Key Points
Question
Are immune-related adverse events associated with improved overall survival in patients with locally advanced or metastatic non–small cell lung cancer receiving immune checkpoint inhibitor (ICI) therapy?
Findings
In this cohort study of 803 patients, developing an immune-related adverse event mandating delay or discontinuation of ICI therapy and/or systemic corticosteroids for management of toxic effects was associated with significantly improved median overall survival. This association was not compromised by hospitalization for management of toxic effects of severe immune-related adverse events.
Meaning
These findings suggest that immune-related adverse events are associated with improved overall survival in patients receiving ICI therapy, even in the context of hospitalization for management of toxic effects.
This cohort study investigates the association of immune-related adverse events with overall survival among patients receiving immune checkpoint inhibitors (ICIs) to treat metastatic non–small cell lung cancer (NSCLC).
Abstract
Importance
Immune-related adverse events (irAEs) secondary to immune checkpoint inhibitor (ICI) therapy reportedly improve overall survival (OS) in patients with non–small cell lung cancer (NSCLC). However, studies have been small and the association between irAE severity and OS remains poorly defined.
Objective
To examine the association between irAEs and their severity with OS in patients with locally advanced or metastatic NSCLC receiving ICIs.
Design, Setting, and Participants
This retrospective observational cohort study included patients with NSCLC receiving ICIs between March 1, 2014, and November 30, 2021, with follow-up until March 31, 2023. Data analysis was completed April 26, 2023. The Alberta Immunotherapy Database, a provincial, multicenter cohort, was used to capture data from patients receiving ICIs in Alberta, Canada. Participants included 803 patients 18 years or older who received at least 1 cycle of ICI (alone or with chemotherapy), agnostic to treatment line.
Exposure
Developing an irAE mandating delay or discontinuation of ICI therapy and/or systematic corticosteroids for management of toxic effects (hereinafter referred to as clinically meaningful irAEs).
Main Outcomes and Measures
The primary outcome was association between irAEs and OS according to Kaplan-Meier analysis. Clinically meaningful irAEs were identified. Patients with poor prognosis (survival <3 months) who may have died prior to irAE development were excluded from OS analysis, mitigating immortal time bias. Adjusted Cox proportional hazards regression analyses ascertained variables associated with OS.
Results
Among the 803 patients included in the analysis, the median age of patients with irAEs was 69.7 (IQR, 63.1-75.2) years and the median age of those without irAEs was 67.5 (IQR, 60.4-73.3) years, with comparable sex distribution (139 of 295 men [47.1%] and 156 of 295 women [52.9%] with irAEs vs 254 of 505 men [50.3%] and 251 of 505 women [49.7%] without irAEs). Mitigating immortal time bias (n = 611), irAEs were associated with OS (median OS with irAEs, 23.7 [95% CI, 19.3-29.1] months; median OS without irAEs, 9.8 [95% CI, 8.7-11.4] months; P < .001). No OS difference was associated with treatment in hospital vs as outpatients for an irAE (median OS, 20.8 [95% CI, 11.7-30.6] vs 25.6 [95% CI, 20.1-29.8] months; P = .33). Developing irAEs remained associated with OS in the total cohort after Cox proportional hazards regression with known prognostic characteristics (hazard ratio, 0.53 [95% CI, 0.40-0.70]; P < .001).
Conclusions and Relevance
In this cohort study of 803 patients with locally advanced or metastatic NSCLC receiving ICIs, developing a clinically meaningful irAE was associated with improved OS. This association was not compromised by hospitalization for severe toxic effects. Whether and how ICI therapy resumption after an irAE is associated with OS warrants further study.
Introduction
Globally, lung cancer remains the leading cause of cancer-related death.1 Improvements are being made, however, and overall mortality has declined over the past decade. This decline has largely been driven by treatment advances in non–small cell lung cancer (NSCLC), with immune checkpoint inhibitors (ICIs) improving clinical outcomes.2 Several clinical trials examining ICIs as monotherapy3,4,5,6,7 or in combination with chemotherapy,8,9,10,11 angiogenesis inhibitors,12 and a second ICI agent13,14,15 have demonstrated improved time to event end points and response rates. The treatment landscape in metastatic NSCLC has thus been transformed.
It is well established that although ICIs improve clinical outcomes in NSCLC, they are complicated by immune-related adverse events (irAEs), the incidence of which varies by study setting, patient selection, treatment parameters, and irAE grading.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 There is growing recognition across solid malignant neoplasms that irAEs affect overall survival (OS). In advanced or metastatic NSCLC, the data suggest that developing an irAE is associated with improved OS. However, the ability to form firm conclusions has been limited by data predominantly arising from small studies, focusing largely on nivolumab monotherapy, and with the clinical effect of irAEs on management decisions poorly described.31,32,33,49,50,51 It has also been challenging to form firm conclusions on the association between irAE severity and OS in advanced or metastatic NSCLC, as published results are conflicting.31,32,33,52
We examined a contemporary multicenter cohort of patients with locally advanced or metastatic NSCLC treated with ICI alone or combined with chemotherapy, agnostic to treatment line. We investigated the association of survival outcomes with (1) irAEs mandating delay or discontinuation of ICI therapy and/or systemic corticosteroids for management of toxic effects (hereinafter termed clinically meaningful irAEs) and (2) hospitalization for irAEs.
Methods
Study Design
In this cohort study, we examined data from the Alberta Immunotherapy Database, a contemporary provincial multicenter observational cohort nested in routine clinical practice. Using a standardized template, the Alberta Immunotherapy Database captures information from patients treated with ICI, including baseline demographic characteristics (age and gender), tumor histology, and clinical, laboratory, and imaging data.53 Study approval was obtained from the Health Research Ethics Board of the Alberta Cancer Committee, which waived the need for patient consent given the low-risk, deidentified, retrospective nature of the study. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients were included if they had metastatic NSCLC or unresectable locally advanced disease ineligible for curative intent chemoradiotherapy and/or durvalumab. Additional study inclusion criteria were age 18 years or older and completion of at least 1 cycle of ICI therapy (atezolizumab, nivolumab, or pembrolizumab alone or combined with chemotherapy) of noncurative intent, agnostic to treatment line. Age and gender are included in the baseline demographic data captured through the Alberta Immunotherapy Database, which relies on information collected through our electronic health records system and currently does not include race or ethnicity.
Patients received treatment between March 1, 2014, and November 30, 2021. The date of last follow-up was March 31, 2023. Study investigators assessed the objective imaging response using Response Evaluation Criteria in Solid Tumours, version 1.1, prior to data analysis.54 Where treatment duration data were missing, patients were excluded from survival analysis.
Outcomes of Interest
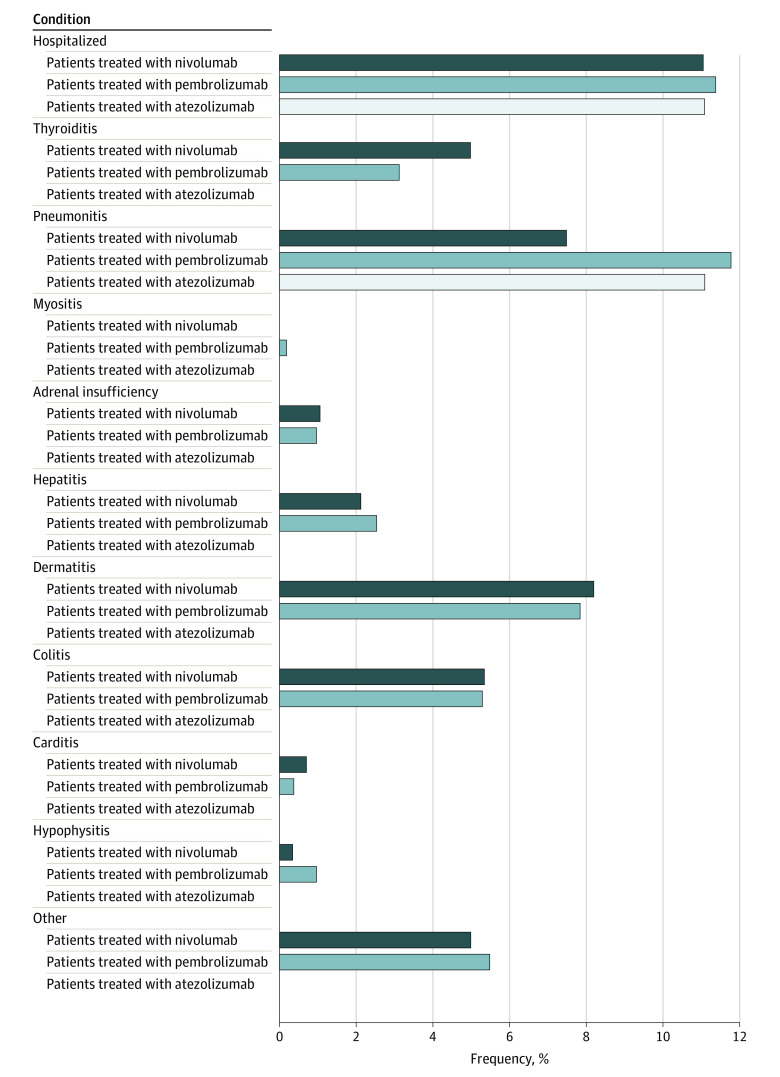
We investigated patients with locally advanced or metastatic NSCLC who met the study inclusion criteria. Clinically meaningful irAEs were identified. As detailed in Figure 1, irAEs were categorized as thyroiditis, pneumonitis, myositis, adrenal insufficiency, hepatitis, dermatitis, colitis, carditis, and hypophysitis. Less commonly occurring irAEs were collectively categorized as “other.” Clinically meaningful irAEs were defined as mandating delay or discontinuation of ICI therapy and/or immunosuppression (oral or intravenous corticosteroids) for management of toxic effects. Patients who did and did not develop irAEs during ICI therapy (alone or combined with chemotherapy) were compared. Our primary end point was the association between developing a clinically meaningful irAE and overall survival (OS). We further examined variables associated with OS, including baseline characteristics (eg, demographic, tumor histology, mutation status, laboratory parameters), irAE development, and hospitalization for irAE management. Our secondary end point was the association between developing a clinically meaningful irAE and time to next treatment (TTNT).
Figure 1. Relative Frequency and Type of Clinically Meaningful Immune-Related Adverse Event by Agent Received.
Electronic medical records were reviewed to identify irAEs during ICI therapy. Only clinically meaningful irAEs as described above were recorded. Where multiple irAEs occurred in the same patient, the most clinically meaningful irAE was included.
Statistical Analysis
Statistical analysis was completed April 26, 2023. Both the χ2 test and the Fisher exact test were used to identify baseline characteristics that were prognostic factors associated with developing an irAE. To mitigate immortal time bias from patients with a poor prognosis who may have died before developing an irAE, a 12-week cutoff was used for OS and TTNT analyses. This cutoff was selected because it corresponds to the median time (3.4 [IQR, 1.4-8.4] months) to irAE development in our study. Thus, patients dying within 12 weeks of ICI therapy initiation were excluded from Kaplan-Meier analysis (compared using a log-rank test). We defined OS as time from ICI therapy initiation to date of death or last censored follow-up. We defined TTNT as time from ICI initiation to starting the next line of systemic therapy, death, or last censored follow-up. A multivariable Cox proportional hazards regression model was applied to OS and TTNT analysis to adjust for significant differences in baseline characteristics, including treatment line and use of ICI alone or combined with chemotherapy.
Where data were missing, the case deletion method was used. All statistical tests were 2 sided. Results were considered statistically significant at P ≤ .05. SAS Cloud of Academics, version 9.4 (SAS Institute Inc) was used for analysis.
Results
Characteristics of Population, Disease, and Treatments Received
A total of 803 patients with locally advanced or metastatic NSCLC treated with ICIs with noncurative intent during the period of interest were included in the analysis. A clinically meaningful irAE was confirmed in 297 patients. The median follow-up was 51.9 (95% CI, 49.4-56.2) months. Patient baseline characteristics are summarized in Table 1. The median age of patients who developed an irAE was 69.7 (IQR, 63.1-75.2) years compared with 67.5 (IQR, 60.4-73.3) years among those who did not. Sex distribution was comparable (139 of 295 men [47.1%] and 156 of 295 women [52.9%] with irAEs vs 254 of 505 men [50.3%] and 251 of 505 women [49.7%] without irAEs). Patients who developed an irAE had a median 10 (IQR, 4-23) ICI cycles compared with 4 (IQR, 2-9) among those who did not. The following baseline characteristics were associated with developing an irAE: 60 years or older (245 of 296 [82.8%] vs 388 of 505 [76.8%]; P = .05), Eastern Cooperative Oncology Group (ECOG) performance status 0 (56 of 294 [19.0%] vs 46 of 500 [9.2%]; P < .001), high expression of programmed cell death ligand 1 (PD-L1) (160 of 240 [66.7%] vs 230 of 404 [56.9%]; P = .05), no metastases to bone (215 of 296 [72.6%] vs 322 of 503 [64.0%]; P = .01), derived neutrophil lymphocyte ratio (DNLR) 3 or less (181 of 260 [69.6%] vs 242 of 452 [53.5%]; P < .001), and levels within reference range for hemoglobin (175 of 265 [66.0%] vs 264 of 457 [57.8%]; P = .03), albumin (189 of 240 [78.8%] vs 263 of 412 [63.8%]; P < .001), and lactate dehydrogenase (152 of 212 [71.7%] vs 217 of 358 [60.6%]; P = .007). Developing an irAE was not associated with patient sex, tumor histology, or the presence of an underlying driver mutation.
Table 1. Baseline Characteristics of Patients With NSCLC With or Without irAEs.
| Characteristics | Patient group (n = 803)a | P valueb | |
|---|---|---|---|
| Without irAEs (n = 506) | With irAEs (n = 297) | ||
| Age, y | |||
| >60 | 388/505 (76.8) | 245/296 (82.8) | .05 |
| ≤60 | 117/505 (23.2) | 51/296 (17.2) | |
| Median (IQR) | 67.5 (60.4-73.3) | 69.7 (63.1-75.2) | NA |
| BMI, median (IQR) | 25.0 (22.0-28.2) | 25.9 (23.0-30.3) | NA |
| Sex | |||
| Men | 254/505 (50.3) | 139/295 (47.1) | .39 |
| Women | 251/505 (49.7) | 156/295 (52.9) | |
| ECOG performance status at ICI therapy startc | |||
| 0 | 46/500 (9.2) | 56/294 (19.0) | <.001 |
| 1 | 273/500 (54.6) | 187/294 (63.6) | |
| 2 | 156/500 (31.2) | 43/294 (14.6) | |
| 3 | 25/500 (5.0) | 8/294 (2.7) | |
| Hemoglobin level | |||
| Within reference range | 264/457 (57.8) | 175/265 (66.0) | .03 |
| Below lower limit of reference | 193/457 (42.2) | 90/265 (34.0) | |
| Lactate dehydrogenase | |||
| Within reference range | 217/358 (60.6) | 152/212 (71.7) | .01 |
| Above upper limit of reference | 141/358 (39.4) | 60/212 (28.3) | |
| Albumin | |||
| Within reference range | 263/412 (63.8) | 189/240 (78.8) | <.001 |
| Below lower limit of reference | 149/412 (36.2) | 51/240 (21.3) | |
| DNLR | |||
| ≤3 | 242/452 (53.5) | 181/260 (69.6) | <.001 |
| >3 | 210/452 (46.5) | 79/260 (30.4) | |
| Histology | |||
| Adenocarcinoma | 362/504 (71.8) | 213/296 (72.0) | .92 |
| Squamous cell carcinoma | 106/504 (21.0) | 64/296 (21.6) | |
| Other | 36/504 (7.1) | 19/296 (6.4) | |
| PD-L1 expression, % | |||
| <1 | 86/404 (21.3) | 42/240 (17.5) | .05 |
| 1-49 | 88/404 (21.8) | 38/240 (15.8) | |
| >50 | 230/404 (56.9) | 160/240 (66.7) | |
| EGFR mutation | |||
| Present | 20/403 (5.0) | 7/227 (3.1) | .26 |
| Absent | 383/403 (95.0) | 220/227 (96.9) | |
| ALK mutation | |||
| Present | 1/387 (0.3) | 0/225 | .45 |
| Absent | 386/387 (99.7) | 225/225 (100) | |
| BRAF mutation | |||
| Present | 0/93 | 2/70 (2.9) | .10 |
| Absent | 93/93 (100) | 68/70 (97.1) | |
| KRAS mutation | |||
| Present | 45/97 (46.4) | 35/70 (50.0) | .65 |
| Absent | 52/97 (53.6) | 35/70 (50.0) | |
| ROS-1 mutation | |||
| Present | 7/43 (16.3) | 2/18 (11.1) | .60 |
| Absent | 36/43 (83.7) | 16/18 (88.9) | |
| PIK3CA mutation | |||
| Present | 1/57 (1.8) | 1/43 (2.3) | .84 |
| Absent | 56/57 (98.2) | 42/43 (97.7) | |
| MET mutation | |||
| Present | 1/1 (100) | 2/4 (50.0) | .36 |
| Absent | 0/1 | 2/4 (50.0) | |
| Metastatic sited | |||
| Lung | 320/504 (63.5) | 130/296 (43.9) | .04 |
| Bone | 181/503 (36.0) | 81/296 (27.4) | .01 |
| Brain | 86/502 (17.1) | 39/296 (13.2) | .14 |
| Liver | 106/503 (21.1) | 50/296 (16.9) | .15 |
| Adrenal | 82/505 (16.2) | 47/296 (15.9) | .89 |
| ICI agent | |||
| Nivolumab | 181/502 (36.1) | 99/296 (33.4) | .18 |
| Pembrolizumab | 313/502 (62.4) | 196/296 (66.2) | |
| Atezolizumab | 8/502 (1.6) | 1/296 (0.3) | |
| ICI treatment line | |||
| First | 260/505 (51.5) | 166/296 (56.1) | .66 |
| Second | 163/505 (32.3) | 89/296 (30.1) | |
| Third | 57/505 (11.3) | 31/296 (10.5) | |
| Fourth or later | 25/505 (5.0) | 10/296 (3.4) | |
| No. of cycles received, median (IQR) | 4 (2-9) | 10 (4-23) | NA |
| Best observed responsee | |||
| Complete response | 3/315 (1.0) | 1/255 (0.4) | <.001 |
| Partial response | 49/315 (15.6) | 103/255 (40.4) | |
| Stable disease | 128/315 (40.6) | 111/255 (43.5) | |
| Progressive disease | 135/315 (42.9) | 40/255 (15.7) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DNLR, derived neutrophil-to-lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; NA, not applicable; PD-L1, programmed cell death ligand 1.
Unless otherwise indicated, data are expressed as No./total No. (%) of patients. Owing to missing data, total numbers may be less than numbers in column headings. Percentages have been rounded and may not total 100.
Two-tailed P ≤ .05 indicates statistical significance.
Lower scores indicate fewer restrictions and capable of more activity.
Patients may have multiple metastatic sites.
Best observed response was categorized per Response Evaluation Criteria in Solid Tumours, version 1.1.
Regarding the ICI agent, 509 patients received pembrolizumab (of whom 62 also received chemotherapy), 280 received nivolumab, and 9 received atezolizumab. Development of an irAE was not associated with the ICI agent or the treatment line. Whether the ICI was used as monotherapy or combined with chemotherapy was also not associated with developing an irAE. A complete or partial response to ICI treatment was associated with developing an irAE (104 of 255 [40.8%] vs 52 of 315 [16.5%]; P < .001).
Characterization of irAEs
A total of 297 patients were diagnosed with a clinically meaningful irAE (37.0%). The median time to developing an irAE was 3.4 (IQR, 1.4-8.4) months. The most common irAEs were pneumonitis (82 [27.6%]), dermatitis (63 [21.2%]), and colitis (42 [14.1%]). Figure 1 further details the types of irAEs and their relative frequency per ICI agent used in our cohort.
No significant differences in the association of irAE relative frequencies were observed between individual ICI agents, or when ICI monotherapy was compared with ICIs combined with chemotherapy. The relative frequency of irAEs was 196 of 509 patients (38.5%) for pembrolizumab, 99 of 280 (35.4%) for nivolumab, 1 of 9 (11.1%) for atezolizumab, and 20 of 62 (32.3%) for ICI combined with chemotherapy.
During the follow-up period, nearly one-third of all clinically meaningful irAEs resulted in hospitalization (90 of 297 [30.3%]), with pneumonitis accounting for over half of hospitalized cases (47 of 90 [52.2%]). All patients with carditis were hospitalized (4 of 4 [100%]), followed in frequency by those with pneumonitis (47 of 82 [57.3%]) and adrenal insufficiency (4 of 8 [50.0%]). No significant differences in the association of hospitalization rates for irAEs were observed between individual ICI agents. The relative frequency of hospitalization for an irAE was 58 of 196 patients (29.6%) receiving pembrolizumab, 31 of 99 (31.3%) receiving nivolumab, and 1 of 1 (100%) receiving atezolizumab.
Survival Outcomes and Time to Next Treatment After irAE Development and Hospitalization
The median OS for the total cohort was 15.7 (95% CI, 13.0-16.6) months. In the 12-week landmark analysis (n = 611), developing a clinically meaningful irAE was associated with a significantly longer median OS compared with those patients who did not (23.7 [95% CI, 19.3-29.1] vs 9.8 [95% CI, 8.7-11.4] months; P < .001) (Figure 2A). For patients with a clinically meaningful irAE, no association with median OS was seen between those hospitalized and those treated as outpatients (20.8 [95% CI, 11.7-30.6] vs 25.6 [95% CI, 20.1-29.8] months; P = .33) (Figure 2B).
Figure 2. Median Overall Survival for Patients With Metastatic Non–Small Cell Lung Cancer .
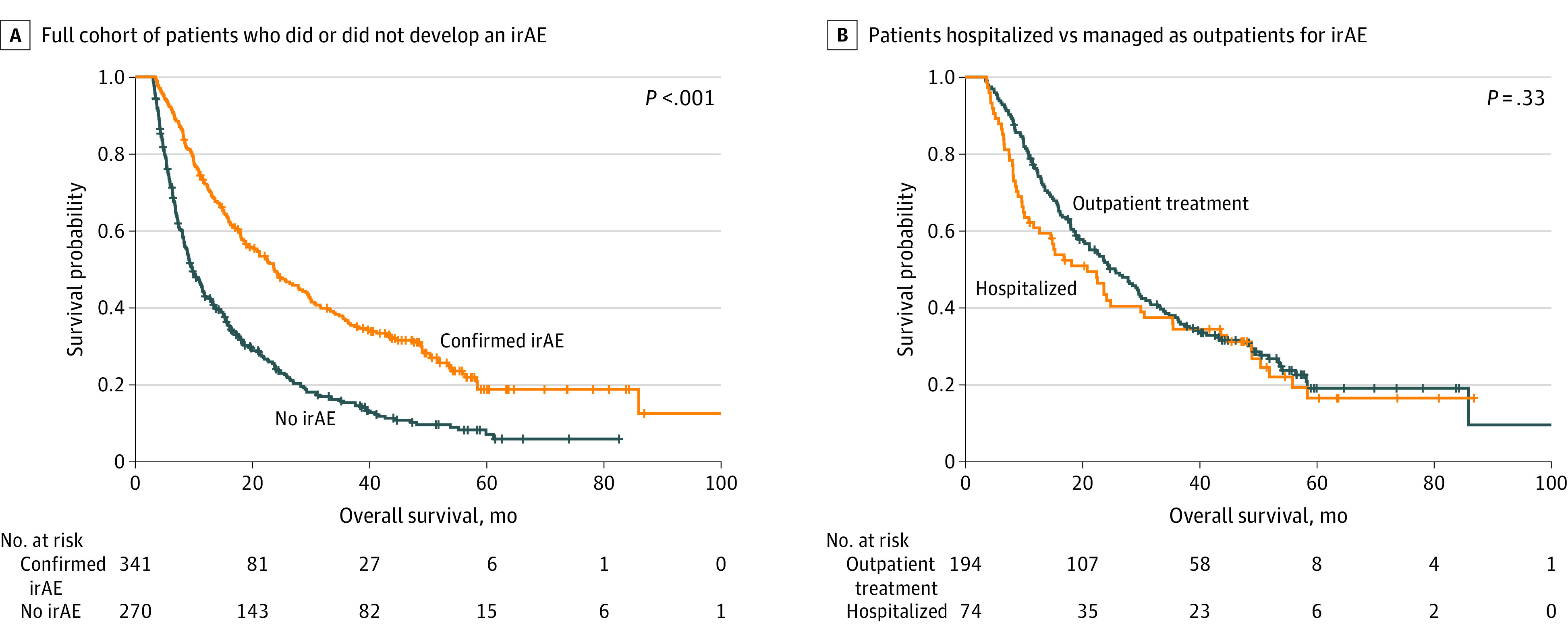
Analysis includes patients who survived to at least 12 weeks. A, Full cohort of patients who did or did not develop an immune-related adverse event (irAE) (median OS, 23.7 [95% CI, 19.3-29.1] vs 9.8 months [95% CI, 8.7-11.4 months]; P < .001). B, Patients hospitalized compared with those treated as outpatients for irAEs (median OS, 20.8 [95% CI, 11.7-30.6] vs 25.6 [95% CI, 20.1-29.8] months; P = .33).
Time to next treatment was the secondary outcome of interest. In the 12-week landmark analysis (n = 611), developing a clinically meaningful irAE was associated with a significantly longer median TTNT compared with those patients who did not (18.0 [95% CI, 15.6-22.9] vs 7.3 [95% CI, 6.6-8.4] months; P < .001) (Figure 3A). For patients with a clinically meaningful irAE, no association in median TTNT was seen between those hospitalized and those treated as outpatients (15.3 [95% CI, 9.6-24.8] vs 15.9 [95% CI, 13.6-18.0] months; P = .67) (Figure 3B).
Figure 3. Median Time to Next Treatment for Patients With Metastatic Non–Small Cell Lung Cancer .
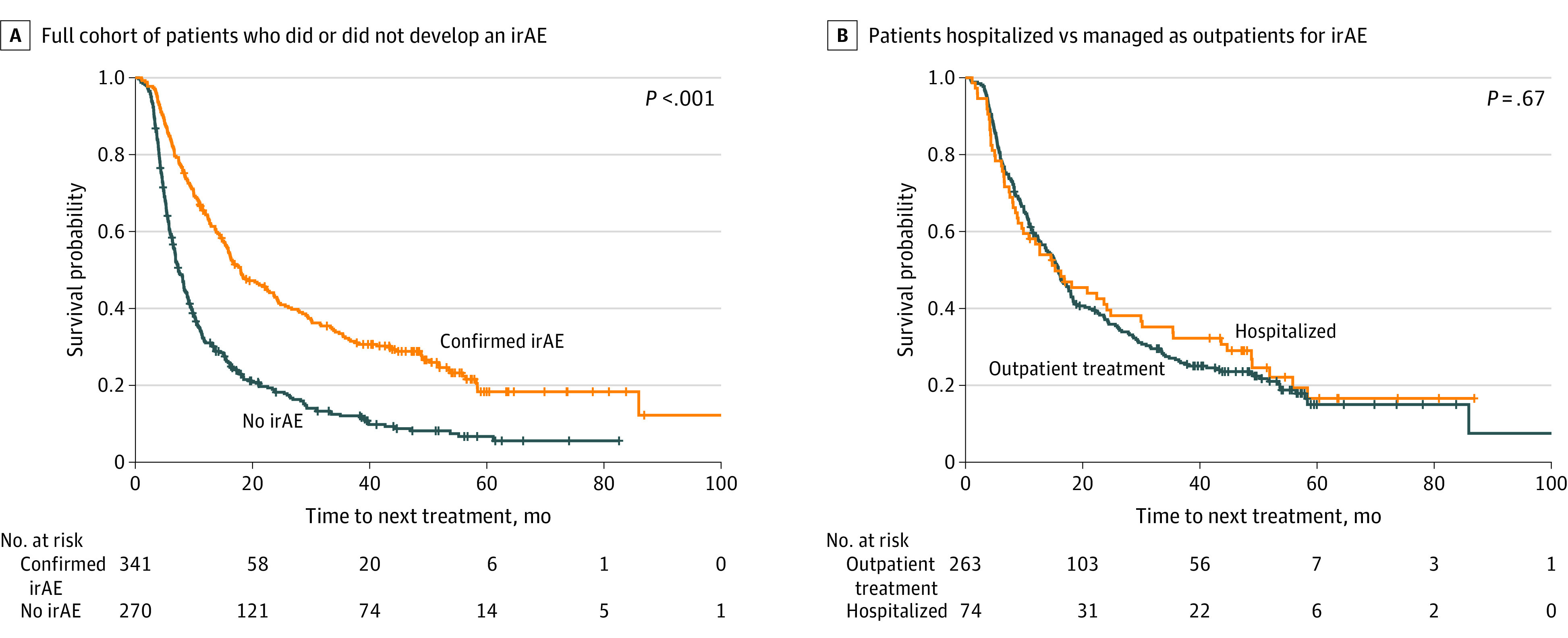
Analysis includes patients who survived to at least 12 weeks. A, Full cohort of patients who did or did not develop an immune-related adverse event (irAE) (median time to next treatment, 18.0 [95% CI, 15.6-22.9] vs 7.3 [95% CI, 6.6-8.4] months; P < .001). B, Patients hospitalized compared with those treated as outpatients for irAEs (median time to next treatment, 15.3 [95% CI, 9.6-24.8] vs 15.9 [95% CI, 13.6-18.0] months; P = .67).
Variables Associated With OS by Cox Proportional Hazards Regression Analysis
To further evaluate the association between potential prognostic factors and OS among patients surviving to 12 weeks, Cox proportional hazards regression analysis was performed (Table 2). On multivariable analysis adjusted for all established prognostic factors (ECOG performance status, PD-L1 expression, organ sites of metastatic disease, DNLR, irAE development, and levels of hemoglobin, albumin, and LDH), developing an irAE remained independently associated with longer OS (hazard ratio, 0.53 [95% CI, 0.40-0.70]; P < .001).
Table 2. Multivariable Adjusted Cox Proportional Hazards Regression Analysis of Factors Associated With Overall Survival.
| Factor | Multivariable analysis | |
|---|---|---|
| HR (95% CI) | P valuea | |
| irAE | 0.53 (0.40-0.70) | <.001 |
| Treatment line ICI | ||
| Second (vs first) | 1.27 (0.93-1.74) | .14 |
| Third (vs first) | 1.33 (0.80-2.21) | .27 |
| Fourth (vs first) | 1.77 (0.54-5.81) | .35 |
| ECOG performance status at ICI therapy start | ||
| 1 (vs 0) | 1.79 (1.24-2.60) | .002 |
| 2 (vs 0) | 2.71 (1.68-4.35) | <.001 |
| 3 (vs 0) | 4.18 (1.67-10.47) | .002 |
| LDH level above reference range | 1.23 (0.91-1.67) | .17 |
| Albumin level below reference range | 1.07 (0.78-1.46) | .68 |
| Hemoglobin below reference range | 0.82 (0.62-1.08) | .15 |
| DNLR >3 | 1.19 (0.89-1.58) | .24 |
| Histology | ||
| Squamous cell carcinoma (vs adenocarcinoma) | 1.31 (0.95-1.81) | .10 |
| Other (vs adenocarcinoma) | 1.44 (0.84-2.48) | .19 |
| PD-L1 expression | ||
| 1%-49% (vs <1%) | 1.06 (0.71-1.58) | .78 |
| >50% (vs <1%) | 0.64 (0.45-0.91) | .01 |
| Metastatic site | ||
| Bone | 1.31 (0.97-1.77) | .08 |
| Brain | 1.28 (0.88-1.86) | .20 |
| Liver | 1.30 (0.91-1.86) | .15 |
Abbreviations: DNLR, derived neutrophil-to-lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; LDH, lactate dehydrogenase; PD-L1, programmed cell death ligand 1.
Two-tailed P ≤ .05 indicated statistical significance.
Discussion
To our knowledge, this study represents the largest contemporary clinical dataset evaluating the association between irAEs and survival in locally advanced or metastatic NSCLC, agnostic to ICI agent, delivery as monotherapy or combined with chemotherapy, and treatment line. In our multicenter, retrospective population-based cohort, developing a clinically meaningful irAE was associated with longer OS in locally advanced or metastatic NSCLC. Association with improved OS continued among patients requiring hospitalization for irAE management.
It is clear ICI therapy is commonly complicated by irAEs. However, the mechanisms underlying irAE development are less clear, with 4 processes tentatively described: (1) increasing T-cell activity against antigens shared by tumor cells and healthy tissue; (2) accumulating levels of preexisting host autoantibodies; (3) rising levels of inflammatory cytokines; and (4) direct binding of anti–CTLA-4 antibodies with CTLA-4 found on healthy tissue, intensifying complement-mediated inflammation. Ultimately irAE development suggests the host immune system has been activated.55 Whether and how this translates to activation of antitumoral host immunity, with clinical outcomes subsequently affected, remains uncertain across solid malignant neoplasms. Cytokines, particularly interleukin 17, have been postulated to play a role in the antitumoral effects of ICIs, but further research is needed.56,57,58 Certainly, in our large cohort, irAE development was associated with improved OS. This supports previously published studies in NSCLC,31,32,33,49,50,51 recognizing they have largely been small, mainly examined nivolumab monotherapy, and inconsistently defined clinical impact of irAEs.
The role of irAE severity in the survival of patients with NSCLC remains contentious. This may reflect differences in toxicity grading, immunosuppressant use, and hospitalization thresholds. Like other published experiences,32,33 we found that irAE severity (reflected by management of toxic effects as an outpatient or hospitalized inpatient) did not compromise the survival gains seen with irAE development. However, this finding is not universally supported. A single study52 demonstrated only high-grade irAEs improve OS in NSCLC. In contrast, pooled data from phase 3 randomized clinical trials evaluating atezolizumab31 reported only low-grade irAEs are associated with improved survival in advanced NSCLC. Patients with high-grade irAEs had inferior survival except at a 12-month landmark analysis, where median OS was longer than among patients without irAEs.31 Improved OS being limited to patients with low-grade irAEs has been corroborated by several other groups34,35,59 and may be explained by the life-threatening potential of high-grade events. High-grade irAEs can result in death, albeit rarely. They may also require (permanent) treatment cessation and/or high-dose corticosteroids that can suppress antitumor efficacy of ICIs.31,55
The median time to developing an irAE in our study population was 3.4 months, longer than that typically cited in the NSCLC literature.24,36,37,38 However, we only recorded clinically meaningful irAEs per our definition, and studies have shown low-grade events develop earlier than high-grade events.34,39 The incidence of reported irAEs in NSCLC varies widely, ranging from 24.0% to 70.5%. This reflects heterogeneity including in study setting (observational vs randomized clinical trial), patient characteristics (cancer stage, PD-L1 status, geographical location, race and ethnicity), treatment parameters (ICI agent used either alone or combined with chemotherapy, treatment line), and irAE grading.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 In our cohort, the incidence of clinically meaningful irAEs was 37.0%. Nearly one-third of all clinically meaningful irAEs resulted in hospitalization (30.3%) for the management of toxic effects. Given our definition, grade 1 toxic effects, topically treated dermatitis, and mild hypothyroidism would not have been captured. Our incidence rates are thus best compared with irAEs higher than grade 2 and hospitalization rates with irAEs higher than grade 3.
Why irAEs occur in some patients but not others remains poorly understood, but a variety of predictive factors in NSCLC have been described. Demographic characteristics (age, sex, race, body mass index, and ECOG), concomitant medications (corticosteroids, proton pump inhibitors, and antibiotics), peripheral laboratory markers (hemoglobin, albumin, C-reactive protein, and others), tumor characteristics (histology, stage, PD-L1 expression, driver mutation status, and disease burden), and treatment-related factors (treatment line, response, ICI agent, concurrent chemotherapy, time to starting ICI, cumulative dose, and cumulative cycles of ICI) are inconsistently reported as predictive factors associated with irAE development.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,48,60,61,62 In our cohort, we identified several baseline patient characteristics associated with irAE development: 60 years or older, ECOG performance status 0, high expression of PD-L1, absence of bone metastases, DNLR of 3 or less, and levels of hemoglobin, albumin, and LDH within reference range. Other than response to ICI therapy, irAE development was not associated with treatment-related characteristics (ICI agent, ICI alone or in combination with chemotherapy, and treatment line) in our study population.
Strengths and Limitations
Our study has several strengths. The sample size of 803 patients represents, to our knowledge, the largest contemporary clinical dataset examining irAEs and survival in locally advanced or metastatic NSCLC. As we used a 12-week landmark analysis, immortal time bias from patients with poor prognosis dying earlier than median onset of irAE development was minimized. Our multivariable Cox proportional hazards regression analysis adjusted for all established irAE predictive factors. Therefore, we can trust the association seen between irAE development and improved survival.
This study also has some limitations. How we defined an irAE differs from other studies, and focusing on clinically meaningful events may have influenced our results. We did not specifically capture grade 5 irAEs and thus could not differentiate death due to toxic effects compared with underlying disease in our 12-week landmark analysis. Although some patients may have died of grade 5 irAEs within 12 weeks, irAEs are very rarely fatal, so most excluded patients likely died of NSCLC.63 Where multiple irAEs occurred in the same patient, only the most clinically meaningful irAE was recorded. Thus, the association between the number of (clinically meaningful) irAEs and OS was not evaluated. We also did not evaluate the association between ICI rechallenge and OS. The existing evidence suggests rechallenge increases risk of further irAEs but, when compared with permanent ICI discontinuation, is not associated with survival.64 There are also limitations associated with retrospective studies, including unaccounted biases in patient selection, irAE capture and management, missing data, and incorporation of subjective assessments, including ECOG.
Conclusions
In our large cohort of patients with locally advanced or metastatic NSCLC, developing a clinically meaningful irAE was associated with improved survival. This association was not compromised by hospitalization for management of severe toxic effects. Several patient baseline characteristics were associated with irAE development in our cohort, including age, ECOG performance status, sites of metastasis, PD-L1 expression, and laboratory parameters. Response to ICI therapy was the only treatment-related characteristic associated with irAE development.
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. doi: 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 4.Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(18)(suppl):LBA4. doi: 10.1200/JCO.2018.36.18_suppl.LBA4 [DOI] [Google Scholar]
- 5.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N Engl J Med. 2020;383(14):1328-1339. doi: 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 6.Jassem J, de Marinis F, Giaccone G, et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1–selected NSCLC. J Thorac Oncol. 2021;16(11):1872-1882. doi: 10.1016/j.jtho.2021.06.019 [DOI] [PubMed] [Google Scholar]
- 7.Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non–small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592-604. doi: 10.1016/S0140-6736(21)00228-2 [DOI] [PubMed] [Google Scholar]
- 8.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Luft A, Vicente D, et al. ; KEYNOTE-407 Investigators . Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040-2051. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 10.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non–small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. doi: 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 11.Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657-1669. doi: 10.1016/j.jtho.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group . Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 13.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi: 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non–small cell lung cancer (NSCLC): two-year update from CheckMate 9LA. J Clin Oncol. 2021;39(15)(suppl):9000. doi: 10.1200/JCO.2021.39.15_suppl.9000 [DOI] [Google Scholar]
- 15.Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. doi: 10.1016/S1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 16.Pavan A, Calvetti L, Dal Maso A, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non–small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist. 2019;24(8):1128-1136. doi: 10.1634/theoncologist.2018-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conde-Estévez D, Monge-Escartín I, Ríos-Hoyo A, et al. Prognostic factors and effect on survival of immune-related adverse events in patients with non–small-cell lung cancer treated with immune checkpoint blockage. J Chemother. 2021;33(1):32-39. doi: 10.1080/1120009X.2020.1849488 [DOI] [PubMed] [Google Scholar]
- 18.Sonehara K, Tateishi K, Araki T, et al. Predictive factors correlated with the development of immune-related adverse events in patients with non–small cell lung cancer treated with immune checkpoint inhibitors. Cancer Manag Res. 2022;14:427-435. doi: 10.2147/CMAR.S347852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noguchi S, Suminaga K, Kaki T, et al. Correlation of immune-related adverse events and effects of pembrolizumab monotherapy in patients with non–small cell lung cancer. Lung Cancer (Auckl). 2020;11:53-57. doi: 10.2147/LCTT.S254146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riudavets M, Mosquera J, Garcia-Campelo R, et al. Immune-related adverse events and corticosteroid use for cancer-related symptoms are associated with efficacy in patients with non-small cell lung cancer receiving anti–PD-(L)1 blockade agents. Front Oncol. 2020;10:1677. doi: 10.3389/fonc.2020.01677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen DH, Wei L, Bertino EM, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non–small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e893-e900. doi: 10.1016/j.cllc.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teraoka S, Fujimoto D, Morimoto T, et al. Early immune-related adverse events and association with outcome in advanced non–small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12(12):1798-1805. doi: 10.1016/j.jtho.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Soon YY, Aminkeng F, et al. Risk factors for immune-related adverse events from anti–PD-1 or anti–PD-L1 treatment in an Asian cohort of nonsmall cell lung cancer patients. Int J Cancer. 2022;150(4):636-644. doi: 10.1002/ijc.33822 [DOI] [PubMed] [Google Scholar]
- 24.Toi Y, Sugawara S, Kawashima Y, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non–small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358-1365. doi: 10.1634/theoncologist.2017-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naqash AR, Ricciuti B, Owen DH, et al. Outcomes associated with immune-related adverse events in metastatic non–small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother. 2020;69(7):1177-1187. doi: 10.1007/s00262-020-02536-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortijo-Cascajares S, Cercós-Lletí AC, Ortiz-Pérez S, Caro-Teller JM, Ferrari-Piquero JM. Analysis of immune-mediated reactions in patients with non-small cell lung cancer treated with nivolumab and its association with effectiveness. J Oncol Pharm Pract. 2023;29(2):290-298. doi: 10.1177/10781552211067429 [DOI] [PubMed] [Google Scholar]
- 27.Cathcart-Rake EJ, Sangaralingham LR, Henk HJ, Shah ND, Riaz IB, Mansfield AS. A population-based study of immunotherapy-related toxicities in lung cancer. Clin Lung Cancer. 2020;21(5):421-427.e2. doi: 10.1016/j.cllc.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortellini A, Friedlaender A, Banna GL, et al. Immune-related adverse events of pembrolizumab in a large real-world cohort of patients with NSCLC with a PD-L1 expression ≥50% and their relationship with clinical outcomes. Clin Lung Cancer. 2020;21(6):498-508.e2. doi: 10.1016/j.cllc.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 29.Sugisaka J, Toi Y, Taguri M, et al. Relationship between programmed cell death protein ligand 1 expression and immune-related adverse events in non–small-cell lung cancer patients treated with pembrolizumab. JMA J. 2020;3(1):58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non–small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813-1822. doi: 10.1007/s00262-020-02585-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Socinski MA, Jotte RM, Cappuzzo F, et al. Association of immune-related adverse events with efficacy of atezolizumab in patients with non–small cell lung cancer: pooled analyses of the phase 3 IMpower130, IMpower132, and IMpower150 randomized clinical trials. JAMA Oncol. 2023;9(4):527-535. doi: 10.1001/jamaoncol.2022.7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grangeon M, Tomasini P, Chaleat S, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non–small-cell lung cancer. Clin Lung Cancer. 2019;20(3):201-207. doi: 10.1016/j.cllc.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 33.Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237-247.e1. doi: 10.1016/j.cllc.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Gu X, Wang L, et al. The prognostic impact of mild and severe immune-related adverse events in non–small cell lung cancer treated with immune checkpoint inhibitors: a multicenter retrospective study. Cancer Immunol Immunother. 2022;71(7):1693-1703. doi: 10.1007/s00262-021-03115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morimoto K, Yamada T, Takumi C, et al. Immune-related adverse events are associated with clinical benefit in patients with non–small-cell lung cancer treated with immunotherapy plus chemotherapy: a retrospective study. Front Oncol. 2021;11:630136. doi: 10.3389/fonc.2021.630136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non–small cell lung cancer. JAMA Oncol. 2020;6(12):1952-1956. doi: 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurokawa K, Mitsuishi Y, Shimada N, et al. Association between the efficacy and immune-related adverse events of pembrolizumab and chemotherapy in non–small cell lung cancer patients: a retrospective study. BMC Cancer. 2022;22(1):1047. doi: 10.1186/s12885-022-10133-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non–small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71-74. doi: 10.1016/j.lungcan.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Nie J, Dai L, et al. Immune-related adverse events and their association with the effectiveness of PD-1/PD-L1 inhibitors in non–small cell lung cancer: a real-world study from China. Front Oncol. 2021;11:607531. doi: 10.3389/fonc.2021.607531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sayer MR, Mambetsariev I, Lu KH, et al. Predicting survival of NSCLC patients treated with immune checkpoint inhibitors: impact and timing of immune-related adverse events and prior tyrosine kinase inhibitor therapy. Front Oncol. 2023;13:1064169. doi: 10.3389/fonc.2023.1064169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouhlel L, Doyen J, Chamorey E, et al. Occurrence and number of immune-related adverse events are independently associated with survival in advanced non–small-cell lung cancer treated by nivolumab. Bull Cancer. 2020;107(9):946-958. doi: 10.1016/j.bulcan.2020.04.019 [DOI] [PubMed] [Google Scholar]
- 42.Sonehara K, Tateishi K, Araki T, et al. The role of immune-related adverse events in prognosis and efficacy prediction for patients with non–small cell lung cancer treated with immunotherapy: a retrospective clinical analysis. Oncology. 2021;99(5):271-279. doi: 10.1159/000511999 [DOI] [PubMed] [Google Scholar]
- 43.Valencia Soto CM, Villacañas Palomares MV, Garcia-Avello Fernández-Cueto A, et al. Predictive value of immune-related adverse events during pembrolizumab treatment in non-small cell lung cancer. Eur J Hosp Pharm. Published online April 5, 2022. doi: 10.1136/ejhpharm-2021-003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non–small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479-485. doi: 10.1007/s00432-018-2805-3 [DOI] [PubMed] [Google Scholar]
- 45.Huang L, Li L, Zhou Y, et al. Clinical characteristics correlate with outcomes of immunotherapy in advanced non–small cell lung cancer. J Cancer. 2020;11(24):7137-7145. doi: 10.7150/jca.49213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ksienski D, Wai ES, Croteau N, et al. Efficacy of nivolumab and pembrolizumab in patients with advanced non–small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clin Lung Cancer. 2019;20(1):e97-e106. doi: 10.1016/j.cllc.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Xiong Z, He W, et al. Proximal shift of colorectal cancer with increasing age in different ethnicities. Cancer Manag Res. 2018;10:2663-2673. doi: 10.2147/CMAR.S166548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serino M, Freitas C, Martins M, et al. Predictors of immune-related adverse events and outcomes in patients with NSCLC treated with immune-checkpoint inhibitors. Pulmonology. Published online April 9, 2022. doi: 10.1016/j.pulmoe.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 49.Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 50.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. 2018;4(3):374-378. doi: 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Chen H, Tang L, et al. Association of immune-related adverse events and efficacy in advanced non–small-cell lung cancer: a systematic review and meta-analysis. Immunotherapy. 2023;15(3):209-220. doi: 10.2217/imt-2022-0028 [DOI] [PubMed] [Google Scholar]
- 52.Guezour N, Soussi G, Brosseau S, et al. Grade 3-4 immune-related adverse events induced by immune checkpoint inhibitors in non–small-cell lung cancer (NSCLC) patients are correlated with better outcome: a real-life observational study. Cancers (Basel). 2022;14(16):3878. doi: 10.3390/cancers14163878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyers DE, Stukalin I, Vallerand IA, et al. The Lung Immune Prognostic Index discriminates survival outcomes in patients with solid tumors treated with immune checkpoint inhibitors. Cancers (Basel). 2019;11(11):1713. doi: 10.3390/cancers11111713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 55.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 56.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114(2):357-359. doi: 10.1182/blood-2008-09-177360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esfahani K, Miller WH Jr. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med. 2017;376(20):1989-1991. doi: 10.1056/NEJMc1703047 [DOI] [PubMed] [Google Scholar]
- 58.Gérard A, Doyen J, Cremoni M, et al. Baseline and early functional immune response is associated with subsequent clinical outcomes of PD-1 inhibition therapy in metastatic melanoma patients. J Immunother Cancer. 2021;9(6):e002512. doi: 10.1136/jitc-2021-002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, Wang W, Yuan Q, et al. Correlation between immune-related adverse events and the efficacy of PD-1/PD-L1 inhibitors in the treatment of non–small cell lung cancer: systematic review and meta-analysis. Cancer Chemother Pharmacol. 2022;89(1):1-9. doi: 10.1007/s00280-021-04375-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Y, Li D, Wu M, Yang Y, Shen M, Chen K. Peripheral absolute eosinophil count identifies the risk of serious immune-related adverse events in non–small cell lung cancer. Front Oncol. 2022;12:1004663. doi: 10.3389/fonc.2022.1004663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daniello L, Elshiaty M, Bozorgmehr F, et al. Therapeutic and prognostic implications of immune-related adverse events in advanced non–small-cell lung cancer. Front Oncol. 2021;11:703893. doi: 10.3389/fonc.2021.703893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi Y, Liu X, Liu J, et al. Correlations between peripheral blood biomarkers and clinical outcomes in advanced non–small cell lung cancer patients who received immunotherapy-based treatments. Transl Lung Cancer Res. 2021;10(12):4477-4493. doi: 10.21037/tlcr-21-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu X, Zhang X, Yao T, Zhang Y, Zhang Y. Fatal adverse events associated with immune checkpoint inhibitors in non–small cell lung cancer: a systematic review and meta-analysis. Front Med (Lausanne). 2021;8:627089. doi: 10.3389/fmed.2021.627089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo M, VanderWalde AM, Yu X, Vidal GA, Tian GG. Immune checkpoint inhibitor rechallenge safety and efficacy in stage IV non–small cell lung cancer patients after immune-related adverse events. Clin Lung Cancer. 2022;23(8):686-693. doi: 10.1016/j.cllc.2022.07.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement