Fig. 7) through 96 h postinfection. The negative control with the sense RNA probe did not show any labeling (Fig. 7B). Thus, in situ hybridization provided no evidence that changes in microvillar density result from bacterium-induced changes in β-actin gene expression.
FIG. 6.
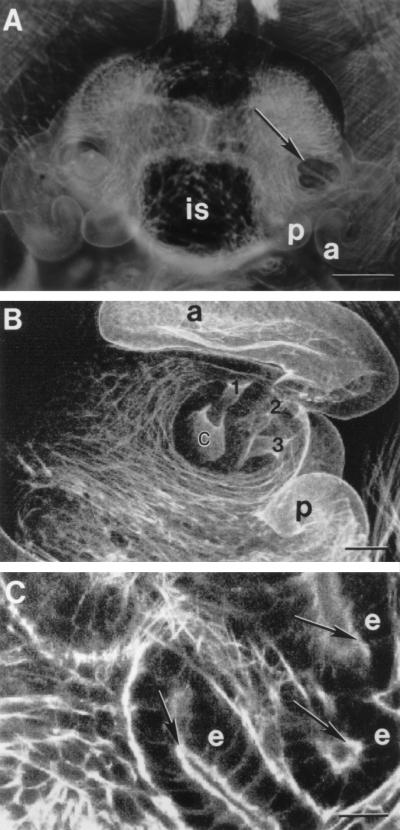
Staining of actin filaments with rhodamine-phalloidin. (A) Light micrograph of the light organ of an aposymbiotic juvenile ventrally observed. Bar, 0.1 mm. a, anterior arm; is, ink sac; p, posterior arm. (B) Confocal microscope image of an aposymbiotic light organ. The fluorescence staining reveals the three pores (indicated by 1, 2, and 3) which lead to the three bacterial crypts (c) on one side of the light organ. Only one crypt is visible in this micrograph. Bar, 50 μm. (C) Confocal microscope image of another animal at a higher magnification. The cytoplasmic F-actin of the brush border is strongly stained with rhodamine-phalloidin (arrows). e, epithelial cell. Bar, 20 μm.
DISCUSSION
In the present study, we sought to describe early changes in the brush border of host crypt epithelia in response to interactions with V. fischeri, identify bacterial signals that mediate these changes, and define the mechanisms underlying the changes in the host cell. In addition, we attempted to determine the usefulness of the E. scolopes-V. fischeri model for the study of alterations in brush border morphology resulting from persistent colonization of animal epithelia by bacteria.
Bacteria exert a wide variety of influences on the morphology of epithelial brush borders in both pathogenic and cooperative associations (11, 25, 43). Among the factors important in determining the nature of these alterations are (i) whether the association is extracellular or intracellular, (ii) whether there is acute or chronic interaction between host and bacterial symbiont, and (iii) the types of biochemical and molecular exchanges that occur between the partners. Changes in the membrane interface of the epithelial cells are central to the symbiosis as they mediate the subsequent relationship between the host and bacteria. In the cooperative association between the squid and its extracellular bacterial symbiont, the relationship is established immediately upon exposure of the host to the environment and persists as a stable relationship throughout the host’s life history (44). The obvious product translocated to the host from the bacteria is luminescence (5), which the host uses in its nocturnal behavior. Studies with V. fischeri auxotrophic mutants indicate that the host provides nutrients in exchange for bacterial light production (44). This dynamic interplay between the host squid and their luminous bacterial symbionts is mediated through the brush border of host epithelial cells. The results presented here indicate that V. fischeri participates in the elaboration of the host brush border, which presumably serves to increase the surface area of exchange between the partners.
At the morphological level, several different types of bacterium-induced changes in animal cell brush borders have been reported. These modifications can be subtle, such as an alteration in the length or width of the microvilli, as reported in the normal development of enterocytes (33). Alternatively, they can be dramatic, such as the membrane ruffling that occurs when Salmonella typhimurium contacts an epithelial cell (14, 15, 20) or the attachment and effacement of host cell brush borders that occurs as a result of interaction of mammalian cells with enteropathogenic Escherichia coli (16, 26, 29, 36). In the E. scolopes-V. fischeri association, we found that the bacteria induce an increase in microvillar density and in the intimacy of the bacteria with the host cell brush border. Our electron micrographs of V. fischeri in association with these cells are reminiscent of ultrastructural analyses of the interaction of Vibrio cholerae with mammalian intestinal cells; in both cases, the bacterial cells appear to nestle down into the brush border (2, 38).
We found that V. fischeri caused a relatively slow yet significant alteration in the brush border during early posthatch development of host epithelial cells. The fourfold increase in microvillar density was achieved over a period of approximately 4 days. This gradual change is in marked contrast with the time courses that have been reported for interactions between host epithelia and various pathogenic bacterial species that cause acute infections. In experiments with both cultured cells and intact host animals, pathogenic microorganisms, whether intracellular or extracellular, often cause changes in host cell brush borders over a period of seconds to hours. For example, the extracellular pathogen Treponema denticola, which is associated with periodontal diseases, causes rearrangement of host cell actin filaments within minutes of exposure to human gingival fibroblasts (3). S. typhimurium adheres to microvilli, is engulfed, and becomes internalized by host cells within 1 h of initiation of the interaction (17, 18).
The temporal program of brush border development is more similar to those of chronic association than it is to the acute ones described in the previous paragraph. The establishment of a long-term relationship may be expected to require a delicate balance in the interaction between the partners. The subsequent development of such associations most likely requires significantly more reciprocal interaction between the partners than that occurring in the progression of an acute pathogenic association. In the best-studied example of the ontogeny of a stable symbiotic state, leguminous plants form a functional association with nitrogen-fixing soil bacteria over a period of weeks, with the modulation of dozens of genes in both partners orchestrating the developmental program (19, 50). In studies that have analyzed the influence of the indigenous microbiota on host brush borders, comparisons of germfree or gnotobiotic animals with control, or naturally colonized, hosts have usually been conducted over a period of weeks or months rather than hours (27, 28, 33, 47). While these studies usually do not focus principally on the early time course of the association, the length of time over which they are conducted suggests that gradual changes may underlie the normal, bacterium-induced alterations of the host epithelial cells.
The gradual, reversible changes that we observed in the host crypt brush border, the tissue with which the bacteria will persistently and directly interact, differ in some respects from other bacterium-induced changes of the symbiotic light organ. Within hours of inoculation, V. fischeri induces an irreversible, massive program of cell death, which results in the loss of the superficial, ciliated epithelium of the organ (9, 35). This tissue, unlike the crypt brush border, is only associated with the inception of the symbiosis and does not directly interact with symbiont cells; i.e., it lies several cell layers away from the crypts where colonization is occurring. Thus, previous studies showed that the bacteria are capable of initiating a quick response in host tissue, but the present study suggests that they may do so only in tissues that have a relatively short-term function in the association.
Despite these differences in the time course and outcome of bacterium-induced changes in the superficial and crypt epithelia of the symbiotic organ, morphogenesis of both tissues requires that the bacterial symbionts enter the organ and interact with crypt cells. Mutant strains that did not fully colonize, but did thinly line the crypt space along the microvilli, induced cell death of the superficial epithelium and increased density of microvilli in the crypt cells. These data suggest that both processes require signaling between host and symbiont at their cell-surface interface and not the accumulation of some excreted bacterial product to some critical level, although this cannot be ruled out because a 20% level of infection may be all that is required.
The mutants chosen for this study were obtained from a growing collection of mutant strains being generated in symbiotic V. fischeri (44). Our results showed that neither luminescence nor complete filling of the crypt space is required to signal brush border development. Analysis of other possible signals, such as adhesion, must await the production of other candidate mutants. Adhesion mutants would be particularly interesting, as studies with germfree animals and cultured epithelial cells have shown that bacterial adhesion induces development of brush borders of intestinal epithelia (4, 6).
The observed increase in microvillar density along the brush border of the crypt cells is most likely to result from changes in β-actin gene expression, protein synthesis, or local induction of the transition from globular to filamentous actin, the principal cytoskeletal protein of animal microvilli (13, 25, 43). The length of time required to produce the observed changes suggested that the increase in microvillar density may result from induction of host β-actin gene expression. In situ hybridization analyses showed no dramatic differences in β-actin gene expression between symbiotic and aposymbiotic juvenile animals. However, this method is not quantitative and would only reveal striking differences. Gradual or slight bacterium-induced changes in expression of this gene cannot be ruled out. These studies did confirm that the mRNA for β-actin concentrates in the microvilli. Thus, microvilli along the apical surfaces of the crypt cells arise similarly to microvilli along intestinal brush borders (7, 13). In many associations of animal and bacterial cells, bacteria induce actin filament formation or rearrangement of existing actin filaments (25, 43). This mechanism may be operating in the E. scolopes-V. fischeri system, but because of the gradual nature of the process, the rearrangements may be difficult to observe and document.
One of the principal challenges in the study of animal-bacterium interactions is to define the critical differences between cooperative and pathogenic associations. Several features of the E. scolopes-V. fischeri relationship render it an attractive and viable complement to studies of germfree and gnotobiotic animals. These characteristics include the ability to study development over a period of a few days, the similarities to other animal-bacterium associations, and the availability of bacterial mutants. The results reported here support the feasibility of the E. scolopes-V. fischeri system as a model for the characterization of bacterium-induced alterations of host brush borders and provide baseline data for future studies of the interactions of extracellular bacteria with animal epithelia.
ACKNOWLEDGMENTS
We thank F. Aeckersberg, J. Doino, J. Foster, L. Gilson, J. Kimbell, M. Nishiguchi, S. Nyholm, E. Ruby, A. Small, and K. Visick for helpful discussions and comments on the manuscript and J. Graf and K. Visick for donation of mutant strains of bacteria.
This work was supported by NSF grant IBN 96-01155 to M.M.N. and E. Ruby and by ONR grant N00019-J-1357 to M.M.N.
REFERENCES
- 1.Angerer L M, Angerer R C. In situ hybridization to cellular RNA with radiolabelled RNA probes. In: Wilkinson D G, editor. In situ hybridization: a practical approach. New York, N.Y: Oxford University Press, Inc.; 1994. pp. 15–32. [Google Scholar]
- 2.Apter F M, Michetti P, Winner III L S, Mack J A, Mekalanos J, Neutra M R. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baehni P C, Song M, McCulloch C A G, Ellen R P. Treponema denticola induces actin rearrangement and detachment of human gingival fibroblasts. Infect Immun. 1992;60:3360–3368. doi: 10.1128/iai.60.8.3360-3368.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benítez J A, Spelbrink R G, Silva A, Phillips T E, Stanley C M, Boesman-Finkelstein M, Finkelstein R A. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect Immun. 1997;65:3474–3477. doi: 10.1128/iai.65.8.3474-3477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boettcher K J, Ruby E G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bry L, Falk P G, Midtvedt T, Gordon J I. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273:1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Bjerknes M. Asymmetric distribution of actin mRNA and cytoskeletal pattern generation in polarized epithelial cells. J Mol Biol. 1989;210:541–549. doi: 10.1016/0022-2836(89)90130-7. [DOI] [PubMed] [Google Scholar]
- 8.Coates M E, Gustafsson B E. The germfree animal in biomedical research. London, United Kingdom: Laboratory Animals, Ltd.; 1984. [Google Scholar]
- 9.Doino J A, McFall-Ngai M J. A transient exposure to symbiosis-competent bacteria induces light organ morphogenesis in the host squid. Biol Bull. 1995;189:347–355. doi: 10.2307/1542152. [DOI] [PubMed] [Google Scholar]
- 10.Duncan H E, Edberg S C. Host-microbe interaction in the gastrointestinal tract. Crit Rev Microbiol. 1995;21:85–100. doi: 10.3109/10408419509113535. [DOI] [PubMed] [Google Scholar]
- 11.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 12.Fasano A. Cellular microbiology: how enteric pathogens socialize their intestinal host. ASM News. 1997;63:259–265. doi: 10.1097/00005176-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Fath K R, Obenauf S D, Burgess D R. Cytoskeletal protein and mRNA accumulation during the brush border formation in adult chicken enterocytes. Development. 1990;109:449–459. doi: 10.1242/dev.109.2.449. [DOI] [PubMed] [Google Scholar]
- 14.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 15.Finlay B B, Leung K Y, Rosenshine I, Garcia-del Portillo F. Salmonella interactions with the epithelial cell. ASM News. 1992;58:486–490. [Google Scholar]
- 16.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis C L, Starnbach M N, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low oxygen conditions. Mol Microbiol. 1992;6:3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 18.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct micropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 19.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-del Portillo F, Pucciarelli M G, Jefferies W A, Finlay B B. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J Cell Sci. 1994;107:2005–2020. doi: 10.1242/jcs.107.7.2005. [DOI] [PubMed] [Google Scholar]
- 21.Gordon H A, Bruckner-Kardoss E. Effects of normal flora on various tissue elements of the small intestine. Acta Anat. 1961;44:210–225. doi: 10.1159/000141723. [DOI] [PubMed] [Google Scholar]
- 22.Gordon H A, Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationship. Bacteriol Rev. 1971;35:390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanlon R T, Claes M F, Ashcraft S E, Dunlap P V. Laboratory culture of the sepiolid squid Euprymna scolopes: a model system for bacteria-animal symbiosis. Biol Bull. 1997;192:364–374. doi: 10.2307/1542746. [DOI] [PubMed] [Google Scholar]
- 24.Heneghan J B, editor. Germfree research: biological effect of gnotobiotic environments. New York, N.Y: Academic Press; 1973. [Google Scholar]
- 25.Higley S, Way M. Actin and cell pathogenesis. Curr Opin Cell Biol. 1997;9:62–69. doi: 10.1016/s0955-0674(97)80153-6. [DOI] [PubMed] [Google Scholar]
- 26.Jepson M K, Clark M A, Simmons N L, Hirst B H. Actin accumulation at sites of attachment of indigenous apathogenic segmented filamentous bacteria to mouse ileal epithelial cells. Infect Immun. 1993;61:4001–4004. doi: 10.1128/iai.61.9.4001-4004.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai Y, Morotomi M. Intestinal enzyme activities in germfree, conventional, and gnotobiotic rats associated with indigenous microorganisms. Infect Immun. 1978;19:771–778. doi: 10.1128/iai.19.3.771-778.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenworthy R, Allen W D. Influence of diet and bacteria on small intestinal morphology, with special reference to early weaning and Escherichia coli: studies with germfree and gnotobiotic pigs. J Comp Pathol. 1966;76:291–296. doi: 10.1016/0021-9975(66)90009-0. [DOI] [PubMed] [Google Scholar]
- 29.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruskal W H, Wallis W A. Use of ranks in one-criterion analysis of variance. J Am Statist Assoc. 1952;47:583–621. [Google Scholar]
- 31.McFall-Ngai M J, Montgomery M K. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae) Biol Bull. 1990;179:332–339. doi: 10.2307/1542325. [DOI] [PubMed] [Google Scholar]
- 32.McFall-Ngai M J, Ruby E G. Symbiotic recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- 33.Meslin J-C, Sacquet E. Effects of microflora on the dimensions of enterocyte microvilli in the rat. Reprod Nutr Dev. 1984;24:307–314. doi: 10.1051/rnd:19840309. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery M K, McFall-Ngai M J. Embryonic development of the light organ of the sepiolid squid Euprymna scolopes Berry. Biol Bull. 1993;184:296–308. doi: 10.2307/1542448. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery M K, McFall-Ngai M J. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development. 1994;120:1719–1729. doi: 10.1242/dev.120.7.1719. [DOI] [PubMed] [Google Scholar]
- 36.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moynihan M. Notes on the behavior of Euprymna scolopes (Cephalopoda: Sepiolidae) Behavior. 1983;85:25–41. [Google Scholar]
- 38.Nelson E T, Clements J D, Finkelstein R A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976;14:527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson W A, Korsmo H A. Sucrase metabolism in germfree rats. Am J Physiol. 1982;242:G650–G653. doi: 10.1152/ajpgi.1982.242.6.G650. [DOI] [PubMed] [Google Scholar]
- 40.Orndorff P E. Bacterial virulence. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The procaryotes. New York, N.Y: Springer-Verlag, Inc.; 1992. pp. 640–658. [Google Scholar]
- 41.Pardue M L. In situ hybridization. In: Hames B D, Higgins S J, editors. Nucleic acid hybridization: a practical approach. New York, N.Y: Oxford University Press, Inc.; 1985. pp. 179–202. [Google Scholar]
- 42.Polotsky Y, Dragunsky E, Khavkin T. Morphologic evaluation of the pathogenesis of bacterial enteric infections. Crit Rev Microbiol. 1994;20:161–208. doi: 10.3109/10408419409114553. [DOI] [PubMed] [Google Scholar]
- 43.Rosenshine I, Finlay B B. Exploitation of host signal transduction pathways and cytoskeletal functions by invasive bacteria. Bioessays. 1993;15:17–24. doi: 10.1002/bies.950150104. [DOI] [PubMed] [Google Scholar]
- 44.Ruby E G. Lessons from a cooperative bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu Rev Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.591. [DOI] [PubMed] [Google Scholar]
- 45.Ruby E G, Asato L M. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch Microbiol. 1993;159:160–167. doi: 10.1007/BF00250277. [DOI] [PubMed] [Google Scholar]
- 46.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C: ASM Press; 1994. [Google Scholar]
- 47.Sharma R, Schumacher U, Ronaason V, Coates M. Rat intestinal mucosal responses to a microbial flora and different diets. Gut. 1995;36:209–214. doi: 10.1136/gut.36.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spurr A. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 49.van der Waaij D. The ecology of the human intestine and its consequences for overgrowth by pathogens such as Clostridium difficile. Annu Rev Microbiol. 1989;43:69–87. doi: 10.1146/annurev.mi.43.100189.000441. [DOI] [PubMed] [Google Scholar]
- 50.van Rhijn P, Vanderleyden J. The Rhizobium-plant symbiosis. Microbiol Rev. 1995;59:124–142. doi: 10.1128/mr.59.1.124-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visick K G, Ruby E G. Construction and symbiotic competence of a luxA− deletion mutant of Vibrio fischeri. Gene. 1996;175:89–94. doi: 10.1016/0378-1119(96)00129-1. [DOI] [PubMed] [Google Scholar]
- 52.Wei S L, Young R E. Development of a symbiotic bacterial bioluminescence in a nearshore cephalopod, Euprymna scolopes. Mar Biol. 1989;103:541–546. [Google Scholar]
- 53.Wilkinson D G. The theory and practice of in situ hybridization. In: Wilkinson D G, editor. In situ hybridization: a practical approach. New York, N.Y: Oxford University Press, Inc.; 1994. [Google Scholar]
- 54.Woolverton C J, Holt L C, Mitchell D, Sartor R B. Identification and characterization of rat intestinal lamina propria cells—consequences of microbial colonization. Vet Immunol Immunopathol. 1992;34:127–138. doi: 10.1016/0165-2427(92)90156-k. [DOI] [PubMed] [Google Scholar]