Abstract
Rectal cancer is one of the most malignant tumors, and postoperative recurrence and metastasis are the main reasons for treatment failure. Lymph node metastasis is the main metastatic pathway of rectal cancer. The present study aimed to investigate the role of lateral lymph node dissection (LLND) in patients with rectal cancer using a meta-analysis. Articles in Chinese and English related to the application of LLND in patients with rectal cancer were retrieved and eligible studies were selected for data analysis. Evaluation indicators included the 5-year survival rate, recurrence rate, urinary system function and operation time. The random-effects model was utilized for the analysis. A total of 10 studies that met the eligibility criteria were selected, comprising 2,272 patients, including 1,101 cases in the LLND group and 1,171 cases in the non-LLND group. No significant difference was found between the two groups in terms of local recurrence rate, 5-year disease-free survival (DFS) rate, and DFS rate at the follow-up. It is noteworthy that cases in the LLND group had no significantly longer overall survival, but had a higher 5-year survival rate. However, cases in the LLND group had a longer operation time and worse urinary dysfunction. The results remained consistent throughout separate analyses for different research quality sources. The present meta-analysis showed that LLND provided a specific advantage in prolonging survival time. However, it was associated with prolonged operation time and an increased incidence of urinary dysfunction.
Keywords: meta-analysis, lateral lymph node dissection, rectal cancer, overall survival, recurrence rate
Introduction
Rectal cancer is a malignant tumor type that ranks second in incidence among all digestive tract cancers. At present, surgical treatment of rectal cancer is the most important and effective treatment approach (1–3). Postoperative recurrence and metastasis are the main reasons for treatment failure (4). Lymph node metastasis is the main metastatic pathway of rectal cancer. Japanese scholars found that nearly 40% of rectal cancer patients had upward lymph node metastasis (upward metastasis along the mesorectal lymphatic vessels) and 10–25% of patients with rectal cancer had lateral lymph node metastasis (mainly along the obturator, internal iliac and external iliac artery) (5,6). Radical resection of rectal cancer following the ‘smash principle’ is routinely performed (7,8). However, whether lateral lymph node dissection (LLND) should be performed has always been the focus of debate (9). European and American scholars routinely do not carry out LLND. It is noteworthy that the presence of lateral lymph node metastasis indicates the breach of the rectum's proper fascia barrier. This occurrence is recognized as one of the signs of advanced rectal cancer and serves as a local indication of the tumor's spread throughout the body (10). Expanding the scope of the operation cannot control local recurrence and improve the 5-year survival rate. On the contrary, it may cause further complications, increase the risk of operation and reduce the quality of life after the operation (9). It is emphasized that attention should be paid to the protection of postoperative function during radical operation and routine preoperative radiotherapy should be performed for advanced rectal cancer with possible lateral metastasis (7). By contrast, in the 1970s, Japanese surgeons began to carry out extended radical resection of rectal cancer mainly by LLND (11). Of note, LLND may significantly improve the survival rate and reduce the recurrence rate, particularly for rectal cancer below the peritoneal reflux (12). In the present study, relevant studies were systematically and quantitatively analyzed in order to evaluate the value of LLND in the treatment of rectal cancer and to provide a reliable reference for further research.
Materials and methods
Eligibility criteria
The study's inclusion criteria were delineated as follows: i) All types of studies, irrespective of their randomization status; ii) comparative studies that assessed the efficacy of total mesorectal excision (TME) accompanied by LLND vs. TME alone in patients who underwent surgical intervention for rectal cancer; iii) adult patients who underwent curative surgery for rectal cancer via laparoscopic, laparoscopic-assisted, or open anterior resection or abdominoperineal resection; iv) the intervention of interest was defined as TME with LLND; v) the control of interest was defined as TME alone; vi) LLND encompassed the dissection of middle and inferior rectal, internal iliac, common iliac and obturator lymph nodes (13).
Primary and secondary outcomes
Primary and secondary endpoints were as follows: The primary outcome measures encompassed overall survival (OS), disease-free survival (DFS) and local recurrence. Secondary outcome measures included postoperative complications, sexual dysfunction, urinary dysfunction and operation time. Survival results were presented in two formats. First, time-to-event outcomes (time-to-event OS and time-to-event DFS) were employed to address uncertainties stemming from varying follow-up durations across the encompassed studies. Second, crude outcomes included OS at the maximum follow-up, 5-year OS, DFS at the maximum follow-up and 5-year DFS. These were used to convey the proportion of patients who survived by the conclusion of specific follow-up periods. In terms of recurrence as an outcome, distinct assessments were conducted for local recurrence, distant recurrence and total recurrence.
Search methods
A total of two investigators (BZ and NN) conducted a comprehensive search across multiple databases, including the Cochrane Central Register of Controlled Trials [CENTRAL (https://www.cochranelibrary.com/central/about-central)], Excerpta Medica database [EMBASE (https://www.embase.com/)], Medical Literature Analysis and Retrieval System Online [MEDLINE, (https://www.nlm.nih.gov/medline/medline_overview.html)], Cumulative Index to Nursing and Allied Health Literature [CINAHL (https://www.ebsco.com/products/research-databases/cinahl-database)], ClinicalTrials.gov, International Clinical Trials Registry Platform [ICTRP (https://www.who.int/clinical-trials-registry-platform)] and International Standard Randomised Controlled Trial Number Registry [ISRCTN (https://www.isrctn.com/)]. The final literature search was performed on November 13, 2019. In addition, to identify further eligible studies, the references cited within the full text of relevant articles were scrutinized. Of note, only studies published in English and Chinese were retrieved and assessed.
Study selection and data extraction
Following the execution of the search strategy in the aforementioned databases, a thorough examination of the titles and abstracts of the located articles was performed. Subsequently, the full texts of these identified studies were acquired and subjected to a rigorous selection process to ensure they met the eligibility criteria. To facilitate this process, a data collection proforma, designed in adherence to Cochrane's guidelines (https://training.cochrane.org/handbook), was developed and assessed using randomly selected studies. The data collection, as depicted in the tables and figures, encompassed various aspects of the eligible studies, including bibliometric parameters (e.g., the first author's name, publication year, journal name, follow-up duration and study design), baseline patient characteristics (such as rectal cancer stage, tumor location, neoadjuvant chemoradiotherapy, adjuvant chemotherapy, age and gender) and outcome measures. The entire procedure of study selection and data extraction was carried out by a pair of reviewers (BZ and NN). In cases of any discrepancies arising during the selection of included studies or the data extraction process, these matters were deliberated upon and resolved through discussion between the two reviewers. If necessary, a third reviewer (YY) was consulted for resolution. Importantly, this meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (http://www.prisma-statement.org/).
Risk of bias assessment
The methodological rigor of randomized controlled trials (RCTs) underwent evaluation utilizing the Cochrane tool, which assesses a study's quality by scrutinizing aspects such as selection, performance, detection, attrition, reporting and other potential sources of bias. Similarly, the methodological quality of nonrandomized comparative studies was appraised using the Risk of Bias In Non-randomized Studies of Interventions assessment tool (https://sites.google.com/site/riskofbiastool/welcome/home?authuser=0). This evaluation scrutinizes the study's quality in relation to potential biases stemming from confounding factors, participant selection, intervention classification, deviations from intended interventions, missing data, outcome measurement and selection of reported results. This comprehensive assessment procedure was conducted collaboratively by two reviewers (BZ and NN). Any disparities that emerged during the selection of included studies or the data extraction process were deliberated upon and resolved through discussion between these two reviewers. If necessary, a third reviewer (YY) was engaged to contribute to the resolution of such matters.
Data analysis
In terms of summary measures, the odds ratio (OR) was computed for dichotomous outcomes and the mean difference was calculated for continuous outcomes. The unit of analysis was the individual patient, and the analyses were conducted based on intent-to-treat information. Data analysis was undertaken using the Review Manager software (RevMan, version 5.3; The Nordic Cochrane Center). Random-effects modeling was employed for the analyses. Heterogeneity was quantified and reported as I2, as determined by the Cochrane Q test. I2 values were interpreted as follows: 0–50% indicating low-level heterogeneity, 50–75% indicating moderate-level heterogeneity and 75–100% indicating high-level heterogeneity. For outcomes reported by a minimum of 10 studies, it was attempted to create funnel plots and assess publication bias by examining the symmetry of these funnel plots.
Uncertainties associated with varying follow-up periods
In the studies that were included, time-to-event outcomes were examined. Initially, the natural logarithm of hazard ratios (HRs) was calculated. Subsequently, the natural logarithm of the upper and lower confidence limits provided for HRs was determined to derive standard errors from confidence intervals (CIs). Finally, the generic inverse variance method was applied to construct meta-analytical models for the computation of HRs on the natural logarithm scale.
Results
Study selection
A total of 2,786 articles were identified after applying the search strategy in the aforementioned databases. Among the studies that were identified through search of electronic databases, 2,755 articles were not relevant to the topic of this study and were excluded. The remaining 31 studies were relevant to the topic of this study. After assessing their full texts, 21 articles were excluded (4 studies were review articles, 6 articles did not use the TME technique, 6 articles were not published in Chinese or English, and 5 articles were a previously published version of an RCT). Finally, 10 studies were selected and involved in the meta-analysis (Fig. 1) (14–24). Tables I and II show the baseline characteristics of the included population. In total, 2,272 patients, including 1,101 cases in the LLND group and 1,171 cases in the non-LLND group, were involved in the pooled analysis. In most of the studies, the intervention involved performing TME along with LLND for rectal cancer (15–19,21–24). However, there was one study (21), in which TME alone was performed as the intervention.
Figure 1.
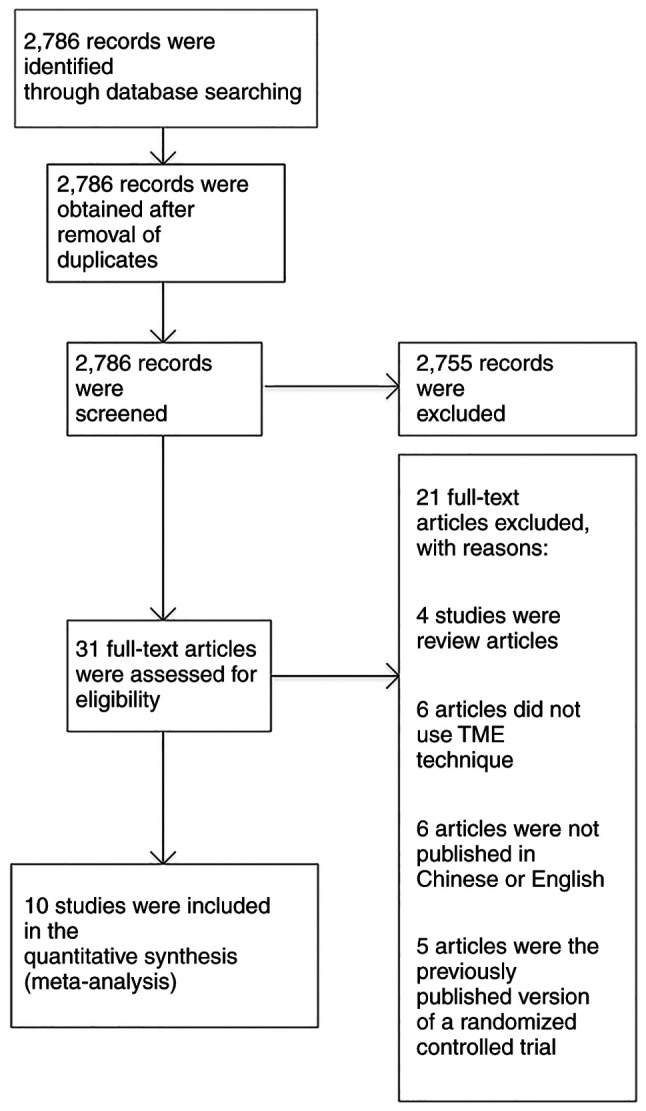
Preferred reporting items for systematic reviews and meta-analyses flow chart. TME, total mesorectal excision.
Table I.
Baseline characteristics of the included population.
| First author, year | Study design | Follow-up, years | Stage | Intervention | Comparison | (Refs.) |
|---|---|---|---|---|---|---|
| Motoki, 2018 | Randomized controlled trial | 5 | II or III | LLND+TME | TME | (15) |
| Zeng, 2019 | Retrospective cohort | 5 | II or III | LLND+TME | TME | (16) |
| Aisu, 2018 | Retrospective cohort | 5 | II or III | LLND+TME | TME | (17) |
| Zhang, 2020 | Retrospective cohort | 3 | II or III | LLND+TME | TME | (18) |
| Fujita, 2012 | Retrospective cohort | 3 | II or III | LLND+TME | TME | (19) |
| Ozawa, 2016 | Retrospective cohort | 5 | II or III | TME | TME | (20) |
| Ogura, 2017 | Retrospective cohort | 5 | II or III | LLND+TME | TME | (21) |
| Akiyoshi, 2014 | Retrospective cohort | 5 | II or III | LLND+TME | TME | (22) |
| Lin, 2020 | Retrospective cohort | 5 | II or III | LLND+TME | TME | (23) |
| Peacock, 2020 | Retrospective cohort | 5 | II or III | LLND+TME | TME | (24) |
Stage II: This stage is divided into IIa and IIb. In stage IIa, the tumor has penetrated through the rectal wall and may have reached the outer layers, while not having spread to the lymph nodes. In stage IIb, the tumor has grown into or through the outer wall of the rectum and there is evidence of involvement of nearby lymph nodes. Stage III: This stage is divided into IIIa, IIIb and IIIc. In stage IIIa, the tumor may or may not have grown into the outer layers of the rectal wall, while nearby lymph nodes are involved. In stage IIIb, the tumor has grown into the outer layers of the rectal wall and has spread to nearby lymph nodes. In stage IIIc, the tumor has penetrated through the outer wall of the rectum and has spread to nearby lymph nodes or tissues. TME, total mesorectal excision; LLND, lateral lymph node dissection.
Table II.
Baseline characteristics of the included population.
| First author | Location of rectal tumor | Age, years' (experimental vs. control group) | Male sex (experimental vs. control group) | (Refs.) |
|---|---|---|---|---|
| Motoki | Low | 67±14 vs. 63±12 | 167/231 vs. 112/156 | (15) |
| Zeng | Low | NR | 214/156 vs. 320/467 | (16) |
| Aisu | Low | 58±12 vs. 61±15 | 56/98 vs. 67/78 | (17) |
| Zhang | Low | 54±13 vs. 62±15 | 126/234 vs. 110/145 | (18) |
| Fujita | Low | 57±15 vs. 66±16 | 56/145 vs. 124/167 | (19) |
| Ozawa | Low | 63±12 | 145/123 vs. 112/134 | (20) |
| Ogura | Low | 58±13 vs. 61±11 | 78/99 vs. 89/102 | (21) |
| Akiyoshi | Low | 51±10 vs. 62±17 | NR | (22) |
| Lin | Low | 70±17 | 75/78 vs. 108/112 | (23) |
| Peacock | Low | 56±12 vs. 60±13 | 66/68 vs. 114/121 | (24) |
Values are expressed as the mean standard ± deviation or n/total. Location of rectal tumor is categorized as low (0–5 cm from the anal verge), middle (5.1–10 cm from the anal verge) and high (10.1–15 cm from the anal verge). NR, not reported.
Risk of bias in the included studies
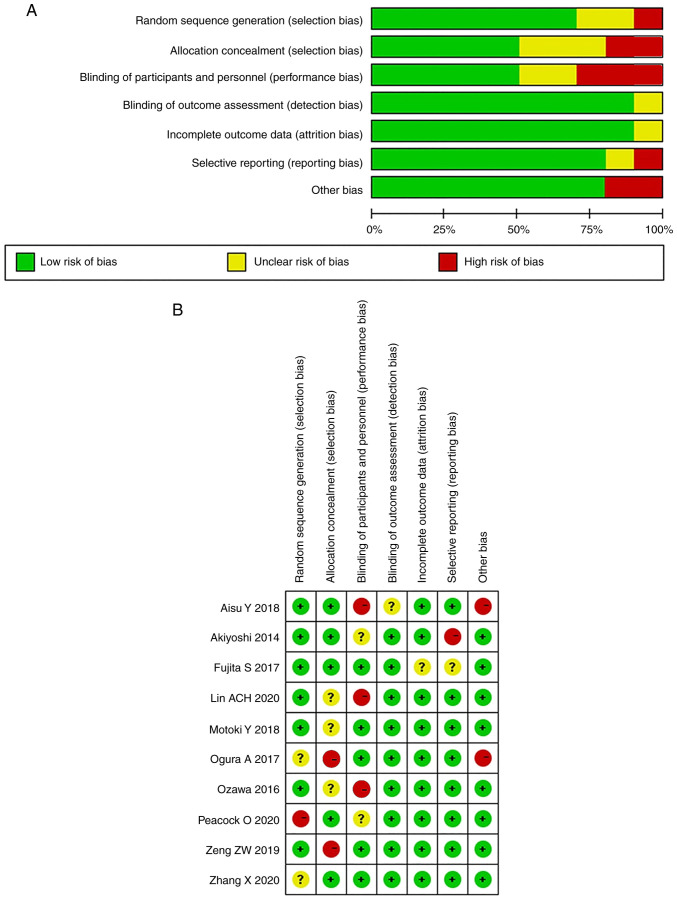
The risk of bias and quality of the evidence of the ROLARR trial (published as conference proceedings) as in a fully published study: Sufficient methodological details were available from the published protocol (25) and authors (contacted) confirmed that no deviations from the protocol had occurred in the conduct of the study. Of the included trials, none had a high risk of bias on all items, while 2 (15,17) were scored as low in 6 out of 7 domains. Three trials (21,23,24) were of unclear or low quality, with a high or unclear risk in at least 1 of 7 domains. Fig. 2 highlights the outcomes of methodological quality assessment based on the Cochrane tool and the Risk of Bias in Nonrandomized Studies of Interventions assessment tool. Of note, as illustrated in Fig. 3, the absence of studies that include inconclusive or negative research findings may contribute to publication bias in the current study.
Figure 2.
Summary of methodological quality assessment: (A) Randomized controlled trials and (B) non-randomized controlled trials.
Figure 3.
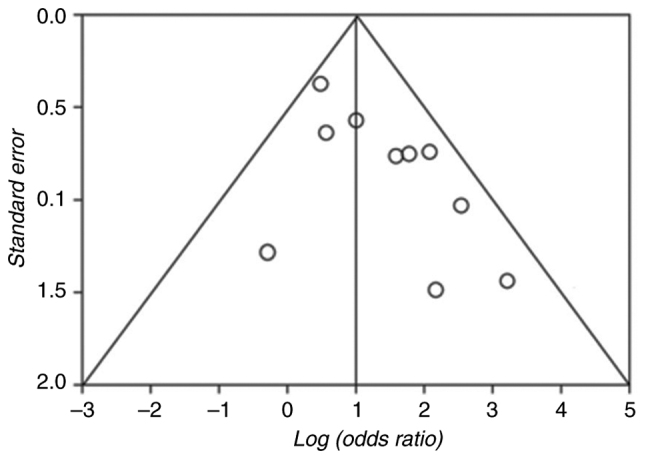
Funnel plot illustrating the results of publication bias assessment.
Outcome synthesis for comparison between the LLND+TME and TME groups
OS
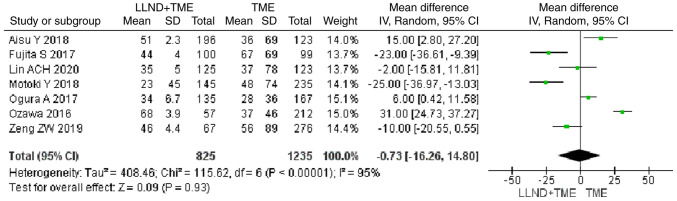
Analysis of time-to-event outcome from 7 studies revealed that cases in the LLND+TME group had longer OS than those in the TME group, but the difference was not significant [OR=−0.73, 95% CI=(−16.26, 14.80), P>0.05]. The reported heterogeneity was judged to be remarkable (Chi2=115.62, I2=95%) (Fig. 4).
Figure 4.
Forest plot comparing the results of analysis of time-to-event outcome between LLND+TME and TME groups [OR=−0.73, 95% CI=(−16.26, 14.80), P>0.05]. LLND, lateral lymph node dissection; df, degrees of freedom; IV, inverse variance; TME, total mesorectal excision.
5-year survival rate
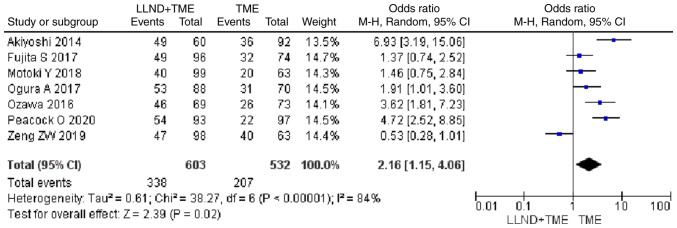
Analysis of 1,135 patients from 7 studies showed that cases in the LLND+TME group had a higher 5-year survival rate than those in the TME group [OR=2.16, 95% CI=(1.15, 4.06), P<0.05]. The reported heterogeneity was judged to be high (Chi2=38.27, I2=84%) (Fig. 5).
Figure 5.
Forest plot comparing the results of analysis of 5-year survival rate between LLND+TME and TME groups [OR=2.16, 95% CI=(1.15, 4.06), P<0.05]. LLND, lateral lymph node dissection; df, degrees of freedom; IV, inverse variance; TME, total mesorectal excision.
Local recurrence
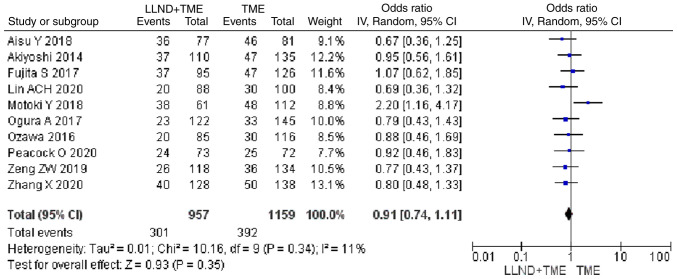
Analysis of 2,116 patients from 10 studies showed that patients in the either LLND+TME group or TME group were similar in terms of local recurrence rate [OR=0.91, 95% CI=(0.74, 1.11), P>0.05]. The reported heterogeneity was judged to be low (Chi2=10.16, I2=11%) (Fig. 6).
Figure 6.
Forest plot comparing the results of analysis of local recurrence rate between LLND+TME and TME groups [OR=0.91, 95% CI=(0.74, 1.11), P>0.05]. LLND, lateral lymph node dissection; df, degrees of freedom; IV, inverse variance; TME, total mesorectal excision.
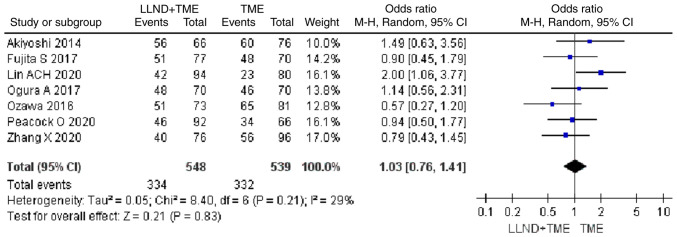
5-year DFS rate
Analysis of 1,931 patients from 7 studies showed that patients in the either LLND+TME group or TME group were similar in terms of 5-year DFS rate [OR=1.03, 95% CI=(0.76, 1.41), P>0.05]. The reported heterogeneity was judged to be low (Chi2=8.4, I2=29%) (Fig. 7).
Figure 7.
Forest plot comparing the results of analysis of 5-year DFS rate between LLND+TME and TME groups [OR=1.03, 95% CI=(0.76, 1.41), P>0.05]. LLND, lateral lymph node dissection; DFS, disease-free survival; df, degrees of freedom; IV, inverse variance; TME, total mesorectal excision.
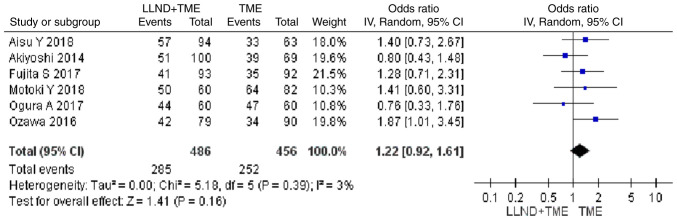
DFS at the maximum follow-up
Analysis of 942 patients from 6 studies indicated that patients in the either LLND+TME group or TME group were similar in terms of DFS at the maximum follow-up [OR=1.22, 95% CI=(0.92, 1.61), P>0.05]. The reported heterogeneity was judged to be low (Chi2=5.18, I2=3%) (Fig. 8).
Figure 8.
Forest plot comparing the results of analysis of DFS at the maximum follow-up between LLND+TME and TME groups [OR=1.22, 95% CI=(0.92, 1.61), P>0.05]. LLND, lateral lymph node dissection; DFS, disease-free survival; df, degrees of freedom; IV, inverse variance; TME, total mesorectal excision.
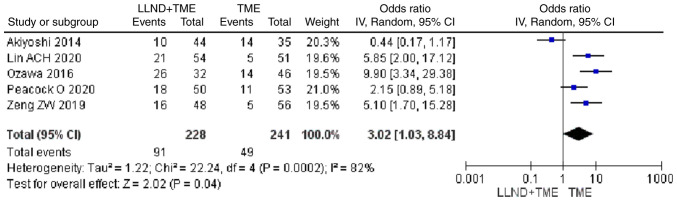
Urinary dysfunction
Analysis of 469 patients from 5 studies showed that patients in the LLND+TME group had a higher risk of urinary dysfunction compared with those in the TME group [OR=3.02, 95% CI=(1.03, 8.84), P<0.05]. The reported heterogeneity was judged to be high (Chi2=22.24, I2=82%) (Fig. 9).
Figure 9.
Forest plot comparing the results of analysis of urinary dysfunction between LLND+TME and TME groups [OR=3.02, 95% CI=(1.03, 8.84), P<0.05]. LLND, lateral lymph node dissection; df, degrees of freedom; IV, inverse variance; TME, total mesorectal excision.
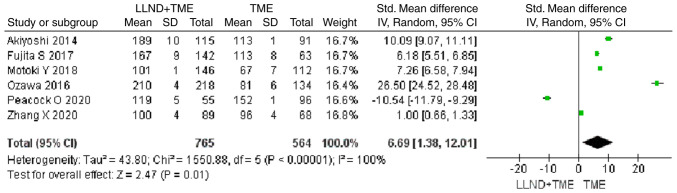
Operation time
Analysis of 1,329 patients from 6 studies revealed that patients in the LLND+TME group had a longer operation time compared with those in the TME group [OR=6.69, 95% CI=(1.38, 12.01), P<0.05]. The reported heterogeneity was judged to be remarkable (Chi2=1,550.88, I2=100%) (Fig. 10).
Figure 10.
Forest plot comparing the results of analysis of operation time between LLND+TME and TME groups [OR=6.69, 95% CI=(1.38, 12.01), P<0.05]. LLND, lateral lymph node dissection; df, degrees of freedom; IV, inverse variance; TME, total mesorectal excision.
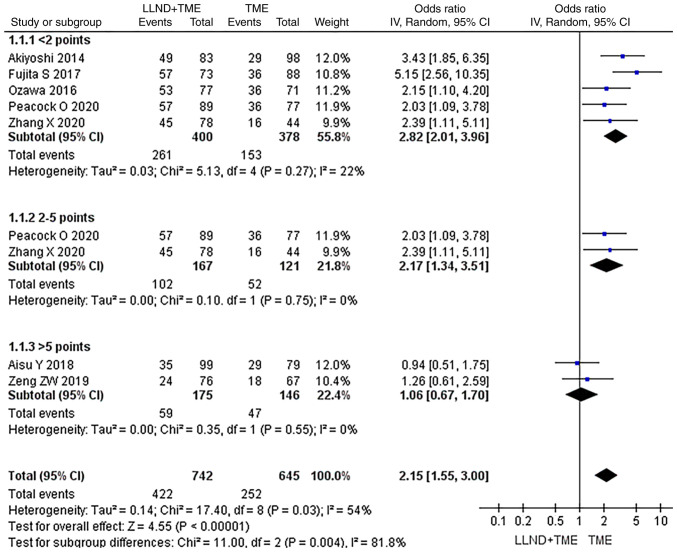
Subgroup analysis of OS data from different research quality sources
In the subgroup analysis, the OS data from different research sources were used and a subgroup analysis was performed according to the influential factors of OS. They were divided into subgroups with scores of <2, 2–5 and >5 points. In each subgroup, patients' OS was analyzed and it was found that in all subgroups, OS in the LLND+TME group was not significantly longer than that in the TME group (P>0.05), indicating high reliability of the analysis (Fig. 11).
Figure 11.
Forest plot for subgroup analysis of OS in the two groups. In all subgroups, OS in the LLND+TME group was not significantly longer than that in the TME group, indicating reliability of the results. df, degrees of freedom; IV, inverse variance; OS, overall survival; TME, total mesorectal excision; LLND, lateral lymph node dissection.
Discussion
The results of the present meta-analysis indicated that LLND did not increase OS, but increased the 5-year survival rate compared to the non-LLND group. The results of the sensitivity analysis were consistent, which suggested that the reliability of these results was reasonable. However, Lin et al (23) also conducted a meta-analysis and found that LLND could not improve the 5-year survival rate of patients with rectal cancer. The different results may be explained by the fact that the included subjects by Lin et al (23) were mainly from Japan, which were not representative, while the present study included eligible patients across the world, including China. The surgical criteria for rectal cancer in Japan were different from those in other regions and countries. Of note, the surgical criteria for rectal cancer in various countries can differ in several aspects. In the US, the standard approach to surgical criteria for rectal cancer primarily includes a combination of neoadjuvant therapies, such as chemotherapy and radiation, followed by surgical intervention. The objective is to shrink the tumor and increase the likelihood of complete resection. Depending on factors, such as tumor size, location and patient health, surgeons may perform surgical procedures [e.g., low anterior resection (LAR) or abdominoperineal resection (APR)]. The decision on whether to preserve the anal sphincter or create a permanent colostomy depends on the extent of the tumor and the patient's overall health (26). European countries, such as the UK, Germany and France, generally follow similar principles to the US. They emphasize neoadjuvant therapies to downsize the tumor before surgery. Minimally invasive techniques (e.g., laparoscopic or robotic-assisted surgeries) are frequently utilized to preserve sphincter function whenever possible. The decision between sphincter-preserving surgery and abdominoperineal resection depends on the tumor's location, size and patient preference (27). In South Korea, there is a strong emphasis on preserving anal sphincter function. Neoadjuvant chemoradiotherapy is a common approach, followed by sphincter-preserving surgeries, such as LAR. Surgeons often utilize techniques that prioritize maintaining bowel continuity while ensuring complete tumor removal (28). In Brazil, surgical criteria for rectal cancer focus on individualized treatment plans. Neoadjuvant therapies are employed and sphincter-preserving surgeries are preferred whenever possible. The decision between LAR and APR depends on the tumor's proximity to the anal sphincter and the patient's overall health (29). In Japan, the emphasis is often on sphincter-preserving surgeries to maintain bowel function and quality of life. They tend to prioritize preoperative chemoradiotherapy, followed by minimally invasive techniques (30). Second, the heterogeneity of the meta-analysis by Lin et al (23) was high. Thus, the findings of the present study are more convincing and representative of the general world population, and the results of Lin et al (23) are more representative of the Japanese population. Besides, the compared results of the perioperative situation in the present study showed that operation time in the LLND group was longer and this may be associated with a higher incidence of perioperative complications compared with the non-LLND group.
Georgiou et al (31) compared conventional surgery and expanded lymphadenectomy for rectal cancer in a meta-analysis in 2009, and in addition, various studies have summarized the effects of generalized lymph node dissection on the prognosis of colorectal cancer (32–34), including survival, DFS, operation time, and urinary tract function. Certain studies included in the meta-analysis by Georgiou et al (31) were excluded from the present study, as they did not use extended lymphadenectomy. Surprisingly, although the research by Georgiou et al (31) was conducted more than a decade ago, the level of evidence remains unchanged due to the lack of comparative data from RCTs. The data of non-RCTs may be complicated by certain bias factors, as it was assumed that patients who received LLND may have more severe disease than those who received TME alone (35,36). Yang et al (37) conducted a meta-analysis of the role of LLND in patients with rectal cancer after surgery. They found that LLND increased the risk of urinary dysfunction and yielded a longer operation time, which was consistent with the present findings. However, their results suggested that LLND did not contribute to longer 3 and 5-year cumulative OS. By contrast, the present study indicated that the LLND group had longer OS and a higher 5-year survival rate. This may be due to the inclusion of different studies and populations. The present study included the largest sample size and heterogeneity was controlled. There was no language restriction related to Chinese. Furthermore, in the present study, a subgroup analysis of different article quality sources was also performed and the same conclusion was drawn. It appears that the present findings were more promising. However, the present results remain to be further verified by more well-designed, large-scale studies.
The treatment of rectal cancer is different between the West and the East (38,39). In Western countries with low-density lipoprotein deficiency, it is common to use neoadjuvant chemotherapy and radiation therapy before TME. Although the use of neoadjuvant radiotherapy and chemotherapy has decreased the local recurrence rate of low rectal cancer after surgery, metastasis to lymph nodes in the lateral pelvis is still a major problem. In Western countries, the standard approach for treating rectal cancer is typically chemoradiotherapy followed by TME instead of LLND (40,41). However, in Eastern countries, particularly Japan, LLND is considered the preferred surgical procedure for locally advanced lower rectal cancer (31). Numerous analyses conducted by different authors have shown that LLND enhances OS rates and reduces local recurrence, as evidenced by historical control studies (42–44). The present study found that LLND has a certain beneficial effect on the survival of patients. The present subgroup analysis also confirmed that similar findings may be obtained from data sources of articles with different quality. The present subgroup analysis is currently the first one in which the impact factors of articles and journals are considered, which may decrease publication bias to a large extent. The current study also confirmed that LLND is associated with a longer operation time and the prognosis of urinary function may be adversely affected, consistent with the results of Yang et al (37) and Georgiou et al (31). It is thought that urinary dysfunction may be improved by minimally invasive procedures. This suggests that LLND is recommended for colorectal cancer.
The present study also has certain limitations that were summarized as follows: i) Due to the limitation of the number and level of existing clinical trials, only two studies included in the present analysis were prospective RCTs, and the remaining eight were non-RCTs, which may produce selection bias, implementation bias and measurement bias; ii) these studies were conducted in different clinical centers and the surgery was performed by different surgeons, which may produce bias; iii) the lack of studies containing inconclusive or negative research results may be a cause of publication bias in the present study. Consequently, the present findings remain to be further verified by additional studies.
In conclusion, LLND provided a specific advantage in terms of increasing 5-year survival rate, while LLND was associated with prolonged operation time and increased incidence of urinary dysfunction.
Acknowledgements
Not applicable.
Funding Statement
This study was funded by the Youth Fund of Peking University International Hospital Research Grant (YN2020QN08).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Conception and design: BZ and YZ. Administrative support: YZ; Provision of study materials or patients: BZ and YZ. Collection and assembly of data: BZ, NN and YY. Data analysis and interpretation and manuscript writing: BZ, NN, YY and YZ. BZ and YZ confirm the authenticity of all the raw data (the pooled data). All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Silva-Fisher JM, Dang HX, White NM, Strand MS, Krasnick BA, Rozycki EB, Jeffers GGL, Grossman JG, Highkin MK, Tang C, et al. Long non-coding RNA RAMS11 promotes metastatic colorectal cancer progression. Nat Commun. 2020;11:2156. doi: 10.1038/s41467-020-15547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones WF, Ahnen DJ, Schroy PC., III Improving on-time colorectal cancer screening through lead time messaging. Cancer. 2020;126:247–252. doi: 10.1002/cncr.32535. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho MR, Reis RL, Oliveira JM. Dendrimer nanoparticles for colorectal cancer applications. J Mater Chem B. 2020;8:1128–38. doi: 10.1039/C9TB02289A. [DOI] [PubMed] [Google Scholar]
- 4.Azzam N, AlRuthia Y, Alharbi O, Aljebreen A, Almadi M, Alarfaj M, Alsaleh K, Almasoud A, Alsharidah M, Alseneidi S, et al. Predictors of survival among colorectal cancer patients in a low incidence area. Cancer Manag Res. 2020;12:451–459. doi: 10.2147/CMAR.S233215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahm SH, Blinman P, Butler S, Tan SYC, Vardy J. Factors associated with fear of cancer recurrence in breast and colorectal cancer survivors: A cross-sectional study of cancer survivors. Asia Pac J Clin Oncol. 2021;17:222–229. doi: 10.1111/ajco.13434. [DOI] [PubMed] [Google Scholar]
- 6.He E, Lew JB, Egger S, Banks E, Ward RL, Beral V, Canfell K. Factors associated with participation in colorectal cancer screening in Australia: Results from the 45 and up study cohort. Prev Med. 2018;106:185–193. doi: 10.1016/j.ypmed.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Shen MH, Chen LP, Ho TF, Shih YY, Huang CS, Chie WC, Huang CC. Validation of the Taiwan Chinese version of the EORTC QLQ-CR29 to assess quality of life in colorectal cancer patients. BMC Cancer. 2018;18:353. doi: 10.1186/s12885-018-4312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng K, Chen X, Xu MU, Liu X, Hu X, Xu T, Sun H, Pan Y, He B, Wang S. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018;9:417. doi: 10.1038/s41419-018-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Crespo A, García-Suárez O, Fernández-Vega I, Solis-Hernandez MP, García B, Castañón S, Quirós LM. Heparan sulfate proteoglycans undergo differential expression alterations in left sided colorectal cancer, depending on their metastatic character. Bmc Cancer. 2018;18:687. doi: 10.1186/s12885-018-4597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Li XY, Hu P, Ding YS. lncRNA NORAD contributes to colorectal cancer progression by inhibition of miR-202-5p. Oncol Res. 2018;26:1411–1418. doi: 10.3727/096504018X15190844870055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Chen X, Cheng R, Yang F, Yu M, Wang C, Cui S, Hong Y, Liang H, Liu M, et al. The Jun/miR-22/HuR regulatory axis contributes to tumourigenesis in colorectal cancer. Mol Cancer. 2018;17:11. doi: 10.1186/s12943-017-0751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Wang C, Yu Y, Singh D, Yang L, Zhou Z. Lateral lymph node dissection reduces local recurrence of locally advanced lower rectal cancer in the absence of preoperative neoadjuvant chemoradiotherapy: A systematic review and meta-analysis. World J Surg Oncol. 2020;18:304. doi: 10.1186/s12957-020-02078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHSN Patient Safety Component Manual, U.S, corp-author. Centers for disease control and prevention, national healthcare safety network. New York; USA: 2023. [Google Scholar]
- 14.Hajibandeh S, Hajibandeh S, Matthews J, Palmer L, Maw A. Meta-analysis of survival and functional outcomes after total mesorectal excision with or without lateral pelvic lymph node dissection in rectal cancer surgery. Surgery. 2020;168:486–496. doi: 10.1016/j.surg.2020.04.063. [DOI] [PubMed] [Google Scholar]
- 15.Motoki Y, Sugimoto K, Sakisaka H, Nakano K, Kan K, Nakaguchi K, Doi S. A case of laparoscopic surgery for advanced rectal cancer with lateral lymph node metastasis resected after neoadjuvant chemotherapy. Gan To Kagaku Ryoho. 2018;45:2357–2359. (In Japanese) [PubMed] [Google Scholar]
- 16.Zeng ZW, Zhang XW, Chen JJ, Huang L, Luo SL, Kang L. Transanal lateral lymph node dissection surgery for 5 cases of mid-low rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22:781–785. doi: 10.3760/cma.j.issn.1671-0274.2019.08.014. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 17.Aisu Y, Kato S, Kadokawa Y, Yasukawa D, Kimura Y, Takamatsu Y, Kitano T, Hori T. Feasibility of extended dissection of lateral pelvic lymph nodes during laparoscopic total mesorectal excision in patients with locally advanced lower rectal cancer: A single-center pilot study after neoadjuvant chemotherapy. Med Sci Monit. 2018;24:3966–3977. doi: 10.12659/MSM.909163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Zhang Y, Wei M, Wang M, Yang X, Deng X, Wang Z. Letter to the editor regarding ‘Does adding lateral pelvic lymph node dissection to neoadjuvant chemotherapy improve outcomes in low rectal cancer?’. Int J Colorectal Dis. 2020;35:2139–2140. doi: 10.1007/s00384-020-03683-y. [DOI] [PubMed] [Google Scholar]
- 19.Fujita S, Mizusawa J, Kanemitsu Y, Saito N, Kinugasa Y, Kanemitsu Y, Ohue M, Fujii S, Shiozawa M, Yamaguchi T, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): Results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012;13:616–621. doi: 10.1016/S1470-2045(12)70158-4. [DOI] [PubMed] [Google Scholar]
- 20.Ozawa H, Kotake K, Hosaka M, Hirata A, Sugihara K. Impact of lateral pelvic lymph node dissection on the survival of patients with T3 and T4 low rectal cancer. World J Surg. 2016;40:1492–1499. doi: 10.1007/s00268-016-3444-y. [DOI] [PubMed] [Google Scholar]
- 21.Ogura A, Akiyoshi T, Nagasaki T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Ueno M, Kuroyanagi H. Feasibility of laparoscopic total mesorectal excision with extended lateral pelvic lymph node dissection for advanced lower rectal cancer after preoperative chemoradiotherapy. World J Surg. 2017;41:868–875. doi: 10.1007/s00268-016-3762-0. [DOI] [PubMed] [Google Scholar]
- 22.Akiyoshi T, Ueno M, Matsueda K, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Unno T, Kano A, Kuroyanagi H, et al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol. 2014;21:189–196. doi: 10.1245/s10434-013-3216-y. [DOI] [PubMed] [Google Scholar]
- 23.Lin ACH, Hakim A, Kellish AS, Singh P, Wozniak M, Kwiatt M, Gaughan J, Hong YK. Inguinal lymph node dissection does not improve overall survival in anal cancer nodal disease. J Surg Res. 2020;255:13–22. doi: 10.1016/j.jss.2020.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Peacock O, George JC. The landmark series: Management of lateral lymph nodes in locally advanced rectal cancer. Ann Surg Oncol. 2020;27:2723–2731. doi: 10.1245/s10434-020-08639-8. [DOI] [PubMed] [Google Scholar]
- 25.Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Edlin R, Hulme C, Brown J. Southampton (UK): NIHR Journals Library; 2019. Robotic-assisted surgery compared with laparoscopic resection surgery for rectal cancer: The ROLARR RCT. [DOI] [PubMed] [Google Scholar]
- 26.Xynos E, Tekkis P, Gouvas N, Vini L, Chrysou E, Tzardi M, Vassiliou V, Boukovinas I, Agalianos C, Androulakis N, et al. Clinical practice guidelines for the surgical treatment of rectal cancer: A consensus statement of the hellenic society of medical oncologists (HeSMO) Ann Gastroenterol. 2016;29:103–126. doi: 10.20524/aog.2016.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babaei M, Balavarca Y, Jansen L, Gondos A, Lemmens V, Sjövall A, Brge Johannesen T, Moreau M, Gabriel L, Gonçalves AF, et al. Minimally invasive colorectal cancer surgery in Europe: Implementation and outcomes. Medicine (Baltimore) 2016;95:e3812. doi: 10.1097/MD.0000000000003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong SY. Surgical management of colorectal cancer. J Korean Med Assoc. 2010;53:569–581. doi: 10.5124/jkma.2010.53.7.569. [DOI] [Google Scholar]
- 29.Valadão M, Cesar D, Véo CAR, Araújo RO, do Espirito Santo GF, Oliveira de Souza R, Aguiar S, Jr, Ribeiro R, de Castro Ribeiro HS, de Souza Fernandes PH, Oliveira AF. Brazilian society of surgical oncology: Guidelines for the surgical treatment of mid-low rectal cancer. J Surg Oncol. 2022;125:194–216. doi: 10.1002/jso.26676. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, Tekkis P. Extended lymphadenectomy versus conventional surgery for rectal cancer: A meta-analysis. Lancet Oncol. 2009;10:1053–1062. doi: 10.1016/S1470-2045(09)70224-4. [DOI] [PubMed] [Google Scholar]
- 32.Yokota M, Morikawa A, Nagahisa Y, Okabe M, Kitagawa H, Kawamoto K. Combined use of curved scissors and the soft coagulation system in robot-assisted lateral lymph node dissection for rectal cancer-a video vignette. Colorectal Dis. 2020;22:2359–2360. doi: 10.1111/codi.15371. [DOI] [PubMed] [Google Scholar]
- 33.Wong KY, Tan AM. Short term outcomes of minimally invasive selective lateral pelvic lymph node dissection for low rectal cancer. World J Gastrointest Surg. 2020;12:178–189. doi: 10.4240/wjgs.v12.i4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabra H, Alimoradi M, El-Helou E, Azaki R, Khairallah M, Kfoury T. Perforated sigmoid colon cancer presenting as an incarcerated inguinal hernia: A case report. Int J Surg Case Rep. 2020;72:108–111. doi: 10.1016/j.ijscr.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hojo D, Murono K, Nozawa H, Kawai K, Hata K, Tanaka T, Ishihara S. Utility of a three-dimensional printed pelvic model for lateral pelvic lymph node dissection. Int J Colorectal Dis. 2020;35:905–910. doi: 10.1007/s00384-020-03534-w. [DOI] [PubMed] [Google Scholar]
- 36.Tsukamoto S, Fujita S, Kanemitsu Y. Author response to: Beyond T, N and M: Can lateral lymph node dissection treat tumour deposits in advanced low rectal carcinoma? Br J Surg. 2020;107:e291. doi: 10.1002/bjs.11743. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Yang S, Hu T, Gu C, Wei M, Deng X, Wang Z, Zhou Z. What is the role of lateral lymph node dissection in rectal cancer patients with clinically suspected lateral lymph node metastasis after preoperative chemoradiotherapy? A meta-analysis and systematic review. Cancer Med. 2020;9:4477–4489. doi: 10.1002/cam4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young R, Rajkomar A, Smart P, Warrier S. Robotic-assisted complete mesocolic excision, central vascular ligation and para-aortic lymph node dissection in multifocal carcinoid: A case report and technical description. Int J Surg Case Rep. 2020;67:262–266. doi: 10.1016/j.ijscr.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asari M, Aoyama T, Koumori K, Uchiyama M, Maezawa Y, Sawazaki S, Numata M, Tamagawa H, Sato T, Osaragi T, et al. The efficacy of D3 lymph node dissection in elderly patients with colorectal cancer. Gan To Kagaku Ryoho. 2020;47:259–261. (In Japanese) [PubMed] [Google Scholar]
- 40.van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ, Dutch Colorectal Cancer Group Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-Year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 41.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Ueno M, Azekura K, Ohta H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43((10 Suppl)):S59–S68. doi: 10.1007/BF02237228. [DOI] [PubMed] [Google Scholar]
- 43.Moriya Y, Sugihara K, Akasu T, Fujita S. Importance of extended lymphadenectomy with lateral node dissection for advanced lower rectal cancer. World J Surg. 1997;21:728–732. doi: 10.1007/s002689900298. [DOI] [PubMed] [Google Scholar]
- 44.Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Miyoshi M, Kajiwara Y, Sato T, Shimazaki H, Hase K. Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg. 2007;245:80–87. doi: 10.1097/01.sla.0000225359.72553.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.