Abstract
Mycobacterium tuberculosis H37Rv causes progressive disease in animals, whereas the H37Ra strain does not. The relevance of this difference in virulence to human infection is uncertain because these strains have been shown to have similar growth rates in human macrophages. To evaluate the intracellular growth of M. tuberculosis strains in macrophages under conditions similar to those encountered in vivo, we infected human monocyte-derived macrophages with H37Ra, H37Rv, or one of four isolates from tuberculosis patients at a low bacillus-to-macrophage ratio. H37Rv and the patient isolates grew significantly faster than H37Ra, based on the numbers of CFU and acid-fast bacilli. These findings did not result from extracellular mycobacterial growth, differential macrophage viability, or bacillary clumping. In contrast to other published results, these findings indicate that the virulence characteristics of M. tuberculosis strains in animal models are relevant to human tuberculosis infection.
A central issue in the pathogenesis of tuberculosis is the characterization of virulence determinants of Mycobacterium tuberculosis that are relevant to human disease. In guinea pigs and mice, the M. tuberculosis H37Rv strain is substantially more virulent than the attenuated H37Ra strain (1, 7). Because the manifestations of tuberculosis in guinea pigs and mice differ significantly from those in humans, it is uncertain if the difference in virulence between these two strains is relevant to human infection with M. tuberculosis. Furthermore, a recent study found that H37Ra and H37Rv grew at similar rates in human monocyte-derived macrophages, whereas H37Rv grew more rapidly in murine macrophages (8).
Quantitation of the intracellular growth of M. tuberculosis in human macrophages is technically demanding for several reasons. First, M. tuberculosis can destroy macrophages during the culture period, artifactually reducing the number of CFU in macrophage lysates. Second, the extracellular growth of M. tuberculosis may complicate the interpretation of results. Third, the clumping of M. tuberculosis is most marked for virulent strains and can artifactually reduce the number of CFU. To determine the growth characteristics of virulent and attenuated M. tuberculosis strains in human macrophages under conditions that mimic those in vivo, we infected monocyte-derived macrophages with very low concentrations of M. tuberculosis. This strategy minimized the effects of M. tuberculosis on macrophage viability and ensured that an adequate population of macrophages would allow continued intracellular mycobacterial growth.
MATERIALS AND METHODS
Preparation of monocyte-derived macrophages.
Peripheral blood mononuclear cells were isolated from buffy coat preparations by differential centrifugation, first over Ficoll-Paque (Pharmacia Fine Chemicals, Piscataway, N.J.) and then on Percoll gradients (Pharmacia, Uppsala, Sweden), and the monocyte fraction was plated in 24-well plates (Becton Dickinson, Lincoln Park, N.J.) at 5 × 105 cells/well in RPMI medium (GIBCO, Grand Island, N.Y.) containing 10% heat-inactivated human serum. After 2 h, cells were washed three times with RPMI medium to remove nonadherent cells. Adherent cells were 90 to 95% monocytes, based on Giemsa and nonspecific esterase stains. Adherent cells were cultured in 1 ml of RPMI medium with 10% heat-inactivated human serum without antibiotics and were allowed to mature into macrophages prior to infection with M. tuberculosis. At this point, each well contained approximately 4 × 105 cells, with >95% cell viability, based on Trypan blue exclusion.
In experiments for which acid-fast staining was performed, monocytes were cultured on slides in removable chambers (SonicSeal Slide wells; Nunc Inc., Naperville, Ill.) for 6 days prior to infection with M. tuberculosis.
Measurement of intracellular mycobacterial growth.
The M. tuberculosis strains used were two H37Ra isolates (no. 25177 from the American Type Culture Collection, Rockville, Md., and a second isolate kindly provided by John Belisle, Colorado State University, Fort Collins), one H37Rv isolate (provided by John Belisle), and four clinical isolates from tuberculosis patients (AS, ET, S29, and S51). Single-cell suspensions of mycobacteria were obtained by a modification of standard methods. Briefly, aliquots of frozen bacilli were cultured in 7H9 media for 7 days, pelleted at 4,500 × g for 15 min, and resuspended in RPMI medium. Clumps of bacilli were dispersed with an ultrasonic cell disrupter (Virtis Co., Gardiner, N.Y.). The sample was centrifuged at 400 × g, and the upper bacterial suspension was used in all experiments. Bacteria were counted in a Petroff-Hausser chamber. With this technique, 90 to 95% of the organisms were single bacilli, with the remaining 5 to 10% in small aggregates of up to five organisms. Mycobacterial viability, based on the number of CFU, was 50 to 60% for all M. tuberculosis strains.
Macrophages were infected in triplicate with M. tuberculosis at 104 bacilli/well. Based on 50% bacterial viability and an estimated 4 × 105 cells/well, the estimated multiplicity of infection was one live M. tuberculosis organism per 80 cells. After 24 h, monolayers were washed to remove extracellular bacilli, and this time point was considered day 0. Cells were cultured at 37°C and 5% CO2 for six additional days. Fresh RPMI medium and 10% human serum were added at day 6, and the cells were cultured for four more days. No antibiotics were ever added to the cultures.
We evaluated the growth of M. tuberculosis in macrophages on day 10 because this provided sufficient time for bacilli to grow by 2 log units and because macrophage viability was not reduced during this period. The numbers of CFU were determined on day 10 in all experiments. In some cases, the numbers of CFU were also measured on day 3 and day 6. The supernatant was aspirated, and macrophages were lysed with distilled water for 10 min, incubated with 0.07% sodium dodecyl sulfate (Sigma Chemical Co., St. Louis, Mo.) for 10 min, and neutralized with 20% bovine serum albumin (Sigma). Bacterial suspensions in cell lysates and supernatants were ultrasonically dispersed, serially diluted, and plated in triplicate on 7H10 agar plates, and CFU were counted after 3 weeks. The numbers of CFU reported for days 0, 3, and 6 represent CFU obtained from cell lysates. For days 3 and 6, the numbers of CFU in supernatants were 10- to 20-fold lower than those in cell lysates. CFU numbers reported for day 10 include CFU from both the supernatant and cell lysate, because at this time point a significant proportion of the macrophages were nonadherent and were present in the supernatant. These macrophages were viable, as assessed by Trypan blue exclusion, and when the supernatant was centrifuged at 200 × g to pellet the cells but not the extracellular bacilli, it was found that >90% of the CFU arose from the cell pellet. This indicates that essentially all mycobacteria were growing intracellularly in our experimental system.
In some experiments, macrophage viability after the culture period was assessed by using 0.25% trypsin (GIBCO BRL, Gaithersburg, Md.) and 1 mM EDTA (GIBCO BRL) for 30 min at 37°C to detach the macrophages, followed by Trypan blue exclusion to estimate the number and percentage of viable cells. Acid-fast staining was performed with the fluorescent auramine-rhodamine stain, according to the manufacturer’s instructions (Medical Chemical Corporation, Santa Monica, Calif.), and mycobacteria were counted with a fluorescence microscope.
Cytokine production.
In some experiments, supernatants of macrophages infected with M. tuberculosis were harvested, aliquoted, and frozen at −70°C. Concentrations of tumor necrosis factor alpha (TNF-α), interleukin 10 (IL-10), and IL-12 were measured by enzyme-linked immunosorbent assay, with antibodies from Pharmingen, San Diego, Calif. (for TNF-α and IL-10) and from Maurice Gately, Hoffmann-La Roche, Nutley, N.J. (for IL-12). The assay sensitivity was 20 pg/ml for TNF-α and IL-10 and 10 pg/ml for IL-12.
Statistical analysis.
The nonparametric Mann-Whitney rank sum test was used to compare different groups because the data were not normally distributed.
RESULTS
Infection of macrophages with M. tuberculosis.
To estimate the intracellular inoculum of M. tuberculosis, macrophages were lysed after 24 h of incubation with H37Ra (obtained from John Belisle), H37Rv, and two clinical isolates (day 0). The washes used to remove extracellular bacilli were pelleted and resuspended. Measurement of the numbers of CFU in washes and cell lysates revealed that approximately 80% of the bacteria were in the washes and 20% were intracellular. Acid-fast staining revealed that 0.1 to 0.2% of the macrophages contained bacilli. Sixty to eighty percent of the macrophages each contained one bacillus, and the remainder each contained two bacilli.
Mycobacterial growth in monocyte-derived macrophages, as measured by CFU.
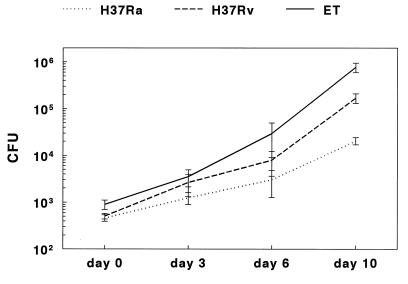
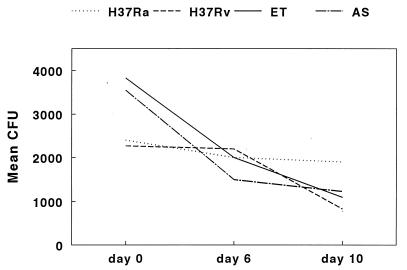
Figure 1 shows the growth curves for H37Ra, H37Rv, and clinical isolate ET in macrophages over 10 days, based on nine experiments. The numbers of CFU in macrophage lysates were comparable at day 0 for all M. tuberculosis strains (Fig. 1). After 10 days, the mean number of CFU (± the standard error) of H37Ra was 2.1 × 104 ± 0.4 × 104, compared to 1.7 × 105 ± 0.4 × 105 for H37Rv (P = 0.0001). The mean number of CFU for clinical isolate ET was 7.7 × 105 ± 1.8 × 105, which was significantly higher than mean number of CFU for H37Ra (P < 0.0001) or H37Rv (P = 0.0001). The mean numbers of CFU for the strains at days 3 and 6 paralleled the findings for day 10. To determine if the slower intracellular growth of strain H37Ra was an anomaly of that specific isolate, we evaluated a second H37Ra isolate (strain 25177 from the American Type Culture Collection) and obtained identical results (data not shown). As an alternative measure of mycobacterial growth rates, we calculated the generation times for these isolates, based on the mean numbers of CFU at day 0 and day 10. The mean generation time for H37Ra was 43.9 h, that for H37Rv was 28.6 h, and that for clinical isolate ET was 24.6 h.
FIG. 1.
Growth of M. tuberculosis in monocyte-derived macrophages. Monocyte-derived macrophages were cultured with H37Ra, H37Rv, and clinical isolate ET for 10 days, and numbers of CFU were measured at different time points. The values shown are the means and standard errors for nine separate experiments for days 0 and 10 and for four experiments for days 3 and 6.
In seven experiments, the mean number of CFU after 10 days of culture of a second clinical isolate, AS, was 4.0 × 105 ± 0.6 × 105, which was intermediate between values for H37Rv and strain ET. In three experiments, the mean numbers of CFU for two additional clinical strains, S29 and S51, were similar to those for strain AS and intermediate between those for H37Rv and strain ET (data not shown).
Mycobacterial growth in monocyte-derived macrophages, as measured by acid-fast staining.
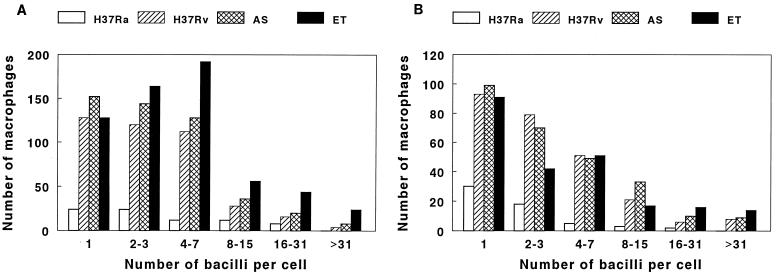
As an alternative measure of mycobacterial growth, we performed acid-fast staining on macrophages after infection with four M. tuberculosis strains for 10 days. Approximately 10,000 macrophages were counted in each of two experiments, and the numbers of macrophages infected with different numbers of mycobacteria were counted. The numbers of cells infected with single bacilli were three- to fivefold higher after infection with the virulent strains than after infection with H37Ra, and similar findings were noted for macrophages infected with 2 to 31 bacilli per cell. Macrophages containing 32 or more bacilli per cell were found only after infection by H37Rv or the clinical isolates (Fig. 2). The highest numbers of macrophages infected with 16 or more bacilli were found after infection with clinical isolate ET, paralleling results obtained by measurement of CFU.
FIG. 2.
Frequency distribution of the numbers of bacilli in macrophages infected with M. tuberculosis. Monocyte-derived macrophages were cultured with H37Ra, H37Rv, and clinical isolates AS and ET for 10 days. Acid-fast staining was performed, and approximately 10,000 macrophages were counted in each experiment. Panels A and B each represent a separate experiment and show the numbers of macrophages containing the indicated numbers of bacilli per cell after 10 days.
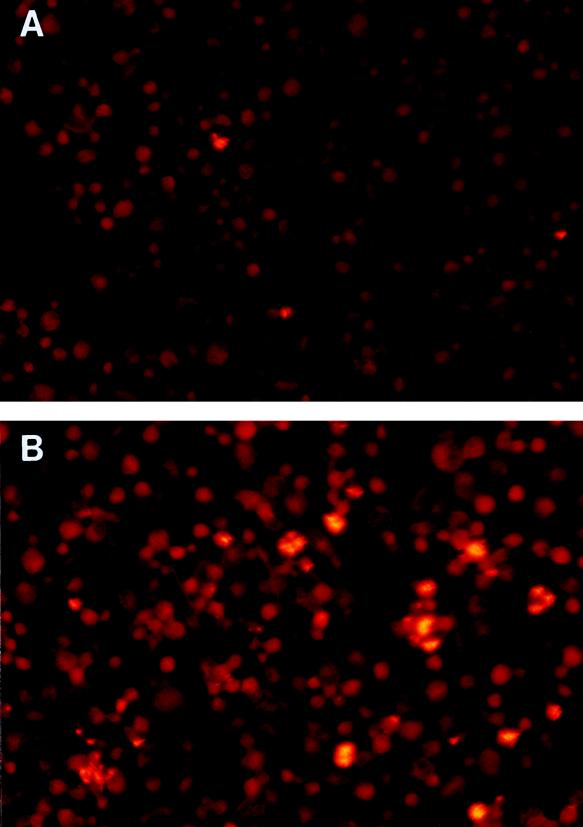
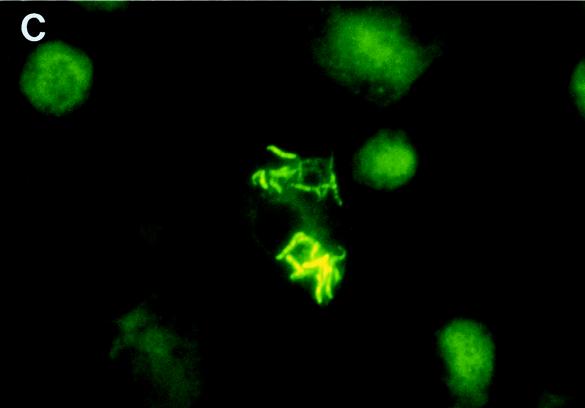
Figure 3 shows representative photomicrographs of macrophages infected with H37Ra and H37Rv. At low power, it was seen that H37Rv infected far more macrophages than H37Ra (Fig. 3A and B). The bacilli were associated with the cells and were not in the intercellular spaces. Higher-magnification views confirmed that the large clumps of H37Rv were intracellular (Fig. 3C).
FIG. 3.
Photomicrographs of M. tuberculosis H37Ra (A) and H37Rv (B and C) after 10 days of culture in monocyte-derived macrophages. Panels A and B are low-power-magnification views (×100) of M. tuberculosis cells in macrophages after staining with auramine-rhodamine. The photomicrographs were taken with a fluorescence microscope equipped with a 470- to 490-nm filter (U-MNB; Olympus, Tokyo, Japan). Bacilli are bright yellow, and macrophages are dull red. Panel C shows a high-power-magnification view (×1,000) of M. tuberculosis H37Rv in macrophages after staining with auramine-rhodamine. A 510- to 550-nm filter (U-MWG; Olympus) was used to view the slide. The bacilli are bright yellow, and the macrophages are green. A different filter was used for panel C because it permitted a more distinct view of cell and bacillary outlines at high magnification.
Evaluation of factors that could cause apparent differences in intracellular mycobacterial growth.
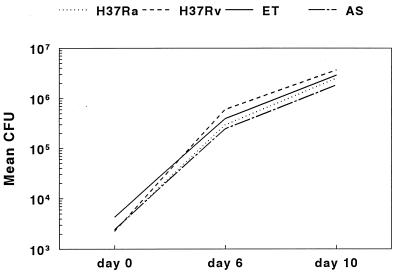
We considered the possibility that the differences in growth between H37Ra and virulent M. tuberculosis strains resulted from extracellular growth in culture medium. In three experiments, mean numbers of CFU declined over 10 days for H37Ra, H37Rv, and clinical isolates ET and AS grown in RPMI medium and 10% serum (Fig. 4). Standard errors were 12 to 40% of mean values and are not shown for clarity. To determine if macrophage-conditioned medium supported mycobacterial growth, we cultured the four M. tuberculosis strains in Transwell inserts in 2-ml wells containing macrophages. The inserts contained 0.4-μm pores that allow solute diffusion but no contact between bacilli and macrophages. The numbers of CFU of all mycobacterial strains declined during 10 days of culture, similar to results shown in Fig. 4 (data not shown).
FIG. 4.
Extracellular growth of M. tuberculosis in culture medium. H37Ra, H37Rv, and clinical isolates ET and AS were cultured in RPMI medium and 10% serum for 10 days. Mean numbers of CFU are shown for three experiments. Standard errors ranged from 12 to 40% of mean values and are not shown for the sake of clarity.
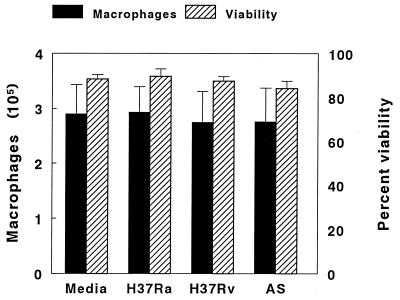
Because our experimental system did not support the extracellular growth of M. tuberculosis, reduced macrophage viability could decrease the number of cells available and artifactually lower the number of CFU isolated. Four experiments were performed to assess macrophage viability after 10 days of culture with medium alone and after infection with H37Ra, H37Rv, or clinical isolate AS. The mean numbers of macrophages recovered and their viabilities were similar for cells cultured in media alone or with the three M. tuberculosis strains (Fig. 5). Therefore, the reduced growth of H37Ra did not result from excessive destruction of infected macrophages.
FIG. 5.
Recovery and viability of macrophages after culture with M. tuberculosis. Monocyte-derived macrophages (4 × 105 per well) were cultured for 10 days in the presence of media alone or with H37Ra, H37Rv, or clinical isolate AS. The numbers of macrophages recovered and their percent viabilities, as assessed by Trypan blue exclusion, were determined. The values shown are the means and standard errors for four experiments.
Clumping of mycobacteria can artifactually reduce the number of CFU, so we evaluated the degree of clumping of H37Ra, H37Rv, and clinical isolates ET and AS. After growth of these strains in macrophages, aliquots of bacterial suspensions were stained with auramine-rhodamine prior to plating. For all strains, 95 to 99% of the bacilli were single, with the remainder in groups of two or three bacilli. Thus, the reduced growth of H37Ra was not due to excessive clumping of this strain.
To determine if there was an intrinsic difference in the growth characteristics of H37Ra, we cultured H37Ra, H37Rv, and clinical isolates ET and AS in 7H9 media. In four experiments, mean numbers of CFU for all strains were similar at 6 and 10 days (Fig. 6). Standard errors were 12 to 32% of mean values and are not shown for clarity.
FIG. 6.
Growth of M. tuberculosis in 7H9 media. H37Ra, H37Rv, and clinical isolates ET and AS were cultured in 7H9 media for 10 days. Mean numbers of CFU are shown for four experiments. Standard errors ranged from 12 to 32% of mean values and are not shown for the sake of clarity.
Cytokine production.
To determine if the intracellular growth rates of virulent and avirulent M. tuberculosis were related to patterns of cytokine production, we measured concentrations of TNF-α, IL-10, and IL-12 in supernatants of macrophages infected with H37Ra, H37Rv, and clinical isolate ET. At a ratio of one live M. tuberculosis organism to 80 cells, no detectable cytokine concentration was measured in cell culture supernatants after 1 to 10 days of culture, and reverse transcription-PCR showed cytokine mRNA at background levels (data not shown). To obtain measurable cytokine concentrations, we repeated the experiments with a ratio of five live M. tuberculosis organisms per cell. Maximal cytokine concentrations were present at 24 h. In three experiments, mean concentrations of TNF-α (± standard errors) were 3,607 ± 407 pg/ml for H37Ra, 3,283 ± 523 pg/ml for H37Rv, and 3,408 ± 707 pg/ml for clinical isolate ET (P > 0.10 for all comparisons). IL-10 and IL-12 concentrations were also similar in supernatants of macrophages infected with the three strains (data not shown).
DISCUSSION
Comprehensive knowledge of the genetic and molecular bases of virulence is central to understanding the pathogenesis of diseases due to intracellular pathogens. The virulent M. tuberculosis strain H37Rv and its avirulent counterpart H37Ra have been studied extensively in animal models, and the genetic and phenotypic differences between these strains are under intensive investigation. Nevertheless, no published data indicate that the differences in virulence between these strains that are observed in animals are applicable to humans. The current data demonstrate that H37Rv and M. tuberculosis isolates from patients grow more rapidly in human macrophages than avirulent H37Ra. This conclusion was arrived at by measuring numbers of CFU and by counting acid-fast bacilli in macrophages. Rigorous controls confirmed that these findings did not result from differential extracellular mycobacterial growth, macrophage viability, or clumping or from the intrinsic growth characteristics of the strains. These findings provide strong evidence that the virulence characteristics of M. tuberculosis strains in animal models are relevant to human tuberculosis.
In guinea pigs and mice, H37Ra and H37Rv both establish infection in the lung. Host defenses limit the growth of H37Ra and eventually clear the infection. In contrast, H37Rv replicates very rapidly and the infection is not contained (1, 7). H37Rv also grows more rapidly than H37Ra in murine peritoneal macrophages (8), a murine macrophage cell line (6), human HeLa cells (10), and human alveolar cell line A549 (2). Nevertheless, it is uncertain if the increased virulence of the H37Rv strain in animal models and in human cell lines is relevant to human tuberculosis because the growth of H37Rv and H37Ra has rarely been evaluated in human macrophages.
The limited published information on the growth characteristics of M. tuberculosis strains in human macrophages reflects the difficulties in establishing a reproducible assay system that eliminates confounding factors. Paul and colleagues observed similar growth rates for H37Ra and H37Rv in human macrophages (8). They used a 1:1 ratio of M. tuberculosis to macrophages, and numbers of CFU for both M. tuberculosis strains increased by approximately 1 log unit in 6 days. In contrast, we found that H37Rv and clinical M. tuberculosis isolates grew significantly more rapidly than H37Ra in human monocyte-derived macrophages. We used a bacillary inoculum which was 80-fold lower than that in the study of Paul et al., which minimized mycobacterial clumping during initial infection and which more closely mimicked conditions in vivo, where small numbers of M. tuberculosis cells can establish infection. The low percentage of infected macrophages minimized decreases in macrophage viability and ensured an adequate cell population to permit continued intracellular growth during culture. We observed mean increases in numbers of CFU of approximately 2 log units with H37Rv and 3 log units with a clinical isolate. The increased availability of macrophages relative to bacilli in our experimental system may have facilitated intracellular growth and enhanced detection of differences in intracellular growth rates between strains. In addition, Paul et al. reported that the percentage of infected macrophages did not change during culture, suggesting that they measured growth within individual cells but not the spread to adjacent cells. In our study, the percentage of infected macrophages increased 20- to 50-fold during culture, indicating that we measured the capacity of M. tuberculosis to grow intracellularly, lyse macrophages, and invade uninfected cells.
All four M. tuberculosis patient isolates evaluated in this study proliferated in human macrophages more rapidly than H37Rv, and this difference in proliferation was statistically significant for isolate ET (Fig. 1). This finding may parallel the increased virulence of M. tuberculosis strains observed after passage in animals. Additional studies are needed to determine if the growth rates of patient isolates correlate with disease severity or contagiousness.
The mechanisms underlying the differential growth rates of virulent and avirulent M. tuberculosis strains in human macrophages remain speculative. H37Ra and H37Rv are similar in their capacities to enter human macrophages (9), and phagocytosis is mediated through complement receptors. However, only H37Rv utilizes mannose receptors during phagocytosis, and this pathway for cell entry may influence bacillary intracellular growth rates.
Several monokines influence intracellular mycobacterial growth in mononuclear phagocytes. TNF-α inhibits the intracellular growth of M. tuberculosis in human alveolar macrophages (5), and IL-10 inhibits the growth of Mycobacterium avium in murine macrophages (3) but not in human monocytes (11). IL-12 plays a central role in initiating effective immunity against mycobacteria (4, 12), although the direct effects of IL-12 on intracellular mycobacterial growth are unknown. We found that avirulent and virulent M. tuberculosis strains elicited the production of similar amounts of TNF-α, IL-10, and IL-12, suggesting that these cytokines do not mediate the differences we observed in intracellular growth rates. Further studies are needed to characterize the genetic and molecular mechanisms that control the growth of M. tuberculosis in human macrophages. These studies will improve our understanding of the virulence of intracellular pathogens and facilitate development of novel measures to treat and prevent infectious diseases.
ACKNOWLEDGMENTS
We thank John Belisle for provision of M. tuberculosis H37Ra and H37Rv and Maurice Gately for materials to measure IL-12 concentrations.
This work was supported by the National Institutes of Health (AI27285 and AI36069). Mycobacterial products were provided through contract AI05074 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Alsaadi A, Smith D W. The fate of virulent and attenuated mycobacteria in guinea pigs infected by the respiratory route. Am Rev Respir Dis. 1973;107:1041–1046. doi: 10.1164/arrd.1973.107.6.1041. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez L E, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulton S A, Johnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch C S, Ellner J J, Russell D G, Rich E A. Complement receptor-mediated uptake and tumor necrosis factor-α-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–753. [PubMed] [Google Scholar]
- 6.McDonough K A, Kress Y, Bloom B R. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North R J, Izzo A A. Mycobacterial virulence. Virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul S, Laochumroonvorapong P, Kaplan G. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J Infect Dis. 1996;174:105–112. doi: 10.1093/infdis/174.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 10.Shepard C C. Growth characteristics of tubercle bacilli and certain other mycobacteria in HeLa cells. J Exp Med. 1957;105:39–48. doi: 10.1084/jem.105.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiratsuchi H, Hamilton B, Toosi Z, Ellner J J. Evidence against a role for interleukin-10 in the regulation of growth of Mycobacterium avium in human monocytes. J Infect Dis. 1996;173:410–417. doi: 10.1093/infdis/173.2.410. [DOI] [PubMed] [Google Scholar]
- 12.Sieling P A, Wang X-H, Gately M K, Oliveros J L, McHugh T, Barnes P F, Wolf S F, Golkar L, Yamamura M, Yogi Y, Uyemura K, Rea T H, Modlin R L. IL-12 regulates T helper type 1 cytokine responses in human infectious disease. J Immunol. 1994;153:3639–3647. [PubMed] [Google Scholar]