Abstract
The 43-kDa glycoprotein of Paracoccidioides brasiliensis is the major diagnostic antigen of paracoccidioidomycosis, the prevalent systemic mycosis of Latin America. Apart from eliciting high antibody titers, gp43 is also immunodominant in delayed-type hypersensitivity reactions in infected animals and humans. The cellular immune response in mice to gp43 administered in complete Freund’s adjuvant involves CD4+ Th-1 lymphocytes, secreting gamma interferon (IFN-γ) and interleukin 2 (IL-2) but not IL-4 and IL-10. The T-cell epitope of this antigen was mapped to a 15-amino-acid peptide (P10) based on lymphoproliferations with primed cells from three different haplotypes and on a computer-assisted protein analysis. The structural requirements of the T-cell epitope were determined by assaying a series of P10 analogous and truncated peptides. Only 12-mer or longer sequences were active, confirming presentation by major histocompatibility complex II. The HTLAIR inner core of P10 is the essential domain of the epitope, with various flanking regions possible. Immunization of mice with both gp43 and P10 led to vigorous protection against intratracheal challenge by virulent P. brasiliensis, with a >200-fold decrease in lung CFU and halting of dissemination to the spleen and liver. The protective effect of P10 is mainly attributed to an IFN-γ-mediated cellular immune response. Unlike gp43, which induces an antibody response compatible with both Th-1 and Th-2 activation in infected BALB/c mice, P10 does not induce a humoral response. Protection by gp43 and P10 was characterized by a few well-demarcated lung granulomas with numerous nonviable yeast forms or resolved lesions with no detectable fungal cells.
Paracoccidioidomycosis (PCM) is a systemic granulomatous disease caused by Paracoccidioides brasiliensis, a thermal dimorphic fungus. It is widespread in South and Central America, mainly affecting rural workers. According to McEwen et al. (22) approximately 10 million people may be infected with this fungus and up to 2% of them may develop the disease. The incidence may increase due to forest destruction and a rise in iatrogenic immunosuppression procedures (41). The acute or subacute form of PCM affects both sexes and chiefly involves the reticuloendothelial system. The chronic form affects adult males with predominantly pulmonary and/or mucocutaneous involvement (15). Cellular rather than humoral immunity is the defense mechanism against both experimental and human PCM (8, 24), and a correlation has been found between the severity of the disease and impaired delayed-type hypersensitivity (DTH) response (27).
The glycoprotein gp43 from P. brasiliensis, which is secreted exocellularly by the infective yeast phase (30, 38), is the main PCM diagnostic antigen (28), being recognized by virtually all sera from infected patients by different serological methods (9, 39). In addition to being immunodominant for antibody production, gp43 also contains epitopes which elicit positive DTH in both guinea pigs (31) and human patients (35). Depletion of gp43 by immunoaffinity chromatography from a complex antigenic preparation generally used for skin tests is followed by negative reactions, implying that the T-cell epitopes of the glycoprotein are also immunodominant (31).
gp43 may act as a virulence factor, since it is a receptor for laminin-1 (40). Yeast cells coated with laminin are more invasive than untreated cells in a hamster intratesticular infection model, and this response can be modulated by anti-gp43 monoclonal antibodies (40, 16). A strong antibody response against gp43, as observed in PCM, may counteract this antigen-mediated virulence, but as a whole, an increased antibody response against P. brasiliensis antigens, whether associated with polyclonal B-cell activation or not, does not induce protection (26).
Considering the immunodominant cellular immune response elicited by gp43, which may halt the progression of the infection, along with a locally effective but generally unprotective antibody response to this antigen, we aimed in the present investigation at determining the epitopes mediating these immune responses and at selecting those that could be used in vaccination trials. We show here that a 15-amino-acid peptide (P10) contained in gp43 is responsible for glycoprotein-mediated T-cell activation and protection against PCM in BALB/c mice without eliciting antibodies directed against the native antigen.
MATERIALS AND METHODS
Purification of gp43 from P. brasiliensis.
P. brasiliensis B-339, originally obtained from A. Restrepo-Moreno, Medellin, Colombia, was grown in yeast extract-peptone-dextrose (YPD) liquid medium for 7 days at 36°C with shaking. The culture was killed by adding 0.2 g of merthiolate/liter filtered through a paper filter, concentrated in a vacuum at 40°C, and dialyzed against distilled water. Purification of gp43 was done by affinity chromatography on Affi-gel (Bio-Rad) bound to anti-gp43 monoclonal antibody (17C) as previously described (29). Elution was carried out with 50 mM citrate buffer, pH 2.8. The eluate was concentrated in Amicon 10K cells, and the antigen preparation was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed by silver stain. The protein content was determined by Bradford’s method (5).
Peptide synthesis and purification.
Peptides were synthesized by the 9-fluoroenylmethoxycarbonyl technique (19) based on the method described by Atherton and Sheppard (3) with an automated benchtop simultaneous multiple solid-phase peptide synthesizer (PSSM 8 system; Shimadzu, Tokyo, Japan). Peptides were purified by high-performance liquid chromatography with a C-R7A Shimadzu UV-vis detector and a Shimadzu RF-535 fluorescence detector coupled to a Vydac C18 analytical column. Amino acid analysis was carried out in a Beckman 6,300 amino acid analyzer following hydrolysis in 6 N HCl with 5% phenol at 110°C for 48 h. Matrix-assisted laser deabsorption ionization-mass spectrometry was performed on a TofSpec E instrument from Micromass, with a matrix of α-cyano-4-hydroxycinnamic acid.
Graphic analyses of antigenicity and structural parameters of the gp43 sequence containing P10.
The Jameson-Wolff antigenic index, the Kyte-Doolittle hydrophilicity plot, Eisenberg’s alpha helix amphipathic regions, Emini’s surface probability plot, and the Sette major histocompatibility complex (MHC)II motif method were graphically analyzed by using the Protean program (protein sequence analysis) of Lasergene biocomputing software for Windows, 1994 (DNASTAR Inc., Madison, Wis.). The Jameson-Wolff antigenic index predicts potential antigenic determinants for antibody recognition by combining existing methods for protein structural predictions, starting with Hopp-Woods hydrophilicity values, then the Emini method for surface probabilities, and, finally, methods for the prediction of backbone or chain flexibility. Flexibility parameters and hydropathy or solvent accessibility are combined to determine the antigenic index. In the Kyte-Doolittle hydrophilicity plot, the regional hydropathy of proteins is predicted from their amino acid sequences. Positive values correspond in this plot to hydrophilic structures, and negative values correspond to hydrophobic structures. Hydropathy values are assigned for all amino acids and are then averaged over a defined window. The window we used averaged 11 amino acids. Amphipathic regions in helices define one polar and one apolar face. A majority of the known helper T-cell antigenic sites involve amphipathic helices. The hydrophobic moment of Eisenberg et al. (14) detects periodicity in protein hydrophobicity. The Sette MHC II motif method predicts peptide epitopes interacting with mouse MHC II haplotype d proteins. The method for I-Ad based on a sequence pattern derived from hexapeptide residues of chicken ovalbumin protein was used.
Immunization of mice.
BALB/c (H-2d), A/Sn (H-2a), and C57BL (H-2b) 15-g male or female mice were subcutaneously immunized with 50 μg of gp43 or 20 μg of P10 in complete Freund’s adjuvant (CFA). The emulsion (50 μl) was inoculated in one of the rear footpads. Control mice were injected with CFA alone.
Lymphoblast proliferation.
Inguinal and popliteal lymph nodes (LN) from control and gp43-immunized mice were removed after 7 days, and the cells were dispersed manually and centrifuged at 1,500 rpm (Sorvall RT 6,000 D centrifuge) for 5 min; this process was repeated twice. The cell pellet was suspended in 1 ml of RPMI 1640 supplemented with 20 mM NaHCO3, 10 mM HEPES, 100 U of penicillin/ml, 100 mg of streptomycin/ml, 2 mM l-glutamine, 50 mM β-mercaptoethanol, 5 mM sodium pyruvate, and 100 mM nonessential amino acids with 1% normal human serum. The cells were counted in 1:1 dilutions in 0.1% trypan blue. Viable cells were cultured at a density of 4 × 105/well in 96-well Costar plates. Different concentrations of gp43 and constituent peptides in 20% dimethyl sulfoxide from 2- and 1-mg/ml stock solutions, respectively, were incubated at 37°C and 5% CO2 for 144 h. Controls were run with complete culture medium or with 2 mg of concanavalin A. The LN cells from animals immunized with CFA alone were incubated with gp43 at 10 μg/well. Sixteen to 24 h before the cells were collected, 1 mCi of [3H]thymidine, (Amersham)/well, 84 Ci/mmol, was added. Proliferation was determined by incorporation of the radioactivity by the cells, and the results (expressed in counts per minute) are the means of triplicate determinations. All antigenic solutions were tested for endotoxin with the E-toxate kit (Sigma, St. Louis, Mo.). Initial proliferations with gp43 were also carried out in the presence of polymyxin B.
Lymphocyte proliferation induced by gp43 and P10.
Lymphocyte proliferation induced by gp43 and P10 was analyzed in the presence or absence of anti-CD4+ {clone GK1.5, isotype immunoglobulin G2b(κ) [IgG2b(κ)]} and anti-CD8+ [clone 53-6.7, isotype IgG2a(κ)] monoclonal antibodies (Pharmingen, San Diego, Calif.). Antibodies were added at the beginning of LN cell culture. Experiments were also conducted with LN cells from mice immunized either with gp43 or P10 and were tested for proliferation with both molecules.
Cytokine detection in culture supernatants.
LN cells from gp43- and P10-immunized mice were suspended in supplemented RPMI medium as described above, but with 10% fetal calf serum and the addition of 30 U of human recombinant interleukin 2 (rIL-2)/ml. Cell suspensions of 4 × 106/ml were distributed in 24-well plates, and gp43 at 50 μg/ml and P10 at 20 μg/ml were added. After 6 days of incubation the culture supernatants were collected and the presence of gamma interferon (IFN-γ), IL-4, IL-5, IL-6, IL-9, IL-10, and IL-12 was analyzed by sandwich enzyme-linked immunosorbent assay (ELISA) and with a Pharmingen kit, following all directions except for the substrate, which was replaced by orthophenylenediamine (Sigma) at 500 μg/ml, and the final reading at 492 nm. Recombinant cytokines (Pharmingen) were used for standard curves with the respective monoclonal antibodies.
For IL-2 detection, supernatant fluids from cell cultures (1 × 107 cells/ml) in supplemented RPMI with 10% fetal calf serum but without rIL-2 and stimulated with gp43 and P10 were collected after 24 and 48 h of stimulation. These supernatants were added to 104 cells/well of an IL-2-responding murine tumor-specific cytotoxic T-lymphocyte line (CTLL) as described by Gillis et al. (17). Cells were incubated for 24 h, and 0.5 mCi of [3H]thymidine was added 6 h before collecting the cells on a fiberglass filter for radioactivity counting. Counts per minute are averages of three determinations. Negative controls were run with the RPMI medium alone or with rat monoclonal anti-mouse IL-2 antibody (clone S4B6, isotype IgG2a; Pharmingen). Another negative control included the supernatant of a LN cell culture stimulated by CFA alone. The positive control had the CTLL cells incubated with rIL-2 at 30 U/ml.
Intratracheal infection of BALB/c mice and immunization with gp43 and P10.
BALB/c mice were inoculated intratracheally with 3 × 105 yeast forms of virulent P. brasiliensis Pb18, grown in Sabouraud agar and suspended in sterile 0.85% NaCl saline solution, per animal. A maximal volume of 50 μl was inoculated per mouse. CFU were determined after 1 and 3.5 months of infection in the lungs, spleens, and livers, which were removed, weighed, homogenized, and washed three times in phosphate-buffered saline (PBS) by centrifugation. The final suspension in PBS was plated on brain heart infusion agar supplemented with 4% fetal calf serum and 5% spent culture medium of P. brasiliensis as a growth factor. Gentamicin (garamycin) and cycloheximide were added at 40 and 500 mg/liter, respectively. The plates were incubated at 36°C and read after 20 days.
Parallel lots of BALB/c mice were immunized with gp43 and P10 by the following protocol. Antigens were emulsified with CFA, and 50 μl of this emulsion containing either 50 or 20 μg of gp43 or P10, respectively, were inoculated in one footpad of each animal. After 15 days, antigens at the same concentrations but solubilized in incomplete Freund’s adjuvant were subcutaneously inoculated into the dorsal emergence of the tail, and 15 days later, the same emulsion was inoculated intraperitoneally. Control mice were inoculated with CFA and incomplete Freund’s adjuvant only. The infection of immunized animals was carried out 15 days after the final immunization step.
Antibody response to gp43 in immunized, infected BALB/c mice.
Antibody response to gp43 was determined 1 and 3.5 months after intratracheal infection of mice with virulent P. brasiliensis. Microtiter plates sensitized with 500 ng of purified gp43 were incubated with 100 μl of mouse serum, serially diluted starting at 1:200 or 1:500, at 37°C for 1 h. The reaction was quantitated with goat anti-mouse Ig, depending on the isotype, for 1 h at 37°C, followed by donkey anti-goat Ig–biotin conjugate and reaction with streptavidin-peroxidase for 30 min at 37°C. After addition of the orthophenylenediamine reagent (500 μg/ml) the reactions were read at 492 nm. The antibodies, conjugates, and substrate were from Sigma Chemical Co.
Histopathology of control and immunized mice.
Groups of 5 to 7 BALB/c mice immunized either with gp43 or P10 were infected intratracheally and killed after 1 or 3.5 months. The lungs, spleens, and livers were excised, fixed in 10% buffered formalin, and embedded in paraffin for sectioning. The sections were stained with hematoxylin-eosin and examined microscopically (Optiphot-2; Nikon, Tokyo, Japan).
RESULTS
Primed lymphoblast proliferation induced by gp43 and constituent peptides.
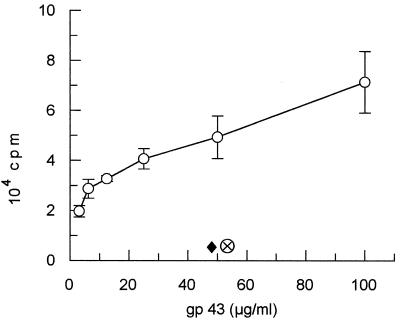
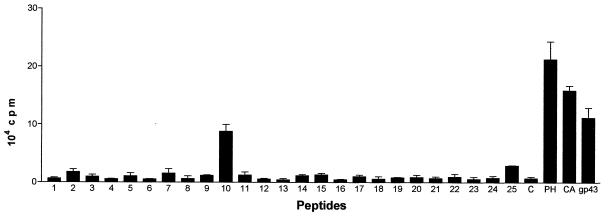
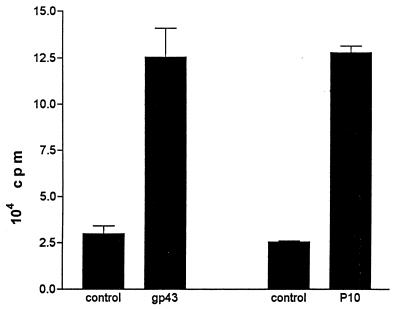
BALB/c mice subcutaneously immunized with gp43 (50 μg/animal) in CFA provided, after 7 days inguinal and popliteal LN cells which proliferated in the presence of gp43 in a dose-dependent manner (Fig. 1). No proliferation was observed when mice were immunized with CFA alone. Proliferation of LN cells from gp43-sensitized BALB/c mice was also observed with a 15-amino-acid peptide (P10) from a series of 25 chemically synthesized peptides spanning the entire sequence of gp43 (Fig. 2). Popliteal and inguinal LN cells recruited by injection of CFA into the footpads of mice infected intraperitoneally with virulent P. brasiliensis 18 also proliferated with both gp43 and P10 (Fig. 3).
FIG. 1.
gp43-stimulated dose-dependent proliferation (± standard deviation) of LN cells from BALB/c mice sensitized with a single injection of gp43 in CFA (open circles). Lymphoblasts from mice sensitized with CFA only (diamond) and cells from mice sensitized with gp43 and stimulated with the culture medium (supplemented RPMI) only (circle with cross) were the negative controls.
FIG. 2.
Proliferation (plus standard deviation) of gp43-sensitized BALB/c LN cells induced by 25 peptides spanning the whole sequence of the native gp43 molecule. P10, the only responding peptide, has 15 amino acids. The peptides were tested at 10 μg/ml. C, control with no antigen added; PH, phytohemagglutinin at 5 μg/ml; CA, concanavalin A at 2 μg/ml (positive controls); gp43 was at 50 μg/ml.
FIG. 3.
Proliferation (plus standard deviation) with gp43 (12.5 μg/ml) and P10 (2 μg/ml) of popliteal and inguinal LN cells recruited by injection of CFA into the footpads of BALB/c mice infected intraperitoneally with 109 yeast forms/ml/mouse of virulent P. brasiliensis 18 for 45 days. The controls were LN cells from infected animals with no antigen added.
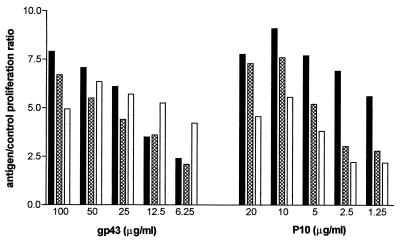
Lymphoproliferative response in mice of different lineages.
LN cells from gp43- or P10-sensitized mice of different lineages (BALB/c H-2d, A/Sn H-2a, and C57/BL H-2b) proliferated when stimulated with the homologous antigen (Fig. 4). Immunization of mice with P10 (20 μg/footpad in CFA) also rendered LN cells responsive to gp43 (not shown). LN cells from control animals immunized only with CFA did not proliferate with either gp43 or P10.
FIG. 4.
Comparative proliferative response to gp43 and P10 of LN cells from A/Sn (black bars), BALB/c (cross-hatched bars), and C57-BL (open bars) mice sensitized subcutaneously with both gp43 and P10 in CFA.
Lymphocyte populations induced by gp43 and P10.
LN cells from BALB/c mice primed with gp43 proliferated when induced by gp43 and P10 to form populations of CD4+ T lymphocytes inhibitable by anti-CD4+ but not by anti-CD8+ antibodies (not shown). LN cells primed with gp43 or P10, when stimulated in vitro with the homologous antigen, produce the Type 1 cytokines IL-2 and IFN-γ but not Type 2 cytokines, at least within the detection limits of the ELISA method (Table 1). With 4 × 106 cells/ml, IFN-γ was only detected when rIL-2 was added at 30 U/ml. With 107 cells/ml, 140 and 163 U/ml of IFN-γ were induced by P10 and gp43, respectively, without further addition of IL-2. Lymphocytes restimulated with P10 for 10 days produced 560 U/ml of IFN-γ, which is a 10-fold-higher concentration than that of singly stimulated cells after 6 days. Restimulation with P10 was not followed by IL-4 and IL-10 detection in this system. Therefore, the cellular immune response induced by gp43 and by the immunodominant P10 in vitro involves predominantly CD4+ T cells of the Th-1 subtype. An IFN-γ-producing Th-1 response to gp43 was also obtained in the H-2a haplotype (200 and 60 pg of IFN-γ/ml was produced by A/Sn and B10A strains, respectively).
TABLE 1.
Cytokine detection in the supernatant fluids of lymphocyte cultures from sensitized BALB/c micea
| Immunogen | Cytokine content (pg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IL-2b | IL-4 | IL-5 | IL-6 | IL-9 | IL-10 | IL-12 | IFN-γc | |
| gp43 | 1,615 | −d | − | − | − | − | − | 390 |
| P10 | 3,501 | − | − | − | − | − | − | 450 |
| CFA only | 300 | − | − | − | − | − | − | − |
Similar results were obtained with the A/Sn mouse lineage.
IL-2 content (in counts per minute) determined by methyl-[3H]thymidine incorporation by CTLL cells; 24-h culture supernatants were used. Negative controls and counts were as follows: culture medium only, 117 cpm; with monoclonal anti-IL-2 antibody, 74 cpm (gp43) and 100 cpm (P10). The positive control (30 IU of rIL-2/ml) had 7,144 cpm.
IFN-γ contents in 6-day culture supernatants are the averages of two determinations and were calculated from a standard curve for rIFN-γ (Pharmingen) (105 U/10 μg). With 4 × 106 cells/ml, IFN-γ was only produced in the presence of 30 IU of IL-2/ml.
−, not detectable by the ELISA method.
Graphic analysis of a 60-amino-acid sequence in gp43 containing P10.
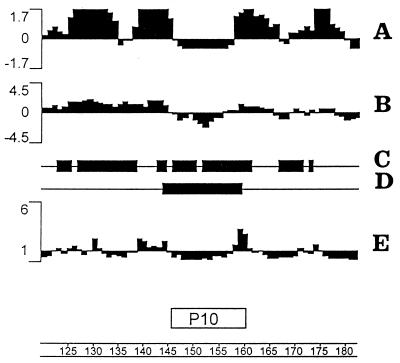
The Jameson-Wolff antigenic index showed several potential hydrophilic determinants for antibody recognition. P10 was contained in a 16-amino-acid sequence in gp43 with a negative antigenic index (Fig. 5A). All but one of the other sequences in gp43 with negative antigenic indexes had fewer than 12 amino acids (data not shown). The prediction of the lack of antigenicity of P10 for antibody binding is coherent with its average hydrophobicity in the Kyte-Doolittle plot and with the low probability of its being expressed at the surface of the protein (Fig. 5B and E). The C-terminal residue of P10, which is a glycosylation site in the native glycoprotein according to Emini’s plot, is expressed at the surface of the protein. There are at least two amphipathic regions in P10 and flanking sequences which are consistent with a CD4+ T-cell-reacting epitope (Fig. 5C). The Sette MHC II motif method predicts peptide sequences which can be presented by MHC II haplotype d I-A molecules. As shown in Fig. 5D a sequence of 16 amino acids was selected that encompasses the entire P10 sequence. A few other sequences indicated in the graph had fewer than 12 amino acids (not shown) and therefore are not effectively presented in the MHC II context.
FIG. 5.
Graphic analysis of a 60-amino-acid fragment (represented by the scale at the bottom) from the gp43 sequence containing P10. (A) Jameson-Wolff antigenic index; (B) Kyte-Doolittle hydrophilicity plot (averaged to 11 amino acid sequences; negative values indicate hydrophobicity); (C) Eisenberg alpha, amphipathic regions; (D) I-Ad regions, Sette MHC II motifs; (E) Emini surface probability plot.
Mapping of the CD4+ T-cell epitope in the P10 amino acid sequence.
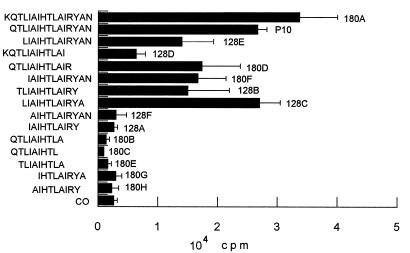
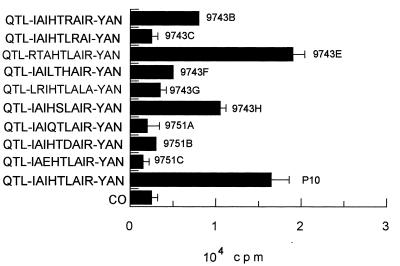
In accordance with the CD4+ T-cell nature of the immune cellular response to gp43 and P10, which involves antigen presentation by MHC class II, only those peptides analogous to P10 of 12 or more amino acids were active for proliferation of primed lymphocytes (Fig. 6). A set of 15 peptides were synthesized, all but one containing truncated sequences in relation to those of P10 (Table 2). Another set of peptides, based on the minimal nine-amino-acid core and with the same flanking amino acids as P10, was tested in an attempt to determine the basic requirements of the epitope for lymphocyte proliferation. Twelve- to 16-mer peptides analogous to P10 were only active in inducing proliferation when the Arg residue before Tyr was present. In mapping the nine-amino-acid putative core, those sequences in which Arg was replaced by hydrophobic amino acids (9743C and 9743G) were not epitopes. The amino acids YAN of the C-terminal sequence of P10 are not essential, since peptide 180D, which lacks them, is active. As for the N-terminal sequence, isoleucine-4 is not essential because peptide 9743E has the IAI sequence replaced by RTA and is as active as P10 itself (Fig. 7). The three flanking amino acids QTL are not essential (peptide 180F is active), but the core sequence IAIHTLAIR needs additional amino acids for presentation in the MHC II context. The following combinations of flanking amino acids allow biological activity: QTL-YAN, KQTL-YAN, only QTL at the N terminus, only YAN at the C terminus, L-YA, TL-Y, and L-YAN; however, the first two combinations, present in P10 and 180A, are the best enhancers of the core-induced proliferation. The 12-amino-acid peptide LIAIHTLAIRYA was as effective as P10, showing that the terminal asparagine residue is not part of the T-cell epitope. The analysis of nine peptides with variable nine-amino-acid core sequences (Table 2) suggested that the sequence HTLAIR best fits the pattern of an epitope. Replacing Leu by Arg or Thr by Ser in the hexapeptide provides sequences which are still recognized but are less active as inducers of LN cell proliferation (Fig. 7). Other alterations in the hexapeptide abolished its activity. Flanking the hexapeptide with Glu but not with hydrophobic amino acids inhibited its activity. In summary, the HTLAIR hexapeptide seems to be the essential sequence, which has to be flanked by a variable combination of amino acids for presentation in the MHC II context. Furthermore, the HTLAIR hexapeptide contains the 5-mer sequence of amino acids, polar-hydrophobic-hydrophobic-hydrophobic-polar (TLAIR), predicted by Rothbard and Taylor (33) for potential T-lymphocyte antigenic determinants.
FIG. 6.
Proliferation response (plus standard deviation) of LN cells from BALB/c mice primed with P10 to analogous and truncated peptides based on the P10 amino acid sequence. Only the peptides with 12 or more amino acids were active. CO, control with no antigen.
TABLE 2.
Peptide sequences for mapping the P10 epitope for T-cell proliferation
| Peptide code no. | Sequence |
|---|---|
| P10 analogous and truncated sequences | |
| 128A | IAIHTLAIRY |
| 128B | TLIAIHTLAIRY |
| 128C | LIAIHTLAIRYA |
| 128D | KQTLIAIHTLAI |
| 128E | LIAIHTLAIRYAN |
| 128F | AIHTLAIRYAN |
| 180A | KQTLIAIHTLAIRYAN |
| 180B | QTLIAIHTLA |
| 180C | QTLIAIHTL |
| 180D | QTLIAIHTLAIR |
| 180E | TLIAIHTLA |
| 180F | IAIHTLAIRYAN |
| 180G | IHTLAIRYA |
| 180H | AIHTLAIRY |
| Peptides with variable nine-amino-acid cores | |
| 9743B | QTL-IAIHTRAIR-YAN |
| 9743C | QTL-IAIHTLRAI-YAN |
| 9743E | QTL-RTAHTLAIR-YAN |
| 9743F | QTL-IAILTHAIR-YAN |
| 9743G | QTL-LRIHTLALA-YAN |
| 9743H | QTL-IAIHSLAIR-YAN |
| 9751A | QTL-IAIQTLAIR-YAN |
| 9751B | QTL-IAIHTDAIR-YAN |
| 9751C | QTL-IAEHTLAIR-YAN |
| P10 | QTL-IAIHTLAIR-YAN |
FIG. 7.
Requirement (plus standard deviations) in the nine-amino-acid core sequence for proliferation of P10-primed lymphocytes. Maximal responses were obtained with structures containing the HTLAIR hexapeptide. CO, control with no antigen.
Protection by gp43 and P10 against P. brasiliensis challenge in BALB/c mice.
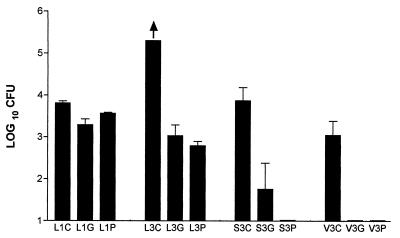
BALB/c mice were immunized with gp43 and P10 and, after 45 days, were challenged by intratracheal inoculation of 3 × 105 yeast forms of the virulent strain Pb 18. Control animals were immunized with Freund’s adjuvant alone following the same protocol. One and 3.5 months after being infected, the mice were killed and CFU were quantitated in the lungs, spleens, and livers (CFU/g of tissue). After 1 month, counts were obtained in the lungs of infected animals without dissemination to the spleens and livers. Unimmunized infected mice had positive DTH reactions (an average of 0.3-mm swelling of the footpad) with gp43 (5 μg) and P10 (5 μg), with no increase in the size of the opposite footpad, which had been injected with PBS. The differences in the CFU of the immunized animals after 1 month were small but significant (P < 0.05). After 3.5 months the protection by gp43 and P10 was quite remarkable, with a difference in relation to the nonimmunized mice of at least 200-fold fewer CFU (Fig. 8). Dissemination to the spleen and liver observed in the control mice was virtually abolished with P10 immunization and much reduced with gp43.
FIG. 8.
Protective effect of immunization with gp43 and P10 against intratracheal infection by virulent P. brasiliensis Pb18. 1 and 3, 1 and 3.5 months of infection, respectively; L, lung; S, spleen; V, liver; C, control with Freund’s adjuvant alone; G, gp43 immunization; P, P10 immunization. In each system five to six BALB/c mice were used. The arrow indicates that with five animals, one had 195,000 CFU/g of lung tissue and the others had more than 200,000 CFU/g of lung tissue. Error bars indicate the standard deviations.
Antibody response to gp43 in infected, immunized mice.
BALB/c mice infected intratracheally with virulent P. brasiliensis had a discrete antibody response to gp43 after 1 month and a much higher response after 3.5 months. Isotypes were compatible with combined Th-1 and Th-2 interleukin-mediated helper effects. Previous immunization with gp43 was followed by a polyclonal B-cell activation and high titers of antibodies against this glycoprotein. In contrast, immunization with P10, which is as protective as gp43 against intratracheal infection, was followed by a very limited antibody response against the glycoprotein in the infected animals (Table 3).
TABLE 3.
Anti-gp43 antibodies in sera from BALB/c mice immunized with gp43 and P10 and infected with 3 × 105 yeast forms of P. brasiliensis
| Immunizationa | Infection time (mo) | ELISA titerb | Immunoglobulins | IDe for gp43 |
|---|---|---|---|---|
| Nonimmunized | 1 | 1:800 | BRc | Negative |
| gp43 | 1 | 1:409,600 | IgG1 > IgE > Ig2a > IgG2b > IgM > IgG3 = IgA | Positive |
| P10 | 1 | 1:200 | BR | Negative |
| Nonimmunized | 3.5 | 1:51,200 | IgG1 = IgG2a > IgE > IgG2b | Positive |
| gp43 | 3.5 | 1:409,600 | IgG1 > IgG2a > IgG2b > IgE > IgM > IgG3 | Positive |
| P10 | 3.5 | 1:3,200 | IgG1d | Negative |
As described in Materials and Methods.
Cutoff for the ELISAs was set at A492 = 0.100.
BR, background reactivity (A492 = 0.100) at the middle of the titration curve.
Very small amounts of anti-gp43 IgG1 detected.
ID, double immunodiffusion test with gp43 at 1 μg/well.
Histopathology of infected, immunized BALB/c mice.
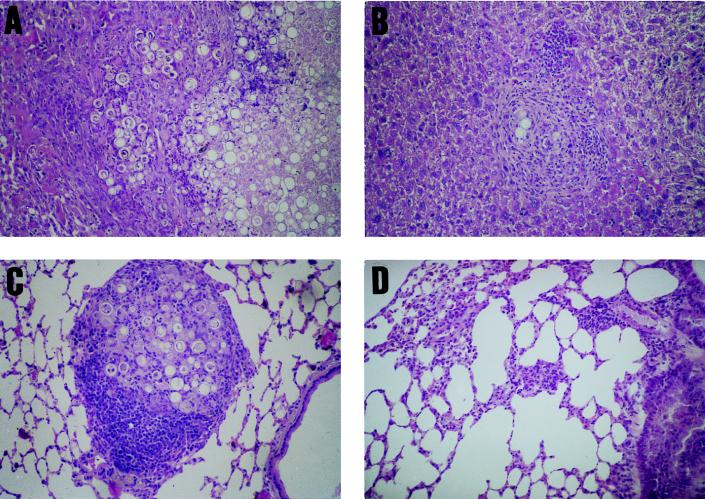
Control nonimmunized mice infected intratracheally showed multiple pulmonary foci of epithelioid granulomatous inflammation, with a loose, edematous arrangement, containing a great number of neutrophils and fungal cells in active multiplication. At 3.5 months postinoculation the overgrowth of the fungus and the confluence of the granulomata led to a paracoccidioma-like appearance with extensive destruction of the lung tissue (Fig. 9A). Fungal hematogenous dissemination produced miliary granulomatous infiltrates in the liver and spleen (Fig. 9B). In the gp43-immunized animals, the pulmonary lesions were few, small, and well demarcated, comprised of epithelioid, compact, pauciparasitic granulomas, with prominent lymphomonocytic halos (Fig. 9C). In the P10-immunized mice, very few and small granulomas in the lungs were seen, with no detectable fungal cells in any sections examined; the lesions had the appearance of a resolved process (Fig. 9D). In both groups of immunized animals there was no hematogenous dissemination of the infection.
FIG. 9.
Protective effect of gp43 and P10 immunization in intratracheally infected mice (3 × 105 virulent P. brasiliensis yeast forms). (A) Lung section from a control nonimmunized BALB/c mouse showing confluent epithelioid granulomata forming a paracoccidioma-like lesion with a great number of viable yeast cells. (B) Dissemination to the liver of P. brasiliensis in a control nonimmunized mouse; confluent epithelioid granulomas with fungal cells are shown. (C) A single, well-demarcated epithelioid granuloma, with a prominent mononuclear cellular halo, encircling viable and nonviable yeast forms in a lung section from a gp43-immunized animal. (D) Apparently resolved granuloma, containing few macrophages and no fungal cells, in a lung section of a P10-immunized mouse.
DISCUSSION
The immunodominant properties of gp43 as an antibody-eliciting antigen, recognized in virtually 100% of sera from patients with PCM, suggested at first that this molecule would not have any role in protection against infection, since high antibody titers against specific components of P. brasiliensis are generally correlated with poor prognosis and aggravation of the disease. In a susceptible-resistant experimental murine model, Calich et al. (8) also correlated the high specific-antibody response to P. brasiliensis (mainly of the IgG1, IgG2B, and IgA subtypes) and the polyclonal B-cell activation with progressive infection in the susceptible B10.A mice. In contrast, resistant A/Sn mice developed a strong DTH response to intraperitoneally inoculated P. brasiliensis and produced low levels of specific antibodies with no evidence of polyclonal B-cell activation.
The observation, however, that the gp43 present in a crude antigenic preparation of P. brasiliensis was immunodominant in eliciting DTH reactions in infected guinea pigs (31) stimulated subsequent work to identify T-cell epitopes in this molecule. In fact, the cell-mediated immune response that leads to positive DTH reactions and acquired resistance to infection is mediated chiefly by T-helper cells of the Th-1 subtype (4, 10, 11, 21, 32, 36, 37). In the present study, we have shown that short-term immunization with gp43 renders LN cells able to proliferate in a dose-dependent way with the homologous antigen. This response is compatible with both mouse haplotypes H-2a and H-2d. Stimulated cells are CD4+ T lymphocytes with no evidence of proliferation of CD8+ cells. CD4+ T lymphocytes are of the Th-1 subtype, since they produce IFN-γ and IL-2 but not IL-4 or IL-10. IFN-γ production is much enhanced in the presence of rIL-2 and can be detected in the sera of some animals immunized with the immunodominant epitope of gp43. These results show that gp43 contains T-cell as well as B-cell epitopes and suggest that the immunological response to these epitopes can be relevant to the progress of the infection.
The search for the immunodominant T-cell epitope in gp43 involved analysis of 25 chemically synthesized peptides spanning the entire amino acid sequence of gp43, deduced from the gene sequence recently determined in our laboratory (10). This set of peptides included 13- to 18-mer species. As has been seen, only the 15-mer peptide P10 was able to induce proliferation of LN cells primed by previous immunization with gp43. Moreover, mice immunized with P10 in Freund’s adjuvant had LN cells that proliferated with either P10 or the native gp43 molecule. LN cells stimulated by P10 were also CD4+ T cells of the Th-1 subtype, forming IFN-γ and IL-2.
Mapping of the T-cell epitope in gp43 also benefited from the Protean computer program of DNASTAR. The Jameson-Wolff antigenic index predicted several antibody binding regions and only two sequences with negative values in the 15- to 18-amino-acid range. One of these corresponded exactly to that of P10. This peptide domain was not expressed at the surface of the protein (lower-than-one values in the Emini probability plot) except for the C-terminal residues that contained the terminal asparagine residue, which is the sole N-glycosylation site of gp43 and which should be at the protein surface. The same region contained an average hydrophobic sequence for an 11-amino-acid window by the Kyte-Doolittle method, unlike most of the other regions of the molecule, which had average hydrophilic natures. Furthermore, the P10 region (residues 146 to 160 in the gp43 sequence) had two amphipathic regions which are compatible with T-cell epitopes and, more remarkably, P10 was the only sequence with 12 or more amino acids that was selected by the Sette MHC II motif method, implying that it fits into the MHC II, I-Ad haplotype for presentation to CD4+ T lymphocytes. These predictions were fully confirmed by the proliferation experiments, also suggesting that P10 is the only epitope in gp43 responsible for T-cell activation in BALB/c mice. Although P10 is also an inducer of T-cell proliferation in two other haplotypes, it is still not known whether additional peptides could also induce proliferation of these lineages.
To determine the structural requirements of the P10 15-mer sequence, and assuming that, theoretically, analogous peptides derived from it could be presented by both MHC classes I and II, a series of fragments ranging from 9- to 16-mer were synthesized (Table 2) and tested for their capacity to induce proliferation of LN cells primed with P10. As shown, only 12-mer and longer peptides were active, in accordance with a presentation by MHC II. A 9-mer amino acid sequence was initially defined as the epitope core, since the six amino acids flanking this sequence, half on each side, were not essential per se, provided three of them on one side or the other were kept to add to the 9-mer core to make a 12-amino-acid reactive sequence. Interestingly, the peptide in which the flanking N-terminal sequence contained only Leu and the flanking C-terminal sequence contained only Tyr and Ala (128C) was as active as P10, suggesting that gp43 is proteolyzed at the Ala-Asn site, since Asn is glycosylated in the native molecule (2, 18). The core sequence was then investigated, keeping constant the flanking six-amino-acid regions. Based on the lymphoproliferative response, we have shown that the amphipathic sequence HTLAIR is the essential inner core of the epitope, in agreement with Rothbard and Taylor’s (33) prediction of five-amino-acid determinants reactive with T cells. The possibility that the epitope included the four hydrophobic amino acids before His was ruled out by replacing the IAI sequence with RTA (9743E). A helical conformation for the epitope is suggested by the inhibitory effect of glutamic acid substitution adjacent to the inner core (9751C), probably interacting with Arg, which is an essential residue in the peptide. Alterations that eliminated the amphipathic nature of the inner core (9743G and 9751B) also precluded reactivity of the peptide. Comparison of P10 with the corresponding sequence of the related glucanase from Candida albicans (10) showed that in the latter the HTLAIR region was replaced by NTIFKK flanked by VTINVL and YGG, displaying a distribution of charged to hydrophobic residues different from that of the P10 epitope. The exoglucanases from Saccharomyces cerevisiae (EXG1 and SPR1) also showed important differences in this region.
Native or recombinant proteins, or fragments thereof, may induce proliferation of T cells from sensitized animals, but this property may or may not result in protection against infection. For instance, the recombinant hsp60 from Histoplasma capsulatum has domains that elicit a cellular immune response (cell-mediated immunity) and is protective against sublethal and lethal challenges by the fungus (13, 18). In contrast, the cell-mediated immune responses to recombinant heat shock protein 70 and the H antigen from the same organism are not associated with protection (1, 12). In P. brasiliensis, the P10 domain of gp43 elicits a strong cell-mediated immunity and is protective against intratracheal infection by a virulent strain of P. brasiliensis. This protective activity is undoubtedly related to the induction of an IFN-γ-secreting Th-1-lymphocyte population. The role of IFN-γ in systemic fungal infections that can be cleared by activated macrophages has been reported previously. IFN-γ is an important mediator of resistance to Cryptococcus neoformans (23, 34) and, together with tumor necrosis factor alpha, contributes to clearance of H. capsulatum cells in the infected host (25). Mice given virulent Blastomyces dermatitidis develop a strong IFN-γ response unlike those of mice that were inoculated with an avirulent isolate (25). IFN-γ has been shown to activate macrophages for increased fungicidal activity against P. brasiliensis or B. dermatitidis (6, 7), and we have recently shown that mice homozygous for the null mutation of the gene encoding the IFN-γ receptor (20) are highly susceptible to infection by P. brasiliensis, compared with the wild type (39a). As an IFN-γ inducer, P10 fosters a protective cellular immunity and has the advantage over gp43 that it does not elicit an antibody response, which is unable to control the infection and is often reported to accompany immunosuppression and anergy in patients with PCM. Mice infected with P. brasiliensis or immunized with gp43 and challenged with a virulent strain produced high IgG1 and IgE titers, and also IgG2a and IgG3, compatible with a mixed Th-1- and Th-2-inducible interleukin-mediated response in vivo. Such an antibody response, however, was not accompanied by anergy in the BALB/c model and did not interfere with the protection effect of gp43 in comparison with that of P10. The mixed Th-1–Th-2 response suggests that the gp43-derived peptides can be presented by different cells in the course of infection, leading to Th-1- and Th-2-lymphocyte activation and thus differing from the immunization response to this antigen and to P10 in CFA, which mainly activates inflammatory, IFN-γ-producing T cells.
Protection by gp43 and P10 against intratracheal infection in mice could also be documented by histopathology of the affected organs. While in the nonimmunized control mice the granulomatous inflammation was exudative and ill organized, containing numerous viable fungal cells and forming paracoccidioma-like masses in the lungs with dissemination to the liver and spleen, immunization with gp43 remarkably reduced the number, size, and nature of the granulomas; these lesions were well organized and compact, contained many dead fungal cells, and were efficient in preventing the dissemination of the infection to other organs. Immunization with P10 was also highly protective, as demonstrated by the presence of very few granulomas in the lungs with no fungal cells inside and the absence of dissemination lesions in the liver and spleen.
These results reinforce the conclusion that gp43, and particularly P10, protects against experimental infection in mice and offer the prospect of this major antigen and its epitopes also being effective in human patients.
ACKNOWLEDGMENTS
The present work was supported by PADCT-CNPq (62.0408/91.0) and FAPESP (95/0559-8). C.P.T. is the recipient of a Doctoral Fellowship from CAPES; R.P., M.A.J. and L.R.T. are Research Fellows of the CNPq.
We thank Mauricio Rodrigues for his advice and support and also Zoilo P. Camargo for laboratory facilities.
REFERENCES
- 1.Allendoerfer R, Maresca B, Deepe G S., Jr Cellular immune responses to recombinant heat shock protein 70 from Histoplasma capsulatum. Infect Immun. 1996;64:4123–4128. doi: 10.1128/iai.64.10.4123-4128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida I C, Neville D C A, Mehlert A, Treumann A, Ferguson M A J, Previato J O, Travassos L R. Structure of the N-linked oligosaccharide of the main diagnostic antigen of the pathogenic fungus Paracoccidioides brasiliensis. Glycobiology. 1996;6:507–515. doi: 10.1093/glycob/6.5.507. [DOI] [PubMed] [Google Scholar]
- 3.Atherton B, Sheppard R C, editors. Solid phase peptide synthesis: a practical approach. Oxford, England: Oxford University Press; 1989. [Google Scholar]
- 4.Bloom B R, Modlin R L, Salgame P. Stigma variations: observations on suppressor T-cell and leprosy. Annu Rev Immunol. 1992;10:453–488. doi: 10.1146/annurev.iy.10.040192.002321. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brummer E, Hanson L H, Restrepo A, Stevens D A. In vivo and in vitro activation of pulmonary macrophages by IFN-γ for enhanced killing of Paracoccidioides brasiliensis or Blastomyces dermatitidis. J Immunol. 1988;140:2786–2789. [PubMed] [Google Scholar]
- 7.Brummer E, Hanson L R, Stevens D A. IFN-γ activation of macrophages for killing Paracoccidioides brasiliensis: evidence for nonoxidative mechanisms. Int J Immunopharmacol. 1988;10:945–952. doi: 10.1016/0192-0561(88)90041-0. [DOI] [PubMed] [Google Scholar]
- 8.Calich V L, Singer-Vermes L M, Russo M, Vaz C A, Burger E. Immunogenetics in Paracoccidioidomycosis. In: Franco M, Lacaz C S, Restrepo A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 151–173. [Google Scholar]
- 9.Camargo Z, Unterkircher C, Campoy S, Travassos L R. Production of Paracoccidioides brasiliensis exoantigen for immunodiffusion tests. J Clin Microbiol. 1988;26:2147–2151. doi: 10.1128/jcm.26.10.2147-2151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cisalpino P S, Puccia R, Yamauchi L M, Cano M I N, Silveira J F, Travassos L R. Cloning, characterization, and epitope expression of the major diagnostic antigen of Paracoccidioides brasiliensis. J Biol Chem. 1996;271:4553–4560. doi: 10.1074/jbc.271.8.4553. [DOI] [PubMed] [Google Scholar]
- 11.Cox F E G, Liew F Y. T-cell subsets and cytokines in parasite infections. Immunol Today. 1992;13:445–448. doi: 10.1016/0167-5699(92)90072-F. [DOI] [PubMed] [Google Scholar]
- 12.Deepe G S, Jr, Durose G G. Immunobiological activity of recombinant H antigen from Histoplasma capsulatum. Infect Immun. 1995;63:3151–3157. doi: 10.1128/iai.63.8.3151-3157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deepe G S, Gibbons R, Brunner G D, Gomez F J. A protective domain of heat-shock protein 60 from Histoplasma capsulatum. J Infect Dis. 1996;174:828–834. doi: 10.1093/infdis/174.4.828. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg D, Weiss R M, Terwilligen T C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci USA. 1984;81:140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco M. Host parasite relationship in paracoccidioidomycosis. J Med Vet Mycol. 1986;25:5–18. doi: 10.1080/02681218780000021. [DOI] [PubMed] [Google Scholar]
- 16.Gesztesi J-L, Puccia R, Travassos L R, Vicentini A P, Moraes J Z, Franco M F, Lopes J D. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidioides brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma. 1996;15:415–422. doi: 10.1089/hyb.1996.15.415. [DOI] [PubMed] [Google Scholar]
- 17.Gillis S, Ferm M M, Ou W, Smith K A. T-cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–2032. [PubMed] [Google Scholar]
- 18.Gomez F J, Allendoerfer R, Deepe G S. Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect Immun. 1995;63:2587–2595. doi: 10.1128/iai.63.7.2587-2595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirata I Y, Cezari M H S, Nakaie C R, Boschkov P, Ito A S, Juliano M A, Juliano L. Internally quenched fluorogenic protease substrates: solid phase synthesis and fluorescence spectroscopy of peptides containing ortho-amino benzoyl/dinitrophenyl groups as donor-acceptor pairs. Lett Pept Sci. 1994;1:299–308. [Google Scholar]
- 20.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 21.Kauffmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 22.McEwen J G, Garcia A M, Ortiz B L, Botero S, Restrepo A. In search of the natural habitat of Paracoccidioides brasiliensis. Arch Med Res. 1995;26:305–306. [PubMed] [Google Scholar]
- 23.Mody C H, Tyler C L, Sitrin R G, Jackson C, Toews G B. Interferon-gamma activates rat alveolar macrophages for anti-cryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Mota N G S, Rezkallah-Iwasso M T, Peraçoli M T S, Aldi R C, Mendes R P, Marcondes J, Marques S A, Dillon N L, Franco M. Correlation between cell-mediated immunity and clinical forms of paracoccidioidomycosis. Trans R Soc Trop Med Hyg. 1985;79:765–772. doi: 10.1016/0035-9203(85)90112-9. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, J. W., B. A. Wu-Hsieh, L. M. Singer-Vermes, A. Ferrante, S. Moser, M. Russo, C. A. C. Vaz, E. Burger, V. L. G. Calich, I. C. Kowanko, D. A. Rathjen, A. J. Martin, R. P. Bucy, and Q. Chen. 1994. Cytokines in the host response to mycotic agents. J. Med. Vet. Mycol. 32(Suppl. I):203–210. [DOI] [PubMed]
- 26.Musatti C C, Rezkallah M T, Mendes E, Mendes N F. In vivo and in vitro evaluation of cell mediated immunity in patients with paracoccidioidomycosis. Cell Immunol. 1976;24:365–378. doi: 10.1016/0008-8749(76)90220-3. [DOI] [PubMed] [Google Scholar]
- 27.Musatti C C, Peraçoli M T S, Soares A M V C, Rezkallah-Iwasso M T. Cell-mediated immunity in patients with paracoccidioidomycosis. In: Franco M, Lacaz C S, Restrepo A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 175–186. [Google Scholar]
- 28.Puccia R, Travassos L R. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo’s disease. J Clin Microbiol. 1991;29:1610–1615. doi: 10.1128/jcm.29.8.1610-1615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puccia R, Travassos L R, Rodrigues E G, Carmona A K, Oliveira M C, Juliano L. Purification of the specific exocellular antigen gp43 from Paracoccidioides brasiliensis: immunological and proteolytic activities. In: Maresca B, Kobayashi G S, editors. Molecular biology of pathogenic fungi. A laboratory manual. New York, N.Y: Telos Press; 1994. pp. 507–515. [Google Scholar]
- 30.Puccia R, Schenkman S, Gorin P A J, Travassos L R. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect Immun. 1986;53:199–206. doi: 10.1128/iai.53.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues E G, Travassos L R. Nature of the reactive epitopes in Paracoccidioides brasiliensis polysaccharide antigen. J Med Vet Mycol. 1994;32:77–81. [PubMed] [Google Scholar]
- 32.Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothbard J B, Taylor W R. A sequence pattern common to T cell epitopes. EMBO J. 1988;7:93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salkowski C A, Balish E. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect Immun. 1991;59:486–493. doi: 10.1128/iai.59.2.486-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraiva E C O, Altemani A, Franco M F, Unterkircher C S, Camargo Z P. Paracoccidioides brasiliensis—gp43 used as paracoccidiodin. J Med Vet Mycol. 1996;34:155–161. doi: 10.1080/02681219680000261. [DOI] [PubMed] [Google Scholar]
- 36.Sell S, Hsu P L. Delayed hypersensitivity, immune deviation, antigen processing and T-cell subset selection in syphilis pathogenesis and vaccine design. Immunol Today. 1993;14:576–582. doi: 10.1016/0167-5699(93)90196-R. [DOI] [PubMed] [Google Scholar]
- 37.Smith J M B, Griffin J F T. Strategies for the development of a vaccine against ringworm. J Med Vet Mycol. 1995;33:87–91. doi: 10.1080/02681219580000201. [DOI] [PubMed] [Google Scholar]
- 38.Stambuk B U, Puccia R, Almeida M L C, Travassos L R, Schenkman S. Secretion of the 43 kDa glycoprotein antigen by Paracoccidioides brasiliensis. J Med Vet Mycol. 1988;26:367–373. doi: 10.1080/02681218880000521. [DOI] [PubMed] [Google Scholar]
- 39.Taborda C P, Camargo Z P. Diagnosis of paracoccidioidomycosis by passive hemagglutination assay for antibody using a purified and specific antigen gp43. J Med Vet Mycol. 1993;31:155–160. doi: 10.1080/02681219380000171. [DOI] [PubMed] [Google Scholar]
- 39a.Taborda, C. P., L. F. Reis, and L. R. Travassos. Unpublished data.
- 40.Vicentini A P, Gesztesi J-L, Franco M F, de Souza W, de Moraes J Z, Travassos L R, Lopes J D. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun. 1994;62:1465–1469. doi: 10.1128/iai.62.4.1465-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wanke B, Londero A T. Epidemiology and paracoccidiodomycosis infection. In: Franco M, Lacaz C S, Restrepo A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 109–120. [Google Scholar]