Abstract
Immune checkpoint inhibitors (ICIs) are commonly utilized in tumor treatment. However, they still have limitations, including insufficient effectiveness and unavoidable adverse events. It has been demonstrated that gut microbiota can influence the effectiveness of ICIs, although the precise mechanism remains unclear. Gut microbiota plays a crucial role in the formation and development of the immune system. Gut microbiota and their associated metabolites play a regulatory role in immune balance. Tumor occurrence and development are linked to their ability to evade recognition and destruction by the immune system. The purpose of ICIs treatment is to reinitiate the immune system's elimination of tumor cells. Thus, the immune system acts as a communication bridge between gut microbiota and ICIs. Varied composition and characteristics of gut microbiota result in diverse outcomes in ICIs treatment. Certain gut microbiota-related metabolites also influence the therapeutic efficacy of ICIs to some extent. The administration of antibiotics before or during ICIs treatment can diminish treatment effectiveness. The utilization of probiotics and fecal transplantation can partially alter the outcome of ICIs treatment. The present review synthesized previous studies to examine the association between gut microbiota and ICIs, elucidated the role of gut microbiota and its associated factors in ICIs treatment, and offered direction for future research.
Keywords: gut microbiota, immune checkpoint inhibitors, immune system, immunotherapy, probiotics, fecal transplantation
1. Introduction
Immunotherapy has emerged as a rapidly advancing treatment for tumors in recent years. Immunotherapy is based on the tumor immune escape mechanism, which manipulates the immune system to reactivate the antitumor immune response and overcome the pathways that lead to tumor escape (1). Current immunotherapy methods encompass immune checkpoint inhibitors (ICIs), adoptive cell therapy, oncolytic viruses and cancer vaccines. Among them, ICIs, including antibodies against programmed cell death protein 1 (PD-1), its ligand PD-L1, cytotoxic T lymphocyte antigen-4 (CTLA-4), lymphocyte activation gene 3 (LAG3), T cell immunoglobulin and mucin domain 3 (TIM3), and indoleamine 2, 3-dioxygenase 1 (IDO), have been widely and rapidly developed in clinical practice, achieving satisfactory results (2). However, there are still several shortcomings in the treatment with ICIs. Only a small number of tumor patients respond to ICIs, and there is still the possibility of drug resistance. Moreover, it is unable to address the disease progression and life-threatening nature for most cancer patients. Additionally, ICIs rely on the activation of autoimmune function to eliminate tumors, and these mechanisms may affect the self-tolerance of healthy tissues, leading to immune side effects known as immune-related adverse events (irAEs) (3). Gut microbiota plays a significant role in the physiological and pathological processes of the human organism. As a current research hotspot, it has made substantial progress in various fields. The potential connection between gut microbiota and ICIs has been extensively investigated in recent years, encompassing the relationship between gut microbiota and its associated metabolites, the clinical efficacy of ICIs, the correlation between gut microbiota and adverse events related to ICIs, the impact of antibiotic application on ICIs, and the application and effectiveness of probiotics and fecal transplantation in clinical practice (4). While the specific mechanism by which gut microbiota influences the treatment of ICIs remains unclear, the current research indicates that gut microbiota may serve as a crucial target for regulating the efficacy of ICIs, making its practical application in clinical settings highly promising (5). The present review examined the relationship between gut microbiota and ICIs, provided a summary of the current research progress, and explored the potential interaction mechanisms and future prospects between these factors.
2. Gut microbiota and the immune system
Gut microbiota
The gut microbiota is a vast microecosystem that includes bacteria, archaea, fungi and viruses. Each person carries up to 1014 microbial species, ~99% of which are bacteria. The primary species are Firmicutes and Bacteroidetes, followed by Actinobacteria and Verrucomicrobiota (6,7). The gut microbiota is closely linked to the activities of life and plays a crucial role in numerous metabolic processes. For instance, the microbiota in the colon encodes a plethora of carbohydrate-active enzymes, allowing them to break down non-digestible dietary residues and release short-chain fatty acids. These microbes assist in the synthesis of micronutrients such as vitamin K, vitamin B12, biotin, folic acid and pantothenic acid, in addition to aiding in the absorption of calcium, magnesium and iron. The gut microbiota can also regulate intestinal endocrine function, nerve signals, bone mineral density, provide biogenic energy, synthesize neurotransmitters, and metabolize bile. Furthermore, the gut microbiota plays a crucial role in the maturation and sustained expression of the host immune response (8,9).
Gut microbiota and the development of the immune system
The influence of gut microbiota on the immune system begins during early life. The immune system develops in a relatively sterile fetal environment during early life, with its primary exposure to antigens derived from the newly established microbial community on the mucosal surface of the newborn. Exposure to microbes during early life can result in lifelong changes in the immune system (10). The infant receives natural passive immunity from the mother through the placental route during pregnancy. The maternal gut microbiota profile can influence the composition of immune cells in infants. The enrichment of Dialister, Escherichia and Ruminococcus in the maternal gut microbiota is associated with a lower proportion of granulocytes and a higher proportion of central naïve CD4+ T cells (CD4+/CD45RA+/CD31−) and naïve regulatory T cells (Treg) (CD4+/CD45RA+/FoxP3low) in cord blood (11). Maternal dietary habits and breastfeeding after birth can also impact the regulation of immune factors in infants (12,13). The gut microbiome undergoes significant changes before the age of 2.5 years under the influence of various factors, but it gradually stabilizes afterward and remains relatively constant throughout the lifetime of an individual (14). Different age groups exhibit distinct gut microbiota profiles, with Bifidobacterium being more prevalent in infants and children, while Megamosna and Peptoniphilus are relatively enriched in the elderly (15). For instance, Akkermansia is more abundant in the gut microbiota of frail elderly individuals. It is positively correlated with the elevation of interleukin 6 and can elevate serum inflammatory factor levels, as well as increase intestinal permeability (16). In conclusion, the influence of gut microbiota on the immune system plays a crucial role in life development and may affect both physiological and pathological processes.
Gut microbiota influence the immune balance
Increasing evidence suggests that gut microbiota can regulate the proliferation and expression of immune cells, particularly the balance between T helper cell 17 (Th17) and Treg cells (17). Th17 cells contribute to autoimmunity and inflammation, while Treg cells inhibit immune responses and maintain immune homeostasis. Both cell types initially differentiate from naive CD4 T cells under the influence of tumor growth factor (TGF)-β (18). A previous study demonstrated that the balance between Th17 and Treg cells in the lamina propria of the mouse small intestine is influenced by the presence of Cytophaga-Flavobacter-Bacteroidetes bacteria. Specifically, Th17 cell differentiation is associated with the presence of these bacteria, while germ-free mice exhibit an increase in Treg cells in the lamina propria (19). Furthermore, a previous study has demonstrated that the presence of mixed Clostridium in mice leads to an upregulation of Treg cell abundance and function in the colon. This effect is attributed to the creation of a transforming growth factor-β enriched environment (20). Thus, it is plausible that Th17/Treg cells are regulated and proliferated by various species of gut microbiota. Research has demonstrated that Bacteroides fragilis (B. fragilis) can stimulate the proliferation of Treg cells through Toll-like receptor 2 (TLR2), consequently suppressing the activity of Th17 cells. As for the mechanism of action, a symbiotic factor known as polysaccharide A (PSA) produced by B. fragilis has been identified as a key player. PSA, a representative immunomodulatory molecule of symbiotic nature, can activate CREB-dependent transcription of anti-inflammatory genes through the coordinated activation of TLR2 and Dectin-1. This activation leads to the production of the immunomodulatory cytokine IL-10 by CD4+ Treg cells. Consequently, immune tolerance is achieved, and it may serve as a mechanism for intestinal commensal bacteria to evade the immune system (21,22). In conclusion, gut microbiota plays a significant role in regulating the balance between proinflammatory responses and immune regulation, despite the precise underlying mechanisms remaining unclear.
Gut microbiota-associated metabolites affect the immune system
Increasing evidence supports the role of gut microbiota-derived metabolites in immune system regulation. The majority of metabolites associated with gut microbiota have been found to be involved in immune regulation, attenuating immune responses, and potentially contributing to immune tolerance. Extensive research on short-chain fatty acids (SCFAs), a well-studied group of metabolites, has demonstrated that SCFAs derived from mouse gut microbiota can activate STAT3 and mTOR in Th1 cells, upregulate the transcription factor B lymphocyte-induced maturation protein 1 (Blimp-1), and stimulate the production of IL-10 to preserve intestinal homeostasis (23). Butyrate, a metabolite produced by Firmicutes and Fusobacteria, can activate the expression of TGFB1 in human intestinal epithelial cells through the transcription factor SP1. This activation leads to the accumulation of Treg cells in the intestine, contributing to its immunomodulatory role (24). Following the consumption of propionic acid by patients with multiple sclerosis, there is a significant and sustained increase in Treg cells. Additionally, the mitochondrial function and morphology of Treg cells normalize, whereas the levels of Th1 and Th17 cells markedly decrease, indicating the immunomodulatory effects (25). Metabolites associated with the microbiota, including taurine, histamine, spermine and bile acids, contribute to the maintenance of intestinal homeostasis through the regulation of NLRP6/NLRP3 inflammasomes (26,27). Probiotics such as Lactobacillus rhamnosus GG and factors derived from LGG broth culture supernatant can activate Akt, alleviate TNF-induced colonic epithelial injury, suppress cytokine-induced epithelial cell apoptosis, and foster intestinal epithelial homeostasis. Furthermore, LGG cell-free supernatant (LGG-SN) has been observed to enhance the sensitivity of human tumor cells to 5-fluorouracil and irinotecan (28,29). The outer membrane protein Amuc_1100 derived from Akkermansia muciniphil stimulates the production of IL-10 by activating TLR2 and TLR4 (30). The human gut Actinobacterium Eggerthella lenta disrupts the inhibition of the Th17 transcription factor Rorγt by cardiac glycoside reductase 2 enzyme, leading to Th17 activation in the intestine and the initiation of autoimmunity (31). Overall, certain metabolites associated with gut microbiota contribute to the maintenance of intestinal immune balance, safeguarding the survival of gut microbiota and protecting the intestinal tract from immune-related harm. Consequently, the intricate mechanism through which gut microbiota regulate the immune system via their metabolites necessitates further investigation.
3. ICIs therapy for cancer
Tumor immunotherapy is initiated by the mechanisms through which tumor cells evade the human immune system. Typically, the immune system can identify and eliminate tumor cells in healthy tissues based on tumor-associated antigens. Tumors, however, employ various immune processes to evade the immune system, including targeted modulation of Tregs function or secretion, antigen presentation processes, modification of immunosuppressive mediator production, development of immune tolerance, and evasion of immune system-mediated killing (32). Immune checkpoints play a crucial role in regulating the host's antitumor immunity. Currently, extensively studied immune checkpoints include PD-1, PD-L1, CTLA-4, LAG3, TIM3 and IDO. ICIs based on these targets have significantly enhanced the efficacy of tumor treatment and made substantial progress in recent years (33). PD-1, a receptor in the (immunoglobulin) Ig superfamily, negatively regulates T-cell antigen receptor signaling through its interaction with the specific ligand PD-L1. PD-L1, also referred to as B7-H1 or CD274, is expressed in numerous tumors, including lung cancer, ovarian cancer, colon cancer and melanoma. This expression reduces the sensitivity of tumor cells to cytotoxic T cell lysis mediated by specific T cell antigen receptors, leading to increased tumorigenicity and aggressiveness (34–36). CTLA-4, a member of the CD28-B7 immunoglobulin superfamily, is expressed on activated T cell surfaces, inhibiting their activity by competing with the costimulatory receptor CD28 for binding to B7-1 and B7-2, thereby downregulating immune responses (37). In vivo, anti-PD-1 and anti-CTLA-4 antibodies have varying immune effects, whether administered alone or in combination. CTLA-4 blockade primarily induced partial proliferation of transitional memory T cells in the blood/tumor tissue analysis of patients undergoing immune checkpoint blockade, whereas PD-1 blockade resulted in changes in cytolysis and NK-cell function-related genes. Blockade of both resulted in non-overlapping changes in gene expression patterns, including proliferation-related and chemokine genes (38). LAG3 comprises four external immunoglobulin superfamily domains in the cellular domain, a long linker peptide in the transmembrane domain, and a serine phosphorylation site in the intracellular domain. It is expressed on the surfaces of CD4+, CD8+, natural killer (NK), NKT and Treg cells, and inhibits T cell function. LAG3 is expressed in various tumors and is associated with patient prognosis. Blockade of LAG3 is also a new antitumor idea (39). TIM3 is an inhibitory checkpoint protein expressed on Th1, Th17, Tregs, CD8+ T, NK and dendritic cells. It is associated with antitumor immunity, and blocking it is a promising approach to cancer therapy (40). IDO is an immunomodulatory enzyme that metabolizes the essential amino acid tryptophan to its downstream kynurenine, thereby inhibiting T cell immunity. Inhibiting IDO is also a way to enhance tumor immunity (32). Furthermore, there has been an increasing use of ICIs and targeted therapies in combination, such as anti-PD-1/PD-L1 and anti-CTLA-4 combination therapy, as well as anti-PD-1/PD-L1 and anti-vascular endothelial growth factor combination therapy (41). ICIs have achieved favorable results in clinical applications. However, some patients initially respond to ICIs therapy but later exhibit drug resistance, which is related to the abundant mutation function of tumor cells, enabling them to evade T cell-mediated immune surveillance once again (42). Moreover, the primary focus of immunotherapy is to enhance the immune activation mechanism. This ‘immune enhancement’ strategy often causes frequent irAEs, although with the advancement of immunotherapy and therapy design, related adverse events are being gradually reduced (43). Common adverse effects of CTLA-4 and/or PD-1 inhibition occur in the skin, gastrointestinal tract, liver and endocrine system, such as pruritus, rash, nausea, diarrhea and thyroid disorders (44). When irAEs occur in ICIs-treated patients, they may need to discontinue ICIs and treat irAEs, compromising treatment efficiency (45). The clinical studies conducted in previous years are included in Table I (46–61). In these clinical studies, a variety of common tumor types were included. Their efficacy in ICIs as monotherapy as in combination therapy is very limited. Response rates were modest, and a substantial proportion of patients developed grade 3–4 irAEs. Despite the progress made with ICIs, their inefficiency and the inevitability of irAEs remain significant challenges. Therefore, more treatment and prevention methods need to be developed to address the deficiencies of ICIs.
Table I.
Clinical studies of ICIs.
| Author/s | Year | Number of patients | ICI | Diagnosis | Trial name | Main conclusion | (Refs.) |
|---|---|---|---|---|---|---|---|
| Topalian et al | 2012 | 296 | BMS-936558, (anti-PD-1) | Advanced solid tumors | NCT00730639 | The ORR was ~1 in 4 to 1 in 5 and 14% of patients experienced grade 3 or 4 irAEs | (46) |
| Ott et al | 2017 | 75 | Pembrolizumab, (anti-PD-1) | Advanced endometrial cancer | NCT02054806 | The PR rates was achieved in 13.0% of the patients, and irAEs occurred in 54.2% of the patients | (47) |
| Antonia et al | 2019 | 304 | Durvalumab, (anti-PD-L1) | Stage IIIB-IV non-small cell lung cancer (NSCLC) | NCT01693562 | The ORR of patients with PD-L1 expression greater than or equal to 25% was 21.8%, and the ORR of patients with PD-L1 expression less than 25% was 6.4% irAEs occurred in 57.2% of patients | (48) |
| Schöffski et al | 2022 | 255 | Ieramilimab (anti-LAG-3) ± Spartalizumab (anti-PD-1) | Advanced solid tumors | NCT02460224 | Tumor responses occurred in 10% of patients. And irAEs occurred in 56 and 69% of patients in the ieramimab monotherapy and ieramimab plus Spartalizumab groups, respectively | (49) |
| Curigliano et al | 2021 | 219 | Sabatolimab (anti-TIM-3) ± Spartalizumab (anti-PD-1) | Advanced solid tumors | NCT02608268 | The partial response rates occurred in 6% of patients receiving combination therapy, and irAEs occurred in 48% | (50) |
| Kelly et al | 2023 | 30 | Epacadostat (anti-IDO1-) Pembrolizumab4 (anti-PD-1) | Advanced sarcoma | N | The ORR was 3.3, and 23% of patients experienced grade 3 irAEs | (51) |
| Zakharia et al | 2021 | 131 | Indoximod (anti-IDO1-) + Pembrolizumab4 (anti-PD-1) | Advanced melanoma | N | The ORR of the evaluable population was 51%, and the most common irAE was fatigue, with an incidence of 62.3% | (52) |
| Lynch et al | 2012 | 204 | Ipilimumab (anti-CTLA-4) + Paclitaxel and Carboplatin | Stage IIIB/IV NSCLC | N | The immune-related best response rates for staged ipilimumab, concurrent ipilimumab, and control therapy were 32, 21, and 18%, respectively. The overall incidence of grade 3 and 4 irAEs was 15, 20, and 6% in the staged ipilimumab, concurrent ipilimumab, and control groups, respectively | (53) |
| Wolchok et al | 2018 | 945 | Ipilimumab (anti-CTLA-4) ± Nivolumab (anti-PD-1) | Advanced melanoma | N | At 3 years, the OS rates were 58% with nivolumab plus ipilimumab, 52% with nivolumab alone, and 34% with ipilimumab alone | (54) |
| Hellmann et al | 2018 | 2,877 | Ipilimumab (anti-CTLA-4) + Nivolumab (anti-PD-1) | Stage IV or recurrent NSCLC | NCT02477826 | The ORR for nivolumab plus ipilimumab was 45.3%, and the incidence of grade 3 or 4 irAEs related to nivolumab plus ipilimumab was 31.2% | (55) |
| Tannir et al | 2021 | 1,096 | Ipilimumab (anti-CTLA-4) + Nivolumab (anti-PD-1) vs. Sunitinib | Advanced renal cell carcinoma with sarco-matoid features | N | The ORR with ipilimumab plus nivolumab was 60.8%, and grade 3 or 4 irAEs occurred in 49% | (56) |
| Rini et al | 2019 | 915 | Atezolizumab (anti-PD-L1) + Bevacizumab vs. Sunitinib | Metastatic renal cell carcinoma | NCT02420821 | Grade 3–4 irAEs occurred in 40% of the atezolizumab plus bevacizumab group | (57) |
| Garon et al | 2019 | 550 | Pembrolizumab (anti-PD-1) | Advanced programmed PD-L1 NSCLC | N | The estimated 5-year OS was 23.2% for treatment-naive patients and 15.5% for previously treated patients, and the incidence of irAEs was 71% | (58) |
| Yuan et al | 2023 | 72 | Camrelizumab (anti-PD-1) + Apatinib | Recurrent/metastatic nasopharyngeal carcinoma | NCT04547088 NCT04548271 | The ORR of the platinum-resistant cohort was 65%, and the ORR of the PD-1 inhibi-tor-resistant cohort was 34.3, and 65.3% of the patients developed ≥ grade 3 irAEs | (59) |
| Liu et al | 2023 | 20 | Camrelizumab (anti-PD-1) + Apatinib | Relapsed or refractory peripheral T-cell lymphoma | N | The ORR for all patients was 30%, and grade 3 or higher adverse events were hyperlipidemia (15%), hypokalemia (15%), and anemia (15%) | (60) |
| Zhao et al | 2023 | 53 | Cadonilimab (anti PD-1 and CTLA-4 bispecific antibody) | Metastatic NSCLC | NCT04172454 | The ORR of patients who had previously received platinum-based two-agent chemotherapy failure was 10, and 11.3% of patients had grade 3–4 irAEs | (61) |
ICI, immune checkpoint inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed cell cell death protein ligand 1; PFS, progression-free survival; NSCLC, non-small cell lung cancer; OS, overall survival. ORR, objective response rate; PR, partial response.
4. Gut microbiota and ICIs
Application and mechanism of gut microbiota in the treatment of ICIs
Recent studies have demonstrated that gut microbiota plays a crucial regulatory role in ICIs therapy, offering a novel approach to enhance the clinical effectiveness of ICIs. Assessing the gut microbiota of patients can provide guidance and regulation for the subsequent clinical implementation of ICIs (62–65). Previous studies exploring the association between gut microbes and ICIs are presented in Table II. Generally, patients with higher levels of Firmicutes and Verrucomicrobiota in their gut microbiota exhibited a more favorable response to ICIs, whereas those with an abundance of Proteobacteria showed a diminished response. The relationship between Bacteroidetes and treatment response was found to be varied. Regarding the occurrence of adverse reactions, Firmicutes exhibited higher levels, whereas Bacteroidetes displayed lower levels. Furthermore, the administration of antibiotics is typically negatively correlated with the clinical response to ICIs (66). A previous study encompassing clinical and animal research demonstrated a correlation between clinical responses to ICIs targeting the PD-1/PD-L1 axis and the relative abundance of Akkermansia muciniphila (67). An investigation into the impact of ICIs treatment on patients with non-small cell lung cancer (NSCLC) revealed a higher prevalence of Akkermansiaceae in individuals demonstrating stable disease and partial response to immunotherapy, as opposed to those with progressive disease (68). The study conducted by Grenda et al (69) demonstrated that Bacteroidaaceae, Barnesiellaceae and Tannerellaceae were capable of extending progression-free survival (PFS) in patients with NSCLC. Newsome et al (70) obtained similar results in their study involving patients with stage III/IV NSCLC who received ICIs treatment, revealing significant enrichment of Ruminococcus, Akkermansia and Faecalibacterium among responders. In the context of melanoma-based ICIs therapy, response was also linked to Bifidobacterium pseudatenulatum, Roseburia spp. and Akkermansia muciniphila (71). The aforementioned multiple similar studies demonstrated the more favorable effects of Akkermansia muciniphila on ICIs. Akkermansia muciniphila, a strictly anaerobic gut bacterium, thrives on intestinal mucin as its exclusive carbon and nitrogen source, colonizing the intestine in a manner intricately linked to the host's well-being. It regulates the immune response of the organism, sustains metabolic equilibrium, ameliorates obesity, type 2 and type 1 diabetes, hepatic steatosis, intestinal inflammation, and augments responses of ICIs across various cancer types (72). Concerning the mechanism underlying the treatment of ICIs by Akkermansia muciniphila, an animal experiment revealed that Akkermansia can modulate the therapeutic capacity of PD-1 antibodies in mice with colorectal cancer by influencing the metabolism of glycerophospholipid and the expression of immune-related cytokines (IFN-γ and IL-2) within the tumor microenvironment, thus preserving the normal effectiveness of PD-1 antibodies (73). However, a recent study examining the association between gastrointestinal microbiome composition and ICIs in advanced metastatic castration-resistant prostate cancer found a decrease in levels of Akkermansia muciniphilia in response samples, which contradicts previous findings in other types of tumors. The aforementioned study observed a correlation between the abundance of Streptococcus salivarius in fecal samples and treatment response. It is possible that tumor type is also associated with the mechanisms through which gut microbiota affect ICIs' therapy (74). Additionally, the study design and potential confounding factors may have contributed to these findings. A study conducted with melanoma patients undergoing anti-PD-1 treatment revealed significant disparities in the diversity and composition of the gut microbiota between individuals who responded to the treatment and those who did not. Responders exhibited significantly higher alpha diversity of gut microbiota and greater relative abundance of Ruminococcaceae compared with non-responders. Moreover, fecal transplantation from responders enhanced antitumor immunity in mice (75). Another analogous study, focusing on patients undergoing ICIs treatment for melanoma, demonstrated the abundance of certain bacterial species, such as Bifidobacterium longum, Collinsella aerofaciens and Enterococcus faecium, in individuals who responded to the treatment (76). A study conducted on ICIs in advanced NSCLC demonstrated that the α diversity of gut microbiota was correlated with overall survival (OS), while the presence of Ruminococcaceae UCG 13 and Agathobacter revealed a positive association with favorable objective response rate and PFS (77). In Chinese patients with NSCLC who underwent anti-PD-1 treatment, the gut microbiota exhibited enrichment of Alistipes putredinis, Bifidobacterium longum and Prevotella copri in the responder group, while Ruminococcus was enriched in the non-responder group. Additionally, patients with a higher diversity of gut microbiota demonstrate an enhanced tumor-killing effect when undergoing anti-PD-1 treatment (78). In a study examining the correlation between clinical response to anti-PD-1 therapy and gut microbiota in patients with advanced hepatobiliary cancer, individuals with a higher abundance of Lachnospiraceae bacterium-GAM79 and Alistipes sp. Marseille-P5997 demonstrated longer PFS and OS compared with those with lower abundance. Furthermore, a high abundance of Ruminococcus calidus and Erysipelotichaceae bacterium-GAM147 was linked to extended PFS and improved treatment response. Conversely, patients with higher abundance of Veillonellaceae exhibited poorer PFS and OS (79). Another study investigating the gut microbiota in patients with hepatocellular carcinoma and their response to ICIs revealed an enrichment of Bifidobacterium, Coprococcus and Acidaminococcus in patients with disease control (80). However, the initial abundance of these three taxa did not predict an OS benefit in the aforementioned study. In a study investigating the combination of regorafenib and toripalimab for colorectal cancer, a higher relative abundance of Fusobacterium was linked to lack of response and shorter PFS (81). Additionally, a previous study provided evidence that Helicobacter pylori infection can upregulate the expression of PD-L1 in human gastric epithelial cells. Further investigation into its clinical significance is warranted (82). According to Park et al (83), the mechanism through which gut microbiota influence ICIs involves the downregulation of PD-L2 and its binding partner, repulsive guidance molecule b, thereby enhancing the efficacy of anti-PD-1 treatment. Ongoing studies in this field are continuously being conducted. Generally, future research should focus on investigating the individual species and overall distribution of gut microbiota. The conclusions of the previous studies are not consistent, which may be attributed to differences in study design, tumor type, sample size and potential confounding factors. Among the aforementioned studies, some are animal studies, some are clinical studies, and the tumor types are not exactly the same. In addition, the sample size was between tens to hundreds, with large differences. Finally, confounding factors such as sex, height, weight, diet and ethnicity can further affect the results of experiments. Nevertheless, certain specific species and characteristics of gut microbiota, such as a higher abundance of Akkermansia and greater α diversity, have demonstrated a positive effect on ICIs in multiple studies. These findings warrant further exploration as important avenues for future research.
Table II.
Gut microbiota and ICIs.
| Author | Year | Categories of study | Gut microbiota | Study summary | (Refs.) |
|---|---|---|---|---|---|
| Vétizou et al | 2015 | Animal study | Bacteroides thetaiotaomicron, | Bacteroides thetaiotaomicron or Bacteroides fragilis can | (65) |
| Bacteroides fragilis | overcome the condition that tumors from antibiotic-treated or | ||||
| germ-free mice do not respond to anti-cytotoxic T lymphocyte | |||||
| antigen-4 | |||||
| Routy et al | 2017 | Animal study | Akkermansia muciniphila | Increased relative abundance of Akkermansia muciniphila could | (67) |
| increase anti-PD-1 efficacy | |||||
| Grenda et al | 2022 | Clinical study | Akkermansiaceae | Patients with higher abundance of Akkermansiaceae had | (68) |
| favorable response to anti-PD-1 or anti-PD-L1 treatment | |||||
| Grenda et al | 2022 | Clinical study | Bacteroidaaceae, Barnesiellaceae, | Bacteroidaaceae, Barnesiellaceae and Tannerellaceae could | (69) |
| Tannerellaceae | prolong PFS in patients with NSCLC treated with ICIs | ||||
| Newsome et al | 2022 | Clinical and | Ruminococcus, Akkermansia, | Ruminococcus, Akkermansia and Faecalibacterium were | (70) |
| animal study | Faecalibacterium | significantly enriched in responders of NSCLC treated with ICIs | |||
| Lee et al | 2022 | Clinical study | Bifidobacterium pseudatenulatum, | Bifidobacterium pseudatenulatum, Roseburia spp. and | (71) |
| Roseburia spp. Akkermansia muciniphila | Akkermansia muciniphila, were associated with response to | ||||
| ICIs in patients with advanced cutaneous melanoma | |||||
| Xu et al | 2020 | Animal study | Prevotella_sp._CAG:485, Akkermansia | Prevotella_sp._CAG:485 and Akkermansia improved anti-PD-1 | (73) |
| efficacy | |||||
| Peiffer et al | 2022 | Clinical study | Akkermansia muciniphilia | Lower levels of Akkermansia muciniphilia were observed in | (74) |
| advanced metastatic castrate resistant prostate cancer responders | |||||
| treated with pembrolizumab | |||||
| Gopalakrishnan et al | 2018 | Clinical study | Ruminococcaceae | The α diversity of gut microbes and the relative abundance of | (75) |
| Ruminococcaceae bacteria were significantly higher in melanoma | |||||
| responders than in non-responders on anti-PD-1 therapy | |||||
| Matson et al | 2018 | Clinical study | Bifidobacterium longum, Collinsella | Bifidobacterium longum, Collinsella aerofaciens and | (76) |
| aerofaciens, Enterococcus faecium | Enterococcus faecium were more abundant in the gut microbiota | ||||
| of melanoma responders treated with anti-PD-1 | |||||
| Hakozaki et al | 2020 | Clinical study | Ruminococcaceae UCG 13, Agathobacter | Enrichment of Ruminococcaceae UCG 13 and Agathobacter is | (77) |
| associated with favorable objective response rate and PFS in | |||||
| NSCLC patients treated with ICIs | |||||
| Jin et al | 2019 | Clinical study | Alistipes putredinis, Bifidobacterium | The gut microbiota of NSCLC patients who responded to | (78) |
| longum, Prevotella copri, Ruminococcus | anti-PD-1 therapy was enriched for Alistipes putredinis, | ||||
| Bifidobacterium longum, and Prevotella copri, whereas | |||||
| Ruminococcus was enriched in non-responders | |||||
| Mao et al | 2021 | Clinical study | Ruminococcus calidus, Erysipelotichaceae | In anti-PD-1 therapy for non-resectable hepatocellular carcinoma | (79) |
| bacterium-GAM147, Veillonellaceae | or advanced biliary tract cancers, Patients with higher abundance | ||||
| of Ruminococcus calidus and Erysipelotichaceae bacterium- | |||||
| GAM147 had longer PFSand OS, whereas those with higher | |||||
| abundance of Veillonellaceae had worse PFS and OS | |||||
| Shen et al | 2021 | Clinical study | Bifidobacterium, Coprococcus, | The enrichment of Bifidobacterium, Coprococcus and | (80) |
| Acidaminococcus | Acidaminococcus was associated with the efficiency of ICIs | ||||
| treatment in patients with hepatocellular carcinoma | |||||
| Wang et al | 2021 | Clinical study | Fusobacterium | Fusobacterium was more abundant in colorectal cancer patients | (81) |
| who did not respond to anti-PD-1 treatment |
ICI, immune checkpoint inhibitors; PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; PFS, progression-free survival; NSCLC, non-small cell lung cancer; OS, overall survival.
Gut microbiota-associated metabolites and ICIs
The role of gut microbiota in the treatment of ICIs may be attributed to their associated metabolites. A study conducted on patients with gastrointestinal cancers receiving anti-PD-1/PD-L1 therapy revealed that those who exhibited a higher Prevotella/Bacteroides ratio or higher abundance of Prevotella, Ruminococcaceae and Lachnospiraceae demonstrated improved responses to anti-PD-1/PD-L1 therapy. These findings may be linked to the metabolites produced by the gut microbiota. Specifically, gut microbiota capable of producing SCFAs, such as Eubacterium, Lactobacillus and Streptococcus, were found to be positively associated with anti-PD-1/PD-L1 responses in gastrointestinal cancers (84). Another study focusing on patients with solid cancer tumors treated with anti-PD-1 therapy demonstrated that higher concentrations of certain SCFAs, including fecal acetic acid, propionic acid, butyric acid, valine and plasma isovaleric acid, were associated with longer PFS (85). The aforementioned study also suggested that SCFAs may serve as the link between gut microbiota and the efficacy of anti-PD-1 therapy. Furthermore, it was found that the gut microbiota metabolite butyrate can directly enhance the response of antitumor cytotoxic CD8+ T cells in vitro and in vivo by promoting IL-12 signaling, thereby improving the efficacy of antitumor therapy (86). However, another study indicated that elevated levels of butyrate and propionate in the blood led to an increase in the proportion of Treg cells, which resulted in a diminished anti-CTLA-4 blockade effect and limited the activity of anti-CTLA-4 therapy (87). Additionally, a study focusing on ICIs for unresectable hepatocellular carcinoma demonstrated that ursodeoxycholic acid and ursocholic acid were significantly enriched in the feces of patients who exhibited an objective response, and these metabolites were correlated with the abundance of Lachnoclostridium (88). Jiang et al (89) study revealed that Fusobacterium nucleatum and increased succinic acid hindered the efficacy of anti-PD-1 therapy in patients with colorectal cancer. However, it is important to note that these studies have yielded conflicting conclusions, emphasizing the need for further exploration into the role of gut microbiota metabolites in ICIs treatment. Currently, there is no further study on how gut microbiota metabolites affect the efficiency of ICIs application by regulating the immune system. The underlying mechanisms are likely to be highly complex, involving interactions between different gut microbiota and various metabolites. Additionally, investigating the intricate mechanisms of downstream gene regulation, immune cell modulation, and regulation of inflammatory factors presents a significant challenge.
Antibiotics and ICIs
The use of antibiotics can affect the composition of gut microbiota, subsequently influencing the modulating role of gut microbiota in the effectiveness of ICIs. Generally, antibiotic treatment is associated with poor OS (90). The utilization of antibiotics emerged as an independent negative predictor of PFS and OS in patients with advanced cancer undergoing ICI treatment. Patients who underwent repetitive or prolonged antibiotic use exhibited a poorer treatment response (91). In a retrospective analysis of nivolumab-treated patients with NSCLC, the median PFS was 1.2 months for patients receiving antibiotics compared with 4.4 months for those who did not, although no difference in OS was observed (92). Another study demonstrated that antibiotic use diminished PFS and OS in patients with advanced renal cell carcinoma and NSCLC, and it exacerbated disease progression in patients with renal cell carcinoma who received antibiotics within 30 days of commencing ICIs, in comparison with those who did not receive antibiotics (93). The mechanism underlying the impact of antibiotics on ICI effectiveness may lie in the disruption of gut microbiota's ecological stability, which compromises the immune homeostasis maintained by gut microbiota, subsequently leading to dysregulation of intestinal immune responses. At present, no further studies have examined how antibiotics specifically affect gut microbiota and the immune system to alter the efficiency of ICIs. This area requires further exploration. Nevertheless, based on the collective results of current studies, the use of antibiotics in patients receiving ICIs should be more strictly regulated to ensure the efficacy of ICIs.
Gut microbiota regulates tumor proliferation
Gut microbiota can directly impact tumors, regulating their occurrence and development. For instance, Propionibacterium acidipropionici and Freudenreichii produce cytotoxic compounds, namely SCFAs propionate and acetate, which induce apoptosis in colorectal cancer cell lines. Similarly, Lactobacilli stimulate immune response, and Lactobacillus casei ATCC334 produces a killing effect on tumor cells through its metabolite ferrichrome (94). The probiotic LGG-SN selectively reduces cancer cell viability by inducing mitotic arrest in the G2/M phase of the cell cycle in tumor cells (29). On the contrary, Fusobacterium nucleatum activates beta-catenin through Fusobacterium adhesin A, and Peptostreptococcus anaerobius promotes tumor cell proliferation by activating the PI3K-Akt pathway in tumor cells and NF-κB activation in tumor-associated macrophages (94). SCFAs, which are common metabolites of gut microbiota, have demonstrated antitumor activity in various types of tumors. They control the proliferation and metastasis of colorectal, gastric, lung, cervical, breast and bladder cancer, and other common tumors through the regulation of epigenetic modifications, inhibition of tumor cell proliferation, and regulation of antitumor immunity (95). The impact of gut microbiota on tumor development is closely related to the tumor microenvironment. Gut microbiota and their metabolites modify the tumor microenvironment by preserving the integrity of the intestinal mucosal barrier, regulating inflammatory factors, and controlling immune cell activation, among other aspects. These mechanisms collectively limit the progression of tumors (96).
Gut microbiota and irAEs and application of probiotics
The relationship between irAEs and gut microbiota has been the subject of investigation in several studies. Andrews et al (97) demonstrated a significant association between a higher abundance of Bacteroides and irAEs in melanoma patients receiving ICIs. Another clinical trial evaluating ipilimumab for the treatment of metastatic melanoma found an inverse association between the increase of specific bacteria in the Bacteroidetes phylum and colitis after immunotherapy (98). Similarly, a study focusing on immune-related diarrhea in lung cancer patients treated with anti-PD-1 antibodies revealed that patients without diarrhea had higher levels of Bacteroidetes and lower levels of Firmicutes (99). At present, there are few studies on the relationship between gut microbiota and irAEs, no specific dominant bacteria have been found, and no studies have further elucidated the underlying mechanism. Further exploration of the relationship between irAEs and gut microbiota is warranted. The underlying mechanism may be linked to the unique properties of certain gut microbiota, necessitating further investigation. Future studies should aim to accurately identify and analyze the relationship between dominant strains and specific irAEs. Nevertheless, supplementing probiotics to modulate the microecological environment of gut microbiota may alleviate the occurrence of irAEs, particularly intestinal-related symptoms. Probiotic supplementation represents a clinical approach that capitalizes on the role of gut microbiota in ICI treatment. Sivan et al (100) animal study demonstrated that oral administration of Bifidobacterium alone achieved comparable melanoma control to anti-PD-L1 treatment. Proton pump inhibitors, which facilitate the migration of oral microbiota to the gut, generally have a negative effect on the efficacy of ICIs in cancer patients. In a trial involving advanced or recurrent patients with NSCLC treated with PD-1/PD-L1, treatment with Clostridium butyricum MIYAIRI 588 (CBM588) improved the efficacy of ICIs in patients receiving proton pump inhibitors, potentially through modulation of specific microbiota richness (101). Another study revealed that CBM588 supplementation increased the response rate and prolonged PFS in the treatment of metastatic renal cell carcinoma with nivolumab plus ipilimumab (102). Similarly, a retrospective analysis demonstrated that CBM588 treatment significantly prolonged PFS and OS in patients with NSCLC treated with PD-1/PD-L1 (103). Probiotic supplementation also reduced immune-related intestinal inflammation. An animal study indicated that Bifidobacterium attenuated intestinal immunopathology in mice without significantly affecting anti-melanoma immunity induced by anti-CTLA-4 treatment (104). However, the role of probiotics in ICI treatment is not always beneficial. A clinical study involving patients with melanoma treated with ICIs suggested that higher dietary fiber intake was associated with significantly improved PFS, particularly in patients who consumed adequate dietary fiber without probiotic use. Consistent with findings in mice, low-fiber diets or probiotics (Bifidobacterium longum- or LGG) impaired anti-PD-1-based treatment responses (105). Gao et al (106) revealed that supplementation with Lacticaseibacillus rhamnosus Probio-M9 enhanced the therapeutic efficiency in colorectal cancer of anti-PD-1 treatment through subsequent metabolism. This supplementation of probiotics may regulate the immune balance by producing beneficial metabolites such as SCFAs in the gut, thereby promoting the infiltration and activation of cytotoxic T lymphocytes and inhibiting the function of Tregs in the tumor microenvironment during ICI treatment. However, the effectiveness of probiotics in ICIs can be influenced by different types of probiotics used in various studies, different tumor types, and diverse patient populations. Inappropriate supplementation can yield contradictory outcomes. Therefore, future research should focus on individualizing probiotic supplementation.
Fecal transplantation
Fecal transplantation is employed as a clinical approach to assess the role of gut microbiota in ICIs treatment. Fecal transplantation entails transferring stool from individuals who respond to non-responders' gut. An animal study demonstrated the superiority of combining fecal transplantation with anti-PD-1 therapy over either therapy alone (107). Experiments involving fecal transplantation of human-germ-free mice revealed that mice receiving response-derived fecal transplantation exhibited enhanced antitumor responses to anti-PD-L1 treatment compared with those receiving non-response-derived fecal transplantation (108). In clinical trials, Baruch et al (109) reported that out of 10 patients with refractory metastatic melanoma to anti-PD-1 therapy who underwent fecal transplantation from responders, 3 patients exhibited a clinical response. Another clinical study demonstrated that fecal transplantation from patients with melanoma who responded to anti-PD-1 therapy provided clinical benefit to 6 out of 15 patients with anti-PD-1 resistance (110). Moreover, fecal microbiota transplantation has been utilized to treat certain cases of ICIs-associated colitis, resulting in clinical benefit (111,112). Koo and Morrow (113) revealed individual variation in fecal dominant donor microbes among recipients following fecal transplantation, which is unrelated to the response to anti-PD-1 therapy. The success of fecal transplantation demonstrates the clinical feasibility of the gut microbiota's significant role in ICIs treatment. Patients who underwent fecal transplantation acquired a gut microecosystem that enhanced the efficacy of ICIs. However, the impact of fecal transplantation is highly limited and does not improve the non-response of the majority of patients to ICIs. This could be attributed to variations in the ecological environment of gut microbiota among individuals, thus suggesting the necessity for additional experiments to explore more precise methods in the application of fecal transplantation (114). Future research should address the need for more accurate donor selection, more effective gut microbiota transplantation methods, as well as the ethical challenges and potential risks associated with fecal transplantation.
5. Conclusion
ICIs have been extensively utilized in clinical practice for cancer therapy, and there is a growing body of evidence supporting the impact of gut microbiota on enhancing the effectiveness of ICIs treatment. The immune system, serving as the communication bridge between these entities, plays a pivotal role in their mechanism of action. ICIs primarily eliminate tumor cells by modulating the activation of the immune system, which is similarly influenced by gut microbiota. In general, gut microbiota, particularly symbiotic bacteria, primarily uphold immune tolerance to preserve their own ecological niche, whereas the principle of ICIs treatment operates in contrast. Conversely, the activation of the immune system by pathogenic bacteria may inflict harm on the body itself. Consequently, achieving a balance between gut microbiota and ICIs treatment may prove to be a highly intricate task. Nevertheless, this equilibrium could potentially serve as the pivotal factor in enhancing the efficiency of ICIs, thereby significantly impacting the prognosis of cancer patients. Currently, despite the ongoing nature of these investigations and the absence of definitive conclusions, the clinical utilization of probiotics and the exploration of fecal transplantation have provided additional perspectives supporting the viability of this approach (Fig. 1). Moving forward, future research can delve into the molecular intricacies of how gut microbiota and their downstream metabolites influence the efficacy of ICIs. Endeavoring to elucidate the precise mechanism underlying the maintenance of balance between gut microbiota and ICIs, as well as identify pivotal species. Ultimately, in clinical practice, precise and individualized implementation of specific probiotic supplementation and fecal transplantation is warranted to enhance the effectiveness of ICIs and optimize patient prognosis.
Figure 1.
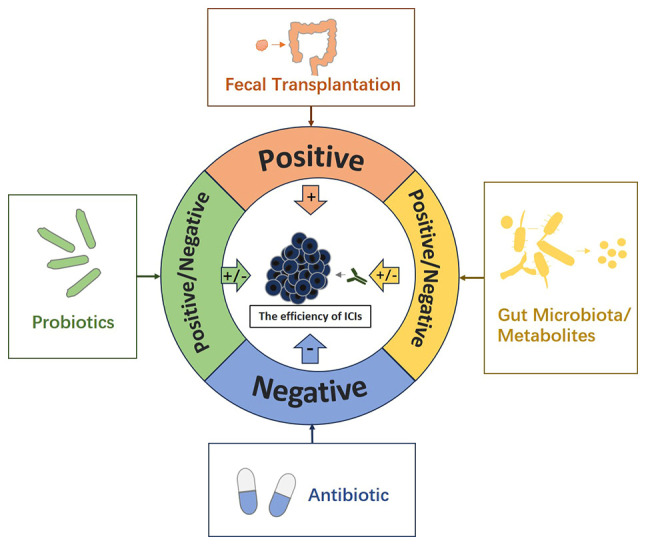
The role of gut microbiota in ICIs. Some members and characteristics of gut microbiota can enhance the efficiency of ICIs, while others can weaken it. According to different reports, some metabolites related to gut microbiota can enhance the efficiency of ICIs, while others can attenuate the efficiency of ICIs. The application of antibiotics in general attenuates the efficiency of ICIs. In general, the application of probiotics can enhance the efficiency of ICIs, but some studies have reported that the application of probiotics can weaken the efficiency of ICIs. Fecal transplantation can enhance the efficiency of ICIs. ICIs, immune checkpoint inhibitors.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HJ proposed the overall idea. HJ and QZ participated in the collection and collation of relevant data and the writing and revision of the manuscript. Data authentication is not applicable. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current perspectives in cancer immunotherapy. Cancers (Basel) 2019;11:1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta SL, Basu S, Soni V, Jaiswal RK. Immunotherapy: An alternative promising therapeutic approach against cancers. Mol Biol Rep. 2022;49:9903–9913. doi: 10.1007/s11033-022-07525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 4.Shi Z, Li H, Song W, Zhou Z, Li Z, Zhang M. Emerging roles of the gut microbiota in cancer immunotherapy. Front Immunol. 2023;14:1139821. doi: 10.3389/fimmu.2023.1139821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G, Libra M. Gut microbiota and cancer: From pathogenesis to therapy. Cancers (Basel) 2019;11:38. doi: 10.3390/cancers11010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumagai T, Rahman F, Smith AM. The microbiome and radiation induced-bowel injury: Evidence for potential mechanistic role in disease pathogenesis. Nutrients. 2018;10:1405. doi: 10.3390/nu10101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuddenham S, Sears CL. The intestinal microbiome and health. Curr Opin Infect Dis. 2015;28:464–470. doi: 10.1097/QCO.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain N. The early life education of the immune system: Moms, microbes and (missed) opportunities. Gut Microbes. 2020;12:1824564. doi: 10.1080/19490976.2020.1824564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, O'Hely M, Quinn TP, Ponsonby AL, Harrison LC, Frøkiær H, Tang MLK, Brix S, Kristiansen K, Burgner D, et al. Maternal gut microbiota during pregnancy and the composition of immune cells in infancy. Front Immunol. 2022;13:986340. doi: 10.3389/fimmu.2022.986340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rio-Aige K, Azagra-Boronat I, Massot-Cladera M, Selma-Royo M, Parra-Llorca A, González S, García-Mantrana I, Castell M, Rodríguez-Lagunas MJ, Collado MC, Pérez Cano FJ. Association of maternal microbiota and diet in cord blood cytokine and immunoglobulin profiles. Int J Mol Sci. 2021;22:1778. doi: 10.3390/ijms22041778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, Olin A, Wang J, Mikes J, Tan Z, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. 2021;184:3884–3898.e11. doi: 10.1016/j.cell.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108((Suppl 1)):S4578–S4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Wang Y, Li H, Dai Y, Chen D, Wang M, Jiang X, Huang Z, Yu H, Huang J, Xiong Z. Altered fecal microbiota composition in older adults with frailty. Front Cell Infect Microbiol. 2021;11:696186. doi: 10.3389/fcimb.2021.696186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng H, Guan X, Chen D, Ma W. The Th17/Treg cell balance: A gut microbiota-modulated story. Microorganisms. 2019;7:583. doi: 10.3390/microorganisms7120583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erturk-Hasdemir D, Oh SF, Okan NA, Stefanetti G, Gazzaniga FS, Seeberger PH, Plevy SE, Kasper DL. Symbionts exploit complex signaling to educate the immune system. Proc Natl Acad Sci USA. 2019;116:26157–26166. doi: 10.1073/pnas.1915978116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Gallausiaux C, Béguet-Crespel F, Marinelli L, Jamet A, Ledue F, Blottière HM, Lapaque N. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci Rep. 2018;8:9742. doi: 10.1038/s41598-018-28048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Dawin E, Bader V, Haase S, Kaisler J, David C, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180:1067–1080.e16. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salemi R, Vivarelli S, Ricci D, Scillato M, Santagati M, Gattuso G, Falzone L, Libra M. Lactobacillus rhamnosus GG cell-free supernatant as a novel anti-cancer adjuvant. J Transl Med. 2023;21:195. doi: 10.1186/s12967-023-04036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M, Boeren S, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander M, Ang QY, Nayak RR, Bustion AE, Sandy M, Zhang B, Upadhyay V, Pollard KS, Lynch SV, Turnbaugh PJ. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe. 2022;30:17–30.e9. doi: 10.1016/j.chom.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35((Suppl)):S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Wei G, Zhang H, Zhao H, Wang J, Wu N, Li L, Wu J, Zhang D. Emerging immune checkpoints in the tumor microenvironment: Implications for cancer immunotherapy. Cancer Lett. 2021;511:68–76. doi: 10.1016/j.canlet.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JJ, Thi EP, Carpio VH, Bi Y, Cole AG, Dorsey BD, Fan K, Harasym T, Iott CL, Kadhim S, et al. Checkpoint inhibition through small molecule-induced internalization of programmed death-ligand 1. Nat Commun. 2021;12:1222. doi: 10.1038/s41467-021-21410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HT, Lee SH, Heo YS. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules. 2019;24:1190. doi: 10.3390/molecules24061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, Dhodapkar KM. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi AP, Tang XY, Xiong YL, Zheng KF, Liu YJ, Shi XG, Lv Y, Jiang T, Ma N, Zhao JB. Immune checkpoint LAG3 and Its Ligand FGL1 in cancer. Front Immunol. 2022;12:785091. doi: 10.3389/fimmu.2021.785091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kandel S, Adhikary P, Li G, Cheng K. The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy. Cancer Lett. 2021;510:67–78. doi: 10.1016/j.canlet.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan AC, Bagley SJ, Wen PY, Lim M, Platten M, Colman H, Ashley DM, Wick W, Chang SM, Galanis E, et al. Systematic review of combinations of targeted or immunotherapy in advanced solid tumors. J Immunother Cancer. 2021;9:e002459. doi: 10.1136/jitc-2021-002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 43.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 45.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, Puzanov I, Mehnert JM, Aung KL, Lopez J, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535–2541. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 48.Antonia SJ, Balmanoukian A, Brahmer J, Ou SI, Hellmann MD, Kim SW, Ahn MJ, Kim DW, Gutierrez M, Liu SV, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol. 2019;14:1794–1806. doi: 10.1016/j.jtho.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Schöffski P, Tan DSW, Martín M, Ochoa-de-Olza M, Sarantopoulos J, Carvajal RD, Kyi C, Esaki T, Prawira A, Akerley W, et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J Immunother Cancer. 2022;10:e003776. doi: 10.1136/jitc-2021-003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM, Sarantopoulos J, Bedard PL, Lin CC, Hodi FS, et al. Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin Cancer Res. 2021;27:3620–3629. doi: 10.1158/1078-0432.CCR-20-4746. [DOI] [PubMed] [Google Scholar]
- 51.Kelly CM, Qin LX, Whiting KA, Richards AL, Avutu V, Chan JE, Chi P, Dickson MA, Gounder MM, Keohan ML, et al. A phase II study of epacadostat and pembrolizumab in patients with advanced sarcoma. Clin Cancer Res. 2023;29:2043–2051. doi: 10.1158/1078-0432.CCR-22-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zakharia Y, McWilliams RR, Rixe O, Drabick J, Shaheen MF, Grossmann KF, Kolhe R, Pacholczyk R, Sadek R, Tennant LL, et al. Phase II trial of the IDO pathway inhibitor indoximod plus pembrolizumab for the treatment of patients with advanced melanoma. J Immunother Cancer. 2021;9:e002057. doi: 10.1136/jitc-2020-002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 54.Wan MT, Ming ME. Nivolumab versus ipilimumab in the treatment of advanced melanoma: A critical appraisal: ORIGINAL ARTICLE. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. Br J Dermatol. 2018;179:296–300. doi: 10.1111/bjd.16785. N Engl J Med 2017; 377: 1345-1356. [DOI] [PubMed] [Google Scholar]
- 55.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tannir NM, Signoretti S, Choueiri TK, McDermott DF, Motzer RJ, Flaifel A, Pignon JC, Ficial M, Frontera OA, George S, et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res. 2021;27:78–86. doi: 10.1158/1078-0432.CCR-20-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 58.Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.2019.37.18_suppl.LBA9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan L, Jia GD, Lv XF, Xie SY, Guo SS, Lin DF, Liu LT, Luo DH, Li YF, Deng SW, et al. Camrelizumab combined with apatinib in patients with first-line platinum-resistant or PD-1 inhibitor resistant recurrent/metastatic nasopharyngeal carcinoma: A single-arm, phase 2 trial. Nat Commun. 2023;14:4893. doi: 10.1038/s41467-023-40402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y, Song Y, Zuo S, Zhang X, Liu H, Wang J, Wang J, Tang Y, Zheng W, Ying Z, et al. Antitumor activity and safety of camrelizumab combined with apatinib in patients with relapsed or refractory peripheral T-cell lymphoma: An open-label, multicenter, phase II study. Front Immunol. 2023;14:1128172. doi: 10.3389/fimmu.2023.1128172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Ma Y, Fan Y, Zhou J, Yang N, Yu Q, Zhuang W, Song W, Wang ZM, Li B, et al. A multicenter, open-label phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific antibody) monotherapy in previously treated advanced non-small-cell lung cancer (AK104-202 study) Lung Cancer. 2023;184:107355. doi: 10.1016/j.lungcan.2023.107355. [DOI] [PubMed] [Google Scholar]
- 62.Shui L, Yang X, Li J, Yi C, Sun Q, Zhu H. Gut microbiome as a potential factor for modulating resistance to cancer immunotherapy. Front Immunol. 2020;10:2989. doi: 10.3389/fimmu.2019.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park EM, Chelvanambi M, Bhutiani N, Kroemer G, Zitvogel L, Wargo JA. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28:690–703. doi: 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, Liu Z, Chen T. Gut microbiota: A promising milestone in enhancing the efficacy of PD1/PD-L1 blockade therapy. Front Oncol. 2022;12:847350. doi: 10.3389/fonc.2022.847350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang C, Li M, Liu B, Zhu H, Dai Q, Fan X, Mehta K, Huang C, Neupane P, Wang F, et al. Relating gut microbiome and its modulating factors to immunotherapy in solid tumors: A systematic review. Front Oncol. 2021;11:642110. doi: 10.3389/fonc.2021.642110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 68.Grenda A, Iwan E, Chmielewska I, Krawczyk P, Giza A, Bomba A, Frąk M, Rolska A, Szczyrek M, Kieszko R, et al. Presence of Akkermansiaceae in gut microbiome and immunotherapy effectiveness in patients with advanced non-small cell lung cancer. AMB Express. 2022;12:86. doi: 10.1186/s13568-022-01428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grenda A, Iwan E, Krawczyk P, Frąk M, Chmielewska I, Bomba A, Giza A, Rolska-Kopińska A, Szczyrek M, Kieszko R, et al. Attempting to identify bacterial allies in immunotherapy of NSCLC patients. Cancers (Basel) 2022;14:6250. doi: 10.3390/cancers14246250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newsome RC, Gharaibeh RZ, Pierce CM, da Silva WV, Paul S, Hogue SR, Yu Q, Antonia S, Conejo-Garcia JR, Robinson LA, Jobin C. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022;14:35. doi: 10.1186/s13073-022-01037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee KA, Thomas AM, Bolte LA, Björk JR, de Ruijter LK, Armanini F, Asnicar F, Blanco-Miguez A, Board R, Calbet-Llopart N, et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med. 2022;28:535–544. doi: 10.1038/s41591-022-01695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19:625–637. doi: 10.1038/s41575-022-00631-9. [DOI] [PubMed] [Google Scholar]
- 73.Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, Wang N, Zhang C, Kong L, Liu Y, et al. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front Microbiol. 2020;11:814. doi: 10.3389/fmicb.2020.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peiffer LB, White JR, Jones CB, Slottke RE, Ernst SE, Moran AE, Graff JN, Sfanos KS. Composition of gastrointestinal microbiota in association with treatment response in individuals with metastatic castrate resistant prostate cancer progressing on enzalutamide and initiating treatment with anti-PD-1 (pembrolizumab) Neoplasia. 2022;32:100822. doi: 10.1016/j.neo.2022.100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benlaïfaoui M, Mimpen I, Terrisse S, Derosa L, Zitvogel L, Routy B, Okuma Y. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol Res. 2020;8:1243–1250. doi: 10.1158/2326-6066.CIR-20-0196. [DOI] [PubMed] [Google Scholar]
- 78.Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, Zheng H, Yao C, Wang Y, Lu S. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol. 2019;14:1378–1389. doi: 10.1016/j.jtho.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Mao J, Wang D, Long J, Yang X, Lin J, Song Y, Xie F, Xun Z, Wang Y, Wang Y, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. 2021;9:e003334. doi: 10.1136/jitc-2021-003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen YC, Lee PC, Kuo YL, Wu WK, Chen CC, Lei CH, Yeh CP, Hsu C, Hsu CH, Lin ZZ, et al. An exploratory study for the association of gut microbiome with efficacy of immune checkpoint inhibitor in patients with hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:809–822. doi: 10.2147/JHC.S315696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, Luo HY, Li JB, Wang FH, Qiu MZ, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: A phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2:100383. doi: 10.1016/j.xcrm.2021.100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu YY, Lin CW, Cheng KS, Lin C, Wang YM, Lin IT, Chou YH, Hsu PN. Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin Exp Immunol. 2010;161:551–559. doi: 10.1111/j.1365-2249.2010.04217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park JS, Gazzaniga FS, Wu M, Luthens AK, Gillis J, Zheng W, LaFleur MW, Johnson SB, Morad G, Park EM, et al. Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy resistance. Nature. 2023;617:377–385. doi: 10.1038/s41586-023-06026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, Zhang X, Gong JF, Li J, Lu M, et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res. 2020;8:1251–1261. doi: 10.1158/2326-6066.CIR-19-1014. [DOI] [PubMed] [Google Scholar]
- 85.Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, Matsumoto S, Inoue K, Muto M. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3:e202895. doi: 10.1001/jamanetworkopen.2020.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, Dong X, Huang J, Wang Q, Mackay CR, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021;33:988–1000.e7. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Coutzac C, Jouniaux JM, Paci A, Schmidt J, Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix L, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020;11:2168. doi: 10.1038/s41467-020-16079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT, Lee IC, Yu-Lun K, Chou SH, Luo JC, Hou MC, Huang YH. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2022;10:e004779. doi: 10.1136/jitc-2022-004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang SS, Xie YL, Xiao XY, Kang ZR, Lin XL, Zhang L, Li CS, Qian Y, Xu PP, Leng XX, et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe. 2023;31:781–797.e9. doi: 10.1016/j.chom.2023.04.010. [DOI] [PubMed] [Google Scholar]
- 90.Yang M, Wang Y, Yuan M, Tao M, Kong C, Li H, Tong J, Zhu H, Yan X. Antibiotic administration shortly before or after immunotherapy initiation is correlated with poor prognosis in solid cancer patients: An up-to-date systematic review and meta-analysis. Int Immunopharmacol. 2020;88:106876. doi: 10.1016/j.intimp.2020.106876. [DOI] [PubMed] [Google Scholar]
- 91.Tinsley N, Zhou C, Tan G, Rack S, Lorigan P, Blackhall F, Krebs M, Carter L, Thistlethwaite F, Graham D, Cook N. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncologist. 2020;25:55–63. doi: 10.1634/theoncologist.2019-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hakozaki T, Okuma Y, Omori M, Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019;17:2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim J, Lee HK. The role of gut microbiota in modulating tumor growth and anticancer agent efficacy. Mol Cells. 2021;44:356–362. doi: 10.14348/molcells.2021.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Son MY, Cho HS. Anticancer effects of gut microbiota-derived short-chain fatty acids in cancers. J Microbiol Biotechnol. 2023;33:849–856. doi: 10.4014/jmb.2301.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li T, Han L, Ma S, Lin W, Ba X, Yan J, Huang Y, Tu S, Qin K. Interaction of gut microbiota with the tumor microenvironment: A new strategy for antitumor treatment and traditional Chinese medicine in colorectal cancer. Front Mol Biosci. 2023;10:1140325. doi: 10.3389/fmolb.2023.1140325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, Khan MAW, Cogdill AP, White MG, Wong MC, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27:1432–1441. doi: 10.1038/s41591-021-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu T, Xiong Q, Li L, Hu Y. Intestinal microbiota predicts lung cancer patients at risk of immune-related diarrhea. Immunotherapy. 2019;11:385–396. doi: 10.2217/imt-2018-0144. [DOI] [PubMed] [Google Scholar]
- 100.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tomita Y, Goto Y, Sakata S, Imamura K, Minemura A, Oka K, Hayashi A, Jodai T, Akaike K, Anai M, et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology. 2022;11:2081010. doi: 10.1080/2162402X.2022.2081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat Med. 2022;28:704–712. doi: 10.1038/s41591-022-01694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S, Sakagami T. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res. 2020;8:1236–1242. doi: 10.1158/2326-6066.CIR-20-0051. [DOI] [PubMed] [Google Scholar]
- 104.Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci USA. 2018;115:157–161. doi: 10.1073/pnas.1712901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao G, Shen S, Zhang T, Zhang J, Huang S, Sun Z, Zhang H. Lacticaseibacillus rhamnosus Probio-M9 enhanced the antitumor response to anti-PD-1 therapy by modulating intestinal metabolites. EBioMedicine. 2023;91:104533. doi: 10.1016/j.ebiom.2023.104533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang J, Zheng X, Kang W, Hao H, Mao Y, Zhang H, Chen Y, Tan Y, He Y, Zhao W, Yin Y. Metagenomic and metabolomic analyses reveal synergistic effects of fecal microbiota transplantation and anti-PD-1 therapy on treating colorectal cancer. Front Immunol. 2022;13:874922. doi: 10.3389/fimmu.2022.874922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaikh FY, Gills JJ, Mohammad F, White JR, Stevens CM, Ding H, Fu J, Tam A, Blosser RL, Domingue JC, et al. Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors. Cancer Immunol Immunother. 2022;71:2405–2420. doi: 10.1007/s00262-022-03169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 110.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fasanello MK, Robillard KT, Boland PM, Bain AJ, Kanehira K. Use of fecal microbial transplantation for immune checkpoint inhibitor colitis. ACG Case Rep J. 2020;7:e00360. doi: 10.14309/crj.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koo H, Morrow CD. Incongruence between dominant commensal donor microbes in recipient feces post fecal transplant and response to anti-PD-1 immunotherapy. BMC Microbiol. 2021;21:251. doi: 10.1186/s12866-021-02312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jamal R, Messaoudene M, de Figuieredo M, Routy B. Future indications and clinical management for fecal microbiota transplantation (FMT) in immuno-oncology. Semin Immunol. 2023;67:101754. doi: 10.1016/j.smim.2023.101754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


