Abstract
Gelatin methacrylate (GelMA) hydrogels have gained significant traction in diverse tissue engineering applications through the utilization of 3D printing technology. As an artificial hydrogel possessing remarkable processability, GelMA has emerged as a pioneering material in the advancement of tissue engineering due to its exceptional biocompatibility and degradability. The integration of 3D printing technology facilitates the precise arrangement of cells and hydrogel materials, thereby enabling the creation of in vitro models that simulate artificial tissues suitable for transplantation. Consequently, the potential applications of GelMA in tissue engineering are further expanded. In tissue engineering applications, the mechanical properties of GelMA are often modified to overcome the hydrogel material's inherent mechanical strength limitations. This review provides a comprehensive overview of recent advancements in enhancing the mechanical properties of GelMA at the monomer, micron, and nano scales. Additionally, the diverse applications of GelMA in soft tissue engineering via 3D printing are emphasized. Furthermore, the potential opportunities and obstacles that GelMA may encounter in the field of tissue engineering are discussed. It is our contention that through ongoing technological progress, GelMA hydrogels with enhanced mechanical strength can be successfully fabricated, leading to the production of superior biological scaffolds with increased efficacy for tissue engineering purposes.
Keywords: GelMA, Hydrogel, Tissue engineering, Mechanical properties, Compression modulus
Graphical abstract
1. Introduction
Hydrogels, which are hydrophilic polymers characterized by their exceptional biocompatibility and biodegradability [[1], [2], [3]], possess a three-dimensional cross-linked network structure. Their porous composition, abundant in water content, facilitates their role as cell adhesion sites akin to the extracellular matrix (ECM), thereby influencing diverse biological processes such as cellular proliferation and differentiation [[4], [5], [6]]. Hydrogels can be fabricated using either natural polymers, such as cellulose, polysaccharides, and proteins, or synthetic polymers, such as ethylene glycol (PEG) and polyvinyl alcohol (PVA), through physical (hydrogen bonding, hydrophobic interaction, etc.) or chemical (free radical polymerization, click chemistry, etc.) cross-linking [[7], [8], [9]]. The manipulation of the physicochemical characteristics of these hydrogels, including mechanical strength, porosity, swelling, and water-absorbing properties, can be achieved by modifying the polymer's composition, preparation method, and degree of cross-linking [10,11]. In contrast to natural hydrogel, Synthetic hydrogels possess a notable advantage in terms of enhanced flexibility in design and adaptability, enabling the creation of hydrogel biomaterials with customized properties [12].
In the year 2000, Gelatin methacryloyl (GelMA) hydrogels were initially synthesized by Bulcke's group [13]. These hydrogels represent innovative substances achieved through the incorporation of methacrylate-based groups into the gelatine side chains via substitution reactions involving amino (-NH2) and hydroxyl (-OH) groups (Fig. 1A) [14]. Gelatin, a naturally occurring hydrophilic polymer, comprises ECM-like constituents and is derived from the partial hydrolysis of native collagen. This biomaterial encompasses RGD sequences (Arg-Gly-Asp peptides), promoting cell adhesion, spreading, and differentiation, alongside matrix metalloproteinase sequences (MMP) that facilitate enzymatic degradation. These attributes ensure remarkable cytocompatibility while facilitating biodegradation [15]. GelMA, a gelatin derivative that has been modified with methacryloyl anhydride (MAAnh), has been found to exhibit a preference for amino acid residues with molar ratios below 5 % [16]. This selective modification allows for the preservation of functional amino acid sequences, such as RGD and MMP, which are important for cell adhesion and biodegradability of GelMA [[17], [18], [19]]. The methacryloyl substituents confer photocrosslinking properties to GelMA, which further enhance the advantages of GelMA photopolymerized hydrogels. These advantages include rapid handling, plasticity, ease of customization, and improved mechanical properties [20]. GelMA hydrogels can undergo photocrosslinking using UV or visible light through the addition of a photoinitiator, such as lithium 2,4,6-trimethylbenzoylphosphonate (LAP) or 2-hydroxy-40-(2-hydroxyethoxy)-2-methylpropiophenone (IC-2959), to the GelMA prepolymer [[21], [22], [23], [24], [25]]. Due to the favorable conditions for photocrosslinking, the majority of RGD and MMP sequences remain unaltered, thereby expanding the potential applications of GelMA hydrogels in the field of bioengineering [26].
Fig. 1.
A) Schematic representation of the formation of GelMA from gelatin by substitution reaction. B) Modification Methods and Tissue Engineering Applications of GelMA Hydrogel. C) Schematic diagram of the formation of IPNs.
GelMA has undergone significant research and application across diverse domains, with a particular emphasis on its utilization in tissue engineering within the field of biomedicine [27]. The utilization of 3D bioprinting, a nascent technology enabling precise spatial arrangement of biological materials and cells, assumes a significant role in the application of GelMA within the field of tissue engineering [28]. The utilization of 3D bioprinting technology enables precise cell and hydrogel distribution within pre-determined “bioink layers,” thereby facilitating the replication of desired tissue structures [29]. GelMA has become a prominent multifunctional hydrogel in the realm of tissue engineering. By utilizing various printing techniques, such as extrusion-based printing (EBP) [30], inkjet-based printing (IBP) [31], and stereolithography (SLA) [32], GelMA hydrogels can attain a diverse range of microstructures, including microfibers [33], microchannels [34], and microgroove and microridge [35] formations, as well as microwells and micropillars [36]. These microstructures play a vital role in the in vitro reconstruction of intricate tissue architectures. The adaptability exhibited by these hydrogels fabricated through 3D printing technology renders them a highly promising platform for the progression of tissue therapies in the next generation. Recent investigations have substantiated the utility of GelMA in diverse realms of tissue engineering, encompassing vascular [37], myocardial [38], cutaneous [39], cartilaginous [40], and neural tissues [41]. Provided that cellular viability can be maintained and functional prerequisites can be fulfilled, bioprinted scaffolds hold the potential to replicate artificial tissues that are amenable for in vitro transplantation [42].
Despite the manifold benefits of GelMA as a bioprinting substrate, the faithful replication of specific local tissues poses difficulties owing to its intrinsic mechanical fragility and swift degradation [43]. Furthermore, GelMA hydrogels exhibiting excessive viscosity tend to obstruct the nozzles during the 3D printing procedure, potentially impeding its utility in the field of tissue engineering [44]. Research studies have revealed that the mechanical characteristics of GelMA, a light-curing hydrogel, such as elasticity, hardness, and compressibility, are intricately connected to the kinetics of cross-linking during the process of photocross-linking [[45], [46], [47], [48]]. To improve the characterization of GelMA bases' mechanical strength, several methods can be utilized, including elevating the concentration of prepolymers [49,50], enhancing the degree of substitution and functionalization [[51], [52], [53]], and modifying the conditions of photocrosslinking [25,[54], [55], [56]]. The degree of substitution of the methacryloyl group is defined as the ratio of the functionalized amino group (-NH2) to the total available amino groups prior to cross-linking, which can be measured using H NMR spectroscopy [57]. Hoch prepared hydrogels with varying degrees of methacrylation (DM) in the degree of 770–194 % for swelling and 5–368 KPa for storage modulus by employing a molar excess of MAAnh relative to the free amino group of gelatin and utilizing Gel-MAAnh ratios of 1:1.5, 1:2, 1:10, and 1:20. The degree of functionalization, as determined by H NMR spectroscopy, was found to be 68.5 % and 85.1 % for Gel-MAAnh ratios of 1:1.5 and 1:2, respectively, while the degree of free amino substitution tended towards 100 % for ratios of 1:10 and 1:20. Subsequent in vitro experiments involving porcine chondrocyte culture provided further evidence of the superior performance of GelMA as a material for tissue engineering applications [58]. The parameters typically involved in photocrosslinking processes encompass light intensity, exposure time, and photoinitiator [54,[59], [60], [61]]. Within a stable light intensity setting, the duration of light exposure emerges as a significant determinant impacting the degree of swelling and mechanical strength of GelMA. Schuurman and his team investigated the effect of irradiation time and observed that the compressive modulus of 30 % GelMA samples crosslinked for 10 min was more than twice that of samples crosslinked for 5 min [62]. The type and concentration of photoinitiators also affect the mechanical properties of GelMA hydrogels. Free radical photoinitiators need excellent biocompatibility and low biotoxicity, and the common ones are lithium 2,4,6-trimethylbenzoylphosphonate (LAP) [63], 2-hydroxy-40-(2-hydroxyethoxy)-2-methylpropiophenone (IC-2959) [64], Irgacure 2955 [65], azo dyes (e.g., VA-086) [66], Type II initiators (e.g., EY/Triethanolamine [67], Ru/SPS [68]). Alexa and colleagues compared the Young's modulus of 3D-printed GelMA hydrogel scaffolds containing 1 % IC-2959 with those containing 0.5 % IC-2959. The former exhibited approximately a 45 % higher Young's modulus compared to the latter (1 % IC-2959: E = 0.393 ± 0.01 GPa, 0.5 % IC-2959: E = 0.22 ± 0.019 GPa) [69]. Additionally, several studies have demonstrated that the mechanical properties of hydrogels for 3D printing applications can be further enhanced through the incorporation of functional groups via chemical modification of GelMA [70]. In their 2013 study, Hoch et al. synthesized two types of GelMA hydrogels with varying degrees of methacrylation: GM10 (10-fold molar excess of MAAnh) and GM2 (2-fold molar excess). Compared to GM2, GM10 exhibited lower viscosity (10 wt%: 3.3.5 mPa s, 37 °C) and higher storage modulus (10 wt%: 15.2 6.4 kPa). Introducing acetyl functional groups into GM2 resulted in a significant reduction in viscosity (<10 mPa s) at room temperature for the obtained dual-functional crosslinked hydrogel (GM2A8). Subsequent experiments involving porcine chondrocyte culture on 3D-printed scaffolds made of GM10 and GM2A8 demonstrated effective cell viability [71]. This further exemplifies the substantial importance of chemical modification in the investigation of GelMA within the field of bioengineering.
Prior studies have emphasized the necessity of expanding the application of GelMA hydrogels in 3D bioprinting for the production of functional three-dimensional biological tissues in vitro [[72], [73], [74], [75]]. To address this, alongside chemical modifications of GelMA, innovative strategies have emerged to enhance the strength of GelMA bioprinting scaffolds at various microscopic levels. These approaches encompass the creation and/or modification of interpenetrating interconnecting networks (IPNs), reinforcement of layer-layer interactions, and modification of ink formulations [65,[76], [77], [78]]. These advancements enhance the structural integrity of GelMA bioprinting scaffolds, thereby facilitating their successful integration into various bioprinting applications. This review aims to elucidate diverse methodologies for enhancing the mechanical robustness of GelMA as a bioprinting substrate in tissue engineering, along with specific application scenarios (Fig. 1C/Table 1). Furthermore, we explore the potentialities and obstacles associated with the sustained utilization of GelMA as an exceptional biomaterial in the field of biomedicine. The objective is to offer rational design concepts and developmental pathways for the pragmatic and intricate implementation of GelMA in bioprinting for tissue engineering purposes.
Table 1.
Modified GelMA and its application scenarios.
| Improvements | Ingredients | Tissue | Cells | Mechanical strength | Ref. |
|---|---|---|---|---|---|
| IPNs | GelMA/HA | Cartilaginous | C28/I2 | 4.2 ± 0.4 kPa (Young's modulus) | [79] |
| sGAG/GelMA/Alginate | Cartilaginous | MSCs | 32.48 ± 4.23 kPa | [80] | |
| CuONPs/GelMA/PVA | Vascular/Skin | ASCs/HUVECs | ∼300 kPa | [81] | |
| BNC/GelMA/Alginate | Nerve | RSC96 | ∼10.92 kPa | [82] | |
| Hybrid bio-ink | LDHs/GelMA | Bone | MC3T3-E1 | ∼1168 kPa | [83] |
| CNCs/GelMA/HAMA | Cartilaginous | ATDC5 | 55.8 ± 2.1 kPa | [84] | |
| HAP/GelMA/Alaiante/Gelatin | Cartilaginous/Vascular | MC3T3/HUVECs | 25∼28.35 kPa | [85] | |
| GelMA/MeHA/dhECM&GelMA/dhECM | Crdiac muscle | hCFs/iCMs | 18.4 ± 2.8 kPa/24.5 ± 2.9 kPa | [86] | |
| ULMA/GelMA/Gelatin | Skin | HDFS | ∼4.2 kPa | [87] | |
| GelMA/HAMA | Cartilaginous | Cartilaginous cell | ∼30 kPa | [88] | |
| Layer-to-layer reinforcement | Kca/GelMA | Muscle | C2C12 | ∼12.5 kPa (Shear stress: USS) | [89] |
| G1Phy/GelMA | Muscle | L929 | [90] |
2. Enhancing the mechanical strength of 3D printed photo-crosslinked GelMA hydrogels
The biocompatibility and cellular microenvironment similarity exhibited by GelMA hydrogels have made them highly sought-after for the advancement of diverse biological alternatives [[91], [92], [93]]. GelMA hydrogels possess the RGD sequence, known to enhance cell adhesion, and the MMP target sequence, which is particularly suitable for cellular remodeling. These distinctive attributes, combined with their remarkable photocrosslinkability, distinguish GelMA hydrogels from conventional hydrogel materials [13,17,94]. Moreover, GelMA hydrogels exhibit exceptional versatility in their processability, enabling various forms of microfabrication and the ability to be combined with diverse materials to achieve a wide array of structures necessary for tissue engineering applications [28,95]. This remarkable flexibility underscores the immense potential of GelMA hydrogels in the field of tissue repair and regeneration. Consequently, there has been a growing need for enhanced GelMA performance in the field of tissue engineering [[96], [97], [98]]. However, despite the existence of multiple physical and chemical modifications, aimed at bolstering the mechanical strength of GelMA hydrogel materials, their mechanical and biological characteristics remain constrained when employed as 3D bioprinting materials. These limitations impede their broader utilization in the field of tissue engineering [[98], [99], [100]]. This limitation has prompted the search for innovative approaches to meet the diverse needs of GelMA as a 3D printing material for tissue engineering.
2.1. Interpenetrating network reinforcement at the monomer level
The synthesis of interpenetrating cross-linked polymerization networks (IPNs) is a highly efficient technique that entails the incorporation of a second monomer, cross-linker, and initiator into an already cross-linked polymer I through physical or chemical cross-linking mechanisms. This procedure results in the in situ polymerization of the second monomer and its subsequent cross-linking, resulting in the formation of polymer II, which becomes intermingled with the existing network of polymer I. The synthesis of interpenetrating cross-linked polymerization networks (IPNs) is a highly efficient technique that entails the incorporation of a second monomer, cross-linker, and initiator into an already cross-linked polymer I through physical or chemical cross-linking mechanisms. This procedure results in the in situ polymerization of the second monomer and its subsequent cross-linking, resulting in the formation of polymer II, which becomes intermingled with the existing network of polymer I [[101], [102], [103]]. The formation of a self-crosslinking and interpenetrating polymer network in GelMA leads to the creation of a monolith that is reinforced at the monomer-to-monomer level (Fig. 1B). This reinforcement enables the material to overcome the inherent mechanical limitations of pure GelMA as a 3D printing material, resulting in a compatible and robust structure. The configuration of the IPN network is contingent upon the distinct phases of the polymer, which can have an influence on the overall mechanical robustness, rate of swelling, and effectiveness of degradation in the crosslinked material [[104], [105], [106]]. GelMA is frequently subjected to cross-linking with various materials, including alginate hydrogels, methacrylate-based hyaluronic acid gelatin (HAMA), silk protein (SF), and several other polymers [76,[107], [108], [109]]. Jonidi Shariatzadeh et al. developed highly porous and mechanically robust gels through the formation of interpenetrating networks (IPNs) combining GelMA and hyaluronic acid (HA) (Fig. 2A and B) [79]. The authors investigated the Young's modulus of pure GelMA compared to IPN with 5 % HA concentration and observed a significant decrease in the latter (2.25 ± 0.5 kPa) compared to pure GelMA (3 kPa) (p < 0.01). However, with increasing HA concentration, the mechanical properties improved, and at a 10 % HA concentration, the Young's modulus (4.25 ± 0.4 kPa) surpassed that of GelMA (p < 0.001). Furthermore, the augmentation of the cross-linked network of GelMA hydrogel IPNs can be achieved through the utilization of polymeric substances like polycaprolactone (PCL) and sulphated glycosaminoglycans (sGAG) [110,111], thereby leading to enhanced mechanical characteristics of the initial IPNs. Wang and colleagues used a sulphated glycosaminoglycan (sGAG) analogue to functionalize an alginate-gelatin (GelMA) intra-penetrating network (IPN) bioink. The mechanical strength of their printed double-cross-linked S-IPN constructs was significantly improved compared to single-printed alginate or GelMA while retaining elasticity and toughness. Ramp compression experiments revealed that the double cross-linking S-IPN constructs (0.7 % alginate, 0.3 % alginate sulphate, 10 % w/v GelMA) had a 3-fold higher compressive modulus (32.48 ± 4.23 kPa) than 10 % GelMA alone (11.07 ± 1.92 kPa) and 5-fold higher than 1 % alginate/aluminium sulphate hydrogel (6.85 ± 1.17 kPa). Moreover, in vitro experiments demonstrated that the ink facilitated the sustained release of transforming growth factor β3 (TGF-β3) while promoting cartilage formation in MSCs (Fig. 2C–G) [80]. Additionally, Wu et al. successfully enhanced the mechanical characteristics of the composite material through the incorporation of nanomaterials within a unique nanocomposite interpenetrating polymer network (IPN) consisting of GelMA, polyvinyl alcohol (PVA), and copper oxide nanoparticles (CuONPs), as opposed to a solitary scaffold. Based on their findings, the authors inferred that these outcomes indicate the promising capability of nanomaterials in reinforcing hydrogel IPN networks [81]. Bipolymer interpenetrating polymer networks (IPNs) frequently exhibit significantly enhanced mechanical properties compared to their individual monomer network components. The charged outer surface of IPN hydrogels has the ability to retain a boundary water layer even under high normal loads [112], effectively reducing the material's coefficient of friction. This property aligns well with the lubrication function of cartilage in joints [113].
Fig. 2.
Reinforcement of hydrogel scaffolds by a network of IPNs. A) The SEM images of the porosity of hydrogel scaffolds with different HA concentration ratios. B) False-colour SEM image of adherent cells on the surface of the scaffold after seeding C28 cells onto the surface of the sample and incubating the scaffold for 24 h after fixation. Reproduced with permission [79]. Copyright 2021, Elsevier. C) Illustration of preparing alginate/alginate sulfateGelMA IPN bioink. D) Schematic of crosslinking processes. The constructs were either cultured in vitro for 6 weeks or subcutaneously implanted for 4 weeks. E) is the hydrogel pattern printed by different bio-inks. F) shows the representative images of live/dead staining of MSCs within constructs at various time points (day 1, 21, and 42) throughout the culture duration. G) Cell survival to 3D printed constructs. Reproduced with permission [80]. Copyright 2021, Elsevier.
2.2. Layer-to-layer reinforcement at the micron level
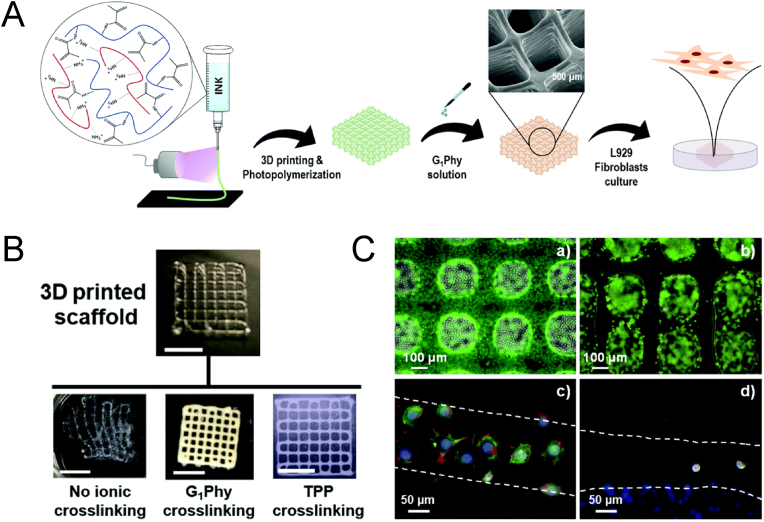
Numerous approaches have been investigated to augment the mechanical characteristics of GelMA, encompassing enhancements in concentration or functionalization, improvements in photocrosslinking density, and the development of innovative crosslinking networks, as previously discussed [114,115]. A fundamental requirement for biomaterials designed for tissue engineering purposes is to offer a microenvironment that is conducive to cellular growth. However, it should be noted that conventional techniques employed for enhancing the strength of GelMA may pose a potential hazard by producing cytotoxic degradation byproducts [[116], [117]]. Considering the layer-by-layer nature of 3D printing and the inherent weakness in adhesion at layer interfaces [[118], [119], [120]], the utilization of ionic action to establish robust interfacial adhesion between printed layers has emerged as a promising strategy for the advancement of GelMA bioprinting materials that are conducive to cellular viability. Li et al. examined the enhancement of adhesion strength between printed layers of 3D printing hydrogels by employing three groups of oppositely charged hydrogels (anionic: alginate, xanthan gum, κ-Carrageenan (Kca)/cationic: chitosan, gelatin, GelMA). Printability and mechanical properties tests identified the Kca-GelMA combination (Kca 2 wt%/GelMA 10 wt%) as the optimal choice among the oppositely charged hydrogels. Additionally, cellular experiments demonstrated that Kca-GelMA constructs exhibited excellent cellular activity (>96 %) [89]. Later, Mora-Boza et al. employed G1Phy, a new ionic cross-linking agent, to reinforce multilayer 3D printed scaffolds with up to 28 layers. Performance tests demonstrated that the ion-treated cross-linked scaffolds exhibited improved shape fidelity, controlled swelling rates, and long-term stability under physiological conditions for a minimum of four weeks (Fig. 3) [90]. These innovative approaches indicate promising prospects for leveraging ionic interactions to augment layer-to-layer stability in GelMA hydrogel 3D printing. The utilization of ionic interactions as a means to augment the stability of layer-to-layer bonding in printed structures represents an unquestionably innovative approach. Its capacity to facilitate more precise print tip applications and enable the formation of intricate print structures suggests its significant potential for implementation in GelMA hydrogel 3D printing.
Fig. 3.
A) Reinforcement of GelMA/chitosan 3D polymer scaffolds with ionic crosslinking agent (G1Phy). B) Visual examination and structural integrity changes that a photochemically 3D printed scaffold (four layers) immediately experienced after immersion in distilled water containing or not the G1Phy or TPP. Scale bar is 0.5 cm. C) Live/dead assay on G1Phy (a) and TPP (b) scaffolds after 24 h of incubation. Pictures were taken of scaffold strands; confocal immunostaining assay performed on G1Phy (c) and TPP (d) scaffolds after 24 h of incubation. Reproduced with permission [90]. Copyright 2020, Royal society of chemistry.
2.3. Structural strengthening at the nano level
Research has indicated that the integration of nanomaterials into GelMA platforms, in comparison to GelMA at equivalent concentration, yields enhanced mechanical robustness, facilitating substantial levels of deformation in compression, tension, and bending. Additionally, this integration leads to improvements in biocompatibility, degradability, porosity, shear thinning capacity, and viscosity modulation [18,[121], [122], [123]]. Due to the inherent drawbacks of traditional natural materials like alginate, gelatin, and hyaluronic acid, which are susceptible to rapid degradation and exhibit limited functionality [[124], [125], [126], [127]], the incorporation of nanoscale modifications into bioinks has emerged as a pivotal approach for bolstering the mechanical robustness of GelMA bioprinted scaffolds. Recent studies have conducted inquiries into the application of various inorganic nanomaterials, such as hydroxyapatite nanoparticles (nHAP) [128], calcium phosphate micro- and nanoparticles (nCNP) [65], bioactive glass (BGn) [129,130], and nano-silicates (nSi) [131], among other substances, to modify bioinks. In a recent investigation, Alarcin et al. presented a novel printing approach for the fabrication of nanocomposite bioinks by incorporating layered double hydroxide nanoparticles (nLDHs) into GelMA. The study demonstrated a notable increase in the compressive strength of the composite hydrogel, from 652 kPa (G-LHD0) to 1168 kPa (G-LHD3), with an LDH concentration of 3 wt%. Furthermore, 3D printed scaffolds derived from this bioink formulation exhibited excellent support for the survival, proliferation, and spreading of osteoblasts, independent of osteoinductive factors. Remarkably, G-LHD3 displayed enhanced spreading efficiency and superior cell morphology compared to cells encapsulated in G-LHD0 [83]. The distinctive network architecture of GelMA, coupled with the effective entrapment of nanomaterials, exerts a substantial impact on the mechanical and biological characteristics of hydrogels via physical and covalent interactions with GelMA. It is noteworthy that the incorporation of specific nanomaterials, including metallic nanomaterials such as gold nanoparticles (GNPs) [132], silver nanoparticles (AgNPs) [133], and titanium [134], as well as carbon nanomaterials like graphene oxide (GO) [135], reduced graphene (rGO) [136,137], and carbon nanotubes (CNTs) [138,139]), imparts a diverse range of exceptional properties to hydrogel materials. These properties encompass electrical conductivity, antimicrobial activity, and other distinctive characteristics [[140], [141], [142], [143], [144], [145]]. The utilization of these special nanomaterials holds great promise for the development of advanced hydrogel systems with multifunctional capabilities. Yu et al. successfully fabricated structurally stable, mechanically sound, and biocompatible bioscaffolds using a composition ratio of 8 % GelMA/1 % HAMA/3 % alginate/0.5 mg/mL graphene oxide (GO) (8/1/3/0.5) via dual-channel printing. In a rat cranial bone defect model, the dual-channel scaffolds encapsulating rat bone marrow-derived macrophages (BMM) and rat bone marrow mesenchymal stem cells (BMSCs) demonstrated superior efficacy compared to single-cell scaffolds and cell-free scaffolds [146]. Several studies have explored the utilization of nanomaterials beyond their direct application as 3D printing bioinks. Fan et al. reinforced GelMA/HAMA bioinks with cellulose nanocrystals (CNCs) using the photo-crosslinking chemistry shared by both inks. The research encapsulated the murine chondrocyte cell line ATDC5 in structures containing CNCs and GelMA/HAMA to explore the material's potential for cartilage tissue application. Notably, an increase in the CNC loading to 10 % (w/v) resulted in a significant increase in the compressive modulus of the GelMA/HAMA cross-linked hydrogel from 22.7 ± 2.8 kPa to 55.8 ± 2.1 kPa. The study demonstrated promising results, with the survival rate of the ATDC5 cell line being 82.4 ± 3.3 % after 1 day of in vitro culture, and cell viability reaching 99.1 ± 2.2 % after 7 days, demonstrating the potential of the material for cartilage repair [84]. The utilization of advanced nanomaterials in the reinforced multifunctional GelMA platform enables it to withstand significant levels of deformation, including compression, bending, elongation, and tearing. This particular attribute proves advantageous in the field of skin tissue engineering, as it necessitates substantial tensile and flexural forces for effective wound healing.
3. Advancements in soft tissue repair engineering
3.1. Vascular engineering
The preservation of the structural and functional integrity of the vasculature holds significant importance in the field of tissue repair engineering. This is primarily due to its role as a conduit for the delivery of oxygen, nutrients, and metabolites to tissues and organs [147,148]. Numerous studies have provided substantial evidence indicating that the vascular network plays a crucial role not only in transportation functions but also in shaping tissue structures [[149], [150], [151], [152], [153]]. It is highly improbable for tissue-engineered structures exceeding 1 mm in size to maintain viability without the presence of a well-developed vascular network [154]. Therefore, the reconstruction of the vascular network to ensure consistent blood perfusion is of utmost importance in achieving successful tissue repair of intricate structures [155,156]. Additionally, the incorporation of vascular structures or the transplantation of microvessels represents a significant utilization of vascular tissue engineering in the management of vascular injuries, including myocardial infarction and peripheral atherosclerosis [157,158]. Consequently, the expeditious attainment of endothelial cell proliferation and the establishment of a vascular network via 3D printing are pivotal elements in the application of GelMA composite hydrogels for vascular network engineering. Shahabipour et al. utilized coaxial 3D printing to create a bone-like model of vascular growth by printing a mixture of gelatin, GelMA, HAPs, and alginate. Osteoblasts (MC3T3) and human umbilical cord vascular endothelial cells (HUVECs) were loaded in the shell and core regions, respectively. The hybrid hydrogel inks demonstrated a higher compressive modulus (25–28.35 kPa) compared to pure GelMA hydrogels (18–21 kPa). Notably, the high concentration scaffold (GelMA 7 % + alginate 2 % + gelatin 1 % + HAp) exhibited a compressive modulus of 30 kPa, surpassing the 25 kPa of the low concentration scaffold (GelMA 5 % + alginate 0.5 % + gelatin 2 % + HAp) due to increased GelMA and alginate content. In vitro incubation of MC3T3 and HUVECs for 21 days in their respective regions demonstrated robust cell growth, as confirmed by confocal images [85]. 3D printing enables rapid fabrication of complex tissue structures in vascular engineering. Improved mechanical properties of GelMA composite hydrogels offer multifunctional bio-scaffolds with suitable strength and biocompatibility for vascular tissues and organs [159]. This approach is a crucial strategy for vascular-based soft tissue repair engineering.
3.2. Myocardial tissue engineering
The composition of human myocardial tissue primarily comprises cardiomyocytes (approximately 30 %) and non-cardiomyocytes, which are closely interconnected [160,161]. In the event of myocardial tissue injury, such as infarction or myocardial ischemia, the surviving myocardial cells undergo fibrosis or hypertrophy [162,163], leading to the formation of scar tissue that exhibits significantly higher stiffness (150 kPa) compared to healthy myocardial tissue (10 kPa) [164]. The utilization of 3D printing in the creation of cardiac regeneration models is a highly effective method for reinstating the intricate distribution of cells [165,166]. Alongside the requirement to replicate the typical stiffness of healthy myocardial tissue, the restoration of intrinsic automaticity and conduction properties of cardiomyocytes is a pivotal concern that necessitates attention in the development of bioprinted materials for myocardial regeneration [167]. These factors collectively pose novel obstacles for GelMA composite hydrogels. Basara et al. successfully developed GelMA-MeHA-dhECM (GME) and GelMA-dhECM (GE) hydrogels using UV light and microbial glutaminase (mTGase) dual cross-linking techniques. These hydrogels, composed of decellularized human cardiac ECM (dhECM) with GelMA or GelMA-MeHA, showed compatibility with human induced pluripotent stem cell-derived cardiomyocytes (iCMs) and human cardiac fibroblasts (hCFs). Double cross-linking reinforcement significantly enhanced the Young's modulus of both GE and GME hydrogels (GM: 14.4 ± 1.8 kPa → 24.5 ± 2.9 kPa/GME: 9.9 ± 2.6 kPa → 18.4 ± 2.8 kPa). GE-loaded iCMs in the printed structures mimicking healthy heart tissue exhibited spontaneous beating (0.65 Hz ± 0.26 Hz) and demonstrated expression of α-actinin and connexin 43 in the transverse myocardial layer, reflecting high cell viability (Fig. 4A–D) [86]. Developing bioinks with optimized mechanical properties for 3D biomodels is a novel approach in myocardial tissue engineering. Rational bioink ratios can address the stiffness limitations of cardiomyocyte-adapted biomaterials, allowing for the investigation of cardiomyocyte myogenic behavior and providing insights into the impact of biomaterials and mechanical stiffness on myogenesis in various muscle tissues, including skeletal muscle.
Fig. 4.
A) Scanning electron microscopic images of lyophilized GelMA, GelMA–dhECM, GelMA–MeHA and GelMA–MeHA–dhECM hydrogels with mTGase treatment. B) Brightfield images of 3D printed 2 layered-grid constructs using these four hydrogels. C) Cell viability analysis of the printed cell-laden hydrogels prepared using these four hydrogels. Reproduced with permission [86]. Copyright 2021, Multidisciplinary Digital Publishing Institute. D) Viability staining of 3D bioprinted GelMA:HAMA (1:1, 2:1 and 3:1) constructs over days 0, 1 and 7. E) Day 0 storage moduli of DoE generated GelMA:HAMA mixtures. (i): 3D surface plot of storage moduli vs crosslinking and material mixtures. (ii): Corresponding normal probability plot of the residuals. Reproduced with permission [88]. Copyright 2022, Elsevier.
3.3. Skin tissue engineering
The skin functions as an inherent safeguard for delicate tissues, capillaries, and organs, serving as the principal defense mechanism of the body [168]. Burns are a frequently encountered type of skin trauma [169], and the presence of open wounds can facilitate the infiltration of bacteria and protracted inflammation, thereby intensifying fluid depletion and hindering the recuperative course. Consequently, the development of autologous epidermal graft substitutes, capable of permanently sealing wounds, is presently acknowledged as the benchmark in burn management [[170], [171], [172], [173]]. However, despite advancements in dermal substitutes, fulfilling the substantial needs of individuals with skin trauma continues to pose difficulties [174,175]. In this regard, 3D printing has emerged as a promising technique for producing bionic skin materials, with GelMA hydrogels garnering attention as a viable material for 3D printing of bioscaffolds [176]. Chen et al. isolated ulvan type polysaccharide UI84 from cultured Ulvacea macroalgae and modified it into methacrylate form (ULMA) for skin engineering materials in conjunction with GelMA hydrogels. Using 3D bioprinting, they fabricated four hydrogel scaffolds: U0 (ULMA 0 %+GelMA 6 %+Gelatin), U2 (ULMA 2 %+GelMA 4 %+Gelatin), U4 (ULMA 4 %+GelMA 2 %+Gelatin), and U6 (ULMA 6 %+GelMA 0 %+Gelatin). Mechanical testing showed that U2, U4, and U6 exhibited 3.9, 9.6, and 11.4 times higher Young's modulus compared to U0 (∼1.1 kPa), respectively. The scaffolds loaded with dermal fibroblasts (HDFS) demonstrated immunostained deposits of key extracellular matrix (ECM) proteins (e.g., collagen I, collagen III, elastin, fibronectin) after 14 days of in vitro culture. Notably, the U2 formulation showed the highest suitability for HDFS growth among all scaffolds [87]. The GelMA hydrogel bio-scaffold exhibits promise for application in various in vitro skin models, including skin substitutes and wound dressings, due to the numerous advantages offered by GelMA hydrogel bioinks in terms of shear dilution, yield stress, mechanical strength, and bioregeneration.
3.4. Cartilage tissue engineering
The articular cartilage possesses exceptional mechanical characteristics due to its high concentration of type II collagen, water, and glycosaminoglycan (GAG) content. This composition grants the cartilage with tensile strength, stiffness, toughness, and the ability to withstand variations in compound loads. These attributes are crucial for minimizing joint loads and friction during physical activity [[177], [178], [179]]. Regrettably, the constrained cellular composition of cartilage tissue and the lack of vascular nerve distribution present formidable obstacles to the regeneration of damaged cartilage [180,181]. Consequently, the implementation of natural bone grafts or artificial scaffolds at the injury site has been suggested as a viable approach for the successful healing of cartilage injuries [182]. The Gelatin methacryloyl (GelMA) hydrogel is extensively employed in the fabrication of 3D printed cartilage structures due to its remarkable biocompatibility and presence of cell binding sites. Furthermore, the hydrogel's effective hardness to induce stem cell differentiation into osteoblasts is estimated to be approximately 100 kPa [183], thus highlighting the significance of overcoming the mechanical constraints associated with GelMA materials and exploring the development of GelMA hydrogel materials that facilitate chondrocyte growth. These endeavors hold promising potential for the advancement of cartilage tissue repair engineering. Martyniak et al. investigated the optimal GelMA/HAMA bioink concentration ratio for 3D printing chondrocyte carrier scaffolds. Three bioinks with GelMA (15 %)/HAMA (2 %) (vol/vol) were evaluated in ratios of (1:1), (2:1), and (3:1) using dynamic mechanics to measure the storage modulus. Chondrocyte viability was assessed up to 7 days post-bioprinting through live/dead staining. The findings revealed that the (2:1) ratio bioink demonstrated superior cartilage growth development, with a threshold modulus of ∼30 kPa determined by mechanical strength assays. Moreover, cell mobility experiments indicated that excessively rigid bioinks impeded cartilage cell flow and growth (Fig. 4E and F) [88]. In the context of cartilage tissue engineering, it is essential to utilize hydrogels that possess both high shape fidelity and mechanical strength, considering the inherent stiffness and elasticity of human cartilage tissue. Therefore, the development of enhanced GelMA hydrogel bioinks with superior mechanical characteristics and their precise fabrication into 3D-printed bio-scaffolds holds significant importance for cartilage tissue engineering.
3.5. Neural tissue engineering
Peripheral nerves play a crucial role in facilitating motor and sensory functions, and any alteration in their structure or function can give rise to various motor and sensory dysfunctions, encompassing pain and disability [184]. Prolonged impairment of nerve function can additionally lead to the degeneration of muscle and bone tissues, exacerbating the decline in the patient's overall well-being [185,186]. Presently, autografting is widely regarded as the preferred method for addressing nerve injuries exceeding 5 mm; however, it is accompanied by a number of challenges, including morbidity in the donor nerve region [187,188]. In order to facilitate the regeneration and restoration of peripheral nerve tissue, diverse biomanufacturing techniques, including electrostatic spinning and mold embossing, have been devised to establish the necessary microenvironment for nerve repair [168,189,190]. The investigation has revealed that the rigidity of hydrogels, which serve as exceptional bioinks for 3D printing, plays a pivotal role in directing the differentiation of stem cells. Hydrogels with a softness ranging from 100 to 500 Pa are conducive to the differentiation into neuronal cells, whereas hydrogels with a higher stiffness of 1 kPa–10 kPa facilitate the proliferation of glial cells [191,192]. The precise regulation of the mechanical strength of 3D printed GelMA hydrogel scaffolds is crucial in modulating nerve cellular activity, including cell growth and differentiation, due to the high sensitivity of nerves to environmental stiffness. Wu et al. utilized bacterial nanocellulose (BNC) to reinforce calcium alginate-gelatin methacrylate (GelMA) IPNs and 3D-printed hydrogel scaffolds. The scaffold (5 % sodium alginate + 5 % GelMA + 0.3 % BNC) effectively encapsulated rat Shewan cells (RSC96) and achieved a compressive modulus of 10.9193 kPa post light curing. BNC incorporation enhanced the material's electrical conductivity, augmenting its potential for neural tissue repair. Live/dead staining within one week of printing confirmed high RSC96 cell viability. S-100β immunofluorescence staining and cytoskeleton staining demonstrated targeted RSC96 growth, accompanied by elevated mRNA expression of associated neurofactors (ASCL1, NEUROG1, ERBB2, POU3F3, NOTCH1, and DLL1). Remarkably, RSC96 cells exhibited sustained growth even after implantation of the hydrogel scaffold into mice (Fig. 5) [82]. The advancement of innovative composite hydrogels fabricated through 3D printing, possessing appropriate mechanical characteristics, presents a promising approach for the utilization of GelMA in the functionality of neural tissues.
Fig. 5.
Interpenetrating networks (IPNs) of calcium alginate-GelMA fortified by (BNC). A) The schematic illustration of 3D-bioprinting bioink production. B) (i): Printing model of the 2.5 % GelMA group, (ii): Printing model of the 5 % GelMA group, (iii): Printing model of the 10 % GelMA group, (iv): Printing model of the 5 % GelMA +0.3 % BNC group (scale bar = 1 mm). C) (i): Electrical conductivity, (ii): Compressive modulus. D) Cytoskeletal staining of RSC96 cytoskeleton on different hydrogel scaffolds on days 4 and 7 (White line in Day 7 shows the outline of the scaffold, and the arrow marks the direction of the long axis of the scaffold). E) Live/dead cell staining: The viability of RSC96 cells in the 3D-bioprinted scaffold within 7 days of culture. F) In vivo experiment: HE and S100β immunohistochemical staining of paraffin embedded sections at 2 and 4 weeks (Scale bar 50 μm). Reproduced with permission [82]. Copyright 2021, Elsevier.
4. Summary and outlook
GelMA has garnered significant interest in the field of tissue engineering due to its remarkable biocompatibility, degradability, photo-crosslinking capabilities, and adjustable mechanical properties. Additionally, substantial endeavors have been undertaken to enhance GelMA materials in order to produce more bioprintable structures that can accommodate a wider array of tissue engineering printing applications. For example, in order to fulfill the fundamental compression modulus necessary for chondrocyte proliferation, GelMA can be combined with HAMA to create an innovative bio-sink capable of printing appropriate bio-scaffolds for chondrocytes. Due to its exceptional biological properties and processability, modified GelMA has emerged as an indispensable constituent in the advancement of novel materials for tissue engineering.
Despite the manifold applications of modified GelMA in the field of tissue engineering, it encounters several obstacles when applied in practical settings. Primarily, certain modified GelMA hydrogels exhibit incomplete biodegradability owing to their modified composition, thereby constraining their efficacy as carriers for tissue grafts. Secondly, in vitro tissue culture fails to accurately replicate the intricate cellular microenvironment found in normal tissues, which comprises various cellular constituents and intricate interactions between cells and the extracellular matrix that remain incompletely understood. Moreover, the current clinical validation of the effectiveness of 3D printed modified GelMA scaffolds for transplantation is restricted. In the future, the utilization of modified GelMA hydrogels in conjunction with the advanced precision and resolution capabilities of 3D printing could enable the production of finely microstructured hydrogels. These hydrogels have the potential to serve as superior cell carriers, surpassing the mechanical limitations of traditional biological scaffolds in the field of tissue engineering. For instance, Liang et al. utilized hydrogel microfabrication to investigate Gingival regeneration in tissue engineering. They employed coaxial electrostatic microdrop technology to develop GelMA-alginate core-shell microcapsules, encapsulating hDPSCs and HUVECs. After 14 days of in vitro culture, the core-shell microcapsules exhibited significantly higher proliferation rates of HUECs and hDPSCs compared to monoculture groups. In vivo experiments further demonstrated enhanced microvessel formation and myeloid tissue regeneration in the core-shell microcapsules (co-culture group) (Fig. 6) [193]. Hence, it is imperative to prioritize the microstructure design of 3D printed hydrogels, particularly in relation to nanofabrication, for forthcoming research endeavors. Additionally, the integration of biomaterials with distinct properties into modified GelMA composite hydrogels can expand their applicability in tissue engineering, augmenting their physicochemical attributes such as electrical conductivity, antibacterial efficacy, and hemostatic capabilities. Rajabi et al. developed a GelMA/Gel-SH/PD-LAP hybrid haemostat by integrating polydopamine-coated saponite (PD-LAP) as a functional nanomaterial with thiol-functionalized gelatin (Gel-SH) and GelMA polymers via Michael reaction and covalent interaction. Assessment of tissue cytological behavior revealed substantial enhancements in swelling behavior, adhesion, dynamic properties, and hemostatic potential (GelMA/Gel-SH/PD-LAP exhibited a significant reduction in blood clotting time) (Fig. 7) [194].
Fig. 6.
A) The schematic illustration of cell-laden GelMA-alginate core-shell microcapsules fabricated using coaxial electrostatic microdroplet system and their application in endodontic regeneration. B) Highermagnification optical images of the core-shell microcapsules and the GelMA microgels and the corresponding SEM images after lyophilization. The scale bars in i, ii, iv and v are 200 μm. The scale bars in iii, vi are 50 μm. C) H&E and immunohistochemical stained images of paraffin sections from each group under light microscope. (i): Acellular GelMA microgels group (group G), (ii): hDPSCs-laden GelMA microgels group (group DP), (iii): GelMA microgels laden with 3:1 hDPSCs: HUVECs group (group 3:1), and (iv): GelMA microgels laden with 1:1 hDPSCs: HUVECs group (group 1:1). Scale bars in the first, second, third and fourth columns were 10 00, 10 0, 50, and 20 μm, respctively. Reproduced with permission [193]. Copyright 2022, Elsevier.
Fig. 7.
A) Scheme representation of the synthesize of Gel-NH2 and Gel-SH. B) 1H NMR spectra of gelatin, Gel-NH2, Gel-SH (one-step) and Gel-SH (two-steps) confirming the chemical grafting of thiol on the gelatin structure. C) FTIR spectra of gelatin, Gel-NH2 and Gel-SH confirming the thiolation process. D) The photographic images of gelatin based hydrogel, i before and ii after crosslinking with visible light. E) Compressive stress-strain curves. F) Compressive strength, G) elastic modulus, H) tensile strength, I) toughness and J) elongation changes as a function of PD-LAP, with the data shown as means ± SD (n = 3) (*: P < 0.05). Reproduced with permission [194]. Copyright 2020, Elsevier.
In the context of advancing tissue engineering, it is imperative to further investigate appropriate manufacturing processes or bio-ink formulations for GelMA hydrogel. This exploration aims to establish a cross-linked environment that is biologically compatible and a simulated bio-scaffold structure, all while ensuring the necessary mechanical strength for bioprinting and tissue engineering purposes. By achieving these objectives, modified GelMA hydrogel scaffolds would be capable of effectively restoring the conducive environment for cell growth, thereby enabling a more realistic simulation of cell growth and even the construction of complete tissues.
CRediT authorship contribution statement
Ao Guo: Writing - original draft, Investigation, Conceptualization. Shengting Zhang: Writing - review & editing, Resources. Runhuai Yang: Writing - review & editing, Supervision, Project administration, Conceptualization. Cong Sui: Writing - review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the study participants and research staff for their contributions and commitment to the present study. This study was supported by Natural Science Foundation of China (NSFC) [Nos. 62373004,], Anhui Universities Natural Science Research projects [2023AIHO53329] and Scientific research improvement Foundation of Anhui Medical University [2023xkjT002] and Fundamental and Clinical Research Collaboration Project of Anhui Medical University [2022xkjT019] and Research projects of Anhui Medical University [K2022038].
Contributor Information
Ao Guo, Email: guoao18895629003@163.com.
Shengting Zhang, Email: zhangshengting7@163.com.
Runhuai Yang, Email: yangrunhuai@ahmu.edu.cn.
Cong Sui, Email: suicong@ahmu.edu.cn.
Data availability
The data that has been used is confidential.
References
- 1.Lei L., Bai Y., Qin X., Liu J., Huang W., Lv Q. Current Understanding of hydrogel for drug release and tissue engineering. Gels. 2022;8:301. doi: 10.3390/gels8050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong H., Huang J., Wu J., Du J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2022;15:787–804. doi: 10.1007/s12274-021-3593-7. [DOI] [Google Scholar]
- 3.Levato R., Jungst T., Scheuring R.G., Blunk T., Groll J., Malda J. From shape to function: the next step in bioprinting. Adv. Mater. 2020;32 doi: 10.1002/adma.201906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao S., Zhao T., Wang J., Wang C., Du J., Ying L., Lin J., Zhang C., Hu W., Wang L., Xu K. Gelatin methacrylate (GelMA)-Based hydrogels for cell transplantation: an effective strategy for tissue engineering. Stem Cell Rev and Rep. 2019;15:664–679. doi: 10.1007/s12015-019-09893-4. [DOI] [PubMed] [Google Scholar]
- 5.Morgan F.L.C., Moroni L., Baker M.B. Dynamic bioinks to advance bioprinting. Adv. Healthcare Mater. 2020;9 doi: 10.1002/adhm.201901798. [DOI] [PubMed] [Google Scholar]
- 6.Rathan S., Dejob L., Schipani R., Haffner B., Möbius M.E., Kelly D.J. Fiber reinforced cartilage ECM functionalized bioinks for functional cartilage tissue engineering. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201801501. [DOI] [PubMed] [Google Scholar]
- 7.Xue B., Gu J., Li L., Yu W., Yin S., Qin M., Jiang Q., Wang W., Cao Y. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 2021;12:7156. doi: 10.1038/s41467-021-27529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian G., Liu Y., Yu M., Liang C., Yang D., Huang J., Zhao Q., Zhang W., Chen J., Wang Y., Xu P., Liu Z., Qi D. Electrostatic interaction-based high tissue adhesive, Stretchable Microelectrode arrays for the electrophysiological interface. ACS Appl. Mater. Interfaces. 2022;14:4852–4861. doi: 10.1021/acsami.1c18983. [DOI] [PubMed] [Google Scholar]
- 9.Takei T., Yoshihara R., Danjo S., Fukuhara Y., Evans C., Tomimatsu R., Ohzuno Y., Yoshida M. Hydrophobically-modified gelatin hydrogel as a carrier for charged hydrophilic drugs and hydrophobic drugs. Int. J. Biol. Macromol. 2020;149:140–147. doi: 10.1016/j.ijbiomac.2020.01.227. [DOI] [PubMed] [Google Scholar]
- 10.Hu W., Wang Z., Xiao Y., Zhang S., Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019;7:843–855. doi: 10.1039/C8BM01246F. [DOI] [PubMed] [Google Scholar]
- 11.Assmann A., Vegh A., Ghasemi-Rad M., Bagherifard S., Cheng G., Sani E.S., Ruiz-Esparza G.U., Noshadi I., Lassaletta A.D., Gangadharan S., Tamayol A., Khademhosseini A., Annabi N. A highly adhesive and naturally derived sealant. Biomaterials. 2017;140:115–127. doi: 10.1016/j.biomaterials.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvatz S., Wertheim L., Fleischer S., Shapira A., Dvir T. Channeled ECM-based nanofibrous hydrogel for engineering vascularized cardiac tissues. Nanomaterials. 2019;9:689. doi: 10.3390/nano9050689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 14.Nunes T.G., Ceballos L., Osorio R., Toledano M. Spatially resolved photopolymerization kinetics and oxygen inhibition in dental adhesives. Biomaterials. 2005;26:1809–1817. doi: 10.1016/j.biomaterials.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Nazir F., Ashraf I., Iqbal M., Ahmad T., Anjum S. 6-deoxy-aminocellulose derivatives embedded soft gelatin methacryloyl (GelMA) hydrogels for improved wound healing applications: in vitro and in vivo studies. Int. J. Biol. Macromol. 2021;185:419–433. doi: 10.1016/j.ijbiomac.2021.06.112. [DOI] [PubMed] [Google Scholar]
- 16.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Chan-Park M.B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials. 2010;31:1158–1170. doi: 10.1016/j.biomaterials.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 18.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benton J.A., DeForest C.A., Vivekanandan V., Anseth K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. 2009;15:3221–3230. doi: 10.1089/ten.TEA.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q., Chang C., Qian C., Xiao W., Zhu H., Guo J., Meng Z., Cui W., Ge Z. Photo-crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater. 2021;125:197–207. doi: 10.1016/j.actbio.2021.02.043. [DOI] [PubMed] [Google Scholar]
- 21.Curk T., Dobnikar J., Frenkel D. Rational design of molecularly imprinted polymers. Soft Matter. 2016;12:35–44. doi: 10.1039/C5SM02144H. [DOI] [PubMed] [Google Scholar]
- 22.Costa A.M.S., Mano J.F. Highly robust hydrogels via a fast, simple and cytocompatible dual crosslinking-based process. Chem. Commun. 2015;51:15673–15676. doi: 10.1039/C5CC05564D. [DOI] [PubMed] [Google Scholar]
- 23.Lai T.C., Yu J., Tsai W.B. Gelatin methacrylate/carboxybetaine methacrylate hydrogels with tunable crosslinking for controlled drug release. J. Mater. Chem. B. 2016;4:2304–2313. doi: 10.1039/C5TB02518D. [DOI] [PubMed] [Google Scholar]
- 24.Lee S., Tong X., Yang F. The effects of varying poly(ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism on protein accumulation in three-dimensional hydrogels. Acta Biomater. 2014;10:4167–4174. doi: 10.1016/j.actbio.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Hennink W.E., van Nostrum C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012;64:223–236. doi: 10.1016/j.addr.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Bupphathong S., Quiroz C., Huang W., Chung P.-F., Tao H.-Y., Lin C.-H. Gelatin methacrylate hydrogel for tissue engineering applications—a review on material modifications. Pharmaceuticals. 2022;15:171. doi: 10.3390/ph15020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S.H., Park J.Y., Ji Y.B., Ju H.J., Min B.H., Kim M.S. An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020;117:108–120. doi: 10.1016/j.actbio.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 30.Prendergast M.E., Burdick J.A. Computational modeling and experimental characterization of extrusion printing into suspension baths. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Garcia L.A., Rodriguez-Salvador M. Uncovering 3D bioprinting research trends: a keyword network mapping analysis. Int J Bioprint. 2018;4:147. doi: 10.18063/IJB.v4i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hossain Rakin R., Kumar H., Rajeev A., Natale G., Menard F., Li I.T.S., Kim K. Tunable metacrylated hyaluronic acid-based hybrid bioinks for stereolithography 3D bioprinting. Biofabrication. 2021;13 doi: 10.1088/1758-5090/ac25cb. [DOI] [PubMed] [Google Scholar]
- 33.Shao L., Gao Q., Zhao H., Xie C., Fu J., Liu Z., Xiang M., He Y. Fiber-based mini tissue with morphology-controllable GelMA microfibers. Small. 2018;14 doi: 10.1002/smll.201802187. [DOI] [PubMed] [Google Scholar]
- 34.Daly A.C., Pitacco P., Nulty J., Cunniffe G.M., Kelly D.J. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials. 2018;162:34–46. doi: 10.1016/j.biomaterials.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 35.Tsang K.M.C., Annabi N., Ercole F., Zhou K., Karst D., Li F., Haynes J.M., Evans R.A., Thissen H., Khademhosseini A., Forsythe J.S. Facile one-step micropatterning using photodegradable methacrylated gelatin hydrogels for improved cardiomyocyte organization and alignment. Adv. Funct. Mater. 2015;25:977–986. doi: 10.1002/adfm.201403124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen D.T., Fan Y., Akay Y.M., Akay M. Investigating glioblastoma angiogenesis using A 3D in vitro GelMA microwell platform. IEEE Trans. NanoBioscience. 2016;15:289–293. doi: 10.1109/TNB.2016.2528170. [DOI] [PubMed] [Google Scholar]
- 37.Cuvellier M., Ezan F., Oliveira H., Rose S., Fricain J.-C., Langouët S., Legagneux V., Baffet G. 3D culture of HepaRG cells in GelMa and its application to bioprinting of a multicellular hepatic model. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120611. [DOI] [PubMed] [Google Scholar]
- 38.Roche C.D., Sharma P., Ashton A.W., Jackson C., Xue M., Gentile C. Printability, durability, contractility and vascular network formation in 3D bioprinted cardiac endothelial cells using alginate-gelatin hydrogels. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.636257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barros N.R., Kim H.-J., Gouidie M.J., Lee K., Bandaru P., Banton E.A., Sarikhani E., Sun W., Zhang S., Cho H.-J., Hartel M.C., Ostrovidov S., Ahadian S., Hussain S.M., Ashammakhi N., Dokmeci M.R., Herculano R.D., Lee J., Khademhosseini A. Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication. 2021;13 doi: 10.1088/1758-5090/aba503. [DOI] [PubMed] [Google Scholar]
- 40.Jia L., Hua Y., Zeng J., Liu W., Wang D., Zhou G., Liu X., Jiang H. Bioprinting and regeneration of auricular cartilage using a bioactive bioink based on microporous photocrosslinkable acellular cartilage matrix. Bioact. Mater. 2022;16:66–81. doi: 10.1016/j.bioactmat.2022.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi H., Li Y., Xu K., Yin J. Advantages of photo-curable collagen-based cell-laden bioinks compared to methacrylated gelatin (GelMA) in digital light processing (DLP) and extrusion bioprinting. Mater Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daly A.C., Prendergast M.E., Hughes A.J., Burdick J.A. Bioprinting for the biologist. Cell. 2021;184:18–32. doi: 10.1016/j.cell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakr M.A., Sakthivel K., Hossain T., Shin S.R., Siddiqua S., Kim J., Kim K. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. 2022;110:708–724. doi: 10.1002/jbm.a.37310. [DOI] [PubMed] [Google Scholar]
- 44.Maher P.S., Keatch R.P., Donnelly K., Mackay R.E., Paxton J.Z. Construction of 3D biological matrices using rapid prototyping technology. Rapid Prototyp. J. 2009;15:204–210. doi: 10.1108/13552540910960307. [DOI] [Google Scholar]
- 45.Kurian A.G., Singh R.K., Patel K.D., Lee J.-H., Kim H.-W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022;8:267–295. doi: 10.1016/j.bioactmat.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen M., Zhong M., Johnson J.A. Light-controlled radical polymerization: mechanisms, methods, and applications. Chem. Rev. 2016;116:10167–10211. doi: 10.1021/acs.chemrev.5b00671. [DOI] [PubMed] [Google Scholar]
- 47.Fedorovich N.E., Oudshoorn M.H., van Geemen D., Hennink W.E., Alblas J., Dhert W.J.A. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30:344–353. doi: 10.1016/j.biomaterials.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 48.Nunes T.G., Ceballos L., Osorio R., Toledano M. Spatially resolved photopolymerization kinetics and oxygen inhibition in dental adhesives. Biomaterials. 2005;26:1809–1817. doi: 10.1016/j.biomaterials.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Zhu M., Wang Y., Ferracci G., Zheng J., Cho N.-J., Lee B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019;9:6863. doi: 10.1038/s41598-019-42186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X., Lang Q., Yildirimer L., Lin Z.Y., Cui W., Annabi N., Ng K.W., Dokmeci M.R., Ghaemmaghami A.M., Khademhosseini A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthcare Mater. 2016;5:108–118. doi: 10.1002/adhm.201500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miri A.K., Hosseinabadi H.G., Cecen B., Hassan S., Zhang Y.S. Permeability mapping of gelatin methacryloyl hydrogels. Acta Biomater. 2018;77:38–47. doi: 10.1016/j.actbio.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shirahama H., Lee B.H., Tan L.P., Cho N.-J. Precise tuning of facile one-pot gelatin methacryloyl (GelMA) synthesis. Sci. Rep. 2016;6 doi: 10.1038/srep31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y.-C., Lin R.-Z., Qi H., Yang Y., Bae H., Melero-Martin J.M., Khademhosseini A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012;22:2027–2039. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen T., Watkins K.E., Kishore V. Photochemically crosslinked cell‐laden methacrylated collagen hydrogels with high cell viability and functionality. J. Biomed. Mater. Res. 2019;107:1541–1550. doi: 10.1002/jbm.a.36668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Im G.-B., Lin R.-Z. Bioengineering for vascularization: trends and directions of photocrosslinkable gelatin methacrylate hydrogels. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1053491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 57.Aldana A., Malatto L., Rehman M., Boccaccini A., Abraham G. Fabrication of gelatin methacrylate (GelMA) scaffolds with nano- and micro-topographical and morphological features. Nanomaterials. 2019;9:120. doi: 10.3390/nano9010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoch E., Schuh C., Hirth T., Tovar G.E.M., Borchers K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J. Mater. Sci. Mater. Med. 2012;23:2607–2617. doi: 10.1007/s10856-012-4731-2. [DOI] [PubMed] [Google Scholar]
- 59.Mironi-Harpaz I., Wang D.Y., Venkatraman S., Seliktar D. Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012;8:1838–1848. doi: 10.1016/j.actbio.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 60.Monteiro N., Thrivikraman G., Athirasala A., Tahayeri A., França C.M., Ferracane J.L., Bertassoni L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018;34:389–399. doi: 10.1016/j.dental.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy S.V., Skardal A., Atala A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. 2013;101A:272–284. doi: 10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 62.Schuurman W., Levett P.A., Pot M.W., van Weeren P.R., Dhert W.J.A., Hutmacher D.W., Melchels F.P.W., Klein T.J., Malda J. Gelatin-Methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs: gelatin-methacrylamide hydrogels as potential biomaterials for fabrication. Macromol. Biosci. 2013;13:551–561. doi: 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 63.Tigner T.J., Rajput S., Gaharwar A.K., Alge D.L. Comparison of photo cross linkable gelatin derivatives and initiators for three-dimensional extrusion bioprinting. Biomacromolecules. 2020;21:454–463. doi: 10.1021/acs.biomac.9b01204. [DOI] [PubMed] [Google Scholar]
- 64.Montazerian H., Baidya A., Haghniaz R., Davoodi E., Ahadian S., Annabi N., Khademhosseini A., Weiss P.S. Stretchable and bioadhesive gelatin methacryloyl-based hydrogels enabled by in situ dopamine polymerization. ACS Appl. Mater. Interfaces. 2021;13:40290–40301. doi: 10.1021/acsami.1c10048. [DOI] [PubMed] [Google Scholar]
- 65.Bhattacharyya A., Janarthanan G., Kim T., Taheri S., Shin J., Kim J., Bae H.C., Han H.-S., Noh I. Modulation of bioactive calcium phosphate micro/nanoparticle size and shape during in situ synthesis of photo-crosslinkable gelatin methacryloyl based nanocomposite hydrogels for 3D bioprinting and tissue engineering. Biomater. Res. 2022;26:54. doi: 10.1186/s40824-022-00301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Occhetta P., Visone R., Russo L., Cipolla L., Moretti M., Rasponi M. VA-086 methacrylate gelatine photopolymerizable hydrogels: a parametric study for highly biocompatible 3D cell embedding. J. Biomed. Mater. Res. 2015;103:2109–2117. doi: 10.1002/jbm.a.35346. [DOI] [PubMed] [Google Scholar]
- 67.Karaoglu I.C., Kebabci A.O., Kizilel S. Optimization of gelatin methacryloyl hydrogel properties through an artificial neural network model. ACS Appl. Mater. Interfaces. 2023;15:44796–44808. doi: 10.1021/acsami.3c12207. [DOI] [PubMed] [Google Scholar]
- 68.Paul S., Schrobback K., Tran P.A., Meinert C., Davern J.W., Weekes A., Klein T.J. Photo-cross-linkable, injectable, and highly adhesive GelMA-glycol chitosan hydrogels for cartilage repair. Adv. Healthcare Mater. 2023 doi: 10.1002/adhm.202302078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leu Alexa R., Iovu H., Ghitman J., Serafim A., Stavarache C., Marin M.-M., Ianchis R. 3D-Printed gelatin methacryloyl-based scaffolds with potential application in tissue engineering. Polymers. 2021;13:727. doi: 10.3390/polym13050727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zu G., Meijer M., Mergel O., Zhang H., van Rijn P. 3D-Printable hierarchical nanogel-GelMA composite hydrogel system. Polymers. 2021;13:2508. doi: 10.3390/polym13152508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoch E., Hirth T., Tovar G.E.M., Borchers K. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J. Mater. Chem. B. 2013;1:5675–5685. doi: 10.1039/C3TB20745E. [DOI] [PubMed] [Google Scholar]
- 72.Vacanti J.P., Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354:S32–S34. doi: 10.1016/S0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 73.Stock U.A., Vacanti J.P. Tissue engineering: current state and prospects. Annu. Rev. Med. 2001;52:443–451. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 74.Lysaght M.J., Hazlehurst A.L. Tissue engineering: the end of the beginning. Tissue Eng. 2004;10:309–320. doi: 10.1089/107632704322791943. [DOI] [PubMed] [Google Scholar]
- 75.Atala A. Engineering organs. Curr. Opin. Biotechnol. 2009;20:575–592. doi: 10.1016/j.copbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Dhand A.P., Galarraga J.H., Burdick J.A. Enhancing biopolymer hydrogel functionality through interpenetrating networks. Trends Biotechnol. 2021;39:519–538. doi: 10.1016/j.tibtech.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang J., Huang Z., Liang Y., Yuan W., Bian L., Duan L., Rong Z., Xiong J., Wang D., Xia J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021;9:2620–2630. doi: 10.1039/D0BM02103B. [DOI] [PubMed] [Google Scholar]
- 78.Choe R., Devoy E., Kuzemchak B., Sherry M., Jabari E., Packer J.D., Fisher J.P. Computational investigation of interface printing patterns within 3D printed multilayered scaffolds for osteochondral tissue engineering. Biofabrication. 2022;14 doi: 10.1088/1758-5090/ac5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jonidi Shariatzadeh F., Solouk A., Bagheri Khoulenjani S., Bonakdar S., Mirzadeh H. Injectable and reversible preformed cryogels based on chemically crosslinked gelatin methacrylate (GelMA) and physically crosslinked hyaluronic acid (HA) for soft tissue engineering. Colloids Surf. B Biointerfaces. 2021;203 doi: 10.1016/j.colsurfb.2021.111725. [DOI] [PubMed] [Google Scholar]
- 80.Wang B., Díaz-Payno P.J., Browe D.C., Freeman F.E., Nulty J., Burdis R., Kelly D.J. Affinity-bound growth factor within sulfated interpenetrating network bioinks for bioprinting cartilaginous tissues. Acta Biomater. 2021;128:130–142. doi: 10.1016/j.actbio.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Hu Q., Lu R., Liu S., Liu Y., Gu Y., Zhang H. 3D printing GelMA/PVA interpenetrating polymer networks scaffolds mediated with CuO nanoparticles for angiogenesis. Macromol. Biosci. 2022;22 doi: 10.1002/mabi.202200208. [DOI] [PubMed] [Google Scholar]
- 82.Wu Z., Xie S., Kang Y., Shan X., Li Q., Cai Z. Biocompatibility evaluation of a 3D-bioprinted alginate-GelMA-bacteria nanocellulose (BNC) scaffold laden with oriented-growth RSC96 cells. Mater. Sci. Eng. C. 2021;129 doi: 10.1016/j.msec.2021.112393. [DOI] [PubMed] [Google Scholar]
- 83.Alarçin E., İzbudak B., Yüce Erarslan E., Domingo S., Tutar R., Titi K., Kocaaga B., Guner F.S., Bal‐Öztürk A. Optimization of methacrylated gelatin/layered double hydroxides nanocomposite cell‐laden hydrogel bioinks with high printability for 3D extrusion bioprinting. J. Biomed. Mater. Res. 2023;111:209–223. doi: 10.1002/jbm.a.37450. [DOI] [PubMed] [Google Scholar]
- 84.Fan Y., Yue Z., Lucarelli E., Wallace G.G. Hybrid printing using cellulose nanocrystals reinforced GelMA/HAMA hydrogels for improved structural integration. Adv. Healthcare Mater. 2020;9 doi: 10.1002/adhm.202001410. [DOI] [PubMed] [Google Scholar]
- 85.Shahabipour F., Tavafoghi M., Aninwene G.E., Bonakdar S., Oskuee R.K., Shokrgozar M.A., Potyondy T., Alambeigi F., Ahadian S. Coaxial 3D bioprinting of tri‐polymer scaffolds to improve the osteogenic and vasculogenic potential of cells in co‐culture models. J. Biomed. Mater. Res. 2022;110:1077–1089. doi: 10.1002/jbm.a.37354. [DOI] [PubMed] [Google Scholar]
- 86.Basara G., Ozcebe S.G., Ellis B.W., Zorlutuna P. Tunable human myocardium derived decellularized extracellular matrix for 3D bioprinting and cardiac tissue engineering. Gels. 2021;7:70. doi: 10.3390/gels7020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X., Yue Z., Winberg P.C., Lou Y.-R., Beirne S., Wallace G.G. 3D bioprinting dermal-like structures using species-specific ulvan. Biomater. Sci. 2021;9:2424–2438. doi: 10.1039/D0BM01784A. [DOI] [PubMed] [Google Scholar]
- 88.Martyniak K., Lokshina A., Cruz M.A., Karimzadeh M., Kemp R., Kean T.J. Biomaterial composition and stiffness as decisive properties of 3D bioprinted constructs for type II collagen stimulation. Acta Biomater. 2022;152:221–234. doi: 10.1016/j.actbio.2022.08.058. [DOI] [PubMed] [Google Scholar]
- 89.Li H., Tan Y.J., Liu S., Li L. Three-dimensional bioprinting of oppositely charged hydrogels with super strong interface bonding. ACS Appl. Mater. Interfaces. 2018;10:11164–11174. doi: 10.1021/acsami.7b19730. [DOI] [PubMed] [Google Scholar]
- 90.Mora-Boza A., Włodarczyk-Biegun M.K., del Campo A., Vázquez-Lasa B., Román J.S. Glycerylphytate as an ionic crosslinker for 3D printing of multi-layered scaffolds with improved shape fidelity and biological features. Biomater. Sci. 2020;8:506–516. doi: 10.1039/C9BM01271K. [DOI] [PubMed] [Google Scholar]
- 91.Luo Y., Shoichet M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 92.Thiele J., Ma Y., Bruekers S.M.C., Ma S., Huck W.T.S. 25th anniversary article: designer hydrogels for cell cultures: a materials selection guide. Adv. Mater. 2014;26:125–148. doi: 10.1002/adma.201302958. [DOI] [PubMed] [Google Scholar]
- 93.West J.L. Customized cell microenvironments. Nat. Mater. 2011;10:727–729. doi: 10.1038/nmat3132. [DOI] [PubMed] [Google Scholar]
- 94.Van den Steen P.E., Dubois B., Nelissen I., Rudd P.M., Dwek R.A., Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit. Rev. Biochem. Mol. Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 95.Yuan Z., Yuan X., Zhao Y., Cai Q., Wang Y., Luo R., Yu S., Wang Y., Han J., Ge L., Huang J., Xiong C. Injectable GelMA cryogel microspheres for modularized cell delivery and potential vascularized bone regeneration. Small. 2021;17 doi: 10.1002/smll.202006596. [DOI] [PubMed] [Google Scholar]
- 96.Dannert C., Stokke B.T., Dias R.S. Nanoparticle-hydrogel composites: from molecular interactions to macroscopic behavior. Polymers. 2019;11:275. doi: 10.3390/polym11020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu J., Wan H., Zhou H., Feng Y., Zhang L., Lyulin A.V. Formation mechanism of bound rubber in elastomer nanocomposites: a molecular dynamics simulation study. RSC Adv. 2018;8:13008–13017. doi: 10.1039/C8RA00405F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie M., Shi Y., Zhang C., Ge M., Zhang J., Chen Z., Fu J., Xie Z., He Y. In situ 3D bioprinting with bioconcrete bioink. Nat. Commun. 2022;13:3597. doi: 10.1038/s41467-022-30997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y., Chen H., Li J. Recent advances on gelatin methacrylate hydrogels with controlled microstructures for tissue engineering. Int. J. Biol. Macromol. 2022;221:91–107. doi: 10.1016/j.ijbiomac.2022.08.171. [DOI] [PubMed] [Google Scholar]
- 100.Klotz B.J., Gawlitta D., Rosenberg A.J.W.P., Malda J., Melchels F.P.W. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34:394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma H., Zou Y., Zhang S., Liu L., Yu J., Fan Y. Nanochitin and poly(N-isopropylacrylamide) interpenetrating network hydrogels for temperature sensor applications. Carbohydr. Polym. 2022;291 doi: 10.1016/j.carbpol.2022.119544. [DOI] [PubMed] [Google Scholar]
- 102.F M., G Y., J F., L X., T N., Z X., Yy Y., Y P., D X. Interpenetrating polymer network hydrogels formed using antibiotics as a dynamic crosslinker for treatment of infected wounds. Adv. Healthcare Mater. 2022;11 doi: 10.1002/adhm.202200902. [DOI] [PubMed] [Google Scholar]
- 103.Vorwald C.E., Gonzalez-Fernandez T., Joshee S., Sikorski P., Leach J.K. Tunable fibrin-alginate interpenetrating network hydrogels to support cell spreading and network formation. Acta Biomater. 2020;108:142–152. doi: 10.1016/j.actbio.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hong S., Sycks D., Chan H.F., Lin S., Lopez G.P., Guilak F., Leong K.W., Zhao X. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater. 2015;27:4035–4040. doi: 10.1002/adma.201501099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao I.-C., Moutos F.T., Estes B.T., Zhao X., Guilak F. Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv. Funct. Mater. 2013;23:5833–5839. doi: 10.1002/adfm.201300483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Co C., H A., K S., S B., Sh P., R A., Z J. Phototunable interpenetrating polymer network hydrogels to stimulate the vasculogenesis of stem cell-derived endothelial progenitors. Acta Biomater. 2021;122 doi: 10.1016/j.actbio.2020.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pacelli S., Rampetsreiter K., Modaresi S., Subham S., Chakravarti A.R., Lohfeld S., Detamore M.S., Paul A. Fabrication of a double-cross-linked interpenetrating polymeric network (IPN) hydrogel surface modified with polydopamine to modulate the osteogenic differentiation of adipose-derived stem cells. ACS Appl. Mater. Interfaces. 2018;10:24955–24962. doi: 10.1021/acsami.8b05200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao W., He J., Nichol J.W., Wang L., Hutson C.B., Wang B., Du Y., Fan H., Khademhosseini A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011;7:2384–2393. doi: 10.1016/j.actbio.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang X., Kim G., Kang M., Lee J., Seo J., Do J., Hong K., Cha J., Shin S., Bae H. Marine biomaterial-based bioinks for generating 3D printed tissue constructs. Mar. Drugs. 2018;16:484. doi: 10.3390/md16120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okada T., Uto K., Aoyagi T., Ebara M. A biomimetic approach to hormone resistant prostate cancer cell isolation using inactivated Sendai virus (HVJ-E) Biomater. Sci. 2016;4:96–103. doi: 10.1039/C5BM00375J. [DOI] [PubMed] [Google Scholar]
- 111.Schipani R., Scheurer S., Florentin R., Critchley S.E., Kelly D.J. Reinforcing interpenetrating network hydrogels with 3D printed polymer networks to engineer cartilage mimetic composites. Biofabrication. 2020;12 doi: 10.1088/1758-5090/ab8708. [DOI] [PubMed] [Google Scholar]
- 112.Gong J.P., Kurokawa T., Narita T., Kagata G., Osada Y., Nishimura G., Kinjo M. Synthesis of hydrogels with extremely low surface friction. J. Am. Chem. Soc. 2001;123:5582–5583. doi: 10.1021/ja003794q. [DOI] [PubMed] [Google Scholar]
- 113.Ateshian G.A. The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech. 2009;42:1163–1176. doi: 10.1016/j.jbiomech.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gong J.P., Katsuyama Y., Kurokawa T., Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003;15:1155–1158. doi: 10.1002/adma.200304907. [DOI] [Google Scholar]
- 115.Liu S., Li L. Ultrastretchable and self-healing double-network hydrogel for 3D printing and strain sensor. ACS Appl. Mater. Interfaces. 2017;9:26429–26437. doi: 10.1021/acsami.7b07445. [DOI] [PubMed] [Google Scholar]
- 116.Luo K., Yang Y., Shao Z. Physically crosslinked biocompatible silk-fibroin-based hydrogels with high mechanical performance. Adv. Funct. Mater. 2016;26:872–880. doi: 10.1002/adfm.201503450. [DOI] [Google Scholar]
- 117.Wang X., Schröder H.C., Müller W.E.G. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: towards a new paradigm in tissue engineering. J. Mater. Chem. B. 2018;6:2385–2412. doi: 10.1039/c8tb00241j. [DOI] [PubMed] [Google Scholar]
- 118.Gleghorn J.P., Lee C.S.D., Cabodi M., Stroock A.D., Bonassar L.J. Adhesive properties of laminated alginate gels for tissue engineering of layered structures. J. Biomed. Mater. Res. 2008;85A:611–618. doi: 10.1002/jbm.a.31565. [DOI] [PubMed] [Google Scholar]
- 119.Li H., Tan Y.J., Leong K.F., Li L. 3D bioprinting of highly thixotropic alginate/methylcellulose hydrogel with strong interface bonding. ACS Appl. Mater. Interfaces. 2017;9:20086–20097. doi: 10.1021/acsami.7b04216. [DOI] [PubMed] [Google Scholar]
- 120.Lee C.S.D., Gleghorn J.P., Won Choi N., Cabodi M., Stroock A.D., Bonassar L.J. Integration of layered chondrocyte-seeded alginate hydrogel scaffolds. Biomaterials. 2007;28:2987–2993. doi: 10.1016/j.biomaterials.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 121.Shin S.R., Bae H., Cha J.M., Mun J.Y., Chen Y.-C., Tekin H., Shin H., Zarabi S., Dokmeci M.R., Tang S., Khademhosseini A. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano. 2012;6:362–372. doi: 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ou Q., Huang K., Fu C., Huang C., Fang Y., Gu Z., Wu J., Wang Y. Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogel with osteoimmunomodulatory and antibacterial activity for bone regeneration. Chem. Eng. J. 2020;382 doi: 10.1016/j.cej.2019.123019. [DOI] [Google Scholar]
- 123.Zheng J., Zhao F., Zhang W., Mo Y., Zeng L., Li X., Chen X. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Mater. Sci. Eng., C. 2018;89:119–127. doi: 10.1016/j.msec.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 124.Gong J., Schuurmans C.C.L., van Genderen A.M., Cao X., Li W., Cheng F., He J.J., López A., Huerta V., Manríquez J., Li R., Li H., Delavaux C., Sebastian S., Capendale P.E., Wang H., Xie J., Yu M., Masereeuw R., Vermonden T., Zhang Y.S. Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat. Commun. 2020;11:1267. doi: 10.1038/s41467-020-14997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng Y., Chan K.H., Wang X.-Q., Ding T., Li T., Lu X., Ho G.W. Direct-ink-write 3D printing of hydrogels into biomimetic soft robots. ACS Nano. 2019;13:13176–13184. doi: 10.1021/acsnano.9b06144. [DOI] [PubMed] [Google Scholar]
- 126.Liang Q., Gao F., Zeng Z., Yang J., Wu M., Gao C., Cheng D., Pan H., Liu W., Ruan C. Coaxial scale‐up printing of diameter‐tunable biohybrid hydrogel microtubes with high strength, perfusability, and endothelialization. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202001485. [DOI] [Google Scholar]
- 127.Zhou L., Ramezani H., Sun M., Xie M., Nie J., Lv S., Cai J., Fu J., He Y. 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater. Sci. 2020;8:5020–5028. doi: 10.1039/D0BM00896F. [DOI] [PubMed] [Google Scholar]
- 128.Comeau P.A., Willett T.L. Triethyleneglycol dimethacrylate addition improves the 3D-printability and construct properties of a GelMA-nHA composite system towards tissue engineering applications. Mater. Sci. Eng., C. 2020;112 doi: 10.1016/j.msec.2020.110937. [DOI] [PubMed] [Google Scholar]
- 129.Zhu S., Li M., Wang Z., Feng Q., Gao H., Li Q., Chen X., Cao X. Bioactive glasses-based nanozymes composite macroporous cryogel with antioxidative, antibacterial, and pro-healing properties for diabetic infected wound repair. Adv. Healthcare Mater. 2023;12 doi: 10.1002/adhm.202302073. [DOI] [PubMed] [Google Scholar]
- 130.Zhou F., Xin L., Wang S., Chen K., Li D., Wang S., Huang Y., Xu C., Zhou M., Zhong W., Wang H., Chen T., Song J. Portable handheld “SkinPen” loaded with biomaterial ink for in situ wound healing. ACS Appl. Mater. Interfaces. 2023;15:27568–27585. doi: 10.1021/acsami.3c02825. [DOI] [PubMed] [Google Scholar]
- 131.Xavier J.R., Thakur T., Desai P., Jaiswal M.K., Sears N., Cosgriff-Hernandez E., Kaunas R., Gaharwar A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano. 2015;9:3109–3118. doi: 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- 132.Heo D.N., Ko W.-K., Bae M.S., Lee J.B., Lee D.-W., Byun W., Lee C.H., Kim E.-C., Jung B.-Y., Kwon I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B. 2014;2:1584–1593. doi: 10.1039/C3TB21246G. [DOI] [PubMed] [Google Scholar]
- 133.Xiang J., Bai Y., Huang Y., Lang S., Li J., Ji Y., Peng B., Liu G. A zwitterionic silver nanoparticle-incorporating injectable hydrogel with a durable and efficient antibacterial effect for accelerated wound healing. J. Mater. Chem. B. 2022;10:7979–7994. doi: 10.1039/d2tb01493a. [DOI] [PubMed] [Google Scholar]
- 134.Tan G., Zhou L., Ning C., Tan Y., Ni G., Liao J., Yu P., Chen X. Biomimetically-mineralized composite coatings on titanium functionalized with gelatin methacrylate hydrogels. Appl. Surf. Sci. 2013;279:293–299. doi: 10.1016/j.apsusc.2013.04.088. [DOI] [Google Scholar]
- 135.Cha C., Shin S.R., Gao X., Annabi N., Dokmeci M.R., Tang X.S., Khademhosseini A. Controlling mechanical properties of cell-laden hydrogels by covalent incorporation of graphene oxide. Small. 2014;10:514–523. doi: 10.1002/smll.201302182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu S., Yu C., Liu N., Zhao M., Chen Z., Liu J., Li G., Huang H., Guo H., Sun T., Chen J., Zhuang J., Zhu P. Injectable conductive gelatin methacrylate/oxidized dextran hydrogel encapsulating umbilical cord mesenchymal stem cells for myocardial infarction treatment. Bioact. Mater. 2022;13:119–134. doi: 10.1016/j.bioactmat.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu Y., Chen Z., Wang H., Guo J., Cai J., Chen X., Wei H., Qi J., Wang Q., Liu H., Zhao Y., Chai R. Conductive nerve guidance conduits based on morpho butterfly wings for peripheral nerve repair. ACS Nano. 2022;16:1868–1879. doi: 10.1021/acsnano.1c11627. [DOI] [PubMed] [Google Scholar]
- 138.Shin S.R., Jung S.M., Zalabany M., Kim K., Zorlutuna P., bok Kim S., Nikkhah M., Khabiry M., Azize M., Kong J., Wan K., Palacios T., Dokmeci M.R., Bae H., Shirley X., Tang, Khademhosseini A. Carbon-Nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7:2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vashist A., Kaushik A., Vashist A., Sagar V., Ghosal A., Gupta Y.K., Ahmad S., Nair M. Advances in carbon nanotubes-hydrogel hybrids in nanomedicine for therapeutics. Adv. Healthcare Mater. 2018;7 doi: 10.1002/adhm.201701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gloria A., Russo T., D'Amora U., Santin M., De Santis R., Ambrosio L. Customised multiphasic nucleus/annulus scaffold for intervertebral disc repair/regeneration. Connect. Tissue Res. 2020;61:152–162. doi: 10.1080/03008207.2019.1650037. [DOI] [PubMed] [Google Scholar]
- 141.Gaharwar A.K., Peppas N.A., Khademhosseini A. Nanocomposite hydrogels for biomedical applications: nanocomposite Hydrogels. Biotechnol. Bioeng. 2014;111:441–453. doi: 10.1002/bit.25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sällström N., Capel A., Lewis M.P., Engstrøm D.S., Martin S. 3D-printable zwitterionic nano-composite hydrogel system for biomedical applications. J. Tissue Eng. 2020;11 doi: 10.1177/2041731420967294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thoniyot P., Tan M.J., Karim A.A., Young D.J., Loh X.J. Nanoparticle-hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015;2 doi: 10.1002/advs.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee H.Y., Park H.K., Lee Y.M., Kim K., Park S.B. A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chem. Commun. 2007:2959–2961. doi: 10.1039/b703034g. [DOI] [PubMed] [Google Scholar]
- 145.Sasidharan A., Monteiro-Riviere N.A. Biomedical applications of gold nanomaterials: opportunities and challenges: biomedical application of gold nanomaterials. WIREs Nanomed Nanobiotechnol. 2015;7:779–796. doi: 10.1002/wnan.1341. [DOI] [PubMed] [Google Scholar]
- 146.Yu K., Huangfu H., Qin Q., Zhang Y., Gu X., Liu X., Zhang Y., Zhou Y. Application of bone marrow-derived macrophages combined with bone mesenchymal stem cells in dual-channel three-dimensional bioprinting scaffolds for early immune regulation and osteogenic induction in rat calvarial defects. ACS Appl. Mater. Interfaces. 2022;14:47052–47065. doi: 10.1021/acsami.2c13557. [DOI] [PubMed] [Google Scholar]
- 147.Xing F., Xiang Z., Rommens P.M., Ritz U. 3D bioprinting for vascularized tissue-engineered bone fabrication. Materials. 2020;13:2278. doi: 10.3390/ma13102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang P., Sun Y., Shi X., Shen H., Ning H., Liu H. 3D printing of tissue engineering scaffolds: a focus on vascular regeneration. Bio-Des. Manuf. 2021;4:344–378. doi: 10.1007/s42242-020-00109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scotti C., Hirschmann M.T., Antinolfi P., Martin I., Peretti G.M. Meniscus repair and regeneration: review on current methods and research potential. Eur. Cell. Mater. 2013;26:150–170. doi: 10.22203/ecm.v026a11. [DOI] [PubMed] [Google Scholar]
- 150.Maes C., Kobayashi T., Selig M.K., Torrekens S., Roth S.I., Mackem S., Carmeliet G., Kronenberg H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Criswell T.L., Corona B.T., Wang Z., Zhou Y., Niu G., Xu Y., Christ G.J., Soker S. The role of endothelial cells in myofiber differentiation and the vascularization and innervation of bioengineered muscle tissue in vivo. Biomaterials. 2013;34:140–149. doi: 10.1016/j.biomaterials.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hatch J., Mukouyama Y. Spatiotemporal mapping of vascularization and innervation in the fetal murine intestine: intestinal Neurovascular Patterning. Dev. Dynam. 2015;244:56–68. doi: 10.1002/dvdy.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gálvez-Montón C., Fernandez-Figueras M.T., Martí M., Soler-Botija C., Roura S., Perea-Gil I., Prat-Vidal C., Llucià-Valldeperas A., Raya Á., Bayes-Genis A. Neoinnervation and neovascularization of acellular pericardial-derived scaffolds in myocardial infarcts. Stem Cell Res. Ther. 2015;6:108. doi: 10.1186/s13287-015-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaully T., Kaufman-Francis K., Lesman A., Levenberg S. Vascularization—the conduit to viable engineered tissues. Tissue Eng. B Rev. 2009;15:159–169. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 155.Niklason L.E., Lawson J.H. Bioengineered human blood vessels. Science. 2020;370 doi: 10.1126/science.aaw8682. [DOI] [PubMed] [Google Scholar]
- 156.Augustin H.G., Koh G.Y. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357 doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 157.Chang W.G., Niklason L.E. A short discourse on vascular tissue engineering. NPJ Regen Med. 2017;2:7. doi: 10.1038/s41536-017-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bae H., Puranik A.S., Gauvin R., Edalat F., Carrillo-Conde B., Peppas N.A., Khademhosseini A. Building vascular networks. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu B., Wang D., Pan H., Gong T., Ren Q., Wang Z., Guo Y. Three-in-one customized bioink for islet organoid: GelMA/ECM/PRP orchestrate pro-angiogenic and immunoregulatory function. Colloids Surf. B Biointerfaces. 2023;221 doi: 10.1016/j.colsurfb.2022.113017. [DOI] [PubMed] [Google Scholar]
- 160.Souders C.A., Bowers S.L.K., Baudino T.A. Cardiac fibroblast: the renaissance cell. Circ. Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Banerjee I., Fuseler J.W., Price R.L., Borg T.K., Baudino T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 162.Foglia M.J., Poss K.D. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143:729–740. doi: 10.1242/dev.132910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kikuchi K., Poss K.D. Cardiac regenerative capacity and mechanisms. Annu. Rev. Cell Dev. Biol. 2012;28:719–741. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Acun A., Nguyen T.D., Zorlutuna P. In vitro aged, hiPSC-origin engineered heart tissue models with age-dependent functional deterioration to study myocardial infarction. Acta Biomater. 2019;94:372–391. doi: 10.1016/j.actbio.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bejleri D., Streeter B.W., Nachlas A.L.Y., Brown M.E., Gaetani R., Christman K.L., Davis M.E. A bioprinted cardiac patch composed of cardiac‐specific extracellular matrix and progenitor cells for heart repair. Adv. Healthcare Mater. 2018;7 doi: 10.1002/adhm.201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Borovjagin A.V., Ogle B.M., Berry J.L., Zhang J. From microscale devices to 3D printing: advances in fabrication of 3D cardiovascular tissues. Circ. Res. 2017;120:150–165. doi: 10.1161/CIRCRESAHA.116.308538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martins A.M., Eng G., Caridade S.G., Mano J.F., Reis R.L., Vunjak-Novakovic G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules. 2014;15:635–643. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Won Y.-W., Patel A.N., Bull D.A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627–5635. doi: 10.1016/j.biomaterials.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 169.Lin Y.-H., Chuang T.-Y., Chiang W.-H., Chen I.-W.P., Wang K., Shie M.-Y., Chen Y.-W. The synergistic effects of graphene-contained 3D-printed calcium silicate/poly-ε-caprolactone scaffolds promote FGFR-induced osteogenic/angiogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng., C. 2019;104 doi: 10.1016/j.msec.2019.109887. [DOI] [PubMed] [Google Scholar]
- 170.Sun B.K., Siprashvili Z., Khavari P.A. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 171.Yannas I.V., Burke J.F. Design of an artificial skin. I. Basic design principles. J. Biomed. Mater. Res. 1980;14:65–81. doi: 10.1002/jbm.820140108. [DOI] [PubMed] [Google Scholar]
- 172.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 173.Roberts C.D., Leaper D.J., Assadian O. The role of topical antiseptic agents within antimicrobial stewardship strategies for prevention and treatment of surgical site and chronic open wound infection. Adv. Wound Care. 2017;6:63–71. doi: 10.1089/wound.2016.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hussein K.H., Abdelhamid H.N., Zou X., Woo H.-M. Ultrasonicated graphene oxide enhances bone and skin wound regeneration. Mater. Sci. Eng., C. 2019;94:484–492. doi: 10.1016/j.msec.2018.09.051. [DOI] [PubMed] [Google Scholar]
- 175.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874–880. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 176.Zhou F., Hong Y., Liang R., Zhang X., Liao Y., Jiang D., Zhang J., Sheng Z., Xie C., Peng Z., Zhuang X., Bunpetch V., Zou Y., Huang W., Zhang Q., Alakpa E.V., Zhang S., Ouyang H. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120287. [DOI] [PubMed] [Google Scholar]
- 177.Silverberg J.L., Barrett A.R., Das M., Petersen P.B., Bonassar L.J., Cohen I. Structure-function relations and rigidity percolation in the shear properties of articular cartilage. Biophys. J. 2014;107:1721–1730. doi: 10.1016/j.bpj.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Buckley M.R., Gleghorn J.P., Bonassar L.J., Cohen I. Mapping the depth dependence of shear properties in articular cartilage. J. Biomech. 2008;41:2430–2437. doi: 10.1016/j.jbiomech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 179.Almarza A.J., Athanasiou K.A. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 2004;32:2–17. doi: 10.1023/B:ABME.0000007786.37957.65. [DOI] [PubMed] [Google Scholar]
- 180.Chung C., Burdick J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008;60:243–262. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pritzker K.P.H., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.-P., Revell P.A., Salter D., van den Berg W.B. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 182.Mouser V.H.M., Abbadessa A., Levato R., Hennink W.E., Vermonden T., Gawlitta D., Malda J. Development of a thermosensitive HAMA-containing bio-ink for the fabrication of composite cartilage repair constructs. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 184.Faroni A., Mobasseri S.A., Kingham P.J., Reid A.J. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015;82–83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 185.Asplund M., Nilsson M., Jacobsson A., von Holst H. Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology. 2009;32:217–228. doi: 10.1159/000197900. [DOI] [PubMed] [Google Scholar]
- 186.Moskow J., Ferrigno B., Mistry N., Jaiswal D., Bulsara K., Rudraiah S., Kumbar S.G. Review: bioengineering approach for the repair and regeneration of peripheral nerve. Bioact. Mater. 2019;4:107–113. doi: 10.1016/j.bioactmat.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Paprottka F.J., Wolf P., Harder Y., Kern Y., Paprottka P.M., Machens H.-G., Lohmeyer J.A. Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: meta-analysis and systematic review. Plast Surg Int. 2013;2013 doi: 10.1155/2013/704589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Griffin J.W., Hogan M.V., Chhabra A.B., Deal D.N. Peripheral nerve repair and reconstruction. J. Bone Joint Surg. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 189.Vickers N.J. Animal communication: when I'm calling you, will you answer too? Curr. Biol. 2017;27:R713–R715. doi: 10.1016/j.cub.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 190.Cui Y., Zhao Y., Tian Y., Zhang W., Lü X., Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 191.Saha K., Keung A.J., Irwin E.F., Li Y., Little L., Schaffer D.V., Healy K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Discher D.E., Janmey P., Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 193.Liang X., Xie L., Zhang Q., Wang G., Zhang S., Jiang M., Zhang R., Yang T., Hu X., Yang Z., Tian W. Gelatin methacryloyl-alginate core-shell microcapsules as efficient delivery platforms for prevascularized microtissues in endodontic regeneration. Acta Biomater. 2022;144:242–257. doi: 10.1016/j.actbio.2022.03.045. [DOI] [PubMed] [Google Scholar]
- 194.Rajabi N., Kharaziha M., Emadi R., Zarrabi A., Mokhtari H., Salehi S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant. J. Colloid Interface Sci. 2020;564:155–169. doi: 10.1016/j.jcis.2019.12.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.