Abstract
Several species of the genus Borrelia exhibit antigenic variation of variable major proteins on their surface during relapsing fever. We have investigated the African relapsing fever species Borrelia crocidurae during infections in mice and compared it with the thoroughly studied North American species Borrelia hermsii. A major difference between the two species is that B. crocidurae can bind and become completely covered with erythrocytes. In addition, B. crocidurae causes a prolonged spirochetemia which coincides with a delayed appearance of antiborrelial antibodies. We show that the antibody response against an unrelated antigen is not delayed and that antibiotic treatment, which dissociates rosettes and inhibits the spirochetes, also leads to an early antibody response. Taken together, the erythrocyte aggregation and prolonged spirochetemia hint at a new mode of immune evasion where erythrocyte-covered spirochetes may avoid contact with the phagocytic cells and B cells of the immune system, thereby delaying the onset of a specific immune response.
Relapsing fever is a disease caused by spirochetes that belong to the genus Borrelia. The borreliae are transmitted from one vertebrate host to another by the bite of soft-shelled ticks (Argasidae) or lice (Pediculus humanus) (11). Therefore, presence in the blood of a host is a prerequisite for transmission of the bacteria. Antigenic variation is the characteristic virulence mechanism by which relapsing fever Borrelia species are able to persist in a mammalian host. Several other pathogenic microorganisms employ antigenic variation of surface proteins. The most familiar are the etiologic agent of sleeping sickness, Trypanosoma brucei (7), and the malaria parasite Plasmodium falciparum. In addition to antigenic variation the malaria parasite displays an erythrocyte (RBC)-rosetting mechanism (20). Antigenic variation among Borrelia species has mainly been studied in spirochetes of the North American species Borrelia hermsii, whose capacity to periodically express new variable major proteins, Vmp, on their surface leads to the typical symptoms of relapsing fever (5, 9). The clinical manifestations during relapsing fever are very diverse, and different species of relapsing fever Borrelia cause different symptoms in different mammals (11, 12). The African relapsing fever species Borrelia crocidurae displays antigenic variation which is very similar to that of B. hermsii. After infection of a mammalian host with one serotype the bacteria grow and a spirochetemia develops whereby numerous bacteria are visible in the blood. A few of these bacteria switch to express a new, antigenically distinct, Vmp protein. When the immune reaction against the primary infecting serotype occurs the spirochetemia subsides and no spirochetes are detected in the blood of the host, but eventually a second spirochetemia appears with borreliae of the new serotype (9).
During growth of B. crocidurae in mice we observed that RBCs aggregated around the spirochetes, as reported previously (17). This aggregation does not occur during B. hermsii spirochetemias. The RBC aggregates are reminiscent of the rosettes formed by Plasmodium-infected RBCs, although the mechanism behind the aggregation is probably different (20). In the present study, we have examined the ability of two relapsing fever Borrelia spirochetes to adhere to and aggregate RBCs. We have also investigated if the immunogenicity of the Vmp or a general suppression of the immune response is responsible for the longer spirochetemias in the B. crocidurae infection.
Observation of distinct RBC binding phenotypes.
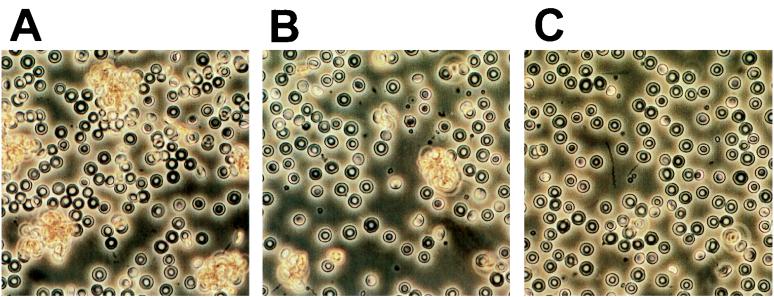
One significant difference between the infection of BALB/c mice (Bomholtgård, Bomholtgård, Denmark) with B. hermsii HS1 serotype 7 (ATCC 35209) versus B. crocidurae serotype C2 (cloned from the strain collection of Alan G. Barbour, Irvine, Calif.) is the apparent RBC binding capacity of B. crocidurae. We found that B. crocidurae binds to RBCs during the spirochetemia so that each bacterium is completely covered by RBCs (Fig. 1A). The aggregation of RBCs around B. crocidurae can be reconstituted in vitro by mixing culture-grown B. crocidurae with diluted blood on a microscopic slide (Fig. 1B). The borreliae were grown in BSKII medium at 34°C for 48 h (3). The bacteria were centrifuged, and the bacterial pellet was resuspended in blood from an uninfected mouse and diluted 1:10 in phosphate-buffered saline (PBS) to a bacterial titer of 108 spirochetes · ml−1. The spirochete-blood mixture was placed on a microscope slide and covered with a cover slip that was sealed along the edges with nail polish. The sealed samples were incubated at 22 or 37°C for 30 min before microscopic examination. Aggregates formed when the spirochete-blood mixture was incubated at 37° but not at 22°C (Fig. 1B and C, respectively). The North American relapsing fever species B. hermsii does not aggregate RBC in vivo, and it did not bind to the cells in vitro at either of the temperatures tested. The in vitro aggregation assay was also applied to mononuclear cells (MNCs). The separation of MNCs was performed with a Lymphoprep kit according to the protocol of the vendor (Nycomed, Oslo, Norway). The cells were diluted in PBS to 106 cells · ml−1. At 22°C neither B. hermsii nor B. crocidurae bound MNCs. At 37°C binding was observed between the MNCs and the borrelial cells; however, the MNCs bound almost as well to B. hermsii as to B. crocidurae. In a competitive assay where equal numbers of RBCs and MNCs were incubated with B. crocidurae, a clear preference for RBC binding was evident. The RBCs formed rosettes around the B. crocidurae cells, and only a few individual MNCs were bound. The displacement of MNCs by RBCs already at a 1:1 ratio suggests that the binding of MNCs is negligible in vivo where the excess of RBCs to MNCs is of several orders of magnitude. The absence of in vitro aggregation of RBCs at 22°C may be a simple developmental strategy of the borreliae. This is the typical temperature of ticks, in which dissociation of the rosette could release the spirochetes for unimpeded development. We have routinely observed rosetting at all spirochetemic peaks in B. crocidurae infections of BALB/c and C3H/Tif mice, suggesting that the phenomenon is independent of antigenic variation and infected mouse strain. Despite the invariable observation of RBC rosetting around B. crocidurae whether blood samples are observed undiluted or diluted with 0.15 M NaCl, PBS, or BSKII culture medium, it is still possible that the rosetting is not an in vivo phenomenon. This may not be undisputedly resolved without microscopic observation of the rosettes in the bloodstream of a living mouse. However, an additional indication of the presence of RBC aggregation in vivo comes from observations of damage on highly vasculated tissues of mice infected with B. crocidurae. It is conceivable that the observed rosetting of RBC may clog the capillaries and thereby cause infarctions, petechiae, or more severe bleedings, explaining some of the clinical symptoms seen in B. crocidurae relapsing fever patients (2, 10). We are currently investigating the role of RBC rosetting in the lesions observed during B. crocidurae infection in mice.
FIG. 1.
Photographs depicting the interaction between B. crocidurae serotype C2 and mouse RBCs. (A) Aggregation of RBC in vivo. The photographed sample was taken early during the massive spirochetemia. (B) Reconstitution of RBC aggregation in vitro after incubation at 37°C for 30 min. (C) No RBC aggregation was seen after incubation at 22°C in vitro.
Analysis of a delayed antibody response.
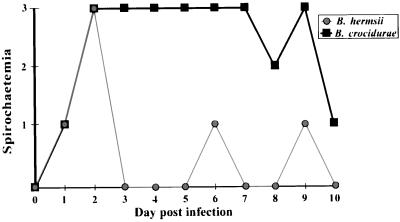
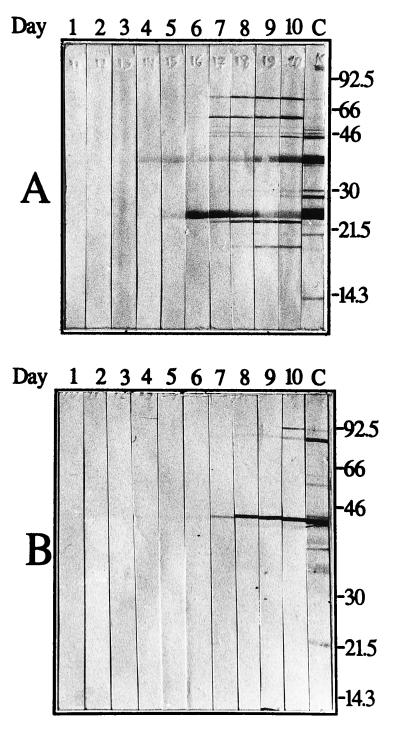
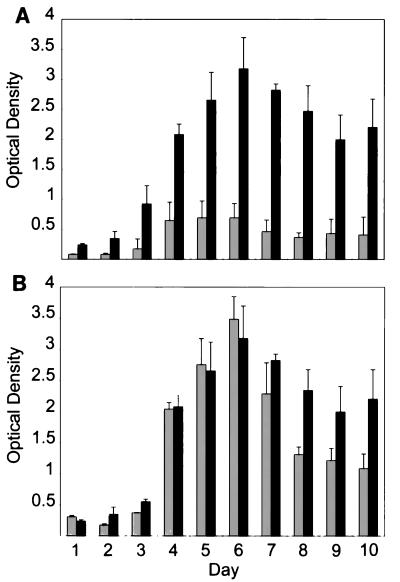
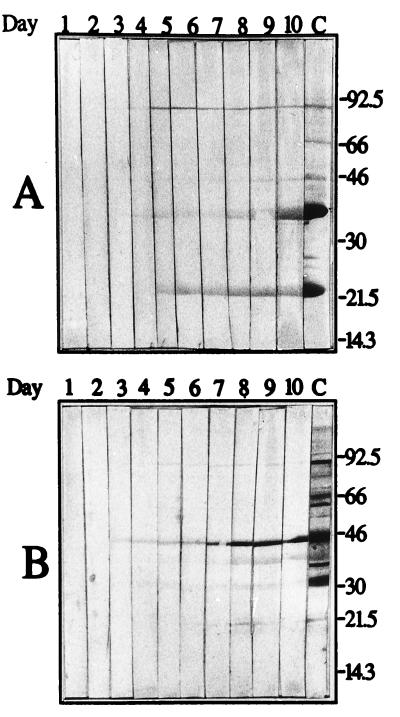
BALB/c mice were inoculated with 106 borreliae by intraperitoneal injection. To monitor the developing spirochetemia, a drop of blood was taken from the tail vein of each mouse, placed on the edge of a drop of PBS with 0.5% bovine serum albumin, covered with a coverslip, and observed by microscopic examination at ×400 magnification. The samples were searched for spirochetes in the boundary between buffer and blood cells. If present, spirochetes could be observed between individual RBC or in RBC aggregates. The first spirochetemia appeared after approximately 2 days for both infections. The B. crocidurae spirochetemia, however, lasted for 4 to 5 days, whereas the B. hermsii spirochetemia lasted for only 1 day (Fig. 2). We suspect that the aggregation of RBCs around the bacteria might cause the prolonged spirochetemia during B. crocidurae infection, by delaying the onset of a specific immune reaction directed against the bacteria. We tested this hypothesis by infecting mice with an equal amount of B. hermsii or B. crocidurae. Presence of spirochetes in the blood was recorded (Fig. 2), and a 30-μl blood sample was collected from each mouse daily for 10 days postinfection. The blood was diluted 1:10 with PBS prior to serum preparation (13). The sera were then tested by Western blotting for the emergence of antibodies specific for the infecting agent. Total protein extracts from B. hermsii and B. crocidurae were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (MSI, Westboro, Mass.). After transfer, each membrane was cut into 5-mm-wide strips, and protein detection was performed as described by Jonsson et al. (15). As a positive control, one strip was incubated for 1 h in a 1:100 dilution of the corresponding mouse anti-Borrelia serum, raised as previously described (19). The procedure was followed by the binding of an alkaline phosphatase-conjugated rabbit anti-mouse antibody (Dako, Älusjö, Sweden), diluted 1:1,000 in 2.5% nonfat milk–PBS. Bound secondary antibody was visualized by detection of alkaline phosphatase activity. Western blot analysis revealed the presence of specific antibodies against B. hermsii 4 days after infection, 2 to 3 days after the start of the visible spirochetemia (Fig. 2 and 3A). The mice infected with B. crocidurae developed specific antibodies at least 7 days after infection, despite the development of the spirochetemia no later than 2 days after infection (Fig. 2 and 3B). This corresponds to a delay in the onset of an immune reaction of 3 days compared to that for B. hermsii infection. Enzyme-linked immunosorbent assay (ELISA) results from the same sera, performed as described by Bunikis et al. (8), agree with the Western blot data inasmuch as a sharp increase of B. hermsii-specific antibodies was apparent from day 3 until day 6. The amount of B. crocidurae-specific antibodies increased only moderately and reached a plateau at a low level already on day 4 (Fig. 4A).
FIG. 2.
Spirochetemia in mice. The development of spirochetemia in BALB/c mice infected with an equal amount (106 spirochetes) of B. hermsii serotype 7 (A) or B. crocidurae serotype C2 (B). The spirochetemia was graded in four groups as follows: 3, one or more spirochetes in each field of vision in the studied sample; 2, one spirochete in every field of vision to one spirochete in 10 fields of vision; 1, less than one spirochete in 10 fields of vision and 0, no spirochete found in the sample.
FIG. 3.
Western blot showing the emergence of a specific immune response against the infecting Borrelia species. (A) Daily serum samples from a mouse infected with B. hermsii serotype 7 reacting against protein extract of B. hermsii serotype 7 cells. (B) Serum from a mouse infected with B. crocidurae serotype C2 reacting against protein extract of B. crocidurae serotype C2 cells. Numbers at the top of each panel correspond to day postinfection. The rightmost lane, marked C, includes a positive control antiserum. Molecular mass standards in kilodaltons are indicated at the right.
FIG. 4.
Serologic responses (immunoglobulin M-ELISA) of mice infected with B. hermsii serotype 7 or B. crocidurae serotype C2. ELISA of total protein from B. hermsii serotype 7 (black bars) or B. crocidurae serotype C2 (grey bars) and either untreated (A) or treated with tetracycline (B) at day 2 postinfection. Error bars indicate standard deviation of the means.
Investigation of Vmp immunogenicity.
The delayed immune response in B. crocidurae infections may have several explanations. If the rosetting and the effect on the immune system are unconnected, the delayed immune response could be accounted for by a lower immunogenicity of the surface molecules of B. crocidurae. To investigate the differences between B. hermsii Vmp7 and B. crocidurae VmpC2, mice were injected with 1 μg of protein extracts enriched for Vmp proteins (6) of B. hermsii serotype 7 and B. crocidurae serotype C2. Serum samples were collected daily for 10 days. Both proteins induced specific antibodies by 2 days postinoculation, as detected by Western blotting (data not shown). The immunogenicity of the Vmp proteins was explored further by treating mice infected with B. hermsii serotype 7 or B. crocidurae serotype C2 with the bacteriostatic antibiotic tetracycline at a concentration of 0.6 g/liter of drinking water as the mice became spirochetemic on day 2 (19). As previously, serum samples were collected daily for 10 days. When the antibiotic inhibits B. crocidurae the spirochetes are no longer able to aggregate RBCs, rendering them accessible to recognition and attack by the immune system. The immune responses in mice infected with B. hermsii were almost indistinguishable between the mice that were not treated and the mice that were treated with tetracycline (compare Fig. 3A with Fig. 5A and Fig. 4A with Fig. 4B). Specific antibodies were detectable from day 4 postinfection, 2 days after the start of the spirochetemia. In contrast, for B. crocidurae there was a clear difference between the antibiotic-treated and untreated mice. With tetracycline treatment, specific antibodies appeared 3 days after infection (Fig. 5B), 1 day after emergence of the spirochetemia. Without treatment, the immune response appeared 7 days postinfection. The corresponding ELISA confirmed that while there is an apparent difference in immune response between B. hermsii and B. crocidurae in the absence of tetracycline (Fig. 4A), the immune reactions to B. hermsii and B. crocidurae are virtually identical upon inhibition of the bacteria early during the spirochetemia (Fig. 4B). The reason for the early immune response to B. crocidurae upon tetracycline treatment may be due to increased lysis. However, the identical immune reactions to B. hermsii with or without tetracycline treatment (Fig. 4) contradicts this, assuming a similar effect of tetracycline on B. hermsii and B. crocidurae. This is in conflict with the hypothesis that a lower immunogenicity of B. crocidurae causes the differences in timing of the immune responses. Similarities of the immune reactions upon interruption of B. crocidurae rosetting with antibiotics further imply an involvement of rosetting in the delay of the immune response.
FIG. 5.
Western blot analysis of serum from mice infected with Borrelia species after treatment with tetracycline. (A) Reactions of serum samples from a mouse infected with B. hermsii serotype 7 against protein extract of B. hermsii serotype 7 cells. (B) Reactions of sera collected daily from a mouse infected with B. crocidurae serotype C2 and tested against protein extract of B. crocidurae serotype C2 cells. The mice were treated with tetracycline at day 2 postinfection. Numbers at the top of each panel correspond to day postinfection. The rightmost lane, marked C, includes a positive control antiserum. Molecular mass standards in kilodaltons are indicated on the right.
Investigation of an immunosuppressing activity.
To assess whether the protracted infection could be explained by a general immune suppression of the host by B. crocidurae, an unrelated antigen was injected into spirochetemic mice. Healthy mice were infected with B. crocidurae serotype C2 or B. hermsii serotype 7. At day 2, when all mice were spirochetemic, each mouse received an intraperitoneal injection of 10 μg of placental alkaline phosphatase (PLAP), purified as described elsewhere (14). Serum samples collected daily from day 1 to day 10 postinfection were used in Western blot assays. A 1:100 dilution of the H7 monoclonal antibody raised against PLAP was used as a positive control (16). Bound monoclonal antibody was detected by incubation with peroxidase-conjugated goat anti-mouse antibody diluted 1:1,000 in 2.5% nonfat milk–PBS. Peroxidase activity was detected with an enhanced chemiluminescence kit (ECL; Amersham, Buckinghamshire, England) and recorded on photographic film. A specific immune response to PLAP became detectable by Western blotting 1 day after injection for uninfected mice and for mice infected with B. hermsii serotype 7 or B. crocidurae serotype C2 (data not shown). The simultaneous appearance of an immune response against the unrelated antigen PLAP in all mice argues against a general immunosuppressing activity of B. crocidurae.
B. crocidurae persists in the bloodstream for a long time without eliciting an evident immune response. This does not seem to be attributable to a particularly low antigenicity of the surface proteins of the bacteria. Neither is there an apparent reduction of the immune response in the host. A potential explanation for the protracted infection is that B. crocidurae may impose a more specific immunosuppressing activity during close interaction with the cells of the host’s immune system. This suppression seems to be dependent on live bacteria, since it is lost upon blocking protein synthesis of B. crocidurae with tetracycline. The assumption that the rosetting and delayed immune response are connected presents an alternative explanation that has been suggested to be involved in malaria infection (1).
It has been shown previously that relapsing fever borreliae are killed by the humoral immune response of the host (4, 18). The initiation of such a response requires the ingestion of bacteria or their antigens by antigen-presenting cells, whose subsequent interaction with B cells exhibiting the correct antiborrelial antibody specificities leads to the proliferation of those B cells and hence to production of anti-Borrelia antibodies. It is possible that binding of RBC to the surface of B. crocidurae can exclude direct interaction of the bacterium with the cells of the immune system. This exclusion would lead to the delayed appearance of a specific immune response during the infection. The RBCs cannot, however, protect the Borrelia from the binding of specific soluble antibodies. This implies that the presence of sufficient numbers of dead or non-RBC binding borreliae in the host eventually would lead to the generation of a delayed immune reaction, which then results in a rapid killing of the borreliae. This immune exclusion, by the aggregation of RBCs around the bacteria, is the favored interpretation of our research group. Experiments designed to elucidate the role of the rosettes in the duration of the infection are currently under way.
Acknowledgments
We thank Alan G. Barbour for kindly providing us with the B. crocidurae isolate and Torgny Stigbrand for the generous gift of PLAP and PLAP monoclonal antibody. David Barry and David Haydon are acknowledged for critically reading the manuscript. We also thank Pierre Martin for skillful technical assistance.
This work was supported by the Swedish Medical Research Council (07922).
REFERENCES
- 1.Allred D R. Immune evasion by Babesia bovis and Plasmodium falciparum: cliff-dwellers of the parasite world. Parasitol Today. 1995;11:100–105. doi: 10.1016/0169-4758(95)80166-9. [DOI] [PubMed] [Google Scholar]
- 2.Aubry P, Renambot J, Teyssier J, Buisson Y, Granic G, Brunetti G, Dano P, Bauer P. Les borrelioses a tiques au Senegal. Dakar Med. 1983;28:413–420. [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochete. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G. Immunobiology of relapsing fever. Contrib Microbiol Immunol. 1987;8:125–137. [PubMed] [Google Scholar]
- 5.Barbour A G. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol. 1990;44:155–71. doi: 10.1146/annurev.mi.44.100190.001103. [DOI] [PubMed] [Google Scholar]
- 6.Barstad P A, Coligan J E, Raum M G, Barbour A G. Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med. 1985;161:1302–1314. doi: 10.1084/jem.161.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borst P, Rudenko G. Antigenic variation in African trypanosomes. Science. 1994;264:1872–1873. doi: 10.1126/science.7516579. [DOI] [PubMed] [Google Scholar]
- 8.Bunikis J, Olsén B, Westman G, Bergström S. Variable serum immunoglobulin responses against different Borrelia burgdorferi sensu lato species in a population at risk for and patients with Lyme disease. J Clin Microbiol. 1995;33:1473–1478. doi: 10.1128/jcm.33.6.1473-1478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffey E M, Eveland W C. Experimental relapsing fever initiated by B. hermsii. II. Sequential appearance of major serotypes in the rat. J Infect Dis. 1967;117:29–34. doi: 10.1093/infdis/117.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Colebunders R, De Serrano P, van Gompel A, Wynants H, Blot K, van Den Enden E, van Den Ende J. Imported relapsing fever in European tourists. Scand J Infect Dis. 1993;25:533–536. doi: 10.3109/00365549309008539. [DOI] [PubMed] [Google Scholar]
- 11.Felsenfeld O. Borrelia: strains, vectors, human and animal borreliosis. Saint Louis, Mo: Warren H. Green Inc.; 1968. [Google Scholar]
- 12.Goubau P F. Relapsing fever. A review. Ann Soc Belge Med Trop. 1984;64:335–364. [PubMed] [Google Scholar]
- 13.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. p. 119. [Google Scholar]
- 14.Holmgren P Å, Stigbrand T. Purification and partial characterization of two genetic variants of placental alkaline phosphatase. Biochem Genet. 1976;14:777–789. doi: 10.1007/BF00485341. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson M, Noppa L, Barbour A G, Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origin. Infect Immun. 1992;60:1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millán J L, Stigbrand T. Antigenic determinants of human placental and testicular placental-like alkaline phosphatase as mapped by monoclonal antibodies. Eur J Biochem. 1983;136:1–7. doi: 10.1111/j.1432-1033.1983.tb07697.x. [DOI] [PubMed] [Google Scholar]
- 17.Mooser H. Erytrozyten-adhäsion und hämagglomeration durch rückfallfieber-spirochäten. Tropenmed Parasitol. 1958;9:93–111. [PubMed] [Google Scholar]
- 18.Newman K, Johnson R. T-cell-independent elimination of Borrelia turicatae. Infect Immun. 1984;45:572–576. doi: 10.1128/iai.45.3.572-576.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoenner H G, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udomsangpetch R, Wahlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]